What is insulin
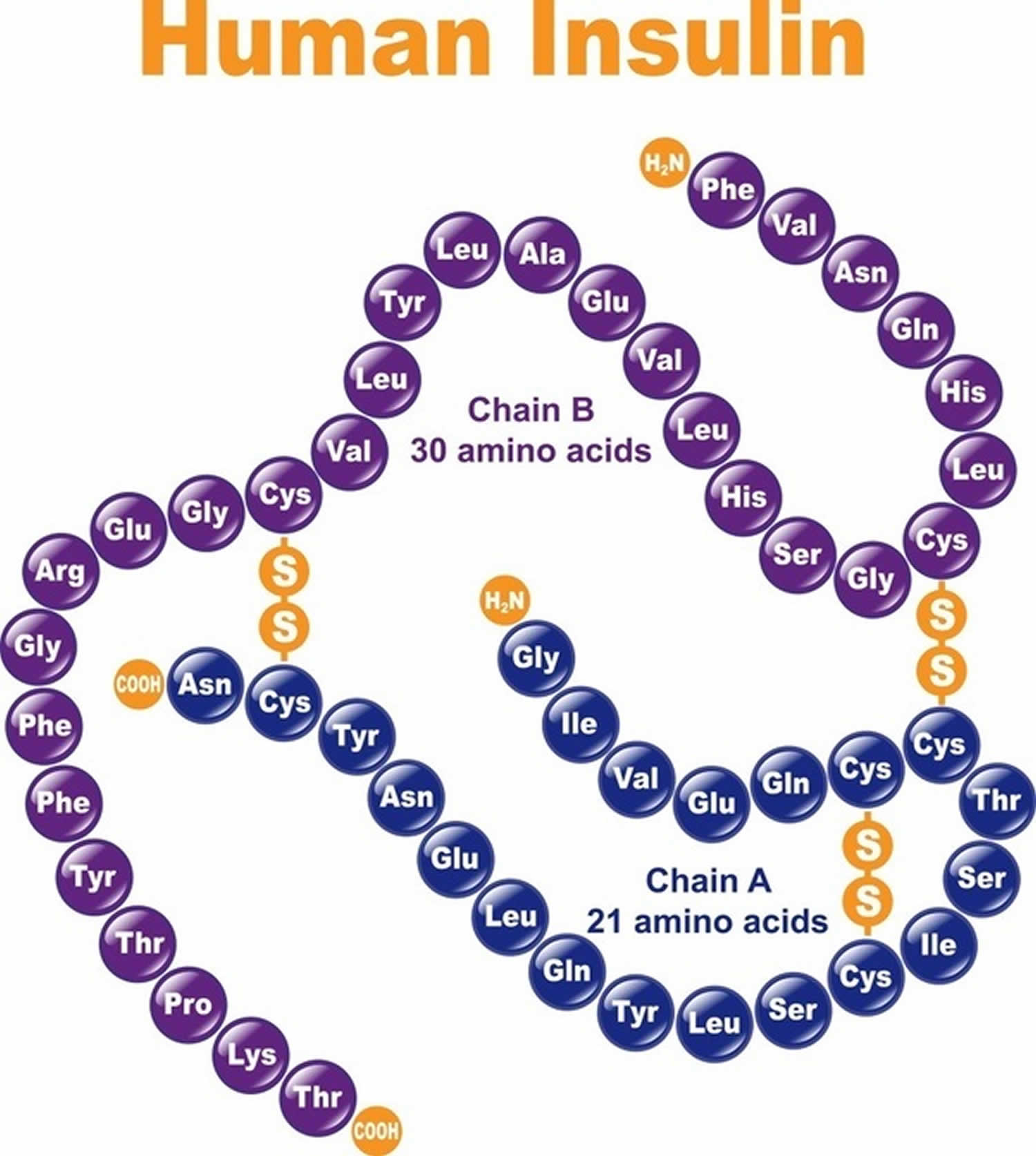
Insulin is a peptide hormone consisting of 51 amino acids arranged in two chains, an A chain (21 amino acids) and B chain (30 amino acids) that are linked by two disulfide bonds 1. Insulin is produced by beta cells of the pancreatic islets (islets of Langerhans); insulin is considered to be the main anabolic hormone of the body. Insulin regulates the metabolism of carbohydrates, fats and protein by promoting the absorption of carbohydrates, especially glucose from the blood into liver, fat and skeletal muscle cells 2. In these tissues the absorbed glucose is converted into either glycogen via glycogenesis or fats (triglycerides) via lipogenesis, or, in the case of the liver, into both. Glucose production and secretion by the liver is strongly inhibited by high concentrations of insulin in the blood 3. Circulating insulin also affects the synthesis of proteins in a wide variety of tissues. It is therefore an anabolic hormone, promoting the conversion of small molecules in the blood into large molecules inside the cells. Low insulin levels in the blood have the opposite effect by promoting widespread catabolism, especially of reserve body fat. When insulin is depleted, impaired or destroyed within the body, it cannot regulate the amount of glucose in the blood.
Laboratory test tube studies have demonstrated the mitogenic effects of insulin at high concentrations, as well as carcinogenic effects of insulin binding to the insulin like growth factor-1 (IGF-1) receptor, suggesting that hyperinsulinemia may promote tumorogenesis 4. Because insulin analogs are modified human insulin, the safety and efficacy profiles of these insulins have been compared to human insulin 5. Insulin and IGF-1 receptor binding affinities, and the metabolic and mitogenic potencies of insulin analogs relative to human insulin have been assessed. Insulin lispro and aspart are similar to human insulin on all of the above parameters, except insulin lispro was found to be 1.5-fold more potent in binding to the IGF-1 receptor compared to human insulin. Insulin glargine was found to have a 6- to 8-fold increase in mitogenic potency and IGF-1 receptor affinity compared to human insulin 4. However, glargine is rapidly degraded to metabolites. The predominant metabolite M1 has been shown to have a 0.4-fold binding affinity to the IGF-1 receptor compared with human insulin 6. In human studies, meta-analyses comparing exogenous insulin to non-insulin antihyperglycemic therapies have shown associations of insulin with several cancers 7, 8. However, there are inherent limitations to such analyses. A more recent review of large epidemiologic studies did not find evidence of an increased risk of malignancy among glargine-treated patients when compared with other insulin therapies 6.
Because pork and beef insulin differ from human insulin by 1 and 3 amino acids respectively, they are more immunogenic than exogenous human insulin 4. Older formulations of insulin were less pure, containing islet-cell peptides, proinsulin, C-peptide, pancreatic polypeptides, glucagon, and somatostatin, which contributed to immunogenicity of insulin 9. Components of insulin preparations (e.g., zinc, protamine) and subcutaneous insulin aggregates are also thought to contribute to antibody formation 9. Commercially available human insulins are now virtually free of contaminants and contain <1 ppm of proinsulin 1. Local or systemic hypersensitivity reactions, lipodystrophy, and antibody production causing insulin resistance, are now rarely seen with exogenous human insulin 1. Because of the availability of human insulin and the increased potential for animal source insulin to be immunogenic, animal source insulins are no longer available in the United States 4.
Rare hypersensitivity responses to insulin can be immediate-type, local or systemic IgE-mediated reactions 9. Patients who experience a true allergic reaction to insulin have typically received insulin in the past, and experience the reaction after insulin is restarted.
Delayed, IgG-mediated allergic reactions also develop with animal insulins 9. Insulin therapy can rarely result in the production of insulin antibodies of the IgG class, which inactivate insulin. Immunological insulin resistance can develop in patients with very high titers of IgG-antibodies.
Lipodystrophy resulting from insulin injections refers to two conditions: lipoatrophy and lipohypertrophy. Lipoatrophy is an immune-mediated condition resulting in loss of fat at insulin injection sites 9 and occurs rarely with purified human insulins. Treatment for patients who developed lipoatrophy due to animal insulin use was injection of human insulin into the atrophied site. Lipohypertrophy is a common, non-immunological side effect of insulin resulting from insulin’s trophic effects following repeated injections of insulin into the same subcutaneous site.
Where is insulin produced?
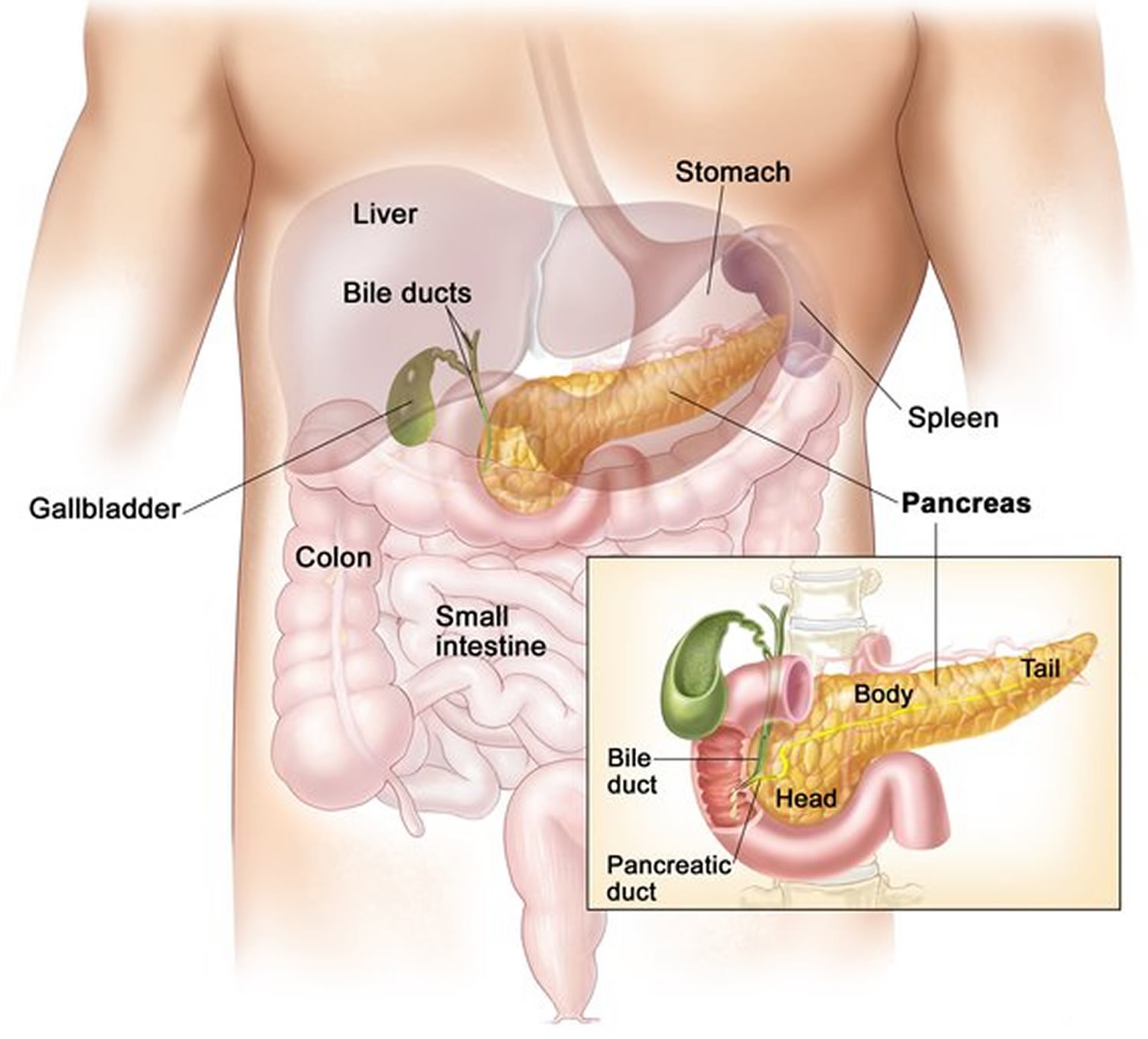
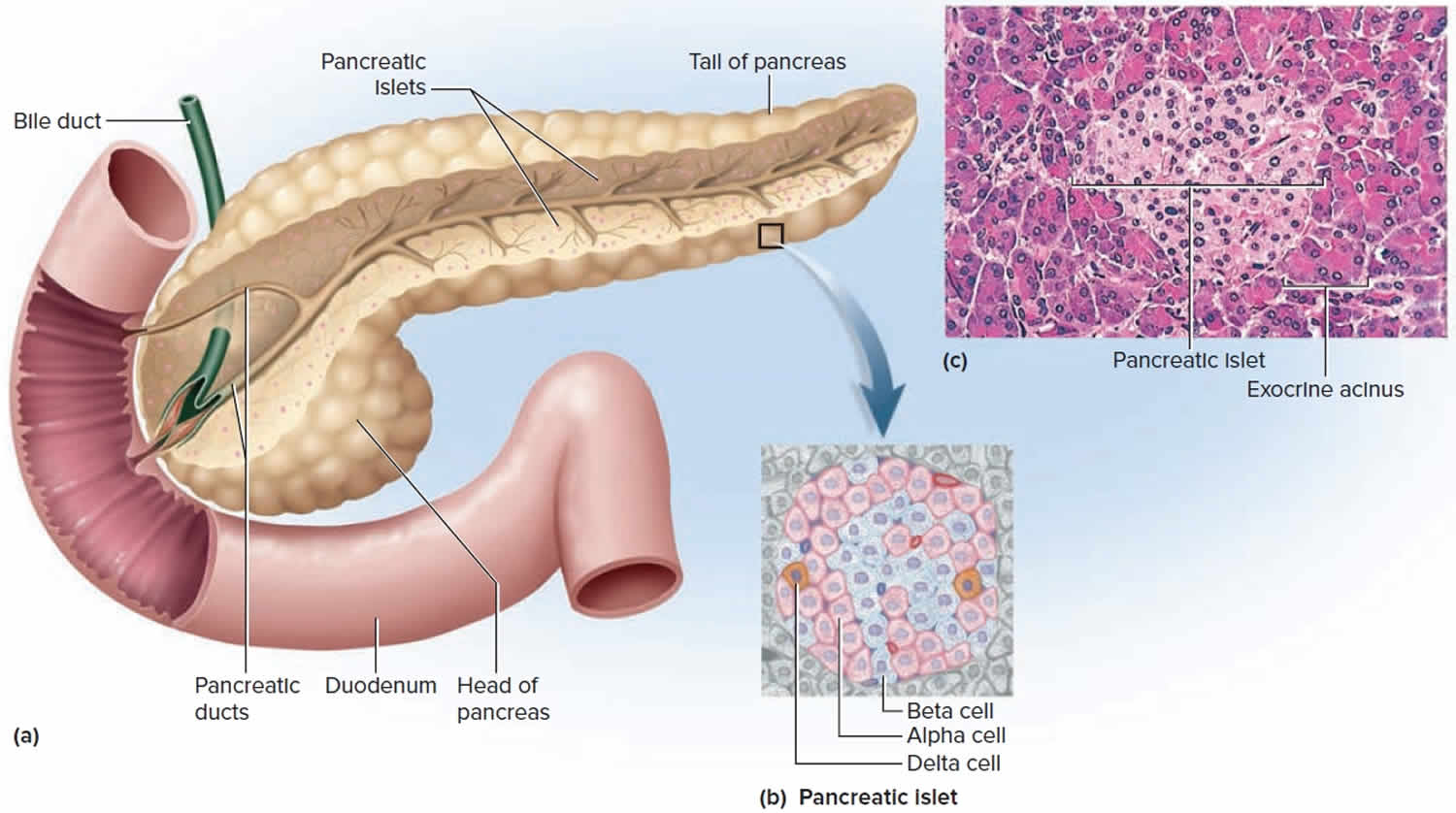
The pancreas is an elongated, spongy gland located below and behind the stomach; most of it is retroperitoneal (see Figure 1). The pancreas is both an endocrine and exocrine gland. The pancreas is primarily an exocrine digestive gland. About 99% of the pancreas is exocrine tissue, which secretes 1,200 to 1,500 mL of pancreatic juice per day. Scattered throughout the exocrine tissue, are 1 to 2 million endocrine groups of cells that are closely associated with blood vessels called pancreatic islets (islets of Langerhans). Although they are less than 2% of the pancreatic tissue, the islets of Langerhans secrete the hormone glucagon and the hormone insulin of vital importance, especially in the regulation of glycemia, the blood glucose concentration. The pancreatic islets of Langerhans include two distinct types of cells—alpha cells, which secrete the hormone glucagon, and beta cells, which secrete insulin hormone. A typical islet measures about 75 × 175 μm and contains from a few to 3,000 cells. Islets of Langerhans main cell types are alpha cells (20%), beta cells (70%), and delta cells (5%). Islets of Langerhans respond directly to blood nutrient levels associated with the cycle of eating and fasting. Their functions are as follows:
- Alpha (α) cells, or A cells, secrete glucagon between meals when the blood glucose concentration falls below 100 mg/dL (5.6 mmol/L). Glucagon exerts two primary actions on the liver: (1) glycogenolysis, the breakdown of glycogen into glucose; and (2) gluconeogenesis, the synthesis of glucose from fats and proteins (see Figure 2). These effects lead to the release of glucose into circulation, thus raising the blood glucose level. In adipose tissue, glucagon stimulates fat catabolism and the release of free fatty acids. Glucagon is also secreted in response to rising amino acid levels in the blood after a high-protein meal. It promotes amino acid absorption and thereby provides cells with the raw material for gluconeogenesis.
- Beta (β) cells, or B cells, secrete two hormones, insulin and amylin. Insulin, “the hormone of nutrient abundance,” is secreted during and immediately following a meal when blood nutrient levels are rising. Osteocalcin, a hormone from the osteoblasts of bone, also stimulates multiplication of beta cells, insulin secretion, and insulin sensitivity of other body tissues. The principal targets of insulin are the liver, skeletal muscles, and adipose tissue. In times of plenty, insulin stimulates cells to absorb glucose, fatty acids, and amino acids and to store or metabolize them; therefore, it lowers the level of blood glucose and other nutrients. It promotes the synthesis of glycogen, fat, and protein, thereby promoting the storage of excess nutrients for later use and enhancing cellular growth and differentiation. It also antagonizes glucagon, thus suppressing the use of already-stored fuels. The brain, liver, kidneys, and red blood cells absorb and use glucose without need of insulin, but insulin does promote glycogen synthesis in the liver. Insulin insufficiency or inaction is well known as the cause of diabetes. The beta cells also secrete another hormone, amylin, simultaneously with insulin. Amylin helps to reduce spikes in blood glucose by slowing the emptying of the stomach; modulating the secretion of gastric enzymes, acid, and bile; inhibiting glucagon secretion; and stimulating the sense of satiety (having had enough to eat).
- Delta (δ) cells, or D cells, secrete somatostatin (growth hormone–inhibiting hormone) concurrently with the release of insulin by the beta cells. Somatostatin works with amylin to limit the secretion of stomach acid.
Other, minor types of pancreatic cells, about 5% of the total, are called PP and G cells. Their functions remain obscure and controversial.
Any hormone that raises blood glucose concentration is called a hyperglycemic hormone. You may have noticed that glucagon is not the only hormone that does so; so do growth hormone, epinephrine, norepinephrine, cortisol, and corticosterone. Insulin is called a hypoglycemic hormone because it lowers blood glucose levels.
Figure 1. Pancreas location
Where is insulin eliminated?
The kidneys and liver account for the majority of insulin degradation. Normally, the liver degrades 50-60% of insulin released by the pancreas into the portal vein, and the kidneys ~35-45% 10. When insulin is injected exogenously, the degradation profile is altered since insulin is no longer delivered directly to the portal vein. The kidney has a greater role in insulin degradation with subcutaneous insulin (~60%), with the liver degrading ~30-40% 11.
Because the kidneys are involved in the degradation of insulin, renal dysfunction will reduce the clearance of insulin and prolong its effect. This decreased clearance is seen with both endogenous insulin production (either normal production or that stimulated by oral agents) and exogenous insulin administration. Renal function generally needs to be greatly diminished before this becomes clinically significant 12. Clinically, a deterioration in renal function leads to a progressive decline in exogenous insulin requirements, and an increased risk of hypoglycemia.
What does insulin do?
Insulin is a hormone which plays a number of key roles in the body’s metabolism. Insulin is a hormone which plays a key role in the regulation of blood glucose levels. Insulin promotes synthesis of glycogen by your liver cells. Key complementary functions of insulin are (a) stimulation of glucose uptake from the systemic circulation and lipid synthesis (lipogenesis) from glucose; (b) suppression of hepatic gluconeogenesis, lipolysis, proteolysis, glycogenolysis and ketogenesis, which together regulating glucose homeostasis 13. Insulin increases glucose utilization in peripheral organs (e.g., skeletal muscle and adipose tissue) and suppresses hepatic glucose production (inhibits gluconeogenesis) and adipose tissue lipolysis. Circulating insulin also affects the synthesis of proteins in a wide variety of tissues. It is therefore an anabolic hormone, promoting the conversion of small molecules in the blood into large molecules inside the cells. Low insulin levels in the blood have the opposite effect by promoting widespread catabolism, especially of reserve body fat.
In addition to these classical metabolic insulin target tissues, there are many other important physiological targets of insulin including the brain, pancreatic beta cells, heart, and vascular endothelium that help to coordinate and couple metabolic and cardiovascular homeostasis under healthy conditions 14.
A lack of insulin, or an inability to adequately respond to insulin, can each lead to the development of the symptoms of diabetes (also called diabetes mellitus). Diabetes is characterized by decreased glucose tolerance resulting from a relative deficiency of insulin or a lack of sensitivity to the endogenous hormone. Insufficient insulin, or decreased insulin sensitivity, results in hyperglycemia. Long-term exposure of tissues to elevated ambient glucose concentrations is associated with the development of complications, including macro- and microvascular disease. Of particular concern are coronary heart disease, cerebrovascular disease, and the characteristic retinopathy, nephropathy, and neuropathy of this disorder .
In addition to its role in controlling blood sugar levels, insulin is also involved in the storage of fat.
Insulin regulates how the body uses and stores glucose and fat. Many of the body’s cells rely on insulin to take glucose from the blood for energy.
What is the role of insulin in diabetes treatment?
The answer to this question varies depending on what type of diabetes you suffer from. Type 1 diabetics, whose natural insulin is inadequate or completely destroyed, are heavily reliant on insulin therapy as their ongoing treatment.
When insulin treatment is irregular or poorly regimented type 1 diabetics may experience rising blood sugar levels. When this occurs, the body relies on its fat. If this condition is not checked, a life-threatening complication known as diabetic acidosis may occur.
Symptoms for this disorder are numerous, and at their worst may include unconsciousness. Usual signs include:
- paleness
- sweating
- increased heartbeat
- blurred vision
- hunger
Type 2 diabetes is a slightly different disease. The body has not actively destroyed its insulin producing pancreatic cells (islets of Langerhans), but a combination of factors lead to increased resistance to the positive benefits of insulin.
Basal insulin
The role of basal insulin, also known as background insulin, is to keep blood glucose levels at consistent levels during periods of fasting.
When fasting, the body steadily releases glucose into the blood to our cells supplied with energy. Basal insulin is therefore needed to keep blood glucose levels under control, and to allow the cells to take in glucose for energy. Basal insulin is usually taken once or twice a day depending on the insulin.
Basal insulin need to act over a relatively long period of time and therefore basal insulin will either be long acting insulin or intermediate insulin.
What is bolus insulin?
A bolus dose is insulin that is specifically taken at meal times to keep blood glucose levels under control following a meal. Bolus insulin needs to act quickly and so short acting insulin or rapid acting insulin will be used.
Bolus insulin is often taken before meals but some people may be advised to take their insulin during or just after a meal if hypoglycemia needs to be prevented.
Your doctor will be able to advise you if you have any questions as to when your bolus insulin should be taken.
What is a basal-bolus insulin regimen?
A basal-bolus routine involves taking a longer acting form of insulin to keep blood glucose levels stable through periods of fasting and separate injections of shorter acting insulin to prevent rises in blood glucose levels resulting from meals.
A basal-bolus regimen, which includes an injection at each meal, attempts to roughly emulate how a non-diabetic person’s body delivers insulin.
A basal-bolus regimen may be applicable to people with type 1 and type 2 diabetes.
Advantages of a basal-bolus regimen
- One of the main advantages of a basal-bolus regimen is that it allows you to fairly closely match how your own body would release insulin if it was able to.
- A second advantage of a basal-bolus regimen is that it allows for flexibility as to when meals are taken.
If you are self-adjusting your insulin doses, it means you also have flexibility over how much carbohydrate you take in for different meals.
Disadvantages of a basal-bolus regimen
One notable disadvantage is that a basal-bolus regimen involves taking more insulin injections each day. This may prove problematic in some people more than others.
Children on a basal-bolus regimen, for example, will need to feel comfortable with injecting at meal times when at school.
Insulin dependent diabetes
Diabetes is a disease in which your blood glucose or blood sugar, levels are too high. Glucose comes from the foods you eat. Insulin is a hormone that helps the glucose get into your cells to give them energy.
There are 2 main types of diabetes:
- Type 1 diabetes – the body’s immune system attacks and destroys the pancreatic beta-cells that produce insulin. With type 1 diabetes, your pancreas does not make insulin. Over 1 million children and adolescents have type 1 diabetes 15.
- Type 2 diabetes – People with type 2 diabetes make insulin, but their bodies don’t respond well to it. Type 2 diabetes arises from a combination of impaired insulin action and defective pancreatic β-cell function. Some people with type 2 diabetes may take pills or insulin shots to help their bodies use glucose for energy. Type 2 diabetes is far more common than type 1. In the US, around 90% of all adults with diabetes have type 2.
Without enough insulin, the glucose stays in your blood. You can also have prediabetes. This means that your blood sugar is higher than normal but not high enough to be called diabetes. Having prediabetes puts you at a higher risk of getting type 2 diabetes.
During pregnancy, some women have such high levels of blood glucose that their body is unable to produce enough insulin to absorb it all. This is known as gestational diabetes. 1 in 6 births is affected by hyperglycaemia in pregnancy 15.
Over time, having too much glucose in your blood can cause serious problems. It can damage your eyes, kidneys, and nerves. Diabetes can also cause heart disease, stroke and even the need to remove a limb.
Blood tests can show if you have diabetes. One type of test, the HbA1C, can also check on how you are managing your diabetes. Exercise, weight control and sticking to your meal plan can help control your diabetes. You should also monitor your blood glucose level and take medicine if prescribed.
Diabetes has reached epidemic proportions throughout the world and will continue to grow and remain the greatest global health challenge the world has ever known: 425 million people have diabetes (1 in 11 adults), and the number of people with the disease is predicted to rise beyond 642 million (55%; 1 in 10 adults) in less than 25 years 15. Approximately 90% of all individuals afflicted have type 2 diabetes, and about 1 in 2 (212 million) remain undiagnosed 15. Individuals with type 1 and type 2 diabetes are at a significantly greater risk for developing microvascular and macrovascular diseases. 12% of global health expenditure ($727 billion) is spent on diabetes.
Type 1 Diabetes
Type 1 diabetes is an autoimmune disease characterized by progressive pancreatic beta-cell destruction resulting in progressive decline in insulin production who eventually require insulin for survival and hyperglycemia 16. Insulin is a hormone that helps glucose get into your cells to give them energy. Without insulin, too much glucose stays in your blood. Over time, high blood glucose can lead to serious problems with your heart, eyes, kidneys, nerves, and gums and teeth.
Type 1 diabetes happens most often in children and young adults but can appear at any age. Symptoms may include:
- Being very thirsty
- Urinating often
- Feeling very hungry or tired
- Losing weight without trying
- Having sores that heal slowly
- Having dry, itchy skin
- Losing the feeling in your feet or having tingling in your feet
- Having blurry eyesight
A blood test can show if you have diabetes. If you do, you will need to take insulin for the rest of your life. A blood test called the HbA1C can check to see how well you are managing your diabetes.
Exogenous insulin therapy is essential to prevent fatal complications from hyperglycemia. The Diabetes Control and Complications Trial and its long-term follow up, the Epidemiology of Diabetes and its Complications study, demonstrated that stringent glycemic control with intensive insulin therapy can prevent or postpone progression of microvascular disease and reduce risk for macrovascular disease and all-cause mortality. In addition, data obtained from the type 1 diabetes Exchange, a registry of type 1 diabetes patients founded in 2010, has become an invaluable resource for scientists worldwide, facilitating collaboration and accelerating understanding of prevailing diabetes practices.
Insulin therapy using rapid- and long-acting insulin analogues is the mainstay of management of type 1 diabetes. Insulin delivery is achieved subcutaneously using multiple daily injections or subcutaneous insulin infusion using insulin pumps. Patients with type 1 diabetes generally require a replacement dose of 0.5-1.0 units per kg of body weight per day of insulin 17. Insulin therapy is often initiated at 0.5-0.75 units/kg/day 18. During the early stages of type 1 diabetes, patients commonly require less insulin because remaining beta cells still produce some insulin; during their “honeymoon period” insulin requirements can be in the range of 0.1-0.6 units per kg per day 17. Intensive insulin therapy (defined as 3 or more insulin injections daily or insulin pump therapy) is indicated for patients with type 1 diabetes to provide better glycemic control with less glucose variability than 1 or 2 daily injections, and reduce the development and progression of microvascular and macrovascular complications 19.
Effective management also involves use of self-monitoring of blood glucose using improved blood glucose meters, continuous glucose monitoring devices, and newer insulin pumps with integrated sensor-augmented systems. Addressing psychosocial aspects of type 1 diabetes plays a crucial role in effective disease management. Strategies to manage type 1 diabetes are rapidly evolving. In addition to newer insulins, adjunctive non-insulin therapies such as use of incretin agents and sodium-glucose transport protein-2 (SGLT-2) and combination SGLT-1/2 inhibitors are being actively pursued 16. Continuous glucose monitoring technology combined with glucose prediction algorithms has allowed for the development of artificial pancreas delivery systems which are actively being tested in clinical trials. Cellular replacement options include pancreas and islet cell transplantation which can restore euglycemia but are limited by donor availability and the need for chronic immunosuppression. Newer strategies under development include islet cell encapsulation techniques, which might obviate the need for immunosuppression. Smart-insulin delivery systems, capable of releasing insulin depending on ambient glucose, are also being evaluated.
Type 2 Diabetes
The slowly progressive beta cell loss in patients with type 2 diabetes means many patients with type 2 diabetes will eventually require insulin therapy to attain adequate glycemic control 4. Currently, there are nine classes of orally available pharmacological agents to treat type 2 diabetes: 1) sulfonylureas, 2) meglitinides, 3) metformin (a biguanide), 4) thiazolidinediones, 5) α-glucosidase inhibitors, 6) dipeptidyl peptidase IV (DPP-IV) inhibitors, 7) bile acid sequestrant, 8) dopamine agonist, and 9) sodium-glucose transport protein (SGLT2) inhibitors 20. Initiation of basal insulin should be considered when a patient has not achieved target glucose levels despite 3 non-insulin hypoglycemic medications, or when glucose levels are very elevated (over 300 mg/dl [16.7 mmol/L] or HbA1C >10-12%) 21. When patients become very symptomatic from hyperglycemia (polyuria and polydipsia) or have signs of catabolism (weight loss), then there is evidence of marked insulin deficiency, and initiation of both basal and bolus insulin therapy should be considered 21. Since type 2 diabetes is associated with insulin resistance, insulin requirements can exceed 1 unit/kg/day. When initiating insulin therapy in patients with type 2 diabetes, basal insulin is often used in combination with other non-insulin antihyperglycemic medications a patient is taking. An intermediate or long-acting insulin (e.g., NPH, insulin glargine, or insulin detemir) is added at bedtime, and the dose titrated to attain a target fasting glucose, usually 80-130 mg/dl [4.4 – 7.2 mmol/L] 22. Besides the many options for insulin, there are also two classes of injectable medications: glucagon like peptide-1 (GLP-1) receptor agonists (incretin mimetics) and an amylin analogue 20.
Basal insulin is effective at lowering HbA1c when added to oral hypoglycemic agents starting at a dose of 10 U daily or 0.2 U/kg. When used in patients uncontrolled on oral agents, basal insulin lowers HbA1c 1.2–1.5% 23. In these treat-to-target studies, patients were instructed to titrate their basal insulin dosages up every 2–3 days by 1–4 units based on treatment target algorithms to achieve fasting plasma glucose levels in the 70- to 126-mg/dl [3.9 – 7.0 mmol/L] range. Those studies targeting a fasting plasma glucose < 108 mg/dl [< 6.0 mmol/L] achieve modestly better success in achieving an HbA1c < 7% (63.2 vs. 52%) than those targeting a fasting plasma glucose of less than 126 mg/dl, with mildly higher rates of hypoglycemia 24.
Alternatively, 70/30 insulin before dinner can be added in patients who have predominantly uncontrolled post-dinner hyperglycemia 25. Basal insulin, by suppressing hepatic glucose output during the night, will control the fasting blood glucose, while the other antihyperglycemic medications continue to control postprandial glucose levels throughout the day 26. A starting dose of 10 units of basal insulin is commonly utilized, though starting a dose of 0.1-0.2 units/kg will more rapidly attain the target fasting glucose level 27. In patients whose fasting glucose levels become well controlled with basal insulin, but whose glucose levels rise significantly high later in the day with a persistently elevated HbA1C, prandial insulin is indicated. At this point, the patient is experiencing beta-cell failure. If the patient is taking an insulin secretagogue (e.g., a sulfonylurea or meglitinide) it should be discontinued, as insulin will now be replaced exogenously. However, other agents not having a predominantly insulin-stimulating effect should be continued.
Types of insulin
With the availability of human insulin by recombinant DNA technology in the 1980s, use of animal insulin declined dramatically. Beef insulin, beef-pork and pork insulin are no longer commercially available in the United States 4. Currently, in the United States, insulins used are either human insulin and/or analogs of human insulin 4. The recombinant DNA technique for human insulin involves insertion of the human proinsulin gene into either Saccharomyces cerevisiae (baker’s yeast) or a non-pathogenic laboratory strain of Escherichia coli (E coli) which serve as the production organism. Human insulin is then isolated and purified 4. The United States FDA may allow for personal importation of beef insulin from a foreign country if a patient cannot be treated with human insulin 28. Beef insulin differs from human insulin by 3 amino acids and pork insulin differs by one amino acid 1.
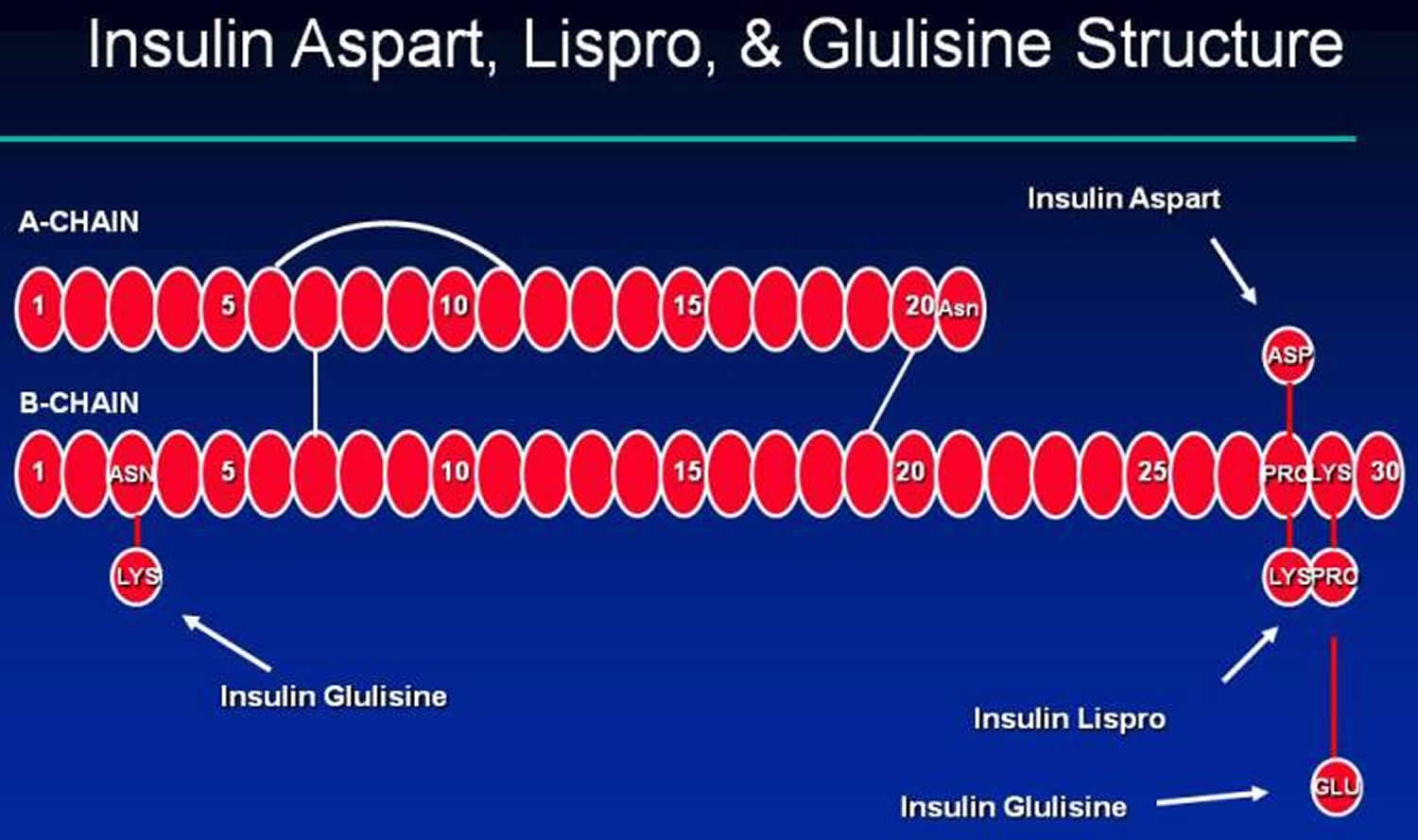
Recombinant DNA technology has allowed for the development and production of analogs to human insulin. With insulin analogs, the insulin molecule structure is modified slightly to alter the pharmacokinetic properties of insulin, primarily affecting the absorption of the drug from the subcutaneous tissue. The B26-B30 region of the insulin molecule is not critical for insulin receptor recognition and it is in this region that amino acids are generally substituted 5. Thus, the insulin analogs are still recognized by and bind to the insulin receptor. The structures of three rapid-acting insulin analogs are shown in Figure 3 (insulin aspart, lispro and glulisine) and the structures of two long-acting insulin analogs are shown in Figure 5 (insulin glargine and detemir). Insulin detemir was found to be more than 5-fold less potent than human insulin in binding to IGF-1 (insulin like growth factor-1) 5. The long term clinical significance of differences in IGF-1 binding among available insulins is not known.
Table 1. Insulins Commercially Available in the US (Recombinant DNA origin)
| Category/Name of Insulin | Brand Name (manufacturer) | Preparation(s) |
| Rapid-Acting | ||
| Insulin Lispro | Humalog (Lilly) | Vial, cartridge, disposable pen |
| Insulin Aspart | Novolog (Novo Nordisk) | Vial, cartridge, disposable pen |
| Insulin Glulisine | Apidra (Sanofi-Aventis) | Vial, disposable pen |
| Technosphere insulin | Afreeza | Inhaler |
| Short-Acting | ||
| Regular Human | Humulin R (Lilly) Novolin R (Novo Nordisk) | Vial |
| Intermediate-Acting | ||
| NPH Human | Humulin N (Lilly) Novolin N (Novo Nordisk) | Vial, disposable pen Vial |
| Long-Acting | ||
| Insulin Detemir | Levemir (Novo Nordisk) | Vial, disposable pen |
| Insulin Glargine | Lantus (Sanofi-Aventis) | Vial, cartridge, disposable pen |
| Insulin Mixtures | ||
| NPH/Regular (70%/30%) | Humulin 70/30 (Lilly) Novolin 70/30 (Novo Nordisk) | Vial, disposable pen Vial |
| Protamine/Lispro (50%/50%) | Humalog Mix 50/50(Lilly) | Vial, disposable pen |
| Protamine/Lispro (75%25%) | Humalog Mix 75/25(Lilly) | Vial, disposable pen |
| Protamine/Aspart (70/30) | Novolog Mix 70/30 (Novo Nordisk) | Vial, disposable pen |
Table 2. Insulin Pharmacodynamics
| Insulin | Onset (hr) | Peak (hr) | Duration (hr) | Appearance |
|---|---|---|---|---|
| Insulin Lispro | within 15 min | ~ 1 | 3-5 | Clear |
| Insulin Aspart | within 15 min | 1-3 | 3-5 | Clear |
| Insulin Glulisine | 0.25-.5 | 0.5-1 | 4 | Clear |
| Regular | ~ 1 | 2-4 | 5-8 | Clear |
| NPH | 1-2 | 4-10 | 14+ | Cloudy |
| Insulin Detemir | 3-4 | 6-8 (though relatively flat) | up to 20-24 | Clear |
| Insulin Glargine | 1.5 | flat | 24 | Clear |
| Lispro Mix 50/50 | 0.25-.5 | 0.5-3 | 14-24 | Cloudy |
| Lispro Mix 75/25 | 0.25-5 | 0.5-2.5 | 14-24 | Cloudy |
| Aspart Mix 70/30 | 0.1-.2 | 1-4 | 18-24 | Cloudy |
Footnote: Patient specific onset, peak, duration may vary from times listed in table.
Peak and duration are dose-dependent, with longer duration of actions seen with large doses.
The pharmacodynamics of regular and NPH are particularly affected by the size of the dose (1). Larger doses can cause a delay in the peak and increase the duration of action.
[Source 4 ]Figure 3. Rapid acting insulin (insulin Aspart, Glulisine and Lispro structures)
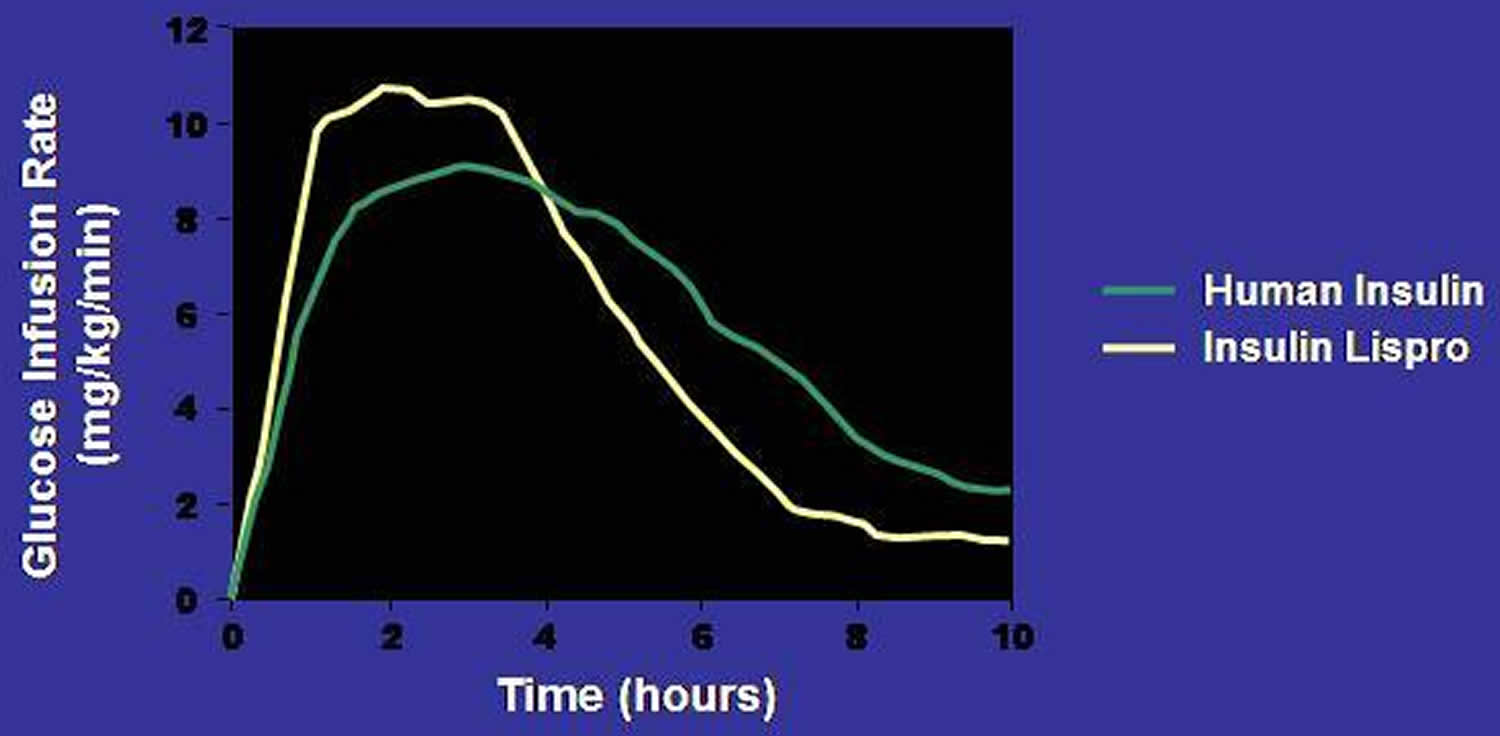
Figure 4. Pharmacodynamic Profiles of a Rapid Insulin Analog (insulin lispro) and Regular Insulin
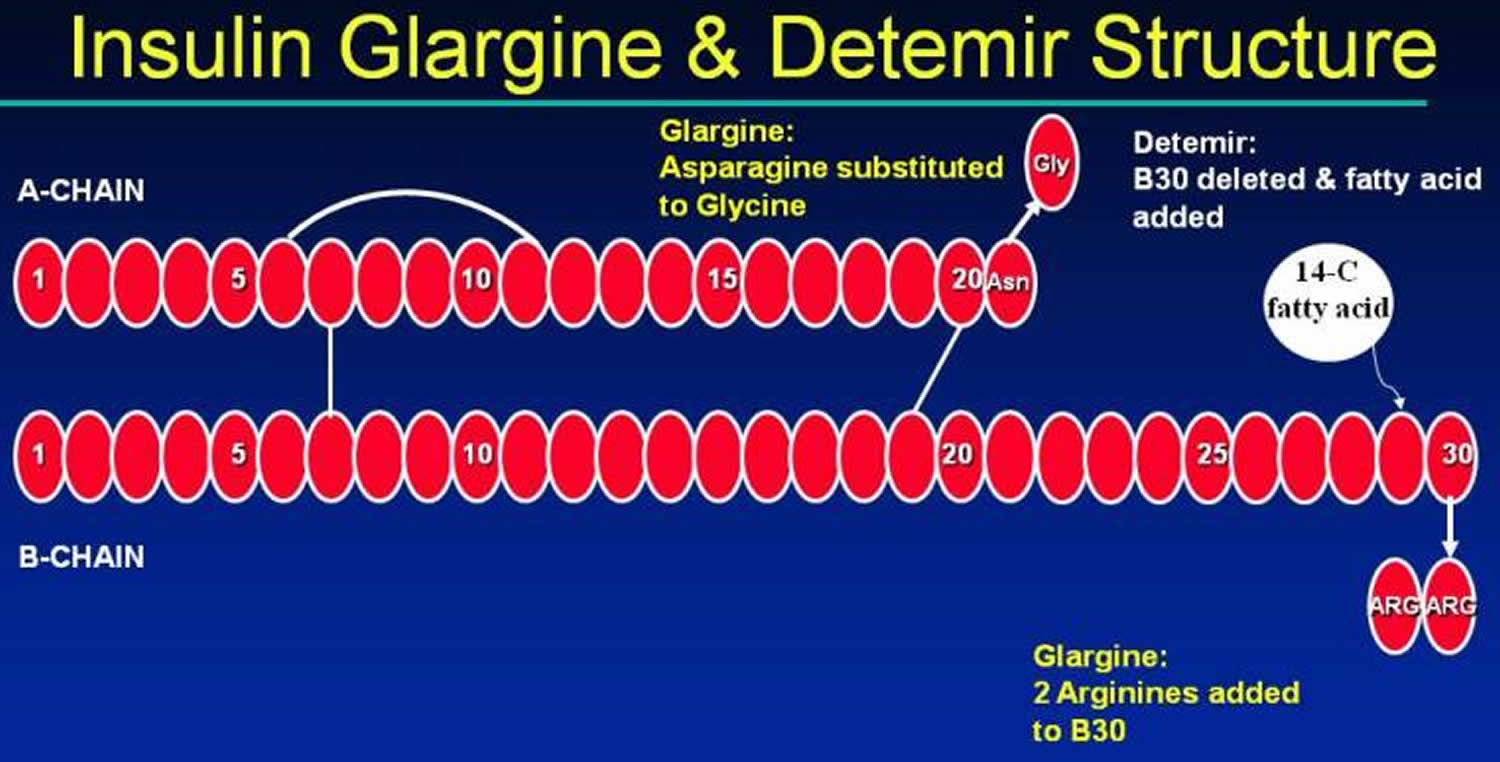
Figure 5. Long acting insulin (insulin Glargine and Detemir structures)
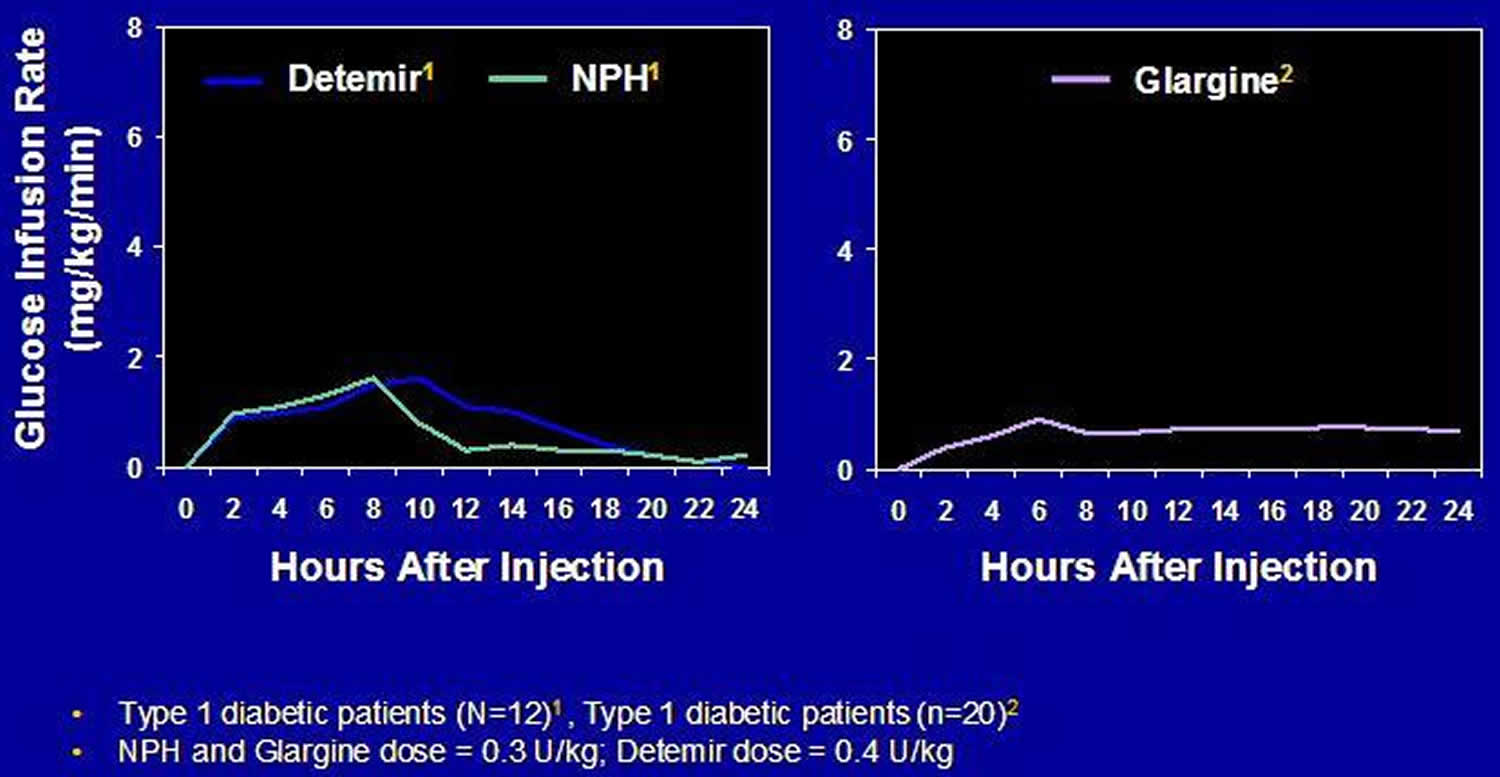
Figure 6. Pharmacodynamic Profiles of Long-Acting and Intermediate-Acting Basal Insulins
Insulin shot concentration
In the United States, all insulins are available in the concentration of 100 units/ml (denoted as U-100) 4. Insulin syringes are designed to accommodate this concentration of insulin. Regular human insulin (Humulin R, Lilly) is available in a more concentrated insulin, U-500 (500 units/ml), and is used primarily in cases of marked insulin resistance, when large doses of insulin (generally > 200 units per day) are required 4. As specific syringes for U-500 insulin are not available, extreme caution must be taken as each marked unit on a U-100 syringe will deliver 5 units of insulin. To avoid errors in insulin dosing when prescribing insulin in concentrations other than U-100, it is recommended that the dose be written both as the number of U-100 units prescribed and the number of ml to draw up on the insulin syringe 4. Insulin glargine is also available in a U-300 concentration, delivering 300 units/ml 4. The dose of insulin a patient dials into the pen device is in U-100 units.
Outside the United States, a less concentrated insulin preparation, U-40, (40 units/ml) is still available and sometimes used 4. Specific U-40 syringes are used with this insulin. It is important that patients traveling from one country to the next be aware of the concentration of insulin they use, and that the appropriate syringe is used.
Insulin injection physical and chemical properties
Regular human insulin is crystalline zinc insulin dissolved in a clear solution. It may be administered by any parenteral route: subcutaneous, intramuscular (IM), or intravenous (IV). Insulin aspart, glulisine and lispro are also soluble crystalline zinc insulin, but are intended for subcutaneous injection. When administered intravenously (IV), the action of these rapid-acting insulin analogs is identical to that of regular insulin. NPH, or neutral protamine Hagedorn, is a suspension of regular insulin complexed with protamine that delays its absorption. Insulin suspensions should not be administered intravenously. All insulins, except insulin glargine, are formulated to a neutral pH.
Long-acting insulin glargine is a soluble, clear insulin, with a pH of 4.0 which affects its subcutaneous absorption characteristics. Insulin glargine should not be mixed with other insulins, and should only be administered subcutaneously 29. Insulin detemir is an insulin analog coupled to an 18-chain fatty acid that binds to albumin in the subcutaneous tissue. This results in delayed absorption and a prolonged duration of action. Insulin detemir should also not be mixed with other insulins, and is intended only for subcutaneous use.
Insulin injection absorption
Insulin administered via subcutaneous injection is absorbed directly into the bloodstream, with the lymphatic system playing a minor role in transport 1. The absorption of human insulin into the bloodstream after subcutaneous absorption is the rate limiting step of insulin activity. Factors that alter insulin absorption do so mostly by changing local blood flow in the subcutaneous tissue. Factors that increase subcutaneous blood flow increase the absorption rate. Table 3 lists factors that affect insulin absorption.
Table 3. Factors Affecting Insulin Absorption
| Factor | Comment |
|---|---|
| Exercise of injected area | Strenuous exercise of a limb within 1 hour of injection will speed insulin absorption. Clinically significant for regular insulin analogs |
| Local massage | Vigorously rubbing or massaging the injection site will speed absorption. |
| Temperature | Heat can increase absorption rate, including use of a sauna, shower, or hot bath soon after injection. Cold has the opposite effect. |
| Site of injection | Insulin is absorbed faster from the abdomen. Less clinically relevant with rapid-acting insulins, insulin glargine and insulin detemir. |
| Lipohypertrophy | Injection into hypertrophied areas delays insulin absorption. |
| Jet injectors | Increase absorption rate. |
| Insulin mixtures | Absorption rates are unpredictable when suspension insulins are not mixed adequately (i.e., they need to be resuspended). |
| Insulin dose | Larger doses delay insulin action and prolong duration. |
| Physical status (soluble vs. suspension) | Suspension insulins must be sufficiently resuspended prior to injection to reduce variability. |
Insulin preparations
Short-Acting Regular Insulin
Regular insulin is injected pre-meal to blunt the postprandial rise in glucose levels. It forms hexamers after injection into the subcutaneous space slowing its absorption. Hexameric insulin progressively dissociates into absorbable insulin dimers and monomers. For this reason, regular insulin has a delayed onset of action of 30-60 minutes, and should be injected approximately 30 minutes before the meal to blunt the postprandial rise in blood glucose. Adherence to a 30 minute pre-meal schedule is inconvenient and difficult for many patients.
Rapid-Acting Insulin Analogs
Rapid-acting analogs result from changes to the amino acid structure of human insulin which lead to decreases in hexameric insulin formation after injection into the subcutaneous space. This leads to more rapid dissolution of insulin into monomers, more rapid insulin absorption into the bloodstream and a shorter duration of action. While on a molar basis rapid-acting insulin analogs have identical in vivo potency compared to regular human insulin, higher peak concentrations are achieved 10. For this reason, when converting from regular to a rapid-acting insulin analog, the dose of insulin may need to be reduced. When compared to regular insulin, the rapid-acting insulin analogs lead to less postprandial hyperglycemia and less late postprandial hypoglycemia 30. Injection of rapid-acting insulin analogs 15-20 minutes pre-meal leads to maximal reduction of postprandial glucose excursions 31, as compared to 30 or more minutes pre-meal for regular insulin. This shorter interval for insulin injection pre-meal is more convenient for patients and leads to greater adherence with prescribed injection timing guidelines. In patients who are unsure of the amount of carbohydrate to be served for a meal, immediate pre-meal dosing allows more accurate dosing, and reduces the risk of hypoglycemia. All rapid-acting insulin analogs are approved for use in pumps.
Insulin Lispro (Humalog)
Insulin lispro (Humalog) results from the reversal of the B28 (proline) and B29 (lysine) amino acid sequence of insulin.
Insulin lispro has been approved for injection before and immediately after a meal. Post-meal insulin dosing is useful for parents of young children with type 1 diabetes, in whom the amount of carbohydrates consumed at a meal can be unpredictable. When compared with pre-meal regular insulin in prepubertal children, post meal insulin lispro showed no significant differences in post meal glucose levels, rates of hypoglycemia or HbA1c 32. In the rare case of severe human insulin allergy, insulin lispro has been shown to be less immunogenic 33.
Insulin Aspart (Novolog)
Insulin aspart differs from human insulin by a substitution of the B28 amino acid proline with aspartic acid. Chemically it is B28-aspartic acid-human insulin.
Insulin Glulisine (Apidra)
Insulin glulisine differs from human insulin by changes in the amino acid asparagine at position B3 to lysine and the lysine at position B29 to glutamic acid. Chemically, it is 3B-lysine-29B-glutamic acid-human insulin.
Inhaled Insulin
Inhaled insulin formulations deliver powdered insulin into the lower airways using an inhaled delivery device. Exubera received FDA approval in 2006 but failed to gain market share and production was discontinued after 1 year. Technosphere insulin (Afrezza) was FDA approved in 2014. Its pulmonary absorption leads to a more rapid absorption than currently available, subcutaneously administered rapid-acting insulin preparations 4. In subjects with type 2 diabetes, serum insulin levels rise within 5 minutes after inhalation and peak after 17 minutes 34. When compared with pre-meal human Regular insulin, inhaled insulin (technosphere insulin) more effectively reduced 4-hour postprandial glucose area under the curve by 52% 35. Only 0.3% of technosphere insulin is detectable in the lungs after 12 hours.
Inhaled insulin (technosphere insulin) leads to a dry cough in 19-30 % of subjects tested 36. Small, reductions in forced expiratory volume (FEV1) are observed in the first 3-6 months of use which are non-progressive for up to 2 years of follow up and reversible after drug discontinuation 37. The use of technosphere is contraindicated in patients who smoke or have COPD because of alterations in drug absorption. Spirometry needs to be performed prior to initiation of technosphere insulin, after 6 months and then annually thereafter, with a 20% or higher decline in FEV1 being an indication for drug discontinuation 38.
Intermediate-Acting Insulins (NPH)
NPH (Neutral Protamine Hagedorn) insulin, was created in 1936 after it was discovered that the effects of subcutaneously injected insulin could be prolonged by the addition of the protein protamine. NPH insulin is an intermediate-acting insulin, whose onset of action is approximately 2 hours, peak effect is 6-14 hours, and duration of action of 10-16 hours (depending on the size of the dose). Because of its broad peak and long duration of action, NPH can serve as a basal insulin only when dosed at bedtime, or a basal and prandial insulin when dosed in the morning. NPH insulin is available in various combinations with either regular insulin or rapid-acting insulins (see Table 1).
Long-Acting (Basal) Insulin Analogs
Long-acting insulins provide basal insulin coverage. Basal insulins suppress hepatic gluconeogenesis to prevent glucose levels from rising during the fasting state in insulin-deficient patients. Among patients with type 1 diabetes, basal insulins additionally prevent ketogenesis.
Insulin Glargine (Lantus)
Insulin glargine (21A-Gly-30Ba-L-Arg-30Bb-L-Arg-human insulin) contains two modifications to human insulin. Two arginines are added to the C-terminus of the B chain shifting the isoelectric point of the insulin from a pH of 5.4 to 6.7 39. This change makes the insulin more soluble at an acidic pH and insulin glargine is formulated at a pH of 4.0. The second modification is at position A21, where asparagine is replaced by glycine. This substitution prevents deamidation and dimerisation that would occur with acid-sensitive asparagine. When insulin glargine is injected into subcutaneous tissue, which is at physiologic pH, the acidic solution is neutralized. Microprecipitates of insulin glargine are formed, from which small amounts of insulin are released throughout a 24-hour period, resulting in a relatively stable level of insulin throughout the day 40. The biological activity of insulin glargine is due to its absorption kinetics and not a different pharmacodynamic activity (e.g., stimulation of peripheral glucose uptake) 41.
Insulin glargine should not be mixed in the same syringe with any another insulin or solution because this will alter its pH and thus affect its absorption profile. Glargine has an onset of action of about 2 hours, and a duration of action of 20-24 hours. It may be given once daily at any time of day, or twice daily at higher doses (typically more than 50 units daily) to better maintain its relatively flat action profile. Its more consistent rate of absorption and lack of a significant peak action result in reduced nocturnal hypoglycemia when insulin glargine is used at bedtime compared with NPH insulin 42.
Insulin Detemir (Levemir)
Insulin detemir (Levemir) is a long-acting insulin analog in which the B30 amino acid is omitted and a C14 fatty acid chain (myristic acid) is bound to the B29 lysine amino acid. Insulin detemir is slowly absorbed due to its strong association with albumin in the subcutaneous tissue. When it reaches the bloodstream it again binds to albumin delaying its distribution to the peripheral tissues. Detemir has an onset of action of about 2 hours, and a duration of action of 16-24 hours. It can be given once or twice daily. Patients who experience a rise in glucose levels in the hours prior to a once daily injection due to the waning action of detemir should use a twice daily dosing regimen.
Insulin degludec is an ultra-long-acting modified human insulin in which the B30 amino acid is omitted and a glutamic acid spacer links a 16-carbon fatty di-acid chain to the B29 amino acid. Deguldec forms multihexamers following subcutaneous injection, leading to a slow release of insulin monomers into the bloodstream and a prolonged duration of action. The half-life of degludec is about 25 hours and its duration of action more than 42 hours. Flat insulin levels are seen within 3 days of the first injection with less daytime variability when compared with glargine insulin 43. With overall similar HbA1c lowering when compared with glargine insulin, reduced rates of hypoglycemia have been seen with degludec use in type 2 diabetes patients, but not in type 1 diabetes patients 44. No differences in local site reactions, weight gain or other adverse reactions have been seen with degludec use. In 2013, the Food and Drug Administration in the United States asked for more cardiovascular safety data before approving degludec, citing an increased risk of cardiovascular events from a meta-analysis of 17 clinical trials which used degludec.
Pre-mixed Intermediate with Short or Rapid-acting insulins (50/50, 70/30 and 75/25)
NPH insulin or protamine added to rapid-acting insulin analogs can be mixed together with regular or rapid-acting insulin analogs in fixed combinations. These insulins thus provide bolus insulin coverage for the meal that follows the injections well as basal coverage from the intermediate-acting component of the insulin. They are given either before a larger breakfast or dinner meal as once daily dosing, or more commonly twice daily before breakfast and dinner. Patients who require basal/bolus insulin replacement but have difficulty with frequently missed insulin dosages may benefit from a regimen utilizing twice daily mixed insulin. However, given the fixed proportions of mixed insulins and their less physiologic action, there is an increased risk of hypoglycemia using these insulin preparations when compared with basal and pre-meal bolus insulin regimens 23.
Biosimilar insulins
Patent protection for a number of insulins will expire in the near future. Biosimilar insulins are in development to match the insulin profiles of a number of reference insulins. As biopharmaceuticals are more complex to manufacture, the cost savings are expected to be less than seen with most generic drugs 45.
Insulin side effects
Hypoglycemia
Hypoglycemia is the most serious adverse effect of insulin therapy and the major barrier to achieving glycemic targets in patients with type 1 diabetes and insulin-requiring type 2 diabetes 46. Intensive insulin therapy in patients with type 1 diabetes in the Diabetes Control and Complications Trial Research was associated with a 2-3 fold increase in severe hypoglycemia, defined as hypoglycemia requiring assistance from others 47. In studies of intensive therapy in type 2 diabetes, including the UKPDS, VADT, ADVANCE, and ACCORD trials, intensive therapy resulted in significantly more common severe hypoglycemia when compared with standard therapy 48. Severe hypoglycemia can cause confusion, motor vehicle accidents, seizures and coma, and is estimated to be a cause of death in 4-6% of patients with type 1 diabetes 49.
The true incidence of hypoglycemia in patients with type 2 diabetes is unknown 4. Patients with type 2 diabetes who have had serious hypoglycemia are at increased risk of death regardless of the intensity of their glycemic control. Hypoglycemia increases heart rate, systolic blood pressure, myocardial contractility and cardiac output, which may adversely affect those with diabetes who frequently have underlying coronary artery disease (coronary heart disease). Glucose levels below 70 mg/dl have been shown to cause ischemic ECG changes in patients with type 2 diabetes and coronary heart disease during continuous glucose and ECG monitoring 50. Hypoglycemia may lead to increased mortality due to the pro-arrhythmic effects of sympathoadrenal activation and hypokalemia 51, or from cardiac repolarization, especially in older patients with underlying cardiac disease.
Risk factors for hypoglycemia among insulin-treated patients include older age, longer duration of diabetes, renal insufficiency, hypoglycemia unawareness, prior hypoglycemia and lower HbA1c 52. Avoidance of hypoglycemia therefore takes on particular importance in older patients, given the greater prevalence of cardiovascular disease, cognitive dysfunction, and higher risk of falls and fractures. To help reduce the incidence of hypoglycemia, the American Diabetes Association 21 advises targeting a higher HbA1c of up to 8% in patients who are older, with a longer duration of disease, more comorbidities, frequent hypoglycemia and underlying cardiovascular disease.
All patients receiving insulin should learn to recognize the symptoms of hypoglycemia and how best to treat low glucose levels
Weight gain
Weight gain is common side effect of insulin therapy. In part, the weight gain can be a result of frequent hypoglycemic episodes in which patients consume extra calories to treat the low glucose level and often overeat in response to hunger. One of the anabolic effects of insulin is to promote the uptake of fatty acids into adipose tissue. The amount of weight gain in the Diabetes Control and Complications Trial (type 1 patients) 47 and UKPDS (type 2 patients) 53 associated with insulin therapy was 4.6 kg and 4.0 kg respectively. Less weight gain is encountered with levemir insulin than with NPH or glargine insulin 24. The etiology of lower weight gain with detemir when compared with NPH or glargine is unknown. Basal insulin added to oral antihyperglycemic agents leads to less weight gain than either biphasic insulin aspart or prandial aspart insulin 23. Lispro mix 75/25 insulin leads to greater weight gain than glargine insulin when added to oral antihyperglycemic agents 54.
Local reactions
True allergic reactions and cutaneous reactions are rare with human insulin and insulin analogs. Hypersensitivity reactions can rarely develop in response to the insulin or one of its additives (protamine for example) and can result in local erythema, pruritus, a wheal or more systemic reactions including anaphylaxis. Successful approaches to insulin allergies include continuous subcutaneous insulin infusions, and use of lispro insulin which appears to be less allergenic 55.
Lipoatrophy was common with use of less pure and animal insulins, but is no longer encountered. To avoid the lipohypertrophic effects of insulin, patients should be instructed to rotate their insulin injection sites, preferably rotating within one area and not reusing for one week
Mitogenic properties
Several retrospective, observational studies have shown correlations between insulin dose and cancer risk for most insulin types (human insulin, aspart, lispro or glargine) 56. These observational studies assessed large patient databases and have significant, inherent limitations, such as the potential for different pre-treatment characteristics of the groups, selection bias, the small numbers of cancer cases found, and short duration of follow-up. Meta-analyses of studies comparing exogenous insulin to non-insulin antihyperglycemic therapies have shown associations of insulin with several cancers 7. However, there are also inherent limitations to such analyses. In a randomized, 5-year, open-label trial comparing the progression of retinopathy of NPH and insulin glargine users, no increased risk of cancer was found in the 1,017 patient sample 57. In an analysis of 31 randomized controlled trials from the Sanofi-Aventis safety database (phase 2, 3, and 4 studies), insulin glargine was not associated with an increased risk of cancer 58. The 7-year, randomized ORIGIN trial assessed the cardiovascular effects of insulin glargine versus standard care in more than 12,500 individuals with diabetes or pre-diabetes and found no increased risk of all-cancer-combined or of cancer mortality among glargine-treated individuals 59. A recent review of large epidemiologic studies did not find evidence of an increased risk of malignancy among glargine-treated patients when compared with other insulin therapies 8.
Cardiovascular disease
Among intensively controlled patients in the VADT (Veterans Affairs Diabetes Trial), ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation), and ACCORD (Action to Control Cardiovascular Risk in Diabetes), a higher proportion (41–90%) required insulin therapy than among the standard control groups (24–74%) 60. Nonsignificant reductions in cardiovascular events were seen with intensive diabetes control when compared with standard control in ADVANCE, ACCORD, and VADT respectively. An increased mortality rate was observed in ACCORD (Action to Control Cardiovascular Risk in Diabetes) after 3.5 years of intensive therapy when patients were targeted to an HbA1c of less than 6.0%, 73% of whom received insulin. However, mortality was not temporally associated with severe hypoglycemia. The results of ACCORD support less aggressive diabetes management among patients at high risk for a cardiovascular event. The 7-year, randomized ORIGIN trial assessed the cardiovascular effects of insulin glargine versus standard care in more than 12,500 individuals with diabetes or pre-diabetes and found no increased risk of cardiovascular events or of cardiovascular mortality among glargine-treated individuals 59.
References- Binder C, Brange J 1997 Insulin chemistry and pharmacokinetics. In: Porte D, Jr., Sherwin R (eds) Ellenberg’s and Rifkin’s Diabetes Mellitus, 5th edition ed. Appleton and Lange, Stamford, CT, p 689
- Stryer L (1995). Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 773–74. ISBN 0 7167 2009 4.
- Insulin: understanding its action in health and disease. British Journal of Anaesthesia , Volume 85 , Issue 1 , 69 – 79. https://bjanaesthesia.org/article/S0007-0912(17)37337-3/fulltext
- Donner T. Insulin – Pharmacology, Therapeutic Regimens And Principles Of Intensive Insulin Therapy. [Updated 2015 Oct 12]. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278938
- Kurtzhals P, Schaffer L, Sorensen A, et 2000 Correlations of receptor binding andmetabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 49:999-1005
- Müssig K, Staiger H, Kantartzis K, Fritsche A, Kanz L, Häring H-U 2011 Type 2 diabetes mellitus and risk of malignancy: is there a strategy to identify a subphenotype of patients with increased susceptibility to endogenous and exogenous hyperinsulinism? Diabet Med 28:276-286
- Karlstad O, Starup-Linde J, Vestergaard P, Hjellvik V, Bazelier MT, Schmidt MK, Andersen M, Auvinen A, Haukka J, Furu K, de Vries F, De Bruin ML 2013 Use of insulin and insulin analogs and risk of cancer – systematic review and meta-analysis of observational studies. Curr Drug Saf 8(5):333-48
- Janghorbani M, Dehghani M, Salehi-Marzijarani M 2012 Systematic review and meta-analysis of insulin therapy and risk of cancer. Horm Cancer 3(4):137-46
- Schernthaner G 1993 Immunogenicity and allergenic potential of animal and human Diabetes Care 16 Suppl 3:155-65
- Duckworth WC, Bennett RG, Hamel FG 1998 Insulin degradation: progress and potential. Endocr Rev 19:608–624
- Nolte MS, Karam, JH. 2001 Pancreatic Hormones & Antidiabetic Drugs. In: Katzung B (ed) Basic and Clinical Pharmacology, 8th ed. Lange Medical Books/McGraw Hill, New York, pp 711-734
- Rabkin R, Ryan MP, Duckworth WC 1984 The renal metabolism of insulin. Diabetologia 27:351-7
- Weiss M, Steiner DF, Philipson LH. Insulin Biosynthesis, Secretion, Structure, and Structure-Activity Relationships. [Updated 2014 Feb 1]. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279029
- Accili, Domenico. “Lilly Lecture 2003.” Diabetes 53.7 (2004): 1633-1642. http://diabetes.diabetesjournals.org/content/53/7/1633.long
- http://www.diabetesatlas.org/
- Subramanian S, Baidal D, Skyler JS, et al. The Management of Type 1 Diabetes. [Updated 2016 Nov 16]. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279114
- Hirsch IB 1999 Type 1 diabetes mellitus and the use of flexible insulin Am Fam Physician 60:2343-52, 2355-6
- February 2015 Novo Nordisk Novolog Mix 70/30 Package Insert.
- Chiang JL, Kirkman MS, Laffel LMB, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014;37:2034–2054
- Evans JL, Balkan B, Chuang E, et al. Oral and Injectable (Non-insulin) Pharmacological Agents for Type 2 Diabetes. [Updated 2016 Jul 20]. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279141
- 2015. American Diabetes Association Standards of Medical Care in Diabetes. Diabetes Care 2015;38:Supplement 1 S1-S93
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. 2009. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 32:193–203
- Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF, Paul SK 2009 Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 361:1736-1747
- Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner 2008. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 51(3):408-416
- Raskin P, Matfin G, Schwartz SL, Chaykin L, Chu P-L, Braceras R, Wynne A 2009 Addition of biphasic insulin aspart 30 to optimized metformin and pioglitazone treatment of type 2 diabetes mellitus: The ACTION Study (Achieving Control Through Insulin plus Oral agents). Diabetes Obes Metab 11:27-32
- Hermann 2000 Optimising therapy for insulin-treated type 2 diabetes mellitus. Drugs Aging 17:283-94
- DeWitt DE, Dugdale 2003 Using new insulin strategies in the outpatient treatment of diabetes: clinical applications. JAMA 289:2265-9
- Questions and Answers on Importing Beef or Pork Insulin for Personal Use. https://www.fda.gov/drugs/resourcesforyou/consumers/questionsanswers/ucm173909.htm
- February 2015 Sanofi-Aventis S. Lantus Package Insert.
- Lalli C, Ciofetta M, Del Sindaco P, et al. 1999 Long-term intensive treatment of type 1 diabetes with the short-acting insulin analog lispro in variable combination with NPH insulin at mealtime. Diabetes Care 22:468-77
- Cobry E, McFann K, Messer L, Gage V, VanderWel B, Horton L, Chase HP 2010 Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with type 1 diabetes. Diabetes Technol Ther. 12(3):173-7
- Tupola S, Komulainen J, Jääskeläinen J, Sipilä I 2001 Post-prandial insulin lispro vs. human regular insulin in prepubertal children with Type 1 diabetes mellitus. Diabet Med. 18(8):654-8
- Kumar D 1997 Lispro analog for treatment of generalized allergy to human insulin. Diabetes Care 20:1357-9
- Rave K, Heise T, Heinemann L, Boss AH 2008 Inhaled Technosphere insulin in comparison to subcutaneous regular human insulin: time action profile and variability in subjects with type 2 diabetes. J Diabetes Sci Technol. 2(2):205-12
- Rave K, Heise T, Pfutzner A, Boss AH 2007 Coverage of postprandial blood glucose excursions with inhaled technosphere insulin in comparison to subcutaneously injected regular human insulin in subjects with type 2 diabetes. Diabetes Care 2007;30:2307-8
- Rosenstock J, Lorber DL, Gnudi L, Howard CP, Bilheimer DW, Chang PC, Petrucci RE, Boss AH, Richardson PC 2010 Prandial inhaled insulin plus basal insulin glargine versus twice daily biaspart insulin for type 2 diabetes: a multicentre randomised trial. 2010 Jun 26;375(9733):2244-53
- Raskin P, Heller S, Honka M, Chang PC, Boss AH, Richardson PC, Amin N 2012 Pulmonary function over 2 years in diabetic patients treated with prandial inhaled Technosphere Insulin or usual antidiabetes treatment: a randomized trial. Diabetes Obes Metab. 14(2):163-73
- June 2014 Mannkind Corporation Afrezza package insert
- Bolli GB, Owens DR 2000 Insulin Lancet 356:443-5
- Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T 2000 Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 23:644-9
- Mudaliar S, Mohideen P, Deutsch R, et 2002. Intravenous glargine and regular insulin have similar effects on endogenous glucose output and peripheral activation/deactivation kinetic profiles. Diabetes Care 25:1597-602
- Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA 2000 Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care 23:639-43
- Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859–864
- Ratner RE, Gough SC, Mathieu C, Del Prato S, Bode B, Mersebach H, Endahl L, Zinman B. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15(2):175-84
- DeVries JH, Gough SCL, Kiljanski J, Heinemann L. Biosimilar insulins: a European perspective. Diabetes Obes Metab. 2015;17: 445–451
- Cryer PE 2013 Mechanisms of Hypoglycemia-Associated Autonomic Failure in Diabetes N Engl J Med 369:362-372
- The Diabetes Control and Complications Trial Research 1993. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977-86
- Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560-2572
- Cryer PE. The Barrier of Hypoglycemia in Diabetes. Diabetes. 2008;57(12):3169-3176
- Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care 2003;26:1485–1489
- Frier BM, Schernthaner G, Heller SR 2011 Hypoglycemia and cardiovascular risks. Diabetes Care 34 (Suppl 2):S132-7.
- Donner T, Muñoz M. Update on Insulin Therapy for Type 2 Diabetes. J Clin Endocrinol Metab 2012;97(5):1405-1413
- UK Prospective Diabetes Study (UKPDS) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53
- Buse JB, Wolffenbuttel BHR, Herman WH, Hippler S, Martin SA, Jiang HH, Shenouda SK, Fahrbach JL 2011 The DURAbility of Basal versus Lispro mix 75/25 insulin Efficacy (DURABLE) trial: comparing the durability of lispro mix 75/25 and glargine. Diabetes Care 34:249-255
- Radermecker RP, Scheen AJ. Allergy reactions to insulin: effects of continuous subcutaneous insulin infusion and insulin analogues. Diabetes Metab Res Rev 2007; 23:348
- Colhoun HM, on behalf of the SDRN Epidemiology Group. 2009. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 52:1755-1765
- Rosenstock J, Fonseca V, McGill JB et al. 2009. Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: findings from a 5 year randomized, open-label study. Diabetologia 52:1778-1788
- Home PD, Lagarenne 2009. Combined randomised controlled tiral experience of malignancies in studies using insulin glargine. Diabetologia 52(12):2499-506
- The ORIGIN Trial Investigators. Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia. N Engl J Med 2012; 367:319-328
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129-139