IUD birth control
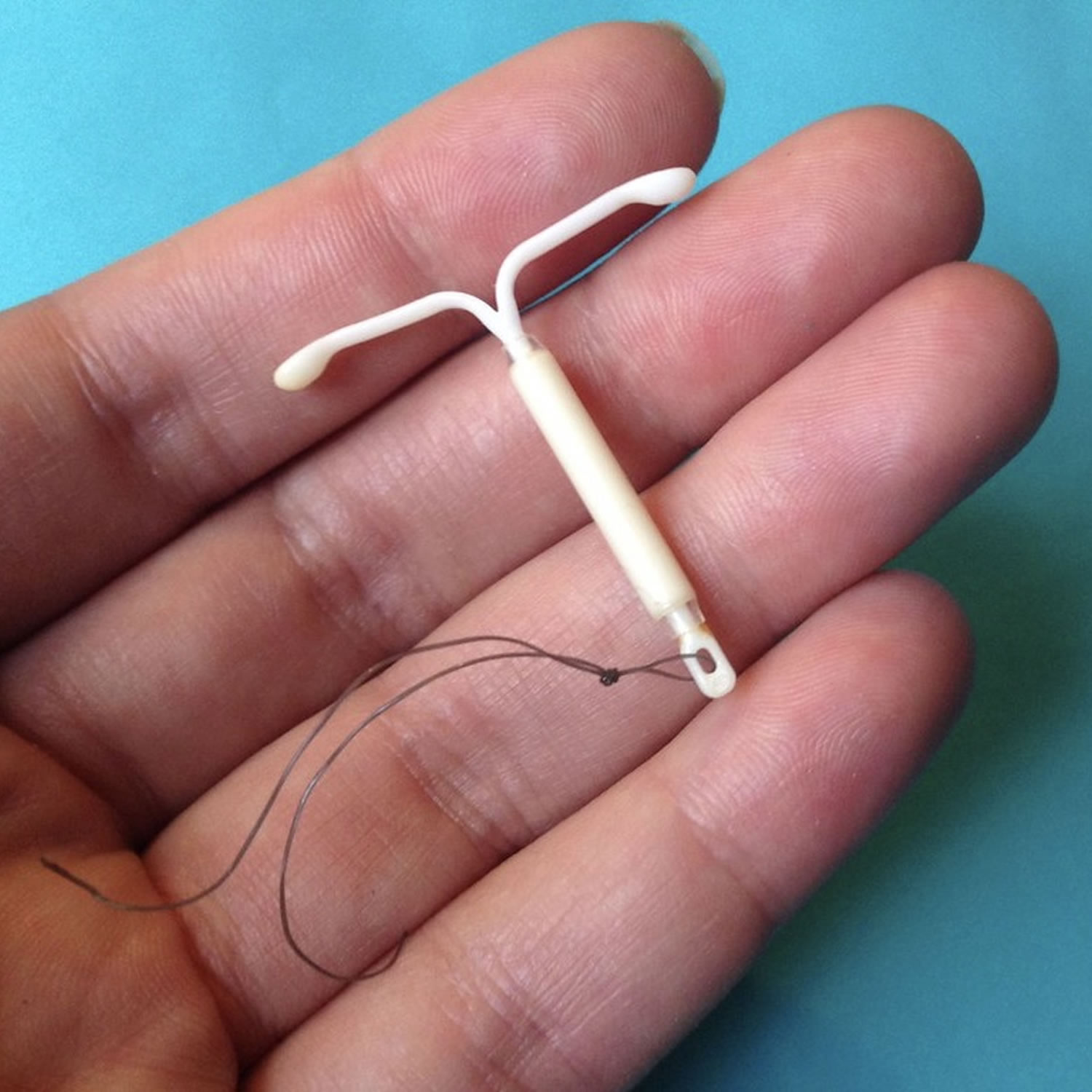
IUD is short for Intrauterine Device or intrauterine contraceptive device, is a highly effective and reliable birth control (contraception) T-shaped plastic device of that can lasts for 3 to 10 years (depending on the type of IUD you are using). IUD contraceptive are small plastic T-shaped devices that are placed inside your uterus (womb) to prevent pregnancy. IUDs can also be used as an emergency contraception. It must be inserted within 5 days of having unprotected sex. IUDs are also known as Long Acting Reversible Contraceptives (LARCs), meaning they are long acting and don’t require you to do anything to prevent pregnancy every day or every time you have sex for 3 to 10 years (depending on the type of IUD you are using). IUDs do not have any impact on your fertility, when they are stopped your fertility returns to normal immediately. In the United States, there has been an increased use of long-acting reversible contraception since 1995. This use has continued to increase from year to year, with 14% of women who use contraception, choosing to use a form of long-acting reversible contraceptive (IUDs and hormonal implants) 1. There has also been a decrease in the number of unplanned pregnancies with the increased use of long-acting reversible contraceptive (LARC) 1. Additionally, there are many benefits of IUDs, including efficacy, ease of use, reversible nature, and patient satisfaction, especially with time commitment for long-term use and cost 2. The IUD works very well if inserted properly and is one of the most effective reversible methods of contraception method available with greater than 99% effective and are suitable for most people 1. If 100 women use an IUD for a year, fewer than 1 will become pregnant. However, an IUD won’t protect you against sexually transmitted infections (STIs) so you will need to use condoms for added protection.
There are 2 types of IUD — the hormonal IUD and the copper IUD. You need a prescription to get an IUD. It needs to be put in place by a specially trained doctor or nurse.
Women can choose from many different types of birth control. Some work better than others at preventing pregnancy. The type of birth control you use depends on your health, your desire to have children now or in the future, and your need to prevent sexually transmitted infections (STIs). Your doctor can help you decide which type is best for you right now.
Three intrauterine devices are available in the United States, the copper-containing IUD (copper T 380A marketed as Paragard IUD) and two levonorgestrel-releasing IUDs (marketed as Mirena IUD and Skyla IUD), one that releases 20 mcg of levonorgestrel per 24 hours (Mirena IUD) and a new low-dose device that releases 14 mcg per 24 hours (Skyla IUD) 3. Failure rates within the first year after insertion are 0.6% to 0.8% for the copper T 380A IUD, 0.2% for the 20-mcg Mirena-IUD, and 0.9% for the 14-mcg Skyla-IUD 4. In contrast to that of many forms of contraception, the effectiveness of IUDs is not heavily dependent on user compliance 4.
There is a normal return to fertility after discontinuation of the copper T 380A IUD (Paragard IUD) or the 20-mcg Mirena-IUD, with a pregnancy rate of 82% one year after device removal and 89% two years after device removal 5. Clinical trials for the 14-mcg Skyla-IUD have found that 77% of women attempting to become pregnant within the first year after IUD removal are successful 6. Even with increasing costs of IUDs, they are one of the most cost-effective forms of long-term contraception 7.
IUDs may be placed immediately after childbirth within 10 minutes of delivery of the placenta or delayed post-partum within 4-6 weeks of delivery or during post-abortion, so long as it was not a septic abortion 8.
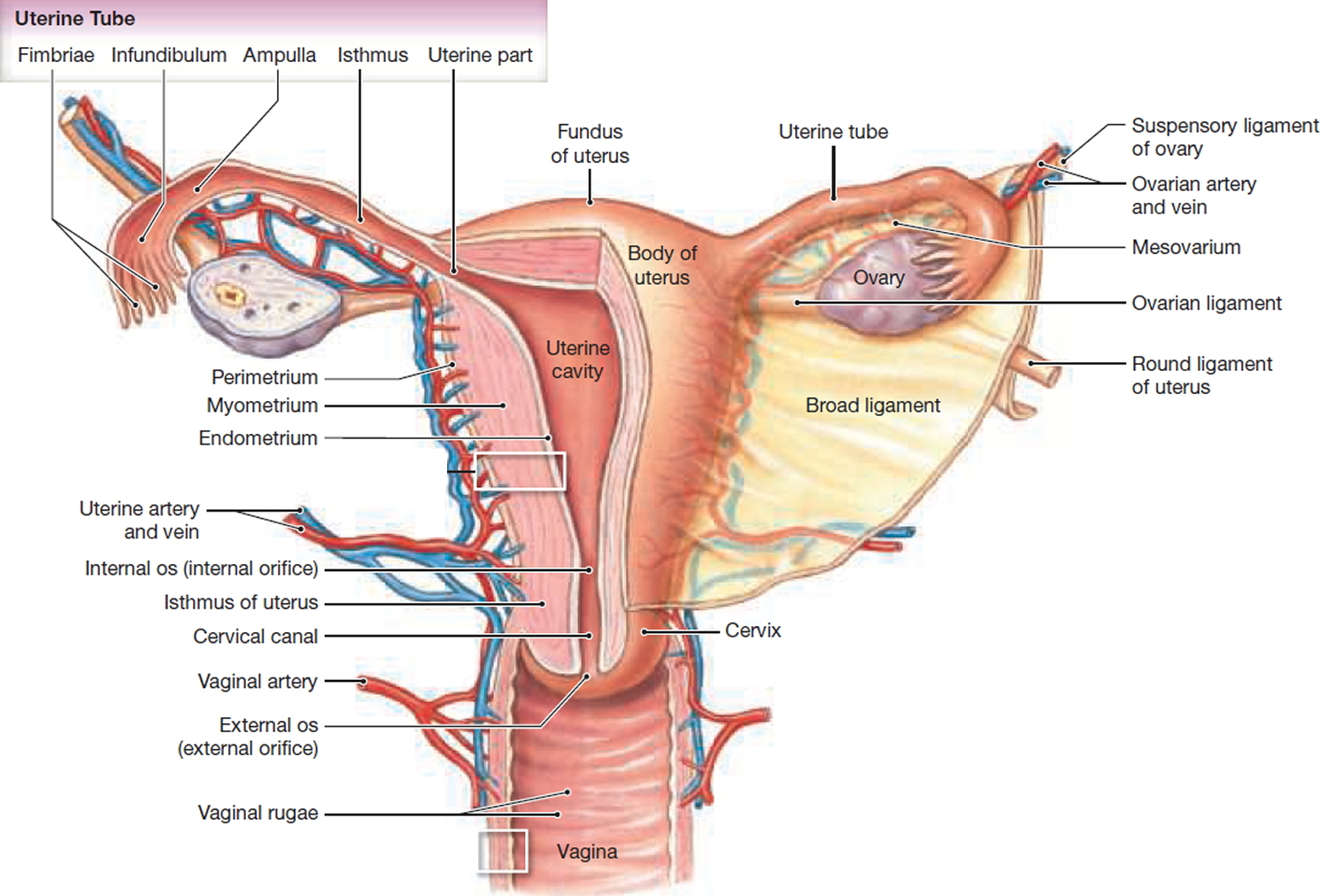
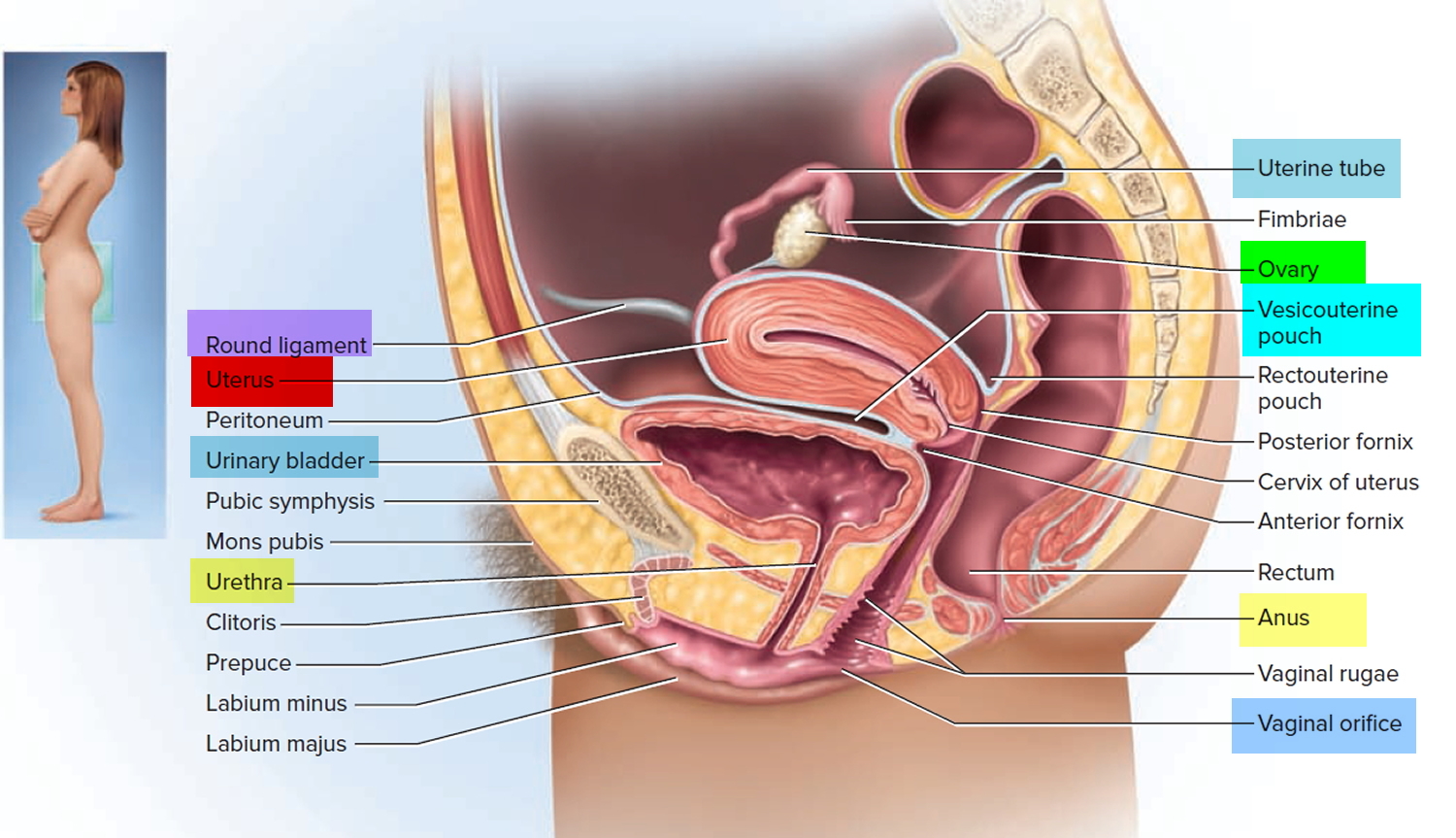
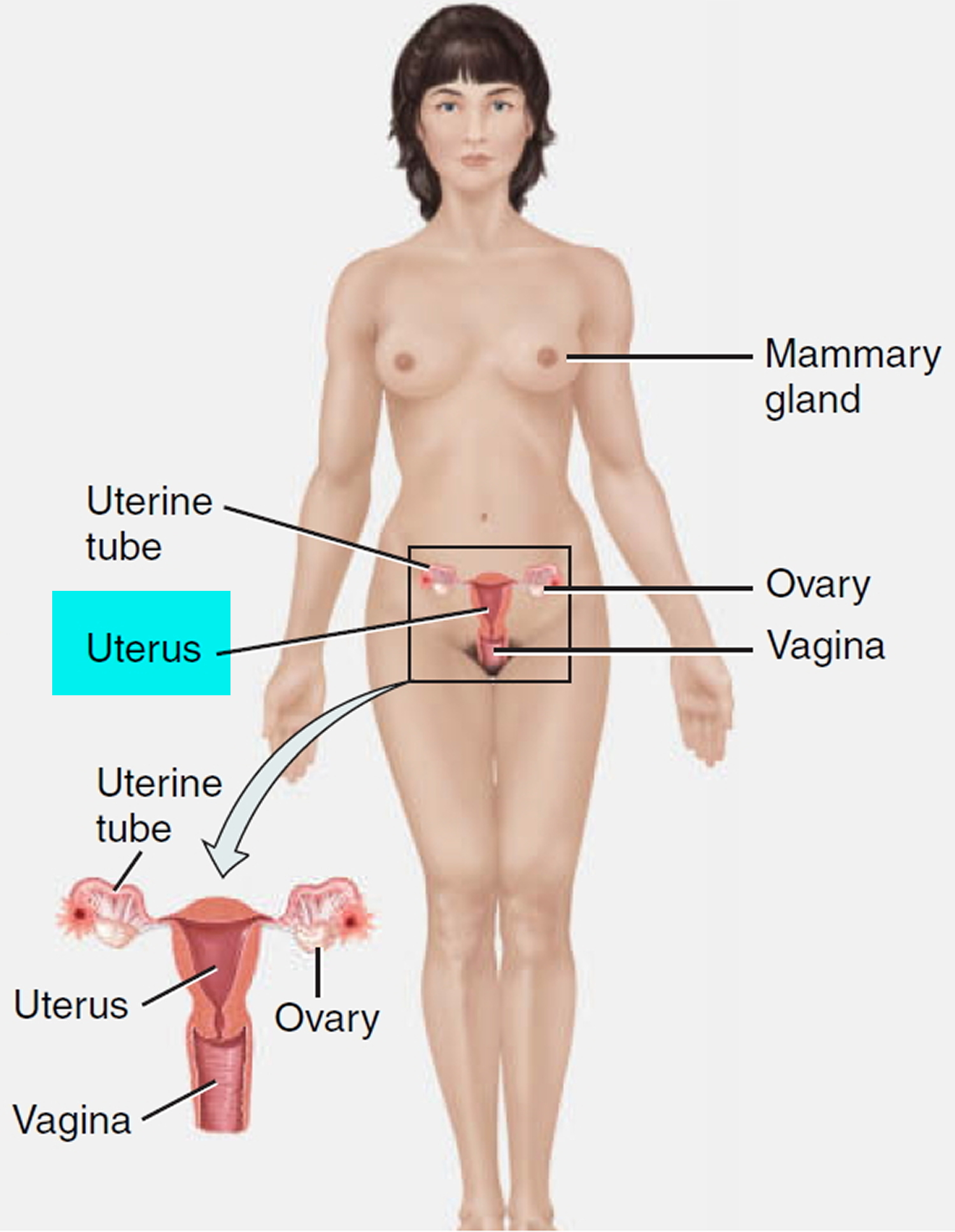
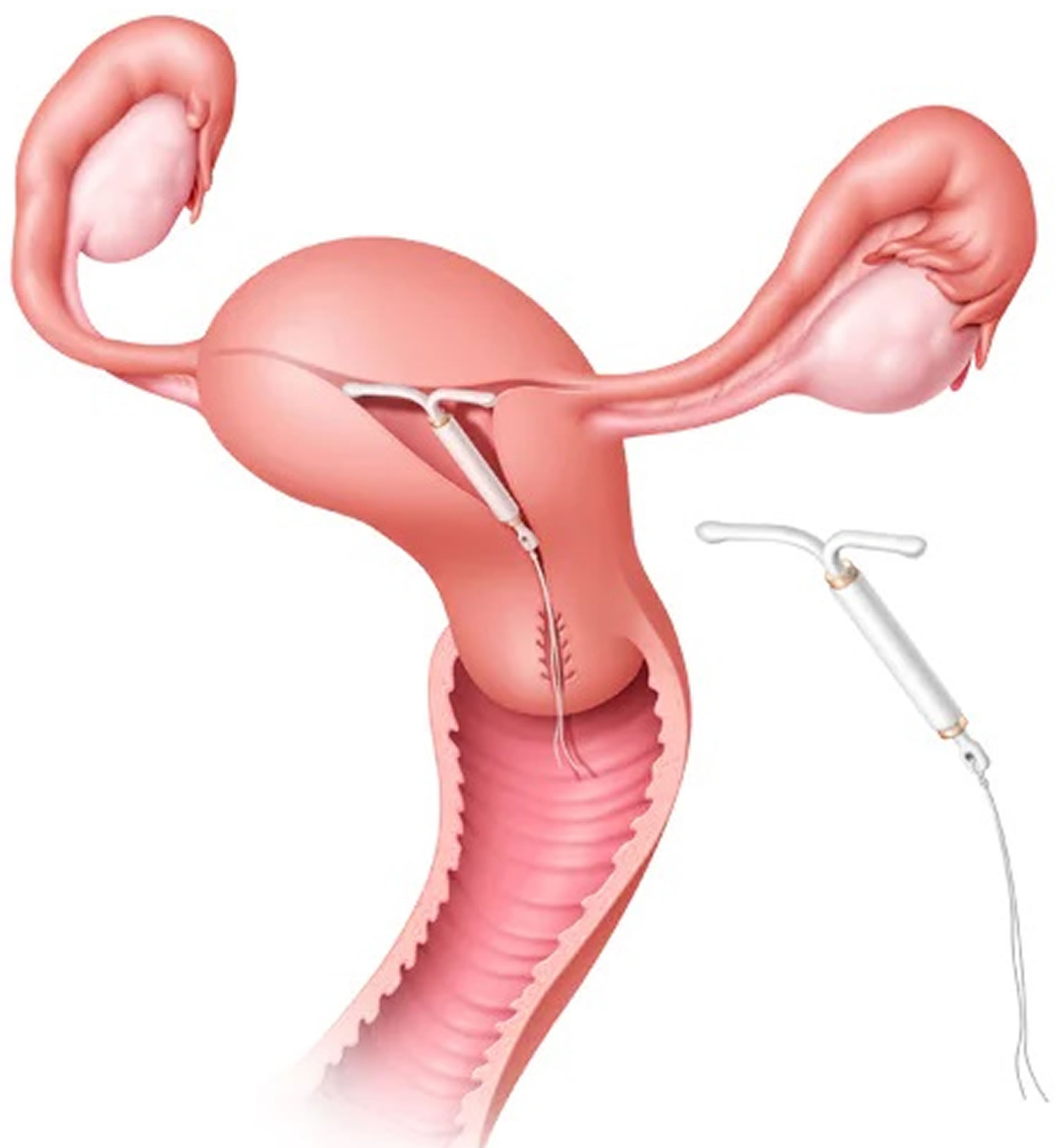
When performing IUD insertion and removal, the primary anatomical landmarks that need to be identified are the cervix and uterus (see Figure 1 to 3). The uterus will be identified by the bimanual exam to assess for size, shape, position, and to identify any anatomical abnormalities 9. The cervix will be identified during the speculum examination 9.
An IUD is often inserted by your health care provider during your monthly period. Either type can be inserted quickly and easily in the provider’s office or clinic. Before placing the IUD, the provider washes the cervix with an antiseptic solution. After this, your doctor:
- Slides a plastic tube containing the IUD through the vagina and into the uterus.
- Pushes the IUD into the uterus with the help of a plunger.
- Removes the tube, leaving two small strings that dangle outside the cervix within the vagina.
The IUD strings have two purposes:
- They let your healthcare provider and you to check that the IUD stays properly in position.
- They are used to pull the IUD out of the uterus when it is time to remove it. This should only be done by a doctor.
The IUD insertion procedure can cause discomfort and pain, but not all women have the same side effects. During insertion, you may feel:
- Little pain and some discomfort
- Cramping and pain
- Dizzy or lightheaded
Some women have cramps and backaches for 1 to 2 days after insertion. Other may have cramps and backaches for weeks or months. Over-the-counter pain relievers can ease the discomfort.
IUD important information:
- IUD is more than 99% effective in preventing pregnancy
- IUD can last between five to ten years, depending on type chosen
- Your chances of getting pregnant return to normal as soon as the IUD is removed
- IUD is cost-effective when compared to other birth control methods due to how long contraception lasts
- IUD is a ‘set-and-forget’ method, so great for regular travelers and women would prefer not to adhere to a daily contraceptive method
- IUD’s nylon strings can be adjusted to suit your body and should not be felt by you or your partner during sex
- IUD does not protect against sexually transmitted infections (STIs) or HIV/AIDS and condoms should be used if you are at risk.
- See your doctor if you experience severe pain during the first few hours after your IUD is inserted. This may be a sign of a serious infection.
Figure 1. Uterus anatomy
Figure 2. Uterus location
Figure 3. Uterus location in the female pelvis
How effective is the IUD?
The IUD works very well if inserted properly and is one of the most effective reversible methods of contraception available. The hormonal IUD is 99.8% effective. Copper IUD are 99.2% effective. If 100 women use an IUD for a year, fewer than 1 will become pregnant.
Copper IUDs are effective for 5 or 10 years depending on the type. Hormonal IUDs are effective for 3 or 5 years depending on the type.
An IUD won’t protect you against sexually transmitted infections (STIs) so you will need to use condoms for added protection.
How does an IUD birth control work?
Copper IUD affects sperm movement to the egg and changes the lining of the uterus to make it difficult for a fertilized egg to take hold.
Hormonal IUD thickens the mucus at the neck of the uterus, blocking the sperm. Hormonal IUD may also affect ovulation by changing the hormones that cause an egg to be released each month.
What are the advantages and disadvantages of the IUD?
Both copper IUD and hormonal IUD offer several benefits, which include being:
- extremely good at preventing pregnancy
- easy to use and maintain
- long-lasting and cost-effective
- immediately reversible if you decide you want to get pregnant
The hormonal IUD also makes periods lighter and less painful. Sometimes they stop altogether.
The main disadvantages of IUDs include:
- A small risk of problems after insertion, such as pelvic infection
- They can move out of place
- Mirena IUD can cause side effects such as irregular bleeding and sore breasts
- Copper IUDs can initially make periods heavier and more painful
How can I compare the different types of birth control?
Table 1. Types of birth control
| Method | Number of pregnancies per 100 women within their first year of typical use 10 | Side effects and risks* *These are not all of the possible side effects and risks. Talk to your doctor or nurse for more information. | How often you have to take or use |
|---|---|---|---|
| Abstinence (no sexual contact) | Unknown (0 for perfect use) | No medical side effects | No action required, but it does take willpower. You may want to have a back-up birth control method, such as condoms. |
| Permanent sterilization surgery for women (tubal ligation, “getting your tubes tied”) | Less than 1 | Possible pain during recovery (up to 2 weeks) Bleeding or other complications from surgery Less common risk includes ectopic (tubal) pregnancy | No action required after surgery |
| Permanent sterilization implant for women (Essure®) The Essure® birth control device will no longer be sold or distributed in the United States after December 31, 2018. | Less than 1 | Pain during the insertion of Essure; some pain during recovery Cramping, vaginal bleeding, back pain during recovery Implant may move out of place Less common but serious risk includes ectopic (tubal) pregnancy | No action required after surgery |
| Permanent sterilization surgery for men (vasectomy) | Less than 1 | Pain during recovery Complications from surgery | No action required after surgery |
| Implantable rod (Implanon®, Nexplanon®) | Less than 1 | Headache Irregular periods Weight gain Sore breasts Less common risk includes difficulty in removing the implant | No action required for up to 3 years before removing or replacing |
| Copper intrauterine device (IUD) (ParaGard®) | Less than 1 | Cramps for a few days after insertion Missed periods, bleeding between periods, heavier periods Less common but serious risks include pelvic inflammatory disease (PID) and the IUD being expelled from the uterus or going through the wall of the uterus. | No action required for up to 10 years before removing or replacing |
| Hormonal intrauterine devices (IUDs) (Liletta, Mirena®, and Skyla®) | Less than 1 | Irregular periods, lighter or missed periods Ovarian cysts Less common but serious risks include pelvic inflammatory disease (PID) and the IUD being expelled from the uterus or going through the wall of the uterus. | No action required for 3 to 5 years, depending on the brand, before removing or replacing |
| Shot/injection (Depo-Provera®) | 6 | Bleeding between periods, missed periods Weight gain Changes in mood Sore breasts Headaches Bone loss with long-term use (bone loss may be reversible once you stop using this type of birth control) | Get a new shot every 3 months |
| Oral contraceptives, combination hormones (“the pill”) | 9 | Headache Upset stomach Sore breasts Changes in your period Changes in mood Weight gain High blood pressure Less common but serious risks include blood clots, stroke and heart attack; the risk is higher in smokers and women older than 35 | Take at the same time every day |
| Oral contraceptives, progestin-only pill (“mini-pill”) | 9 | Spotting or bleeding between periods Weight gain Sore breasts Headache Nausea | Take at the same time every day |
| Skin patch (Xulane®) | 9 May be less effective in women weighing 198 pounds or more 11 | Skin irritation Upset stomach Changes in your period Changes in mood Sore breasts Headache Weight gain High blood pressure Less common but serious risks include blood clots, stroke and heart attack; the risk is higher in smokers and women older than 35 | Wear for 21 days, remove for 7 days, replace with a new patch |
| Vaginal ring (NuvaRing®) | 9 | Headache Upset stomach Sore breasts Vaginal irritation and discharge Changes in your period High blood pressure Less common but serious risks include blood clots, stroke and heart attack; the risk is higher in smokers and women older than 35 | Wear for 21 days, remove for 7 days, replace with a new ring |
| Diaphragm with spermicide (Koromex®, Ortho-Diaphragm®) | 12 If you gain or lose than 15 pounds, or have a baby, have your doctor check you to make sure the diaphragm still fits. | Irritation Allergic reactions Urinary tract infection (UTI) Vaginal infections Rarely, toxic shock if left in for more than 24 hours Using a spermicide often might increase your risk of getting HIV | Insert each time you have sex |
| Sponge with spermicide (Today Sponge®) | 12 (among women who have never given birth before) or 24 (among women who have given birth) 12 | Irritation Allergic reactions Rarely, toxic shock if left in for more than 24 hours Using a spermicide often might increase your risk of getting HIV | Insert each time you have sex |
| Cervical cap with spermicide (FemCap®) | 23 12 | Vaginal irritation or odor Urinary tract infections (UTIs) Allergic reactions Rarely, toxic shock if left in for more than 48 hours Using a spermicide often might increase your risk of getting HIV | Insert each time you have sex |
| Male condom | 18 | Irritation Condom may tear, break or slip off Allergic reactions to latex condoms | Use each time you have sex |
| Female condom | 21 | Irritation Condom may tear or slip out Allergic reaction | Use each time you have sex |
| Withdrawal — when a man takes his penis out of a woman’s vagina (or “pulls out”) before he ejaculates (has an orgasm or “comes”) | 22 | Sperm can be released before the man pulls out, putting you at risk for pregnancy | Use each time you have sex |
| Natural family planning (rhythm method) | 24 | Can be hard to know the days you are most fertile (when you need to avoid having sex or use back-up birth control) | Depending on method used, takes planning each month |
| Spermicide alone | 28 Works best if used along with a barrier method, such as a diaphragm | Irritation Allergic reactions Urinary tract infection Frequent use of a spermicide might increase your risk of getting HIV | Use each time you have sex |
Who should not use IUD?
IUDs may not be suitable for people:
- who have a current sexually transmitted infection (STI) or other pelvic infection
- with abnormal vaginal bleeding (this may need to be checked by a doctor first)
- with certain known abnormalities of the uterus such as large fibroids
- think you might be pregnant
- with cervical or uterine cancer
- who are or may be allergic to copper (ParaGard copper IUD only)
A copper IUD may not be your first choice of contraception if you have:
- heavier periods
- anemia (low blood iron)
The hormonal IUD is not recommended for people with past or current breast cancer.
Which is the best IUD for me?
The best IUD for you depends on your health, your desire to have children now or in the future, your lifestyle, and your need to prevent sexually transmitted infections (STIs), as well as any side effects that you may experience. Your doctor can help you decide which type is best for you right now.
The birth control method that is right for you and your partner depends on many things, and may change over time.
Before choosing a birth control method, talk to your doctor or nurse about:
- Whether you want to get pregnant soon, in a few years, or never
- How well each method works to prevent pregnancy
- Possible side effects
- How often you have sex
- The number of sex partners you have
- Your overall health
- How comfortable you are with using the method (For example, can you remember to take a pill every day? Will you have to ask your partner to put on a condom each time?)
How can I get an IUD?
If you decide to use an IUD you will usually need to have an appointment with your doctor to discuss what type of IUD suits you. An IUD may not be suitable for a small number of people and this may mean you are recommended to use a different form of contraception. If an IUD is suitable, you can then make an appointment to get an IUD inserted. You may need to purchase the IUD and bring it with you to your appointment.
When can an IUD be used after childbirth?
An IUD can be inserted from 4-6 weeks after childbirth.
What are the benefits of having an IUD?
IUD contraceptive devices are a very effective, reliable method of contraception (greater than 99% effective) and work for a long time. Copper IUDs are effective for 5 or 10 years depending on the type. Hormonal IUDs are effective for 5 years.
- IUD contraception are a ‘set and forget’ method. You do not have to remember to take it every day/week/month or every time you have sex.
- While the initial cost is more expensive, over time IUDs are the least expensive and longest lasting method of birth control.
- IUD contraceptive devices can be taken out at any time and they are easily removed.
- IUD contraceptive devices are fully reversible, fertility is considered returned as soon as the IUD is removed. In other word your ability to become pregnant quickly returns after the IUD is removed.
- Copper IUDs (Load and Copper T) can be used as highly effective emergency contraception.
- An IUD is a good option for users who cannot use estrogen or who are breastfeeding.
- The ParaGard copper IUD does not affect your hormone levels.
- Hormonal IUDs may reduce cramps and make your period lighter. Periods may stop altogether, which some users consider a benefit.
- The Mirena hormonal IUD can be used to treat heavy menstrual bleeding.
- The Mirena IUD can be used as part of menopause hormone treatment.
What are the side effects of an IUD?
The side effects and complications with all IUDs include:
- a small risk of developing pelvic infection in the first 3 weeks following insertion (this is easily treated with antibiotics). Risk of infection that decreases after the first 20 days after IUD insertion.
- a small risk of perforation of the uterus during insertion, but this is uncommon (less than 1% of users).
- a small risk of the IUD moving from its position or coming out (expulsion). If your IUD comes out even a little bit, it must be removed. If the IUD slips out, pregnancy can happen.
- a very small risk of ectopic pregnancy (pregnancy developing in the tubes)
- cramps and backache for the first month after the IUD is inserted. With time, these decrease in intensity and frequency.
All IUDs do not protect against sexually transmitted infections (STIs). External condoms or internal condoms can be used to prevent STI.
People using copper IUDs may have:
- spotting, cramping, heavier and/or longer periods, which may settle after the first 3–6 months
People using hormonal IUDs may have:
- persistent or irregular bleeding and/or spotting for the first 3–5 months
- no periods at all or occasional light periods by 12 months
- hormonal side effects are rare and can include hair change, acne, headache and breast tenderness, which usually settle after the first 3 months
- ovarian cysts may occur when using the hormonal IUD; however, most cysts will not cause pain and will settle without any treatment
When can an IUD be inserted?
- Hormonal IUDs should be inserted during the first 7 days of your menstrual cycle, which starts with the first day of bleeding.
- Copper IUDs should be inserted during the first 12 days of your menstrual cycle, which starts with the first day of bleeding.
If inserted at any other time in the cycle then pregnancy needs to be completely excluded.
If you are using other methods of contraception and are changing to an IUD you need to discuss with the doctor when the IUD should be inserted.
To decrease cramping a medicine used for period pain, such as ibuprofen (Advil, Motrin IB, others) should be taken one hour before insertion.
Make sure you have eaten breakfast or lunch on the day the IUD is being inserted and have drunk plenty of fluids.
When does the IUD start working?
If the IUD is inserted as recommended above, the contraceptive effect will start immediately. It may be inserted at another time in your cycle if pregnancy can be ruled out; in that case it will be effective after 7 days.
What do I do after my IUD has been put in?
To reduce your risk of infection, avoid sex, tampons, swimming and baths for 48 hours after insertion. If cramps occur, take medicine used for period pain to reduce pain and the risk of the IUD coming out. Heat packs can also be beneficial to reduce cramping.
You should check your IUD strings about 1 week after insertion. People using copper IUDs are recommended to check their strings after every period. To check that your IUD is still in place insert one or two fingers into your vagina to feel the string.
Contact your doctor if you:
- cannot feel the string
- feel the string has lengthened
- can feel the hard stem of the IUD
Use another form of contraception (e.g. a condom) until you have had a check-up.
You should also contact your doctor if you have any of the following symptoms:
- a fever or you’re feeling unwell, weak or tired
- persistent or excessive cramps or back pain
- unusual pelvic pain or tenderness
- unusual vaginal discharge or odor
- pain during sex
If you have a copper IUD and your period is more than a week overdue you should do a pregnancy test or see your doctor.
How do I check my IUD?
You should check your IUD every month to make sure it has not slipped out. An IUD is most likely to slip out of place during your period. IUDs have strings attached to them. Between periods you can check for the strings by following these steps:
- Wash your hands.
- Sit or squat down.
- Put your index or middle finger up into the vagina until you touch the cervix.
- Feel for the string ends that should be coming through. If you find them, then the IUD is in place and working.
- If the strings feel longer or shorter than before, or you feel the hard, plastic part of the IUD against your cervix, the IUD may have moved.
- If the IUD has slipped out or moved, do not try to put it back in place on your own. Be sure to use a back-up method of birth control until you see your health care provider.
When can the IUD be removed?
An IUD can be removed at any time but there is a risk of pregnancy if you have had unprotected sexual intercourse within the last week. It’s a good idea to use condoms or avoid sex for 7 days before removal, unless you are planning a pregnancy.
How is IUD removed?
Although IUDs provide protection for many years, but they can be removed earlier. It is a quick procedure, in which a doctor gently pulls on the string, and the IUD’s arms fold up so it slips out. Some women find it uncomfortable while others don’t feel very much.
What are the different types of birth control?
Women can choose from many different types of birth control methods. These include, in order of most effective to least effective at preventing pregnancy:
- Female and male sterilization (female tubal ligation or occlusion, male vasectomy) — Birth control that prevents pregnancy for the rest of your life through surgery or a medical procedure.
- Long-acting reversible contraceptives (LARC) methods (IUDs and hormonal implants) — Birth control your doctor inserts one time and you do not have to remember to use birth control every day or month. LARCs last for 3 to 10 years, depending on the method.
- Short-acting hormonal methods (pill, mini pills, patch, shot, vaginal ring) — Birth control your doctor prescribes that you remember to take every day or month. The shot requires you to get a shot from your doctor every 3 months.
- Barrier methods (condoms, diaphragms, sponge, cervical cap) — Birth control you use each time you have sex.
- Natural rhythm methods — Not using a type of birth control but instead avoiding sex and/or using birth control only on the days when you are most fertile (most likely to get pregnant). An ovulation home test kit or a fertility monitor can help you find your most fertile days.
Which types of birth control help prevent sexually transmitted infections (STIs)?
Only two types can protect you from sexually transmitted infections (STIs), including HIV: male condoms and female condoms 14.
While condoms are the best way to prevent sexually transmitted infections (STIs) if you have sex, they are not the most effective type of birth control. If you have sex, the best way to prevent both sexually transmitted infections (STIs) and pregnancy is to use what is called “dual protection.” Dual protection means you use a condom to prevent sexually transmitted infections (STIs) each time you have sex, and at the same time, you use a more effective form of birth control, such as an IUD, implant, or shot.
Which types of birth control can I get without a prescription?
You can buy these types of birth control over the counter at a drugstore or supermarket:
- Male condoms
- Female condoms
- Sponges
- Spermicides
- Emergency contraception pills. Plan B One-Step® and its generic versions are available in drugstores and some supermarkets to anyone, without a prescription. However you should not use emergency contraception as your regular birth control because it does not work as well as regular birth control. Emergency contraception is meant to be used only when your regular birth control does not work for some unexpected reason.
IUD types
There are 2 types of IUD — the hormonal IUD (levonorgestrel-containing IUD) and the copper-containing IUD (copper T 380A) – that are presently used in the United States. Both the hormonal IUD (levonorgestrel-containing IUD) and the copper-containing IUD have similar rates of preventing pregnancy with failure rates of 0.08% and 0.02%, respectively 15.
All IUDs currently available in the United States are T-shaped with the top of the T resting across the top of the endometrial cavity. IUDs are between 28 mm to 32 mm wide and 30 mm to 36 mm long 15. Uterine width traditionally has been assumed to be adequate in all patients; however, recent ultrasound studies have indicated that cavity width in nulliparous women (a female of reproductive age who has never had a live delivery; being nulliparous does not mean that a female has never been pregnant) may be narrower than device width 16. Therefore, it is important to consider the available IUD options available. The smallest IUDs measure 28 mm wide, and 30 mm long and are best suited for nulliparous and young women.
Hormonal IUD
Hormonal IUD is a small T-shaped plastic device that is fitted inside your uterus (womb) and slowly releases a progestogen hormone called levonorgestrel, which is a synthetic version of the hormone progesterone. There are 4 types of hormonal IUDs: Mirena IUD and Kyleena IUD, both of which can be used for up to 5 years; Liletta and Skyla both last 3 years. Kyleena IUD contains a lower dose of this hormone than Mirena IUD. The levonorgestrel hormone thickens the mucus at the entrance to your uterus so sperm cannot get through, and thins the lining of the uterus, making it hard for a fertilized egg to take hold and cause a pregnancy. Your periods usually become lighter or may stop when using a hormonal IUD. This is similar to the way in which the mini pill and the contraceptive implant work.
For the levonorgestrel-containing IUD, there are three different strengths of levonorgestrel available, 13.5 mg, 19.5 mg, and 52 mg 15. They are all equally effective at providing reliable contraception 17. However, the higher dose IUD, 52 mg device (Mirena/Liletta/Levosert), is also approved for the treatment of menorrhagia (abnormally heavy or prolonged menstrual bleeding) and endometrium protection during hormone replacement therapy 18. The 13.5 mg IUD (Jaydess/Skyla) is approved for use for up to 3 years, while the 19.5 mg (Kyleena) and 52 mg (Mirena/Liletta/Levosert) IUDs are approved for up to 5 years 17.
There are four different brands of hormonal IUDs in the United States 19:
- Mirena IUD (52 mg levonorgestrel) can prevent pregnancy for up to 6 years
- Kyleena IUD (19.5 mg levonorgestrel) can work for up to 5 years
- Liletta IUD (52 mg levonorgestrel) works for as long as 4 years
- Skyla IUD (13.5 mg levonorgestrel) prevents pregnancy for up to 3 years
Things to remember with your hormonal IUD:
- Check the threads each month to make sure the IUD is in the right place
- See your doctor straight away if you have any unusual symptoms such as:
- Discharge from your vagina
- Pain low in your tummy
- Deep pain during sex
- Protect yourself from sexually transmitted infections (STIs) by using a condom at the same time
- Keep a record of the date to take out your IUD – it shouldn’t stay in for longer than the recommended time
Figure 4. Hormonal IUD
How does hormonal IUD work?
Hormonal IUD is placed inside the uterus (womb) by a doctor or nurse. The hormonal IUD:
- stops sperm from fertilizing the egg
- makes the mucus in the cervix thicker so that sperm can’t get into the uterus
- changes the wall of the uterus, making it hard for an egg to attach to the wall
- sometimes stops your ovaries from releasing an egg.
How effective is hormonal IUD?
Hormonal IUDs are very effective. The Mirena hormonal IUD is 99.9% effective; the Kyleena IUD is 99.7% effective.
What are the advantages and disadvantages of hormonal IUD?
Advantages of hormonal IUD:
- It’s very effective
- It protects you against pregnancy for up to 5 years
- The initial cost of a hormonal IUD can be more than for other methods, but it lasts for 5 years
- Your fertility goes back to normal once the IUD is taken out
- Hormonal IUDs usually makes bleeding much lighter and sometimes periods stop all together (this is more likely with Mirena than Kyleena IUD)
- It can reduce period pain
Disadvantages of hormonal IUD:
- It doesn’t protect against sexually transmitted infections (STIs)
- It requires a procedure to be put in and removed from the uterus; putting it in can be uncomfortable
- When the IUD is put in there is a small chance of:
- difficulty inserting the IUD
- damage to the wall of the uterus
- infection
- it can sometimes come out by itself
- You can have some irregular bleeding or spotting in the first few months
- You can get hormonal side effects like:
- bloating
- headaches
- mood changes
- acne
- lowered interest in sex
Who can use a hormonal IUD?
- Most women who want a reliable, long-term contraceptive
- Women who haven’t had a pregnancy
- Women who have decided to not have any more children
- Women who would like to space out their pregnancies
- Women who are breastfeeding
Hormonal IUDs can be used by women who have heavy periods but Mirena IUD is proven to be very effective at reducing bleeding during your period.
You should NOT use the hormonal IUD if:
- you might be pregnant
- you have a recent infection called pelvic inflammatory disease (PID)
- you have unusual bleeding from your vagina
- you have a history of breast cancer or some serious liver conditions
Talk to your doctor before deciding to use a hormonal IUD if you have:
- a recent sexually transmitted infection (STI)
- fibroids or other conditions that change the shape of your uterus
- previous problems with an IUD (for instance the IUD has come out by itself)
- you are unable to have a follow-up check-up after the IUD is put in
Hormonal IUD risks and side effects
Hormonal IUD may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- headache
- acne
- breast tenderness
- nausea
- weight gain
- cramps or pain during menstruation
- decreased sexual desire
- depression
- changes in mood
- hair loss
- unwanted hair growth
Some side effects can be serious. If you experience any of these symptoms, call your doctor immediately or get emergency medical treatment:
- foul-smelling or unusual vaginal discharge
- vaginal pain
- pain during sex
- sores on the genital area
- yellowing of the skin or eyes
- sudden weakness of an arm or leg
- drooping of one side of the face
- difficulty speaking or understanding
- crushing chest or shoulder pain
- swelling of the lips, tongue, throat, arms, hands, legs, or feet
- rash
- hives
Hormonal IUD may increase the risk that you will develop a cyst on your ovary. This type of cyst may cause pain but will usually disappear in 2-3 months. In rare cases, surgery to remove the cyst may be needed. Talk to your doctor about the risks of using a hormonal IUD.
Hormonal IUD may cause other side effects. Call your doctor if you have any unusual problems while your IUD is in place.
Hormonal IUD insertion
Your health care provider will evaluate your overall health and do a pelvic exam before inserting hormonal IUD. You may be screened for sexually transmitted infections (STIs).
Hormonal IUD can be inserted:
- Anytime during your menstrual cycle if you’re not pregnant. You might need to take a pregnancy test to confirm you’re not pregnant.
- Immediately after a pregnancy termination.
- Immediately after delivering a baby vaginally or by cesarean section — although insertion immediately after vaginal delivery increases the risk of expelling Mirena.
For a pregnancy test to be accurate, one of the following criteria must be met 20:
- Less than seven days after the start of regular menses
- No sexual intercourse since the start of last menses
- Consistently using another form of contraception reliably
- Less than 7 days after spontaneous or induced abortion
- 4 weeks postpartum
- Fully or nearly fully breastfeeding and amenorrheic, and less than six months postpartum
If these criteria are not met, it is an acceptable practice to bridge the patient with a non-implantable form of contraception, including oral contraceptives, vaginal rings, transdermal patches, condoms, or medroxyprogesterone acetate injections 21. If the patient still desires long-acting reversible contraception insertion after starting one of these bridge methods, a repeat pregnancy test may be conducted in 3-4 weeks, and if negative, the patient may undergo long-acting reversible contraception placement 21. If placing a copper IUD, the prior form of birth control used does not need to be continued, as it will be effective immediately. However, if the levonorgestrel IUD is not placed within 7 days of the start of menses, an additional form of birth control should be used for 7 days 20.
If you have hormonal IUD inserted more than seven days after the start of your period, be sure to use backup contraception for one week.
Taking a nonsteroidal anti-inflammatory medication, such as ibuprofen (Advil, Motrin IB, others), one to two hours before the procedure can help reduce cramping.
Hormonal IUD is typically inserted in a health care provider’s office. Hormonal IUD insertion is a sensitive procedure for which a chaperone may be provided. Your doctor will:
- Put a speculum into your vagina
- Cleanse the area with antibacterial solution to minimize the chance of infection
- Put an instrument on your cervix to stabilize it; this will cause cramping
- Put an instrument into your uterus to measure its depth
- Insert the IUD
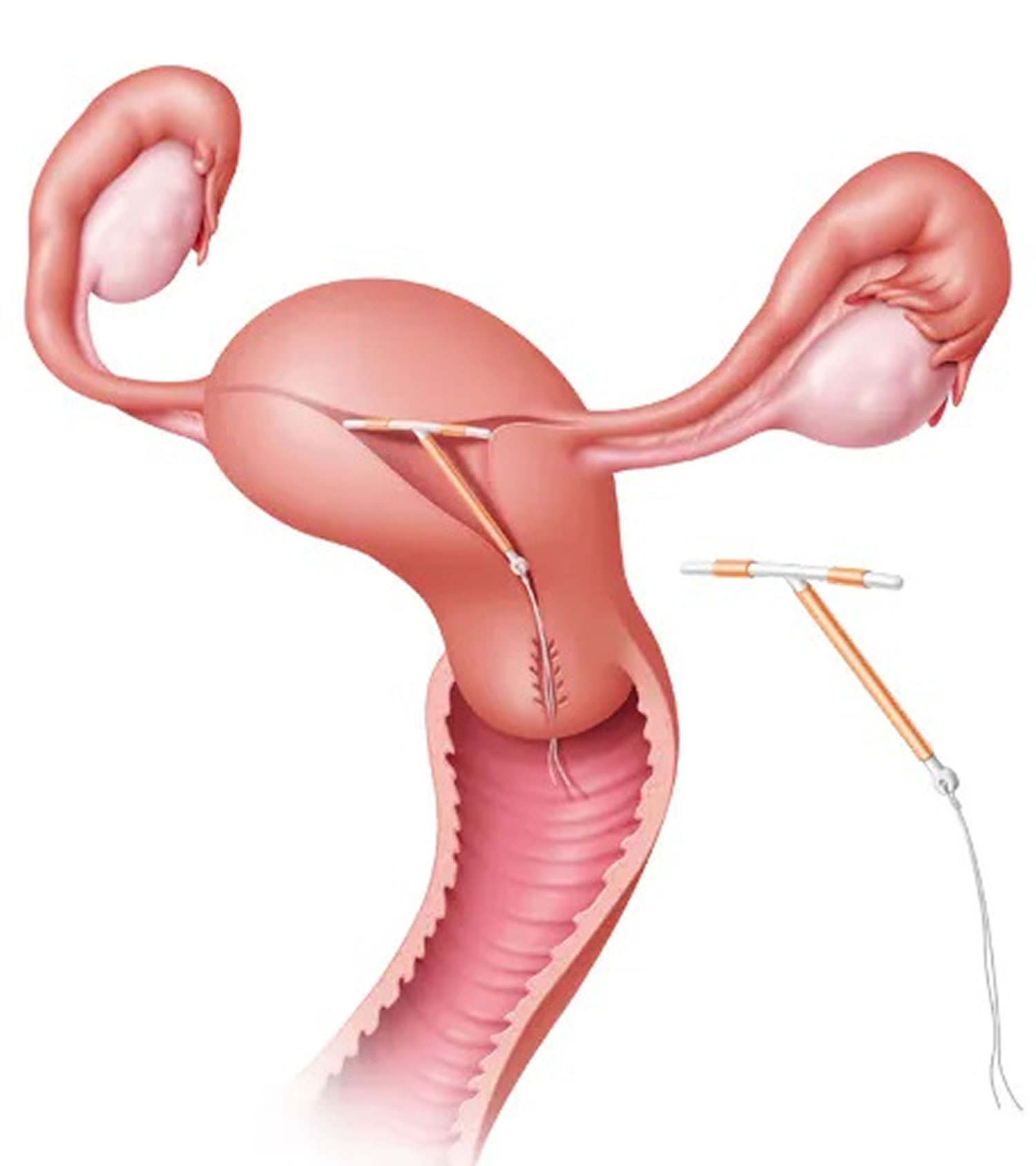
Figure 5. Mirena IUD insertion
Footnote: Hormonal IUD is inserted into the uterus by a health care provider using a special applicator. Short strings connected to the IUD extend beyond the cervix into the vagina and allow for device removal.
After the hormonal IUD insertion procedure
You may want to have someone drive you home after the procedure. Some women have mild cramping, low backache, and spotting for a couple of days.
If you have a progestin-releasing IUD, it takes about 7 days for it to start to work. You do not need to wait to have sex. But you should use a backup form of birth control, such as a condom, for the first week. You should avoid sex, tampons, swimming and baths for 2 days to reduce the risk of infection.
Your doctor will want to see you 2 to 4 weeks after the procedure to be sure the IUD is still in place, re-examine you to make sure the IUD hasn’t moved and to check for signs and symptoms of infection. Ask your doctor to show you how to check that the IUD is still in place, and how often you should check it.
In rare cases, an IUD can slip partly or all of the way out of your uterus. This is generally seen after pregnancy. If this happens, contact your doctor right away. DO NOT try to remove an IUD that has come part of the way out or has slipped out of place.
Once a month, check to feel that the IUD’s strings are protruding from your cervix. Be careful not to pull on the strings.
While you’re using a IUD, contact your health care provider immediately if you:
- Think you may be pregnant
- Have unusually heavy, persistent vaginal bleeding
- Have abdominal pain or pain during sex
- Have an unexplained fever
- Have chills
- Have cramps
- Have flu-like symptoms
- Have unusual or foul-smelling vaginal discharge, lesions or sores
- Develop very severe headaches or migraines
- Have yellowing of the skin or eyes (jaundice)
- Were exposed to an sexually transmitted infection (STI)
- Can no longer feel the IUD strings, or they suddenly seem longer
It’s also important to contact your health care provider immediately if you think the IUD is no longer in place. Your doctor will check the location of the IUD and, if it’s displaced, remove it if necessary.
Hormonal IUD Removal
The hormonal IUD can lasts for 3 to 10 years (depending on the type of hormonal IUD you are using), but it can be taken out sooner. To remove a hormonal IUD, your health care provider will likely use forceps to grasp the device’s strings and gently pull. The hormonal IUD’s arms will fold upward as it’s withdrawn from the uterus.
Light bleeding and cramping is common during removal. Rarely, removal can be more complicated.
If you want another hormonal IUD, the old one can be taken out and a new one put in at the same visit.
Mirena IUD
Mirena IUD offers effective, long-term contraception. It can be used in premenopausal women of all ages, including teenagers.
Mirena IUD benefits:
- Eliminates the need to interrupt sex for contraception
- Doesn’t require partner participation
- Can remain in place for up to five years
- Can be removed at any time, followed by a quick return to your normal fertility
- Can be used while breast-feeding — although your health care provider will likely recommend waiting six to eight weeks after childbirth because earlier placement increases the risk of injuring the uterus during placement
- Doesn’t carry the risk of side effects related to birth control methods containing estrogen
Mirena IUD can decrease menstrual bleeding after three or more months of use. About 20 percent of women stop having periods after one year of using Mirena.
Mirena IUD can also decrease:
- Severe menstrual pain and pain related to the abnormal growth of uterine-lining tissue outside the uterus (endometriosis)
- The risk of pelvic infection
- The risk of endometrial cancer
Because of these noncontraceptive benefits, Mirena IUD is often prescribed for women with:
- Heavy menstrual bleeding
- Cramping or pain with periods
- Endometriosis
- Abnormal growth of the lining of the uterus (endometrial hyperplasia)
- Abnormal growth of uterine-lining tissue into the muscular wall of the uterus (adenomyosis)
- Anemia
- Fibroids
Mirena IUD isn’t appropriate for everyone. Your health care provider may discourage use of Mirena if you have:
- Breast cancer, or have had it
- Uterine or cervical cancer
- Liver disease
- Uterine abnormalities, such as fibroids, that interfere with the placement or retention of Mirena
- A pelvic infection or current pelvic inflammatory disease
- Unexplained vaginal bleeding
Tell your health care provider if you:
- Take any medications, including nonprescription and herbal products
- Have diabetes or high blood pressure
- Have a heart condition or have had a heart attack
- Have migraines
- Have blood-clotting problems or have had a stroke
- Recently gave birth or are breast-feeding
Mirena IUD risks and side effects
Less than 1 percent of women who use Mirena IUD will get pregnant in a year of typical use. If you do conceive while using Mirena IUD, you’re at higher risk of an ectopic pregnancy — when the fertilized egg implants outside the uterus, usually in a fallopian tube. However, because Mirena IUD prevents most pregnancies, women who use it are at lower risk of having an ectopic pregnancy than are other sexually active women who are not using contraception.
Mirena IUD is generally safe. But it’s important to remember that:
- Mirena IUD doesn’t protect against sexually transmitted infections (STIs).
- Rarely, insertion of Mirena IUD causes perforation of the uterus. The risk of perforation might be higher when inserted during the postpartum period.
Side effects associated with Mirena IUD include:
- Headache
- Acne
- Breast tenderness
- Irregular bleeding, which can improve after six months of use
- Mood changes
- Cramping or pelvic pain
It’s also possible to expel Mirena IUD from your uterus. You may be more likely to expel Mirena IUD if you:
- Have never been pregnant
- Have heavy or prolonged periods
- Have severe menstrual pain
- Previously expelled an IUD
- Are younger than age 20
- Had Mirena inserted immediately after childbirth
Your health care provider may recommend removal of Mirena IUD if you develop:
- A pelvic infection
- Inflammation of the endometrium (endometritis)
- Endometrial or cervical cancer
- Pelvic pain or pain during sex
- Very severe migraine
- A significant increase in blood pressure, or have a stroke or heart attack
- Possible exposure to an sexually transmitted infection (STI)
Copper IUD
The copper IUD sometimes referred to as a nonhormonal IUD, is made of copper and plastic (fine copper wire wrapped around a plastic frame), and does not release a hormone. Copper IUD is placed inside your uterus to prevent pregnancy. A fine nylon thread is attached to the IUD – the thread comes out through the cervix into the top end of the vagina. Copper is toxic to sperm, so the copper IUD can also be used as an emergency contraceptive within 5 days of unprotected sex.
There are 2 copper IUDs: Multiload IUD lasts for up to 5 years and the Copper T IUD (or copper T 380A IUD) lasts for up to 10 years. ParaGard (copper T 380A) is the only copper IUD available in the United States. It can prevent pregnancy for up to 10 years after insertion. The copper IUD is 99.5% effective.
The copper IUD works by:
- copper IUD is toxic to the egg and sperm which stops sperm from fertilizing the egg
- copper IUD slows the transport of the egg to delay chance of sperm and egg meeting
- producing an inflammatory reaction in the lining of the uterus to make it unable to support a fertilized egg.
The copper IUD works by preventing sperm motility and viability within the uterine cavity by causing a localized cytotoxic inflammatory response 22. Because of this mechanism, copper IUDs are also an extremely effective form of emergency contraception, if placed within 5 days of unprotected intercourse 22.
ParaGard copper IUD isn’t appropriate for everyone. Your health care provider may discourage use of ParaGard copper IUD if you:
- Have uterine abnormalities — such as large fibroids — that interfere with the placement or retention of ParaGard
- Have a pelvic infection, such as pelvic inflammatory disease (PID)
- Have uterine or cervical cancer
- Have unexplained vaginal bleeding
- Are allergic to any component of ParaGard
- Have a disorder that causes too much copper to accumulate in your liver, brain and other vital organs (Wilson’s disease)
ParaGard copper IUD offers effective, long-term contraception. It can be used in premenopausal women of all ages, including teenagers.
ParaGard copper IUD benefits:
- Eliminates the need to interrupt sex for contraception
- Can remain in place for up to 10 years
- Can be removed at any time
- Can be used while breast-feeding
- Doesn’t carry the risk of side effects, such as blood clots, related to hormonal birth control methods
- Can be used for emergency contraception if inserted within five days after unprotected sex
Things to remember with your copper IUD:
- check the threads each month after your period to make sure the IUD is still in the right place
- if you have any unusual symptoms (discharge from your vagina, pain low in your tummy or deep pain during sex) see your doctor straight away
- if you are worried you could be pregnant, have a pregnancy test – this includes if your period is more than a week overdue or you have a change in your bleeding pattern
- if you or your partner have a new sexual partner, use a condom every time until you both have been checked for STIs
- keep a record of the date to take out your IUD – it shouldn’t stay in for longer than the recommended time
Figure 6. Copper IUD
What are the advantages and disadvantages of copper IUD?
Advantages copper IUD:
- It’s very effective
- It can stay in place for 5 or 10 years depending on which one you choose
- The initial cost of a coper IUD is more than for other methods, but it lasts for 5 or 10 years so it works out to be a reasonable price
- It does not contain hormones so it has no hormonal side-effects
- Your fertility goes back to normal straight away when you take it out
- It can be used as emergency contraception if it is put in up to 5 days after unprotected sex.
Disadvantages copper IUD:
- It doesn’t protect against sexually transmissible infections (STIs)
- It requires a procedure to be put in and removed from the uterus; putting it in can be uncomfortable
- When the IUD is put in there is a small chance of:
- difficulty inserting the IUD
- damage to the wall of the uterus (perforation)
- infection
- It can sometimes come out by itself
- Your periods can be heavier and last longer
- You can have some irregular spot bleeding in the first few months
Who can use the copper IUD?
- most women who want a reliable, long-term contraceptive
- women who haven’t had a pregnancy, have completed their families, or are spacing their pregnancies
- women who are breastfeeding
- women who cannot, or do not want to, use hormonal contraception because of medical conditions or personal preference
You should NOT use the copper IUD if:
- you think you might be pregnant
- you have had a recent infection called pelvic inflammatory disease (PID)
- you have unusual bleeding from your vagina
- you have Wilson’s disease or you are allergic to copper
It is important to talk to your doctor before deciding to use the copper IUD if:
- you have painful or heavy long-lasting periods
- you have anemia (not enough iron in your blood)
- you have had a recent sexually transmissible infection (STI)
- you have fibroids or other conditions that change the shape of your uterus
- you have had problems in the past with an IUD (for instance it has come out by itself)
- you are unable to have a follow-up check-up after the IUD is put in
Copper IUD risks and side effects
Less than 1 percent of women who use ParaGard copper IUD will get pregnant in the first year of typical use. Over time, risk of pregnancy in women who use ParaGard copper IUD remains low.
If you do conceive while using ParaGard copper IUD, you’re at high risk of an ectopic pregnancy — when the fertilized egg implants outside the uterus, usually in a fallopian tube. But because ParaGard copper IUD prevents most pregnancies, the overall risk of having an ectopic pregnancy is lower than it is for sexually active women who don’t use contraception.
ParaGard copper IUD doesn’t offer protection from sexually transmitted infections (STIs).
Side effects associated with ParaGard copper IUD include:
- Bleeding between periods
- Cramps
- Severe menstrual pain and heavy bleeding
- Rarely, it’s possible for the copper IUD to perforate your uterine wall or cervix. If this complication occurs, your health care provider will discuss the appropriate management.
It’s also possible to expel ParaGard copper IUD from your uterus. You might not feel the copper IUD expulsion if it occurs.
You may be more likely to expel ParaGard copper IUD if you:
- Have never been pregnant
- Have heavy or prolonged periods
- Have severe menstrual pain
- Previously expelled an IUD
- Are younger than age 25
- Had the IUD inserted immediately after childbirth
Copper IUD insertion
ParaGard copper IUD is typically inserted in a health care provider’s office. ParaGard copper IUD can be inserted anytime during a normal menstrual cycle. If you just had a baby, your doctor might recommend waiting about eight weeks after delivery before inserting ParaGard copper IUD.
Before inserting ParaGard copper IUD, your health care provider will evaluate your overall health and do a pelvic exam. You may have a pregnancy test to confirm you’re not pregnant, and you may be screened for sexually transmitted infections (STIs).
Taking a nonsteroidal anti-inflammatory drug (NSAID), such as ibuprofen (Advil, Motrin IB, others), one to two hours before the procedure can help reduce cramping.
During the procedure
Your health care provider will insert a speculum into your vagina and clean your vagina and cervix with an antiseptic solution. A special instrument might be used to gently align your cervical canal and uterine cavity. Next, your health care provider will fold down ParaGard’s copper IUD horizontal arms and place the device inside an applicator tube. The tube is inserted into your cervical canal and ParaGard copper IUD is carefully placed in your uterus. When the applicator tube is removed, ParaGard copper IUD will remain in place. Your health care provider will trim ParaGard’s copper IUD strings so that they don’t protrude too far into the vagina and may record the length of the strings.
During ParaGard copper IUD insertion, you may experience dizziness, fainting, nausea, low blood pressure or a slower than normal heart rate. Your health care provider will likely suggest that you stay lying down for a few minutes to allow these side effects to pass.
Figure 7. Copper IUD insertion
Footnote: The ParaGard copper IUD can remain in place in your uterus for up to 10 years. Short strings connected to the device extend beyond the cervix into the vagina and allow for device removal.
After the copper IUD insertion procedure
About a month after ParaGard copper IUD is inserted, your health care provider may re-examine you to make sure the IUD device hasn’t moved and to check for signs and symptoms of infection.
While you’re using ParaGard copper IUD, contact your health care provider immediately if you have:
- Signs or symptoms of pregnancy
- Unusually heavy vaginal bleeding
- Foul vaginal discharge
- Worsening pelvic pain
- Severe abdominal pain or tenderness
- Unexplained fever
- Possible exposure to an sexually transmitted infection (STI)
It’s also important to contact your health care provider immediately if you think your ParaGard copper IUD is no longer in place. Call your doctor if:
- You have breakthrough bleeding or bleeding after sex
- Sex is painful for you or your partner
- The strings are missing or suddenly seem longer
- You feel part of the device at your cervix or in your vagina
Your health care provider will check the location of ParaGard copper IUD and remove it if necessary.
Copper IUD Removal
The copper IUD can stay in place for 5 or 10 years, depending on the type you choose. ParaGard copper IUD is usually removed in a health care provider’s office. Your provider will likely use forceps to grasp the device’s strings and gently pull. The copper IUD device’s arms will fold upward as it’s withdrawn from the uterus. Light bleeding and cramping are common during removal. Rarely, removal can be more complicated. It only takes a couple of minutes. If you want another copper IUD, the old one can be taken out and the new one put in at the same time.
If you are aged 40 years or more when it is put in your doctor may advise you that it can be kept in place until menopause. If you want to get pregnant or you no longer want the IUD it can be taken out earlier.
IUD insertion
The copper T 380A IUD (Paragard IUD) may be placed at any time during the menstrual cycle, as long as the patient is not pregnant 23. The prescribing information for the 14- and 20-mcg levonorgestrel-IUDs recommends insertion during the first seven days of the menstrual cycle 24. Placement of the copper T 380A IUD or the 20-mcg levonorgestrel-IUD is also considered safe and effective immediately after vaginal or cesarean delivery (within 10 minutes after placental separation), although the risk of expulsion is considerably higher than if insertion is delayed 25. One study comparing placement of the levonorgestrel-IUD immediately after placental delivery vs. six months postpartum found expulsion rates of 24% and 4%, respectively 26. Two other studies comparing expulsion rates with immediate and delayed insertion of the copper T 380A IUD following delivery (vaginal or cesarean) found expulsion rates of 12% and 17%, respectively 27, 25. Immediate insertion after a pregnancy loss in the second trimester has a higher risk of expulsion compared with loss in the first trimester 3. The prescribing information for both types of the levonorgestrel-IUD advises a waiting period of six weeks postpartum or following second-trimester pregnancy loss 28.
Although the expulsion rate is lower, one disadvantage of delayed insertion, for example at the postpartum visit, is that some women may not return for the follow-up 28. Therefore, women who might benefit most from IUD insertion immediately after delivery would be those who are less likely to return for insertion.
Women who develop peripartum infections, such as chorioamnionitis, endometritis, or puerperal sepsis, should not undergo IUD placement for a least three months postpartum 3. IUDs may be placed immediately after completion of a spontaneous or induced abortion, except in cases of septic abortion, in which a waiting period of at least three months is advised 29.
Your health-care provider will place your intrauterine contraception device into your uterus, but will leave two threads dangling through your cervix. You should check for these threads once a month so that you will know whether your IUD is still in place. To check for the threads, you should wash your hands with soap and water. Then, reach up to the top of your vagina with clean fingers to feel the threads. If you cannot feel the threads or if you feel any part of the IUD other than the threads, your intrauterine contraceptive device may not be in place and may not prevent pregnancy. If this happens, see your doctor and use a non-hormonal birth control method such as condoms and spermicide to prevent pregnancy until you are seen by your doctor.
You will need a follow-up appointment with your health-care provider 4-6 weeks after your IUD is inserted to be sure that your system is properly in place. After this appointment, you will need to be examined once every year or more often if you have any problems or concerns.
If your hormonal IUD (levonorgestrel IUD) must be removed, talk to your doctor about the best time to remove it. You are not protected from pregnancy once your IUD is removed, so if you do not want to become pregnant, you will need to be sure that you have effective birth control as soon as your IUD is removed. If you plan to have your IUD replaced with a new IUD, you can have the old IUD removed and the new IUD inserted at any time during your menstrual cycle. If you have chosen to use a different form of birth control instead of your IUD and you have regular menstrual cycles, you should have the IUD removed during the first 7 days after your menstrual period begins and begin using your new form of birth control right away. If you have chosen to use a different form of birth control and you do not have regular cycles, you do not menstruate at all, or you are not able to have your IUD removed during the first 7 days of your menstrual period, you should start using your new form of birth control 7 days before your IUD is removed.
IUD insertion procedure
Your health care provider will insert a speculum into your vagina and clean your vagina and cervix with an antiseptic solution. Special instruments might be used to gently align your cervical canal and uterine cavity and to measure the depth of your uterine cavity.
Next, your health care provider will fold the IUD’s horizontal arms and place the device inside an applicator tube. The tube is inserted into your cervical canal, and the IUD is carefully placed in your uterus. When the applicator tube is removed, the IUD will remain in place.
Your health care provider will trim the IUD’s strings so that they don’t protrude too far into the vagina, and may record the length of the strings.
During the IUD insertion, you may experience cramping, dizziness, fainting or a slower than normal heart rate.
After IUD insertion procedure
Once a month, check to feel that the IUD’s strings are protruding from your cervix. Be careful not to pull on the strings.
About a month after the IUD is inserted, your health care provider may re-examine you to make sure the IUD hasn’t moved and to check for signs and symptoms of infection.
While you’re using a IUD, contact your health care provider immediately if you:
- Think you may be pregnant
- Have unusually heavy, persistent vaginal bleeding
- Have abdominal pain or pain during sex
- Have an unexplained fever
- Have unusual or foul-smelling vaginal discharge, lesions or sores
- Develop very severe headaches or migraines
- Have yellowing of the skin or eyes
- Were exposed to an sexually transmitted infection (STI)
- Can no longer feel the IUD strings, or they suddenly seem longer
It’s also important to contact your health care provider immediately if you think the IUD is no longer in place. Your doctor will check the location of the IUD and, if it’s displaced, remove it if necessary.
IUD removal
The copper IUD is approved for contraceptive use for up to 10 years. There are 4 types of hormonal IUDs (levonorgestrel-containing IUDs): Mirena IUD and Kyleena IUD, both of which can be used for up to 5 years; Liletta and Skyla both last 3 years. Additionally, there are also indications for removal of the IUD. The primary indication for removal is the patient’s preference for any reason including, but not limited to, desire for pregnancy, irregular bleeding pattern, heavy vaginal bleeding, and discomfort or pain, which may represent the displacement of the device 30. Bleeding changes, especially heavier bleeding, were more likely to occur in the copper-containing IUDs rather than the levonorgestrel-IUD prompting patient’s desire for removal 31. Another indication for removal is an intrauterine pregnancy. However, the IUD should only be removed if the strings are visible or easily found within the cervical os with no devices entering the uterine cavity 32. Leaving the IUD in place increases the risk of spontaneous abortion by 40% to 50%. However, there is no risk of teratogenesis with leaving the IUD in place. Removing the IUD decreases the risk of spontaneous abortion to 20% 32. For the levonorgestrel-containing IUD, additional indications for removal include the diagnosis of a cervical or uterine malignancy or jaundice 33. The last indication for removal is if the IUD has been in for longer than the approved efficacy period. For the 19.5 mg and 52 mg hormonal IUDs, the approved duration is 5 years. For the 13.5 mg hormonal IUDs, the approved duration is 3 years. For some, the approved duration is 10 years. These approved durations are constantly changing, and it is best practice to refer to the individual product’s package insert for the most up-to-date prescribing information.
IUD contraindications
Given that there are two classes of IUDs available, there are specific contraindications for each type of IUD. However, there are also universal contraindications that are specific to both types.
Universal contraindications for the use of IUD 34:
- Pregnancy, or suspected pregnancy
- Sexually transmitted infection (STI) at the time of insertion, including cervicitis, vaginitis, or any other lower genital tract infection
- A congenital uterine abnormality that distorts the shape of the uterine cavity making insertion difficult
- Acute pelvic inflammatory disease (PID)
- History of pelvic inflammatory disease (PID), unless a subsequent successful intrauterine pregnancy has occurred
- History of septic abortion or history of postpartum endometritis within the last 3 months
- Confirmed or suspicion of uterine or cervical malignancy/neoplasia
- Abnormal uterine bleeding of unknown origin
- Any condition that increases the risk of pelvic infection
- History of previously inserted IUD that has not been removed
- Hypersensitivity to any component of the device
For the hormonal IUD, additional contraindications include 34:
- Confirmed or suspicion of breast malignancy or other progestin-sensitive cancer
- Liver tumors, benign or malignant
- Acute liver disease
For the copper IUD, additional contraindications include 34:
- Wilson disease
- Sensitivity to copper
IUD in Special Populations
Nulliparous women
Many physicians are reluctant to recommend an IUD to nulliparous women (female of reproductive age who has never had a live delivery but has been pregnant before) because of perceived concerns about safety 35 and because earlier studies suggested that younger, nulliparous women have a higher risk of IUD device expulsion 36. Although good-quality data on expulsion rates in nulliparous women are lacking, one study of the 20-mcg hormonal IUD found that the expulsion rate in nulliparous adolescents was lower than the overall expulsion rate for all the adolescents in the study (4% vs. 8%, respectively) 37. Another study comparing IUD use in nulliparous and parous women (women who have given birth) found similar rates of complications, patient discontinuation, and expulsion (expulsion rates were 0% to 0.2% per year for hormonal IUDs and 0% to 1.2% per year for copper IUDs) 38. A third study found that although most nulliparous women reported pain during insertion of an hormonal IUD, they had continuation rates similar to those of women using oral contraceptives, confirming that the device is well tolerated in nulliparous women 39.
The U.S. Medical Eligibility Criteria for Contraceptive Use guidelines, developed by the World Health Organization (WHO) and adapted by the Centers for Disease Control and Prevention (CDC), assign the 20-mcg hormonal IUD and the copper T 380A IUD a category 2 rating for use in nulliparous women, which indicates that the advantages generally outweigh the theoretical or proven risks 40. The 14-mcg hormonal IUD was not available when the U.S. Medical Eligibility Criteria for Contraceptive Use guidelines were written. Only the copper T 380A and the 14-mcg hormonal IUD are approved by the U.S. Food and Drug Administration (FDA) for nulliparous women 3.
Regardless of the IUD selected or the parity of the patient, clinicians should attempt safe, fundal placement to lower the risk of displacement and expulsion. Patients should be taught how to check for proper positioning of the IUD strings 41.
Adolescents
The use of IUDs in adolescents (i.e., females from menarche to younger than 21 years) has been questioned because of the increased prevalence of sexually transmitted infections (STIs) in this group, and the resulting increased risk of pelvic inflammatory disease (PID) and subsequent infertility. Adolescents who choose long-acting reversible contraceptives (LARC) methods are advised to use male or female condoms consistently (dual method use) to decrease the risk of sexually transmitted infections (STIs), including human immunodeficiency virus (HIV). A 2018 American College of Obstetricians and Gynecologists committee opinion states that complications of intrauterine devices (IUDs) and contraceptive implants are rare and differ little between adolescents and women, which makes these methods safe for adolescents 42. A recent study on the effectiveness of long-acting contraception found the IUD to be more effective than the contraceptive pill, patch, or ring for prevention of pregnancy, including in adolescents 43. The U.S. Medical Eligibility Criteria for Contraceptive Use guidelines state that the advantages of using the IUD in adolescents generally outweigh the risks 40.
Women at risk of sexually transmitted infection (STI)
A history of sexually transmitted infection (STI) does not preclude IUD insertion 44. In a study of patients in an urban university clinic, IUDs were used safely in women with a history of STI, and the incidence of STI in these women decreased following IUD insertion 44. However, clinicians should screen patients following Centers for Disease Control and Prevention (CDC) guidelines for all women 45. For women with a known STI, it is recommended that IUD insertion be delayed for at least three months after resolution of the infection. Women should also be rescreened for STI three to six months after treatment 3.
If a woman does not have a known STI or active signs or symptoms of a genital tract STI, it is safe to screen for STI on the same day as IUD placement, although the rate of pelvic inflammatory disease will be slightly increased if it turns out that an STI was present at the time of IUD insertion (0% to 5% with STI present vs. 0% to 2% without) 46. The prescribing information for the hormonal IUDs states that it is usually appropriate to remove an IUD if an STI is diagnosed; however, the USMEC guidelines state that in a patient who tests positive for an STI but has no symptoms, antibiotics may be prescribed and, if clinically appropriate, the IUD may be left in place 40, 3.
Pregnancy in women with an IUD in place
The risk of ectopic pregnancy is higher in women who become pregnant with an IUD in place, compared with women who do not have an IUD 3. However, because IUDs are highly effective at preventing pregnancy, ectopic pregnancies occur less often in IUD users than in women using other methods of contraception or no contraception 47.
Once pregnancy is confirmed in a woman with an IUD, ectopic pregnancy should be excluded and the IUD removed 3. If the strings are not visible, ultrasonography can be used to confirm whether the IUD is present in the uterus. Invasive procedures, such as hysteroscopy, are not recommended for IUD removal during pregnancy 3. Patients who become pregnant with an IUD in place should be informed that there is a substantial risk of pregnancy loss even when the IUD is removed. One study found that among 89 women who became pregnant with an IUD in place and desired pregnancy continuation, 40% had spontaneous abortion following IUD removal 48. IUD removal is recommended because pregnancy loss and complications are less likely if the IUD is removed than if it is left in place. Later pregnancy complications, including placental abruption, placenta previa, preterm delivery, low birth weight, chorioamnionitis, and need for cesarean delivery, are also more likely in women who become pregnant with an IUD in place 3.
Women who are breastfeeding
Use of IUDs is considered acceptable in women who are breastfeeding, although there are limited data on whether rates of breastfeeding success differ among women using the copper T 380A vs. an hormonal IUD. One study found no difference in infant growth and development or in overall breastfeeding success between the two types of IUD 49. The American Academy of Family Physicians supports the use of IUDs in women who are breastfeeding 28.
Medical conditions
Some women may not be appropriate candidates for IUD placement because of chronic medical conditions 3. Table 2 shows the U.S. Medical Eligibility Criteria for Contraceptive Use risk categories for IUD use in women with common medical conditions 40.
Table 2. Guide for initiating and continuing IUD use in women with Common Medical Conditions
| Medical condition | Levonorgestrel-releasing IUD (Mirena) | Copper T 380A IUD (Paragard) | ||
|---|---|---|---|---|
| Initiation | Continuation | Initiation | Continuation | |
| Cardiovascular conditions | ||||
| Deep venous thrombosis (history of) | 2 | 2 | 1 | 1 |
| Deep venous thrombosis (on anticoagulants) | 2 | 2 | 2 | 2 |
| Hypertension, poorly controlled (≥ 160 mm Hg systolic or ≥ 100 mm Hg diastolic) | 2 | 2 | 1 | 1 |
| Ischemic heart disease | 2 | 3 | 1 | 1 |
| Multiple risk factors for cardiovascular disease | 2 | 2 | 1 | 1 |
| Stroke | 2 | 2 | 1 | 1 |
| Thrombophilia | 2 | 2 | 1 | 1 |
| Gastrointestinal disorders | ||||
| Cirrhosis (decompensated) | 3 | 3 | 1 | 1 |
| Gallbladder disease | 2 | 2 | 1 | 1 |
| Infections | ||||
| AIDS | 3 | 2 | 3 | 2 |
| Human immunodeficiency virus infection | 2 | 2 | 2 | 2 |
| Tuberculosis (pelvic) | 4 | 3 | 4 | 3 |
| Metabolic disorders | ||||
| Diabetes mellitus | 2 | 2 | 1 | 1 |
| Hyperlipidemia | 2 | 2 | 1 | 1 |
| Neoplastic disorders | ||||
| Breast cancer (current) | 4 | 4 | 1 | 1 |
| Breast cancer (history of; no disease for 5 years) | 3 | 3 | 1 | 1 |
| Cervical cancer (awaiting treatment) | 4 | 2 | 4 | 2 |
| Endometrial cancer | 4 | 2 | 4 | 2 |
| Hepatocellular adenoma | 3 | 3 | 1 | 1 |
| Hepatocellular carcinoma | 3 | 3 | 1 | 1 |
| Neurologic disorders | ||||
| Migraine with aura | 2 | 3 | 1 | 1 |
| Migraine without aura | 2 | 2 | 1 | 1 |
| Rheumatologic disorders | ||||
| Rheumatoid arthritis (on immunosuppression) | 2 | 1 | 2 | 1 |
| SLE (on immunosuppression) | 2 | 2 | 2 | 1 |
| SLE (uncomplicated) | 2 | 2 | 1 | 1 |
| SLE (with positive or unknown antiphospholipid antibodies) | 3 | 3 | 1 | 1 |
| SLE (with severe thrombocytopenia) | 2 | 2 | 3 | 2 |
| Other | ||||
| Anemia (any cause) | 1 | 1 | 2 | 2 |
| Solid organ transplant (uncomplicated) | 2 | 2 | 2 | 2 |
| Solid organ transplant with graft failure or rejection | 3 | 2 | 3 | 2 |
Footnotes: The low-dose levonorgestrel-releasing IUD (Skyla) was not available at the time the U.S. Medical Eligibility Criteria for Contraceptive Use guidelines were published.
U.S. Medical Eligibility Criteria for Contraceptive Use categories: 1 = No restrictions on IUD use; 2 = There are theoretical or proven risks of IUD use, but the advantages generally outweigh those risks; 3 = Theoretical or proven risks generally outweigh advantages of an IUD; 4 = IUD use is an unacceptable health risk. The U.S. Medical Eligibility Criteria for Contraceptive Use guidelines provide more information about the risks of IUD use with these and other medical conditions and with certain medications.
Abbreviations: IUD = intrauterine device; SLE = systemic lupus erythematosus; USMEC = U.S. Medical Eligibility Criteria.
Antibiotic prophylaxis before IUD insertion
There is a slightly increased risk of pelvic inflammatory disease (PID) within the first 20 days after IUD insertion, likely related to insertion technique, although the risk is low thereafter 50. Antibiotic prophylaxis before or at the time of IUD insertion does not have a major effect on reducing the risk of pelvic infection, and does not alter the need for IUD removal in the months after insertion,30 even in women at risk of sexually transmitted infections (STIs) 51.
Use of misoprostol before insertion
A 2007 study suggested that the use of misoprostol (Cytotec) before IUD insertion allowed for easier insertion 52. However, more recent studies show no benefit and increased side effects with misoprostol 53. The American College of Obstetricians and Gynecologists makes no recommendation regarding the use of misoprostol before IUD insertion.
IUD risks and side effects
All types of IUDs are well tolerated, with continuation rates higher than those for all other forms of reversible contraception except the contraceptive implant (78% for the copper T 380A, 80% for the 20-mcg hormonal IUD, and 82% for the 14-mcg hormonal IUD) 54. However, there are possible side effects that patients should be aware of.
While uncommon, IUDs carry some risks, such as:
- There is a small chance of getting pregnant while using an IUD. If you do get pregnant, your provider can remove the IUD to lower the risk for miscarriage or other problems.
- A very small risk of ectopic pregnancy (pregnancy developing in the tubes), but only if you do get pregnant while using an IUD. An ectopic pregnancy is one that occurs outside the womb. It can be serious, even life-threatening.
- A small risk of perforation of the uterus during insertion, but this is uncommon (less than 1% of users).
- A small risk of developing pelvic infection in the first 3 weeks following insertion (this is easily treated with antibiotics). Risk of infection that decreases after the first 20 days after IUD insertion.
- A small risk of the IUD moving from its position or coming out (expulsion). If your IUD comes out even a little bit, it must be removed. If the IUD slips out, pregnancy can happen.
- Cramps and backache for the first month after the IUD is inserted. With time, these decrease in intensity and frequency.
All IUDs do not protect against sexually transmitted infections (STIs). External condoms or internal condoms can be used to prevent STI.
People using copper IUDs may have:
- spotting, cramping, heavier and/or longer periods, which may settle after the first 3–6 months
People using hormonal IUDs may have:
- persistent or irregular bleeding and/or spotting for the first 3–5 months
- no periods at all or occasional light periods by 12 months
- hormonal side effects are rare and can include hair change, acne, headache and breast tenderness, which usually settle after the first 3 months
- ovarian cysts may occur when using the hormonal IUD; however, most cysts will not cause pain and will settle without any treatment.
Side effects of the hormonal IUD are similar to those of other progestin-based contraceptives and include headaches, nausea, hair loss, breast tenderness, depression, decreased libido, and ovarian cysts 55. Women who use the 14-mcg or 20-mcg hormonal IUD also experience vulvovaginitis at rates of 20.2% and less than 5%, respectively, as well as abdominal/pelvic pain at rates of 18.9% and 12.8%, respectively 6. Women may have amenorrhea (absence of menstruation) or irregular spotting throughout use of the hormonal IUD, although the amount of bleeding decreases for most women the longer the IUD is in place because of thinning of the endometrium 3. Up to 70% of women using the 20-mcg hormonal IUD report oligomenorrhea (infrequent menstrual periods defined as fewer than six to eight periods per year) or amenorrhea after two years of use 56, 57. Because the 20-mcg hormonal IUD reduces endometrial thickness, it has been successfully used for the treatment of menorrhagia (heavy periods or menstrual bleeding that lasts more than 7 days) 58.
The copper T 380A IUD can cause irregular, heavy bleeding. Unlike the hormonal IUD, bleeding, including painful intermenstrual bleeding, may continue throughout use 59. Nonetheless, discontinuation rates are similar between the IUD types 60. Because the copper device does not contain hormonal agents, it does not cause the progestin-related side effects possible with hormonal IUDs 3.
References- Kavanaugh, M. L., & Jerman, J. (2018). Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception, 97(1), 14–21. https://doi.org/10.1016/j.contraception.2017.10.003
- Peipert, J. F., Zhao, Q., Allsworth, J. E., Petrosky, E., Madden, T., Eisenberg, D., & Secura, G. (2011). Continuation and satisfaction of reversible contraception. Obstetrics and gynecology, 117(5), 1105–1113. https://doi.org/10.1097/AOG.0b013e31821188ad
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404.
- Sivin I, Stern J, Diaz S, et al. Rates and outcomes of planned pregnancy after use of Norplant capsules, Norplant II rods, or levonorgestrel-releasing or copper TCu 380Ag intrauterine contraceptive devices. Am J Obstet Gynecol. 1992;166(4):1208–1213.
- Skyla (levonorgestrel-releasing intrauterine system) [package insert]. Wayne, N.J.: Bayer HealthCare Pharmaceuticals, Inc.; 2013. http://labeling.bayerhealthcare.com/html/products/pi/Skyla_PI.pdf
- Trussell J. Update on and correction to the cost effectiveness of contraceptives in the United States. Contraception. 2012;85(2):218.
- Chen, B. A., Reeves, M. F., Hayes, J. L., Hohmann, H. L., Perriera, L. K., & Creinin, M. D. (2010). Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstetrics and gynecology, 116(5), 1079–1087. https://doi.org/10.1097/AOG.0b013e3181f73fac
- Insertion and Removal of Intrauterine Devices. Am Fam Physician. 2005 Jan 1;71(1):95-102. https://www.aafp.org/afp/2005/0101/p95.html
- Centers for Disease Control and Prevention. (2016). U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morbidity & Mortality Weekly Report; 65(RR-4): 1–66.
- Curtis, K.M., Tepper, N.K., Jatlaoui, T.C., et al. (2016). U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep; 65(RR-3):1–104.
- BARRIER METHODS https://www.fda.gov/consumers/free-publications-women/birth-control#BarrierMethods
- Birth control methods. https://www.womenshealth.gov/a-z-topics/birth-control-methods
- Birth Control. https://www.fda.gov/consumers/free-publications-women/birth-control
- Lanzola EL, Ketvertis K. Intrauterine Device. [Updated 2021 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557403
- Wildemeersch D, Hasskamp T, Nolte K, Jandi S, Pett A, Linden S, van Santen M, Julen O. A multicenter study assessing uterine cavity width in over 400 nulliparous women seeking IUD insertion using 2D and 3D sonography. Eur J Obstet Gynecol Reprod Biol. 2016 Nov;206:232-238. doi: 10.1016/j.ejogrb.2016.09.023
- Apter D, Gemzell-Danielsson K, Hauck B, Rosen K, Zurth C. Pharmacokinetics of two low-dose levonorgestrel-releasing intrauterine systems and effects on ovulation rate and cervical function: pooled analyses of phase II and III studies. Fertil Steril. 2014 Jun;101(6):1656-62.e1-4. doi: 10.1016/j.fertnstert.2014.03.004
- Grandi G, Farulla A, Sileo FG, Facchinetti F. Levonorgestrel-releasing intra-uterine systems as female contraceptives. Expert Opin Pharmacother. 2018 May;19(7):677-686. doi: 10.1080/14656566.2018.1462337
- Giovanni Grandi, Antonino Farulla, Filomena Giulia Sileo & Fabio Facchinetti (2018) Levonorgestrel-releasing intra-uterine systems as female contraceptives, Expert Opinion on Pharmacotherapy, 19:7, 677-686, DOI: 10.1080/14656566.2018.1462337
- Min, J., Buckel, C., Secura, G. M., Peipert, J. F., & Madden, T. (2015). Performance of a checklist to exclude pregnancy at the time of contraceptive initiation among women with a negative urine pregnancy test. Contraception, 91(1), 80–84. https://doi.org/10.1016/j.contraception.2014.08.002
- Secura, G. M., Allsworth, J. E., Madden, T., Mullersman, J. L., & Peipert, J. F. (2010). The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. American journal of obstetrics and gynecology, 203(2), 115.e1–115.e1157. https://doi.org/10.1016/j.ajog.2010.04.017
- Bahamondes L, Fernandes A, Monteiro I, Bahamondes MV. Long-acting reversible contraceptive (LARCs) methods. Best Pract Res Clin Obstet Gynaecol. 2020 Jul;66:28-40. doi: 10.1016/j.bpobgyn.2019.12.002
- Paragard T 380 A. Intrauterine copper contraceptive [package insert]. Sellersville, Pa.; 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/018680s066lbl.pdf
- U.S. Food and Drug Administration. Mirena (levonorgestrel-releasing intrauterine system). https://www.fda.gov/media/92554/download
- Çelen S, Şucak A, Yıldız Y, Danişman N. Immediate postplacental insertion of an intrauterine contraceptive device during cesarean section. Contraception. 2011;84(3):240–243.
- Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116(5):1079–1087.
- Celen S, Möröy P, Sucak A, Aktulay A, Danişman N. Clinical outcomes of early postplacental insertion of intrauterine contraceptive devices. Contraception. 2004;69(4):279–282.
- Intrauterine Devices: An Update. Am Fam Physician. 2014 Mar 15;89(6):445-450. https://www.aafp.org/afp/2014/0315/p445.html
- International Medical Advisory Panel. IMAP statement on intrauterine devices. IPPF Med Bull. 2003;37(2):1–4.
- Strasser J, Borkowski L, Couillard M, Allina A, Wood SF. Access to Removal of Long-acting Reversible Contraceptive Methods Is an Essential Component of High-Quality Contraceptive Care. Womens Health Issues. 2017 May-Jun;27(3):253-255. doi: 10.1016/j.whi.2017.04.003
- Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin No. 186: Long-Acting Reversible Contraception: Implants and Intrauterine Devices. Obstet Gynecol. 2017 Nov;130(5):e251-e269. doi: 10.1097/AOG.0000000000002400
- Kaneshiro, B., & Aeby, T. (2010). Long-term safety, efficacy, and patient acceptability of the intrauterine Copper T-380A contraceptive device. International journal of women’s health, 2, 211–220. https://doi.org/10.2147/ijwh.s6914
- Farmer M, Webb A. Intrauterine device insertion-related complications: can they be predicted? J Fam Plann Reprod Health Care. 2003 Oct;29(4):227-31. doi: 10.1783/147118903101197854
- Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016 Jul 29;65(3):1-103. doi: 10.15585/mmwr.rr6503a1
- Tyler CP, Whiteman MK, Zapata LB, Curtis KM, Hillis SD, Marchbanks PA. Health care provider attitudes and practices related to intrauterine devices for nulliparous women. Obstet Gynecol. 2012;119(4):762–771.
- Hubacher D. Copper intrauterine device use by nulliparous women: review of side effects. Contraception. 2007;75(6 suppl):S8–S11.
- Paterson H, Ashton J, Harrison-Woolrych M. A nationwide cohort study of the use of the levonorgestrel intrauterine device in New Zealand adolescents. Contraception. 2009;79(6):433–438.
- Veldhuis HM, Vos AG, Lagro-Janssen AL. Complications of the intrauterine device in nulliparous and parous women. Eur J Gen Pract. 2004;10(3):82–87.
- Suhonen S, Haukkamaa M, Jakobsson T, Rauramo I. Clinical performance of a levonorgestrel-releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception. 2004;69(5):407–412.
- Centers for Disease Control and Prevention. U.S. Medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86.
- Johnson BA. Insertion and removal of intrauterine devices. Am Fam Physician. 2005;71(1):95–102.
- Adolescents and Long-Acting Reversible Contraception: Implants and Intrauterine Devices. Number 735 (Replaces Committee Opinion Number 539, October 2012. Reaffirmed 2021). https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/05/adolescents-and-long-acting-reversible-contraception-implants-and-intrauterine-devices
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998–2007.
- Campbell SJ, Cropsey KL, Matthews CA. Intrauterine device use in a high-risk population: experience from an urban university clinic. Am J Obstet Gynecol. 2007;197(2):193.e1–193.e6.
- Sexually Transmitted Infections Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/default.htm
- Mohllajee AP, Curtis KM, Peterson HB. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception. 2006;73(2):145–153.
- Xiong X, Buekens P, Wollast E. IUD use and the risk of ectopic pregnancy: meta-analysis of case-control studies. Contraception. 1995;52(1):23–34.
- Inal MM, Ertopçu K, Ozelmas I. The evaluation of 318 intrauterine pregnancy cases with an intrauterine device. Eur J Contracept Reprod Health Care. 2005:10(4):266–271.
- Shaamash AH, Sayed GH, Hussien MM, Shaaban MM. A comparative study of the levonorgestrel-releasing intrauterine system Mirena versus the Copper T380A intrauterine device during lactation: breast-feeding performance, infant growth and infant development. Contraception. 2005;72(5):346–351.
- Grimes, D. A., Lopez, L. M., & Schulz, K. F. (1999). Antibiotic prophylaxis for intrauterine contraceptive device insertion. The Cochrane Database of Systematic Reviews, 1999(3), CD001327. https://doi.org/10.1002/14651858.CD001327
- Walsh T, Grimes D, Frezieres R, et al.; IUD Study Group. Randomised controlled trial of prophylactic antibiotics before insertion of intrauterine devices. Lancet. 1998;351(9108):1005–1008.
- Sääv I, Aronsson A, Marions L, Stephansson O, Gemzell-Danielsson K. Cervical priming with sublingual misoprostol prior to insertion of an intrauterine device in nulliparous women: a randomized controlled trial. Hum Reprod. 2007;22(10):2647–2652.
- Espey E, Singh RH, Leeman L, Ogburn T, Fowler K, Greene H. Misoprostol for intrauterine device insertion in nulliparous women: a randomized controlled trial [published ahead of print November 8, 2013]. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2013.11.018
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404
- Paterson H, Clifton J, Miller D, Ashton J, Harrison-Woolrych M. Hair loss with use of the levonorgestrel intrauterine device. Contraception. 2007;76(4):306–309.
- Sivin I, Stern J, Diaz J, et al. Two years of intrauterine contraception with levonorgestrel and with copper: a randomized comparison of the TCu 380Ag and levonorgestrel 20 mcg/day devices. Contraception. 1987;35(3):245–255.
- Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49(1):56–72.
- Kaunitz AM, Meredith S, Inki P, Kubba A, Sanchez-Ramos L. Levonorg-estrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113(5):1104–1116.
- Hubacher D, Chen PL, Park S. Side effects from the copper IUD: do they decrease over time? Contraception. 2009;79(5):356–362.
- Luukkainen T. Levonorgestrel-releasing intrauterine device. Ann N Y Acad Sci. 1991;626:43–49.