Locked in Syndrome
Locked-in Syndrome is rare a neurological disorder that occurs due to damage in the brainstem, most commonly (at least 60%) due to a brain stem stroke, that is characterized by quadriplegia (complete paralysis of all of the voluntary muscles of the legs and arms) and bulbar palsy (impairment of function of the glossopharyngeal, vagus, accessory and hypoglossal nerves) with preserved vertical eye and eyelid movements, self-awareness and normal if not near-normal cognitive abilities 1, 2, 3, 4, 5, 6, 7. Locked-in Syndrome patients are conscious, alert, aware of their environment, have preserved cognitive functions (thinking, reasoning and memory), but cannot speak or move their limbs, usually communicate through eye movements and blinking 5. Also, despite their quadriplegia, locked-in syndrome patients retain tactile sensitivity 8. Some treating physician may erroneously diagnose the Locked-in Syndrome patient as being in a coma, or as occupying one of several disorders of consciousness 9. These include the Minimally Conscious State (i.e., with discernible but fluctuating evidence of consciousness without effective communication) 10, 11 or the unresponsive wakefulness syndrome (i.e., eye opening period but no sign of consciousness) 12, 13. The American Congress of Rehabilitation Medicine defined Locked-in Syndrome as neurologic impairment characterized by sustained eye opening, preserved cognitive function, severe hypophonia (soft speech, especially resulting from a lack of coordination in the vocal muscles) or aphonia (loss of voice), quadriplegia (quadriparesis) and ability to communicate through vertical or lateral eye movement or blinking the upper eyelid 14. The disability associated with Locked-in Syndrome is most similar to upper cervical spine injury and advanced amyotrophic lateral sclerosis (ALS) 5.
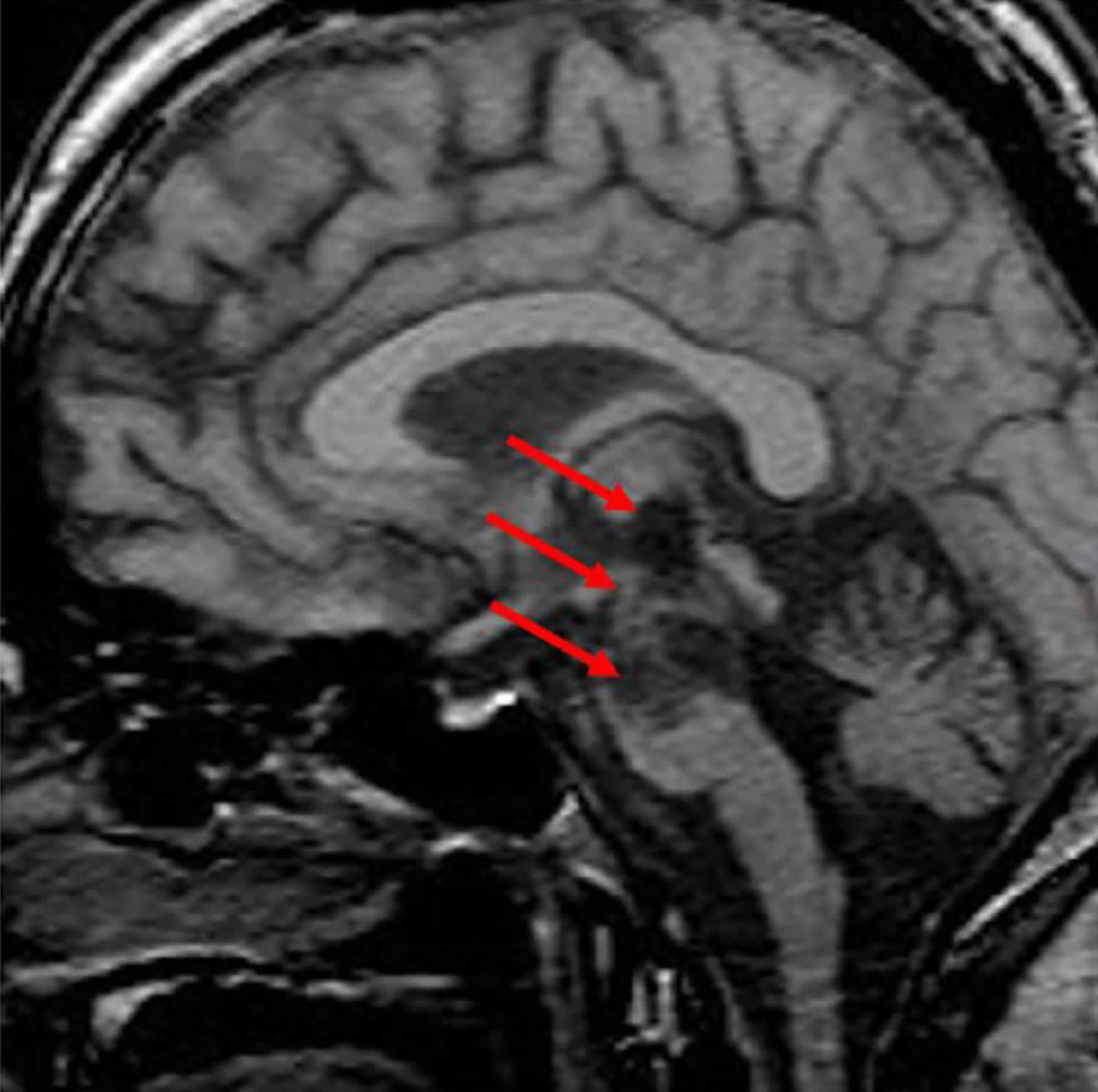
Locked-in Syndrome was first described by Plum and Posner 1972 1. In 1979, Bauer et al. 15 introduced further Locked-in Syndrome subcategorizations: they distinguished the classical Locked-in Syndrome from the complete and incomplete Locked-in Syndrome to describe patients whose capacity of voluntary movements went below or above the classical Locked-in Syndrome motor symptoms. Figure 1 shows a typical MRI of a classical or incomplete Locked-in syndrome patient.
- Classic Locked-in Syndrome: Quadriplegia and a complete loss of speech (anarthria) with preserved consciousness and vertical eye movement. The person with Locked-In Syndrome hears everything, understands everything, but cannot move or speak.
- Complete Locked-in Syndrome: Total immobility (no voluntary movements are achievable at all) and inability to communicate directly, with full consciousness, which makes further diagnostics indispensable.
- Incomplete Locked-in Syndrome is a less severe form, which can also be a transitory stage of recovery, where patients are able to execute more voluntary movements than in the classical form of Locked-in Syndrome.
- For patients who additionally suffer from disturbances of consciousness, the term Locked-in Plus Syndrome (LiPS) was introduced by the Salzburg Coma Group 16.
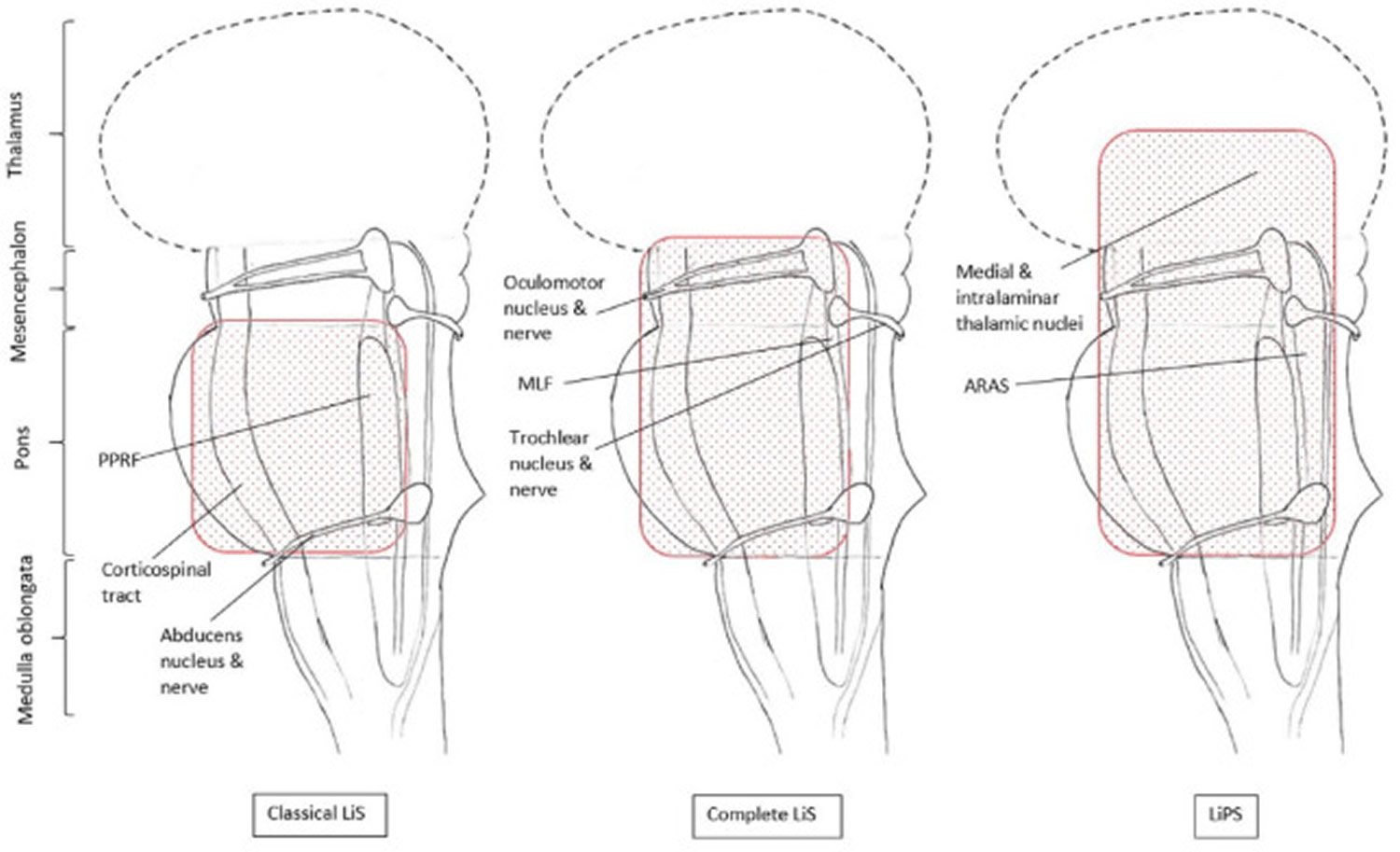
- Figures 1 to 3 display the regions of the brainstem and thalamus that can be affected in different forms of Locked-in Syndrome (LiS), while Figure 4 shows the MRI of a Locked-in Plus Syndrome (LiPS) patient.
Unless the physician is familiar with the signs and symptoms of the Locked-in Syndrome, the diagnosis may be missed and the patient may erroneously be considered as being in a coma, vegetative state, or akinetic mustism 17. Locked-in Syndrome diagnosis can be missed if voluntary vertical eye movement is not assessed in patients who seem unresponsive. When magnetic resonance imaging (MRI) shows a ventral pontine insult in an otherwise unresponsive patient, the assessor should re-examine vertical eye movement. Locked-in Syndrome can be difficult to diagnose because some patients emerge from coma into a locked-in state after a variable delay. In a recent survey of 44 Locked-in Syndrome patients belonging to the French Association Locked in Syndrome (ALIS), the first person to realize the patient was conscious and could communicate via eye movements most often was a family member (55% of cases) and not the treating physician (23% of cases) 18, 19.
Locked-in Syndrome diagnosis usually takes between 2.5 months and 4 to 6 years, and in the majority of the cases (55%) diagnosis is made by the patient’s relative, not the treating physicians (23%) 20. In that study the mean time to diagnosis was 78.8 days 18.
Pain management using pharmacological treatments in post-comatose patients can have deleterious side effects, such as increasing fatigue or decreasing awareness 9. This can lead to misdiagnosis and have important consequences on the continuation of care. The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” 6, 13, 21. Locked-in Syndrome patients can suffer from acute pain, which usually occurs suddenly, most often due to inflammation or severe medical condition 21 and/or chronic pain (i.e., pain lasting longer than 3 months or beyond the expected period of healing of tissue pathology) 22. Locked-in Syndrome patients can also suffer from neuropathic pain, which is defined as a “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” 23. In addition, Locked-in Syndrome patients are likely to develop spasticity (muscle spasms), the severity of which correlates with pain intensity 24. Pain perception has been previously investigated in disorders of consciousness patients. Neuroimaging studies have shown that painful stimulations applied to minimally conscious state patients can lead to the activation of brain regions involved in the sensorial and affective processing of pain (i.e., insula, anterior cingulate cortex and secondary somatosensory cortex), similar to observations in healthy subjects 25, 26. Meanwhile, in unresponsive wakefulness syndrome patients, only the primary somatosensory cortex (i.e., involved in the sensory processing of pain) was activated following a painful stimulation, suggesting that pain processing in unresponsive wakefulness syndrome could be compromised 27, 28.
There are minimal scientific studies investigating the processing of pain in Locked-in Syndrome. A survey conducted in 2008 showed that despite the fact that 46% of the Locked-in Syndrome patients reported moderate or extreme pain, 72% of patients reported a good quality of life 29. Another study showed that pain was associated with a decrease of quality of life in post-stroke patients 30. Furthermore, it has been shown that patients’ mental health and the presence of physical pain correlate with the frequency of suicidal thoughts, suggesting that pain has a significant impact on the quality of life of these patients as in other diseases like cancer, fibromyalgia syndrome or stroke 19, 30, 31, 32, 33, 34.
Although Locked-In Syndrome survivors frequently experience motor loss that is debilitating and permanent, prompt recognition of and supportive treatment for this neurological disorder is critical. The acute management of patients with Locked-In Syndrome is similar to that for patients with other acute brain stem insults. The initial emphasis is on maintaining an airway and adequate oxygenation. Managing reversible medical causes and reducing risk factors are essential while preventing the complications of immobility, dysphagia, and incontinence. Chest physiotherapy, including deep breathing exercises, frequent positional changes, postural drainage, and suctioning, may limit pulmonary complications. Corneal ulceration, due to impaired eye closure, can be treated by lateral tarsorrhaphy or botulinum therapy. Avoiding full eye closure is important because it will prevent communication. Pathological laughing and crying, which are not symptoms of a mood disorder, do not respond to pharmacological treatment and should be treated with a cognitive-behavior approach 35. Intrathecal baclofen is used frequently to treat spasticity in Locked-in Syndrome patients and has also the potential to increase the motor recovery 36, 37. Early rehabilitation can enhance patient quality of life and improve recovery outcomes 5.
Locked-in Syndrome is a very rare syndrome and no exact incidence or prevalence is known 2. Locked-in Syndrome affects males and females in equal numbers. Locked-in Syndrome can affect individuals of all ages including children, but most often is seen in adults who are more at risk for brain stroke and bleeding 38. Because cases of Locked-in Syndrome may go unrecognized or misdiagnosed, it is difficult to determine the actual number of individuals who have had the disorder in the general population. A 2013 study estimated a prevalence of 0.7 per 10,000 patients with classical Locked-in Syndrome in Dutch nursing homes 39. The French Association Locked in Syndrome (ALIS) states on its website that more than 500 French people live in Locked-in Syndrome in 2022 40. Life expectancy has improved in the last decades for Locked-in Syndrome patients, some living for many decades in Locked-in Syndrome 41, 42.
In 1986, Patterson and Grabois 43 obtained a sample of 139 cases including six own cases and cases from a literature review. They found a mean age of onset of 52 years (range: 16–90) with more males than females affected (85 to 52) 43. A total of 89 patients were diagnosed with classical, 46 incomplete and 3 complete Locked-in Syndrome (one unclassified); 10 patients showed transient states 43. Eighty-two patients suffered an infarction of the pons, other vascular and non-vascular causes being less common. Roughly one third of the patients had a history of high blood pressure (hypertension). A mortality rate of 60% was reported, but no time frame given, although the majority of deaths occurred within the first 4 months after onset 43. A newer study reports a mortality rate of 75% in the acute phase 44. The most reported causes of death are pulmonary complications and further brainstem damage 43, 44. Sensory perception varied wildly in the population from normal to absent. It was also shown that substantial recovery is possible and can continue over years, possibly by reorganization of the descending pathways 43, 45, 46, 47, 48, 49, 50, 51, 52, 53. One survey of chronic Locked-in Syndrome patients showed a recovery of functional movements in 72% 54. Distal motor functions are more likely to recover 55 and even when large brainstem strokes are apparent in magnetic resonance imaging (MRI), substantial recovery is possible 56.
Figure 1. Locked-in Syndrome pathology
Footnotes: Regions of the brainstem and thalamus which can be lesioned in classical Locked-in Syndrome (LiS), complete Locked-in Syndrome (LiS) and Locked-in Plus Syndrome (LiPS), including the most important structures causing the symptoms and function losses.
Abbreviations: ARAS = ascending reticular activating system; LiPS = locked-in plus syndrome; LiS = Locked-in Syndrome; MLF =, medial longitudinal fasciculus; PPRF = paramedian pontine reticular formation.
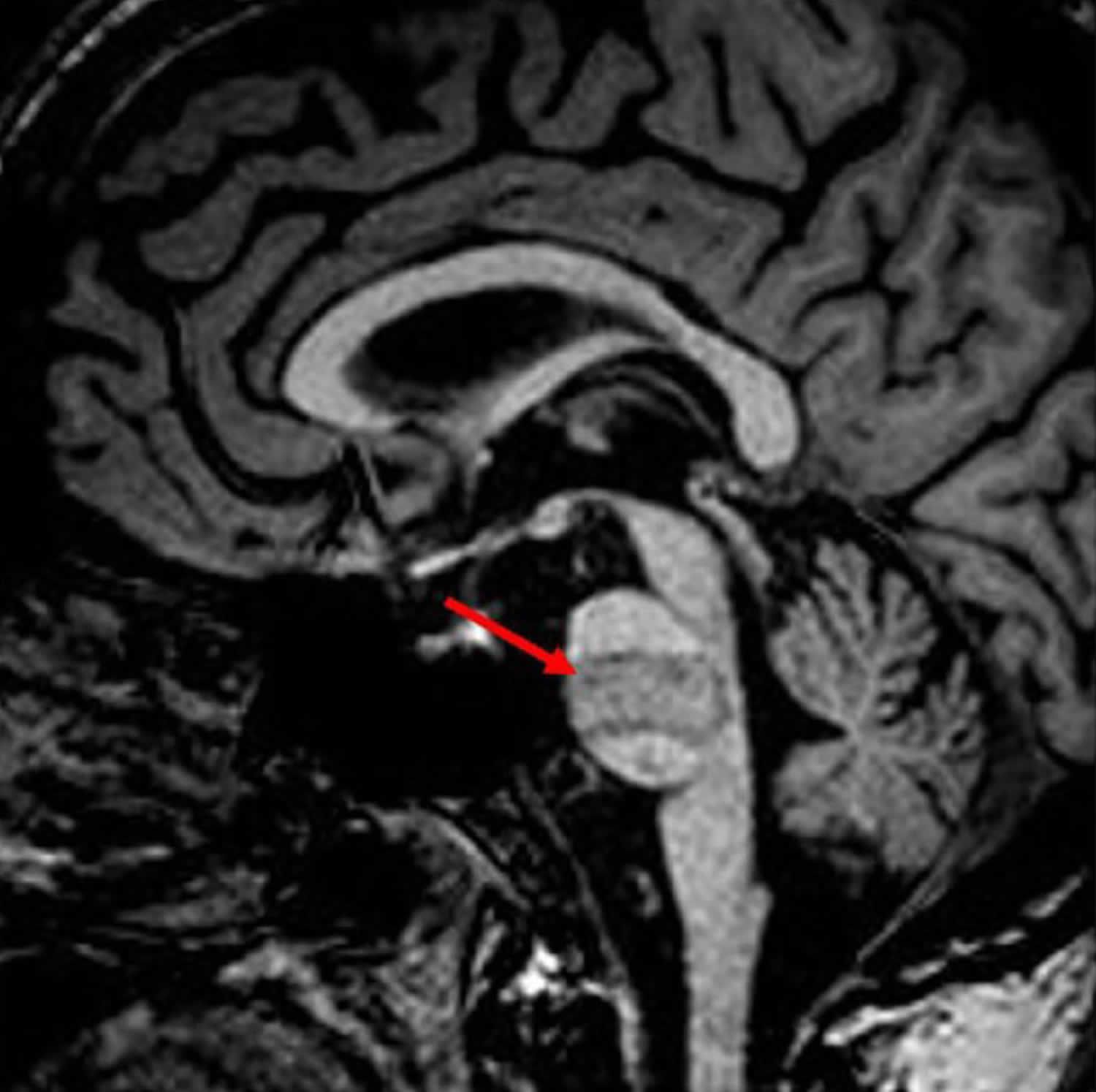
[Source 2 ]Figure 2. Locked-in syndrome MRI
Footnote: MRI of a patient with locked-in syndrome due to basilar thrombosis showing lesions involving the ventral pons (arrow).
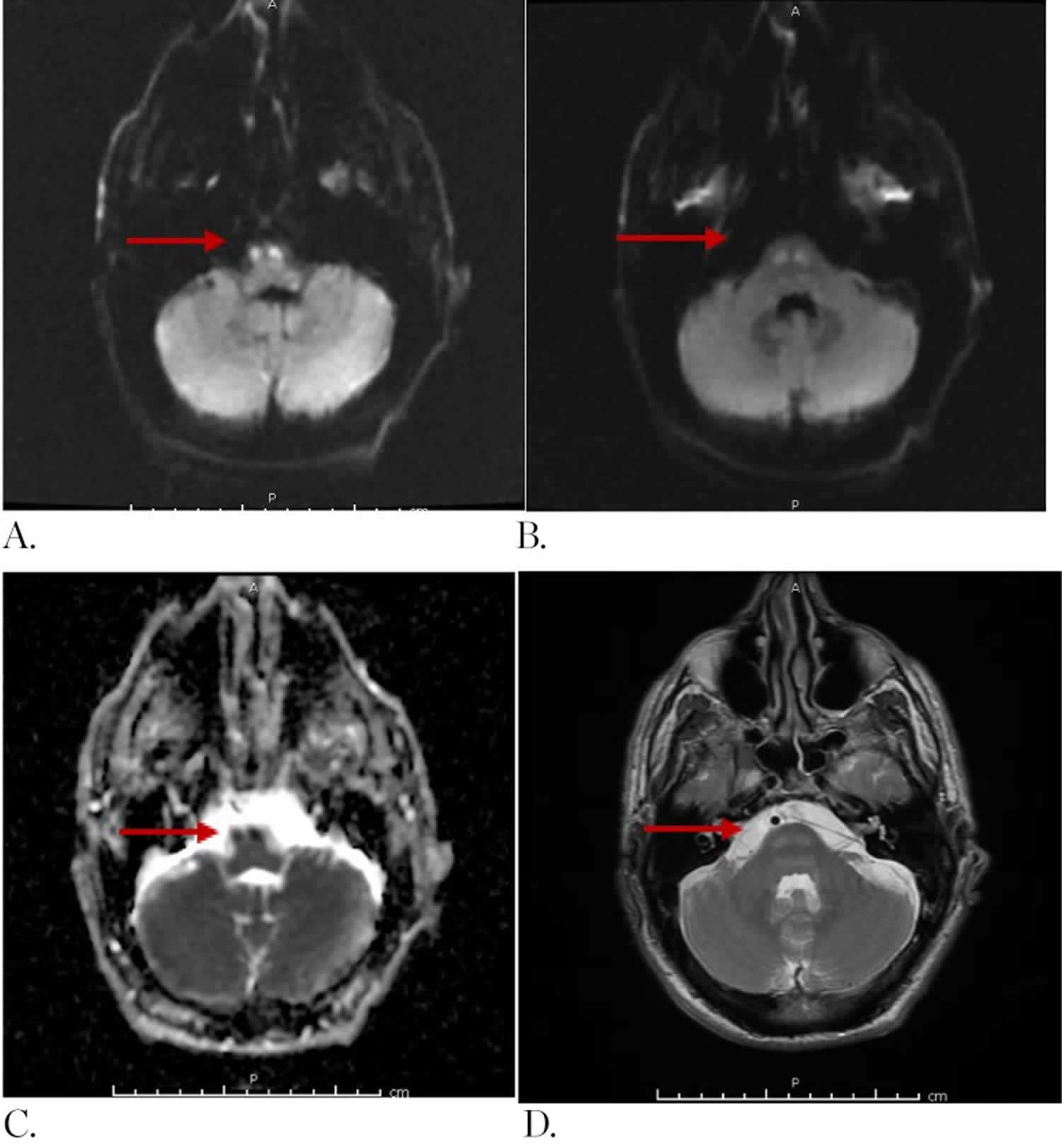
[Source 2 ]Figure 3. Locked-in Syndrome MRI
Footnote: A 56-year-old male with history of alcohol abuse was admitted following a motor vehicle collision, where he was a passenger. The patient was found unresponsive with a Glasgow Coma Score of 3 (E1, V1, M1). Initial brain imaging demonstrated small subdural hematoma along the falx cerebri as well as the frontal convexities. A cervical spine magnetic resonance imaging (MRI) demonstrated severe post-traumatic changes at C5 – C6 level without evidence of cord compression. Axial MRI of the brain obtained 36 hours after admission demonstrates (by red arrows) symmetric areas of increased signal in diffusion weighted imaging (DWI) (A, B) with decreased signal in apparent diffusion coefficient (C) and high T2 signal (D) at the level of the medullary pyramids and caudal pons involving predominantly white matter tracts.
[Source 57 ]Figure 4. Locked-in Plus Syndrome (LiPS) MRI
Footnote: MRI of a patient with Locked-in Plus Syndrome (LiPS) due to basilar artery thrombosis, the arrows showing the lesions involving the brainstem (pons and mesencephalon) and thalamus.
[Source 2 ]What is Locked-in Plus Syndrome?
Impairment of consciousness in Locked-in Plus Syndrome patients may be caused either by (1) a lesion extending rostral through the mesencephalon into the medial thalamus, or (2) a lesion that stretches dorsal affecting the ascending reticular activating system, or (3) combinations thereof 2.
A study investigating the cognitive functioning of Locked-in Syndrome patients found that lesions can occur in locations beyond the pons (e.g. the thalamus) and that these were related to a worse cognitive outcome 58. Schnakers et al. 7 showed that additional lesions on top of the brainstem lesions are often related with cognitive deficits. Other studies also reported cognitive impairments in locked-in patients 59, 60. However, these studies did not investigate patients with impaired consciousness, but they show a continuum on which Locked-in Plus Syndrome (LiPS) could represent the most extreme form.
The top of the basilar syndrome is an event that occludes the most distal part of the basilar artery usually involving damage of the thalamus 61. Percheron 62 was the first to describe the arterial supply of the thalamus including the paramedian arteries. These originate shortly after the bifurcation of the basilar artery, although there are varying patterns of origin 62. The paramedian arteries supply the medial and intralaminar thalamic nuclei, which play an important role for consciousness and other cognitive functions. Their bilateral occlusion therefore can cause impairment of consciousness and even deep coma 63, 64, 65.
The central thalamus including the intralaminar nuclei play a key role in awareness and consciousness 66. In an experimental study, consciousness was restored in an anesthetised macaque by stimulating the central lateral thalamus (which belongs to the intralaminar thalamic nuclei) but not any other surrounding region 67.
In addition, a study demonstrated that patients with Locked-in Syndrome and severely impaired awareness had lesions, which extended into the midbrain and/or pontine tegmentum, whereas purely thalamic lesions alone did not severely impair consciousness 68. It was also shown that a small region of the left rostral dorsolateral pontine tegmentum is significantly more often associated with coma compared with controls 69.
One structure of key importance involved in these cases is the ascending reticular activating system (ARAS). Moruzzi and Magoun 70 proposed in 1949, the ascending reticular activating system (ARAS) is important for arousal. Wakefulness in turn is needed for awareness and consciousness. Ascending reticular activating system (ARAS) consists of various diffuse brainstem nuclei, which are mostly located in the midline of the dorsal part of the brainstem and project inter alia to the thalamus and the cortex. A tractography study demonstrated that the intralaminar thalamic nuclei are an important connection of the ascending reticular activating system (ARAS) 71.
A lesion of the ascending reticular activating system (ARAS) or its connections to the thalamus or other structures like the hypothalamus and basal forebrain will therefore also cause an impairment of consciousness.
To summarize, the impairment of consciousness in Locked-in Plus Syndrome (LiPS) patients can be explained by brainstem lesions, which not only involve the ventral pons but also the mesencephalon, thalamus and dorsal regions of the brainstem 2.
Locked-in Syndrome causes
Locked-in syndrome is most often caused by infarction and bleeding in the vertebrobasilar artery territory to a specific part of the brainstem known as the pons (most importantly the ventral part of the pons), although extensive bilateral destruction of corticobulbar and corticospinal tracts in the cerebral peduncles may also be responsible 72, 73, 18, 74, 43, 75. This is sometimes preceded by a ‘pontine warning syndrome’, which describes recurrent, fluctuating motor or sensory symptoms associated with a high risk of basilar infarction 51, 76, 77, 78. The pons contains important neuronal pathways between the cerebrum, spinal cord and cerebellum. In Locked-in syndrome there is an interruption of all the motor fibers running from grey matter in the brain via the spinal cord to the body’s muscles and also damage to the centers in the brainstem important for facial control and speaking.
Damage to the pons most often results from tissue loss due to lack of blood flow (infarct) or bleeding (hemorrhage) – less frequently it can be caused by trauma. An infarct can be caused by several different conditions such as a blood clot (thrombosis) or stroke.
Additional conditions that can cause locked-in syndrome include infection in certain portions of the brain, tumors, loss of the protective insulation (myelin) that surrounds nerve cells (myelinolysis), inflammation of the nerves (polymyositis), and certain disorders such as amyotrophic lateral sclerosis (ALS).
Although in most cases the brainstem and especially the ventral pons is the primary site of injury, recent studies have shown that other brain areas may be involved in addition. Diffuse supra- and infratentorial white matter fiber tract injuries have been reported 79, as well as atrophy in cortical areas 80 (e.g. dorsomedial prefrontal cortex) 81 and changes in cortical rhythms in resting-state electroencephalography (EEG) experiments compared with healthy controls 82. However, one study also showed increased fiber density and connectivity in some parts of the brain 83.
Locked-in Syndrome causes 42:
- Ischemic: Basilar artery occlusion, hypotensive or hypoxic events
- Bleeding: Hemorrhage originating within or infiltrating into the pons
- Traumatic brain injury (TBI): Direct brain stem contusion or vertebrobasilar axis dissection (axonal injury & secondary infarction) 47, 57, 103, 104, 105, 87, 106, 107, 108
- Mechanical brain herniation 109, 110, 111, 112, 113
- Tumor: Primary or secondary (metastatic) infiltration of the ventral pons 114, 115, 116, 117, 118
- Demyelination: Diseases that destroy the myelin sheath (a protective covering that surrounds nerve cells). For example, multiple sclerosis (MS) affecting the ventral pons 119, 120, 121 or central pontine myelinolysis 122, 123, 124
- Infections: Abscess infiltrating the ventral pons, brain stem encephalitis, meningovascular syphilis 125, 126, 127, 128
- A medication overdose
- Hyperhomocysteinemia 129
- Cocaine abuse 130
- Prolonged hypoglycemia 131
- Locked-in like states
- Complications during
Locked-in Syndrome pathophysiology
In the early literature, classical Locked-in syndrome was also called the ventral pons syndrome 2. Being ‘locked in’ within a body, all voluntary movements are impaired by the damage of the corticospinal and corticobulbar tracts, which both run side by side through the cerebral peduncles of the midbrain and are located in the very ventral part of the pons and the medulla oblongata.
The corticospinal tract is crucial for voluntary movements of the body and extremities, whereas corticobulbar fibres are responsible for voluntary, non-oculomotor movements of muscles innervated by caudal cranial nerves (e.g. speech, tongue movement) 2.
Eye movements are spared as they are controlled by different centers in the brainstem. The paramedian pontine reticular formation (PPRF) is responsible for horizontal eye movements and saccades. The paramedian pontine reticular formation (PPRF) signals the abducens nucleus an ipsilateral horizontal eye movement whereupon the nucleus contracts the ipsilateral rectus lateralis muscle via the abducens nerve. Simultaneously, the contralateral oculomotorius nucleus signals via the medial longitudinal fasciculus (MLF) to contract the ipsilateral rectus medialis muscle. This results in a horizontal gaze movement, which has its control center in the pons 150.
Vertical eye movements are controlled by mesencephalic structures [the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF), interstitial nucleus of Cajal, the posterior commissure, oculomotorius and trochlearis nucleus and nerve] 151. The levator palpebrae superioris responsible for retraction of the upper eyelid and therefore blinking is also innervated by the oculomotor nerve. Therefore, damage of paramedian mesencephalic structures can cause vertical gaze paralysis and ptosis. A patient who suffered from top of the basilar artery occlusion which could be recanalized but still resulted in bilateral infarctions in the midbrain tegmentum, especially affecting the oculomotor nuclei, showed bilateral ptosis, non-reactive pupils and ophthalmoplegia 152.
The additional involvement of the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) and other mesencephalic structures in brainstem damage in Locked-in syndrome leads to the clinical presentation of complete Locked-in syndrome therefore formerly also referred to as mesencephalic Locked-in syndrome 153. Otherwise, the sparing of paramedian pontine reticular formation (PPRF), and/or parts of the corticospinal tracts results in incomplete Locked-in syndrome presenting without oculomotor disturbances and/or preserved body movements.
Involuntary movements have been reported in Locked-in syndrome patients by Bauer et al. 154 who described stimulus evoked oral automatisms like sucking, chewing and swallowing, most likely apparent due to lost pyramidal control of the motor centres in the brainstem. Moreover, involuntary movements like flexor and extensor spasms 97, pain reactions, compulsory mimic reactions, cat crying, whining, moaning, groaning, yawning, sighing and coughing were reported 155. Many of these motor behaviors have also been described in newborns suffering from anencephaly, which shows that the cortex is not needed for these complex movements 156. Trismus and yawning were described in one patient 157. A possible explanation for the excessive yawning could be its role in thermoregulation as a mechanism to cool the brain 158, 159. Besides orofacial stereotypes, palatal tremor has also been observed 160. These motor phenomena have to be kept in mind when examining a patient and distinguished from voluntary movements that can serve as communication.
Locked-in Syndrome signs and symptoms
People with Locked-in Syndrome are:
- Paralyzed except for the muscles that control eye movement. Some affected individuals can move their eyes up and down (vertically), but not side-to-side (horizontally).
- Conscious (aware) and can think and reason, but cannot move or speak; although they may be able to communicate with blinking eye movements.
Locked-in Syndrome signs and symptoms include:
- Quadriplegia or tetraplegia: Paralysis of all four limbs, and trunk of the body.
- Muscle spasticity: Involuntary muscle stiffness
- Hypertonia: Increased muscle tone so that arms or legs, for example, are stiff and difficult to move.
- Abnormality of the voice
- A defect in the motor ability that enables speech (anarthria).
- Double vision (diplopia)
- Respiratory impairment with frequent respiratory infections
- Feeding difficulties
- Hearing impairment
- Behavioral changes
People with Locked-in Syndrome are conscious and can think and reason, but are unable to speak or move. Vertical eye movements and blinking can be used to communicate. People with Locked-in Syndrome classically cannot consciously or voluntarily chew, swallow, breathe, speak, or produce any movements other than those involving the eyes or eyelids. Some affected individuals can move their eyes up and down (vertically), but not side-to-side (horizontally). Affected individuals are bedridden and completely reliant on caregivers. Despite physical paralysis, cognitive function is unaffected.
Individuals with Locked-in Syndrome are fully alert and aware of their environment. They can hear, see and have preserved sleep-wake cycles. Affected individuals can communicate through purposeful movements of their eyes or blinking or both. They can comprehend people talking or reading to them.
Individuals with Locked-in Syndrome often initially are comatose before gradually regaining consciousness, but remain paralyzed and unable to speak.
Additional symptoms under investigation
Patients with Locked-in Syndrome are traditionally considered cognitively intact as all the cerebral structures with the exception of the ventral portion of the pons of the brain are apparently preserved. However, recent evidence suggests that Locked-in Syndrome patients can develop some non-motor symptoms including motor imagery defects, pathological laughter and crying, and difficulties in the recognition of some facial expressions. The interruption of the cortico-ponto-cerebellar pathways, by means of the the same lesion causing the Locked-in Syndrome, may be responsible for the appearance of these clinical manifestations. However, these symptoms are not detected in all affected individuals and are currently under further investigation. The recognition of motor imagery defects deserves special attention because these symptoms, whenever present, may interfere with the success of rehabilitation strategies.
Locked-in Syndrome diagnosis
A diagnosis of Locked-in Syndrome is usually made clinically. A variety of tests may be performed to rule out other conditions. Such tests include magnetic resonance imaging (MRI), which shows the damage to the pons, and magnetic resonance angiography (MRA), which can show the blood clot in the arteries of the brainstem. An MRI uses a magnetic field and radio waves to produce cross-sectional images of particular organs and bodily tissues such as the brain. Magnetic resonance angiography (MRA) uses a magnetic field and radio waves to produce cross-sectional images of blood vessels inside the body. These tests can also rule out damage elsewhere in the brain.
An electroencephalogram (EEG), a test that measures the electrical activity of the brain, may reveal normal brain activity and sleep-wake cycles in individuals with Locked-in Syndrome.
Evoked potentials, tests that average the EEG signal in response to stimulation (pain or auditory or visual), permit a look at the damaged responses in the brainstem and the preserved responses in the brain.
Electromyography (EMG) and nerve conduction study (NCS) can be used to rule out damage to the muscles and nerves. An electromyography (EMG) is a test that records electrical activity in the skeletal (voluntary) muscles at rest and during muscle contraction. Nerve conduction study determines the ability of specific nerves to relay nerve impulses to the muscles.
Locked-in Syndrome differential diagnosis
Locked-in Syndrome is often misdiagnosed due to its severe symptoms. The most important differential diagnoses that have to be considered are unresponsive wakefulness syndrome, minimally conscious state, cognitive motor dissociation and akinetic mutism 2.
Without careful clinical investigation and EEG, Locked-in syndrome can be misinterpreted as a disorder of consciousness, for example, the unresponsive wakefulness syndrome if signs of consciousness such as voluntary movements are very subtle or missing 2. The unresponsive wakefulness syndrome formerly known as vegetative state 161 or apallic syndrome 162 is the first remission stage of a coma and marked by spontaneous eye opening and mostly resumed autonomic function but a lack of conscious behavior 163.
More recently, the minimally conscious state was proposed as an interim state in which patients show reproducible behavioral signs of minimal consciousness even though these can be very inconsistent for instance due to high vigilance fluctuations. Behaviors like following simple commands, gestural or verbal yes/no answers, intelligible verbalizations or purposeful behavior have to be present to diagnose a patient as suffering from minimally conscious state 164. Minimally conscious state has also been subcategorized into minimally conscious state plus (MCS+ patients showing command following) and minimally conscious state negative (MCS− patients with minimal behavioral interaction) based on the complexity of the behavioral responses of the patient, for example, command following versus minimal behavioral interaction 165. Moreover, the term ‘emergence from minimally conscious state’ has been defined by a functional interactive communication or functional object use 164.
The gold standard for diagnosing these disorders of consciousness (unresponsive wakefulness syndrome, minimally conscious state) is the Coma Recovery Scale–Revised (CRS-R) 2. This is a standardized, behavioural scale which is able to discriminate unresponsive wakefulness syndrome, minimally conscious state and conscious patients according to their behavioral output 166. Unresponsive wakefulness syndrome patients show reflexive behaviors only, whereas minimally conscious state patients exhibit signs of minimal consciousness as described above. However, patients who are not able to show their consciousness behaviorally are missed by the Coma Recovery Scale–Revised (CRS-R).
Another term that has gained more and more importance recently is cognitive motor dissociation, also known as functional locked-in syndrome, nonbehavioral minimally conscious state or covert cognition 167, 168. Cognitive motor dissociation describes patients who, although showing a preserved yet possibly limited capacity of rational thoughts, have no capability of creating any output to the environment due to complete paralysis. The state of these patients can only be detected by further diagnostics such as functional MRI or event-related EEG techniques 169.
Akinetic mutism is a clinical picture in which a patient neither moves (akinetic) nor speaks (mutism). Akinetic mutism clinical picture was first described by Cairns in a patient with an epidermoid cyst of the third ventricle in 1941 170. Akinetic mutism was also described in a case of paramedian thalamic infarction where the lesions included dorsomedian and intralaminar nuclei 171, 172. According to Arnts et al. 173, akinetic mutism is the most severe form of a motivational disorder and caused by a disruption of the frontal subcortical circuit which can occur when the anterior cingulate cortex, the striatum, the internal pallidal complex or the medial thalamus are damaged.
Although the classical locked-in syndrome is not a disorder of consciousness or motivation, it is often difficult to differentiate Locked-in syndrome from unresponsive wakefulness syndrome, minimally conscious state, cognitive motor dissociation or akinetic mutism due to quadriplegia and complete loss of speech (anarthria) 14. However, besides structured, precise and repeated bedside examination, it can be differentiated with further diagnostics, for example, imaging like MRI, as the location of the lesions differ. Unresponsive wakefulness syndrome, minimally conscious state, cognitive motor dissociation and akinetic mutism are characterized by lesions found supratentorial, whereas the lesions of all forms of Locked-in syndrome are dominantly in the brainstem 2. In cognitive motor dissociation for instance, the connectivity between subcortical and cortical areas is impaired 174, in contrast to patients suffering from Locked-in syndrome where the main locus of the injury is the ventral pons 4. However, one patient with supratentorial lesions, which caused an incomplete locked-in like syndrome, has been described 48, and Locked-in syndrome due to traumatic causes can also present with lesions outside of the typical location 175. Locked-in syndrome should therefore always be considered. Moreover, a clear definition of cognitive motor dissociation and its related terms is needed to differentiate it from complete Locked-in syndrome and allow a consistent nomenclature 168.
Moreover, there are patients who show lesions in the brainstem, which are typical for Locked-in syndrome, but no clinical signs of consciousness can be found. One possible explanation is that the patient suffers from complete Locked-in syndrome, where no voluntary movement is possible at all and therefore, patients seem to be in deep non-reactive coma at bedside examination. To diagnose Locked-in syndrome, functional magnetic resonance imaging (fMRI) and EEG-based active paradigms are mandatory 176, 177. Still, there are patients who show no or only minimal signs of consciousness even when using these diagnostic measures. Keep in mind that a negative fMRI outcome by far does not mean that the patient is not conscious as bad timing, movement artefacts or other problems can lead to an erroneous result. Furthermore, many paradigms need a high level of cognitive functioning and can be tiring or too complicated for the patients. Therefore, the European Academy of Neurology suggests using not only a repeated standardized clinical assessment but also a multimodal evaluation for patients suffering from disorders of consciousness 177.
Still, there are patients in Locked-in syndrome who also suffer from a dysfunction of consciousness and are therefore in Locked-in Plus Syndrome 16.
To summarize, to correctly diagnose Locked-in syndrome and its subgroups, repeated, clinical bedside evaluation, imaging and, in case no communication can be established, fMRI or EEG-based functional examinations should be performed.
Locked-in Syndrome treatment
The acute management of patients with Locked-In Syndrome is similar to that for patients with other acute brain stem insults aimed at the underlying cause of the disorder. The initial emphasis is on maintaining an airway and adequate oxygenation. Managing reversible medical causes and reducing risk factors are essential while preventing the complications of immobility, difficulty swallowing (dysphagia), and incontinence. For example, reversal of a basilar artery blood clot (thrombosis) with intraarterial thrombolytic therapy may be attempted up to six hours after symptoms onset. Tumors may be treated with intravenous steroids or radiation. Affected individuals often need an artificial aid for breathing and will have a tracheotomy (a tube going in the airway via a small hole in the throat) in the beginning.
Feeding and drinking will not be possible via the mouth (it may cause respiratory infection by running into the lungs rather than stomach) and hence will need to be assured via a small tube inserted in the stomach called gastrostomy.
To avoid pulmonary complications, which are the main cause of death, chest physiotherapy like deep breathing exercises, frequent position changes, postural drainage, and suctioning is recommended 178. Diaphragmatic pacing, which is established for spinal cord injured patients, is also an option that showed benefits for a Locked-in Syndrome patient already 179.
An ophthalmologic examination is recommended as visual function is frequently impaired in Locked-in Syndrome patients, and appropriate treatment can improve comfort and aid with communication 180. If a patient suffers from opsoclonus-myoclonus syndrome, gabapentin can ameliorate the symptoms and enable communication through eye movements 181, 182. In cases of corneal ulceration due to impaired eye closure, lateral tarsorrhaphy or botulinum therapy is indicated 178. Avoiding full eye closure is important because it will prevent communication.
It is important to establish an eye-coded communication as soon as possible. Healthcare providers and family and friends should try to find out what is the easiest code for the affected individual and consequently all use the same code. This can be ‘look up’ for ‘Yes’ and ‘look down’ for ‘No’ or whatever is the easiest movement for the specific case. Communication is then limited to closed yes-no questions and can next be replaced by eye-coded letter spellers such as saying the alphabet and having the affected individual look down to choose her or his letter. There are many variations on this way of communication presenting the letters in frequency of use in the English language (A, B, C to Z) or using letter boards with different columns and lines for vowels and consonants for example.
Next, treatment should be aimed at the early rehabilitation of the small voluntary movements that remain or recover (often in a finger or foot or swallowing and sound production). Rehabilitation and various supportive therapies are very beneficial and should be started as early as possible even if it needs to be stressed that recovery of near-normal motor control, speaking, swallowing and walking are extremely unusual. After the acute stabilization and treatment of the underlying cause, an intensive and early rehabilitation (begun on average 1 month after onset) improves the functional outcome of Locked-in Syndrome patients and reduces the mortality rate 183. The currently available recommendations on the rehabilitation of Locked-in Syndrome patients suggest that the treatment and management of pain, spasticity, incontinence, nutrition, tracheostomy, vision, vegetative and vestibular functions and communication are very important 184. Moreover, the benefit of early interdisciplinary therapy including physical, speech, occupational therapy and the use of assistive devices are critical 185. Furthermore, based on a case report, multisensory, progressive, multimodal and technology based interventions are suggested to fill the gap in motor retraining 186. A multidisciplinary team approach including nursing interventions is also recommended 187, 188. Although very rare, Locked-in Syndrome in children poses special challenges especially in the areas of communication, treatment and end-of-life decisions 189.
Devices to aid in communication and other assistive technologies have proven beneficial as well as allowing individuals to become active members of society. Infrared eye tracking devices now permit affected individuals to use a computer with artificial voice, control their environment, surf on the internet and send email. In rare cases, some individuals have recovered limited motor abilities, however, in most people such recovery does not occur. Those who recover some motor control in hand or head (as will over half of the patients) can use this to communicate with a computer and sometimes control their wheelchair.
Pathological laughing and crying, which are not symptoms of a mood disorder, do not respond to pharmacological treatment and should be treated with a cognitive-behavior approach 35. Intrathecal baclofen is used frequently to treat spasticity in Locked-in Syndrome patients and has also the potential to increase the motor recovery 36, 37.
Other specific interventions that were proposed were occupational therapy in combination with new technologies, treadmill therapy and repetitive sensor motor training.164,187 The improvement of feeding motivation and oral intake in one Locked-in Syndrome patient treated with transcranial direct current stimulation of the prefrontal area suggests the usefulness of this application.188 Moreover, spinal cord stimulation was also suggested for Locked-in Syndrome patients.189 Bispectral monitoring was used to assess postoperative analgesia in a Locked-in Syndrome patient.190
Although a systematic review found insufficient evidence for the benefits of physical exercise in physical recovery of Locked-in Syndrome patients, a positive trend was seen for muscle strength, tone, walking ability and activities in daily living and the need for well-designed studies was stated 190. As in stroke patients, repetitive sensorimotor training could be beneficial for Locked-in Syndrome patients 191. Sildenafil citrate is hypothesized to support recovery 192. To raise the chances for a Locked-in Syndrome patient to return home, the involvement of relatives in rehabilitation should be considered 42.
Furthermore, psychological and psychiatric support and treatment is essential. Although the depression rate among Locked-in Syndrome patients is lower than many would expect, if present, it must be treated adequately 193. Offering coping strategies to patients who have difficulties in adapting to the new situation are essential 193. Hallucinations and delusions are another most likely underreported symptom of Locked-in Syndrome patients, which often improves when the patient is repeatedly informed about the illusory nature 80.
The rehabilitation of Locked-in Syndrome patients with cognitive impairments is still a complex problem 58, and also patients who suffer from the Locked-in Plus Syndrome (LiPS) need special treatment adapted to their level of functioning. Assessing cognitive functions is important to improve communication 7, evaluate the possible usage of different communication devices 194 and to assess the patients decision-making capacity 195.
Tasks like complex sentence comprehension, mental calculation and problem solving are often impaired 196. Motor imagery 197, 198 and the recognition of negative facial expressions can also be disturbed in Locked-in Syndrome patients 199. Specific rehabilitation can benefit selected patients with cognitive deficits 200. Complicating factors like central deafness also have to be considered when evaluating a patient to avoid underestimating the patient’s capacity 201. Nevertheless, even after years in Locked-in Syndrome, patients can show preserved cognitive abilities 202, 203, 204.
Brain computer interfaces (BCIs) and other technical support systems are also highly important in the care of Locked-in Syndrome patients 178. EEG is the most commonly used method in brain computer interfaces (BCIs), and the control signals are either evoked, spontaneous or hybrid 205. Especially patients with less residual movements possibilities consider the use of brain computer interfaces (BCIs), the most important application being direct personal communication 206. In one survey, 63% of patients used high-tech assistive devices as their main way of communication 54. Personalized brain computer interfaces (BCIs) 207 and even controlling a humanoid robot 208 or exoskeleton 209 with brain computer interface are investigated. However, brain computer interfaces (BCIs) have some usability challenges as a lot of time is needed for training, the usage can be very fatiguing and restricted to special settings 205. Moreover, motor impairments were associated with difficulties in motor imagery, which could cause problems with some brain computer interface applications 210 and even in healthy individuals, motor imagery caused significant power changes in EEG only in half of the sample 211. It was shown in spinal cord injury patients that brain activity can change after injury which might interfere with brain computer interface (BCI) application designed for healthy brains and not the individually altered brains 212. Nevertheless, progress in brain computer interface technology is rapidly advancing and promises advantages for many patients 213, 214.
Follow-ups and re-assessments should be done periodically as new technologies are constantly emerging and to adapt and improve the current treatment and care of a Locked-in Syndrome patient 194. As one study stated 18, nearly half of the patients included did not receive any kind of treatment, which indicates that the full potential of recovery might often be missed. Studies found that areas of unfulfilled needs especially concerned the fields of information, respect and specialized technical aids 215, 216. It is important to emphasize that treatment must not be withhold, but should be as vigorous as for other patients 4.
Finally, recent studies and articles in the medical literature have noted that despite significant motor disability Locked-in Syndrome individuals can retain a good quality of life. In addition, quality of life is unrelated to the degree of physical impairment. With advances in care and assistive technologies, individuals with Locked-in Syndrome can become productive members of society.
Locked-in Syndrome prognosis
Early literature on Locked-in Syndrome prognosis, primarily relied on autopsy findings, reported that long term survival was rare without neurological recovery. In 1986, Locked-in Syndrome prognosis mortality was estimated at 60%, being greatest in the first four months and higher in patients with vascular insult than non-vascular causes 43. Survivors tended to be younger at age of onset. Early rehabilitation and more effective nursing care have recently been reported to reduce mortality from acute Locked-in Syndrome 183. Casanova et al 183 reported that patients with Locked-in Syndrome who began rehabilitation within one month of the acute event had a mortality of only 14% at five years. Although most survivors remain either in a chronic locked-in state or severely impaired, early signs of recovery can be exploited through multidisciplinary rehabilitation 72, 73, 55, 43, 75, 183. In a rehabilitation programme, therapists monitor for recovery of thumb, finger, head, and neck movement; evidence of independent swallow; and improvement in respiratory function. Any movement that may enable the patient to use a buzzer, an environmental control, or a communication device is targeted 217.
Patients initially tire quickly when using vertical eye movement to communicate 42. Furthermore, their attention span may be severely limited in the first few weeks or months 42. An agreed system of interpretation is necessary, where one upward movement signifies “Yes” and two rapid upward movements, “No”. Effective questioning skills must be developed, avoiding open ended questions and confirming answers by repeating questions when necessary.
Aggressive treatment of infections, respiratory difficulties, pain, or localized problems such as corneal abrasions can enhance physical stamina and communication 42. Because patients with Classic Locked-in Syndrome are unable to call for attention or initiate conversation, they should frequently be given the opportunity to communicate and end dialogue 42.
The only aspect of locked-in syndrome recovery for which a classification has been found is motor recovery 43.
Classification of recovery of motor function in Locked-in Syndrome 43:
- No recovery: No return of motor function, total dependence for all activities of daily living
- Minimal recovery: Minimal motor return, total dependence for all activities of daily living
- Moderate recovery: Moderate motor return, independence in some but not all activities of daily living
- Full recovery: Independence in all activities of daily living but some minimal neurological deficit
- No neurological deficit: No reported residual deficits
No specific classification systems exist for vocal, dysphagia, cognitive, emotional, or behavioural recoveries. A retrospective review of 53 patients with other brain stem strokes used the modified Barthel index to measure functional outcomes such as limb weakness, ataxia, dysarthria, dysphasia, and urinary continence, but emotional and behavioral recoveries remain unclear 218.
Table 1 summarises the findings from three studies examining life expectancy and functional recovery in patients with locked-in syndrome 183, 55, 75. With return of greater respiratory effort, some swallowing ability, and improved continence, the need for tracheostomies, gastrostomies, and urinary catheters reduces with time 183, 55. All patients with locked-in syndrome should be rehabilitated in a national or regional specialist center that has specific multidisciplinary rehabilitation experience with locked-in syndrome.
Many patients choose to return to live at home, which presumably enables greater social interaction with family and friends 183, 55, 75. Return to home life may positively influence the patient’s desire to live 75. However, it places a long term physical and psychological burden on the family. Limited funding means that community care is often scarce and the carers are poorly supported.
Only two references to patients who returned to work. The first was a lawyer who used morse code blinks to provide legal opinions and the second was someone who taught maths and spelling using a mouth stick to trigger an electronic voice device 75.
Table 1. Findings from three case series of patients with Locked-in Syndrome
| Variable | Doble 2003 (USA) | Casanova 2003 (Italy) | Richard 1995 (France) |
|---|---|---|---|
| Mean age of onset (years) | 33.6 | 44.7 | 45.3 |
| Age range at onset (years) | 01/01/70 | 16-71 | 17-73 |
| No of males/females | 19/10/23 | 09/05/23 | 09/02/23 |
| Cause: | |||
| Vascular | 15 (52%) | 11 (79%) | 10 (91%) |
| Traumatic | 9 (31%) | 3 (21%) | 1 (9%) |
| Other | 5 (17%) | 0 | 0 |
| Survival rates (%): | |||
| 5 years | 83 | 86 | NA |
| 10 years | 83 | NA | NA |
| 20 years | 40 | NA | NA |
| Motor recovery | 16 (55%) | 11 (79%) | 10 (91%) |
| Respiratory improvement | NA | 8 (58%) | 10 (91%) |
| Emergence of swallow | 20 (69%) | 6 (42%) | 8 (73%) |
| Basic verbal communication | 9 (31%) | 4 (28%) | 4 (36%) |
| Bowel continence | 4 (14%) | 5 (35%) | NA |
| Bladder continence | 7 (24%) | 5 (35%) | 10 (100%) |
| Returned to live with family | 19 (65%) | 8 (57%) | 8 (73%) |
Footnotes: Values are numbers (percentages) unless stated otherwise.
[Sources 183, 55, 75 ]Quality of life
Although the Locked-in Syndrome appears as the most dramatic form of motor disability one can imagine, some scientific reports indicate that the quality of life of patients is not so poor as expected. A recent survey investigated the self-reported quality of life of chronic patients with locked-in syndrome and concluded that many patients have a happy and meaningful life, especially when proper social services help patients to have a normal role at home as well as in the community 183, 55, 75.
Locked-in Syndrome survival rate
Given the severity of the neurologic impairment in Locked-in Syndrome, clinicians are frequently confronted with questions regarding Locked-in Syndrome long-term morbidity and mortality. Once a Locked-in Syndrome patient becomes medically stable and receives appropriate medical care, life expectancy increases by several decades 19. One study revealed that 87% of Locked-in Syndrome patients die within the first four months 43. Other studies have shown long-term survival with a 10 and 20-year survival of 83% and 40%, respectively 75, 219, 19. Advances in medical technology, such as mechanical ventilation, allow prolongation of life in Locked-in Syndrome patients 220. The elapsed time between brain injury and Locked-in Syndrome diagnosis is precious, and finding the appropriate treatment both efficiently and effectively is of the upmost importance 9. Available resources and cultural differences likely contribute to the discrepancy in Locked-in Syndrome long-term morbidity and mortality.
The French Association for Locked-In Syndrome database (n = 250) showed that 65% of patients eventually removed their tracheostomy tube, and 58% eventually removed their gastrostomy tube 19. However, patients with Locked-in Syndrome often have limited motor recovery of their arms and legs, even with long-term survival 43, 75.
Despite the presence of persistent neurologic disability and low health-related quality of life, global quality of life studies have shown that Locked-in Syndrome patients have comparable quality of life to healthy controls as well as chronically ill patients 19, 221, 29. In addition, most studies have not found a correlation between the extent of physical impairment and depression 19, 222 although one study did 221. It appears that both global quality of life and depression may not be affected by the physical impairment in Locked-in Syndrome 19, 222.
References- Plum F, Posner JB. The diagnosis of stupor and coma. Contemp Neurol Ser. 1972;10:1-286.
- Schnetzer L, McCoy M, Bergmann J, Kunz A, Leis S, Trinka E. Locked-in syndrome revisited. Ther Adv Neurol Disord. 2023 Mar 29;16:17562864231160873. doi: 10.1177/17562864231160873
- Johncy N., Shabaraya A.R. Review on Locked-in Syndrome. IJRR. 2020;7:392–396.
- Laureys S, Pellas F, Van Eeckhout P, Ghorbel S, Schnakers C, Perrin F, Berré J, Faymonville ME, Pantke KH, Damas F, Lamy M, Moonen G, Goldman S. The locked-in syndrome : what is it like to be conscious but paralyzed and voiceless? Prog Brain Res. 2005;150:495-511. doi: 10.1016/S0079-6123(05)50034-7
- Maiser S., Kabir A., Sabsevitz D., Peltier W. Locked-In Syndrome: Case Report and Discussion of Decisional Capacity. J. Pain Symptom Manag. 2016;51:789–793. https://doi.org/10.1016/j.jpainsymman.2015.10.021
- Gallo U.E., Fontanarosa P.B. Locked-in Syndrome: Report of a Case. Am. J. Emerg. Med. 1989;7:581–583. doi: 10.1016/0735-6757(89)90278-7
- Schnakers C, Majerus S, Goldman S, Boly M, Van Eeckhout P, Gay S, Pellas F, Bartsch V, Peigneux P, Moonen G, Laureys S. Cognitive function in the locked-in syndrome. J Neurol. 2008 Mar;255(3):323-30. doi: 10.1007/s00415-008-0544-0
- León-Carrión J., Van Eeckhout P., Domínguez-Morales M.d.R., Pérez-Santamaría F.J. Survey: The Locked-in Syndrome: A Syndrome Looking for a Therapy. Brain Inj. 2002;16:571–582. doi: 10.1080/02699050110119781
- Bonin EAC, Delsemme Z, Blandin V, Alnagger NL, Thibaut A, Faymonville ME, Laureys S, Vanhaudenhuyse A, Gosseries O. French Survey on Pain Perception and Management in Patients with Locked-In Syndrome. Diagnostics (Basel). 2022 Mar 21;12(3):769. doi: 10.3390/diagnostics12030769
- Demertzi A., Racine E., Bruno M.-A., Ledoux D., Gosseries O., Vanhaudenhuyse A., Thonnard M., Soddu A., Moonen G., Laureys S. Pain Perception in Disorders of Consciousness: Neuroscience, Clinical Care, and Ethics in Dialogue. Neuroethics. 2013;6:37–50. doi: 10.1007/s12152-011-9149-x
- Giacino J.T., Ashwal S., Childs N., Cranford R., Jennett B., Katz D.I., Kelly J.P., Rosenberg J.H., Whyte J., Zafonte R.D., et al. The Minimally Conscious State: Definition and Diagnostic Criteria. Neurology. 2002;58:349–353. doi: 10.1212/WNL.58.3.349
- Laureys S., Celesia G.G., Cohadon F., Lavrijsen J., León-Carrión J., Sannita W.G., Sazbon L., Schmutzhard E., von Wild K.R., Zeman A., et al. Unresponsive Wakefulness Syndrome: A New Name for the Vegetative State or Apallic Syndrome. BMC Med. 2010;8:68. doi: 10.1186/1741-7015-8-68
- Schnakers C., Majerus S., Laureys S. Diagnostic et évaluation des états de conscience altérée. Diagnosis and investigation of altered states of consciousness. Reanimation. 2004;13:368–375. doi: 10.1016/j.reaurg.2004.03.019
- Recommendations for use of uniform nomenclature pertinent to patients with severe alterations in consciousness. American Congress of Rehabilitation Medicine. Arch Phys Med Rehabil. 1995 Feb;76(2):205-9. doi: 10.1016/s0003-9993(95)80031-x. Erratum in: Arch Phys Med Rehabil 1995 Apr;76(4):397.
- Bauer G, Gerstenbrand F, Rumpl E. Varieties of the locked-in syndrome. J Neurol. 1979 Aug;221(2):77-91. doi: 10.1007/BF00313105
- Seidl M, Golaszewski S, Kunz A, et al. The locked-in plus syndrome. J Neurol Sci 2013; 333: e263–e264.
- Gallo UE, Fontanarosa PB. Locked-in syndrome: report of a case. Am J Emerg Med. 1989 Nov;7(6):581-3. https://doi.org/10.1016/0735-6757(89)90278-7
- León-Carrión J, van Eeckhout P, Domínguez-Morales Mdel R, Pérez-Santamaría FJ. The locked-in syndrome: a syndrome looking for a therapy. Brain Inj. 2002 Jul;16(7):571-82. doi: 10.1080/02699050110119781
- Laureys S, Pellas F, Van Eeckhout P, Ghorbel S, Schnakers C, Perrin F, Berré J, Faymonville ME, Pantke KH, Damas F, Lamy M, Moonen G, Goldman S. The locked-in syndrome : what is it like to be conscious but paralyzed and voiceless? Prog Brain Res. 2005;150:495-511. https://doi.org/10.1016/S0079-6123(05)50034-7
- León-Carrión J., van Eeckhout P., Domínguez-Morales M.d.R. Review of Subject: The Locked-in Syndrome: A Syndrome Looking for a Therapy. Brain Inj. 2002;16:555–569. doi: 10.1080/02699050110119466
- Michaelides A., Zis P. Depression, Anxiety and Acute Pain: Links and Management Challenges. Postgrad. Med. 2019;131:438–444. doi: 10.1080/00325481.2019.1663705
- Turk D.C., Wilson H.D., Cahana A. Treatment of Chronic Non-Cancer Pain. Lancet. 2011;377:2226–2235. doi: 10.1016/S0140-6736(11)60402-9
- Bouhassira D. Neuropathic Pain: Definition, Assessment and Epidemiology. Rev. Neurol. 2019;175:16–25. doi: 10.1016/j.neurol.2018.09.016
- Pistoia F., Sacco S., Sarà M., Franceschini M., Carolei A. Intrathecal Baclofen: Effects on Spasticity, Pain, and Consciousness in Disorders of Consciousness and Locked-in Syndrome. Curr. Pain Headache Rep. 2015;19:466. doi: 10.1007/s11916-014-0466-8
- Boly M., Faymonville M.-E., Schnakers C., Peigneux P., Lambermont B., Phillips C., Lancellotti P., Luxen A., Lamy M., Moonen G., et al. Perception of Pain in the Minimally Conscious State with PET Activation: An Observational Study. Lancet Neurol. 2008;7:1013–1020. doi: 10.1016/S1474-4422(08)70219-9
- Laureys S., Faymonville M.E., Peigneux P., Damas P., Lambermont B., Del Fiore G., Degueldre C., Aerts J., Luxen A., Franck G., et al. Cortical Processing of Noxious Somatosensory Stimuli in the Persistent Vegetative State. NeuroImage. 2002;17:732–741. doi: 10.1006/nimg.2002.1236
- Giacino J.T., Katz D.I., Schiff N.D., Whyte J., Ashman E.J., Ashwal S., Barbano R., Hammond F.M., Laureys S., Ling G.S.F., et al. Practice Guideline Update Recommendations Summary: Disorders of Consciousness. Arch. Phys. Med. Rehabil. 2018;99:1699–1709. doi: 10.1016/j.apmr.2018.07.001
- Calabrò R.S., Naro A., Manuli A., Leo A., De Luca R., Lo Buono V., Russo M., Bramanti A., Bramanti P. Pain Perception in Patients with Chronic Disorders of Consciousness: What Can Limbic System Tell Us? Clin. Neurophysiol. 2017;128:454–462. doi: 10.1016/j.clinph.2016.12.011
- Bruno M.-A., Bernheim J.L., Ledoux D., Pellas F., Demertzi A., Laureys S. A Survey on Self-Assessed Well-Being in a Cohort of Chronic Locked-in Syndrome Patients: Happy Majority, Miserable Minority. BMJ Open. 2011;1:e000039. doi: 10.1136/bmjopen-2010-000039
- Bergés I.-M., Ottenbacher K.J., Kuo Y.-F., Smith P.M., Smith D., Ostir G.V. Satisfaction With Quality of Life Poststroke: Effect of Sex Differences in Pain Response. Arch. Phys. Med. Rehabil. 2007;88:413–417. doi: 10.1016/j.apmr.2006.12.022
- Skevington S.M. Investigating the Relationship between Pain and Discomfort and Quality of Life, Using the WHOQOL. Pain. 1998;76:395–406. doi: 10.1016/S0304-3959(98)00072-4
- Glare P.A., Davies P.S., Finlay E., Gulati A., Lemanne D., Moryl N., Oeffinger K.C., Paice J.A., Stubblefield M.D., Syrjala K.L. Pain in Cancer Survivors. JCO. 2014;32:1739–1747. doi: 10.1200/JCO.2013.52.4629
- Westerlind E., Singh R., Persson H.C., Sunnerhagen K.S. Experienced Pain after Stroke: A Cross-Sectional 5-Year Follow-up Study. BMC Neurol. 2020;20:4. doi: 10.1186/s12883-019-1584-z
- Staud R., Rodriguez M.E. Mechanisms of Disease: Pain in Fibromyalgia Syndrome. Nat. Rev. Rheumatol. 2006;2:90–98. doi: 10.1038/ncprheum0091
- Sacco S, Sarà M, Pistoia F, Conson M, Albertini G, Carolei A. Management of pathologic laughter and crying in patients with locked-in syndrome: a report of 4 cases. Arch Phys Med Rehabil. 2008 Apr;89(4):775-8. doi: 10.1016/j.apmr.2007.09.032
- Pistoia F, Sacco S, Sarà M, Franceschini M, Carolei A. Intrathecal baclofen: effects on spasticity, pain, and consciousness in disorders of consciousness and locked-in syndrome. Curr Pain Headache Rep. 2015 Jan;19(1):466. doi: 10.1007/s11916-014-0466-8
- Cairns K, Stein J. Motor function improvement following intrathecal baclofen pump placement in a patient with locked-in syndrome. Am J Phys Med Rehabil. 2002 Apr;81(4):307-9. doi: 10.1097/00002060-200204000-00013
- Locked In Syndrome. https://rarediseases.org/rare-diseases/locked-in-syndrome
- Kohnen RF, Lavrijsen JC, Bor JH, Koopmans RT. The prevalence and characteristics of patients with classic locked-in syndrome in Dutch nursing homes. J Neurol. 2013 Jun;260(6):1527-34. doi: 10.1007/s00415-012-6821-y
- Association du Locked-in Syndrome (ALIS). Qu’est-ce que le Locked-In Syndrome? https://alis-asso.fr/quest-ce-que-le-lis
- Thadani VM, Rimm DL, Urquhart L, Fisher L, Williamson PD, Enriquez R, Kim JH, Levy LL. ‘Locked-in syndrome’ for 27 years following a viral illness: clinical and pathologic findings. Neurology. 1991 Apr;41(4):498-500. doi: 10.1212/wnl.41.4.498
- Smith E, Delargy M. Locked-in syndrome. BMJ. 2005 Feb 19;330(7488):406-9. doi: 10.1136/bmj.330.7488.406
- Patterson JR, Grabois M. Locked-in syndrome: a review of 139 cases. Stroke. 1986 Jul-Aug;17(4):758-64. https://doi.org/10.1161/01.STR.17.4.758
- Nikić PM, Jovanović D, Paspalj D, Georgievski-Brkić B, Savić M. Clinical characteristics and outcome in the acute phase of ischemic locked-in syndrome: case series of twenty patients with ischemic LIS. Eur Neurol. 2013;69(4):207-12. doi: 10.1159/000345272
- Rae-Grant AD, Lin F, Yaeger BA, Barbour P, Levitt LP, Castaldo JE, Lester MC. Post traumatic extracranial vertebral artery dissection with locked-in syndrome: a case with MRI documentation and unusually favourable outcome. J Neurol Neurosurg Psychiatry. 1989 Oct;52(10):1191-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1031707/pdf/jnnpsyc00532-0071.pdf
- Barbieri F, Sinisi L, Cirillo S, Delehaje L, Mansi D. Long-term observation of two cases of locked-in syndrome with recovery. Clinical and CT-scan features. Clin Neurol Neurosurg. 1987;89(3):177-80. doi: 10.1016/s0303-8467(87)80051-3
- Sedney CL, Coger BR, Bailes JE. Posterior fossa subdural hematoma resulting in locked-in syndrome: case report. Neurosurgery. 2011 Aug;69(2):E497-500. doi: 10.1227/NEU.0b013e318218cf85
- Horan J, Tromp S, Mankahla N. Transient Incomplete Locked-In Syndrome Secondary to Supratentorial Gunshot Wound. World Neurosurg. 2019 Jun;126:560-563. doi: 10.1016/j.wneu.2019.03.163
- Buchman AS, Wichter MD. Recovery following the “locked-in” syndrome. Stroke. 1986 May-Jun;17(3):558.
- Khurana RK, Genut AA, Yannakakis GD. Locked-in syndrome with recovery. Ann Neurol. 1980 Oct;8(4):439-41. doi: 10.1002/ana.410080418
- Fujimoto Y, Ohnishi Y, Wakayama A, Yoshimine T. Transient total mesencephalic locked-in syndrome after bilateral ptosis due to basilar artery thrombosis. J Stroke Cerebrovasc Dis. 2012 Nov;21(8):909.e7-8. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.016
- McCusker EA, Rudick RA, Honch GW, Griggs RC. Recovery from the ‘locked-in’ syndrome. Arch Neurol. 1982 Mar;39(3):145-7. doi: 10.1001/archneur.1982.00510150015004
- Kwon HG, Jang SH. Motor recovery mechanism in a quadriplegic patient with locked-in syndrome. NeuroRehabilitation. 2012;30(2):113-7. doi: 10.3233/NRE-2012-0734
- Lugo ZR, Bruno MA, Gosseries O, Demertzi A, Heine L, Thonnard M, Blandin V, Pellas F, Laureys S. Beyond the gaze: Communicating in chronic locked-in syndrome. Brain Inj. 2015;29(9):1056-61. doi: 10.3109/02699052.2015.1004750
- Richard I, Péreon Y, Guiheneu P, Nogues B, Perrouin-Verbe B, Mathe JF. Persistence of distal motor control in the locked in syndrome. Review of 11 patients. Paraplegia. 1995 Nov;33(11):640-6. doi: 10.1038/sc.1995.135
- Tomycz ND, Holm MB, Horowitz MB, Wechsler LR, Raina K, Gupta R, Jovin TG. Extensive brainstem ischemia on neuroimaging does not preclude meaningful recovery from locked-in syndrome: two cases of endovascularly managed basilar thrombosis. J Neuroimaging. 2008 Jan;18(1):15-7. doi: 10.1111/j.1552-6569.2007.00147.x
- Tung RC, Vivar-Cruz PW. Locked-In Syndrome. Kans J Med. 2019 May 15;12(2):56. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6527196
- Kumral E, Dorukoğlu M, Uzunoğlu C, Çetin FE. The clinical and cognitive spectrum of locked-in syndrome: 1-year follow-up of 100 patients. Acta Neurol Belg. 2022 Feb;122(1):113-121. doi: 10.1007/s13760-021-01675-5
- Garrard P, Bradshaw D, Jäger HR, Thompson AJ, Losseff N, Playford D. Cognitive dysfunction after isolated brain stem insult. An underdiagnosed cause of long term morbidity. J Neurol Neurosurg Psychiatry. 2002 Aug;73(2):191-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1737986/pdf/v073p00191.pdf
- New PW, Thomas SJ. Cognitive impairments in the locked-in syndrome: a case report. Arch Phys Med Rehabil. 2005 Feb;86(2):338-43. doi: 10.1016/j.apmr.2004.09.005
- Ortiz de Mendivil A, Alcalá-Galiano A, Ochoa M, Salvador E, Millán JM. Brainstem stroke: anatomy, clinical and radiological findings. Semin Ultrasound CT MR. 2013 Apr;34(2):131-41. doi: 10.1053/j.sult.2013.01.004
- Percheron G. The anatomy of the arterial supply of the human thalamus and its use for the interpretation of the thalamic vascular pathology. Z Neurol. 1973 Aug 29;205(1):1-13. doi: 10.1007/BF00315956
- Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003 Sep;34(9):2264-78. doi: 10.1161/01.STR.0000087786.38997.9E
- Powell R, Hughes T. A chamber of secrets. The neurology of the thalamus: lessons from acute stroke. Pract Neurol. 2014 Dec;14(6):440-5. doi: 10.1136/practneurol-2014-000852
- Carvalho V, Cruz VT. Clinical presentation of vertebrobasilar stroke. Porto Biomed J. 2020 Nov 24;5(6):e096. doi: 10.1097/j.pbj.0000000000000096
- Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105-18. doi: 10.1196/annals.1417.029
- Redinbaugh MJ, Phillips JM, Kambi NA, Mohanta S, Andryk S, Dooley GL, Afrasiabi M, Raz A, Saalmann YB. Thalamus Modulates Consciousness via Layer-Specific Control of Cortex. Neuron. 2020 Apr 8;106(1):66-75.e12. doi: 10.1016/j.neuron.2020.01.005
- Hindman J, Bowren MD, Bruss J, Wright B, Geerling JC, Boes AD. Thalamic strokes that severely impair arousal extend into the brainstem. Ann Neurol. 2018 Dec;84(6):926-930. doi: 10.1002/ana.25377
- Fischer DB, Boes AD, Demertzi A, Evrard HC, Laureys S, Edlow BL, Liu H, Saper CB, Pascual-Leone A, Fox MD, Geerling JC. A human brain network derived from coma-causing brainstem lesions. Neurology. 2016 Dec 6;87(23):2427-2434. doi: 10.1212/WNL.0000000000003404
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949 Nov;1(4):455-73.
- Edlow BL, Takahashi E, Wu O, Benner T, Dai G, Bu L, Grant PE, Greer DM, Greenberg SM, Kinney HC, Folkerth RD. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012 Jun;71(6):531-46. doi: 10.1097/NEN.0b013e3182588293
- Haig AJ, Katz RT, Sahgal V. Mortality and complications of the locked-in syndrome. Arch Phys Med Rehabil. 1987 Jan;68(1):24-7.
- Katz RT, Haig AJ, Clark BB, DiPaola RJ. Long-term survival, prognosis, and life-care planning for 29 patients with chronic locked-in syndrome. Arch Phys Med Rehabil. 1992 May;73(5):403-8. https://alis-asso.fr/wp-content/uploads/2019/03/long-term_su4dae1.pdf
- Casanova E, Lazzari RE, Lotta S, Mazzucchi A. Locked-in syndrome: improvement in the prognosis after an early intensive multidisciplinary rehabilitation. Arch Phys Med Rehabil. 2003 Jun;84(6):862-7. doi: 10.1016/s0003-9993(03)00008-x
- Doble, Jennifer E. MD; Haig, Andrew J. MD; Anderson, Christopher DO; Katz, Richard MD. Impairment, Activity, Participation, Life Satisfaction, and Survival in Persons With Locked-In Syndrome for Over a Decade: Follow-Up on a Previously Reported Cohort. Journal of Head Trauma Rehabilitation 18(5):p 435-444, September 2003. doi: 10.1097/00001199-200309000-00005
- García-Esperón C, López-Cancio E, Martín-Aguilar L, Millán M, Castaño C, Munuera J, Dávalos A. Fluctuating locked-in syndrome as a presentation of a bilateral pontine infarction. Neuroradiol J. 2016 Oct;29(5):347-9. doi: 10.1177/1971400916658896
- Saposnik G, Noel de Tilly L, Caplan LR. Pontine warning syndrome. Arch Neurol. 2008 Oct;65(10):1375-7. doi: 10.1001/archneur.65.10.1375
- Lynch JM, Hennessy MJ. Third nerve palsy: Harbinger of basilar artery thrombosis and locked-in syndrome? J Stroke Cerebrovasc Dis. 2005 Jan-Feb;14(1):42-3. doi: 10.1016/j.jstrokecerebrovasdis.2004.09.003
- Leonard M, Renard F, Harsan L, Pottecher J, Braun M, Schneider F, Froehlig P, Blanc F, Roquet D, Achard S, Meyer N, Kremer S. Diffusion tensor imaging reveals diffuse white matter injuries in locked-in syndrome patients. PLoS One. 2019 Apr 10;14(4):e0213528. doi: 10.1371/journal.pone.0213528
- Sarà M, Cornia R, Conson M, Carolei A, Sacco S, Pistoia F. Cortical Brain Changes in Patients With Locked-In Syndrome Experiencing Hallucinations and Delusions. Front Neurol. 2018 May 17;9:354. doi: 10.3389/fneur.2018.00354
- Cotton F, Ciancia S, Tell L, Lachaise L, Braun M, Rode G. Degeneration of the Arnold’s prefrontopontocerebellar tract in a case of locked-in syndrome over a 23-year period. J Neuroradiol. 2011 May;38(2):118-24. doi: 10.1016/j.neurad.2010.09.004
- Babiloni C, Pistoia F, Sarà M, Vecchio F, Buffo P, Conson M, Onorati P, Albertini G, Rossini PM. Resting state eyes-closed cortical rhythms in patients with locked-in-syndrome: an EEG study. Clin Neurophysiol. 2010 Nov;121(11):1816-24. doi: 10.1016/j.clinph.2010.04.027
- Tan X, Sun Y, Gao J. Investigating Structure-Function Connectivity in a Patient With Locked-In Syndrome by 7 T Magnetic Resonance Imaging: A Case Report. Neurologist. 2022 Nov 1;27(6):367-372. doi: 10.1097/NRL.0000000000000424
- Inatomi Y, Nakajima M, Yonahara T. Transient total locked-in syndrome due to vertebral and basilar artery dissection. BMJ Case Rep. 2021 Feb 22;14(2):e238912. doi: 10.1136/bcr-2020-238912
- Nadour W, Goldwasser B, Biederman RW, Taffe K. Silent aortic dissection presenting as transient locked-in syndrome. Tex Heart Inst J. 2008;35(3):359-61. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2565549
- Fox C, Lavin M. Vertebral artery dissection resulting in locked-in syndrome. J Neurosci Nurs. 1991 Oct;23(5):287-9. doi: 10.1097/01376517-199110000-00003
- Odabaşi Z, Kütükçü Y, Gökçil Z, Vural O, Yardim M. Traumatic basilar artery dissection causing locked-in syndrome. Minim Invasive Neurosurg. 1998 Mar;41(1):46-8. doi: 10.1055/s-2008-1052015
- Arunodaya GR, Vani S, Shankar SK, Taly AB, Swamy HS, Sarala D. Fibromuscular dysplasia with dissection of basilar artery presenting as “locked-in-syndrome”. Neurology. 1997 Jun;48(6):1605-8. doi: 10.1212/wnl.48.6.1605
- Orsini GG, Metaxas GE, Legros V. Locked-In Syndrome Following Cervical Manipulation by a Chiropractor: A Case Report. J Crit Care Med (Targu Mures). 2019 Aug 9;5(3):107-110. doi: 10.2478/jccm-2019-0014
- Povlsen UJ, Kjaer L, Arlien-Søborg P. Locked-in syndrome following cervical manipulation. Acta Neurol Scand. 1987 Dec;76(6):486-8. doi: 10.1111/j.1600-0404.1987.tb03607.x
- Horn SW 2nd. The “Locked-In” syndrome following chiropractic manipulation of the cervical spine. Ann Emerg Med. 1983 Oct;12(10):648-50. doi: 10.1016/s0196-0644(83)80217-0
- Ke JQ, Yin B, Fu FW, Shao SM, Lin Y, Dong QQ, Wang XT, Zheng GQ. A Case Report of Locked-in Syndrome Due to Bilateral Vertebral Artery Dissection After Cervical Spine Manipulation Treated by Arterial Embolectomy. Medicine (Baltimore). 2016 Feb;95(5):e2693. doi: 10.1097/MD.0000000000002693
- Lacroix G, Couret D, Combaz X, Prunet B, Girard N, Bruder N. Transient locked-in syndrome and basilar artery vasospasm. Neurocrit Care. 2012 Feb;16(1):145-7. doi: 10.1007/s12028-011-9655-z
- Sulkava R, Kovanen J. Locked-in syndrome with rapid recovery: a manifestation of basilar artery migraine? Headache. 1983 Sep;23(5):238-9. doi: 10.1111/j.1526-4610.1983.hed2305238.x
- Kolić Z, Kukuljan M, Vukas D, Bonifačić D, Vrbanec K, Franić IK. Locked-in syndrome in a patient with acute obstructive hydrocephalus, caused by large unruptured aneurysm of the basilar artery (BA). Br J Neurosurg. 2017 Dec;31(6):738-740. doi: 10.1080/02688697.2016.1229755
- Fujiyama K, Motomura M, Shirabe S, Nakamura T, Isomoto I, Shibayama K, Nagasato K, Yoshimura T, Tsujihata M, Nagataki S. Locked-in syndrome and abnormal orientation of the right vertebral artery in a young man. Intern Med. 1994 Aug;33(8):476-80. doi: 10.2169/internalmedicine.33.476
- Maramattom BV, Bhattacharjee S. Decerebrate rigidity with preservation of consciousness in pontine hemorrhage with complete neurologic recovery. Neurol India. 2019 May-Jun;67(3):881-883. doi: 10.4103/0028-3886.263170
- Shanmuganathan M, Warlow CP, Al-Shahi Salman R, Keston P, Sellar RJ, Fouyas IP. Unlocking the ‘locked-in syndrome’. Br J Neurosurg. 2011 Feb;25(1):109-10. doi: 10.3109/02688697.2010.542838
- Schoenmaker RT. Locked-in syndrome caused by a megadolicho vascular malformation of the basilar artery. Clin Neurol Neurosurg. 1984;86(3):159-62. doi: 10.1016/0303-8467(84)90191-4
- Al-Sardar H, Grabau W. Locked-in syndrome caused by basilar artery ectasia. Age Ageing. 2002 Nov;31(6):481-2. doi: 10.1093/ageing/31.6.481
- Lui YW, Law M, Jafar JJ, Douglas A, Nelson PK. Perfusion and diffusion tensor imaging in a patient with locked-in syndrome after neurosurgical vascular bypass and endovascular embolization of a basilar artery aneurysm: case report. Neurosurgery. 2006 Apr;58(4):E794; discussion E794. doi: 10.1227/01.NEU.0000204893.07192.1F
- Khan SI, Beaujon W, Ross ED. Interpeduncular basilar aneurysm causing progressive locked-in syndrome: to coil or not to coil. Eur Neurol. 2008;60(3):159-61. doi: 10.1159/000145335
- Golubović V, Muhvić D, Golubović S. Posttraumatic locked-in syndrome with an unusual three day delay in the appearance. Coll Antropol. 2004 Dec;28(2):923-6.
- Lagrand TJ, Bruijnes VAJ, Van der Stouwe AMM, Deckers EA, Mazuri A, Jacobs B. Locked-In Syndrome after Traumatic Basilar Artery Entrapment within a Clivus Fracture: A Case Report and Review of the Literature. Neurotrauma Rep. 2020 Sep 14;1(1):73-77. doi: 10.1089/neur.2020.0015
- Golubović V, Muhvić D, Golubović S, Juretić M, Tokmadzić VS. Two different manifestations of locked-in syndrome. Coll Antropol. 2013 Mar;37(1):313-6.
- Cabezudo JM, Olabe J, Lopez-Anguera A, Bacci F. Recovery from locked-in syndrome after posttraumatic bilateral distal vertebral artery occlusion. Surg Neurol. 1986 Feb;25(2):185-90. doi: 10.1016/0090-3019(86)90292-2
- Chang B, Morariu MA. Transient traumatic “locked-in” syndrome. Eur Neurol. 1979;18(6):391-4. doi: 10.1159/000115110
- Ahn ES, Aarabi B. Posttraumatic locked-in syndrome from a pontomedullary contusion: case report. J Trauma. 2007 Aug;63(2):420-3. doi: 10.1097/01.ta.0000174727.59241.cb
- Luxenberg EL, Goldenberg FD, Frank JI, Loch Macdonald R, Rosengart AJ. Locked-in syndrome from rostro-caudal herniation. J Clin Neurosci. 2009 Feb;16(2):333-5. doi: 10.1016/j.jocn.2007.10.009
- Jang SH, Chang CH, Jung YJ, Seo JP. Locked-in Syndrome Due to Transtentorial Herniation and Kernohan Notch Phenomenon. Am J Phys Med Rehabil. 2017 Apr;96(4):e77. doi: 10.1097/PHM.0000000000000596
- Wijdicks EF, Miller GM. Transient locked-in syndrome after uncal herniation. Neurology. 1999 Apr 12;52(6):1296-7. doi: 10.1212/wnl.52.6.1296-a
- Keane JR, Itabashi HH. Locked-in syndrome due to tentorial herniation. Neurology. 1985 Nov;35(11):1647-9. doi: 10.1212/wnl.35.11.1647
- Habre W, Caflisch M, Chaves-Vischer V, Delavelle J, Haenggeli CA. Locked-in syndrome in an adolescent patient with pneumococcal meningitis. Neuropediatrics. 1996 Dec;27(6):323-5. doi: 10.1055/s-2007-973802
- Masuzawa H, Sato J, Kamitani H, Kamikura T, Aoki N. Pontine gliomas causing locked-in syndrome. Childs Nerv Syst. 1993 Aug;9(5):256-9. doi: 10.1007/BF00306266
- Cherington M, Stears J, Hodges J. Locked-in syndrome caused by a tumor. Neurology. 1976 Feb;26(2):180-2. doi: 10.1212/wnl.26.2.180
- Inci S, Ozgen T. Locked-in syndrome due to metastatic pontomedullary tumor–case report. Neurol Med Chir (Tokyo). 2003 Oct;43(10):497-500. doi: 10.2176/nmc.43.497
- Breen P, Hannon V. Locked-in syndrome: a catastrophic complication after surgery. Br J Anaesth. 2004 Feb;92(2):286-8. doi: 10.1093/bja/aeh034
- Poston JN, Dorer R, Aboulafia DM. The Diving Bell and the Butterfly Revisited: A Fatal Case of Locked-in Syndrome in a Man With Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma, Not Otherwise Specified. Clin Med Insights Blood Disord. 2018 Mar 26;11:1179545X18762799. doi: 10.1177/1179545X18762799
- Keme-Ebi IK, Asindi AA. Locked-in Syndrome in a Nigerian male with Multiple Sclerosis: a case report and literature review. Pan Afr Med J. 2008 Oct 30;1:4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2984268
- Blunt SB, Boulton J, Wise R, Kennard C, Lewis PD. Locked-in syndrome in fulminant demyelinating disease. J Neurol Neurosurg Psychiatry. 1994 Apr;57(4):504-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1072887/pdf/jnnpsyc00034-0104.pdf
- Forti A, Ambrosetto G, Amore M, De Maria R, Michelucci R, Omicini E, Rizzuto N, Fenzi F, Tassinari CA. Locked-in syndrome in multiple sclerosis with sparing of the ventral portion of the pons. Ann Neurol. 1982 Oct;12(4):393-4. doi: 10.1002/ana.410120413
- Sohn MK, Nam JH. Locked-in Syndrome due to Central Pontine Myelinolysis: Case Report. Ann Rehabil Med. 2014 Oct;38(5):702-6. doi: 10.5535/arm.2014.38.5.702
- Heckmann JG, Dinkel HP. Recovery of locked-in syndrome in central pontine myelinolysis. Am J Case Rep. 2013 Jun 26;14:219-20. doi: 10.12659/AJCR.889378
- El Moghazy W, Gala-Lopez B, Wong W, Kneteman N. Recovery of locked-in syndrome following liver transplantation with calcineurin inhibitor cessation and supportive treatment. Am J Case Rep. 2013;14:16-9. doi: 10.12659/AJCR.883748
- Murphy MJ, Brenton DW, Aschenbrener CA, Van Gilder JC. Locked-in syndrome caused by a solitary pontine abscess. J Neurol Neurosurg Psychiatry. 1979 Nov;42(11):1062-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC490403/pdf/jnnpsyc00091-0090.pdf
- Young NP, Dyck PJ, Wijdicks EF. Locked-in syndrome due to invasive fungal rhinosinusitis in an immunosuppressed patient. Neurologist. 2007 May;13(3):158-60. doi: 10.1097/01.nrl.0000263701.15487.97
- Yokota Y, Ishihara M, Ninomiya S, Mitsuke K, Kamei S, Nakajima H. Locked-in Syndrome Due to Meningovascular Syphilis: A Case Report and Literature Review. Intern Med. 2022 May 15;61(10):1593-1598. doi: 10.2169/internalmedicine.8269-21
- Hantson P, Wittebole X, Rombaux P, Cosnard G. Locked-in syndrome as an unusual complication of acute otitis media. B-ENT. 2012;8(2):131-4.
- Sonkar SK, Kumar S, Singh NK, Tandon R. Hyperhomocysteinemia induced locked-in syndrome in a young adult due to folic acid deficiency. Nutr Neurosci. 2021 Oct;24(10):781-783. doi: 10.1080/1028415X.2019.1681064
- Ali O, Bueno MG, Duong-Pham T, Gunawardhana N, Tran DH, Chow RD, Verceles AC. Cocaine as a rare cause of locked-in syndrome: a case report. J Med Case Rep. 2019 Nov 19;13(1):337. doi: 10.1186/s13256-019-2278-2
- Negreiros dos Anjos M. “Locked in” syndrome following prolonged hypoglycemia. Diabetes Care. 1984 Nov-Dec;7(6):613. doi: 10.2337/diacare.7.6.613
- Han D, Connelly NR, Weintraub A, Kanev P, Solis E. Conversion locked-in syndrome after implantation of a spinal cord stimulator. Anesth Analg. 2007 Jan;104(1):163-5. doi: 10.1213/01.ane.0000250363.40356.b8
- Kuzma-Kozakiewicz M, Andersen PM, Ciecwierska K, Vázquez C, Helczyk O, Loose M, Uttner I, Ludolph AC, Lulé D. An observational study on quality of life and preferences to sustain life in locked-in state. Neurology. 2019 Sep 3;93(10):e938-e945. doi: 10.1212/WNL.0000000000008064
- Medici C, Gonzalez G, Cerisola A, Scavone C. Locked-in syndrome in three children with Guillain-Barré syndrome. Pediatr Neurol. 2011 Aug;45(2):125-8. doi: 10.1016/j.pediatrneurol.2011.03.005
- Korinthenberg R, Eckenweiler M, Fuchs H. Severe Locked-In-Like Guillain-Barré’s Syndrome: Dilemmas in Diagnosis and Treatment. Neuropediatrics. 2021 Feb;52(1):19-26. doi: 10.1055/s-0040-1715480
- Loeb C, Mancardi GL, Tabaton M. Locked-in syndrome in acute inflammatory polyradiculoneuropathy. Eur Neurol. 1984;23(2):137-40. doi: 10.1159/000115692
- Prakash S, Mathew C, Bhagat S. Locked-in syndrome in snakebite. J Assoc Physicians India. 2008 Feb;56:121-2.
- Kutiyal AS, Malik C, Hyanki G. Locked-in syndrome post snake bite: a rare presentation. Trop Doct. 2018 Jan;48(1):68-69. doi: 10.1177/0049475517726262
- Azad C, Mahajan V, Jat KR. Locked-in syndrome as a presentation of snakebite. Indian Pediatr. 2013 Jul;50(7):695-7. doi: 10.1007/s13312-013-0174-1
- Kenny DJ, Luke DA. ‘Locked-in’ syndrome. Occurrence after coronary artery bypass graft surgery. Anaesthesia. 1989 Jun;44(6):483-4. doi: 10.1111/j.1365-2044.1989.tb11374.x
- Chen JA, Driver J, Segar D, Bernstock JD, Gupta S, Gormley W. Medullary Infarction Leading to Locked-In Syndrome Following Lumbar Puncture in a Patient with Basilar Invagination. World Neurosurg. 2020 May;137:292-295. doi: 10.1016/j.wneu.2020.02.040
- McGuire TJ, Hodges DL, Medhat MA, Redford JB. Transient locked-in syndrome and phenobarbital. Arch Phys Med Rehabil. 1987 Sep;68(9):566-7.
- Tüz M, Erodlu F, Dodru H, Uygur K, Yavuz L. Transient locked-in syndrome resulting from stellate ganglion block in the treatment of patients with sudden hearing loss. Acta Anaesthesiol Scand. 2003 Apr;47(4):485-7. doi: 10.1034/j.1399-6576.2003.00076.x
- Chaturvedi A, Dash H. Locked-in syndrome during stellate ganglion block. Indian J Anaesth. 2010 Jul;54(4):324-6. doi: 10.4103/0019-5049.68376
- Dukes RR, Alexander LA. Transient locked-in syndrome after vascular injection during stellate ganglion block. Reg Anesth. 1993 Nov-Dec;18(6):378-80.
- Durrani Z, Winnie AP. Brainstem toxicity with reversible locked-in syndrome after intrascalene brachial plexus block. Anesth Analg. 1991 Feb;72(2):249-52. doi: 10.1213/00000539-199102000-00020
- Lee P. Locked-in syndrome as a result of cyclizine administration. J Pain Symptom Manage. 2013 May;45(5):e5-7. doi: 10.1016/j.jpainsymman.2013.02.005
- Sabzi F, Moloudi A. Locked-in Syndrome and Blue Toe Syndrome Caused by Cardiopulmonary Bypass. J Tehran Heart Cent. 2010 Summer;5(3):150-2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3466837
- Biyani N, Silbiger A, Ben-Ari J, Constantini S. Postoperative brain stem tension pneumocephalus causing transient locked-in syndrome. Pediatr Neurosurg. 2007;43(5):414-7. doi: 10.1159/000106394
- Bae YJ, Kim JH, Choi BS, Jung C, Kim E. Brainstem pathways for horizontal eye movement: pathologic correlation with MR imaging. Radiographics. 2013 Jan-Feb;33(1):47-59. doi: 10.1148/rg.331125033
- Yang Y, Qidwai U, Burton BJL, Canepa C. Bilateral, vertical supranuclear gaze palsy following unilateral midbrain infarct. BMJ Case Rep. 2020 Nov 4;13(11):e238422. doi: 10.1136/bcr-2020-238422
- Raibagkar P, Chavali RV, Kaplan TB, Kim JA, Nitka MV, Chou SH, Edlow BL. Reverse Locked-In Syndrome. Neurocrit Care. 2017 Aug;27(1):108-114. doi: 10.1007/s12028-017-0391-x
- Meienberg O, Mumenthaler M, Karbowski K. Quadriparesis and nuclear oculomotor palsy with total bilateral ptosis mimicking coma: a mesencephalic ‘locked-in syndrome”? Arch Neurol. 1979 Nov;36(11):708-10. doi: 10.1001/archneur.1979.00500470078016
- Bauer G, Prugger M, Rumpl E. Stimulus evoked oral automatisms in the locked-in syndrome. Arch Neurol. 1982 Jul;39(7):435-6. doi: 10.1001/archneur.1982.00510190053017
- Bauer G, Gerstenbrand F, Hengl W. Involuntary motor phenomena in the locked-in syndrome. J Neurol. 1980;223(3):191-8. doi: 10.1007/BF00313183
- Gamper E. Bau und Leistungen eines menschlichen Mittelhirnwesens (Arhinencephalie mit Encephalocele) Zugleich ein Beitrag zur Teratologie und Fasersystematik. Z Gesamte Neurol Psychiatr 1926; 102: 154–235.
- Krasnianski M, Gaul C, Neudecker S, Behrmann C, Schlüter A, Winterholler M. Yawning despite trismus in a patient with locked-in syndrome caused by a thrombosed megadolichobasilar artery. Clin Neurol Neurosurg. 2003 Dec;106(1):44-6. doi: 10.1016/s0303-8467(03)00045-3
- Prasad H. Amelioration of pathological yawning after tracheostomy in a patient with locked-in syndrome: a thermoregulatory approach. Eur J Neurol. 2008 Dec;15(12):e114; author reply e115. doi: 10.1111/j.1468-1331.2008.02323.x
- Gallup AC, Gallup GG Jr. Medical implications of excessive yawning in relation to thermoregulatory dysfunction. Eur J Neurol. 2009 Jun;16(6):e120. doi: 10.1111/j.1468-1331.2009.02638.x
- Duffey P, Jenkins N, King D. Palatal tremor and orofacial stereotypies within the locked-in syndrome. Mov Disord. 2007 Mar 15;22(4):593-5. doi: 10.1002/mds.21327
- Jennett B, Plum F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet. 1972 Apr 1;1(7753):734-7. doi: 10.1016/s0140-6736(72)90242-5
- Gerstenbrand F. Das traumatische apallische Syndrom. Cham: Springer Verlag, 1967.
- Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state (1). N Engl J Med. 1994 May 26;330(21):1499-508. doi: 10.1056/NEJM199405263302107
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, Kelly JP, Rosenberg JH, Whyte J, Zafonte RD, Zasler ND. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002 Feb 12;58(3):349-53. doi: 10.1212/wnl.58.3.349
- Bruno MA, Majerus S, Boly M, Vanhaudenhuyse A, Schnakers C, Gosseries O, Boveroux P, Kirsch M, Demertzi A, Bernard C, Hustinx R, Moonen G, Laureys S. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J Neurol. 2012 Jun;259(6):1087-98. doi: 10.1007/s00415-011-6303-7
- Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004 Dec;85(12):2020-9. doi: 10.1016/j.apmr.2004.02.033
- Schiff ND. Cognitive Motor Dissociation Following Severe Brain Injuries. JAMA Neurol. 2015 Dec;72(12):1413-5. doi: 10.1001/jamaneurol.2015.2899
- Schnakers C, Bauer C, Formisano R, Noé E, Llorens R, Lejeune N, Farisco M, Teixeira L, Morrissey AM, De Marco S, Veeramuthu V, Ilina K, Edlow BL, Gosseries O, Zandalasini M, De Bellis F, Thibaut A, Estraneo A. What names for covert awareness? A systematic review. Front Hum Neurosci. 2022 Aug 5;16:971315. doi: 10.3389/fnhum.2022.971315
- Edlow BL, Claassen J, Schiff ND, Greer DM. Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol. 2021 Mar;17(3):135-156. doi: 10.1038/s41582-020-00428-x
- Cairns H, Oldfield RC, Pennybacker JB, et al. Akinetic mutism with an epidermoid cyst of the 3rd ventricle. Brain 1941; 64: 273–290.
- Cavanna AE, Bertero L, Cavanna S, Servo S, Strigaro G, Monaco F. Persistent akinetic mutism after bilateral paramedian thalamic infarction. J Neuropsychiatry Clin Neurosci. 2009 Summer;21(3):351. doi: 10.1176/jnp.2009.21.3.351
- Shetty AC, Morris J, O’Mahony P. Akinetic mutism–not coma. Age Ageing. 2009 May;38(3):350-1. doi: 10.1093/ageing/afp005
- Arnts H, van Erp WS, Lavrijsen JCM, van Gaal S, Groenewegen HJ, van den Munckhof P. On the pathophysiology and treatment of akinetic mutism. Neurosci Biobehav Rev. 2020 May;112:270-278. doi: 10.1016/j.neubiorev.2020.02.006
- Fernández-Espejo D, Rossit S, Owen AM. A Thalamocortical Mechanism for the Absence of Overt Motor Behavior in Covertly Aware Patients. JAMA Neurol. 2015 Dec;72(12):1442-50. doi: 10.1001/jamaneurol.2015.2614
- Kartum TA, Baş G, Kemerdere R, Hanci MM. Posttraumatic locked-in syndrome from combined brainstem and upper cervical injury in childhood: A case report. Neurochirurgie. 2022 Dec;68(6):e104-e106. doi: 10.1016/j.neuchi.2022.05.003
- Schnakers C, Perrin F, Schabus M, Hustinx R, Majerus S, Moonen G, Boly M, Vanhaudenhuyse A, Bruno MA, Laureys S. Detecting consciousness in a total locked-in syndrome: an active event-related paradigm. Neurocase. 2009 Aug;15(4):271-7. doi: 10.1080/13554790902724904
- Kondziella D, Bender A, Diserens K, van Erp W, Estraneo A, Formisano R, Laureys S, Naccache L, Ozturk S, Rohaut B, Sitt JD, Stender J, Tiainen M, Rossetti AO, Gosseries O, Chatelle C; EAN Panel on Coma, Disorders of Consciousness. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020 May;27(5):741-756. doi: 10.1111/ene.14151
- Papadopoulou SL, Dionyssiotis Y, Krikonis K, Lаgopati N, Kamenov I, Markoula S. Therapeutic Approaches in Locked-in Syndrome. Folia Med (Plovdiv). 2019 Sep 30;61(3):343-351. doi: 10.3897/folmed.61.e39425
- DiTommaso C, Berliner J, Cruz D, Wenzel LR. Diaphragmatic pacing and protocol with locked-in syndrome. PM R. 2012 Feb;4(2):152-3. doi: 10.1016/j.pmrj.2011.11.011
- Graber M, Challe G, Alexandre MF, Bodaghi B, LeHoang P, Touitou V. Evaluation of the visual function of patients with locked-in syndrome: Report of 13 cases. J Fr Ophtalmol. 2016 May;39(5):437-40. doi: 10.1016/j.jfo.2016.01.005
- Pistoia F, Conson M, Sarà M. Opsoclonus-myoclonus syndrome in patients with locked-in syndrome: a therapeutic porthole with gabapentin. Mayo Clin Proc. 2010 Jun;85(6):527-31. doi: 10.4065/mcp.2010.0042
- Pistoia F, Sarà M. Gabapentin therapy for ocular opsoclonus-myoclonus restores eye movement communication in a patient with a locked-in syndrome. Neurorehabil Neural Repair. 2010 Jun;24(5):493-4. doi: 10.1177/1545968309353330
- Casanova E, Lazzari RE, Lotta S, Mazzucchi A. Locked-in syndrome: improvement in the prognosis after an early intensive multidisciplinary rehabilitation. Arch Phys Med Rehabil. 2003 Jun;84(6):862-7. https://doi.org/10.1016/S0003-9993(03)00008-X
- Farr E, Altonji K, Harvey RL. Locked-In Syndrome: Practical Rehabilitation Management. PM R. 2021 Dec;13(12):1418-1428. doi: 10.1002/pmrj.12555
- Halan T, Ortiz JF, Reddy D, Altamimi A, Ajibowo AO, Fabara SP. Locked-In Syndrome: A Systematic Review of Long-Term Management and Prognosis. Cureus. 2021 Jul 29;13(7):e16727. doi: 10.7759/cureus.16727
- McNair K, Lutjen M, Langhamer K, Nieves J, Hreha K. Comprehensive, technology-based, team approach for a patient with locked-in syndrome: A case report of improved function & quality of life. Assist Technol. 2019;31(1):53-58. doi: 10.1080/10400435.2017.1352052
- Mauss-Clum N, Cole M, McCort T, Eifler D. Locked-in syndrome: a team approach. J Neurosci Nurs. 1991 Oct;23(5):273-85. doi: 10.1097/01376517-199110000-00002
- Palmieri RL. Unlocking the secrets of locked-in syndrome. Nursing. 2009 Jul;39(7):22-9; quiz 29-30. doi: 10.1097/01.NURSE.0000357263.81710.51
- Bruno MA, Schnakers C, Damas F, Pellas F, Lutte I, Bernheim J, Majerus S, Moonen G, Goldman S, Laureys S. Locked-in syndrome in children: report of five cases and review of the literature. Pediatr Neurol. 2009 Oct;41(4):237-46. doi: 10.1016/j.pediatrneurol.2009.05.001
- Law YM, Feng LF, Liang Q, Meng LJ, Shen P, Yu SJ, Pao WY. Effect of Exercise on Physical Recovery of People with Locked-In Syndrome after Stroke: What Do We Know from the Current Evidence? A Systematic Review. Cerebrovasc Dis Extra. 2018;8(2):90-95. doi: 10.1159/00049031
- Hummelsheim H, Eickhof C. Repetitive sensorimotor training for arm and hand in a patient with locked-in syndrome. Scand J Rehabil Med. 1999 Dec;31(4):250-6. doi: 10.1080/003655099444434
- Silver B, Grover KM, Arcila X, Mitsias PD, Bowyer SM, Chopp M. Recovery in a patient with locked-in syndrome. Can J Neurol Sci. 2006 May;33(2):246-9. doi: 10.1017/s0317167100005084
- Lulé D, Zickler C, Häcker S, Bruno MA, Demertzi A, Pellas F, Laureys S, Kübler A. Life can be worth living in locked-in syndrome. Prog Brain Res. 2009;177:339-51. doi: 10.1016/S0079-6123(09)17723-3
- Schjolberg A, Sunnerhagen KS. Unlocking the locked in; a need for team approach in rehabilitation of survivors with locked-in syndrome. Acta Neurol Scand. 2012 Mar;125(3):192-8. doi: 10.1111/j.1600-0404.2011.01552.x
- Maiser S, Kabir A, Sabsevitz D, Peltier W. Locked-In Syndrome: Case Report and Discussion of Decisional Capacity. J Pain Symptom Manage. 2016 Apr;51(4):789-793. doi: 10.1016/j.jpainsymman.2015.10.021
- Rousseaux M, Castelnot E, Rigaux P, Kozlowski O, Danzé F. Evidence of persisting cognitive impairment in a case series of patients with locked-in syndrome. J Neurol Neurosurg Psychiatry. 2009 Feb;80(2):166-70. doi: 10.1136/jnnp.2007.128686
- Conson M, Sacco S, Sarà M, Pistoia F, Grossi D, Trojano L. Selective motor imagery defect in patients with locked-in syndrome. Neuropsychologia. 2008 Sep;46(11):2622-8. doi: 10.1016/j.neuropsychologia.2008.04.015
- Conson M, Pistoia F, Sarà M, Grossi D, Trojano L. Recognition and mental manipulation of body parts dissociate in locked-in syndrome. Brain Cogn. 2010 Aug;73(3):189-93. doi: 10.1016/j.bandc.2010.05.001
- Pistoia F, Conson M, Trojano L, Grossi D, Ponari M, Colonnese C, Pistoia ML, Carducci F, Sarà M. Impaired conscious recognition of negative facial expressions in patients with locked-in syndrome. J Neurosci. 2010 Jun 9;30(23):7838-44. doi: 10.1523/JNEUROSCI.6300-09.2010
- Trojano L, Moretta P, Estraneo A, Santoro L. Neuropsychologic assessment and cognitive rehabilitation in a patient with locked-in syndrome and left neglect. Arch Phys Med Rehabil. 2010 Mar;91(3):498-502. doi: 10.1016/j.apmr.2009.10.033
- Smart CM, Giacino JT, Cullen T, Moreno DR, Hirsch J, Schiff ND, Gizzi M. A case of locked-in syndrome complicated by central deafness. Nat Clin Pract Neurol. 2008 Aug;4(8):448-53. doi: 10.1038/ncpneuro0823
- Cappa SF, Pirovano C, Vignolo LA. Chronic ‘locked-in’ syndrome: psychological study of a case. Eur Neurol. 1985;24(2):107-11. doi: 10.1159/000115769
- Allain P, Joseph PA, Isambert JL, Le Gall D, Emile J. Cognitive functions in chronic locked-in syndrome: a report of two cases. Cortex. 1998 Sep;34(4):629-34. doi: 10.1016/s0010-9452(08)70520-3
- Cappa SF, Vignolo LA. Locked-in syndrome for 12 years with preserved intelligence. Ann Neurol. 1982 May;11(5):545. doi: 10.1002/ana.410110521
- Mridha MF, Das SC, Kabir MM, Lima AA, Islam MR, Watanobe Y. Brain-Computer Interface: Advancement and Challenges. Sensors (Basel). 2021 Aug 26;21(17):5746. doi: 10.3390/s21175746
- Branco MP, Pels EGM, Sars RH, Aarnoutse EJ, Ramsey NF, Vansteensel MJ, Nijboer F. Brain-Computer Interfaces for Communication: Preferences of Individuals With Locked-in Syndrome. Neurorehabil Neural Repair. 2021 Mar;35(3):267-279. doi: 10.1177/1545968321989331
- Ma Y, Gong A, Nan W, Ding P, Wang F, Fu Y. Personalized Brain-Computer Interface and Its Applications. J Pers Med. 2022 Dec 26;13(1):46. doi: 10.3390/jpm13010046
- Chamola V, Vineet A, Nayyar A, Hossain E. Brain-Computer Interface-Based Humanoid Control: A Review. Sensors (Basel). 2020 Jun 27;20(13):3620. doi: 10.3390/s20133620
- Colucci A, Vermehren M, Cavallo A, Angerhöfer C, Peekhaus N, Zollo L, Kim WS, Paik NJ, Soekadar SR. Brain-Computer Interface-Controlled Exoskeletons in Clinical Neurorehabilitation: Ready or Not? Neurorehabil Neural Repair. 2022 Dec;36(12):747-756. doi: 10.1177/15459683221138751
- Thomschewski A, Ströhlein A, Langthaler PB, Schmid E, Potthoff J, Höller P, Leis S, Trinka E, Höller Y. Imagine There Is No Plegia. Mental Motor Imagery Difficulties in Patients with Traumatic Spinal Cord Injury. Front Neurosci. 2017 Dec 11;11:689. doi: 10.3389/fnins.2017.00689
- Höller Y, Bergmann J, Kronbichler M, Crone JS, Schmid EV, Thomschewski A, Butz K, Schütze V, Höller P, Trinka E. Real movement vs. motor imagery in healthy subjects. Int J Psychophysiol. 2013 Jan;87(1):35-41. doi: 10.1016/j.ijpsycho.2012.10.015
- Thomschewski A, Höller Y, Höller P, Leis S, Trinka E. High Amplitude EEG Motor Potential during Repetitive Foot Movement: Possible Use and Challenges for Futuristic BCIs That Restore Mobility after Spinal Cord Injury. Front Neurosci. 2017 Jun 23;11:362. doi: 10.3389/fnins.2017.00362
- Saha S, Mamun KA, Ahmed K, Mostafa R, Naik GR, Darvishi S, Khandoker AH, Baumert M. Progress in Brain Computer Interface: Challenges and Opportunities. Front Syst Neurosci. 2021 Feb 25;15:578875. doi: 10.3389/fnsys.2021.578875
- Höller Y, Thomschewski A, Uhl A, Bathke AC, Nardone R, Leis S, Trinka E, Höller P. HD-EEG Based Classification of Motor-Imagery Related Activity in Patients With Spinal Cord Injury. Front Neurol. 2018 Nov 19;9:955. doi: 10.3389/fneur.2018.00955
- Svernling K, Törnbom M, Nordin Å, Sunnerhagen KS. Locked-in syndrome in Sweden, an explorative study of persons who underwent rehabilitation: a cohort study. BMJ Open. 2019 Apr 20;9(4):e023185. doi: 10.1136/bmjopen-2018-023185
- Snoeys L, Vanhoof G, Manders E. Living with locked-in syndrome: an explorative study on health care situation, communication and quality of life. Disabil Rehabil. 2013 May;35(9):713-8. doi: 10.3109/09638288.2012.705950
- Söderholm S, Meinander M, Alaranta H. Augmentative and alternative communication methods in locked-in syndrome. J Rehabil Med. 2001 Sep;33(5):235-9. doi: 10.1080/165019701750419644
- Chua KS, Kong KH. Functional outcome in brain stem stroke patients after rehabilitation. Arch Phys Med Rehabil. 1996 Feb;77(2):194-7. doi: 10.1016/s0003-9993(96)90167-7
- León-Carrión J, van Eeckhout P, Domínguez-Morales Mdel R, Pérez-Santamaría FJ. The locked-in syndrome: a syndrome looking for a therapy. Brain Inj. 2002 Jul;16(7):571-82. https://doi.org/10.1080/02699050110119781
- Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006 Feb;5(2):140-7. doi: 10.1016/S1474-4422(05)70326-4
- Rousseau MC, Pietra S, Nadji M, Billette de Villemeur T. Evaluation of quality of life in complete locked-in syndrome patients. J Palliat Med. 2013 Nov;16(11):1455-8. doi: 10.1089/jpm.2013.0120
- Lulé D, Zickler C, Häcker S, Bruno MA, Demertzi A, Pellas F, Laureys S, Kübler A. Life can be worth living in locked-in syndrome. Prog Brain Res. 2009;177:339-51. https://orbi.uliege.be/bitstream/2268/91902/1/lule_PBR_coma_science_2009.pdf