Haldane effect
Haldane effect is a property of hemoglobin where increase in the concentration of carbon dioxide (CO2) will displace oxygen from hemoglobin (Hb) and binding of oxygen with hemoglobin in turn will displace carbon dioxide from blood. In other words, deoxygenation of the blood increases its ability to carry carbon dioxide. Conversely, oxygenated blood has a reduced capacity for carbon dioxide. This is a consequence of the fact that reduced (deoxygenated) hemoglobin is a better proton acceptor than the oxygenated form. Haldane effect was first described by the British physician John Scott Haldane.
In red blood cells, the enzyme carbonic anhydrase catalyzes the conversion of dissolved carbon dioxide to carbonic acid, which rapidly dissociates to bicarbonate and a free proton:
- CO2 + H2O -> H2CO3 -> H+ + HCO3-
By Le Chatelier’s principle, anything that stabilizes the proton produced will cause the reaction to shift to the right, thus the enhanced affinity of deoxyhemoglobin for protons enhances synthesis of bicarbonate and accordingly increases capacity of deoxygenated blood for carbon dioxide. The majority of carbon dioxide in the blood is in the form of bicarbonate. Only a very small amount is actually dissolved as carbon dioxide, and the remaining amount of carbon dioxide is bound to hemoglobin.
In addition to enhancing removal of carbon dioxide from oxygen-consuming tissues, the Haldane effect promotes dissociation of carbon dioxide from hemoglobin in the presence of oxygen. In the oxygen-rich capillaries of the lung, this property causes the displacement of carbon dioxide to plasma as venous blood enters the alveolus and is vital for alveolar gas exchange.
The general equation for the Haldane Effect is:
- H+ + HbO2 <-> H+.Hb + O2
In patients with lung disease, lungs may not be able to increase alveolar ventilation in the face of increased amounts of dissolved CO2.
This partially explains the observation that some patients with emphysema might have an increase in PaCO2 (arterial dissolved carbon dioxide) following administration of supplemental oxygen.
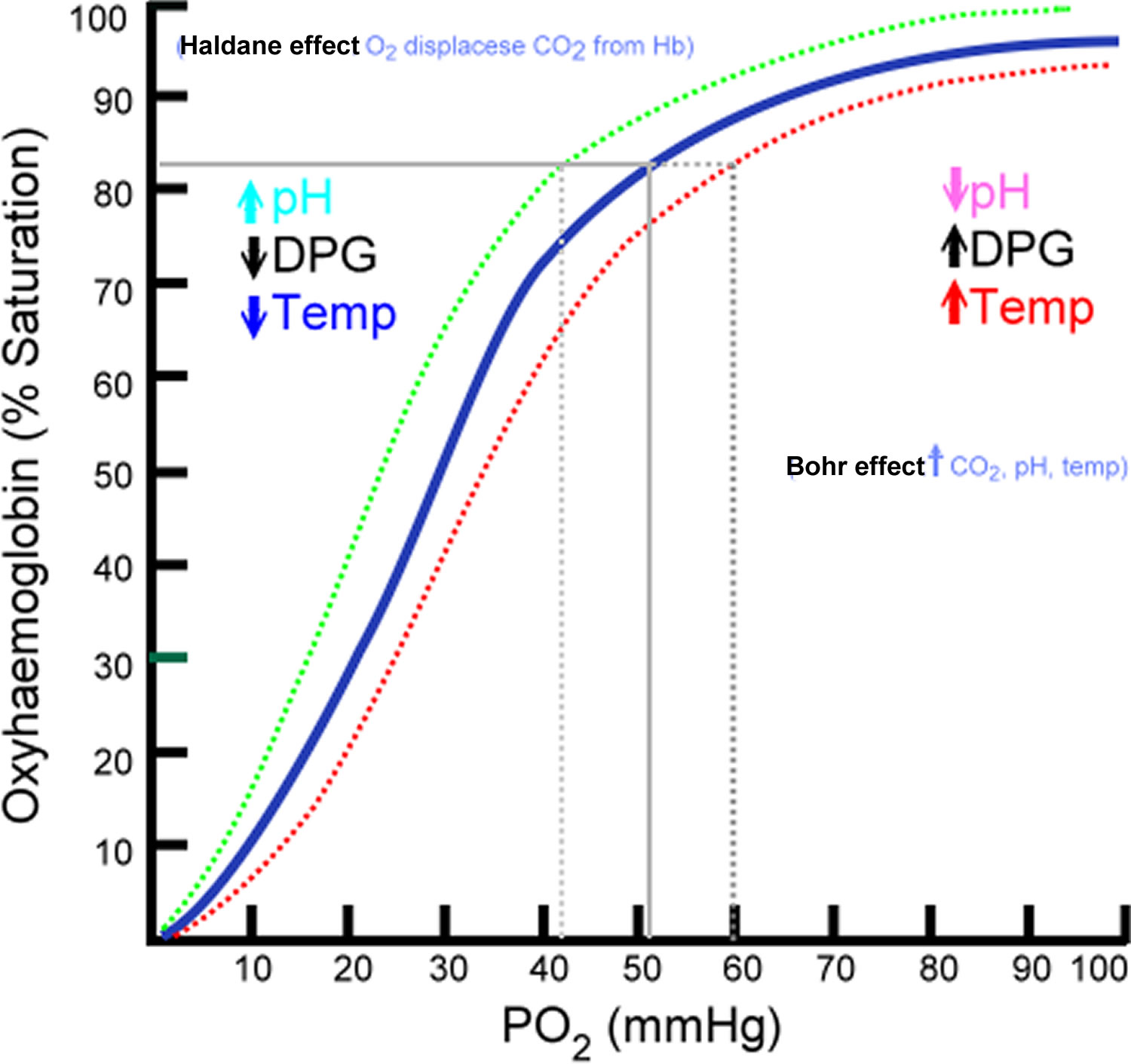
Figure 1. Oxyhemoglobin Dissociation Curve.
Footnote: Dotted green line corresponds with shift to the right caused by Haldane effect. Dotted red line corresponds with shift to the right caused by Bohr effect.
Bohr effect
The Bohr effect describes hemoglobin’s lower affinity for oxygen secondary to increases in the partial pressure of carbon dioxide (PaCO2) and decreases in blood pH. This lower affinity, in turn, enhances the unloading of oxygen into tissues to meet the oxygen demand of the tissue 1. Through the Bohr effect, more oxygen is released to those tissues with higher carbon dioxide concentrations. Bohr effect is a property of hemoglobin first described by the Danish physiologist Christian Bohr in 1904, which states that in the presence of carbon dioxide (CO2), the oxygen affinity for dissociation of respiratory pigments, such as hemoglobin decreases; because of the Bohr effect, an increase in blood carbon dioxide level or a decrease in pH causes hemoglobin to bind to oxygen with less affinity.
In addition to hypercarbia and acidemia described by the Bohr effect, other factors that cause a right shift in the oxy-hemoglobin dissociation curve include increases in temperature, 2,3-Bisphosphoglyceric acid (2-3-BPG). The summation of these factors determines the level of the influence on the hemoglobin oxygen binding capacity. For example, in the setting of exercising skeletal muscle, high metabolic demands require maximal oxygen delivery for cellular respiration. Increased metabolic rates at skeletal muscle results in both carbon dioxide and lactic acid from aerobic and anaerobic cellular respiration, respectively, drastically lowering the surrounding blood pH in addition to temperature increases as a result of exothermic reactions. The environmental alterations from the summation of these factors and resulting hemoglobin conformational optimize hemoglobin oxygen delivery to peripheral tissues. Similarly, under the stress of chronic hypoxic conditions ranging from high altitude to chronic lung disease or congestive heart failure, the body relies on glycolysis to meet metabolic demands. Through increased levels of glycolysis under hypoxic conditions, the resulting 2,3-BPG byproduct further shifts the oxy-hemoglobin dissociation curve to the right, in favor of oxygen unloading 2.
Bohr effect facilitates oxygen transport as hemoglobin binds to oxygen in the lungs, but then releases it in the tissues, particularly those tissues in most need of oxygen. When a tissue’s metabolic rate increases, its carbon dioxide production increases. The carbon dioxide is quickly converted into bicarbonate molecules and acidic protons by the enzyme carbonic anhydrase:
- CO2+ H2O ⇌ H+ + HCO3−
This causes the pH of the tissue to decrease, and so increases the dissociation of oxygen from hemoglobin, allowing the tissue to obtain enough oxygen to meet its demands.
The dissociation curve shifts to the right when carbon dioxide or hydrogen ion concentration is increased. This facilitates increased oxygen dumping. This makes sense because increased CO2 concentration and lactic acid build-up occur when the muscles need more oxygen. Changing hemoglobin’s oxygen affinity is the body’s way of adapting quickly to this problem.
The Bohr effect describes the ability of red blood cells to adapt to changes in the biochemical environment, maximizing hemoglobin-oxygen binding capacity in the lungs while simultaneously optimizing oxygen delivery to tissues with the greatest demand.
The sensitivity to Bohr effect can be suppressed in chronic diseases, leading to decreased oxygenation of peripheral tissues. Chronic conditions such as asthma, cystic fibrosis, or even diabetes mellitus, can lead to a chronic state of hyperventilation in order to maintain adequate tissue oxygenation. These states can have ventilation of up to 15 L per minute compared to the average normal minute ventilation of 6 L per minute. This hyperventilation minimizes the potential of the Bohr effect through excess exhalation of carbon dioxide resulting in hypocapnia, causing a left shift the oxygen dissociation and unnecessarily increased oxygen-hemoglobin binding affinity with impaired oxygen release to peripheral tissues, including our most vital organs (brain, heart, liver, kidney). Thus, the Bohr effect is essential in maximizing oxygen transport capabilities of hemoglobin and functionally dynamic oxygen-binding/release secondary to carbon dioxide equilibrium 3.
In the Hiroshima variant hemoglobinopathy the Bohr effect is diminished so the hemoglobin has a higher affinity for oxygen and tissue may suffer minor oxygen starvation during high work.
Carbon Monoxide Effect
While the presence of carbon dioxide leads to the greater unloading of oxygen, carbon monoxide has the opposite effect. Carbon monoxide (CO) has a 200-times greater affinity for hemoglobin than oxygen, out-competing oxygen for available binding sites in a nearly irreversible fashion (reversible, but very minimally). Carbon monoxide further decreases oxygen delivery through the stabilization of hemoglobin in the R-form. Counter-intuitively, although this facilitates oxygen loading to the remaining binding sites, hemoglobin becomes resistant to environmental influences that would normally encourage conformational changes into taut-form, limiting the potential for unloading of oxygen. Under the influence of carbon monoxide, the oxy-hemoglobin dissociation curve significantly shifts left in addition to the reduction of the sigmoidal curve shape as a result of blunted positive cooperativity response of hemoglobin. In the presence of significant carbon monoxide inhalation, tissue hypoxia occurs despite normal pO2 levels, as carbon monoxide competitively binds hemoglobin while inhibiting the release of oxygen from the remaining binding sites. Carbon monoxide poisoning is treated with hyperbaric oxygen therapy, delivering 100% O2 at increased atmospheric pressures to facilitate hemoglobin oxygen binding in the presence of highly competitive carbon monoxide 4.
Bohr effect and Haldane effect
In Bohr Effect, binding of oxygen to hemoglobin in lungs takes place because carbon dioxide concentration is less in lung alveoli, which results in leftward shift of oxyhemoglobin dissociation curve (see Figure 1). In contrast to it, release of oxygen from hemoglobin in body tissues due to increased amount of carbon dioxide will cause rightward shift of the curve.
The Bohr effect maintains significant clinical relevance within the field of Anesthesiology, as it directly influences patient outcomes throughout the perioperative process. Whether through hypo or hyperventilation, the alterations in carbon dioxide content and acid-base status results in shifts in the oxy-hemoglobin dissociation curve, either amplifying or dampening the magnitude of the Bohr Effect regarding hemoglobin re-oxygenation at the alveoli and delivery/release at peripheral tissues.
The Haldane effect, on the other hand describes how oxygen concentration can determine hemoglobin’s affinity towards carbon dioxide.
In patients with lung disease, lungs may not be able to increase alveolar ventilation in the face of increased amounts of dissolved carbon dioxide.
This partially explains the observation that some patients with emphysema might have an increase in PaCO2 (arterial dissolved carbon dioxide) following administration of supplemental oxygen.
References- Patel AK, Benner A, Cooper JS. Physiology, Bohr Effect. [Updated 2019 Jul 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526028
- Jendroszek A, Malte H, Overgaard CB, Beedholm K, Natarajan C, Weber RE, Storz JF, Fago A. Allosteric mechanisms underlying the adaptive increase in hemoglobin-oxygen affinity of the bar-headed goose. J. Exp. Biol. 2018 Sep 17;221(Pt 18).
- Tsuji B, Filingeri D, Honda Y, Eguchi T, Fujii N, Kondo N, Nishiyasu T. Reply to Parkes: Effect of hypocapnia on the sensitivity of hyperthermic hyperventilation and the cerebrovascular response in resting heated humans. J. Appl. Physiol. 2018 May 01;124(5):1213.
- Kuo SC, Hsu CK, Tsai CT, Chieh MJ. [Hyperbaric Oxygen Therapy and Acute Carbon Monoxide Poisoning]. Hu Li Za Zhi. 2018 Aug;65(4):11-17.