What is hydroxyproline
Hydroxyproline is a major part of collagen and has an essential role in collagen stability. Small amounts of hydroxyproline are also found in the following 1:
- Elastin
- Acetylcholinesterase
- The C1q component of the complement system
- The macrophage scavenger proteins
- Ectodysplasin A
Two isomeric forms of hydroxyproline have been identified in collagen. Trans-4-hydroxy-L-proline is found in type 1 and type 3 collagen, whereas higher amounts of trans-3-hydroxy-L-proline are found in type 4 collagen 1. Normal secretion of procollagen molecules out of the cells requires a critical amount of trans-4-hydroxy-L-proline. Therefore, without hydroxyproline, no functional collagen fibers appear in the extracellular space 1.
Hydroxyproline can be tested in the serum and in the urine. The serum level of hydroxyproline excretion correlates well with its urinary level 2. However, urinary hydroxyproline better discriminates (x 100) between normal and metastatic disease of the bone 3.
In addition to the substrate, hydroxylation of peptidyl prolyl and peptidyl lysyl residues, catalyzed by prolyl hydroxylase and lysyl hydroxylase of skin, skeletal muscle, and granulating wounds, requires molecular oxygen, ascorbate, Fe2+, and alpha-ketoglutarate. A vitamin C deficiency can lead to scurvy, which produces bleeding gums, swelling joints, and impaired wound healing result due to the impaired collagen stability 1.
Collagen quantification has long been relevant to biomedical research and clinical practice to characterize tissues and determine disease states.
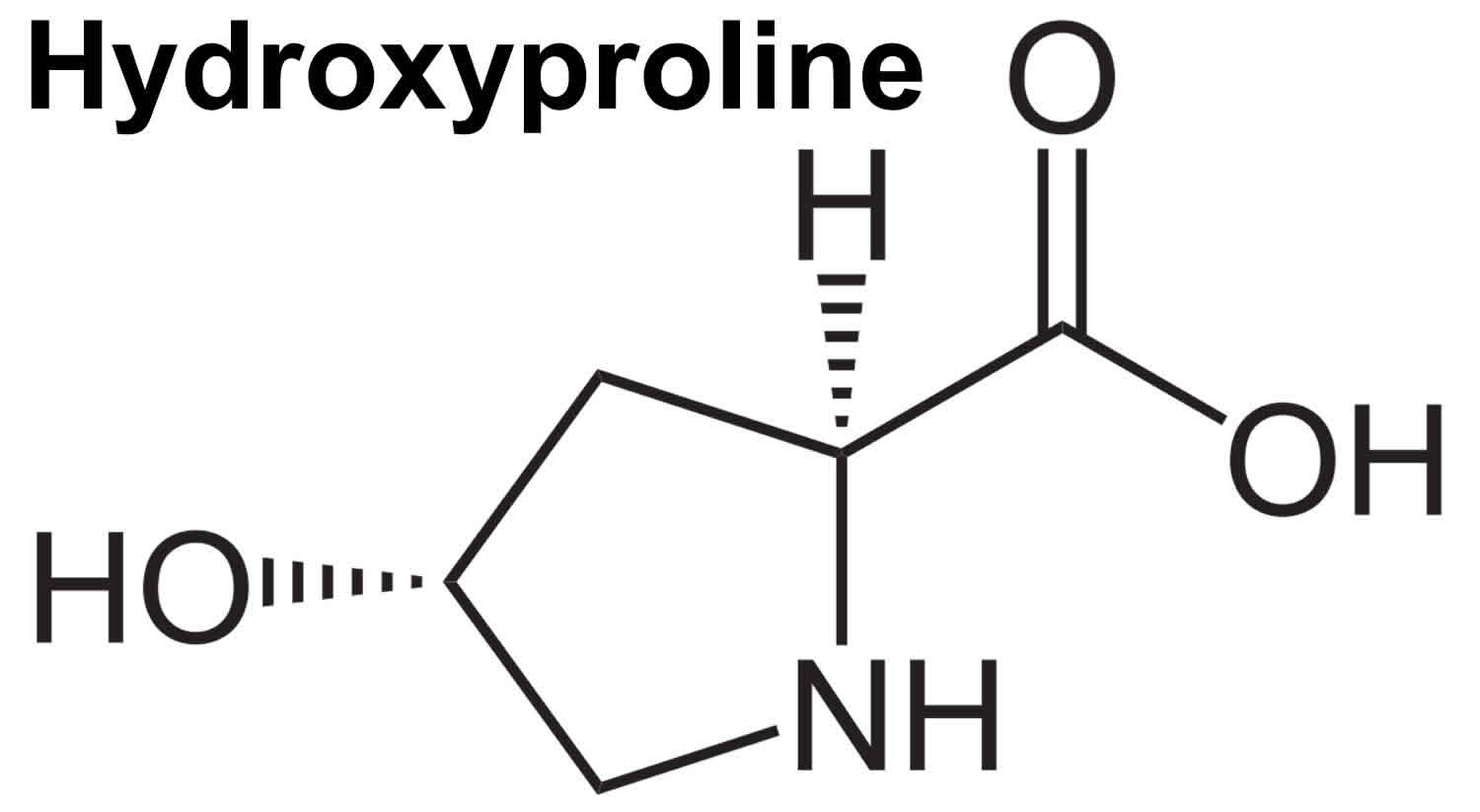
Figure 1. Hydroxyproline structure
Hydroxyproline assay
Hydroxyproline testing is indicated for the following:
- Paget disease of bone or to detect the inflammatory or neoplastic involvement of the bone
- As an indicator of activity in acromegaly and response to treatment to growth hormone 2
- As a diagnostic aid in Marfan syndrome 2
Testing hydroxyproline in the serum and in the urine is common. The gold standard for measuring hydroxyproline is chromatographic amino acid analysis, but this technique is not practical for all experiments due to its low-throughput nature and relatively high cost 4. As such, Neuman and Logan 5 devised a simple colorimetric assay that generates a chromophore from hydroxyproline via reaction with p-dimethylaminobenzaldehyde (DMAB, a.k.a. Ehrlich’s reagent). Uniquely, their assay required relatively small amounts of protein, which represented a marked advance in hydroxyproline quantification compared to previous methods. Since that initial breakthrough, hydroxyproline quantification, and its use toward quantifying collagen, has been continually improved 6 and was eventually commercialized. An alternative approach to quantifying collagen, based on Sirius Red dye binding, was later introduced 7 and is also available as a commercial assay. More recently, a method was described for quantifying collagen in collagenase-digested tissue based on binding of a fluorescent molecule to peptides containing N-terminyl glycine residues 8. Each of these biochemical methods for measuring collagen exploits a different, unique attribute of collagen’s amino acid sequence or structure.
The reference range of hydroxyproline is as follows 2:
- Total hydroxyproline in the urine among those aged 18-21 years – 13-28 mg/24/m²
- Total hydroxyproline in the urine among those aged 22-55 years – 8.5-23.5 mg/24/m²
- Free hydroxyproline in the serum of males – 0.7-1.55 µg/mL
- Free hydroxyproline in the serum of females – 0.7-1.40 µg/mL
The following conditions are associated with increased hydroxyproline levels 3:
- Paget disease
- Rheumatoid arthritis
- Hyperparathyroidism
- Osteomyelitis
- Polyarteritis nodosa
- Marfan syndrome
- Acromegaly
- Hyperthyroidism
- Turner syndrome
- Skeletal metastases
- Pregnancy
Hyperhydroxyprolinemia is a benign condition caused by a defect in 4-hydroxyproline dehydrogenase. No impairment of proline catabolism is associated with this condition 9.
The ratio of hydroxyproline/creatinine in a fresh specimen of early morning urine, considered the total urinary hydroxyproline, is the best index of collagen breakdown in metastatic bone disease. This measurement is preferable to free serum hydroxyproline measurement in this setting 3.
Although serum hydroxyproline and urinary hydroxyproline measurements are strongly correlated, urinary hydroxyproline measurement in 24-hour or spot urine is easier to collect. Spot urine testing is measurement of choice in screening for bony involvement by malignancy because collagen intake does not influence hydroxyproline/creatinine.
In individuals with bony metastases (most notably those with prostate cancer), increases in serum and urine hydroxyproline levels are observed prior to radiographic evidence of metastatic lesions; thus, these measurements are potential markers of skeletal metastatic disease and may be recommended in settings where advanced imaging modalities are not readily available 10. Moreover, hydroxyproline level measurements may permit illness monitoring and assessment of treatment response in such settings 10.
References- Uitto J CM, Gallo R, Eisen AZ. 7th ed. Collagen, Elastic Fibers, and Extracellular Matrix of the Dermis. Wolff K GL, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, ed. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw-Hill; 2008.
- Laitinen O, Nikkila EA, Kivirikko KI. Hydroxyproline in the serum and urine. Normal values and clinical significance. Acta medica Scandinavica. Mar 1966. 179(3):275-284.
- Gasser AB, Depierre D, Courvoisier B. Total urinary and free serum hydroxyproline in metastatic bone disease. Br J Cancer. Mar 1979. 39(3):280-283.
- Cissell DD, Link JM, Hu JC, Athanasiou KA. A Modified Hydroxyproline Assay Based on Hydrochloric Acid in Ehrlich’s Solution Accurately Measures Tissue Collagen Content. Tissue Eng Part C Methods. 2017;23(4):243–250. doi:10.1089/ten.tec.2017.0018 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5397204
- Neuman R.E., and Logan M.A. The determination of hydroxyproline. J Biol Chem 184, 299, 1950
- Colgrave M.L., Allingham P.G., and Jones A. Hydroxyproline quantification for the estimation of collagen in tissue using multiple reaction monitoring mass spectrometry. J Chromatogr A 1212, 150, 2008
- Junquiera L.C., Junqueira L.C., and Brentani R.R. A simple and sensitive method for the quantitative estimation of collagen. Anal Biochem 94, 96, 1979
- Yasmin H., Kabashima T., Rahman M.S., Shibata T., and Kai M. Amplified and selective assay of collagens by enzymatic and fluorescent reactions. Sci Rep 4, 4950, 2014
- VW R. Biosynthesis of the Nutritionally Nonessential Amino Acids. Bender DA BK, Weil PA, Kennelly PJ, Murray RK, Rodwell VW, ed. Harper’s Illustrated Biochemistry. 29 ed. New York: McGraw-Hill; 2011.
- Romics I, Lengyel L. Study of serum and urine hydroxyproline level of patients with prostatic cancer. European urology. 1984. 10(6):395-397.