Impella device
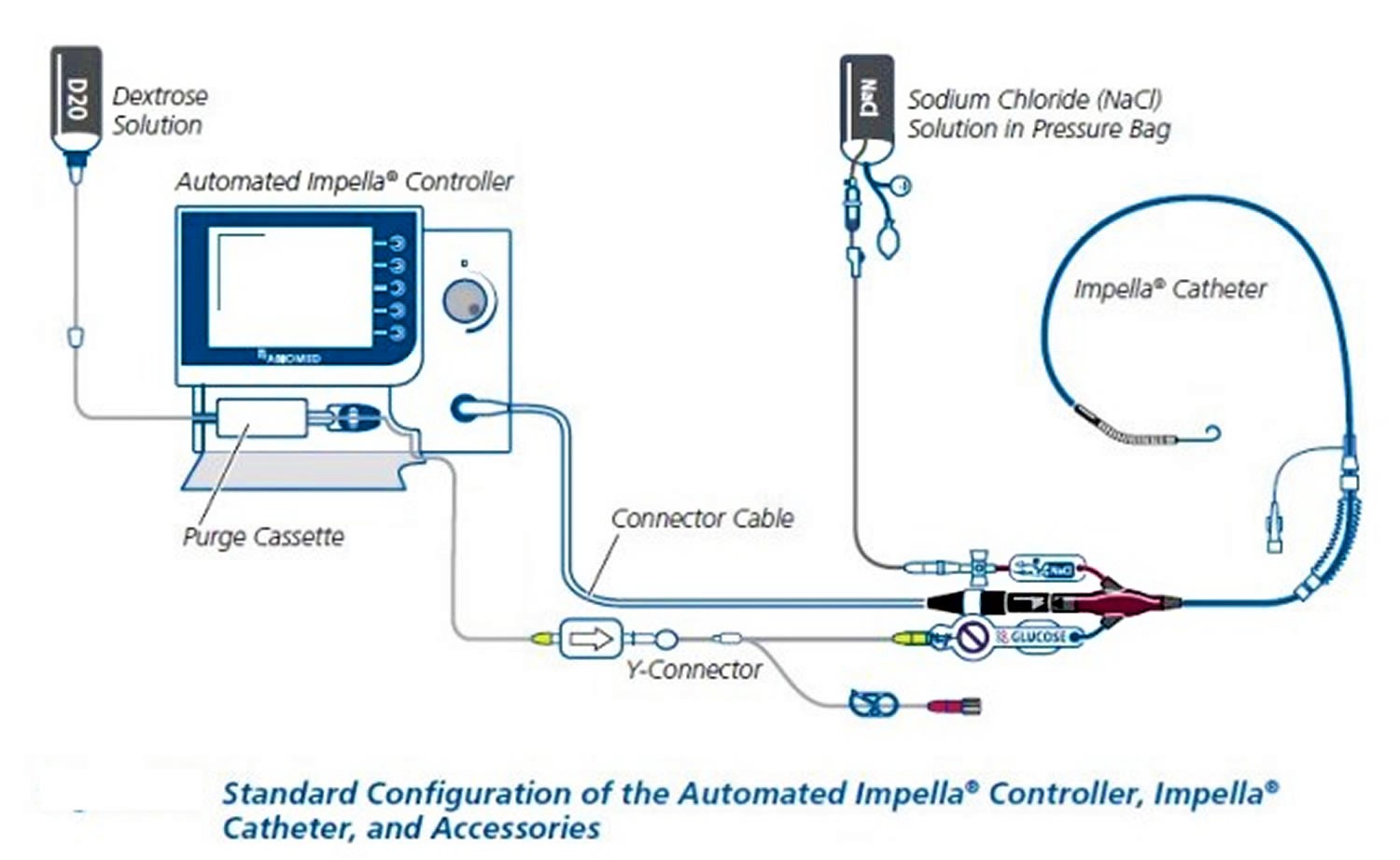
Impella device also called the Impella Ventricular Support Systems, is a mini left-side heart pump systems intended to help pump blood in patients who need short-term support (up to 6 days) 1. The Impella ventricular assist device includes a mini heart pump (Impella 2.5, Impella CP or cardiac power, or Impella 5.0/LD) mounted at the end of a thin, flexible tube (catheter), a console that drives the pump, and an infusion system that flushes the pump. Both American College of Cardiology and American Heart Association and European Society of Cardiology guidelines give Impella a Class IIb (Level of Evidence C) indication for use in refractory cardiogenic shock complicating myocardial infarction. When using Impella ventricular assist device, the ejection fraction increases while simultaneously reducing the heart’s work. This unloads the ventricle and coronary circulation improves. Thus, myocardial oxygen consumption and demand are better balanced. The Impella ventricular assist device also increases the systemic aortic pressure and mean arterial pressure (MAP). Overall, the changes induced by the Impella heart pump optimize the conditions for a natural recovery of the heart 2.
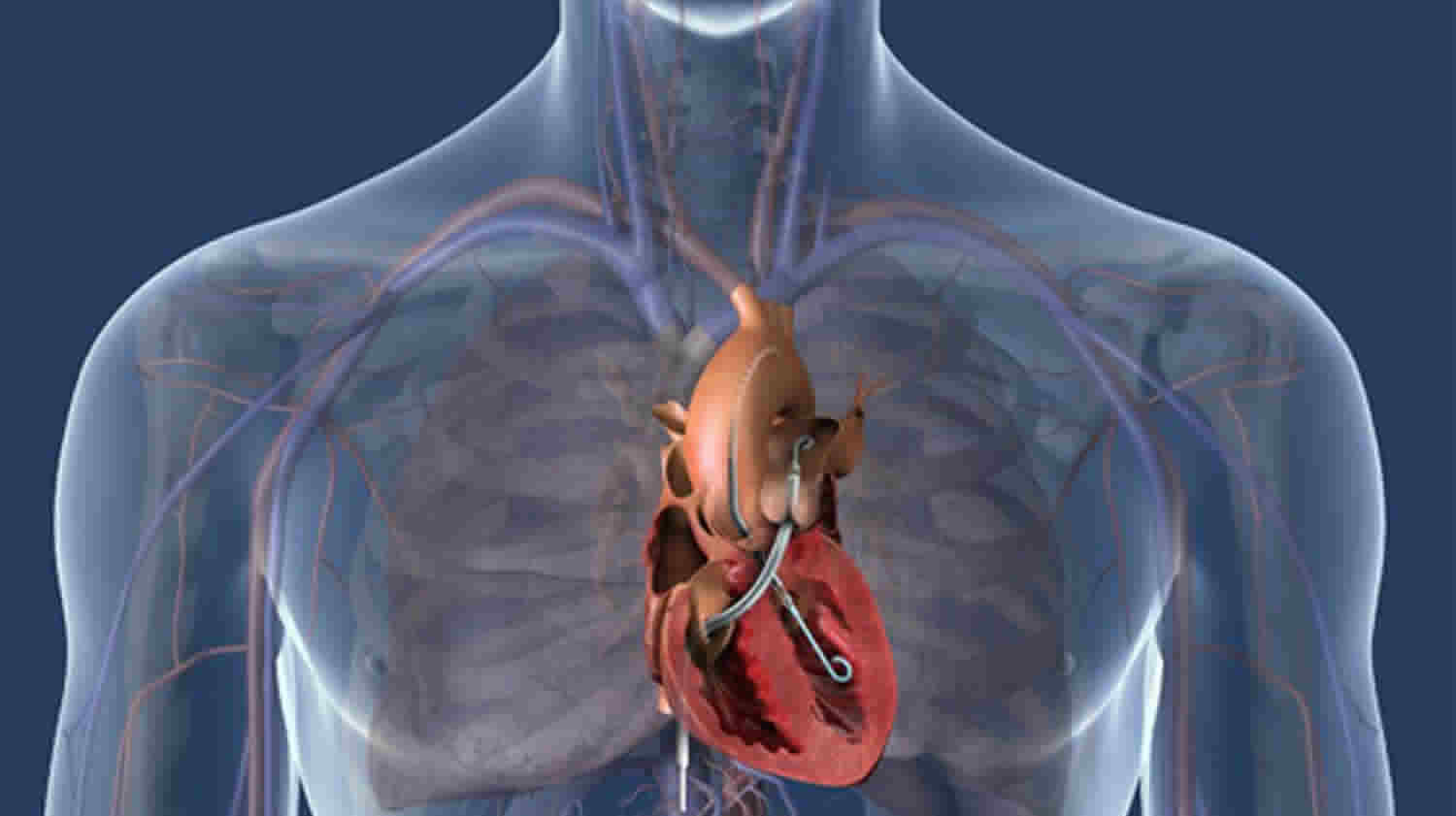
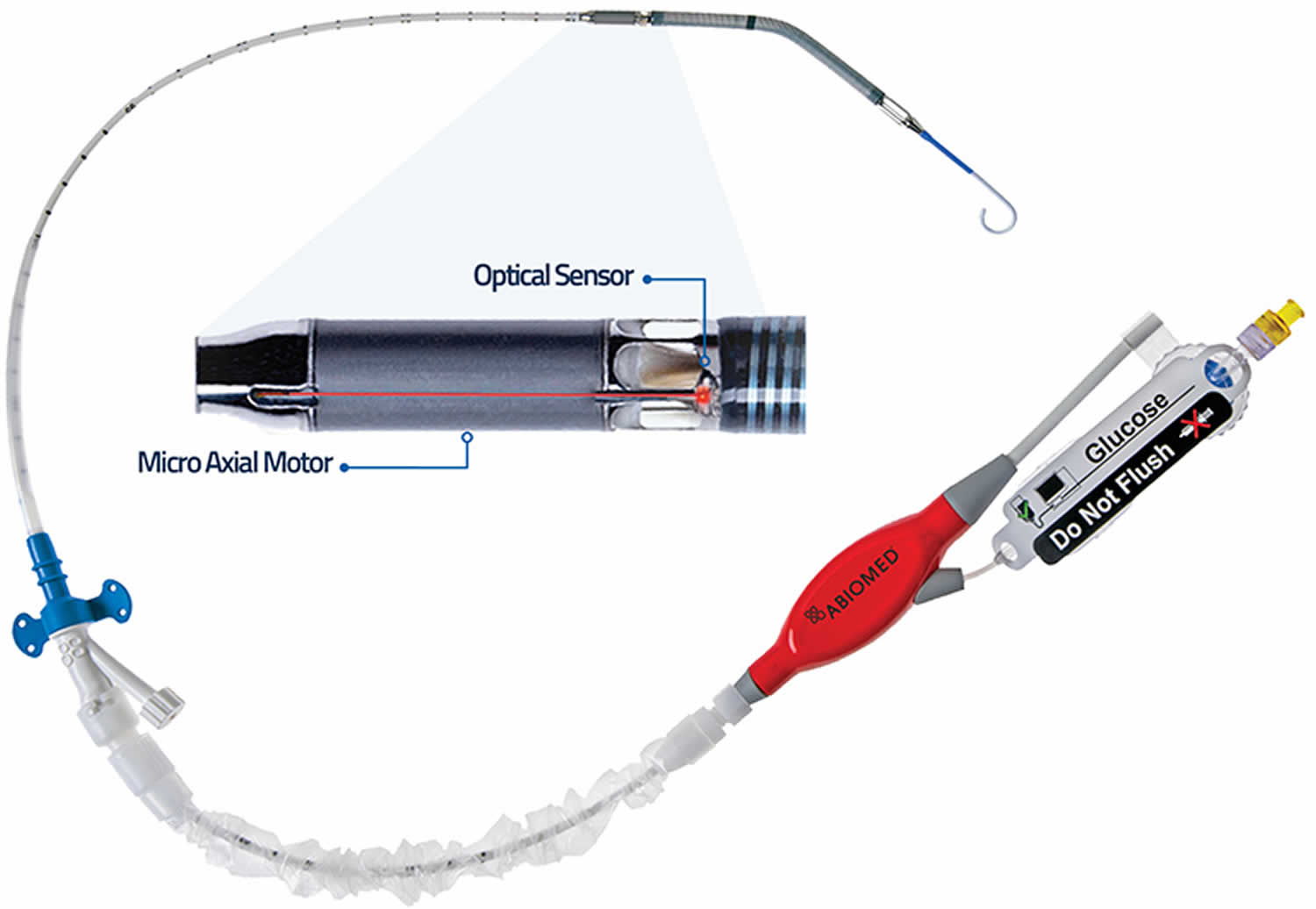
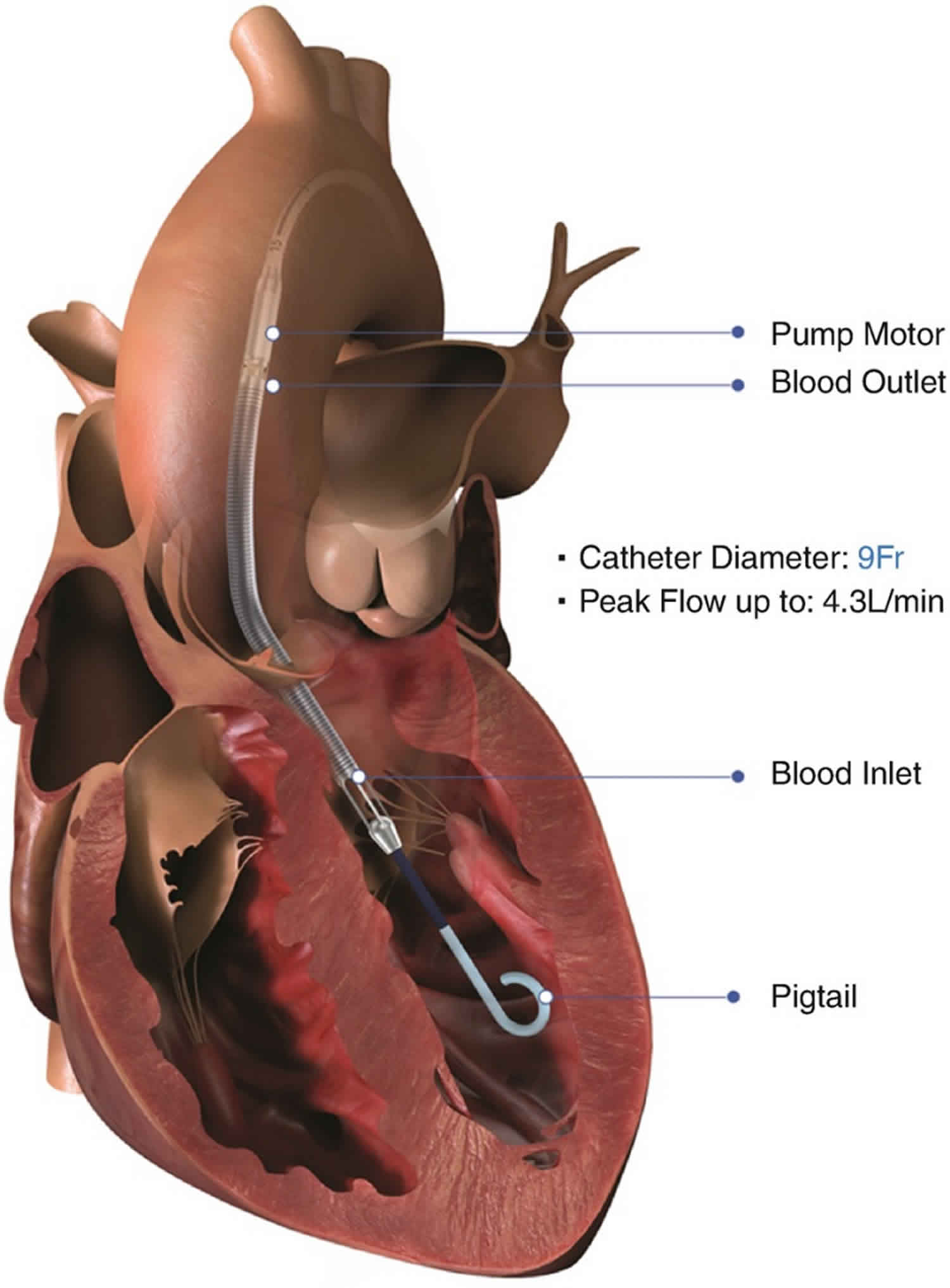
The Impella consists of a miniature axial-flow pump system that continuously pulls blood from the left ventricle into the ascending aorta, thus augmenting cardiac output (Figure 1) 3. The pump is mounted on a catheter that is inserted retrograde across the aortic valve, usually via the femoral artery. The tip of the catheter is in the shape of a pigtail for stabilization and safety of the device in the left ventricle. Near the tip of the catheter is an inlet area, from which blood is pulled to be expelled through the outlet area into the aorta. While three versions of the device are available, Impella 2.5 and the Impella cardiac power (CP) are most commonly used in ST-elevation myocardial infarction (STEMI) since they can both be inserted percutaneously. The third version, Impella 5.0, requires surgical cutdown. Although all three devices have a 9 French (Fr) catheter, the size of the femoral artery sheath varies due to different sizes of the pump motor (Table 1):
- Impella 2.5: 12 Fr pump motor requiring a 13 Fr femoral arterial sheath
- Impella CP: 14 Fr pump motor requiring a 14 Fr femoral arterial sheath
- Impella 5.0: 21 Fr pump motor requiring a 22 Fr femoral arterial sheath
Table 1. Comparison of mechanical circulatory support devices
| Intra-aortic balloon pump | Impella 2.5, CP, 5.0 | TandemHeart | Venoarterial Extracorporeal Membrane Oxygenation | |
|---|---|---|---|---|
| Pump mechanism | Pneumatic | Axial | Centrifugal | Centrifugal |
| Sheath size | 7.5–8.0 Fr | 13 Fr (2.5); 14 Fr (CP); 21 Fr (5.0) | 15–19 Fr arterial; 21 Fr venous | 15–17 Fr arterial; 18–21 Fr venous |
| Hemodynamic support | 0.5 L/min | 2.5 L/min (2.5); 4.0 L/min (4.0); 5.0 L/min (5.0) | 3.5–5.0 L/min | 4.0–6.0 L/min |
| Afterload | ↓ | ↓ | ↑ | ↑↑↑ |
| Risk of limb ischemia and bleeding | ↑ | ↑↑ | ↑↑↑ | ↑↑↑ |
| Complexity of insertion | ↑ | ↑↑ | ↑↑↑↑ | ↑↑↑ |
By directly removing blood from the left ventricle, the Impella reduces left ventricle end-diastolic volume and end-diastolic pressure. The blood is expelled into the ascending aorta, which increases aortic root pressure and increases coronary perfusion during diastole. The net effect is an increase in cardiac output, mean arterial pressure (MAP), and coronary perfusion, with a decrease in wall stress. The Impella more markedly reduces left ventricle volumes and preload compared with an intra-aortic balloon pump, thus reducing myocardial oxygen demands of the left ventricle which can be visualized as the area inside the loop. It is also important to note that the width of the pressure-volume loop only shows the stroke volume produced by the heart and not by the contributions of the Impella. Hemodynamic support is ~2.5 L/minute for the Impella 2.5, ~4.0 L/minute for the Impella CP, and ~5.0 L/min for the Impella 5.0 3.
Heart surgeons also use the Impella to support your heart during interventional procedures like percutaneous coronary interventions, angioplasty, or stenting. Imagine you have two or three arteries blocked because of coronary artery disease. Opening those arteries takes time, and if you do not tolerate the procedure very well, your blood pressure can drop to dangerous levels. To prevent that, your medical team can insert the Impella to keep your blood pressure and flow in a safe zone while your physician opens your arteries.
Impella device use in high-risk patients
The patients who benefit the most are those at high risk for complications. Doctors consider the use of the Impella device in patients with:
- Weakened heart muscle. If you have congestive heart failure, a low ejection fraction, or diminished heart function, your heart’s pumping capacity may be so low as to make heart procedures risky.”
- Co-morbidities. This includes people with advanced age, diabetes, kidney disease, prior stroke, and blockages in their leg arteries.
- Advanced heart disease. These patients have significant blockages in all three arteries.
If you have one or more of these conditions, the Impella may provide effective heart support throughout your angioplasty or stenting procedure.
The Impella is inserted through an artery in your leg, across the aortic valve, and into the left ventricle of your heart. The Impella is placed retrograde across the aortic valve, with the pigtail and inlet area in the left ventricle and the outlet area in the ascending aorta. Blood is continuously pulled from the left ventricle into the ascending aorta. There is a console (imagine an iPad on a stand) that doctors use to monitor things like blood flow and pressure. The console allows your medical team to dial the flow up and down as appropriate. It also has many alerts for your medical team to monitor.
Heart surgeons generally insert the Impella at the beginning of the procedure and remove it at the end. The patient often returns to the room without the Impella device in place. However, in very sick patients, the Impella device could be kept in place for two to three days as your doctor determines the right therapy for you.
Before the Impella, heart surgeons used a balloon pump inserted through the groin. Over the years, doctors learned that the balloon pump was not great at maintaining blood pressure and flow for high-risk patients. The heart typically pumps about 4 liters of blood to the rest of your body; balloon pumps can handle about 0.5 liters, but the Impella device can pump 2 to 3 liters. The larger Impella devices can pump 3.5 to 4 liters.
Figure 1. Impella device
How does Impella ventricular assist device work?
The Impella Ventricular Support System helps pump blood by drawing blood out of your heart and pumping it into your aorta, partially or fully bypassing the left ventricle. It is implanted into the left side of a patient’s heart through a small incision in the femoral artery (major artery in the leg). It can also be implanted through a small incision in a subclavian artery (an artery in the chest).
When is Impella ventricular assist device used?
Cardiogenic shock is a condition in which the left ventricle of the heart fails to pump enough blood to the body, resulting in inadequate circulation, progressive severe damage to other organs, and even death. When patients with cardiogenic shock are not responsive to conventional treatment measures, additional short term mechanical support of the circulation may be required. The Impella Ventricular Support System may be used temporarily (four days or less for the Impella 2.5 and Impella cardiac power, and 6 days or less for Impella 5.0 and LD) to treat cardiogenic shock. Impella device therapy is intended to provide adequate circulation of blood (replace or supplement left ventricle pumping) while also allowing the damaged heart muscle the opportunity to rest and recover. Depending on the severity of the underlying damage to the heart, the Impella device may either be weaned (slowly turned down and removed), or may provide a bridge to additional, more permanent forms of circulatory support. The Impella device has been approved for use in the treatment of sudden episodes of cardiogenic shock that may occur immediately (within 48 hours) after a heart attack or open heart surgery, or in the setting of acute left heart failure in an already diseased heart (cardiomyopathy), during pregnancy, post-partum (following childbirth) (post-partum), or as the result of myocarditis (a viral heart infection).
The Impella device is also beneficial for your physician. It makes the heart interventional procedures more predictable, allows for better control of blood pressure, and leads to a reduction in stress for the medical team.
The Impella heart pump is a relatively safe device. Like any other procedure in cardiology, the biggest risk is bleeding from the insertion site. Also, the device can move, which is when the monitors become very important. Your cardiology team will keep a close eye on the monitors to be sure we’re in the right area of your heart, doing the right thing. The monitors will alert us when there’s something wrong. Your nursing staff and medical team know what to look for to ensure a safer, more effective outcome for you.
What is cardiogenic shock?
A cardiogenic shock is a life-threatening condition which is caused by the massive reduction of the heart’s pumping function.
This may lead to:
- A lower ejection fraction
- Reduced contractility
- Low blood pressure
- Coronary insufficiency
The heart is no longer able to provide the required cardiac output for the supply of vital organs.
Untreated, a cardiogenic shock can lead to multiorgan failure and ultimately to the death of a patient.
The cause of a cardiogenic shock is usually a pre-existing heart condition. In about 80% of cases, a heart attack is caused by the failure of the left ventricle. Rarely, heart valve damage or myocarditis trigger a cardiogenic shock.
During a cardiogenic shock the blood volume flowing through the body suddenly decreases. Thus, the heart’s inability to function leads to an oxygen deficiency within vital organs, which in turn leads to increased anaerobic decomposition processes within the body.
Because this metabolic pathway does not require oxygen, the process is not fully completed. This leads to acidosis (hyperacidity of the blood), which causes the arterioles to slacken and damages the blood capillaries.
In addition, a loss of fluid takes place, that increases hypovolaemia (volume deficit). Furthermore, this leads to an accumulation of blood within the capillaries, which might lead to microthromboses. The entire process continues to intensify independently of its cause and is therefore referred to as a shock spiral.
Of all the patients suffering from cardiogenic shock, 50 to 80 % die because of circulatory or end-organ failure. Systems for circulatory support such as the Impella heart pump can improve hemodynamics and promote the recovery of the heart muscle.
The therapeutic standard for a cardiogenic shock is an early revascularization as well as the treatment with inotropes and catecholamines. However, drugs are only effective when sufficient healthy myocardium remains.
Alternatively, percutaneous mechanical support can provide a way out of the life-threatening situation. Impella heart pumps can be used for this purpose. They improve hemodynamics and stimulate the recovery of the heart muscle.
What will Impella ventricular assist device accomplish?
Because the Impella Ventricular Support System acts as a blood pump that effectively bypasses the left ventricle, it supports blood pressure and provides increased blood flow to critical organs in patients with cardiogenic shock. This allows the left ventricle to rest and hopefully recover and resume pumping without support. Clinical evidence demonstrated that 58% of patients with cardiogenic shock who received the Impella device survived the procedure, and 67% of patients who left the hospital experienced myocardial recovery (they did not have another heart failure event) within 30 days of the procedure.
Impella ventricular assist device contraindications
The Impella Ventricular Support System should not be used if the patient has any of the conditions noted below:
- Clot(s) in the left side of the heart, which may break off while the pump is in use and result in harm;
- A replacement heart valve or other heart device, which could block the open area available for the pump to pass;
- Mechanical aortic valve;
- Critical severe aortic stenosis;
- Moderate to severe aortic regurgitation;
- Ventricular septal defect;
- Severe peripheral arterial disease;
- Severe sepsis;
- Severe narrowing of one of the heart valves, which could block the open area available for the pump to pass;
- A problem with the aortic valve that allows blood to leak back into the left ventricle from the aortic artery—this can cause the heart to work harder and over time may decrease the ability of the heart to supply enough fresh blood to the rest of the body;
- Defects in the patient’s veins and arteries, including calcium deposits or hardening of the vessel walls, which could block the open area available for the pump to pass;
- A severe problem with the right side of the heart, which caused it to fail to efficiently pump blood;
- A severe problem with the lungs which prevents the lungs from providing oxygen to circulating blood;
- A defect, such as a small channel in the heart, which could shunt the blood flow between its chambers and reduce pump output;
- A split or rupture of the left side of the heart which results in the leakage of blood out of the left side of the heart;
- A build-up of blood into the space surrounding the heart which blocks its ability to fill with blood.
Impella ventricular assist device complications
The most common complication of Impella is bleeding, given the need for anticoagulation and the 13–22 Fr size catheters needed for arterial access. In the Impella-EUROSHOCK-registry that retrospectively evaluated 120 patients receiving Impella 2.5 for cardiogenic shock complicating myocardial infarction, bleeding requiring transfusion occurred in 24.2% of patients, and bleeding requiring surgery occurred in 4.2% of patients. Other complications of Impella include:
- Limb ischemia
- Stroke
- Left ventricle injury or perforation
- Cardiac tamponade
- Hemolysis
- Arrhythmias
- Acquired von Willebrand syndrome
- Mitral regurgitation secondary to chordal rupture
- Functional mitral stenosis.
- Impella Ventricular Support Systems – P140003/S018. https://www.fda.gov/medical-devices/recently-approved-devices/impella-ventricular-support-systems-p140003s018
- Anderson MB et al. Benefits of a novel percutaneous ventricular assist device for right heart failure. The prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant. 2015, 34: 1549-60.
- Lo N, Magnus Ohman E. Mechanical Circulatory Support in ST-Elevation Myocardial Infarction. 2018 Jul 14. In: Watson TJ, Ong PJL, Tcheng JE, editors. Primary Angioplasty: A Practical Guide [Internet]. Singapore: Springer; 2018. Chapter 19. Available from: https://www.ncbi.nlm.nih.gov/books/NBK543576/ doi: 10.1007/978-981-13-1114-7_19