Gray matter heterotopia
Gray matter heterotopia also called grey matter heterotopia, is a relatively common malformation of cortical development, where cortical cells (grey matter) are present in inappropriate locations in the brain, due to interruption in their migration to their correct location in the cerebral cortex 1. Gray matter heterotopia is characterized by the presence of small or extended portions of grey matter in areas of the brain or spinal cord that should physiologically be occupied by white matter. Heterotopia means “out of place”. Grey matter heterotopia is a condition in which nerve cells (neurons) do not migrate properly during the early development of the fetal brain, from about the 6th week to the 24th week of pregnancy. In normal brain development, neurons form in the periventricular region, located around fluid-filled cavities (ventricles) near the center of the brain. The neurons then migrate outward to form the exterior of the brain (cerebral cortex) in six onion-like layers. Grey matter heterotopias are believed to be due interruption of the normal migration of neurons from the periventricular telencephalic germinal matrix to the cortex and may be due to either genetic abnormalities or infection/trauma 2. Gray matter heterotopia may be unilateral or bilateral, singular or multiple, separate or contiguous. The most common form is bilateral periventricular nodular heterotopia (grey matter heterotopia lining the lateral ventricles). Grey matter heterotopia can also occur in subcortical white matter (subcortical nodular heterotopia). Grey matter heterotopia can co-occur with other structural abnormalities. Unilateral periventricular nodular heterotopia can co-occur with subcortical nodular heterotopia and with polymicrogyria. Bilateral periventricular nodular heterotopia can co-occur with cerebellar vermis hypoplasia and hypoplasia of the corpus callosum. Hippocampal sclerosis can co-occur with heterotopia.
Gray matter heterotopias can be divided macroscopically into:
- Nodular heterotopias
- Subependymal heterotopia: most common
- Subcortical heterotopia
- Diffuse heterotopias
- Band heterotopia also known as double cortex heterotopia and X-linked lissencephaly (chromosome Xq22.3)
- Lissencephaly: types 1 and 2
- Laminar heterotopia (a problematic term variably defined)
Gray matter heterotopia patients most commonly present with partial seizures in the second decade of life, frequently resistant to medication. Additionally, and depending on the extent, children may demonstrate developmental delay or intellectual disability, although symptoms range from absent to profound 3. Clinical investigations so far conducted failed to identify the epileptogenic focus in grey matter heterotopia patients, but it is proposed that reactive changes in peri‐ectopic areas are instrumental 4. This precludes surgery and urges investigations of the pathophysiological changes leading to hyperexcitability in gray matter heterotopia.
Certain types of gray matter heterotopia have also been found to be associated with metabolic disorders such as neonatal adrenoleukodystrophy, connective tissue disorders such as Ehlers-Danlos syndrome and a number of congenital central nervous system malformations and these associated anomalies will also affect symptomatology. Associated anomalies include 3:
- agenesis of the corpus callosum
- encephalocoeles
- myelomeningocoeles
- pachygyria
- schizencephaly
- polymicrogyria
- Chiari 2 malformation
- basilar cephalocoeles.
Gray matter heterotopia may constitute about 15% of cortical developmental malformations and may be found in about 2% of patients with epilepsy 5. In a study of 16 patients who were being evaluated for intractable epilepsy, all of them were found to have gray matter heterotopia – both laminar and nodular forms were discovered 6. The prevalence of gray matter heterotopia in the general population is not known 5. There are conflicting reports as to whether the phenomenon has been found in normal persons 7. In another study investigating the prevalence of gray matter heterotopia using magnetic resonance imaging (MRI) scans for diagnostic purposes, none of the 75 normal persons, who made up the control group of the study, had gray matter heterotopia 7.
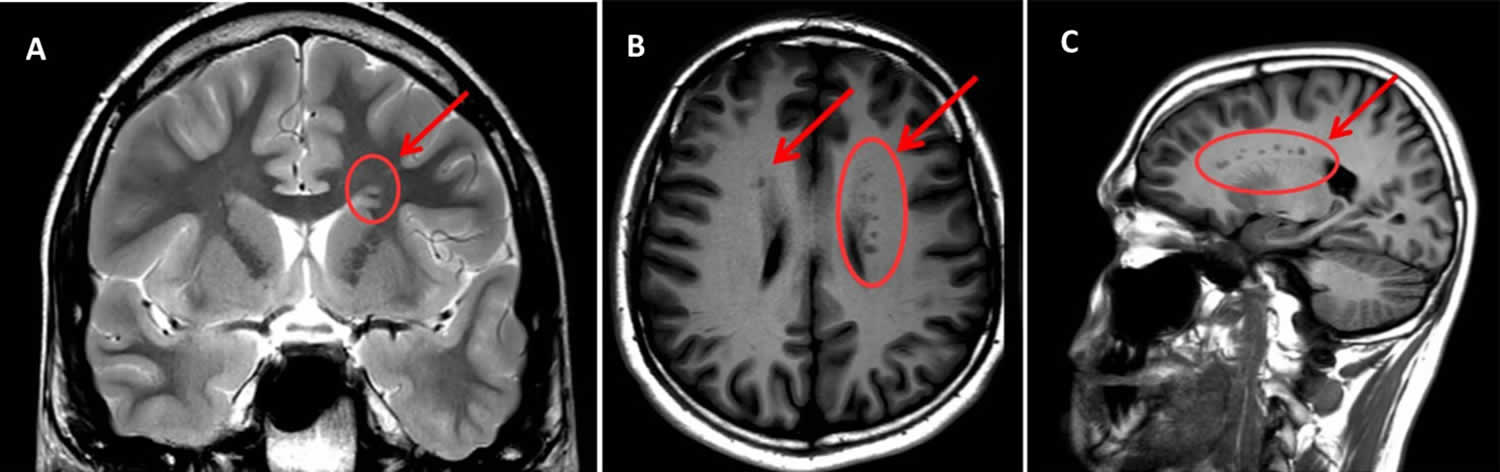
Figure 1. Gray matter heterotopia
Footnote: Magnetic resonance imaging (MRI) scan sections of the patient’s brain reporting the presence of gray matter heterotopia (indicated by arrows and circles). (A) T2W – coronal section; (B) T1W- assial section; (C) T1W – sagittal section. Arrows indicate areas of subependimal alterated signal (bilateral and asymmetrical periventricular nodular heterotopia—with heterotopic grey matter stretching all along the ventricular walls, maximum diameter = 5 mm). Note that the shade of grey is the same as that of the cortical grey matter (the same signal intensity), which confirms that it is grey matter—the pathognomonic finding in Gray matter heterotopia.
[Source 1 ]Periventricular gray matter heterotopia
Periventricular gray matter heterotopia also called periventricular nodular heterotopia, subependymal gray matter heterotopia or familial nodular heterotopia, some neurons fail to migrate to their proper position and form clumps around the ventricles and it is characterized by the presence of ectopic neuronal nodules lining the walls of the lateral ventricles 4. Theses nodules can readily be detected with magnetic resonance imaging (MRI). There is a wide spectrum of anatomic and clinical presentations of periventricular nodular heterotopia, ranging from asymptomatic small unilateral or bilateral nodules to extensive agglomerates of heterotopia lining the lateral ventricles in patients with intractable epilepsy and intellectual disabilities 8. There is also a range of associated cerebral and systemic malformations. To date, 13 distinct periventricular nodular heterotopia disorders have been described but for the majority of them the etiology remains unknown 9.
Patients affected by periventricular nodular heterotopia have been classified on the basis of MRI features 10. Five different groups of periventricular nodular heterotopia have been distinguished 11: (1) bilateral and symmetrical; (2) bilateral single-noduled; (3) bilateral and asymmetrical; (4) unilateral; and (5) unilateral with extension to neocortex.
Periventricular gray matter heterotopia is the most common form of grey matter heterotopia in adulthood. Periventricular gray matter heterotopia usually becomes evident when seizures (epilepsy) first appear, often during the teenage years 12. The nodules around the ventricles are then typically discovered when magnetic resonance imaging (MRI) studies are done. Affected individuals usually have normal intelligence, although some have mild intellectual disability. Difficulty with reading and spelling (dyslexia) and movement problems have been reported in some people with periventricular gray matter heterotopia.
Less commonly, individuals with periventricular gray matter heterotopia may have other features including more severe brain malformations, small head size (microcephaly), developmental delays, recurrent infections, blood vessel abnormalities, stomach problems, or lung disease 13. Periventricular gray matter heterotopia may also occur in association with other conditions such as Ehlers-Danlos syndrome, which results in extremely flexible joints, skin that stretches easily, and fragile blood vessels.
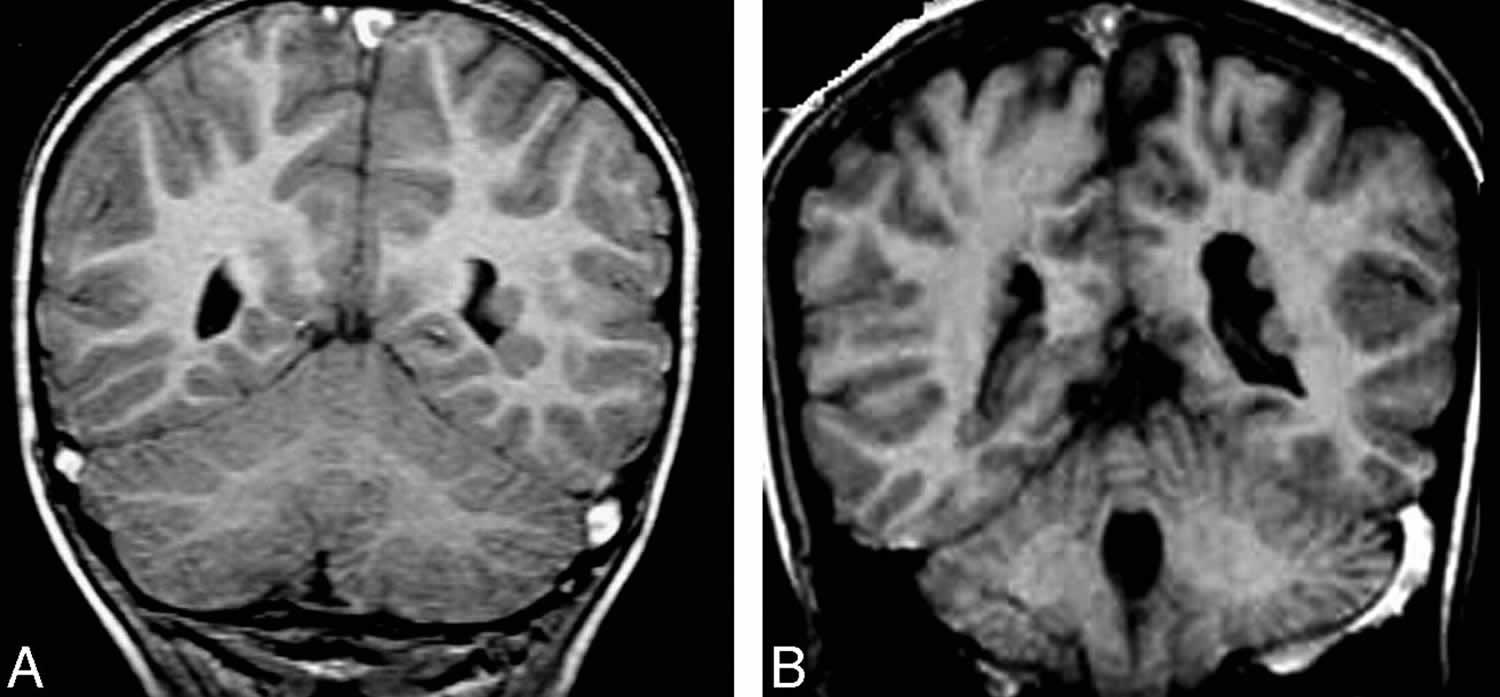
Figure 2. Periventricular gray matter heterotopia
Footnote: A and B, Coronal T1-weighted images show a few small periventricular nodules, isointense to the gray matter, along the lateral ventricular wall.
[Source 3 ]Periventricular gray matter heterotopia causes
In most cases, periventricular gray matter heterotopia is caused by mutations in the FLNA gene. This gene provides instructions for producing the protein filamin A, which helps build the network of protein filaments (cytoskeleton) that gives structure to cells and allows them to change shape and move. Certain mutations in the FLNA gene result in an impaired FLNA protein that cannot perform this function, disrupting the normal migration patterns of neurons during brain development.
Periventricular gray matter heterotopia can also be caused by mutations in the ARFGEF2 gene. This gene provides instructions for making a protein that is involved in the movement (trafficking) of small sac-like structures (vesicles) within the cell. Vesicle trafficking is important in controlling the migration of neurons during the development of the brain. Mutations in the ARFGEF2 gene may disrupt this function, which could result in the abnormal neuronal migration seen in periventricular gray matter heterotopia.
Researchers believe that mutations in the FLNA or ARFGEF2 genes may also result in weakening of the attachments (adhesion) between cells that form the lining of the ventricles. A weakened ventricular lining could allow some neurons to form clumps around the ventricles while others migrate normally to the exterior of the brain, as seen in periventricular gray matter heterotopia.
In a few cases, periventricular gray matter heterotopia has been associated with abnormalities in chromosome 5. In each case, the affected individual had extra genetic material caused by an abnormal duplication of part of this chromosome. It is not known how this duplicated genetic material results in the signs and symptoms of periventricular gray matter heterotopia.
Periventricular gray matter heterotopia inheritance pattern
Periventricular gray matter heterotopia can have different inheritance patterns. When this condition is caused by mutations in the FLNA gene, it is inherited in an X-linked dominant pattern.
A condition is considered X-linked if the mutated gene that causes the disorder is located on the X chromosome, one of the two sex chromosomes. The inheritance is dominant if one copy of the altered gene in each cell is sufficient to cause the condition. A characteristic of X-linked inheritance is that fathers cannot pass X-linked traits to their sons.
In X-linked periventricular gray matter heterotopia, males experience much more severe symptoms of the disorder than females, and in most cases die before birth.
In about 50 percent of cases of X-linked periventricular gray matter heterotopia, an affected person inherits the mutation from a mother who is also affected. Other cases may result from new mutations in the gene. These cases occur in people with no history of the disorder in their family.
Periventricular gray matter heterotopia caused by mutations in the ARFGEF2 gene is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. Individuals with periventricular gray matter heterotopia in whom ARFGEF2 gene mutations have been identified have a severe form of the disorder, including microcephaly, severe developmental delay, and seizures beginning in infancy. Most often, the parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but do not show signs and symptoms of the condition.
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Subcortical band heterotopia
Subcortical band heterotopia also known as double cortex syndrome or subcortical laminar heterotopia, is a condition in which nerve cells (neurons) do not move (migrate) to their proper locations in the fetal brain during early development. Most affected individuals are female 14. Normally, the neurons that make up the outer surface of the brain (cerebral cortex) are distributed in a well-organized and multi-layered way. In people with subcortical band heterotopia, some neurons that should be part of the cerebral cortex do not reach it. These neurons stop their migration process in areas of the brain where they are not supposed to be and form band-like clusters of tissue. Since these bands are located beneath the cerebral cortex, they are said to be subcortical. In most cases, the bands are symmetric, which means they occur in the same places on the right and left sides of the brain.
The abnormal brain development causes neurological problems in people with subcortical band heterotopia. The signs and symptoms of the condition depend on the size of the bands and the lack of development of the cerebral cortex. The signs and symptoms can vary from severe intellectual disability and seizures that begin early in life and affect both sides of the brain (generalized seizures) to normal intelligence with seizures occurring later in life and affecting only one side of the brain (focal seizures). Some affected individuals also have weak muscle tone (hypotonia), loss of fine motor skills such as using utensils, or behavioral problems. Subcortical band heterotopia is typically found when brain imaging is done following the onset of seizures, usually in adolescence or early adulthood.
More than 200 cases of subcortical band heterotopia have been reported in the scientific literature 14.
Subcortical band heterotopia causes
Mutations in the DCX or PAFAH1B1 gene cause subcortical band heterotopia. Both genes provide instructions for making proteins that are involved in the movement of neurons to their proper locations in the developing brain, a process called neuronal migration. Neuronal migration is essential for normal brain development and function.
Most individuals with subcortical band heterotopia have DCX gene mutations. These mutations impair the protein’s function or alter the protein’s structure or stability. PAFAH1B1 gene mutations are less common. Mutations in this gene reduce the protein’s function.
Altered structure or function of the proteins produced by the DCX or PAFAH1B1 gene impairs important interactions that are needed for neuronal migration. Without proper neuronal migration, neurons in the developing brain can be misplaced, forming abnormal bands of tissue beneath the cerebral cortex.
Subcortical band heterotopia inheritance pattern
The inheritance pattern of subcortical band heterotopia depends on its genetic cause.
When subcortical band heterotopia is caused by mutations in the DCX gene, it is inherited in an X-linked pattern. The DCX gene is located on the X chromosome, which is one of the two sex chromosomes. In females, who have two copies of the X chromosome, one altered copy of the gene in each cell can lead to the condition, sometimes with less severe symptoms than affected males. In males, who have only one X chromosome, a mutation in the only copy of the gene in each cell usually causes a more severe condition called isolated lissencephaly sequence (ILS). Most males with subcortical band heterotopia have a DCX gene mutation that is not inherited and is present in only some of the body’s cells, a situation known as mosaicism. A characteristic of X-linked inheritance is that fathers cannot pass X-linked traits to their sons.
When subcortical band heterotopia is caused by a PAFAH1B1 gene mutation, it is generally not inherited but arises from a mutation in the body’s cells that occurs after conception, which leads to mosaicism. This alteration is called a somatic mutation. PAFAH1B1 gene mutations that occur in all of the body’s cells (germline mutations) usually cause isolated lissencephaly sequence.
Gray matter heterotopia causes
Although the exact causes for grey matter heterotopia are not yet fully elucidated, studies demonstrate that genetic and epigenetic factors may contribute to the cause of gray matter heterotopia. The first include mutations of the X-linked FNLA gene (Xq28), coding for the Filamin, an actin-binding protein that crosslinks actin filaments and links actin filaments to membrane glycoproteins 1. Glycoproteins are involved in remodeling of the cytoskeleton and effect changes in cell shape and migration. Rare mutations or microdeletion of autosomal genes 15 may result further in syndromic disorders. Among epigenetic factors, perinatal stressors, such as hypoxic-ischemic events occurring during migration of neuroblasts, at 7-16 weeks of fetal development, may play a crucial role in the etiopathogenesis of gray matter heterotopia 16.
Genetic causes
Mutations in the FLNA gene, on Xq28, were found in 100% of families with X‐linked bilateral periventricular nodular heterotopia and in 26% of sporadic patients with periventricular nodular heterotopia 8. The FLNA gene encodes a very large (280 kD) cytoplasmic protein that binds to actin and a wide range of cytoplasmic signaling proteins involved in cell adhesion and migration 17. In the brain, FLNA is expressed at high levels in prenatal and neonatal stages and these levels diminish during adolescence to reach moderate expression in adulthood 18. FLNA is also expressed in pyramidal neurons in the neocortex where it localizes in somatodendritic compartments 19. Heterozygous females have normal to borderline intelligence and epilepsy 20. A few living male patients with bilateral periventricular nodular heterotopia due to FLNA mutations have been reported; however, most male fetuses are not viable 21. Coagulopathy and cardiovascular abnormalities have been observed in some patients 21.
Other genes can cause periventricular nodular heterotopia
Neuroblasts proliferate in the germinal matrix between 7 and 8 weeks of gestation. Migration take place from 8 to 26 weeks gestation, and is maximal between 8 and 16 weeks 22.
A rare recessive form caused by mutations in the ARFGEF2 gene, on 20q13.1, has been reported in two consanguineous families 23. ARFGEF2 encodes a protein called BIG2 (brefeldin A‐inhibited guanine nucleotide exchange factor 2 protein) localized along the Golgi and recycling endosomes 22. BIG2 is thought to carry out ARF‐dependent vesicle trafficking along these subcellular compartments 24. Recently, it has been reported that biallelic mutations in genes encoding the receptor‐ligand cadherin pair DCHS1 and FAT4 lead to a multisystem disorder that includes periventricular nodular heterotopia 25. Periventricular nodular heterotopia has also been observed in patients with chromosomal rearrangements, such as deletions of the 5q14.3‐15 9 or 6q27 26 regions. For the latter, a de novo missense mutation in the C6orf70 gene, mapping the minimal critical deleted 6q27 region, was identified in a sporadic patient with developmental delay, epilepsy, and periventricular nodular heterotopia 26.
The mechanism involved in the genesis of periventricular nodular heterotopia remains elusive although it is widely accepted that it results from a defective migration of neurons which remain blocked in the ventricular zone (VZ)–subventricular zone (SVZ). Although two FLNA knockout mice strains have been developed, progress has been hindered by the fact that none of them showed the presence of ectopic nodules 27. In contrast, in utero knockdown of FLNA expression has succeeded in reproducing a periventricular nodular heterotopia phenotype in rat similar to the one observed in human patients and represents an appropriate model to investigate pathogenetic mechanisms underlying periventricular nodular heterotopia associated to mutations in FLNA gene 28. In this model, periventricular nodular heterotopia is associated with an impairment of radial glial integrity in the ventricular zone. Thus, the phenotype would associate a cell‐autonomous migration defect as largely proposed and an alteration of RGCs and radial glial scaffold. Interestingly, Carabalona et al 28 demonstrated a similar disruption of radial glial cells in human PH brains from a 35‐week fetus and a 3‐month‐old child, harboring distinct FLNA mutations. Other studies have shown that mice mutant for MEKK4, a MAP kinase that regulates the CSBP2 and JNK‐MAPK pathways, showed a periventricular nodular heterotopia phenotype 29. Interestingly, phosphorylation of FLNA at serine 2152 depends on MEKK4 signaling and phosphorylation at this site regulates FLNA localization at the cell membrane. Mice with mutations in the Napa gene, which encodes for the vesicle trafficking protein αSnap, also replicate the periventricular nodular heterotopia phenotype 30. The αSnap protein is involved in SNAP receptor (SNARE)‐mediated vesicle fusion thus suggesting that it plays a role in vesicle trafficking in periventricular nodular heterotopia formation. Finally, it has been shown that deletion of the RhoGTPase Cdc42 gene in mouse disrupts the neuroependymal lining, local adherens junctions, and proliferation of basal progenitors, which may lead to neuronal heterotopia 31. Overall, as the majority of periventricular nodular heterotopia genes are required for some forms of vesicle trafficking, it has been proposed that an overriding defect in the vesicle trafficking machinery may contribute to periventricular nodular heterotopia formation 32.
Experimental periventricular nodular heterotopia can also be modeled in rodents using various nongenetic manipulations, including prenatal exposure to ionizing radiations, methylazoxymethanol (MAM), carmustine (1‐3‐bis‐chloroethylnitrosourea or BCNU) in rats, or postnatal exposure to ibotenate in hamsters. These teratogens produce damages within the proliferative neuroepithelium, affecting both the genesis of newborn neurons and their migration along the radial glial scaffold 33. As a consequence, animals generated with these treatments invariably have microcephaly and altered cortical structure and exhibit various types and combination of gray matter heterotopia, including periventricular nodular heterotopia, layer 1 ectopia, intracortical and subcortical heterotopia, and intrahippocampal heterotopia.
Gray matter heterotopia symptoms
The clinical presentation of grey matter heterotopia depends on the extent of the cortical malformation and whether the cortical malformation is unilateral or bilateral, or associated with other brain malformations or congenital malformations elsewhere in the body. Gray matter heterotopia cause a variety of symptoms mainly epilepsy or seizures that are seen in 80-90% of cases and the seizures are frequently resistant to medication. Absence seizures are the most common 34. Epilepsy usually develops during the second decade of life 34.
Along with epilepsy, further manifestations of gray matter heterotopia are represented by mild intellectual disability, developmental delay and motor impairments and dyslexia involving impairments in reading ability, processing speed, and executive functioning 35. Developmental delay is common but neurological deficits are usually only seen in more severe cases of gray matter heterotopia with mild motor, sensory and visual defects being reported 6. Dyspraxia (difficulties with coordinated movement) and problems with fine motor skills have also been found 36. Neurological deficits and developmental delay, in particular, depend on the type and severity of the gray matter heterotopia as well as the location of the lesions. Symptoms range from absent to profound, the more severe are seen in those with bilateral or extensive heterotopia or other brain abnormalities. Patients with severe unilateral subcortical gray matter heterotopia often present with hemiplegia, yet it is not uncommon for a person with gray matter heterotopia to present without neurological deficits or developmental delay 7.
There is a female predominance in cases of bilateral periventricular nodular heterotopia. Moreover, because of the potentially disruptive impact of heterotopic neuronal migration, a number of neurodevelopmental disorders, such as autism 37 and ADHD 38, are associated with gray matter heterotopia. Less frequently, reports of depression 39, anxiety 35, and schizophrenia 40 have been found in comorbidity with grey matter heterotopia.
List of principal neurological and psychiatric conditions associated with gray matter heterotopia 41, 42, 43:
- Principal neurological conditions associated with gray matter heterotopia
- Epilepsy
- Partial, complex, atypical absence
- Drop attacks
- Motor skill loss
- Principal psychiatric conditions associated with gray matter heterotopia
- Delirium
- Schizophrenia
- Schizoaffective disorder
- Unspecified schizophrenia spectrum disorder
- Bipolar disorder
- Major depressive disorder
- Autism spectrum disorder
- Attention-deficit hyperactivity disorder (ADHD)
- Intellectual disability
- Language disorder
- Autism spectrum disorder
- Somatic symptom disorder
The most common neuropsychiatric clinical picture is that of intellectual disability, which ranges from mild to severe in nature, even though many patients with gray matter heterotopia present with normal intellectual functioning 44.
- Neuropsychiatric symptoms of grey matter heterotopia commonly presenting during childhood 45:
- Dyslexia and other learning difficulties
- Deficits in:
- Reading fluency
- Spatial orientation
- Planning
- Constructional abilities
- Attention
- Processing speed
- Adaptive skills
- Other areas of executive functioning
- Problems in areas of:
- Social skills
- Leadership
- Functional communication
- Hyperactivity
- Rage
- Aggression
- Violence
- Lack of remorse for problematic behaviour
- Mood symptoms
- Mood lability
- Depressive symptoms
- Low mood
- Low self-esteem
- Insomnia and nightmares
- Social withdrawal
- Hopelessness
- Deliberate self-harm
- Suicidal ideation and suicide attempts
- Manic symptoms
- Elevated mood
- Racing thoughts
- Decreased need for sleep
- Distractibility
- Irritability
- Agitation
- Impulsivity
- Sexual inhibition and sexually inappropriate behavior
- Financial and social indiscretions
- Psychotic symptoms:
- Thought form disorder
- Auditory and visual hallucinations
- Paranoid ideation
- Referential thinking
- Vague delusions (more so than systematised delusions)
- Inappropriate affect
- Other, less common symptoms:
- Anxiety
- Panic attacks
- Obsessive-compulsive symptoms
- Phobias
- Disorientation and ‘confusion’
Neuropsychiatric symptoms are less well researched and possibly underrecognised 36. The development of all these symptoms can be independent of epilepsy because they were found to be present before the onset of seizures in many children with gray matter heterotopia 44.
Gray matter heterotopia diagnosis
Gray matter heterotopia affected individuals typically have seizures and/or developmental delay or behavioral problems. Detection of heterotopia generally occurs when a patient receives brain imaging—usually an MRI or CT scan—to diagnose seizures that are resistant to medication. Gray matter heterotopia cannot always be seen on a CT scan 5, MRI is the modality of choice in assessing heterotopic grey matter due to MRI’s ability to accurately discriminate between grey and white matter 46.
Gray matter heterotopia treatment
Treatment of epilepsy generally follows principles for a seizure disorder caused by a known structural brain abnormality. Carbamezipine is most often used, because most people with gray matter heterotopia have focal seizures. However, the choice of antiepileptic drug may be selected based on side effects, tolerability, and efficacy 12.
It is recommended that people with the X-linked form of periventricular nodular heterotopia have studies evaluating the carotid artery and an abdominal ultrasound, due to the risk for aortic or carotid dissection or other vascular anomalies 12.
Treatment may also include surgery for removal of the lesion and more recently, laser ablation guided with magnetic resonance 47.
In individuals with subcortical band heterotopia, antiepileptic drugs for epileptic seizures; deep brain stimulation may improve the seizure disorder; special feeding strategies in newborns with poor suck; physical therapy to promote mobility and prevent contractures; special adaptive chairs or positioners as needed; occupational therapy to improve fine motor skills and oral-motor control; participation in speech therapy, educational training, and enrichment programs 48.
Gray matter heterotopia prognosis
In general, gray matter heterotopia is fixed in both its occurrence and symptoms; that is, once symptoms occur, it does not tend to progress. Varying results from surgical resection of the affected area have been reported. Although such surgery cannot reverse developmental disabilities, it may provide full or partial relief from seizures.
References- Curci A, Rampino A. Grey Matter Heterotopia and Criminal Responsibility in a Case of Personal Injury Defense. Front Psychiatry. 2020;11:261. Published 2020 Apr 1. doi:10.3389/fpsyt.2020.00261 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7139628
- Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain 2012;135:1348–1369.
- Abdel Razek AA, Kandell AY, Elsorogy LG, Elmongy A, Basett AA. Disorders of cortical formation: MR imaging features. AJNR Am J Neuroradiol. 2009;30(1):4-11. doi:10.3174/ajnr.A1223 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7051699
- Watrin, F., Manent, J.‐B., Cardoso, C. and Represa, A. (2015), Causes and Consequences of Gray Matter Heterotopia. CNS Neurosci Ther, 21: 112-122. doi:10.1111/cns.12322
- Pridmore S, McInerney G, Rybak M, Archer S. Psychotic disorder NOS with heterotopia. J Pychiatr Intensive Care. 2007;2(2):118–21. 10.1017/S1742646407000386
- Stafford Johnson DB, Brennan P, Dwyer AJO, Toland J. Grey matter heterotopia: An unusual association of intractable epilepsy. Ir J Med Sci. 1997;166(3):135–8. 10.1007/BF02943590
- Nopoulos PC, Swayze VW, Flaum M, Andreasen NC. Incidence of ectopic gray matter in patients with schizophrenia and healthy control subjects studied with MRI. J Neuropsychiatry Clin Neurosci. 1998;10:351–3. 10.1176/jnp.10.3.351
- Parrini E, Ramazzotti A, Dobyns WB, et al. Periventricular heterotopia: phenotypic heterogeneity and correlation with Filamin A mutations. Brain 2006;129:1892–1906.
- Cardoso C, Boys A, Parrini E, et al. Periventricular heterotopia, mental retardation, and epilepsy associated with 5q14.3‐q15 deletion. Neurology 2009;72:784–792.
- Classification system for malformations of cortical development: Update 2001. A. J. Barkovich, R. I. Kuzniecky, G. D. Jackson, R. Guerrini and W. B. Dobyns. Neurology 2001;57;2168-2178 https://geiselmed.dartmouth.edu/radiology/wp-content/uploads/sites/47/2019/04/Classification-System-for-Malformations-of-Cortical-Development-2001-Update-Barkovich.pdf
- Battaglia, G., Chiapparini, L., Franceschetti, S., Freri, E., Tassi, L., Bassanini, S., Villani, F., Spreafico, R., D’Incerti, L. and Granata, T. (2006), Periventricular Nodular Heterotopia: Classification, Epileptic History, and Genesis of Epileptic Discharges. Epilepsia, 47: 86-97. doi:10.1111/j.1528-1167.2006.00374.x
- Chen MH, Walsh CA. FLNA-Related Periventricular Nodular Heterotopia. 2002 Oct 8 [Updated 2015 Sep 17]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1213
- Periventricular heterotopia. https://ghr.nlm.nih.gov/condition/periventricular-heterotopia
- Subcortical band heterotopia. https://ghr.nlm.nih.gov/condition/subcortical-band-heterotopia
- Rezazadeh A, Bercovici E, Kiehl TR, Chow EW, Krings T, Bassett AS, et al. Periventricular nodular heterotopia in 22q11. 2 deletion and frontal lobe migration. Ann Clin Trans Neurol (2018) 5(11):1314–22. 10.1002/acn3.641
- Badrfam R, Zandifar A, Abhari SAA. Subependymal Heterotopia With Psychosis and Imperforate Anus in a Female Patent. Case Rep Clin Pract (2018) 3(4):113–7. 10.16966/2471-4925.172
- Robertson SP. Filamin A: phenotypic diversity. Curr Opin Genet Dev 2005;15:301–307.
- Stossel TP, Condeelis J, Cooley L, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol 2001;2:138–145.
- Noam Y, Phan L, McClelland S, et al. Distinct regional and subcellular localization of the actin‐binding protein filamin A in the mature rat brain. J Comp Neurol 2012;520:3013–3034.
- Fox JW, Lamperti ED, Eksioglu YZ, et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron 1998;21:1315–1325.
- Sheen VL, Dixon PH, Fox JW, et al. Mutations in the X‐linked filamin 1 gene cause periventricular nodular heterotopia in males as well as in females. Hum Mol Genet 2001;10:1775–1783.
- Charych EI, Yu W, Miralles CP, et al. The brefeldin A‐inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem 2004;90:173–189.
- Sheen VL, Ganesh VS, Topcu M, et al. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat Genet 2004;36:69–76.
- Shin H, Shinotsuka C, Nakayama K. Expression of BIG2 and analysis of its function in mammalian cells. Methods Enzymol 2005;404:206–215.
- Cappello S, Gray MJ, Badouel C, et al. Mutations in genes encoding the cadherin receptor‐ligand pair DCHS1 and FAT4 disrupt cerebral cortical development. Nat Genet 2013;45:1300–1308.
- Conti V, Carabalona A, Pallesi‐Pocachard E, et al. Periventricular heterotopia in 6q terminal deletion syndrome: role of the C6orf70 gene. Brain 2013;136:3378–3394.
- Feng Y, Chen MH, Moskowitz IP, et al. Filamin A (FLNA) is required for cell‐cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci U S A 2006;103:19836–19841.
- Carabalona A, Beguin S, Pallesi‐Pocachard E, et al. A glial origin for periventricular nodular heterotopia caused by impaired expression of Filamin‐A. Hum Mol Genet 2012;21:1004–1017.
- Sarkisian MR, Bartley CM, Chi H, et al. MEKK4 signaling regulates filamin expression and neuronal migration. Neuron 2006;52:789–801.
- Chae TH, Kim S, Marz KE, Hanson PI, Walsh CA. The hyh mutation uncovers roles for alpha Snap in apical protein localization and control of neural cell fate. Nat Genet 2004;36:264–270.
- Cappello S, Attardo A, Wu X, et al. The Rho‐GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci 2006;9:1099–1107.
- Sheen VL. Periventricular heterotopia: shuttling of proteins through vesicles and actin in cortical development and disease. Scientifica (Cairo) 2012;2012:480129.
- Roper SN, Abraham LA, Streit WJ. Exposure to in utero irradiation produces disruption of radial glia in rats. Dev Neurosci 1997;19:521–528.
- Barkovich AJ, Kuzniecky RI. Gray matter heterotopia. Neurology. 2000;55:1603–8. 10.1212/WNL.55.11.1603
- Felker MV, Walker LM, Sokol DK, Edwards-Brown M, Chang BS. Early cognitive and behavioral problems in children with nodular heterotopia. Epilepsy Behav (2011) 22(3):523–6. 10.1016/j.yebeh.2011.08.010
- Fry AE, Kerr MP, Gibbon F, et al. . Neuropsychiatric disease in patients with periventricular heterotopia. J Neuropsychiatry Clin Neurosci. 2013;25:26–31. 10.1176/appi.neuropsych.11110336
- Blackmon K, Ben-Avi E, Wang X, Pardoe HR, Di Martino A, Halgren E, et al. Periventricular white matter abnormalities and restricted repetitive behavior in autism spectrum disorder. NeuroImage Clin (2016) 10:36–45. 10.1016/j.nicl.2015.10.017
- Nopoulos P, Berg S, Castellenos FX, Delgado A, Andreasen NC, Rapoport JL. Developmental brain anomalies in children with attention-deficit hyperactivity disorder. J Child Neurol (2000) 15(2):102–8. 10.1177/088307380001500208
- Maruyama Y, Onishi H, Miura T, Kosaka K. A case of depressive disorder with neuronal heterotopia. Psychiatry Clin Neurosci (1998) 52(3):361–2. 10.1046/j.1440-1819.1998.00385.x
- Nopouulos PC, Flaum M, Andreasen NC, Swayze VW. Gray matter heterotopias in schizophrenia. Psychiatry Res (1995) 61(1):11–4. 10.1016/0925-4927(95)02573-G
- Fry AE, Kerr MP, Gibbon F, Turnpenny PD, Hamandi K, Stoodley N, et al. Neuropsychiatric disease in patients with periventricular heterotopia. J Neuropsychiatry Clin Neurosci (2013) 25(1):26–31. 10.1176/appi.neuropsych.11110336
- Lippi G. Neuropsychiatric symptoms and diagnosis of grey matter heterotopia: a case-based reflection. South Afr J Psychiatry (2017) 23(1):1–6. 10.4102/sajpsychiatry.v23i0.923
- Watrin F, Manent JB, Cardoso C, Represa A. Causes and consequences of gray matter heterotopia. CNS Neurosci Ther (2015) 21(2):112–22. 10.1111/cns.12322
- Felker MV, Walker LM, Sokol DK, Edwards-Brown M, Chang BS. Early cognitive and behavioral problems in children with nodular heterotopia. Epilepsy Behav. 2011;22(3):523–6. 10.1016/j.yebeh.2011.08.010
- Lippi G. Neuropsychiatric symptoms and diagnosis of grey matter heterotopia: A case-based reflection. S Afr J Psychiatr. 2017;23:923. Published 2017 Mar 28. doi:10.4102/sajpsychiatry.v23i0.923 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6138094
- Bourgeois JA, Nisenbaum J, Drexler KG, Dobbins KM, Hall MJ. A case of subcortical grey matter heterotopia presenting as bipolar disorder. Compr Psychiatry. 1992;33(6):407–10. 10.1016/0010-440X(92)90063-V
- Esquenazi Y, Kalamangalam GP, Slater JD, Knowlton RC, Friedman E, Morris SA, Shetty A, Gowda A, Tandon N. Stereotactic laser ablation of epileptogenic periventricular nodular heterotopia. Epilepsy Res. 2014 Mar;108(3):547-54. https://doi.org/10.1016/j.eplepsyres.2014.01.009
- Hehr U, Uyanik G, Aigner L, et al. DCX-Related Disorders. 2007 Oct 19 [Updated 2019 Feb 7]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1185