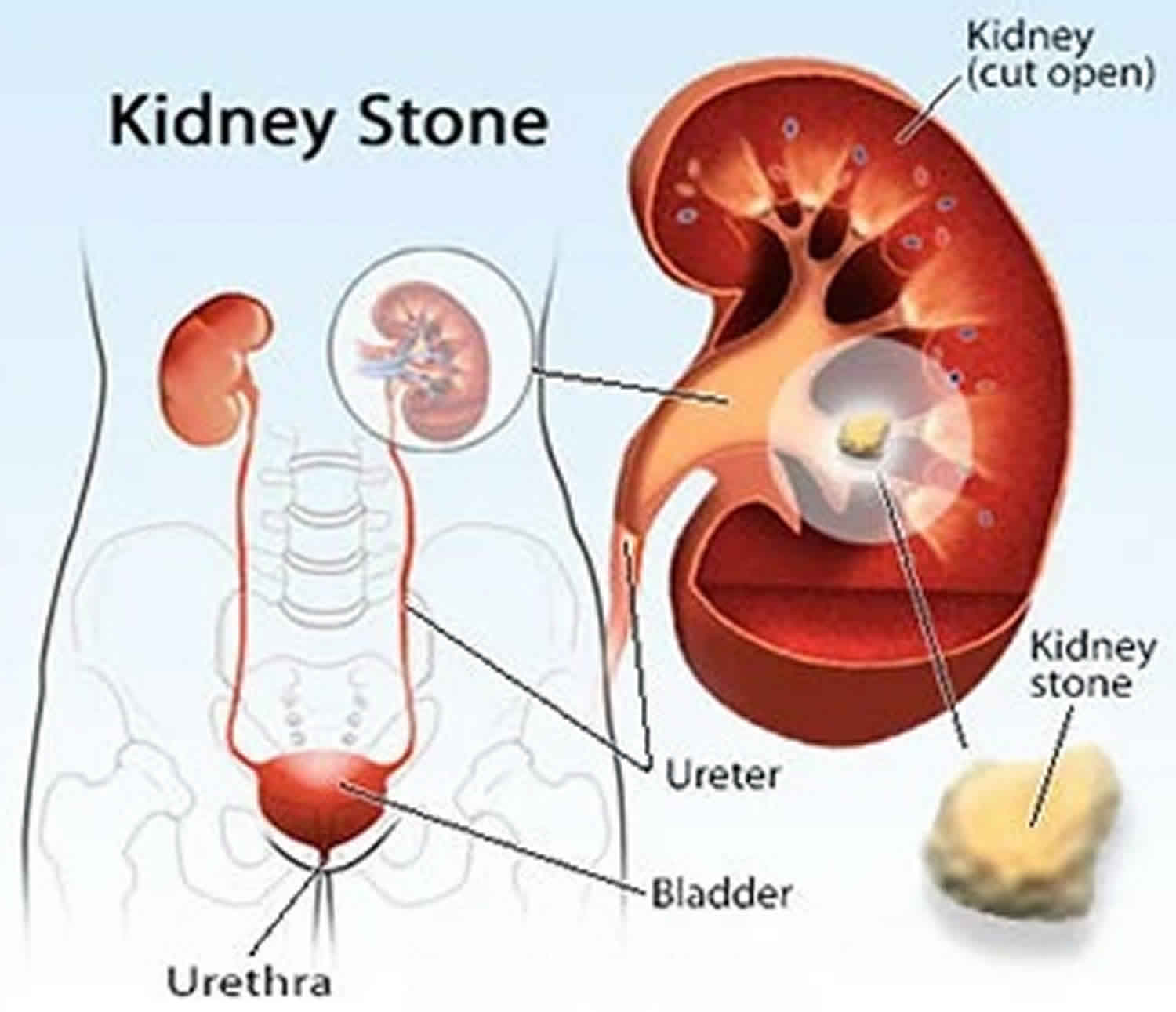
Hypocitraturia
Hypocitraturia means low amount of citrate in the urine, is an important risk factor for kidney stone formation (nephrolithiasis) 1. Hypocitraturia is a common metabolic abnormality found in 20% to 60% of kidney stone formers. Citrate in the urine has long been recognized as an inhibitor of calcium salt crystallization. Citrate is a known inhibitor of kidney stone formation, working through a variety of mechanisms. In the renal tubule, citrate complexes with calcium in the urine, increasing its solubility and reducing the concentration of free calcium in the urine. This calcium-citrate complex limits calcium supersaturation and prevents nucleation of both calcium oxalate and calcium phosphate, at least partly through interactions with Tamm-Horsfall protein 2. Additionally, citrate prevents crystal agglomeration and growth through its ability to bind to the crystal’s surface and may also prevent adhesion of calcium oxalate to renal epithelial cells 3.
Citrate is the dissociated anion of citric acid, a weak acid that is ingested in the diet and produced endogenously in the tricarboxylic acid cycle. Hypocitraturia is generally defined as urinary citrate excretion of less than 320 mg (1.67 mmol) per day for adults 4. In healthy individuals the mean urinary citrate excretion is 640 mg/day 5.
The excretion of citrate in the urine is a function of filtration, reabsorption, peritubular transport, and synthesis by the renal tubular cell. The proximal tubule reabsorbs most (70-90%) of the filtered citrate through the sodium-citrate cotransporter, and citrate secretion is negligible.
Acid-base status plays the most significant role in citrate excretion. Alkalosis enhances citrate excretion, while acidosis decreases it. In acidosis, increased citrate utilization by the mitochondria in the tricarboxylic acid cycle occurs. This results in lower intracellular levels of citrate, facilitating citrate reabsorption and hence reducing citrate excretion. Citrate excretion is impaired by acidosis, hypokalemia (causing intracellular acidosis), a high–animal protein diet (with an elevated acid-ash content), and urinary tract infection (UTI).
In summary, hypocitraturia enhances urine calcium salt supersaturation and reduces calcium crystallization inhibition, increasing the risk of calcium nephrolithiasis. It also may play a role in uric acid solubility and uric acid stone formation.
Urinary calculi secondary to hypocitraturia are typically composed of some hydroxyapatite (calcium phosphate), along with calcium oxalate.
Hypocitraturia commonly is observed in patients with kidney stone (nephrolithiasis), metabolic acidosis, and chronic diarrheal syndromes 5. Hypocitraturia itself may not be associated with significant mortality or morbidity; however, potential complications of kidney stone (nephrolithiasis) secondary to hypocitraturia can be significant. Potential morbidity due to nephrolithiasis includes hematuria, ureteral obstruction, urinary tract infection (UTI), urosepsis, and loss of kidney function.
Hypocitraturia should be managed through a combination of dietary modifications, oral alkali, and possibly lemonade or other citrus juice based therapy.
Currently, the preferred treatment for hypocitraturia is with potassium citrate (eg, Urocit-K, Polycitra-K) supplementation. The sodium-containing forms of citrate (eg, Bicitra, Polycitra) and sodium bicarbonate do not have the same beneficial effects, because the excess sodium in these preparations actually aggravates hypercalciuria and hyperuricosuria 6.
The objective of treatment with potassium citrate (eg, Urocit-K, Polycitra-K) is to provide potassium citrate in sufficient dosage to restore normal urinary citrate (ie, > 320 mg/d and as close to the normal mean of 640 mg/day as possible) and to increase urinary pH to a level between 6.0 and 7.0. Urinary citrate and/or urinary pH measurements should be evaluated every 4 months. Those results should be used to determine the adequacy of the initial dosage and to evaluate the effectiveness of any dosage change.
Potassium citrate is available in 5- or 10-mEq tablets (eg, Urocit-K) or as a liquid, powder, or syrup combining potassium citrate and citric acid (eg, Polycitra-K). The powder and syrup are mixed with water before ingestion.
The tablet formulation has been shown to produce less variability in the level of urinary citrate throughout the day, but the liquid form is better in short-bowel syndromes, in which absorption is a problem, and in more severe cases because of its higher citrate dose.
Note that doses of Urocit-K greater than 100 mEq/d have not been studied and should be avoided when possible. Obviously, the risk of hyperkalemia is increased when these higher dosages are used, so serum potassium levels should be monitored, particularly in cases of renal failure.
The pH change after institution of potassium citrate therapy generally is small, although reduced doses may be required, with frequent pH monitoring, to maintain the pH below 7.0-7.2. At pH levels above 7.2, the risk of calcium phosphate crystallization increases significantly. Calcium phosphate can coat an otherwise dissolvable uric acid stone and prevent it from dissolving, so avoiding overalkalinization when trying to dissolve pure uric acid calculi is particularly important.
Potassium-containing medications should be used with caution in patients with kidney insufficiency or in those receiving potassium-sparing diuretics.
Citrate therapy may be counterproductive in patients with struvite (infection) stones.
Meta-analysis shows that up to 48% of patients in long-term studies discontinue oral potassium citrate therapy because of side effects 7.
Hypocitraturia key points
- Hypocitraturia is generally defined as urinary citrate excretion less than 320 mg (1.67 mmol) per day for adults, is a common metabolic abnormality in kidney stone formers, occurring in 20% to 60% of cases.
- Modulation of citrate excretion in the kidney is influenced by multiple factors; however, pH (systemic, tubular, and intracellular) has the strongest impact.
- Although the majority of patients have idiopathic hypocitraturia, there are a number of causes for this abnormality, including distal renal tubular acidosis, hypokalemia, bowel dysfunction, and a high-protein, low-alkali diet.
- Other factors associated with altered citrate excretion include genetic factors, certain drugs (eg, acetazolamide, topiramate, angiotensin-converting enzyme inhibitors, and thiazide), renal insufficiency, hyperaldosteronism, type 1 glycogen storage disease, and exercise.
- Dietary modifications benefit the majority of patients with nephrolithiasis. These include high fluid and citrus fruit intake, normal calcium consumption, and restriction of sodium, oxalate, animal protein, and fructose intake.
- The administration of citrate preparations or other alkali has been demonstrated to benefit hypocitraturic stone formers. Although many forms of citrate have been used for these patients (potassium citrate, sodium citrate, potassium-magnesium-citrate), potassium citrate has emerged as the most beneficial.
- Patients who either cannot tolerate or cannot afford potassium citrate may benefit from consuming citrus juices, which contain significant amounts of citrate.
Citrate physiology
Citrate, a tricarboxylic acid, is synthesized in mitochondria from oxaloacetate and acetyl-CoA (coenzyme A) by the enzyme citrate synthase; it is a central component of the tricarboxylic acid (TCA) cycle. After first describing TCA cycle reactions, Krebs and colleagues 8 demonstrated that citrate is excreted in urine. Through its metabolism in the TCA cycle, citrate generates a significant amount of energy through reduced nicotinamide-adenine dinucleotide, guanosine triphosphate, and reduced flavin-adenine dinucleotide, creating CO2 and H2O in this process. If all the citrate in the kidney were metabolized, it would provide 10% of the renal energy requirement 9.
Citrate renal transport
In serum, the concentration of citrate is relatively constant, ranging from 0.05 mM to 0.3 mM 10. The majority of citrate exists complexed to divalent ions, such as calcium and magnesium, and is filtered freely at the glomerulus; reabsorption takes place predominantly in the proximal tubule 11. Because there is currently no evidence that significant citrate secretion occuring in the nephron, the extent of reabsorption largely regulates citrate excretion 11. In humans, 65% to 90% of the filtered load of citrate is reabsorbed 12.
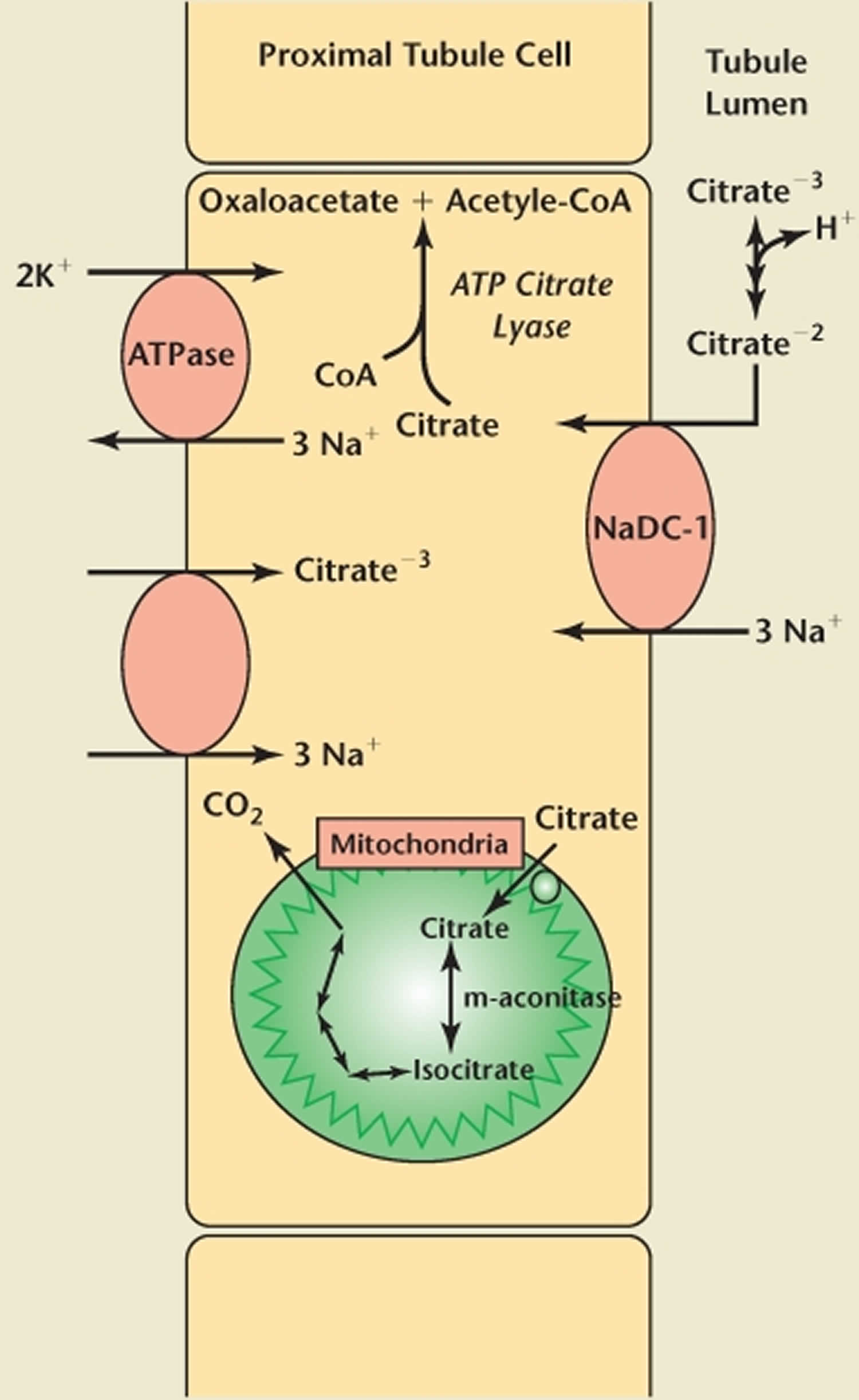
Transport of citrate across the apical membrane in the proximal tubule has been extensively studied. First cloned by Pajor 13, the dominant membrane protein is a sodium-dependent dicarboxylate transporter (NaDC-1) found not only in renal tubules, but also in the small intestine, colon, liver, and brain 14. The NaDC-1 transporter has broad specificity; it is also used by other TCA intermediates, including succinate and α-ketoglutarate (Figure 1) 15.
Apical membrane reabsorption of citrate is an electrogenic process requiring secondary active transport that is highly pH dependent 14. The binding of 3 sodium ions induces a conformational change in the NaDC (sodium-dependent dicarboxylate transporter) protein, which allows their cotransport with 1 uncomplexed, divalent citrate molecule (citrate−2) 16. This movement of ions creates the net transfer of a positive charge into the cell, relying on a basolateral Na/K adenosine triphosphatase to maintain electrical neutrality. Whereas the Km for this reaction is approximately 15 µM/L, the Km for the transport of trivalent citrate (citrate−3) is far greater. Thus, more of the former is transported, whereas the latter acts as a competitive inhibitor 17. The pKa of citrate is 5.6; thus the majority of citrate exists as citrate−3 in the renal tubule 12. These properties reflect the important role that pH has in the regulation of citrate absorption in the nephron.
Once absorbed through the apical membrane of the proximal tubule, citrate does not pass through a basolateral transporter into the interstitium. In fact, there is some evidence that citrate moves in the opposite direction, though transport across this membrane is not well understood 18. Unlike the movement of citrate across the apical membrane, transport on the basolateral side into proximal tubular cells is electroneutral and not affected by changes in pH 19. This transport is also sodium dependent. Citrate is thought to be absorbed across the basolateral membrane and into proximal tubular cells in its trivalent form, reflecting its pH independence 19.
Figure 1. Citrate renal transport
Footnote: Representation of proximal tubule citrate absorption and metabolism.
Abbreviations: CoA = coenzyme A; ATP = adenosine triphosphate.
[Source 1 ]Renal citrate metabolism
A portion of the citrate absorbed in the kidney will pass into the mitochondria for utilization in the TCA cycle.18 Adenosine triphosphate citrate lyase, however, reacts with some of the citrate while it remains in the cytoplasm.26 This enzyme catalyzes the conversion of citrate and CoA to oxaloacetate and acetyl-CoA. Acetyl-CoA produced through this pathway may be used in the synthesis of fatty acids and cholesterol and is also involved in other types of metabolism. Oxaloacetate is a substrate for gluconeogenesis, which is catalyzed by the enzyme phosphoenolpyruvate carboxykinase.26
Gastrointestinal citrate absorption
Information about the intestinal absorption of citrate is limited. It is thought that citrate absorption in the small intestine uses the same NaDC transporter as the proximal tubule.19,27,28 Such transporters have also been found in membranes within the intestines of several animal species.29–34 Clinically, there is strong evidence for intestinal absorption of citrate. Fegan and associates,35 using an intestinal washout technique, reported 96% to 98% absorption of an oral citrate load within 3 hours in both stone-forming and normal subjects. Others have demonstrated a significant increase in serum citrate after an oral citrate load.36 Patients with intestinal malabsorption syndromes tend to have low urinary citrate excretion,37 but this association is thought to be due to gastrointestinal bicarbonate wasting.38,39 In contrast to renal citrate transport, there is evidence for citrate efflux from intestinal enterocytes.32
pH regulation of citrate excretion
Modulation of citrate excretion in the kidney is influenced by multiple factors; however, pH (systemic, tubular, and intracellular) has the strongest impact. It has long been known that acidosis decreases renal citrate excretion, whereas alkalosis increases it 20. There are several mechanisms through which pH exerts these effects. As noted previously, citrate is reabsorbed through the sodium citrate cotransporter as citrate−2 but exists predominately as citrate−3 within renal tubules. Lowering tubular pH increases the concentration of citrate−2 available for transport and reduces the concentration of citrate−3, thereby limiting its competitive inhibition. Even small decreases in tubular pH (7.4 to 7.2) significantly increase tubular reabsorption 21. Acute acidosis is associated with increased activity of the NaDC transporter 22 and chronic acidosis leads to increased transporter messenger ribonucleic acid and the transporter itself 23.
In addition to increased capability to transport citrate in acidotic states, acidosis drives citrate metabolism. This is demonstrated by an increase in citrate reabsorption and decreased citrate tissue levels reported with acidosis 14. Intracellular acidosis has been shown to increase mitochondrial citrate transport and oxidation 14. Alkalosis, which produces the opposite effect, is thought to be modulated at least partly through pH regulation of the mitochondrial enzyme aconitase (m-aconitase) 24. m-Aconitase is the enzyme responsible for the first step in citrate metabolism within the mitochondria. Alkali feeding decreases the activity and amount of this enzyme, thus inhibiting citrate metabolism; acidosis increases the activity of the enzyme 24. In addition to mitochondrial oxidation, intracellular acidosis increases citrate metabolism in the cytoplasm through increased activity of adenosine triphosphate citrate lyase 25. Products of both cytoplasmic and mitochondrial metabolism are further metabolized by phosphoenolpyruvate carboxykinase, whose activity is also increased with acidosis 26. Although these metabolic changes are also seen with reductions in systemic pH, hypokalemia produces similar effects, suggesting a strong intracellular component to pH regulation of citrate metabolism 24.
Hypocitraturia causes
Hypocitraturia is most commonly idiopathic (unknown) in origin but may be caused by distal renal tubular acidosis (RTA), hypokalemia, bowel dysfunction, and a high-protein, low-alkali diet. Genetic factors, medications and other comorbid disorders also play a role.
The following are causes of hypocitraturia:
- Acid-base imbalance
- Distal (type 1) renal tubular acidosis
- Other systemic acidosis
- Diarrhea/malabsorption
- Chronic diarrheal syndrome
- Hypokalemia
- Diet
- Diet high in animal protein
- High sodium intake
- Ketosis promoting diets
- Low fruit/vegetable intake
- Starvation
- Medications
- Thiazide diuretic
- Amiloride
- Acetazolamide therapy
- ACE inhibitors
- Calcitonin
- Calcium
- Ethacrynic acid
- Lithium
- Topiramate
- Vitamin D
- Strenuous physical exercise
- Gout or gouty diathesis
- Active urinary tract infection (UTI)
- Genetic influence
- VDR polymorphisms
- NaDC-1 gene polymorphisms
- Other associated disorders
- Renal insufficiency
- Hyperaldosteronism
- Type 1 glycogen storage disease
- Hypocalciuria, hypomagnesuria
- Precursor compounds
- Metabolic inhibitors
Distal renal tubular acidosis
renal tubular acidosis is a term applied to several clinical syndromes of metabolic acidosis that result from specific defects in renal tubular hydrogen ion secretion and urinary acidification.
One of the more common presentations of hypocitraturia is distal renal tubular acidosis, which can occur in a complete or incomplete form. The complete form is characterized by hyperchloremic metabolic acidosis, hypokalemia, and elevated urine pH, while the incomplete form exhibits normal serum electrolytes but the inability to acidify urine following an ammonium chloride load 27.
Both forms can be associated with hypercalciuria and profound hypocitraturia. Combined with alkaline urine, such abnormalities place patients at high risk for calcium phosphate or, less commonly, calcium oxalate stone formation.
Chronic diarrheal syndrome
Chronic diarrheal syndrome results in fluid loss and intestinal alkali loss. Patients with chronic diarrhea and inflammatory bowel disease frequently have hypocitraturia due to bicarbonate loss from the intestinal tract. Hypocitraturia caused by renal tubular acidosis or chronic diarrheal syndrome is associated with other metabolic abnormalities (eg, hypercalciuria, hyperuricosuria) or may occur alone. In chronic diarrheal syndrome, other risk factors for stone formation often are present (eg, low urinary volume, hyperoxaluria, hypomagnesuria, low urinary pH).
Thiazide diuretic or acetazolamide therapy
Thiazide therapy may induce hypocitraturia owing to hypokalemia with resultant intracellular acidosis. Acetazolamide (a carbonic anhydrase inhibitor used in the treatment of glaucoma) produces changes in urine composition that are similar to those found in distal renal tubular acidosis. It causes hyperchloremic acidosis by inhibiting sodium bicarbonate reabsorption in the proximal tubule. Thus, hypocitraturia often occurs due to metabolic acidosis.
Topiramate
Therapy with topiramate (also a carbonic anhydrase inhibitor), a commonly used anti-seizure medication, has also been associated with an increased risk of formation of calcium phosphate stones. Warner et al have shown that the use of topiramate has a ‘dose-dependent’ effect on renal excretion of citrate. A 40% reduction in urinary citrate has been observed in patients on starting dose of topiramate; reduction of urinary citrate by as much as 65% may result at higher doses 28.
Angiotensin-converting enzyme inhibitors
Angiotensin-converting enzyme inhibitors or ACE inhibitors, by increasing adenosine triphosphate (ATP) citrate lyase activity, have also been shown to reduce urinary citrate concentrations 29.
Diet high in animal protein
A diet rich in animal protein (from elevated acid-ash content) promotes mild metabolic acidosis, leading to reduced citrate excretion, may produce hypocitraturia and a reduction in urine pH 30. Animal proteins contain sulfate and phosphate moieties that are excreted as acids.
Severely carbohydrate-restricted and animal protein-rich diets, such as the Atkins diet, further exacerbate this metabolic acidosis through the creation of ketones. Compared with a normal diet, both the induction and maintenance phases of an Atkins type diet promote lower urine pH and citrate excretion 30. Other forms of dietary protein may also influence citrate excretion. For example, rats fed a high-casein diet have lower citrate and increased calcium excretion. The reduced citrate excretion in these animals is thought to be due to increased activity of the NaDC transporter induced by casein 31. Fruits, vegetables, and dietary fiber, often under-consumed in kidney stone patients, provide a source of alkali with the potential to reverse the effects of protein consumption 32. When these foods are removed from the diets of normal patients, they have decreased urinary citrate excretion and higher saturations of calcium oxalate and calcium phosphate; opposite changes are seen with increased consumption of fruits and vegetables 33. High-sodium diets, possibly through a mild expansion acidosis, decrease urinary citrate 34. Finally, starvation has been shown to increase citrate absorption through an increase in NaDC transporters and systemic acidosis 35.
Strenuous physical exercise
Strenuous physical exercise which causes lactic acidosis can likewise produce hypocitraturia.
Gout and gouty diathesis
Gout and gouty diathesis are conditions that involve excessive serum uric acid, which often is associated with nephrolithiasis. Controlling the uric acid problem and its potential contribution to stone formation may involve limiting purine intake, controlling hepatic uric acid production, monitoring urinary uric acid levels, and checking or altering urinary acidity.
Active urinary tract infection
UTI with bacteria that degrade citrate lowers urinary citrate levels.
Chronic kidney disease (CKD)
As glomerular filtration rate (GFR) decreases, there is a stepwise decrease in the amount of citrate that is filtered; however, in the early stages of chronic kidney disease, the increased fractional excretion of citrate prevents an abrupt decline in urinary citrate, such that overt hypocitraturia is not usually observed until advanced stages of chronic kidney disease 36.
Primary yperaldosteronism
In this disease entity, both hypercalciuria and hypocitraturia occur via Na-dependent volume expansion and chronic hypokalemia 37.
Genetic associations
Vitamin D receptor (VDR) gene polymorphisms are epidemiologically associated with recurrent and familial stone disease 38. In the nephron, 1,25(OH)2D3, the active form of vitamin D, utilizes the vitamin D receptor (VDR) to modulate citrate metabolism and transport 39. It also plays a role in the protein kinase pathway, altering the function of NaDC transporters 40. Recently, certain vitamin D receptor (VDR) gene polymorphisms, specifically the bb and TT subtypes, have been demonstrated with higher frequency in hypocitraturic stone formers when compared with both normocitraturic stone formers and normal controls 39. Certain VDR haplotypes are also associated with higher familial incidence of stone disease and lower mean age of onset 41.
Shah and colleagues 42 suggest further genetic influences on citrate handling. They propose a codominant inheritance of alleles at a single locus based on their trimodality frequency distribution for citrate excretion. This theory would account for the apparent “low,” “medium,” and “high” phenotypic expression found in their study, which is similar to what they found when investigating the genetics of calcium oxalate stone disease 43. There has also been recent identification of a single nucleotide polymorphism in the gene encoding NaDC-1, which may be associated with reduced urinary citrate excretion in recurrent stone formers 44. Ethnic background does not seem to play a role in citrate excretion 45.
Risk factors in hypocitraturia
- Diet and fluid intake
- High meat intake increases the urinary excretion of calcium, oxalate, and uric acid and decreases urinary pH and citric excretion. The use of high-protein, low-carbohydrate diets for weight loss has led to concern about increased risk of stone formation, as these diets have been shown to be associated with decreased urinary citrate and pH levels and increased urine calcium and sodium levels in the induction and maintenance phases 46.
- Excessive dietary sodium can also result in hypocitraturia, leading to increased stone formation.
- Stone-provoking medications
- Hypercalciuria can result from administration of corticosteroids, aluminum-containing antacids, loop diuretics, and vitamin D.
- Hypocitraturia is often associated with thiazide diuretic or acetazolamide administration. Topiramate has also been found to be associated with an increased risk for urinary stones 47 and to cause hypocitraturia by its inhibition of renal carbonic anhydrases 28.
- Genetic risk factors
- Genetic factors play a key role in the pathogenesis of hypocitraturia. The Na+-dicarboxylate cotransporter NaDC1, encoded by the sodium-dicarboxylate cotransporter (SLC13A2) gene, is a major determinant of urinary citrate excretion and its biological functions are regulated also by the vitamin D/vitamin D receptor biological system 48. In a study of Thai patients 49, those with AA genotypes of the rs11567842 (A/G) variant in the SLC13A2 gene were more susceptible to hypocitraturia than those with GG genotypes.
Hypocitraturia symptoms
Hypocitraturia is asymptomatic. A kidney stone usually will not cause symptoms until it moves around within your kidney or passes into your ureters — the tubes connecting the kidneys and the bladder. If it becomes lodged in the ureters, it may block the flow of urine and cause the kidney to swell and the ureter to spasm, which can be very painful. At that point, you may experience these signs and symptoms:
- Severe, sharp pain in the side and back, below the ribs
- Pain that radiates to the lower abdomen and groin
- Pain that comes in waves and fluctuates in intensity
- Pain or burning sensation while urinating
Other signs and symptoms may include:
- Pink, red or brown urine
- Cloudy or foul-smelling urine
- A persistent need to urinate, urinating more often than usual or urinating in small amounts
- Nausea and vomiting
- Fever and chills if an infection is present
Pain caused by a kidney stone may change — for instance, shifting to a different location or increasing in intensity — as the stone moves through your urinary tract.
Abdominal tenderness may develop during renal colic, but peritoneal signs are not commonly found. Renal colic due to kidney stone disease is one of the most common reasons for visits to the emergency department for urologic care.
The following may be signs of kidney stones that need a doctor’s help:
- Extreme pain in your back or side that will not go away
- Blood in your urine
- Fever and chills
- Vomiting
- Urine that smells bad or looks cloudy
- A burning feeling when you urinate
Make an appointment with your doctor if you have any signs and symptoms that worry you.
Seek immediate medical attention if you experience:
- Pain so severe that you can’t sit still or find a comfortable position
- Pain accompanied by nausea and vomiting
- Pain accompanied by fever and chills
- Blood in your urine
- Difficulty passing urine
Hypocitraturia diagnosis
If your doctor suspects that you have a kidney stone, you may have diagnostic tests and procedures, such as:
- Blood testing. Blood tests may reveal too much calcium or uric acid in your blood. Blood test results help monitor the health of your kidneys and may lead your doctor to check for other medical conditions.
- Urine testing. The 24-hour urine collection test may show that you’re excreting too many stone-forming minerals or too few stone-preventing substances. For this test, your doctor may request that you perform two urine collections over two consecutive days.
- Imaging. Imaging tests may show kidney stones in your urinary tract. High-speed or dual energy computerized tomography (CT) may reveal even tiny stones. Simple abdominal X-rays are used less frequently because this kind of imaging test can miss small kidney stones. Ultrasound, a noninvasive test that is quick and easy to perform, is another imaging option to diagnose kidney stones.
- Analysis of passed stones. You may be asked to urinate through a strainer to catch stones that you pass. Lab analysis will reveal the makeup of your kidney stones. Your doctor uses this information to determine what’s causing your kidney stones and to form a plan to prevent more kidney stones.
Laboratory studies in hypocitraturia
24-hour urine collection
Hypocitraturia, defined as less than 320 mg of citrate excreted per 24-hour urine collection, is diagnosed by 24-hour urine collection for metabolic stone risk analysis. Many laboratories, however, have their own definition of normal citrate levels.
The collection should be undertaken in recurrent stone formers, children, patients with solitary kidneys, and, selectively, in first-time stone formers with either increased risk (eg, family history of stones, bone or bowel disease, gout, chronic UTI, nephrocalcinosis) or sufficientl motivation to follow long-term therapy for stone prophylaxis.
A 24-hour urine sample is obtained for analysis of a full stone risk profile while patients maintain their customary activity level, diet, and fluid intake. Some practitioners always use 2 separate 24-hour urine collections for initial evaluation, to avoid clinical confusion due to spurious variations in diet or activity. Patients should not have a metabolic stone risk profile while on a controlled diet in the hospital or while they are experiencing an acute renal colic attack.
Several commercial laboratories provide metabolic kidney stone prevention profiles, including Dianon, LabCorp, Litholink, Mission, Nichols, and Urocor. Some analyze only 24-hour urine data, while others include serum data and/or patient-reported clinical history and medications in their analysis. They calculate the total volume from the dilution of the volume marker and analyze metabolic risk factors (eg, calcium, oxalate, uric acid, citrate, pH), as well as total volume, sodium, phosphorus, and magnesium. Supersaturation ratios are calculated, and the risk of specific stone types is presented.
The 24-hour urine collection usually is deferred for several weeks after stone passage, although several studies have suggested that this may be unnecessary as long as the patient is following regular routine diet and activity.
If the evaluation results are normal, the 24-hour collection may be repeated twice. If the evaluation results of metabolic stone workups remain normal after 2 separate 24-hour urine collections, idiopathic nephrolithiasis is suspected. False-positive 24-hour urine collection results can be caused by the patient altering the usual diet while being tested.
The issue of defining what may be within the reference range versus what is optimal for a stone former also may exist. Increased fluid output almost always is recommended for recurrent stone formers, regardless of the measured amount on a 24-hour urine testing.
In a large database of over 20,000 stone-forming patients with computerized analysis of the urinary and serum laboratory data, fewer than 1% demonstrated no urinary or serum chemistry abnormality related to possible kidney stone disease.
Other studies
Other useful studies include the following:
- Urine analysis and culture
- Sequential multiple analysis of 20 chemical constituents – Serum calcium, phosphorus, electrolyte, uric acid, and creatinine levels
- Parathyroid hormone (PTH)
- Urinary stone/sediment analysis
Laboratory results by underlying condition
Type 1 renal tubular acidosis
Complete type 1 renal tubular acidosis is characterized by the following:
- Urine pH > 6.9
- High serum chloride level
- Low serum potassium level
- Low bicarbonate levels
- In some cases, hypercalciuria
Patients with incomplete type 1 renal tubular acidosis have normal electrolytes but demonstrate a poor response to an acid load (ammonium chloride, 100 mg/kg, administered orally; urine pH does not fall below 5.3). In some cases, hypercalciuria may be present.
Chronic diarrhea
Test results in chronic diarrhea include the following:
- Urine pH < 5.5
- Low urine volume
- Hypokalemia
- Hypomagnesemia
- Hypocalciuria
- Hypomagnesuria
Uric acid lithiasis
Some patients with uric acid stones may demonstrate unusually acidic urine and normal or near-normal uric acid excretion levels. In these cases, appropriate doses of potassium citrate should be used to alkalinize the urine sufficiently to prevent uric acid stone formation.
Imaging studies in hypocitraturia
Imaging studies used in the diagnosis of nephrolithiasis include the following:
- Kidneys, ureters, and bladder (KUB) radiography
- Intravenous pyelography (IVP)
- Renal ultrasonography
- Noncontrast spiral computed tomography (CT) scans
However, no imaging modality is sensitive or specific for hypocitraturia as an etiology for detected stones.
Kidneys, ureters, and bladder (KUB) radiography
Consider the following:
- KUB radiography is the least specific, but most available and inexpensive, imaging modality for stone detection.
- Radiopaque stones imply the presence of calcium oxalate, calcium phosphate, struvite, or cystine.
- Radiolucency may implicate uric acid stones
- Nephrocalcinosis may lead to consideration of type 1 renal tubular acidosis
- Large branching stones are more likely to be infection stones or cystine stones.
- KUB radiography is commonly used for follow-up of stone therapies or to screen for recurrence.
Kidney ultrasonography
This operator-dependent modality is widely available and noninvasive and does not use ionizing radiation. Renal ultrasonography is typically used in pregnant women, in acute screening, and in follow-up in conjunction with KUB radiography.
Intravenous pyelography (IVP) or intravenous urography (IVU)
These modalities are widely available. Their sensitivity and specificity are better than those of plain KUB radiography. However, intravenous pyelography (IVP) and intravenous urography (IVU) are invasive and entail increased radiation exposure. Contrast allergy and nephropathy are associated risks.
Noncontrast spiral computed tomography scanning
Noncontrast spiral CT has the best sensitivity and specificity for stone detection and is typically used in the immediate evaluation of patients with renal colic. In addition, cross-sectional imaging allows for evaluation of non–stone-related causes of flank or abdominal pain. However, the radiation exposure in noncontrast spiral CT scanning is greater than it is in KUB radiography.
Hypocitraturia treatment
The treatment of hypocitraturia should be aimed at correcting the underlying disorder that reduces urine citrate. If the patient has idiopathic hypocitraturia, induce a mild metabolic alkalosis to increase urine citrate. The administration of citrate preparations or other alkali has been demonstrated to benefit hypocitraturic stone formers 50. Although many forms of citrate have been used for these patients (potassium citrate, sodium citrate, potassium-magnesium-citrate), potassium citrate has emerged as the most tolerable and beneficial 51. These citrate preparations raise urinary citrate by providing an alkali load. Approximately 80-90% of patients with hypocitraturia are treated successfully with potassium citrate to raise their urine citrate levels. This reduces the risk of recurrent stone formation 52.
An advantage of the potassium preparations is that they may prevent or correct hypokalemia. They also increase urine pH, which benefits uric acid and cystine stone formers 53. Potassium citrate has also been demonstrated to prevent stone formation in patients with infection-related stones 54. It can be administered in conjunction with thiazide agents being used to reduce calcium excretion. Pak and associates 55 demonstrated a reduction in stone formation from 4.69 to 0.57 stones per patient per year when potassium citrate was added to the medical regimen of thiazide-unresponsive patients. They also showed a significant increase in urinary citrate excretion and pH in these patients. Others have shown that the addition of either potassium citrate or potassium-magnesium-citrate was more beneficial than potassium chloride for stone prevention in thiazide-resistant patients 56. Potassium-magnesium-citrate has not yet been approved for use in the United States.
Consider long-term medical treatment for a patient with recurrent kidney stone disease 57.
There have been 4 prospective randomized controlled trials aimed at preventing stone recurrence through the administration of citrate preparations. Treatment was for 3 years in 3 of these trials and for only 1 year for the other 58. Two of these studies used potassium citrate, 1 used sodium-potassium-citrate, and 1 used magnesium-potassium-citrate. Citrate doses ranged from 60 to 90 mEq/d. Urinary citrate excretion on average increased 31% to 115% in the treatment groups, compared with no change in the control groups. In 3 of the 4 studies, significantly higher rates of stone remission were seen in the treatment groups (72%–100% treatment, 20%–71.4% control). However, Hofbauer and associates 59, using sodium-potassium-citrate, did not see a statistical difference in stone recurrence between the treatment and control groups. Barcelo and colleagues 60 analyzed pre- and posttreatment procedure rates and found a significant 92% reduction in the treatment group, compared with a 20% reduction in the control group. In an intention-to-treat analysis of these 4 trials, Mattle and Hess 61 reported that 53.5% of alkali citrate-treated patients remained stone free through at least 1 year of follow-up, compared with only 35% of control group patients.
Two prospective randomized trials lasting at least 1 year looked at rates of stone clearance after shock wave lithotripsy. Soygur and colleagues 58 used potassium citrate therapy in patients with lower-pole renal stones. Cicerello and associates 54 administered sodium-potassium-citrate to subjects with stones in various locations in the kidney. Both groups found that significantly more patients in the treatment groups were stone free at 1 year of follow-up (44%–86% treatment, 12.5%–40% control). Additionally, they both found that a significantly higher percentage of control group patients had stone growth at 1 year. Less than 5% of the alkali citrate-treated patients, compared with up to 62.5% of the control patients, had an increase in the size of their residual stones on follow-up radiographic and ultrasound examinations. Interestingly, Cicerello and colleagues 54 reported that neither the treatment nor control patients with infection-related stones showed any progression in stone burden. On intention-to-treat analysis, 66% of alkali citrate-treated patients became stone free at 1 year, compared with only 27.5% of control patients, a statistically significant difference 54.
Side effects of alkali citrate therapy are usually minimal and often gastrointestinally related. Among randomized controlled trial patients, approximately 33% of treated patients, compared with 17% of placebo-treated patients, reported side effects from the medication 61. Complaints included abdominal bloating, diarrhea, nausea, and abdominal pain. Potassium citrate is available in 3 formulations: tablets, crystals for dilution, and oral solution. Liquid preparations have been shown to produce more gastrointestinal side effects than tablets (33% vs 9.3%) 62. Tablet potassium citrate, however, is not absorbed as completely as liquid preparations 63 and patients with chronic diarrheal syndromes typically have short gastrointestinal transit, which limits the effectiveness of potassium citrate tablets 64. Sodium-based alkali (sodium citrate, sodium bicarbonate) may need to be used in hypocitraturic patients who have diminished renal function or those who use potassium-sparing medications. This is mandated in those who are hyperkalemic.
The dose of alkali is based on the degree of hypocitraturia and acidosis, as well as on patient size (pediatric patients). Typical doses of potassium citrate for adults with idiopathic hypocitraturia and normal renal function range from 40 to 60 mEq per day. The dose for those with distal renal tubular acidosis may need to be higher because these individuals have profound hypocitraturia and acidosis and are frequently hypokalemic.
Patients being administered alkali therapy warrant follow-up with blood and urine testing. Serum electrolytes should be checked, urine pH measured, and 24-hour urine metabolic testing obtained. Urine pH may increase to a level that places the patient at risk for developing calcium phosphate stones. The latter stone type is becoming more prevalent, and some have questioned whether this could be due to the widespread use of potassium citrate 65. Therefore, if the pH is 7 or greater, the dose of alkali may need to be decreased. An exception is those with distal renal tubular acidosis who have high baseline urine pH. Adjustments of alkali dose in these patients are mainly based on serum electrolytes and 24-hour urine testing.
Patients who either cannot tolerate or cannot afford potassium citrate may benefit from consuming citrus juices, which contain significant amounts of citrate. Wabner and Pak 66 compared orange juice consumption (1.2 L/d) with potassium citrate (60 mEq/d). Increases in urinary pH and citrate excretion were similar for both. Orange juice, however, did not decrease urinary saturation of calcium oxalate, whereas potassium citrate did. Grapefruit juice, although shown to have significantly higher levels of citrate than orange juice 67, does not seem to reduce urinary risk factors for stone formation 68. Additionally, grapefruit inhibits cytochrome p-450, thereby altering the metabolism of many commonly used medications.
Lemonade has been reported to increase citrate consumption. This was initially reported by Seltzer and associates 69 who had hypocitraturic patients consume 2 L of a lemonade preparation per day (120 mL of concentrated lemon diluted up to 2 L with water). Citrate excretion increased 144%. Kang and associates 70 retrospectively compared the outcomes of patients receiving lemonade therapy (120 mL concentrated lemon juice diluted to 2 L with water) with those of patients receiving potassium citrate (40 mEq/d). They reported significant increases in renal excretion of citrate in both the lemonade and the potassium citrate groups; however, compared with lemonade consumption, patients taking potassium citrate had significantly greater increases in urinary citrate excretion, as well as increases in urine pH. Urine pH was not altered by lemonade 70. However, not all studies on lemonade therapy have demonstrated positive results. Penniston and associates 71 compared lemonade therapy (either 120 mL of concentrated lemon juice diluted in water or 1 L of sugar-free lemonade) with lemonade therapy (same preparation) combined with potassium citrate (20–90 mEq per day). Although both regimens initially promoted a significant increase in citrate excretion, only the potassium citrate cohort had a durable response. Koff and colleagues 72 performed a cross-over comparison of potassium citrate (60 mEq/d) and lemonade (90 mL of concentrated lemon juice diluted in 532 mL of water). Potassium citrate consumption significantly increased pH and citrate excretion, whereas this did not occur with lemonade. Odvina and colleagues 56 performed a study in which other nutrients were controlled. Lemonade (1200 mL/d), distilled water (1200 mL/d), and orange juice (1200 mL/d) were administered in a cross-over design. Only orange juice resulted in an increase in urine pH and citrate excretion. This investigator thought that this difference was due to orange juice having high potassium citrate content as compared with lemonade, which has a high level of citric acid. The former would theoretically result in an increased alkali load, whereas with the latter the benefits of citrate would be negated by the accompanying hydrogen proton that neutralizes the effects of citrate.
The citric acid contents of various juice preparations have been measured. Penniston and colleagues 73 quantified citrate content in natural and commercially available fruit juices, using ion chromatography. They found that natural lemon and lime juice contain the greatest quantity of citric acid, followed closely by lemon and lime juice concentrates. Grapefruit juice and orange juice contained significant amounts of citrate; however, both contained less than lemon and lime juices. In a similar study, Haleblian and associates 67 used nuclear magnetic resonance spectroscopy to quantify citrate content. Using this technique, they found grapefruit juice to have the highest citrate content, followed by lemon juice and orange juice. They also found that among low-calorie beverages, Crystal Light® lemonade (Kraft Foods, Glenview, IL) had the highest citrate levels.
Calcium citrate
Calcium citrate may be used in patients with enteric hyperoxaluria and hypocitraturia, as the calcium is available to bind oxalate in the intestinal lumen. This therapy can raise urinary citrate levels and lower urinary oxalate levels, but it can also raise urinary calcium levels. Potassium citrate is often used in addition to calcium citrate in patients with enteric hyperoxaluria and hypocitraturia to further elevate urinary citrate and pH levels.
Calcium citrate is often recommended for calcium supplementation in postmenopausal women and others at risk for osteoporosis. It increases urinary citrate levels in non–stone-forming patients but also raises urine calcium excretion and does not significantly increase or decrease the relative supersaturation of calcium oxalate.
Calcium citrate has not been well studied as therapy for hypocitraturia in idiopathic calcium oxalate stone formers and is not typically used for this purpose. Natural sources of citrate are citrus fruits. Lemons contain the most concentrated form of citrate and, when provided as lemonade, can increase fluid volume and citrate excretion 74.
Potassium-magnesium-citrate
Potassium-magnesium-citrate has been investigated and may be more effective than potassium citrate in the prevention of stones, because it increases not only urinary citrate but also urinary magnesium, which is another well-known inhibitor of stone formation. It is available over-the-counter and on the Internet but has not yet been widely embraced by the urologic community.
Low-normal urinary citrate level
In patients with a low-normal urinary citrate level (320-400 mg/d), administer potassium citrate (10 mEq orally two or three times daily). Consider optimizing 24-hour citrate to 600 mg per 24 hours.
Mild to moderate hypocitraturia
The recommended therapy in cases of mild to moderate hypocitraturia (100-320 mg/d) caused by diet is the administration of supplemental potassium citrate (initial dose 20 mEq oral two or three times daily) and dietary restriction of animal protein. The dose is adjusted based on urinary pH and citrate levels obtained at 2-3 months of treatment.
Once the patient’s condition has stabilized, repeat testing can be performed at 4- to 6-month intervals and then yearly if stable. Performing a complete, 24-hour urine metabolic screening that includes serum potassium, as well as urinary volume, calcium, uric acid, oxalate, phosphate, sodium, and magnesium, in addition to citrate, is important.
New problems may develop as the patient’s diet is adjusted. Adequate fluid volume needs to be maintained, even if this means dilution of the citrate concentration. When that occurs, appropriate adjustment of the potassium citrate dosage should be achieved to ensure an optimal concentration of roughly 300 mg of citrate per liter.
When hypocitraturia results from incomplete renal tubular acidosis, potassium citrate supplementation should be employed as appropriate.
Severe hypocitraturia
Severe hypocitraturia (< 100 mg/d) is treated with the following:
- Chronic diarrheal states – Liquid formulation of potassium citrate
- Complete distal renal tubular acidosis – Potassium citrate 20-40 mEq oral 2-4 times daily as needed to optimize urinary citrate excretion
- Infection stones (magnesium and ammonium phosphate or carbonate apatite) – Potassium citrate, antibiotics, stone removal
With regard to struvite (infection) stones, potassium citrate should be used cautiously in these situations, because alkalinization will increase the formation of these stones.
Hypocitraturia diet
Dietary modifications benefit the majority of patients with nephrolithiasis. These include high fluid and citrus fruit intake, normal calcium consumption, and restriction of sodium, oxalate, animal protein, and fructose intake 75. Increased consumption of fruits and vegetables has been demonstrated to significantly increase citrate excretion in hypocitraturic stone formers 33.
Hypocitraturia dietary approaches include the following:
- High fluid intake – Sufficient to produce 2 L or more of urine per day
- Sodium restriction – Avoidance of saltshaker use and processed or salty foods, with a goal sodium intake of 2400 mg daily
- Oxalate restriction – Patients should avoid nuts, legumes, dark roughage, chocolate, tea, and vitamin C supplements
- Restriction of animal proteins – Limit servings of meat products, especially at the same meal.
Increased citrus fruits, potassium-rich products, and alkalinizing foods
Orange juice is a good source of dietary citrate, containing approximately 50 mEq/L of potassium and 160 mEq/L of citrate.
In a study of the value of orange juice consumption in kidney stone prevention, Wabner and Pak found that, compared with potassium citrate, orange juice delivered an equivalent alkali load and caused a similar increase in urinary pH (6.48 versus 6.75, from 5.71) and urinary citrate (962 versus 944 from 571 mg/d). However, orange juice increased urinary oxalate and did not alter calcium excretion, whereas potassium citrate decreased urinary calcium without altering urinary oxalate 76.
A retrospective study compared outcomes with lemonade therapy (120 mL concentrated lemon juice diluted to 2L with water) versus potassium citrate (40 mEq/day). Although both groups showed a significant increase in the amount of urinary citrate, those taking potassium citrate had greater increases in urinary citrate, as well as increases in urine pH. Lemonade therapy did not alter urine pH 77.
Penniston et al used ion chromatography to perform a quantitative assessment of citrate in natural and commercially available fruit juices. Natural lemon and lime juice contained the highest amount of citric acid, followed by lemon and lime juice concentrates. Among low-calorie beverages, grapefruit juice and orange juice had the highest citrate content 78.
Calcium intake
Dietary calcium should not be restricted, as that can lead to increased mobilization of calcium and hypercalciuria. As well, with decreased calcium in the intestinal lumen, oxylate is more freely available for absorption, which can lead to hyperoxaluria.
References- Zuckerman JM, Assimos DG. Hypocitraturia: pathophysiology and medical management. Rev Urol. 2009;11(3):134-144. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2777061
- Hess B, Zipperle L, Jaeger P. Citrate and calcium effects on Tamm-Horsfall glycoprotein as a modifier of calcium oxalate crystal aggregation. Am J Physiol. 1993;265:F784–F791.
- Sheng X, Jung T, Wesson JA, Ward MD. Adhesion at calcium oxalate crystal surfaces and the effect of urinary constituents. Proc Natl Acad Sci U S A. 2005;102:267–272.
- Chow K, Dixon J, Gilpin S, et al. Citrate inhibitsgrowth of residual fragments in an in vitromodel of calcium oxalate renal stones. KidneyInt.2004;65:1724-1730.
- Hypocitraturia Overview of Potassium Citrate and Calcium Citrate. https://emedicine.medscape.com/article/444968-overview
- Pinheiro VB, Baxmann AC, Tiselius HG, Heilberg IP. The effect of sodium bicarbonate upon urinary citrate excretion in calcium stone formers. Urology. 2013 Jul. 82(1):33-7.
- Phillips R, Hanchanale VS, Myatt A, Somani B, Nabi G, Biyani CS. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst Rev. 2015 Oct 6. CD010057
- Krebs HA, Salvin E, Johnson WA. The formation of citric and alpha-ketoglutaric acids in the mammalian body. Biochem J. 1983;32:113–117.
- Nieth H, Schollmeyer P. Substrate-utilization of the human kidney. Nature. 1966;209:1244–1245.
- Minisola S, Rossi W, Pacitti MT, et al. Studies on citrate metabolism in normal subjects and kidney stone patients. Miner Electrolyte Metab. 1989;15:303–308.
- Brennan TS, Klahr S, Hamm LL. Citrate transport in rabbit nephron. Am J Physiol. 1986;251:F683–F689.
- Hamm LL. Renal handling of citrate. Kidney Int. 1990;38:728–735.
- Pajor AM. Sequence and functional characterization of a renal sodium/dicarboxylate cotransporter. J Biol Chem. 1995;270:5779–5785.
- Simpson DP. Citrate excretion: a window on renal metabolism. Am J Physiol. 1983;244:F223–F234.
- Pajor AM. Sodium-coupled transporters for Krebs cycle intermediates. Annu Rev Physiol. 1999;61:663–682.
- Hirayama B, Wright EM. Coupling between sodium and succinate transport across renal brush border membrane vesicles. Pflugers Arch. 1986;407(suppl 2):S174–S179.
- Barac-Nieto M. Effects of pH, calcium, and succinate on sodium citrate cotransport in renal microvilli. Am J Physiol. 1984;247:F282–F290.
- Chen X, Tsukaguchi H, Chen XZ, et al. Molecular and functional analysis of SDCT2, a novel rat sodium-dependent dicarboxylate transporter. J Clin Invest. 1999;103:1159–1168.
- Jørgensen KE, Kragh-Hansen U, Røigaard-Petersen H, Sheikh MI. Citrate uptake by basolateral and luminal membrane vesicles from rabbit kidney cortex. Am J Physiol. 1983;244:F686–F695.
- Dedmon RE, Wrong O. The excretion of organic anion in renal tubular acidosis with particular reference to citrate. Clin Sci. 1962;22:19–32.
- Brennan S, Hering-Smith K, Hamm LL. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol. 1988;255:F301–F306.
- Jenkins AD, Dousa TP, Smith LH. Transport of citrate across renal brush border membrane: effects of dietary acid and alkali loading. Am J Physiol. 1985;249:F590–F595.
- Aruga S, Wehrli S, Kaissling B, et al. Chronic metabolic acidosis increases NaDC-1 mRNA and protein abundance in rat kidney. Kidney Int. 2000;58:206–215.
- Melnick JZ, Preisig PA, Moe OW, et al. Renal cortical mitochondrial aconitase is regulated in hypo- and hypercitraturia. Kidney Int. 1998;54:160–165.
- Melnick JZ, Srere PA, Elshourbagy NA, et al. Adenosine triphosphate citrate lyase mediates hypocitraturia in rats. J Clin Invest. 1996;98:2381–2387.
- Hwang JJ, Curthoys NP. Effect of acute alterations in acid-base balance on rat renal glutaminase and phosphoenolpyruvate carboxykinase gene expression. J Biol Chem. 1991;266:9392–9396.
- Forni Ogna V, Blanchard A, Vargas-Poussou R, Ogna A, Baron S, Bertocchio JP, et al. Signification of distal urinary acidification defects in hypocitraturic patients. PLoS One. 2017. 12 (5):e0177329
- Warner BW, LaGrange CA, Tucker T, Bensalem-Owen M, Pais VM Jr. Induction of progressive profound hypocitraturia with increasing doses of topiramate. Urology. 2008 Jul. 72(1):29-32; discussion 32-3.
- Melnick JZ, Preisig PA, Haynes S, Pak CY, Sakhaee K, Alpern RJ. Converting enzyme inhibition causes hypocitraturia independent of acidosis or hypokalemia. Kidney Int. 1998 Nov. 54(5):1670-4.
- Reddy ST, Wang CY, Sakhaee K, et al. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002;40:265–274.
- Amanzadeh J, Gitomer WL, Zerwekh JE, et al. Effect of high protein diet on stone-forming propensity and bone loss in rats. Kidney Int. 2003;64:2142–2149.
- Hess B, Michel R, Takkinen R, et al. Risk factors for low urinary citrate in calcium nephrolithiasis: low vegetable fibre intake and low urine volume to be added to the list. Nephrol Dial Transplant. 1994;9:642–649.
- Meschi T, Maggiore U, Fiaccadori E, et al. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004;66:2402–2410.
- Sakhaee K, Harvey JA, Padalino PK, et al. The potential role of salt abuse on the risk for kidney stone formation. J Urol. 1993;150:310–312.
- Windus DW, Cohn DE, Heifets M. Effects of fasting on citrate transport by the brush-border membrane of rat kidney. Am J Physiol. 1986;251:F678–F682.
- Marangella M, Vitale C, Manganaro M, Cosseddu D, Martini C, Petrarulo M, et al. Renal handling of citrate in chronic renal insufficiency. Nephron. 1991. 57(4):439-43.
- Shey J, Cameron MA, Sakhaee K, Moe OW. Recurrent calcium nephrolithiasis associated with primary aldosteronism. Am J Kidney Dis. 2004 Jul. 44(1):e7-12.
- Nishijima S, Sugaya K, Naito A, et al. Association of vitamin D receptor gene polymorphism with urolithiasis. J Urol. 2002;167:2188–2191.
- Mossetti G, Vuotto P, Rendina D, et al. Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J Intern Med. 2003;253:194–200.
- Pajor AM, Sun N. Protein kinase C-mediated regulation of the renal Na(+)/dicarboxylate cotransporter, NaDC-1. Biochim Biophys Acta. 1999;1420:223–230.
- Mossetti G, Rendina D, Viceconti R, et al. The relationship of 3′ vitamin D receptor haplotypes to urinary supersaturation of calcium oxalate salts and to age at onset and familial prevalence of nephrolithiasis. Nephrol Dial Transplant. 2004;19:2259–2265.
- Shah O, Assimos DG, Holmes RP. Genetic and dietary factors in urinary citrate excretion. J Endourol. 2005;19:177–182.
- Goodman HO, Brommage R, Assimos DG, Holmes RP. Genes in idiopathic calcium oxalate stone disease. World J Urol. 1997;15:186–194.
- Okamoto N, Aruga S, Matsuzaki S, et al. Associations between renal sodium-citrate cotransporter (hNaDC-1) gene polymorphism and urinary citrate excretion in recurrent renal calcium stone formers and normal controls. Int J Urol. 2007;14:344–349.
- Maloney ME, Springhart WP, Ekeruo WO, et al. Ethnic background has minimal impact on the etiology of nephrolithiasis. J Urol. 2005;173:2001–2004.
- Reddy ST, Wang CY, Sakhaee K. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002 Aug. 40(2):265-74.
- Vega D, Maalouf NM, Sakhaee K. Increased propensity for calcium phosphate kidney stones with topiramate use. Expert Opin Drug Saf. 2007 Sep. 6(5):547-57.
- Rendina D, De Filippo G, Gianfrancesco F, Muscariello R, Schiano di Cola M, Strazzullo P, et al. Evidence for epistatic interaction between VDR and SLC13A2 genes in the pathogenesis of hypocitraturia in recurrent calcium oxalate stone formers. J Nephrol. 2017 Jun. 30 (3):411-418.
- Udomsilp P, Saepoo S, Ittiwut R, Shotelersuk V, Dissayabutra T, Boonla C, et al. rs11567842 SNP in SLC13A2 gene associates with hypocitraturia in Thai patients with nephrolithiasis. Genes Genomics. 2018 Sep. 40 (9):965-972.
- Pak CY, Fuller C. Idiopathic hypocitraturic calcium-oxalate nephrolithiasis successfully treated with potassium citrate. Ann Intern Med. 1986;104:33–37.
- Ettinger B, Pak CY, Citron JT, et al. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol. 1997;158:2069–2073.
- Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak CY. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol. 1993 Dec. 150(6):1761-4.
- Hamm LL, Hering-Smith KS. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31:885–893.
- Cicerello E, Merlo F, Gambaro G, et al. Effect of alkaline citrate therapy on clearance of residual renal stone fragments after extracorporeal shock wave lithotripsy in sterile calcium and infection nephrolithiasis patients. J Urol. 1994;151:5–9.
- Pak CY, Peterson R, Sakhaee K, et al. Correction of hypocitraturia and prevention of stone formation by combined thiazide and potassium citrate therapy in thiazide-unresponsive hypercalciuric nephrolithiasis. Am J Med. 1985;79:284–288.
- Odvina CV, Mason RP, Pak CY. Prevention of thiazide-induced hypokalemia without magnesium depletion by potassium-magnesium-citrate. Am J Ther. 2006;13:101–108.
- Karsli O, Izol V, Aridogan IA, Borekoglu A, Satar N. Metabolic risk factors and the effect of metaphylaxis in pediatric stone disease with hypocitraturia. Urolithiasis. 2013 Feb. 41(1):9-13.
- Soygur T, Akbay A, Kupeli S. Effect of potassium citrate therapy on stone recurrence and residual fragments after shockwave lithotripsy in lower caliceal calcium oxalate urolithiasis: a randomized controlled trial. J Endourol. 2002;16:149–152.
- Hofbauer J, Höbarth K, Szabo N, Marberger M. Alkali citrate prophylaxis in idiopathic recurrent calcium oxalate urolithiasis-a prospective randomized study. Br J Urol. 1994;73:362–365.
- Barcelo P, Wuhl O, Servitge E, et al. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol. 1993;150:1761–1764.
- Mattle D, Hess B. Preventive treatment of nephrolithiasis with alkali citrate-a critical review. Urol Res. 2005;33:73–79.
- Pak CY, Fuller C, Sakhaee K, et al. Long-term treatment of calcium nephrolithiasis with potassium citrate. J Urol. 1985;134:11–19.
- Fegan J, Khan R, Poindexter J, Pak CY. Gastrointestinal citrate absorption in nephrolithiasis. J Urol. 1992;147:1212–1214.
- Shenoy C. Hypocitraturia despite potassium citrate tablet supplementation. MedGenMed. 2006;8:8.
- Parks JH, Worcester EM, Coe FL, et al. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004;66:777–785.
- Wabner CL, Pak CY. Effect of orange juice consumption on urinary stone risk factors. J Urol. 1993;149:1405–1408.
- Haleblian GE, Leitao VA, Pierre SA, et al. Assessment of citrate concentrations in citrus fruit-based juices and beverages: implications for management of hypocitraturic nephrolithiasis. J Endourol. 2008;22:1359–1366.
- Goldfarb DS, Asplin JR. Effect of grapefruit juice on urinary lithogenicity. J Urol. 2001;166:263–267.
- Seltzer MA, Low RK, McDonald M, et al. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J Urol. 1996;156:907–909.
- Kang DE, Sur RL, Haleblian GE, et al. Long-term lemonade based dietary manipulation in patients with hypocitraturic nephrolithiasis. J Urol. 2007;177:1358–1362. discussion 1362; quiz 1591.
- Penniston KL, Steele TH, Nakada SY. Lemonade therapy increases urinary citrate and urine volumes in patients with recurrent calcium oxalate stone formation. Urology. 2007;70:856–860.
- Koff SG, Paquette EL, Cullen J, et al. Comparison between lemonade and potassium citrate and impact on urine pH and 24-hour urine parameters in patients with kidney stone formation. Urology. 2007;69:1013–1016.
- Penniston KL, Nakada SY, Holmes RP, Assimos DG. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J Endourol. 2008;22:567–570.
- Seltzer MA, Low RK, McDonald M. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J Urol. 1996 Sep. 156(3):907-9.
- Ekeruo WO, Tan YH, Young MD, et al. Metabolic risk factors and the impact of medical therapy on the management of nephrolithiasis in obese patients. J Urol. 2004;172:159–163.
- Wabner CL, Pak CY. Effect of orange juice consumption on urinary stone risk factors. J Urol. 1993 Jun. 149(6):1405-8.
- Kang DE, Sur RL, Haleblian GE, Fitzsimons NJ, Borawski KM, Preminger GM. Long-term lemonade based dietary manipulation in patients with hypocitraturic nephrolithiasis. J Urol. 2007 Apr. 177(4):1358-62; discussion 1362; quiz 1591.
- Penniston KL, Nakada SY, Holmes RP, Assimos DG. Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J Endourol. 2008 Mar. 22(3):567-70.