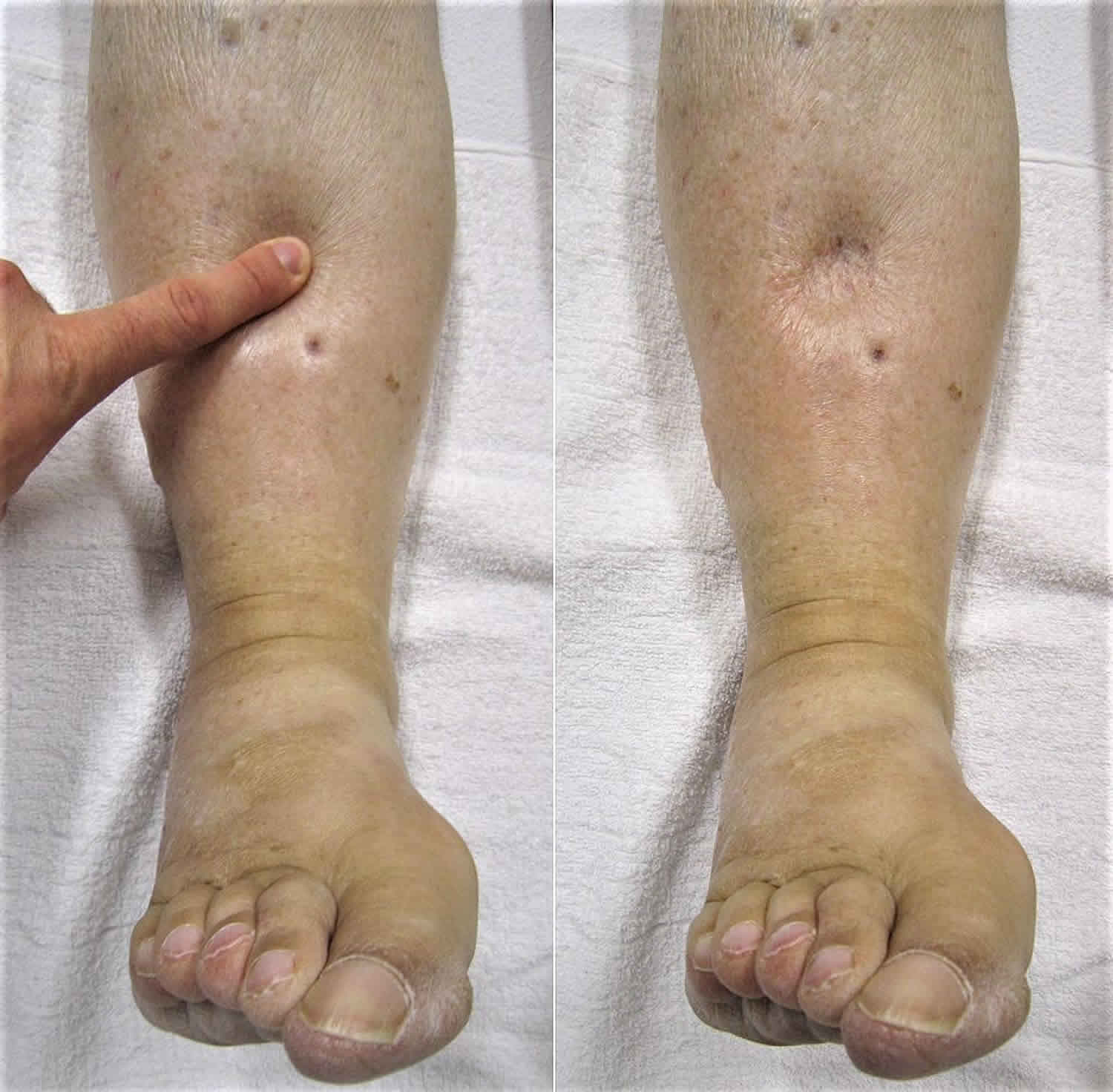
Hypoproteinemia
Hypoproteinemia is a condition where there is an abnormally low level of protein in the blood. Hypoproteinemia is a plasma or serum total protein concentration that is below that expected in people of the same age, sex and physiologic state. Hypoproteinemia can be a result of a reduction in concentration of albumin and globulin, or a reduction in either albumin or globulin concentrations. Abnormalities in plasma protein concentration include the following 1:
- Panhypoproteinemia with hypoalbuminemia and hypoglobulinemia
- Hypoproteinemia with hypoalbuminemia and normal globulin concentration
- Hypoproteinemia with hypoglobulinemia and normal albumin
- Normal total protein concentration with hypoalbuminemia and hyperglobulinemia; less commonly, hyperalbuminemia and hypoglobulinemia
The specific deficiency has important diagnostic significance.
Panhyproteinemia is hypoproteinemia with hypoalbuminemia and hypoglobulinemia can be either relative or absolute.
- Relative hypoproteinemia occurs when plasma protein concentrations are lower than normal, but the absolute content of protein in the vascular space is normal. This is a dilutional hypoproteinemia and is attributable to either excessive fluid therapy or excessive water intake. These causes are readily determined from a review of the history and treatment of the animal, and cases resolve within hours of discontinuation of fluid therapy or restriction of fluid intake.
- Absolute hypoproteinemia occurs when there is a reduction in the amount of plasma proteins in the vascular space in the presence of normal or almost normal plasma volume. The reduced protein concentration can be the result of impaired production or accelerated loss. Reduced production of all plasma proteins occurs only as part of malnutrition and starvation. Liver disease can cause a reduction in the concentration in plasma of those proteins produced by the liver (see following discussion) but in large animals is an unusual cause of hypoproteinemia. Loss of protein is a more common cause of hypoproteinemia.
A specific form of hypoproteinemia is hypoalbuminemia. Hypoalbuminemia is a common problem among persons with acute and chronic medical conditions. At the time of hospital admission, 20% of patients have hypoalbuminemia. Hypoalbuminemia can be caused by various conditions, including nephrotic syndrome, hepatic cirrhosis, heart failure, and malnutrition; however, most cases of hypoalbuminemia are caused by acute and chronic inflammatory responses.
Serum albumin level is an important prognostic indicator. Among hospitalized patients, lower serum albumin levels correlate with an increased risk of morbidity and mortality.
Albumin, the body’s predominant serum-binding protein, has several important functions. Albumin comprises 75-80% of normal plasma colloid oncotic pressure and 50% of protein content. When plasma proteins, especially albumin, no longer sustain sufficient colloid osmotic pressure to counterbalance hydrostatic pressure, edema develops. Although primarily in the intravascular space, albumin has a major trafficking function through the interstitium and lymphatics.
Albumin transports various substances, including bilirubin, fatty acids, metals, ions, hormones, and exogenous drugs. One consequence of hypoalbuminemia is that drugs that are usually protein bound are free in the plasma, allowing for higher drug levels, more rapid hepatic metabolism, or both.
Albumin reference serum values range from 3.5-4.5 g/dL, with a total body albumin content of 300-500 g. Albumin synthesis occurs only in hepatic cells at a rate of approximately 15 g/day in a healthy person, but the rate can vary significantly with various types of physiologic stress. The half-life of albumin is approximately 21 days, with a degradation rate of approximately 4% per day 2.
Alterations in albumin level affect platelet function.
Hypoproteinemia causes
Hypoproteinemia can result from decreased albumin production, defective synthesis because of hepatocyte damage, deficient intake of amino acids, increased losses of albumin via gastrointestinal or renal processes, and, most commonly, acute or chronic inflammation. The loss of proteins can also be from either from the vascular space into the extravascular compartment (e.g., endotoxemia, vasculitis) or from the body (compensated hemorrhage, glomerulonephritis, protein-losing enteropathy). This situation is evident as a reduction in concentrations of both albumin and globulins, and in hemorrhagic disease, by a reduction in hematocrit. Loss because of vascular leakage is usually evident as hypoproteinemia with normal or elevated hematocrit. Some of the many causes are discussed below.
One common cause of hypoproteinemia is due to excess protein in the urine (proteinuria), which can be a medical sign of nephrotic syndrome.
Other causes of hypoproteinemia include:
- Cirrhosis of liver
- Acute and chronic inflammation
- Drug side effect- Cefpodoxime, Cidofovir, Ibuprofen, Olanzapine, Thalidomide
- Endotoxemia: protein loss is secondary to leakage of protein from the vascular space into interstitial spaces because of increased capillary permeability.
- Extensive burns
- Congestive heart failure
- Hemorrhage: hypoproteinemia occurs when plasma volume is restored after severe hemorrhage, or in normovolemic anemia when there is persisting loss of blood. All causes of chronic blood loss can cause hypoproteinemia.
- Liver failure
- Malabsorption syndrome
- Preeclampsia 3
- Lymphatic blockage or mucosal disease
- Menetrier disease
- Protein losing enteropathy 4, the initial change is in plasma albumin concentration, but panhypoproteinemia ensues as the disease progresses. Diseases causing protein-losing enteropathy include the following:
- Water overload
- Nephrotic syndrome
- Intestinal enteropeptidase deficiency
- Kwashiorkor or protein malnutrition
- Malabsorption syndrome
- Capillary leak syndrome
- Atopic dermatitis with hypoproteinemia 5
Hypoalbuminemia
Hypoalbuminemia with normal or elevated plasma globulin concentration occurs in diseases in which there is insufficient production of albumin by the liver or excessive or selective loss of albumin compared with loss of globulin. Insufficient production of albumin occurs in diseases of the liver and in malnutrition or starvation. Diseases of the liver that cause hypoalbuminemia are usually diffuse, severe, and chronic.
Albumin has a lower molecular weight than most globulins, especially the immunoglobulins, and can be lost selectively in renal or gastrointestinal disease. Diseases associated with hypoalbuminemia and normal to elevated globulin concentrations include the following:
- Amyloidosis—loss of albumin into urine or the gastrointestinal tract is sometimes offset, in terms of plasma protein concentration, by increases in plasma globulin concentration.
- Chronic peritonitis or pleuritis—loss of albumin into the inflammatory exudate is offset, in terms of plasma total protein concentration, by increases in plasma globulin concentration.
- Renal disease
- Glomerulonephritis—because of changes in the size and charge on proteins of the glomerular membrane, albumin is not prevented from entering the ultrafiltrate and is lost in urine. Any diseases affecting the glomeruli can cause albumin loss.
- Pyelonephritis
Hypogammaglobulinemia
Hypoglobulinemia with normal plasma albumin concentration occurs in few diseases. Notably, it is a feature of failure of transfer of passive immunity in neonates. Hypoglobulinemia is an unusual isolated defect in other diseases. It can be detectable in immunodeficiencies causing decreased production of gammaglobulins, such as combined variable immunodeficiency.
Hypofibrinogenemia
Hypofibrinogenemia usually only occurs as part of disseminated intravascular coagulation, although it could conceivably occur in chronic liver failure.
Protein malnutrition
Deficient protein intake results in the rapid loss of cellular ribonucleic acid and disaggregation of the endoplasmic reticulum–bound polysomes and, therefore, decreased albumin synthesis. Albumin synthesis can decrease by more than one third during a 24-hour fast. Albumin synthesis may be stimulated by amino acids produced in the urea cycle, such as ornithine.
Defective synthesis
In patients with cirrhosis, synthesis is decreased because of the loss of hepatic cell mass. Also, portal blood flow is often decreased and poorly distributed, leading to maldistribution of nutrients and oxygen. The flow of substrate may affect certain functions of the liver, including protein synthesis, which is decreased in patients with cirrhosis who lack ascites. Albumin synthesis may actually increase in patients with cirrhosis who have ascites, possibly because of a change in hepatic interstitial colloid levels, which may act as an overriding stimulus for albumin production. Although synthesis is increased, the concentration of albumin is decreased because of dilution.
Nephrotic syndrome
This can produce hypoproteinemia by massive proteinuria, with 3.5 g or more of protein lost within 24 hours. Albumin is filtered by the glomerulus and catabolized by the renal tubules into amino acids that are recycled. In patients with chronic renal disease, in whom both glomerular and tubular diseases are present, excessive protein filtration may lead to both increased protein loss and increased degradation. Only at higher rates of albuminuria (>100 mg/kg/day) and only when the diet is adequate is albumin synthesis increased.
Protein-losing enteropathy
Under normal conditions, less than 10% of the total albumin is lost through the intestine. This fact has been confirmed by comparing albumin labeled with chromium-51, which helps measure intestinal losses, to albumin labeled with iodine-125, which helps measure overall degradation. In cases of protein-losing enteropathy related to bacterial overgrowth, hypoproteinemia is exacerbated by peripheral factors that inhibit albumin synthesis by mechanisms similar to those observed with burns, trauma, infection, and carcinoma.
Extensive burns
The skin is the major site for extravascular albumin storage and is the major exchangeable albumin pool needed to maintain plasma levels. Hypoalbuminemia results from direct losses of albumin from tissue damage, from compromised hepatic blood flow due to volume loss, and from inhibitory tissue factors (eg, tumor necrosis factor, interleukin-1, interleukin-6) released at the burn sites.
Lymphatic blockage or mucosal disease
Diseases that result in protein loss from the intestine are divided into 2 main types. The first is lymphatic blockage, which can be caused by constrictive pericarditis, ataxia telangiectasia, and mesenteric blockage due to tumor. The second is mucosal disease with direct loss into the bowel, which is observed with (1) inflammatory bowel disease and sprue and (2) bacterial overgrowth, as in blind loop syndrome after intestinal bypass surgery.
Hemodilution
In the presence of ascites from any cause, the serum albumin level is not a good index of the residual synthetic capacity of the liver unless actual radioisotopic measurements of production are used. With ascites, synthesis may be normal or even increased, but serum levels are low because of the larger volume of distribution. This is true even for ascites due to cirrhosis.
Congestive heart failure
The synthesis of albumin is normal in patients with congestive heart failure. Hypoalbuminemia results from an increased volume of distribution.
Oncotic pressure increase
The serum oncotic pressure partially regulates albumin synthesis. The regulation site may be the oncotic content in the hepatic interstitial volume because albumin synthesis is inversely related to the content of this volume. Conditions that increase other osmotically active substances in the serum tend to decrease the serum albumin concentration by decreasing synthesis. Examples include elevated serum globulin levels in hepatitis and hypergammaglobulinemia.
Acute and chronic inflammation
Albumin levels that are low because of acute inflammation should normalize within weeks of resolution of the inflammation. Persistent hypoproteinemia beyond this point should prompt an investigation for an ongoing inflammatory process. The cytokines (TNF, IL-6) released as part of the inflammatory response to physiologic stress (infection, surgery, trauma) can decrease serum albumin by the following mechanisms:
- Increased vascular permeability (allowing albumin to diffuse into the extravascular space)
- Increased albumin degradation
- Decreased synthesis (among other mechanisms, by activating TNF-a, which decreases transcription of the albumin gene)
Menetrier disease
Ménétrier disease or Menetrier disease, is an uncommon condition characterized by protein-losing and hypertrophic gastropathy 6. In adults (mean age at diagnosis is 55), the disorder of Menetrier disease usually presents with an insidious onset and a progressive clinical course 7. Menetrier disease typical clinical symptoms including nausea, vomiting, diarrhea, epigastric pain, weight loss, malnutrition, fatigue, and peripheral edema due to hypoalbuminemia 8.
The definitive cause of Menetrier disease is still unknown, but Helicobacter pylori infection is believed to have some relation with it 9. Although the definite etiology of Menetrier disease in adults still remains unknown, it often coexists with some specific infections, such as cytomegalovirus, Helicobacter pylori, herpes virus, human immunodeficiency virus, mycoplasma 10 as well as nonspecific ulcerative colitis 11. Nevertheless, Menetrier disease patients can show no remission when specific therapies are employed in these disorders 8. Above all, in order to establish the correct diagnosis of Menetrier disease, the histological findings, endoscopic and clinical features all should be necessary. Since the knowledge regarding its pathogenesis and effective therapeutic management has been lacking so far, Menetrier disease should be paid more attention to with particular treatment and ruled out of other similar diseases.
Menetrier disease should be suspected in cases of upper gastrointestinal tract symptoms and hypertrophied gastric mucosa with or without Helicobacter pylori or hypoalbuminemia. Before Menetrier disease is diagnosed, some other hypertrophic gastropathies, including lymphoma, polyposis, and suspected gastric malignancies, should discriminate from it. Further, it must be based on a comprehensive collection of data concerning clinical, endoscopic, laboratory, and histopathological findings. Menetrier disease is generally shown by huge gastric mucosal folds in the body and fundus, with antral sparing. However, there are some reports showing that Menetrier disease affects the entire stomach 12, the duodenum 13 and the small bowel 14. Ding et al 6 described a patient who bears Helicobacter pylori infection and Menetrier disease, and this disorder is involved not only in stomach, but also in both the small bowel and total colon. However, as for the colon, the lesion is especially obvious in the cecum and ascending colon. Helicobacter pylori eradication treatment was initiated and provided therapeutic benefit to this patient.
Hypoproteinemia symptoms
The potential underlying causes of hypoproteinemia are numerous. The clinical presentation, physical examination findings, and laboratory results associated with hypoproteinemia depends on the underlying disease process.
Gather past medical history for a history of liver or renal failure, hypothyroidism, malignancy, and malabsorption. Evaluate the patient for appropriate dietary intake. Seek potential causes of acute or chronic inflammation that could explain the low albumin levels.
Physical examination
Abnormal physical examination findings may be found in multiple organ systems depending on the underlying disease. The following findings suggest the potential underlying disease processes rather than the underlying hypoalbuminemia, per se:
- Head, eyes, ears, nose, and throat – Facial edema, macroglossia, parotid swelling, conjunctival icterus, temporal wasting
- Integumentary – Loss of subcutaneous fat, delayed wound healing, dry coarse skin, painful dermatoses, peripheral edema, thin hair, spider angiomas, palmar erythema, changes due to surgery and burns, jaundice
- Cardiovascular – Bradycardia, hypotension, cardiomegaly
- Respiratory – Decreased respiratory expansion due to pleural effusion and weakened intercostal muscles
- Gastrointestinal – Hepatosplenomegaly, ascites
- Musculoskeletal – Muscle wasting, growth retardation in children, atrophy of the interosseus hand muscles
- Neurological – Encephalopathy, asterixis
- Genitourinary – Testicular atrophy
- Endocrine – Gynecomastia, hypothermia, thyromegaly
- Other – Various other signs related to associated specific nutrient deficiencies
Hypoproteinemia diagnosis
Clinical suspicion of the underlying disease process should guide appropriate laboratory studies, some of which are as follows:
- Malnutrition: Lymphocyte count and blood urea nitrogen levels are decreased. Transferrin, prealbumin, and retinol-binding protein have shorter half-lives compared with albumin and better reflect short-term changes in nutritional status than albumin, which has a long half-life.
- Inflammation: C-reactive protein levels and increased erythrocyte sedimentation rate are elevated.
- Nephrotic syndrome: The 24-hour urine collection contains more than 3 g of protein in 24 hours.
- Cirrhosis: Liver function test findings (transaminase levels) may be elevated or normal in patients who are cirrhotic. Coagulation studies may be abnormal. Cirrhosis has numerous potential etiologies, and more specific studies, such as hepatitis screening, may be needed.
- Malabsorption: Fecal fat studies including Sudan qualitative stain for fat, 72-hour quantitative fecal fat collection, and fecal a-1-antitrypsin clearance are needed.
Serum protein electrophoresis results help to determine if hypergammaglobulinemia is present.
None of the various correction factors for determining the effects of hypoalbuminemia on the plasma calcium concentration has proven reliable. Corrected calcium (mg/dL) is equal to measured total calcium (mg/dL) plus 0.8 (average normal albumin level of 4.4 minus serum albumin [g/dL]). The only method of identifying true (ionized) hypocalcemia in the presence of hypoalbuminemia is to measure the ionized fraction directly.
Elderly patients living in nursing homes or other institutionalized settings who have hypoalbuminemia should be evaluated for treatable comorbid conditions contributing to the malnutrition (eg, medications causing decreased appetite, thyroid dysfunction, diabetes, malabsorption, depression, cognitive impairment).
Imaging studies
Imaging studies can be performed for the following:
- Liver ultrasound for evidence of cirrhosis
- Small bowel barium series for mucosal abnormalities typical of malabsorption syndromes
- Imaging studies as appropriate to seek infectious causes of inflammation and hypoalbuminemia (eg, chest radiography)
- Echocardiogram for congestive heart failure
Procedures
Procedures can be performed for the following:
- Liver biopsy to confirm cirrhosis. When hypoalbuminemia is due to cirrhosis, liver biopsy findings show a loss of hepatic architecture, fibrosis, and nodular regeneration. The pattern of injury and special stains can help determine the etiology of cirrhosis.
- Kidney biopsy to help evaluate etiology of nephrosis. When hypoalbuminemia is due to nephrotic syndrome secondary to a primary renal disorder, light microscopy may show sclerosis (focal glomerulosclerosis), mesangial immunoglobulin A (immunoglobulin A nephropathy), or no changes (minimal change disease). Electron microscopy may show subepithelial immunoglobulin G deposits (membranous glomerulonephritis).
Hypoproteinemia treatment
Hypoproteinemi treatment should focus on treating the underlying cause of hypoproteinemia. Exogenous albumin is not used for the purpose of raising serum albumin levels.
Depending on the clinical situation, multiple consultations may be necessary.
- Gastroenterologist
- Intensivist
- Nephrologist
- Surgeon
- Endocrinologist
- Registered dietitian
To help optimize fluid resuscitation with colloids in patients who are critically ill, volume status may be monitored with a central venous, pulmonary artery catheter or other minimal invasive techniques.
In patients who are critically ill, low calcium levels can be simply due to hypoalbuminemia, which has no clinical significance because the active fraction (ionized) is not affected. However, to prevent missing a second hypocalcemic disorder, measure the ionized calcium level whenever the albumin level is low.
In end-stage cirrhosis, albumin infusions decrease the incidence of renal insufficiency and decrease the mortality rate. Furthermore, in the setting of spontaneous bacterial peritonitis, the combination of cephalosporin and albumin markedly increases survivorship, presumably by improving toxin clearance.
Indications and the use of albumin administration in critically ill patients is an area of controversy; studies to clarify these issues are ongoing 15. Two major clinical trials compared albumin as a volume expander to crystalloids in the management of circulatory shock. Neither study specifically addressed the management of hypoalbuminemia. Both the SAFE 16 and the ALBIOS study 17 compared crystalloid to albumin infusions, and both documented a small but statically significant increase in serum albumin levels.
A separate question is whether or not albumin as a resuscitation fluid is useful as a volume expander or harmful for unrelated reasons. Although prior meta-analysis of small heterogeneous studies suggested that albumin infusions may be harmful as a volume expansion resuscitation fluid (increasing the mortality rate by 6% compared with crystalloid), the two large multicenter clinical trials (SAFE, ABLIOS) documented that, except in the SAFE trial, patients with neurotrauma had a worse outcome 16, whereas in the ABLIOS trial, patients with septic shock did better with albumin as a volume expander 17. In patients with neurotrauma, these trials found a small, but significant, increase in mortality compared with crystalloid therapy. However, neither trial was focused on treating hypoalbuminemia, but rather resuscitation from circulatory shock. In fact, outcomes are similar regardless of baseline serum albumin concentration; albumin administration for patients with hypoalbuminemia has no added benefit. Based on these studies of patients with septic shock, the benefit of colloid versus crystalloid administration for critically ill patients is not clearly demonstrated. Furthermore, the relative amount of albumin that can be effectively replenished by infusion is minimal, considering the normal albumin turnover rate.
These findings are in contrast to prior studies that also found no difference or increased mortality among those receiving albumin. Preliminary studies, including a favorable study by Dubois 18, examined the effect of albumin on organ function in critically ill patients, but additional work is needed in this area.
For patients with hypoalbuminemia and critical illness, the administration of albumin has not been shown to reduce mortality 19.
Limited indications for albumin supplementation exist, and considerable clinical judgment is required when albumin is administered. Albumin has been used as one part of regimens designed to prevent hepatorenal syndrome in patients with cirrhosis in whom forced diuresis is being performed; however, this is controversial and survival benefit has not been clearly established. However, in general, albumin is not given specifically to treat hypoalbuminemia, which is a marker for serious disease.
Like crystalloids, colloids produce a dilutional effect on hemoglobin and clotting factors. Clinicians need to monitor the appropriate parameters to safeguard against iatrogenic complications.
Considering fluid resuscitation more generally, recent investigation found that 6% hydroxyethyl starch used for resuscitation in patients with severe sepsis was associated with a significant increase in acute renal failure, calling this approach into question.
The most effective method of minimizing hypoalbuminemia and restoring serum oncotic pressure is by creating a positive nitrogen balance. This is usually accomplished by enteral protein feeding and reversing the inflammatory state, if present. Clearly, those patients with nephrotic syndrome need the nephrosis treated as a primary problem. The importance of enteral nutrition as an early and continued treatment for hypoalbuminemia cannot be overemphasized.
Diet
Support the underlying cause with adequate nutrition (sufficient high biological value protein and energy intake for anabolism).
Hypoproteinemia prognosis
Low serum albumin levels are an important predictor of morbidity and mortality. A meta-analysis of cohort studies found that, with every 10 g/L decrease in serum albumin, mortality was increased by 137% and morbidity increased by 89%. Patients with serum albumin levels of less than 35 at 3 months following discharge from the hospital have a 2.6 times greater 5-year mortality than those with a serum albumin levels greater than 40.
Hypoalbuminemia has also been studied as an important prognostic factor among subsets of patients, such as patients with severe sepsis, burns 20 and regional enteritis (Crohn disease) and has recently been associated with an increased risk of reintubation 21.
Whether or not hypoalbuminemia is merely a marker of severe protein malnutrition, which itself is a cause of increased morbidity and mortality, or an independent risk factor for death, is unclear. However, its association with a poor prognosis remains strong.
References- Diseases of the Hemolymphatic and Immune Systems. In Veterinary Medicine (Eleventh Edition), 2017. https://www.sciencedirect.com/book/9780702052460/veterinary-medicine
- Hypoalbuminemia. https://emedicine.medscape.com/article/166724-overview
- Chen H, Tao F, Fang X, Wang X. Association of hypoproteinemia in preeclampsia with maternal and perinatal outcomes: A retrospective analysis of high-risk women. J Res Med Sci. 2016;21:98. Published 2016 Nov 2. doi:10.4103/1735-1995.193170 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5244641
- Braamskamp MJ, Dolman KM, Tabbers MM. Clinical practice. Protein-losing enteropathy in children. Eur J Pediatr. 2010;169(10):1179–1185. doi:10.1007/s00431-010-1235-2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2926439
- Jo SY, Lee CH, Jung WJ, Kim SW, Hwang YH. Common features of atopic dermatitis with hypoproteinemia. Korean J Pediatr. 2018;61(11):348–354. doi:10.3345/kjp.2018.06324 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6258965
- Ding Q, Lu P, Ding S, et al. Ménétrier disease manifested by polyposis and involved in both the small bowel and entire colon: A Case Report. Medicine (Baltimore). 2016;95(36):e4685. doi:10.1097/MD.0000000000004685 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5023884
- Coffey RJ, Washington MK, Corless CL, et al. Menetrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach. J Clin Invest 2007; 117:70–80.
- Rich A, Toro TZ, Tanksley J, et al. Distinguishing Menetrier’s disease from its mimics. Gut 2010; 59:1617–1624.
- Fretzayas A, Moustaki M, Alexopoulou E, et al. Menetrier’s disease associated with Helicobacter pylori: three cases with sonographic findings and a literature review. Ann Trop Paediatr 2011; 31:141–147.
- diSibio G, McPhaul LW, Sarkisian A, et al. Menetrier’s disease associated with Kaposi’s sarcoma. Exp Mol Pathol 2008; 85:160–164.
- Nguyen VX, Nguyen CC, Leighton JA, et al. The association of Menetrier disease with ulcerative colitis: a case report with implications on the pathogenesis of Menetrier disease. Case Rep Gastroenterol 2010; 4:66–70.
- Konstantinidou AE, Morphopoulos G, Korkolopoulou P, et al. Menetrier disease of early infancy: a separate entity? J Pediatr Gastroenterol Nutr 2004; 39:177–182.
- Wu CS, Lin CJ, Chen TC, et al. Menetrier’s disease: a new variant with duodenal involvement. Am J Gastroenterol 1997; 92:1041–1043.
- Duprey KM, Ahmed S, Mishriki YY. Menetrier disease in an acquired immunodeficiency syndrome patient. South Med J 2010; 103:93–95.
- Vincent JL. Relevance of albumin in modern critical care medicine. Best Pract Res Clin Anaesthesiol. 2009 Jun. 23(2):183-91.
- Finfer S, Bellomo R, McEvoy S, Lo SK, Myburgh J, Neal B, et al. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: analysis of data from the saline versus albumin fluid evaluation (SAFE) study. BMJ. 2006 Nov 18. 333(7577):1044.
- Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014 Apr 10. 370 (15):1412-21.
- Dubois MJ, Orellana-Jimenez C, Melot C, De Backer D, Berre J, Leeman M, et al. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study. Crit Care Med. 2006 Oct. 34(10):2536-40.
- Roberts I, Blackhall K, Alderson P, Bunn F, Schierhout G. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. 2011 Nov 9. CD001208
- Eljaiek R, Dubois MJ. Hypoalbuminemia in the first 24h of admission is associated with organ dysfunction in burned patients. Burns. 2013 Feb. 39 (1):113-8.
- Rujirojindakul P, Geater AF, McNeil EB, Vasinanukorn P, Prathep S, Asim W, et al. Risk factors for reintubation in the post-anaesthetic care unit: a case-control study. Br J Anaesth. 2012 Oct. 109 (4):636-42.