Hemorrhagic cystitis
Hemorrhagic cystitis is a diffuse inflammatory condition of the urinary bladder due to an infectious (bacterial, fungal, parasitic and viral bladder infections) or noninfectious cause (anticancer drugs, radiation therapy, exposure to chemicals, such as dyes or insecticides), resulting in bleeding from the bladder mucosa. Infectious causes of hemorrhagic cystitis include bacteria and viruses. Noninfectious hemorrhagic cystitis most commonly occurs in patients who have undergone pelvic radiation, chemotherapy, or both 1. Affected patients may develop asymptomatic microscopic hematuria or gross hematuria with clots, leading to urinary retention. Symptoms include pain and a burning feeling while urinating, feeling a need to urinate often, and being unable to control the flow of urine.
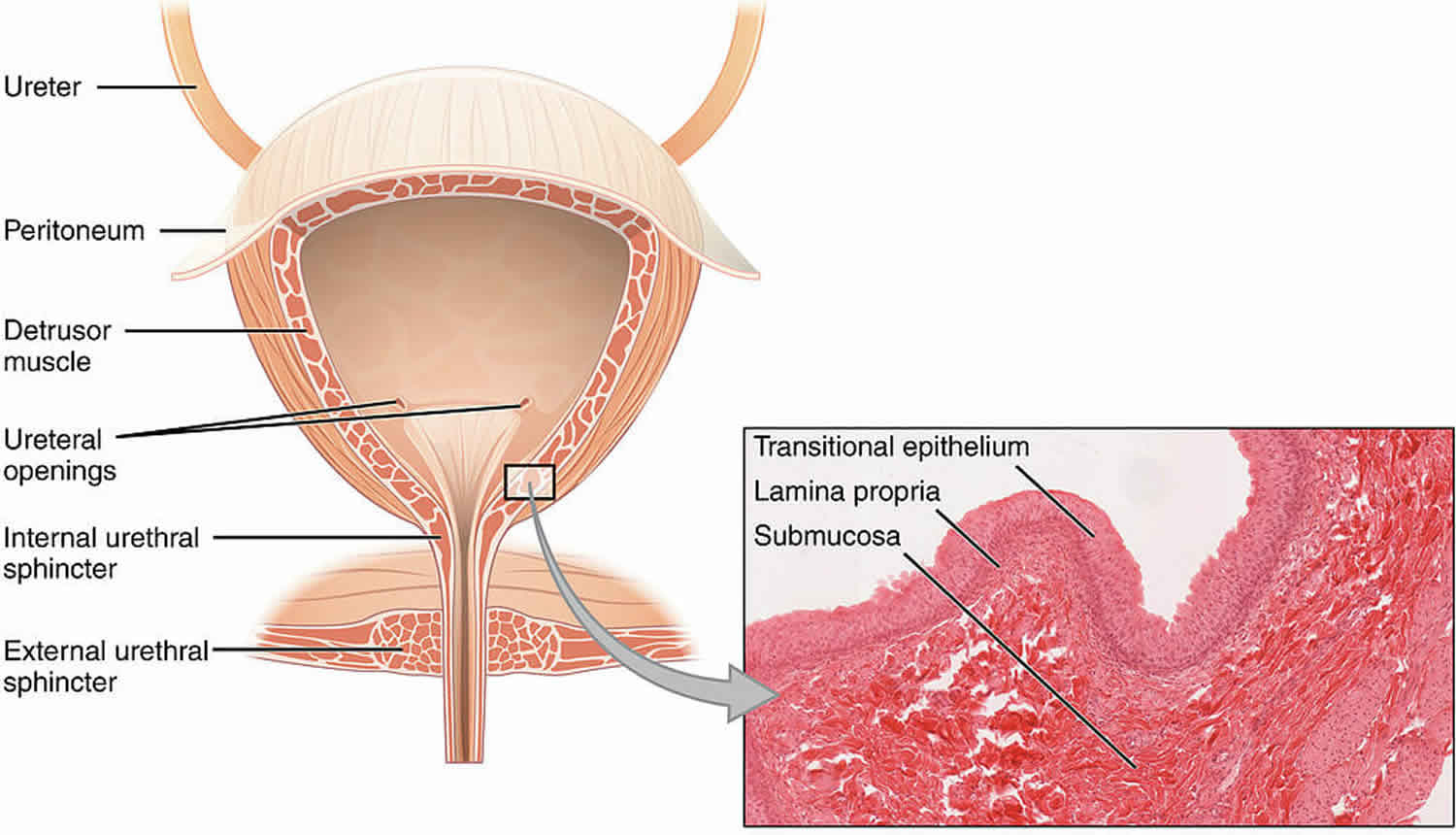
Hemorrhagic cystitis results from damage to the bladder’s transitional epithelium and blood vessels by toxins, viruses, radiation, drugs (in particular, chemotherapeutic drugs), bacterial infections, or other disease processes. Histologically, the bladder wall demonstrates nonspecific findings of intense inflammatory infiltrates, chronic inflammation, and fibrosis 2.
Chronic and recurrent hemorrhagic cystitis often results from anticancer chemotherapy or radiotherapy for the treatment of pelvic malignancies. Patients who undergo bone marrow transplantation frequently have hemorrhagic cystitis because most are exposed to cyclophosphamide, total-body irradiation, or both. Patients with malignancies and those undergoing chemotherapy are often immunocompromised and are at high risk of acquiring bacterial and viral infections that can cause hemorrhagic cystitis. For patients receiving drugs or undergoing procedures that are known to cause hemorrhagic cystitis, prevention is essential. Two standard methods of preventing cyclophosphamide-related bladder toxicity are hyperhydration and mesna administration. Controversial methods include prophylactic bladder irrigation and hourly voiding.
Acute infectious causes (both viral and bacterial) are less common except in immunocompromised hosts like bone marrow transplant patients.
Hemorrhagic cystitis cases can be challenging for the urologist and a source of substantial morbidity (and sometimes mortality) for patients.
Treatment depends on the cause, severity of the bleeding, and symptoms 3.
Hemorrhagic cystitis causes
Hemorrhagic cystitis has both infectious and noninfectious causes. Although the noninfectious causes of hemorrhagic cystitis vary, this condition most commonly develops as a complication of pelvic radiation or from toxicity related to the use of certain chemotherapeutic drugs (eg, cyclophosphamide, ifosfamide). Less commonly, exposure to certain industrial chemicals, such as aniline or toluidine derivatives, causes hemorrhagic cystitis.
Rarely, drugs such as penicillins or danazol can also precipitate hemorrhagic cystitis. Other, extremely rare reports include associations with food poisoning (from Salmonella typhi) 4 and prolonged high-altitude air travel (Boon disease) 5.
Infectious hemorrhagic cystitis
Regardless of the perceived circumstance, an infectious cause should be sought as part of the initial assessment, even in the setting of radiation or chemical exposure, as infection may serve as an exacerbating factor. Bacterial, fungal, parasitic, and especially viral bladder infections in immunocompromised patients are often complicated by hemorrhage. Reported causative infectious agents for hemorrhagic cystitis include the following:
- Escherichia coli
- Adenoviruses 7, 11, 21, and 35
- Papovavirus
- Influenza A
Radiation-induced hemorrhagic cystitis
Nearly 25% of patients who undergo pelvic radiation develop bladder-related complications. Mucosal ischemia secondary to radiation injury results from endarteritis inducing hypoxic surface damage, ulceration, and bleeding. The incidence in the pediatric population is less than that in adults. Slightly less than 50% of these patients develop diffuse hematuria.
Patients with radiation-induced hemorrhagic cystitis have usually undergone radiation therapy for cancer of the prostate, colon, cervix, or bladder. Urgency, frequency, dysuria, and stranguria may develop acutely during radiation or may begin months to years after completion of radiotherapy.
The higher the dose and the wider the field encompassed by the radiation exposure, the more likely radiation cystitis becomes. Because of the cumulative dose effect, patients who have undergone pelvic radiation are at an increased risk for radiation cystitis if additional radiotherapy is performed. Infection, bladder outlet obstruction, and instrumentation are all factors that can exacerbate radiation cystitis 6.
Drug-induced hemorrhagic cystitis
Chemotherapeutic drugs hemorrhagic cystitis
The most common pharmacologic causes of hemorrhagic cystitis are the oxazaphosphorine-alkylating agents cyclophosphamide and ifosfamide 7. Unfortunately, the toxicity of these drugs is not insignificant, and many of the adverse effects are urologic.
Cyclophosphamide (Cytoxan) is used in the treatment of non-Hodgkin lymphoma (as part of the CHOP regimen) and breast cancer, as well as in steroid-resistant nephrotic syndrome, and as an orphan drug in systemic sclerosis. Specifically pediatric uses include juvenile idiopathic arthritis/vasculitis and systemic lupus erythematosus.
The incidence of adverse urologic effects with cyclophosphamide varies from 2-40%, and the toxicity is dose-related. In pediatric patients, adverse effects are typically seen after oral doses greater than 90 g and intravenous doses greater than 18 g, and they occur more commonly in patients receiving intravenous treatment.
Cyclophosphamide can cause microscopic and gross hematuria. The onset of hematuria usually occurs within 48 hours of treatment 8. Cyclophosphamide is also associated with bladder cancer, which is typically aggressive 9.
Cyclophosphamide itself is not toxic; the drug’s toxicity is due to its hepatic conversion to the metabolite acrolein, which is excreted in the urine and causes bladder edema and bladder hemorrhage. Over time, chronic bladder damage manifests as fibrosis with decreased capacity, trabeculations, and telangiectasias. The adverse effects of cyclophosphamide are more likely due to increased bladder exposure to acrolein 10.
Hemorrhagic cystitis secondary to cyclophosphamide therapy is most prevalent in patients who are dehydrated. Thus, patients receiving cyclophosphamide should always receive good hydration, and a Foley catheter is often placed to ensure immediate drainage of the bladder 11. Continuous bladder irrigation is sometimes recommended to hasten acrolein clearance from the bladder 12.
Ifosfamide (Ifex) is approved for use in germ cell testicular cancer, and as an orphan drug for treatment of sarcomas of soft tissue and bone. Hemorrhagic cystitis due to ifosfamide therapy is generally worse than that caused by cyclophosphamide 13. Ifosfamide causes the release of tumor necrosis factor – alpha and interleukin-1 beta, mediating the release of nitric oxide and leading to hemorrhagic cystitis 14.
Penicillins hemorrhagic cystitis
In rare cases, penicillins have been reported to cause hemorrhagic cystitis. Case reports have implicated the following agents:
- Methicillin
- Carbenicillin
- Ticarcillin
- Piperacillin
- Penicillin VK
Most cases of hemorrhagic cystitis in patients taking extended-spectrum penicillins have been reported in individuals with cystic fibrosis who had previously received penicillin antibiotics.
Symptoms can take up to 2 weeks to develop after the drug is started; when symptoms occur, the best treatment is to discontinue the offending drug immediately. Hemorrhagic cystitis in patients taking penicillins is thought to be caused by an immune-mediated hypersensitivity. Examination of the urine frequently reveals eosinophils 15.
Danazol hemorrhagic cystitis
Treatment with danazol, a semisynthetic anabolic steroid, has caused hemorrhagic cystitis in patients with hereditary angioedema. Interestingly, the advent of hemorrhagic cystitis in these patients has followed years of symptom-free treatment with the drug. Hematuria developed after 30-77 months of treatment in one study. In almost all cases, the hematuria resolved after cessation of danazol. The dose of danazol did not correlate with the severity of hemorrhagic cystitis. The etiology of hemorrhagic cystitis from danazol is unclear 16.
Other medications hemorrhagic cystitis
Medications that have been implicated in the development of hemorrhagic cystitis in limited reports include the following:
- Temozolomide 17
- Bleomycin 18
- Tiaprofenic acid 19
- Allopurinol 20
- Methaqualone 21
- Methenamine mandelate 22
- Ether
- Gentian violet inadvertently placed in the urethra 23
- Nonoxynol-9 suppositories inadvertently placed in the urethra 24
- Intravesical acetic acid 25
Risperidone has been associated with hemorrhagic cystitis but is also used as treatment for hemorrhagic cystitis due to JC polyomavirus 26.
A chemical hemorrhagic cystitis can develop when vaginal products are inadvertently placed in the urethra. Gentian violet douching to treat candidiasis has resulted in hemorrhagic cystitis when the drug has been misplaced in the urethra, but this hemorrhagic cystitis has resolved spontaneously with cessation of treatment 23.
Accidental urethral placement of contraceptive suppositories has also caused hemorrhagic cystitis in several patients. In this case, the bladder irritation was thought to be caused by contact of the acidic compound nonoxynol-9 (pH, 3.35) with the bladder. In the acute setting, the bladder can be copiously irrigated with alkalinized normal saline to minimize bladder irritation 24.
Chemically induced hemorrhagic cystitis
Cases of hemorrhagic cystitis with no infectious etiology have been reported in patients who have been in contact with certain urotoxic chemicals, such as derivatives of aniline (found in dyes, marking pens, and shoe polish) and toluidine (found in pesticides and shoe polish). Exposure to these chemicals is usually work-related. Hemorrhagic cystitis caused by these derivatives is self-limiting, and cessation of the exposure usually suffices for cure. Exposure also increases the risk of transitional cell carcinoma. Thus, the workup for gross hematuria should reflect this possibility 27.
Viral causes of hemorrhagic cystitis
Patients undergoing therapy to suppress the immune system—eg, after solid organ, bone marrow, or cord blood transplantation—are at risk for hemorrhagic cystitis due to either the direct effects of chemotherapy or activation of dormant viruses in the kidney, ureter, or bladder 28.
In the early 1990s, infection with polyomavirus or adenoviruses was implicated as the likely etiology of this condition. The BK polyomavirus subclinically infects most of the population in childhood and persists indefinitely in the kidney after primary infection. When the immune system is compromised, as in persons with human immunodeficiency virus (HIV) infection or those undergoing chemotherapy or chemical immunosuppression, the virus can be reactivated, leading to clinical nephritis, ureteritis, or cystitis 29.
The BK polyomavirus 30 and adenovirus types 7, 11, 34, and 35 31 have been the most commonly described viruses in these cases. Cytomegalovirus 32, JC virus 33 and herpesviruses 34 have also been identified as causative agents in these scenarios.
In the pediatric population, the species most commonly isolated is adenovirus type 11, which has a propensity for the urinary tract. It reactivates with profound immunosuppression. It is also the most common cause of hemorrhagic cystitis in the healthy child.
HIV itself does not cause hemorrhagic cystitis, but immune suppression from HIV infection can predispose to other viral infections, such as BK virus, leading to the condition 35. BK virus has also been suggested to be a causal transforming agent for bladder cancer 32.
Hemorrhagic cystitis symptoms
Patients who present with hemorrhagic cystitis usually have a history of radiation or chemical exposure. They are often inpatients or are well known to their respective services.
Noninfectious hemorrhagic cystitis is characterized by inflammation of the bladder associated with hematuria. Patients with this condition usually present with urgency, frequency, dysuria, and, in some cases, abdominal discomfort. A history of new urinary incontinence is frequently noted.
The presence or absence of clotted blood in the urine is not completely helpful in determining the etiology of hemorrhagic cystitis, but the presence of long, stringy clots suggests an upper urinary tract etiology. Symptoms include suprapubic discomfort, urinary frequency, and inability to empty the bladder due to the clots.
Upon examination, the patient often demonstrates suprapubic fullness and discomfort or pain to palpation, as well as costovertebral angle tenderness if the bladder obstruction is chronic.
Patients with hemorrhagic cystitis can present with variable degrees of hematuria, ranging from slightly blood-tinged urine to massive gross hematuria with passing of clots that may cause urinary retention. Clot retention is common and can be very painful. Urinary incontinence is frequently observed.
Hemorrhagic cystitis diagnosis
Documentation of noninfectious hemorrhagic cystitis requires a negative urine culture for bacteria and viruses. If even “insignificant” growth on an adequately collected voided specimen or any growth on a catheterized specimen is present, antibiotics should be initiated. Certain circumstances seem to predispose to urinary tract infections and cause signs and symptoms disproportionate to the amount of pathogen growth, especially in hemorrhagic cystitis due to radiation treatment or chemotherapy. Empiric antibiotics should be switched to culture-directed agents as soon as sensitivities are available.
In all patients, obtain a complete blood count (CBC), basic metabolic profile, and coagulation studies. The hematocrit is rarely below the reference range during an initial occurrence of hemorrhagic cystitis; however, patients with chronic hemorrhagic cystitis may have a lower hematocrit level and prevailing signs of chronic anemia. The white blood cell (WBC) count may be elevated because of a concurrent infection or because of the treatment (eg, chemotherapy) of the underlying malignancy.
Basic metabolic profile (SMA-7) findings are usually normal but may reflect sequelae due to treatment of the primary condition. Liver function test abnormalities related to the primary process may be found but are generally not related to the hemorrhagic cystitis.
Imaging of the upper tracts and bladder is recommended in all cases of hemorrhagic cystitis to assist in ascertaining the etiology and/or confounding variables. At a minimum, perform bladder and renal ultrasonography with a KUB (kidney, ureter, bladder) film to assess for radio-opaque stones (see the diagnosis algorithm below).
In patients with normal renal function, computed tomography (CT) urography is the most helpful imaging test in most cases. Cystoscopy is indicated in all but straightforward cases of uncomplicated bacterial cystitis. If a bacterial infection is documented, voiding cystourethrography (VCUG) may be performed, if indicated, after the infection has been cleared.
Urine studies for viruses, when indicated, include the following:
- Viral culture
- Electron microscopy of bladder biopsy specimens
- Enzyme-linked immunosorbent assay (ELISA)
Cystoscopy
Cystoscopy, with or without retrograde pyelography, is indicated in all cases of hemorrhagic cystitis. This may be delayed until the acute bleeding has been treated with successful use of conservative measures; ie, manual irrigation and continuous bladder irrigation. In this case, outpatient flexible cystoscopy is used typically without need for general anesthesia.
However, cystoscopic clot evacuation is often necessary to facilitate complete clot removal. This allows close inspection of the bladder urothelium and assessment of potential neoplasm(s) as the bleeding source. Use of a rigid cystoscope of the largest possible caliber permits improved removal of clots. Endoscopic inspection is essential in planning treatment and in preventing future episodes.
In the pediatric population, cystoscopy should be considered a first-line therapy in the setting of clot retention or failure of initial conservative therapy. Cystoscopy also allows for evaluation of concomitant neoplasm (fairly uncommon in pediatric population). The endoscopic procedure is performed under general anesthesia in the pediatric population. Complete removal of clot is paramount prior to beginning intravesical irrigation due to the risk of overdistention and potential for bladder rupture in children. Most of the time, cystoscopic findings are nonspecific. The bladder may appear edematous with multiple punctate hemorrhages. Visible areas of active bleeding can be identified and judiciously fulgurated to control bleeding 36.
Urinary tract imaging
Renal and bladder ultrasonography along with a KUB (kidney, ureter, bladder) film is an excellent initial screening test to evaluate many causes of hematuria. Anatomic and pathologic changes in the urothelium can occur in the upper urinary tract, which may result in hematuria and possible hydronephrosis.
Renal ultrasonography is a reliable and cost-effective initial modality to identify hydronephrosis. Dilatation of the upper urinary tract can be secondary to obstruction at the ureteral level, secondary to bladder wall thickening, or secondary to reflux of urine. Ultrasonography of the bladder may also help to identify blood clots and evaluate their size.
Evaluation of the complete ureter and enhanced anatomic detail is limited with ultrasonography. Evidence of hydronephrosis or a high index of suspicion should prompt further evaluation with CT or MR urography or retrograde pyelography.
In general, the evaluation of any patient with gross hematuria should include an assessment of the upper urinary tract. Imaging studies are as follows, in descending order of helpfulness:
- CT urography
- Magnetic resonance (MR) urography
- Intravenous pyelography (IVP)
- CT scanning without contrast (stone protocol, prone scanning)
- Renal ultrasonography
When only renal ultrasonography or noncontrast CT scanning is performed, retrograde intravenous pyelography may be necessary to further evaluate urothelium of the upper urinary tract. Even if upper urinary tract lesions are identified, a bladder etiology for hematuria should be suspected and then checked with cystoscopy 37.
If intravesical sclerotherapy (eg, formalin, silver nitrate) is planned after exhaustion of other control measures, cystography is necessary to determine the bladder capacity and to determine the presence of vesicoureteral reflux (VUR). The administration of sclerotherapy in the presence of VUR can lead to ureteral fibrosis, obstruction, and possible renal failure, as well as systemic absorption of the agent.
Hemorrhagic cystitis treatment
It is best to assume that a bacterial urinary tract infection is present until cultures return as ”no growth.” Empiric antibiotics to cover the usual flora are indicated during initiation of other measures. Regardless of the cause of noninfectious hemorrhagic cystitis, treatment follows the same course. In the absence of obstructing clots and if the patient is voiding well, hydration with careful observation may be the only treatment required. If the patient demonstrates difficulty with urination, clots are likely occluding the bladder outlet and clot evacuation is indicated. Continuous bladder irrigation with normal saline is started after clots are cleared.
If bladder irrigation with a hematuria catheter to clear the clots is not possible, cystoscopy with clot evacuation under anesthesia and antibiotic coverage are necessary. Fulguration of bleeding sites and biopsies of suspicious areas may be performed at that time. Although continuous bladder irrigation with saline solution cannot replace manual irrigation for removal of the clots, it can aid in preventing further clotting. For persistent hematuria, the bladder can be irrigated with a variety of agents. Hyperbaric oxygen therapy has been used with some success in difficult cases 38.
In immunocompromised patients, who are at high risk for infections, results from the bacterial and viral cultures guide the selection of antibiotic and antiviral therapy. In chemically induced cases, elimination of the agent predisposing to cystitis is paramount. Transfusion with platelets or coagulation products may be indicated.
Patients with active gross hematuria should limit their activities until it resolves. These patients are typically hospitalized and put on bed rest during their therapeutic interventions. The patient should remain well hydrated after the resolution of the hematuria. If a urethral catheter is left in place for monitoring purposes, providers must pay careful attention to reinitiation of hematuria, clot formation, and development of catheter-related urinary tract infections.
The oncologic patient with hemorrhagic cystitis should be treated at an institution familiar with this condition. The patient with severe hemorrhagic cystitis should be transferred only after his or her condition is stabilized. Only a few facilities may offer hyperbaric oxygen therapy. Patients requiring this therapy should be transferred early to these facilities.
The best treatment of hemorrhagic cystitis is prevention, especially with cyclophosphamide-induced hemorrhagic cystitis. Adequate hydration to induce brisk diuresis, continuous bladder irrigation, and prophylactic dosing of mesna are important preventive measures. Prompt recognition and treatment of urinary tract infections is also a prudent preventive measure. After hemorrhagic cystitis develops, the treatment follows the same guidelines irrespective of the cause, although most infectious cases resolve with appropriate antibiotics.
Clot evacuation
The first step in the treatment of hemorrhagic cystitis should be directed toward making sure that the bladder does not become overly distended. Bladder outlet obstruction from clots can lead to urosepsis, bladder rupture, and renal failure. Clot evacuation can be performed at the bedside by carefully placing a large, stiff-walled hematuria catheter. In pediatric patients, a catheter with decreased luminal size is needed because of the small urethra. Consideration should be given to the use of a suprapubic tube, but only under the direction of a trained urologist.
Initial irrigations may be performed manually with sterile water; water is preferable to sodium chloride solution because it helps to lyse red blood cells and clots. Care must be taken to not overdistend the bladder and cause a perforation, especially of the small pediatric bladder.
After clot evacuation, if hematuria persists, a 3-way catheter can be inserted and continuous bladder irrigation with saline can be started. All clots must be removed before continuous irrigation is started to avoid overdistention and potential bladder ruptures. The patient should be vigorously hydrated using oral and/or intravenous fluids to keep clots from reforming.
If clot evacuation is unsuccessful with this approach, the patient should undergo cystoscopy in the operating room with clot evacuation and fulguration of bleeding sites. Some have proposed the use of epsilon aminocaproic acid (Amicar) as an aid to stopping small vessel bleeding at this point.
Epsilon aminocaproic acid can be used either orally or parenterally. It works by inhibiting clot lysis by urinary urokinase. It is contraindicated in patients with upper urinary tract bleeding because it can cause extremely dense clots, resulting in ureteral obstruction and potential loss of the kidney. These clots are also often difficult to manually evacuate through a catheter; thus, evacuation via cystoscopy or cystotomy in the operating room may be required 39. Use of this agent in pediatric patients is not recommended.
Bladder irrigation agents
If hematuria persists after the treatments described above, bladder irrigation can be performed with carboprost, 1-2% alum, or silver nitrate. Alum and silver nitrate, which are astringents, work by forming precipitates over the bleeding surfaces of the bladder wall.
These agents are not significantly absorbed through an intact bladder wall but may enter the circulation under pressure through open veins. Rarely, alum irrigation causes encephalopathy and acidosis in patients with renal insufficiency, in whom serum aluminum level monitoring is advisable 40. Both agents are somewhat caustic to the bladder, and alum can cause bladder wall necrosis and even perforation.
Because alum and silver nitrate irrigations are not without risk, the importance of constant monitoring to ensure low-pressure bladder irrigation is paramount and may warrant performing the procedure in an intensive care unit setting. One drawback to alum irrigation is that it imparts a leathery consistency to any clot present in the bladder at the time of irrigation, thus precluding easy clot evacuation. Occlusion is especially likely with a pediatric-sized catheter.
Carboprost
Carboprost tromethamine (prostaglandin F2 [Hemabate]) is approved by the US Food and Drug Administration (FDA) for uterine bleeding and induction of second-trimester abortion. It induces smooth-muscle contraction in blood vessel walls and has been used off-label for bladder irrigation in the treatment of hemorrhagic cystitis due to cyclophosphamide 41. The main advantages of carboprost are that it is easy to use and that it does not cause bladder pain with irrigation.
Prostaglandin E1 and E2
Other prostaglandins have also been used intravesically or parenterally in the treatment of pain associated with hemorrhagic cystitis, including prostaglandin E1 and prostaglandin E2. These agents cause vasodilation, which improves blood flow to the bladder wall, and presumably decrease pain because of their anti-inflammatory properties. These agents are expensive, and their efficacy in bleeding reduction is marginal 42. However, they involve no coagulum formation, have few side effects, and are easily tolerated by patients.
Formalin
In severe, refractory cases of hemorrhagic cystitis, formalin can be instilled in the bladder 43. Formalin coagulates the bleeding bladder surface by hydrolyzing and cross-linking proteins. Prior to instillation, reflux into the ureters must be assessed with cystography. Formalin must not reflux into the upper urinary tract, because this can cause irreversible fibrosis, papillary necrosis, or ureteral obstruction. If reflux is present, formalin can be used if occlusion balloon catheters are inserted into both ureters prior to proceeding.
A 2.5-4% formalin solution is instilled in the bladder and left for up to 30 minutes, after which the bladder is meticulously irrigated with continuous bladder irrigation. Intravesical formalin instillation must be performed with the patient under anesthesia because it is otherwise excruciatingly painful 44. Placement via cutaneous vesicostomy has also been described 45.
Surgery
In patients with refractory hemorrhagic cystitis, surgical intervention is warranted. This may include any of the following:
- Open cystostomy and temporary packing
- Percutaneous nephrostomy drainage
- Selective hypogastric artery embolization (very rarely effective)
- Ileal conduit diversion
- Cutaneous ureterostomy
- Cystectomy
Advances in the management of bleeding related to soft-tissue trauma have had application to hemorrhagic cystitis. These include the following:
- Argon beam coagulators 46
- Activated thrombin and fibrin agents (eg, Evicel, FloSeal) 47
- Angiogenesis inhibitors (for cyclophosphamide cystitis) 48
Hemorrhagic cystitis prognosis
In general, hemorrhagic cystitis caused by exposure to chemotherapeutic drugs can be expected to resolve after discontinuation of the agent and treatment with irrigation/fulguration. Conversely, hemorrhagic cystitis due to pelvic radiation therapy tends to recur for months, or even years, after completion of radiotherapy. Seemingly minor events, such as urinary tract infection or bladder distention, may trigger florid hemorrhage. Close follow-up with periodic urinalysis and urine culture and sensitivity testing, along with aggressive management, may prevent recurrences.
The prognosis in pediatric patients with hemorrhagic cystitis is related to successful treatment of their primary oncologic condition. Most patients are successfully treated, with a resolution of hemorrhagic cystitis. However, long-term effects on the bladder may include increased bladder fibrosis, reduced bladder capacity, and upper tract deterioration.
In a retrospective single-instiution study of children who developed hemorrhagic cystitis after bone marrow transplantation, Au et reported high mortality and significant genitourinary morbidity. Factors associated with higher mortality included Foley catheterization, need for dialysis, and BK viremia 49.
Complications are unusual in patients with chemical cystitis. Wound problems, urinary anastomotic strictures and leaks, and bowel anastomosis problems are more common in patients who have undergone a urinary diversion procedure after radiation therapy 50. Patients with severe hemorrhagic cystitis refractory to medical intervention are at an increased risk for mortality.
References- Payne H, Adamson A, Bahl A, Borwell J, Dodds D, Heath C, et al. Chemical- and radiation-induced haemorrhagic cystitis: current treatments and challenges. BJU Int. 2013 Nov. 112 (7):885-97.
- Haldar S, Dru C, Bhowmick NA. Mechanisms of hemorrhagic cystitis. Am J Clin Exp Urol. 2014. 2 (3):199-208.
- Avidor Y, Nadu A, Matzkin H. Clinical significance of gross hematuria and its evaluation in patients receiving anticoagulant and aspirin treatment. Urology. 2000 Jan. 55(1):22-4.
- Arad E, Naschitz J, Yeshurun D. [Hemorrhagic cystitis as a presenting symptom of acute infection with Salmonella typhi]. Harefuah. 1996 Jun 16. 130(12):815-6.
- Kok LP. Boon’s disease: hemorrhagic cystitis in conjunction with massive exfoliation of degenerated urothelial cells (apoptosis?) during intercontinental flights in an otherwise healthy person. Diagn Cytopathol. 2001 Dec. 25(6):361-4.
- Cardinal J, Slade A, McFarland M, Keihani S, Hotaling JN, Myers JB. Scoping Review and Meta-analysis of Hyperbaric Oxygen Therapy for Radiation-Induced Hemorrhagic Cystitis. Curr Urol Rep. 2018 Apr 13. 19 (6):38.
- Klastersky J. Side effects of ifosfamide. Oncology. 2003. 65 Suppl 2:7-10.
- Stillwell TJ, Benson RC Jr. Cyclophosphamide-induced hemorrhagic cystitis. A review of 100 patients. Cancer. 1988 Feb 1. 61(3):451-7.
- Fernandes ET, Manivel JC, Reddy PK, Ercole CJ. Cyclophosphamide associated bladder cancer–a highly aggressive disease: analysis of 12 cases. J Urol. 1996 Dec. 156(6):1931-3.
- Cox PJ. Cyclophosphamide cystitis: identification of acrolein as the causative agent. Biochem Pharmacology. 1979;28:2045
- Robinson D, Schulz G, Langley R, Donze K, Winchester K, Rodgers C. Evidence-Based Practice Recommendations for Hydration in Children and Adolescents With Cancer Receiving Intravenous Cyclophosphamide. J Pediatr Oncol Nurs. 2014 May 5. 31(4):191-199.
- Turkeri LN, Lum LG, Uberti JP, Abella E, Momin F, Karanes C, et al. Prevention of hemorrhagic cystitis following allogeneic bone marrow transplant preparative regimens with cyclophosphamide and busulfan: role of continuous bladder irrigation. J Urol. 1995 Mar. 153(3 Pt 1):637-40.
- Lima MV, Ferreira FV, Macedo FY, de Castro Brito GA, Ribeiro RA. Histological changes in bladders of patients submitted to ifosfamide chemotherapy even with mesna prophylaxis. Cancer Chemother Pharmacol. 2007 Apr. 59(5):643-50.
- Ribeiro RA, Freitas HC, Campos MC, Santos CC, Figueiredo FC, Brito GA, et al. Tumor necrosis factor-alpha and interleukin-1beta mediate the production of nitric oxide involved in the pathogenesis of ifosfamide induced hemorrhagic cystitis in mice. J Urol. 2002 May. 167(5):2229-34.
- Kim MK, Kang CK, Kim MJ, Jun KI, Lee YK, Jeong SJ, et al. Penicillin G-induced hemorrhagic cystitis: a case and review of the literature. Korean J Intern Med. 2013 Nov. 28 (6):743-5.
- Andriole GL, Brickman C, Lack EE, Sesterhenn IA, Javadpour N, Linehan WM, et al. Danazol-induced cystitis: an undescribed source of hematuria in patients with hereditary angioneurotic edema. J Urol. 1986 Jan. 135(1):44-6.
- Islam R, Isaacson BJ, Zickerman PM, Ratanawong C, Tipping SJ. Hemorrhagic cystitis as an unexpected adverse reaction to temozolomide: case report. Am J Clin Oncol. 2002 Oct. 25(5):513-4.
- Komiya I, Nojiri M, Kuriya S, Saito Y. Hemorrhagic cystitis caused by bleomycin treatment. Jpn J Med. 1991 Jul-Aug. 30(4):392.
- Crawford ML, Waller PC, Wood SM. Severe cystitis associated with tiaprofenic acid. Br J Urol. 1997 Apr. 79(4):578-84.
- Bramble FJ, Morley R. Cystitis associated with allopurinol. Br J Urol. 1997 May. 79(5):817.
- Goldfarb M, Finelli R. Necrotizing cystitis. Secondary to “bootleg” methaqualone. Urology. 1974 Jan. 3(1):54-5.
- Ross RR Jr, Conway GF. Hemorrhagic cystitis following accidental overdose of methenamine mandelate. Am J Dis Child. 1970 Jan. 119(1):86-7.
- Kim SJ, Koh DH, Park JS, Ahn HS, Choi JB, Kim YS. Hemorrhagic cystitis due to intravesical instillation of gentian violet completely recovered with conservative therapy. Yonsei Med J. 2003 Feb. 44(1):163-5.
- Mayersak JS, Viviano CJ. Severe chemical cystitis from the transurethral intravesical insertion of a vaginal contraceptive suppository: a report of 3 cases and proposed method of management. J Urol. 1993 Apr. 149(4):835-7.
- Osorio AV, Simckes AM, Hellerstein S. Hemorrhagic cystitis caused by acetic acid instillation. J Urol. 1996 Feb. 155(2):685.
- Hudson RG, Cain MP. Risperidone associated hemorrhagic cystitis. J Urol. 1998 Jul. 160(1):159.
- Konety BR, Carroll PR. Urothelial Carcinoma: Cancers of the Bladder, Ureter, and Renal Pelvis. McAninch JW, Lue TF, eds. Smith & Tanagho’s General Urology. 18th ed. New York, NY: McGraw-Hill/Lange; 2013. 310-29.
- Shakiba E, Yaghobi R, Ramzi M. Prevalence of viral infections and hemorrhagic cystitis in hematopoietic stem cell transplant recipients. Exp Clin Transplant. 2011 Dec. 9(6):405-12.
- Bogdanovic G, Priftakis P, Giraud G, Kuzniar M, Ferraldeschi R, Kokhaei P, et al. Association between a high BK virus load in urine samples of patients with graft-versus-host disease and development of hemorrhagic cystitis after hematopoietic stem cell transplantation. J Clin Microbiol. 2004 Nov. 42(11):5394-6.
- Schneidewind L, Neumann T, Kranz J, Knoll F, Pelzer AE, Schmidt C, et al. Nationwide survey of BK polyomavirus associated hemorrhagic cystitis in adult allogeneic stem cell transplantation among haematologists and urologists. Ann Hematol. 2017 May. 96 (5):797-803.
- Hatakeyama N, Suzuki N, Yamamoto M, Kuroiwa Y, Hori T, Mizue N, et al. Detection of BK virus and adenovirus in the urine from children after allogeneic stem cell transplantation. Pediatr Infect Dis J. 2006 Jan. 25(1):84-5.
- Geetha D, Tong BC, Racusen L, Markowitz JS, Westra WH. Bladder carcinoma in a transplant recipient: evidence to implicate the BK human polyomavirus as a causal transforming agent. Transplantation. 2002 Jun 27. 73(12):1933-6.
- Focosi D, Kast RE. Hyaluronate and risperidone for hemorrhagic cystitis. Bone Marrow Transplant. 2007 Jan. 39(1):57.
- McClanahan C, Grimes MM, Callaghan E, Stewart J. Hemorrhagic cystitis associated with herpes simplex virus. J Urol. 1994 Jan. 151(1):152-3.
- Barouch DH, Faquin WC, Chen Y, Koralnik IJ, Robbins GK, Davis BT. BK virus-associated hemorrhagic cystitis in a Human Immunodeficiency Virus-infected patient. Clin Infect Dis. 2002 Aug 1. 35(3):326-9.
- Decker DB, Karam JA, Wilcox DT. Pediatric hemorrhagic cystitis. J Pediatr Urol. 2009 Aug. 5(4):254-64.
- McCarville MB, Hoffer FA, Gingrich JR, Jenkins JJ 3rd. Imaging findings of hemorrhagic cystitis in pediatric oncology patients. Pediatr Radiol. 2000 Mar. 30(3):131-8.
- Dautruche A, Delouya G. A contemporary review about the management of radiation-induced hemorrhagic cystitis. Curr Opin Support Palliat Care. 2018 Sep. 12 (3):344-350.
- Lakhani A, Raptis A, Frame D, Simpson D, Berkahn L, Mellon-Reppen S, et al. Intravesicular instillation of E-aminocaproic acid for patients with adenovirus-induced hemorrhagic cystitis. Bone Marrow Transplant. 1999 Dec. 24(11):1259-60.
- Perazella M, Brown E. Acute aluminum toxicity and alum bladder irrigation in patients with renal failure. Am J Kidney Dis. 1993 Jan. 21(1):44-6.
- Ippoliti C, Przepiorka D, Mehra R, Neumann J, Wood J, Claxton D, et al. Intravesicular carboprost for the treatment of hemorrhagic cystitis after marrow transplantation. Urology. 1995 Dec. 46(6):811-5.
- Trigg ME, O’Reilly J, Rumelhart S, Morgan D, Holida M, de Alarcon P. Prostaglandin E1 bladder instillations to control severe hemorrhagic cystitis. J Urol. 1990 Jan. 143(1):92-4.
- Ziegelmann MJ, Boorjian SA, Joyce DD, Montgomery BD, Linder BJ. Intravesical formalin for hemorrhagic cystitis: A contemporary cohort. Can Urol Assoc J. 2017 Mar-Apr. 11 (3-4):E79-E82.
- Sarnak MJ, Long J, King AJ. Intravesicular formaldehyde instillation and renal complications. Clin Nephrol. 1999 Feb. 51(2):122-5.
- Redman JF, Kletzel M. Cutaneous vesicostomy with direct intravesical application of formalin: management of severe vesical hemorrhage resulting from high dose cyclophosphamide in boys. J Urol. 1994 Apr. 151(4):1048-50.
- Quinlan DM, Naslund MJ, Brendler CB. Application of argon beam coagulation in urological surgery. J Urol. 1992 Feb. 147(2):410-2.
- Tirindelli MC, Flammia GP, Bove P, Cerretti R, Cudillo L, De Angelis G, et al. Fibrin Glue Therapy for Severe Hemorrhagic Cystitis after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2014 Jun 20.
- Gomes TN, Santos CC, Souza-Filho MV, Cunha FQ, Ribeiro RA. Participation of TNF-alpha and IL-1 in the pathogenesis of cyclophosphamide-induced hemorrhagic cystitis. Braz J Med Biol Res. 1995 Oct. 28(10):1103-8.
- Au JK, Graziano C, Elizondo RA, Ryan S, Roth DR, Koh CJ, et al. Urologic Outcomes of Children With Hemorrhagic Cystitis After Bone Marrow Transplant at a Single Institution. Urology. 2017 Mar. 101:126-132.
- Nieh, Peter T and Marshall, Fray F. Surgery of Bladder Cancer. In: Wein, Alan J; Kavoussi Louis R; Novick, Andrew C; Partin, Alan W; Peters, Craig A. Campbell-Walsh Urology. 3. 9th. United States of America: Saunders Elsevier; 2007:Chapt 78, pages 2479-2505.