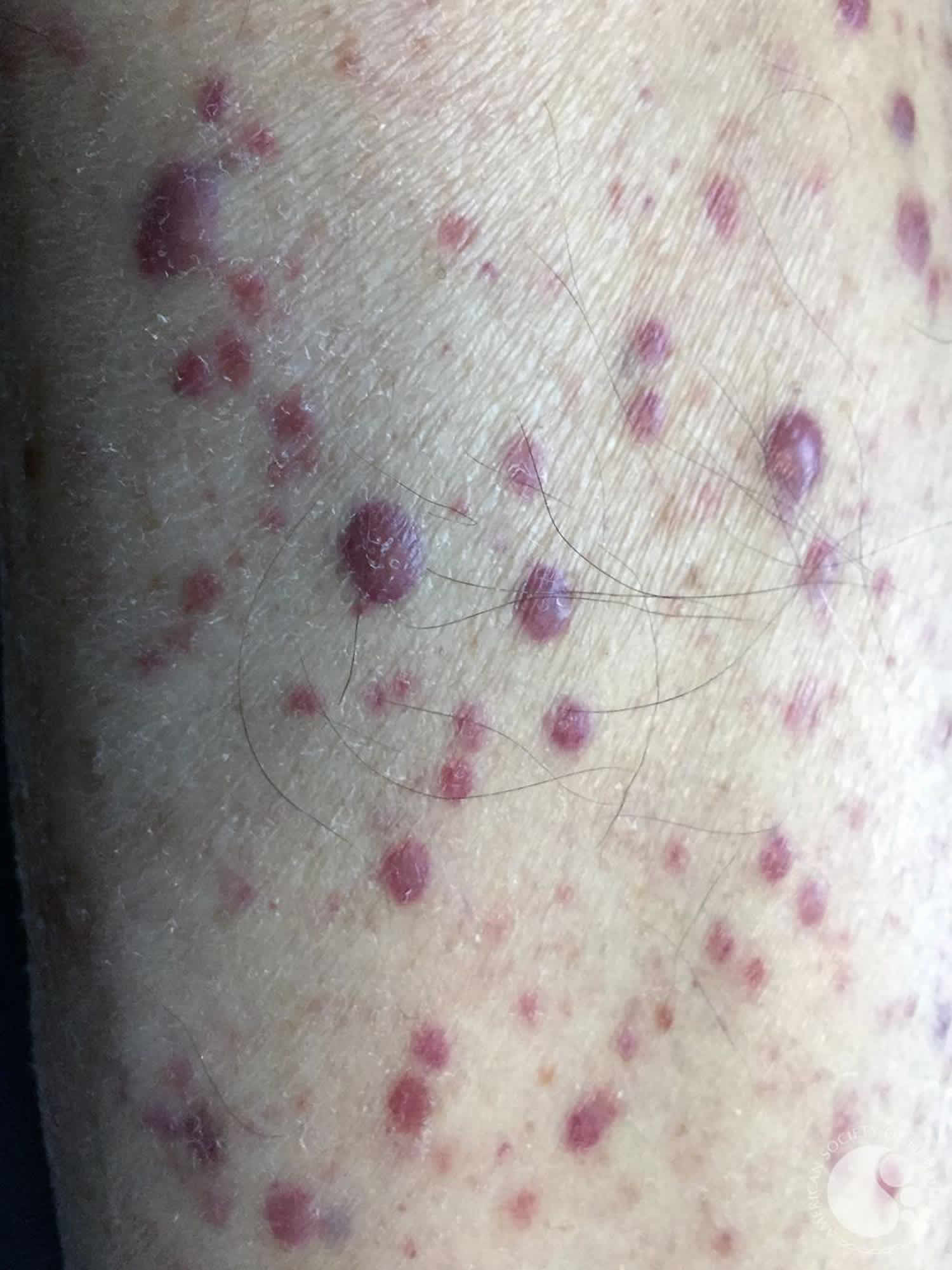
What is leukemia cutis
Leukemia cutis refers to the infiltration of the skin with leukemia cells 1. Leukemia cutis characteristically demonstrates the infiltration of the skin by neoplastic leukocytes 2. While the extramedullary collection of leukemic cells is generally regarded as myeloid sarcoma (previously chloroma/granulocytic sarcoma), leukemia cutis is a generic term to describe specific cutaneous involvement. Although any subtype of leukemia can involve the skin, the most common types seen, in clinical practice, are chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML) with monocytic or myelomonocytic morphology 3. Involvement by chronic myeloid leukemia (CML) is extremely rare and may be indicative of blast phase. Patients usually have a prior diagnosis of systemic leukemia or myelodysplasia. However, cutaneous lesions may present as the primary manifestation of systemic disease in rare cases. Cutaneous involvement is generally indicative of advanced disease and should stipulate investigation of other body sites for extramedullary involvement.
Leukemia is the name given to a group of blood disorders in which there is a malignant proliferation of white cells (leukocytes). The leukemia may be acute or chronic, due to proliferation of lymphocytes or more commonly, myeloid cells (the neutrophils). Clonal expansion or proliferation of identical abnormal cells mainly occurs in the bone marrow.
The most common types of leukemia are:
- Acute lymphoblastic leukemia (ALL)
- Chronic lymphoblastic leukemia (CLL)
- Acute myeloid leukemia (AML)
- Chronic myeloid leukemia (CML)
- Hairy cell leukemia
- Acute T-cell leukemia (ATLL).
Subtypes of leukemia involving skin:
- Myeloid/monocytic disorders:
- Acute myeloid leukemia (monocytic or myelomonocytic) (AML)
- Chronic myeloid leukemia (CML)
- Chronic myelomonocytic leukemia (transformation)
- Myelodysplastic syndrome (transformation)
- Lymphoproliferative disorders:
- B- cell leukemia/lymphomas
- Precursor B-cell acute lymphoblastic leukemia
- Chronic lymphocytic leukemia (CLL)
- Hairy cell leukemia
- T- cell leukemia/lymphomas
- Precursor T-cell acute lymphoblastic leukemia
- Adult T-cell leukemia/lymphoma (ATLL)
- T- cell prolymphocytic leukemia
- B- cell leukemia/lymphomas
Leukemia cutis is rare and the exact data on incidence and demographic predilections do not exist. However, children with congenital leukemia are prone to develop leukemia cutis, ranging from 25 to 30% of cases 4. Although the highest rate of leukemia cutis occurs in patients with adult T-cell leukemia/lymphoma (ATLL), the incidence of this leukemia is quite rare. Thus the most common subtypes seen in clinical practice are acute myeloid leukemia (AML) and chronic lymphocytic leukemia (CLL) involving 13% and 8% of total cases, respectively.
Majority of skin lesions occur in patients already diagnosed with leukemia (55 to 77%), cutaneous lesions may appear at the presentation of systemic leukemia (23 to 44%), or even precede the development of leukemia in the peripheral blood and/or bone marrow in 2 to 3% of cases.[3] This latter condition termed as “aleukemic” cutis eventually develops acute myeloid leukemia (AML) 5.
A skin biopsy and immunophenotyping must be performed in all suspected patients, regardless of prior leukemia history as transformation or development of different leukemia may occur. Identification of leukemia can be performed based on CD markers. Histology of skin biopsy shows nodular or diffuse infiltrates of leukemic cells in the dermis and/or subcutaneous tissue. These lesions spare the epidermis along with a narrow band of uninvolved upper dermis known as Grenz zone. However T-cell leukemia including adult T-cell leukemia/lymphoma (ATLL) may show epidermotropism. The size of cells varies depending on the subtype of leukemia. Chronic lymphocytic leukemia (CLL) cells are smaller, mature-appearing cells that may show perivascular, periadnexal, or nodular distribution. Myeloid leukemia cells are larger with high nuclear to cytoplasmic ratio, often prominent nucleoli and involving dermis and subcutis in a diffuse pattern. Immunohistochemical staining and history remain essential to distinguish reactive from neoplastic infiltrates.
What causes leukemia cutis?
Both genetic and environmental components are involved in the cause of any leukemia and are responsible for maturation arrest and clonal expansion of precursor cells. While the neoplastic cells carry the genetic abnormality of underlying leukemia, aneuploidy of chromosome 8, chromosome 3 translocations and t(6;9) are particularly notable in patients with leukemia cutis 6. Environmental risk factors for leukemia cutis are same as for developing any systemic leukemia and include benzene, ionizing radiation, alkylating and viral agents. Use of all-trans retinoic acid to treat acute promyelocytic leukemia may increase the risk of extramedullary involvement including the skin 7.
Leukemia cutis pathophysiology
The pathophysiology of leukemia cutis involves the migration of leukemic cells into the skin. The exact mechanism is unknown, but recent molecular analysis is beginning to provide more information on cell-cell interaction and the role of adhesion molecules. Mechanisms involving various chemokines and the molecular expression on leukemic cells mediating the migration of leukemic cells to the skin via skin-selective homing processes are the proposed cause 8.
Who gets leukemia cutis?
Leukemia cutis is rare, affecting about 3% of people diagnosed with leukemia. It occurs in males and females of any age, and with almost any kind of leukemia. In most cases, leukemia occurs out of the blue. However, some kinds of leukemia are associated with abnormalities of chromosomes, such as the Philadelphia chromosome.
Proliferation of the abnormal leukocytes is due to activation of oncogenes (cancer-promoting genes) or inactivation of tumour suppressor genes. Thus leukemia is more common in syndromes that have mutations in these genes. These syndromes may be inherited and include Bloom syndrome.
Other known risks for the development of leukemia include:
- Exposure to benzene
- Ionizing radiation
- Alkylating agents used in chemotherapy
- Viral infection e.g., HTLV-1, Epstein-Barr virus
- Other blood diseases such as myelodysplastic syndrome
It is not known why the leukemia cells sometimes choose to grow in the skin.
Leukemia cutis symptoms
In most cases presenting with leukemia cutis, the patient is already known to have leukemia because of abnormal blood count or bone marrow findings.
The skin lesion is the very first sign of the haematological malignancy in 7% of patients with leukemia cutis. This is sometimes called aleukaemic leukemia. Bone marrow infiltration and systemic symptoms occur later.
Sometimes myeloid leukemia relapses in the skin after apparently successful treatment of bone marrow. It is then called ‘extramedullary’ leukemia.
Skin changes
Leukemia cutis can present with various types of skin lesion. They are usually asymptomatic; that is, they are not itchy or sore.
- Firm papules (small bumps), nodules (larger lumps), and plaques (thickened flat patches), which may be skin-coloured, red, brown or purple
- Diffuse eczema-like red dry thickened skin (erythroderma)
- Figurate or annular erythema
- Purpura and petechiae (purple patches and spots due to bleeding into the skin)
- Blisters, erosions and ulcers
- Swollen gums (gingival hyperplasia or gingivitis) in acute leukemia.
- The skin lesions most often arise on the head, neck and trunk.
- They favour sites of injury.
- In babies, leukemia cutis is one of the causes of blueberry muffin syndrome.
Chloroma
‘Chloromas’ sometimes arise in myeloid leukemia or granulocytic sarcoma. These may present as solitary or numerous nodules. They appear blue-green when cut into at the time of biopsy, hence the name. The colour is due to myeloperoxidase granules in the malignant leukocytes. This type of leukemia cutis may precede the development of systemic leukemia by several months.
Blood abnormalities
Pancytopaenia occurs; that is, a reduction in red cells, white cells and platelets — all normally formed in the bone marrow.
- Anemia: pallor, tiredness, breathlessness.
- Reduced neutrophil count: bacterial, viral, fungal infections often with unusual or opportunistic organisms.
- Reduced platelet count (thrombocytopenia): easy bruising, bleeding.
Other organs
Symptoms depend on which organs have been infiltrated by the leukaemic cells.
- Fever, chills, lethargy
- Enlarged liver and spleen: nausea, abdominal fullness, constipation.
- Swollen lymph glands.
- Central nervous system: cranial nerve palsies, stroke, seizures, headache, loss of sensation.
- Musculoskeletal system: painful or swollen bones and joints, fractures, gout.
Leukemia cutis complications
Many of the complications are the result of underlying leukemia or its treatment, and not specifically as a result of cutaneous skin lesions.
- Leukemia patients are prone to a variety of opportunistic infections due to pancytopenia
- Bleeding is a result of thrombocytopenia or as a result of erosion of skin lesions
- Reactions to chemotherapy
- Mass effect if leukemia cutis manifests as a tumor
- Graft versus host disease: Graft versus host disease following bone marrow transplantation is common.
Leukemia cutis diagnosis
Leukemia is suspected from an abnormal blood count and confirmed by sampling the bone marrow by aspiration or trephine.
In the absence of established systemic leukemia, confirming the diagnosis of suspected cases of leukemia cutis proves to be challenging. In all cases, a judicious correlation between clinical features, histopathology, and immunophenotyping is necessary.
A complete blood count and peripheral smear must be performed to assess abnormalities in cell counts (cytopenias generally present) and evidence of circulating leukemic cells. A bone marrow biopsy is imperative for the definitive diagnosis of systemic leukemia. In addition to immunohistochemistry, biopsy samples should have cytogenetics and flow cytometry studies.
Although leukemia cutis may be suspected from the patient’s history and the appearance of the skin, skin biopsy is necessary to confirm the diagnosis. This reveals a diffuse infiltration of malignant leukocytes in the dermis.
- The cells can cluster around blood vessels and sweat glands.
- They may spread as single cells through the collagen bundles.
- Immature or precursor myeloid cells in acute myeloid leukemia (AML) often have cytoplasmic granules.
- Immature lymphoid cells in acute lymphoblastic leukemia (ALL) are large and do not have granules.
- Promyelocytes, metamyelocytes, bands and mature neutrophils are typical in chronic myeloid leukemia (CML).
- Dense uniform-looking small round lymphocytes are seen in chronic lymphocytic leukemia (CLL).
- Kidney-shaped cells are seen in monocytic leukemia.
- Flower cells are seen in adult T-cell leukemia/lymphoma (ATLL).
- There may be considerable bleeding, especially in acute myeloid leukemia (AML).
Further histochemistry (immunostaining and immunotyping) may reveal the specific cell type involved. It is sometimes very difficult to diagnose leukemia cutis.
- Acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) may be confused with lymphoma.
- Acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) may be confused with extramedullary haematopoeisis.
- Similar features may be seen in vasculitis, drug eruptions and infectious emboli.
- If the cells are uniform, the lymphocytic or neutrophilic infiltrate may appear reactive rather than malignant.
Other appropriate tests needed may include coagulation studies, complete metabolic profile, liver and kidney function tests, uric acid and lactate dehydrogenase. If the patient develops high unrelenting fevers, especially during the course of treatment, an infectious workup is necessary as these patients are immunocompromised and prone to infections.
A thorough search for other sites of systemic involvement is crucial. Imaging studies can invariably help to determine the extent of systemic involvement.
Leukemia cutis treatment
Treatment is directed towards the underlying leukemia, so the patient should be under the care of an experienced clinical hematologist and/or medical oncologist. Systemic chemotherapy is the usual choice depending on the overall condition of the patient.
Other treatments include electron beam therapy, localized radiation, and phototherapy.
Fever usually indicates infection and requires investigation and treatment with systemic antibiotics, antifungal and antiviral treatment.
The prognosis is poor.
Radiation therapy
Radiation therapy can be used to palliate symptoms of pain and pruritus in patients with leukemia cutis who are not candidates for systemic chemotherapy. Addition of radiation therapy to skin lesions after complete response to systemic chemotherapy provides no additional benefit. In a rare instance, radiation may be used to treat isolated skin relapse when bone marrow shows complete remission and no other site of extramedullary relapse is evident. If these patients are subsequently treated with chemotherapy, reports detail severe radiation recall phenomenon occurring with chemotherapy drugs like cytarabine and clofarabine 9. Treatment options for leukemia cutis are expanding rapidly. Helical irradiation of the total skin therapy has been modified as simultaneous integrated boost –helical arc radiotherapy of total skin (HEARTS) and used to treat acute myeloid leukemia in someone with disseminated leukemia cutis 10.
Diet
Neutropenic patients may be placed on diet of well-cooked foods with avoidance of deli meats, fresh fruits, and fresh vegetables to avoid associated risk of infection.
Leukemia cutis prognosis
Leukemia cutis is regarded as a systemic manifestation of underlying leukemia and generally carries a poor prognosis 11.
While some patients can experience remission of skin lesions after systemic chemotherapy, a diagnosis of leukemia cutis strongly correlates with additional sites of extramedullary involvement, with meninges being the most common site of spread (40%) 12.
A 2-year follow-up in acute myeloid leukemia (AML) patients showed a 6% survival rate with skin involvement vs. 30% in those without skin infiltrates 13.
Leukemia cutis survival rates
The prognosis is poor, with many patients having other extramedullary disease and poor survival rates. Several studies indicate that, in the presence of leukemia cutis in acute myeloid leukemia (AML) or chronic myeloid leukemia (CML), the disease course is aggressive and the length of survival is short. Even patients with aleukemic leukemia cutis or granulocytic sarcoma progress to systemic disease and should be treated systemically from the time of diagnosis.
A study by Kaddu et al showed an average survival time in acute myeloid leukemia (AML) to be 7.5 months and in chronic myeloid leukemia (CML), 9.4 months 14. Another study by Baer et al revealed that of 18 patients with leukemia cutis in acute myeloid leukemia (AML), 90% had other sites of extramedullary involvement, and, in 40% of these patients, the meninges were involved 15. In a smaller case series by Shaikh et al with only 5 patients with AML, all 5 died within 6 months of their diagnosis of leukemia cutis 16.
The prognostic significance of leukemia cutis in chronic lymphocytic leukemia (CLL) is less clear. In a study by Cerroni et al 17, chronic lymphocytic leukemia (CLL) was associated with advanced stage and a poor prognosis. One series by Su et al 18 of 16 patients with chronic lymphocytic leukemia (CLL) demonstrated a mean survival of 16 months, with 88% of the patients dying within 1 year. Other reports suggest that finding of leukemia cutis in chronic lymphocytic leukemia (CLL) in the absence of large cell transformation or worsening systemic disease, skin infiltration alone does not affect prognosis 19.
References- Leukaemia cutis. https://www.dermnetnz.org/topics/leukaemia-cutis/
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol. Online J. 2015 Aug 15;21(8).
- Wagner G, Fenchel K, Back W, Schulz A, Sachse MM. Leukemia cutis – epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012 Jan;10(1):27-36.
- Zhang IH, Zane LT, Braun BS, Maize J, Zoger S, Loh ML. Congenital leukemia cutis with subsequent development of leukemia. J. Am. Acad. Dermatol. 2006 Feb;54(2 Suppl):S22-7.
- Agis H, Weltermann A, Fonatsch C, Haas O, Mitterbauer G, Müllauer L, Schreiber S, Schwarzinger I, Juretzka W, Valent P, Jäger U, Lechner K, Geissler K. A comparative study on demographic, hematological, and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann. Hematol. 2002 Feb;81(2):90-5.
- Ratnam KV, Khor CJ, Su WP. Leukemia cutis. Dermatol Clin. 1994 Apr;12(2):419-31.
- Giralt S, O’Brien S, Weeks E, Luna M, Kantarjian H. Leukemia cutis in acute promyelocytic leukemia: report of three cases after treatment with all-trans retinoic acid. Leuk. Lymphoma. 1994 Aug;14(5-6):453-6.
- Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011 Oct 06;118(14):3785-93
- Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. Oct, 2011. 118:3785-3793.
- Hsieh CH, Tien HJ, Yu YB, Wu YH, Shueng PW, Lu YF, et al. Simultaneous integrated boost with helical arc radiotherapy of total skin (HEARTS) to treat cutaneous manifestations of advanced, therapy-refractory cutaneous lymphoma and leukemia – dosimetry comparison of different regimens and clinical application. Radiat Oncol. 2019 Jan 28. 14 (1):17.
- Paydaş S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br. J. Dermatol. 2000 Oct;143(4):773-9.
- Baer MR, Barcos M, Farrell H, Raza A, Preisler HD. Acute myelogenous leukemia with leukemia cutis. Eighteen cases seen between 1969 and 1986. Cancer. 1989 Jun 01;63(11):2192-200.
- Kaddu S, Zenahlik P, Beham-Schmid C, Kerl H, Cerroni L. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J. Am. Acad. Dermatol. 1999 Jun;40(6 Pt 1):966-78.
- Kaddu S, Smolle J, Cerroni L, Kerl H. Prognostic evaluation of specific cutaneous infiltrates in B-chronic lymphocytic leukemia. J Cutan Pathol. 1996 Dec. 23(6):487-94.
- Baer MR, Barcos M, Farrell H, Raza A, Preisler HD. Acute myelogenous leukemia with leukemia cutis. Eighteen cases seen between 1969 and 1986. Cancer. 1989 Jun 1. 63(11):2192-200.
- Shaikh BS, Frantz E, Lookingbill DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987 Jan. 39(1):57-60.
- Cerroni L, Hofler G, Bck B, Wolf P, Maier G, Kerl H. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia (B-CLL) at sites typical for Borrelia burgdorferi infection. J Cutan Pathol. 2002 Mar. 29(3):142-7.
- Su WP, Buechner SA, Li CY. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984 Jul. 11(1):121-8.
- Ali L, Cheney R, Merzianu. Subclinical Chronic lymphocytic leukemia with atypical cutaneous presentation. J Cutan Pathol. Feb. 38:236-240.