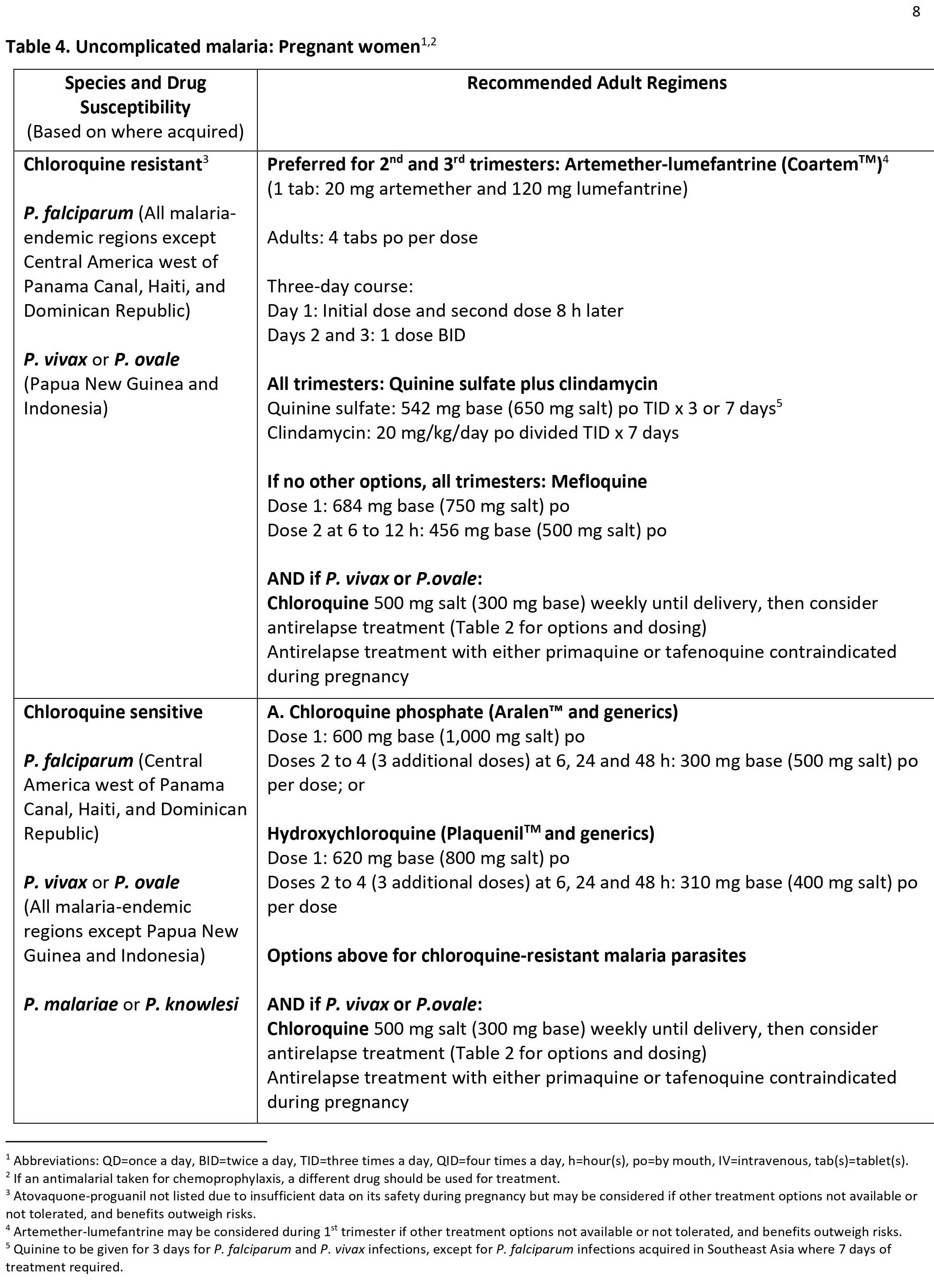
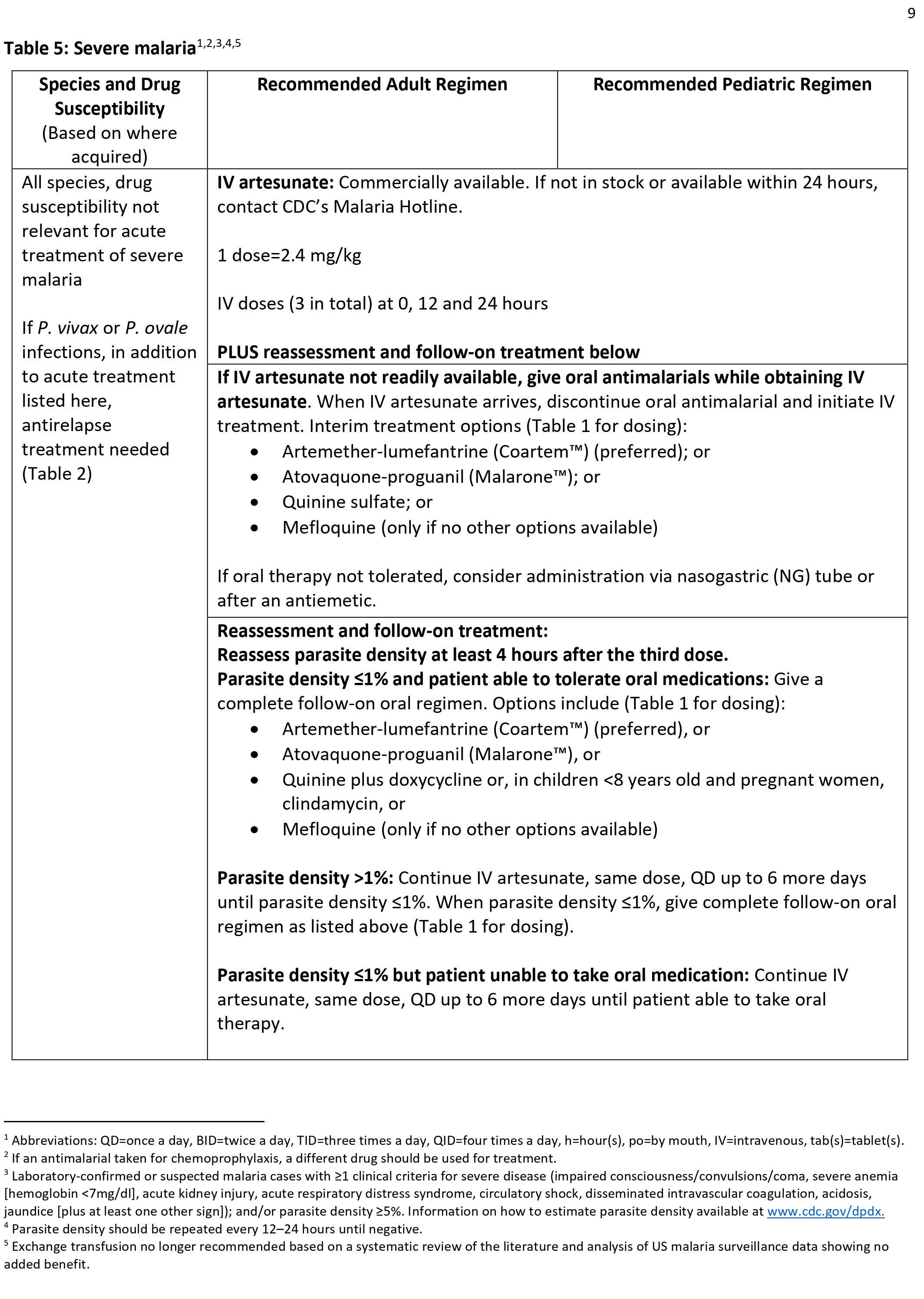
Malaria
Malaria is a mosquito-borne disease caused by a parasite of the genus plasmodium. The plasmodium parasite is transmitted to humans most commonly through mosquito bites. Usually, people get malaria by being bitten by an infective female Anopheles mosquito. You get it when an infected female Anopheles mosquito bites you. People with malaria are typically very sick with high fevers, chills, flu-like illness, vomiting, diarrhea, and jaundice (yellowing of the skin and eyes). Left untreated, they may develop severe complications and die. Four kinds of malaria parasites infect humans: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae. In addition, Plasmodium knowlesi, a type of malaria that naturally infects macaques (Old World monkeys) in Southeast Asia, also infects humans, causing malaria that is transmitted from animal to human (“zoonotic” malaria). Plasmodium falciparum is the type of malaria that is most likely to result in severe infections and if not promptly treated, may lead to death. Although malaria can be a deadly disease, illness and death from malaria can usually be prevented. Malaria is a major cause of death worldwide, but it is almost wiped out in the United States. Globally, the World Health Organization estimates that in 2019, 229 million clinical cases of malaria occurred, and 409,000 people died of malaria, mostly children in the African Region 1. About 2,000 cases of malaria are diagnosed in the United States each year. The vast majority of cases in the United States are in travelers and immigrants returning from countries where malaria transmission occurs, many from sub-Saharan Africa and South Asia 2.
Malaria is mostly a problem in developing countries with warm climates. If you travel to these countries, you are at risk. A blood test can diagnose malaria. In general, malaria is a curable disease if diagnosed and treated promptly and correctly. You can treat malaria with drugs. The type of drug depends on which kind of malaria you have and where you were infected.
Forty percent of the total global population resides in or visits malaria-endemic regions annually 3. Plasmodium falciparum is present in Western and sub-Saharan Africa and displays the highest morbidity and mortality of the Plasmodia species 4. Plasmodium vivax is present in South Asia, the Western Pacific, and Central America 4. Plasmodium ovale and Plasmodium malariae are present in Sub-Saharan Africa 4. Plasmodium knowlesi is present in Southeast Asia 4. As many as 500 million cases of malaria occur annually, with 1.5 to 2.7 million deaths 3. Ninety percent of fatalities occur in Africa 3. Those at highest risk include children under age 5, pregnant women, and disease naïve populations, including refugee populations in Central and Eastern Africa, nonimmune civilian and military travelers, and immigrants returning to their place of origin 5. Of the 125 million travelers who visit endemic locations each year, 10000 to 30000 develop malaria, and 1% of these will die from complications of their disease 5. Rising average global temperatures and changes in weather patterns are projected to expand the burden of malaria; a rise of 3 degrees Celsius is postulated to increase malaria incidence by 50 to 80 million 3.
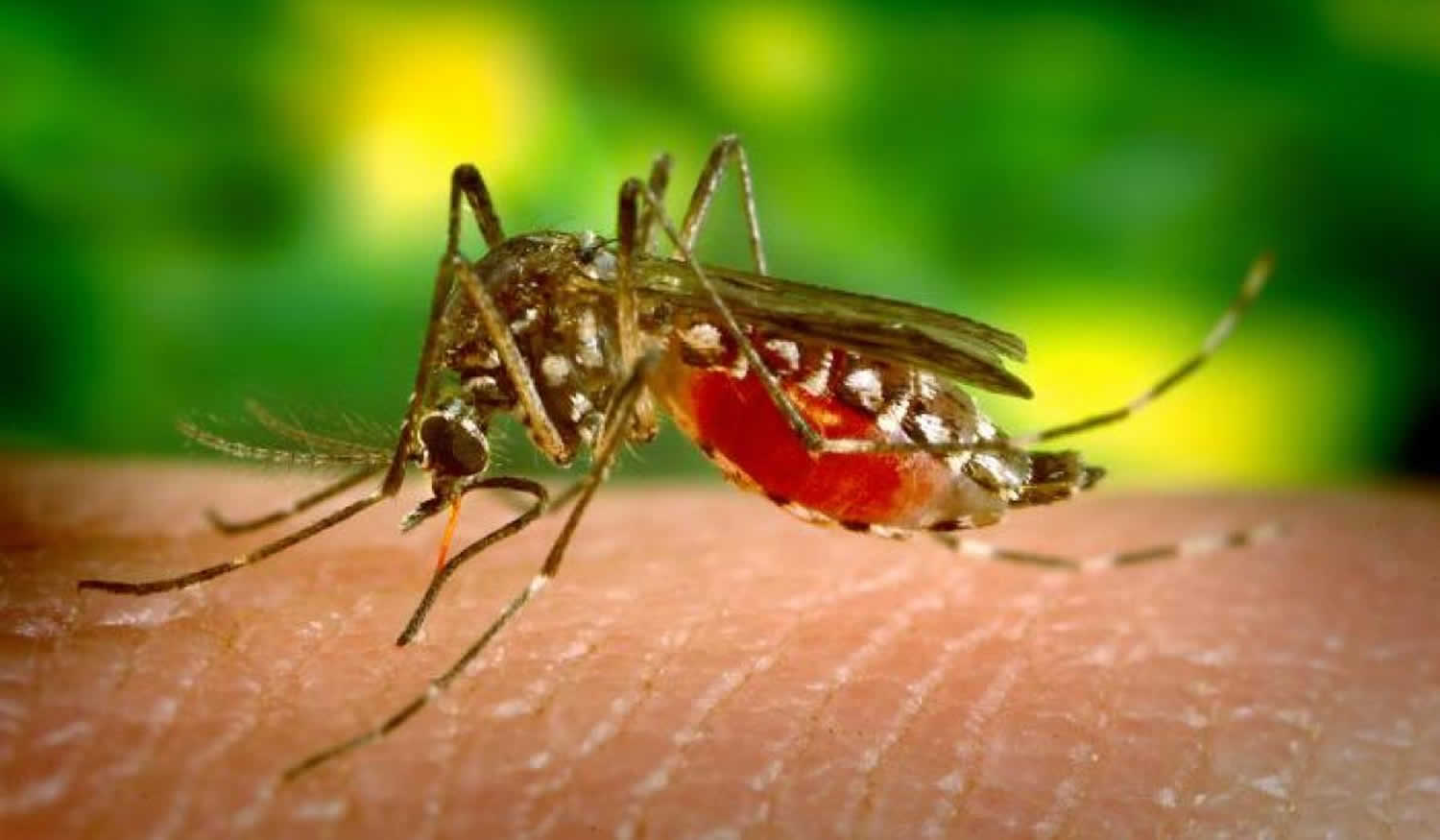
Figure 1. Female Anopheles mosquito
Footnote: There are approximately 3,500 species of mosquitoes grouped into 41 genera. Human malaria is transmitted only by female mosquitoes of the genus Anopheles. Of the approximately 430 Anopheles species, only 30-40 transmit malaria (i.e., are “vectors”) in nature. The rest either bite humans infrequently or cannot sustain development of malaria parasites. Female mosquitoes take blood meals for egg production, and these blood meals are the link between the human and the mosquito hosts in the parasite life cycle.
Where does malaria occur?
Where malaria is found depends mainly on climatic factors such as temperature, humidity, and rainfall. Malaria is transmitted in tropical and subtropical areas, where:
- Anopheles mosquitoes can survive and multiply, and
- Malaria parasites can complete their growth cycle in the mosquitoes (“extrinsic incubation period”).
Temperature is particularly critical. For example, at temperatures below 20°C (68°F), Plasmodium falciparum (which causes severe malaria) cannot complete its growth cycle in the Anopheles mosquito, and thus cannot be transmitted.
Malaria occurs mostly in poor, tropical and subtropical areas of the world. Africa is the most affected due to a combination of factors:
- A very efficient mosquito (Anopheles gambiae complex) is responsible for high transmission.
- The predominant parasite species is Plasmodium falciparum , which is the species that is most likely to cause severe malaria and death.
- Local weather conditions often allow transmission to occur year round.
- Scarce resources and socio-economic instability have hindered efficient malaria control activities.
- In other areas of the world, malaria is a less prominent cause of deaths, but can cause substantial disease and incapacitation, especially in some countries in South America and South Asia.
In many malaria-endemic countries, malaria transmission does not occur in all parts of the country. Even within tropical and subtropical areas, transmission will not occur:
- At very high altitudes;
- During colder seasons in some areas;
- In deserts (excluding the oases); and
- In some countries where transmission has been interrupted through successful control/elimination programs.
Generally, in warmer regions closer to the equator:
- Transmission will be more intense, and
- Malaria is transmitted year-round.
The highest transmission is found in Africa South of the Sahara and in parts of Oceania such as Papua New Guinea.
In cooler regions, transmission will be less intense and more seasonal. There, Plasmodium vivax might be more prevalent because it is more tolerant of lower ambient temperatures.
In many temperate areas, such as western Europe and the United States, economic development and public health measures have succeeded in eliminating malaria. However, most of these areas have Anopheles mosquitoes that can transmit malaria, and reintroduction of the disease is a constant risk.
Figure 2. Countries where malaria is found
Footnote: This map shows an approximation of the parts of the world where malaria transmission occurs.
[Source 6 ]Why is malaria so common in Africa?
In Africa south of the Sahara, the principal malaria mosquito, Anopheles gambiae, transmits malaria very efficiently. The type of malaria parasite most often found, Plasmodium falciparum, causes severe, potentially fatal disease. Lack of resources and political instability can prevent the building of solid malaria control programs. In addition, malaria parasites are increasingly resistant to antimalarial drugs, presenting one more barrier to malaria control on that continent.
In some countries, malaria is said to exist in “rural” areas. How would one know if an area is rural vs urban?
What constitutes a rural area can vary by country. In general, urbanization can be said to involve both population size and economic development of an area in which there is concentrated commercial activity, such as manufacturing, the sale of goods and services, and transportation. Rural areas tend to have less commercial activity, less population density, more green space, and agriculture may be a main feature.
Is malaria a contagious disease?
No. Malaria is not spread from person to person like a cold or the flu, and it cannot be sexually transmitted. You cannot get malaria from casual contact with malaria-infected people, such as sitting next to someone who has malaria.
Who is at risk for malaria?
Anyone can get malaria. Most cases occur in people who live in countries with malaria transmission. People from countries with no malaria can become infected when they travel to countries with malaria or through a blood transfusion (although this is very rare). Also, an infected mother can transmit malaria to her infant before or during delivery.
Who is most vulnerable malaria?
The most vulnerable are persons with no or little immunity against malaria. In areas with high malaria transmission (such as Africa south of the Sahara), the most vulnerable groups are:
- Young children, who have not yet developed partial immunity to malaria
- Pregnant women, whose immunity is decreased by pregnancy, especially during the first and second pregnancies
- Travelers or migrants coming from areas with little or no malaria transmission, who lack immunity.
In areas with lower malaria transmission (such as Latin America and Asia), residents are less frequently infected. Many persons may reach adult age without having built protective immunity and are thus susceptible to malaria, including severe and fatal illness.
Who is most at risk of getting very sick and dying from malaria?
Plasmodium falciparum is the type of malaria that most often causes severe and life-threatening malaria; this parasite is very common in many countries in Africa south of the Sahara desert. People who are heavily exposed to the bites of mosquitoes infected with Plasmodium falciparum are most at risk of dying from malaria. People who have little or no immunity to malaria, such as young children and pregnant women or travelers coming from areas with no malaria, are more likely to become very sick and die. Poor people living in rural areas who lack access to health care are at greater risk for this disease. As a result of all these factors, an estimated 90% of deaths due to malaria occur in Africa south of the Sahara; most of these deaths occur in children under 5 years of age.
How soon will a person feel sick after being bitten by an infected mosquito?
For most people, symptoms begin 10 days to 4 weeks after infection, although a person may feel ill as early as 7 days or as late as 1 year later. Two kinds of malaria, Plasmodium vivax and Plasmodium ovale, can occur again (relapsing malaria). In Plasmodium vivax and Plasmodium ovale infections, some parasites can remain dormant in the liver for several months up to about 4 years after a person is bitten by an infected mosquito. When these parasites come out of hibernation and begin invading red blood cells (“relapse”), the person will become sick.
How long after returning from an area with malaria could I develop malaria?
Any traveler who becomes ill with a fever or flu-like illness while traveling, and up to 1 year after returning home, should immediately seek professional medical care. You should tell your health-care provider that you have been traveling in an area where malaria transmission occurs and ask to be tested for malaria infection.
How do I know if I have malaria for sure?
Most people, at the beginning of the disease, have fever, sweats, chills, headaches, malaise, muscles aches, nausea, and vomiting. Malaria can very rapidly become a severe and life-threatening disease. The surest way for you and your health-care provider to know whether you have malaria is to have a diagnostic test where a drop of your blood is examined under the microscope for the presence of malaria parasites. If you are sick and there is any suspicion of malaria (for example, if you have recently traveled in a country where malaria transmission occurs), the test should be performed without delay.
I was born in a country where malaria is present and had malaria as a child, and then moved to the United States many years ago. Do I need to worry about getting malaria when I return home to visit my friends and relatives?
Yes. Anyone who goes to a country where malaria transmission occurs should take precautions against contracting malaria. During the time that you have spent in the United States, you have lost any malaria immunity that you might have had while living in your native country. Without frequent exposure to malaria parasites, your immune system has lost its ability to fight malaria. You are now as much at risk as someone who was born in the United States (a “nonimmune” person). Please consult with your health-care provider or a travel clinic about precautions to take against malaria (preventive drugs and protection against mosquito bites) and against other diseases.
I live in the United States, am 4 months pregnant, and want to take a 2-week trip to a country where malaria transmission occurs. Is it safe to do so?
The Centers for Disease Control and Prevention (CDC) advises women who are pregnant or likely to become pregnant not to travel to areas where malaria transmission occurs, if possible. Malaria in pregnant women can be more severe than in women who are not pregnant. Malaria can increase the risk for serious pregnancy problems, including prematurity, miscarriage, and stillbirth. If travel to a malarious area cannot be postponed, use of an effective chemoprophylaxis regimen is essential. However, no preventive drugs are completely effective. Please consider these risks (and other health risks as well) and discuss them with your health-care provider.
I plan to become pregnant after I return from an area where malaria transmission occurs. How long does it take it take for antimalarial drugs to clear the body?
Because there is no evidence that chloroquine and mefloquine are associated with congenital defects when used for preventing malaria (prophylaxis), CDC does not recommend that women planning pregnancy need to wait a specific period of time after their use before becoming pregnant. However, if women or their health-care providers wish to decrease the amount of antimalarial drug in the body before conception, the below table provides information on the half-lives of selected antimalarial drugs. After two, four, and six half-lives, approximately 25%, 6%, and 2% of the drug remain in the body.
Half-lives of selected antimalarial drugs
| Drug | Half life |
| Atovaquone | 2–3 days |
| Chloroquine | 6–60 days |
| Doxycycline | 12–24 hours |
| Mefloquine | 2–3 weeks |
| Primaquine | 4–7 hours |
| Proguanil | 14–21 hours |
| Tafenoquine | 2 weeks |
Is it safe for me to breastfeed while taking an antimalarial drug?
There are limited data available about the safety of antimalarial drugs while breastfeeding. However, the amount of antimalarial drug transferred from the nursing mother to her infant is not thought to be harmful to the infant. Very small amounts of the antimalarial drugs chloroquine and mefloquine are excreted in the breast milk of women who are breastfeeding. Although there is limited information about the use of doxycycline in breastfeeding women, most experts consider it unlikely to cause any harm.
No information is available on the amount of primaquine or tafenoquine that enters human breast milk. The mother and infant should be tested for G6PD deficiency before primaquine is given to a woman who is breastfeeding. Because there is no information on the use of tafenoquine in infants, tafenoquine is not recommended during breastfeeding.
It is not known whether atovaquone, which is a component of the antimalarial drug Malarone, is excreted in human milk. Proguanil, the other component of Malarone, is excreted in human milk in small quantities.
Because there is little information available on the safety of atovaquone/proguanil to prevent malaria in infants weighing less than 5 kg (11 lbs), CDC does not currently recommend it for the prevention of malaria in women breastfeeding infants weighing less than 5 kg.
If I am taking an antimalarial drug and breast-feeding, will my baby be protected from malaria because of the medication transferred in my breast milk?
No. Based on experience with other antimalarial drugs, the quantity of drug transferred in breast milk is not likely to be enough to provide protection against malaria for the infant.
Should infants and children be given antimalarial drugs?
Yes, but not all types of malaria drugs. Children of any age can get malaria and any child traveling to an area where malaria transmission occurs should use the recommended prevention measures, which often include an antimalarial drug. However, some antimalarial drugs are not suitable for children. Doses are based on the child’s weight.
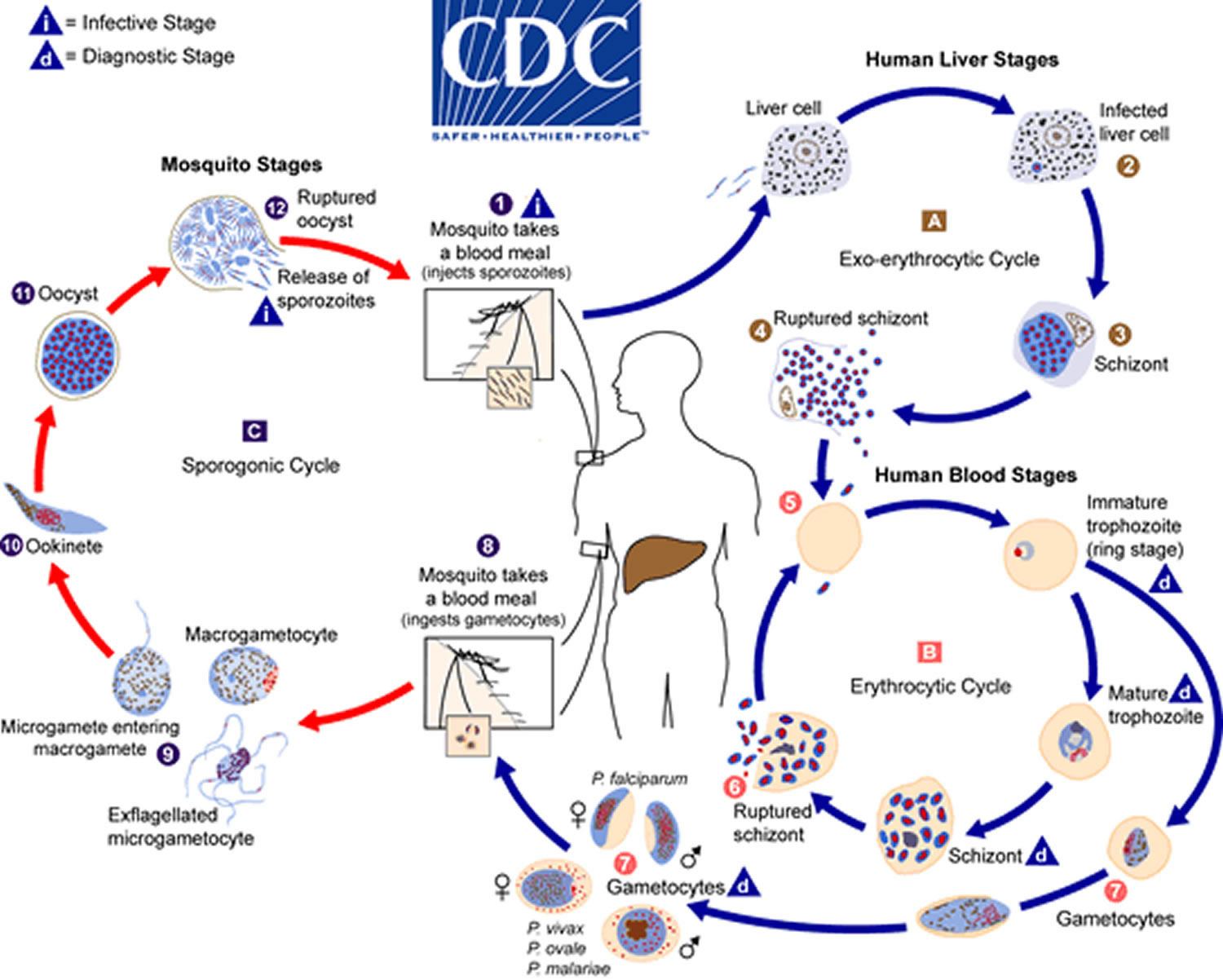
Life cycle of malaria parasite
The life cycle of all Plasmodium species is complex (Figure 3). The natural history of malaria involves cyclical infection of humans and female Anopheles mosquitoes. Infection in humans begins with the bite of an infected female Anopheles mosquito. Sporozoites released from the salivary glands of the mosquito enter the bloodstream during feeding. In humans, the parasites grow and multiply first in the liver cells (hepatocytes) and then in the red blood cells (erythrocytes) of the blood. In the blood, successive broods of parasites grow inside the red cells and destroy them, releasing daughter parasites (“merozoites”) that continue the cycle by invading other red cells.
The blood stage parasites are those that cause the symptoms of malaria. When certain forms of blood stage parasites (gametocytes, which occur in male and female forms) are ingested during blood feeding by a female Anopheles mosquito, they mate in the gut of the mosquito and begin a cycle of growth and multiplication in the mosquito. After 10 to 18 days, a form of the parasite called a sporozoite migrates to the mosquito’s salivary glands. When the Anopheles mosquito takes a blood meal on another human, anticoagulant saliva is injected together with the sporozoites, which migrate to the liver, thereby beginning a new cycle. Thus the infected mosquito carries the disease from one human to another (acting as a “vector”), while infected humans transmit the parasite to the mosquito, In contrast to the human host, the mosquito vector does not suffer from the presence of the parasites.
The successful development of the malaria parasite in the mosquito (from the “gametocyte” stage to the “sporozoite” stage) depends on several factors. The most important is ambient temperature and humidity (higher temperatures accelerate the parasite growth in the mosquito) and whether the Anopheles survives long enough to allow the parasite to complete its cycle in the mosquito host (“sporogonic” or “extrinsic” cycle, duration 9 to 18 days). In contrast to the human host, the mosquito host does not suffer noticeably from the presence of the parasites.
Figure 3. Malaria life cycle
Footnotes: The malaria parasite life cycle involves two hosts. During a blood meal, a malaria-infected female Anopheles mosquito inoculates sporozoites into the human host (number 1). Sporozoites infect liver cells (number 2) and mature into schizonts (number 3), which rupture and release merozoites (number 4). (Of note, in Plasmodium vivax and Plasmodium ovale a dormant stage [hypnozoites] can persist in the liver (if untreated) and cause relapses by invading the bloodstream weeks, or even years later.) After this initial replication in the liver (exo-erythrocytic schizogony [letter A]), the parasites undergo asexual multiplication in the erythrocytes (erythrocytic schizogony [letter B]). Merozoites infect red blood cells (number 5). The ring stage trophozoites mature into schizonts, which rupture releasing merozoites (number 6). Some parasites differentiate into sexual erythrocytic stages (gametocytes) (number 7). Blood stage parasites are responsible for the clinical manifestations of the disease. The gametocytes, male (microgametocytes) and female (macrogametocytes), are ingested by an Anopheles mosquito during a blood meal (number 8). The parasites’ multiplication in the mosquito is known as the sporogonic cycle [letter C]. While in the mosquito’s stomach, the microgametes penetrate the macrogametes generating zygotes (number 9). The zygotes in turn become motile and elongated (ookinetes) (number 10) which invade the midgut wall of the mosquito where they develop into oocysts (number 11). The oocysts grow, rupture, and release sporozoites (number 12), which make their way to the mosquito’s salivary glands. Inoculation of the sporozoites (number 1) into a new human host perpetuates the malaria life cycle.
[Source 7 ]Malaria transmission
Usually, people get malaria by being bitten by an infective female Anopheles mosquito. Only Anopheles mosquitoes can transmit malaria and they must have been infected through a previous blood meal taken from an infected person. When a mosquito bites an infected person, a small amount of blood is taken in which contains microscopic malaria parasites. About 1 week later, when the mosquito takes its next blood meal, these parasites mix with the mosquito’s saliva and are injected into the person being bitten.
Because the malaria parasite is found in red blood cells of an infected person, malaria can also be transmitted through blood transfusion, organ transplant, or the shared use of needles or syringes contaminated with blood. Malaria may also be transmitted from a mother to her unborn infant before or during delivery (“congenital” malaria).
Incubation period of malaria
Following the infective bite by the Anopheles mosquito, a period of time (the “incubation period”) goes by before the first symptoms appear. The incubation period and therefore time to symptom development varies by species in most cases varies from 7 to 30 days: 8 to 11 days for Plasmodium falciparum, 8 to 17 days for Plasmodium vivax, 10 to 17 days for Plasmodium ovale, 18 to 40 days for Plasmodium malariae (though possibly up to several years), and 9 to 12 days for Plasmodium knowlesi 3. The shorter periods are observed most frequently with Plasmodium falciparum and the longer ones with Plasmodium malariae.
The periodicity of the Plasmodium lifecycle creates the classic “malarial paroxysm” of rigors, followed by several hours of fever, followed by diaphoresis and drop to normal body temperature (Plasmodium vivax infection establishes a 48-hour cycle), though this is less commonly seen today due to rapid identification and treatment 3.
Antimalarial drugs taken for prophylaxis (prevention) by travelers can delay the appearance of malaria symptoms by weeks or months, long after the traveler has left the malaria-endemic area. This can happen particularly with Plasmodium vivax and Plasmodium ovale, both of which can produce dormant liver stage parasites; the liver stages may reactivate and cause disease months after the infective mosquito bite. Such long delays between exposure and development of symptoms can result in misdiagnosis or delayed diagnosis because of reduced clinical suspicion by the health-care provider. Returned travelers should always remind their health-care providers of any travel in areas where malaria occurs during the past 12 months.
Where does malaria transmission occur?
For malaria transmission to occur, conditions must be such so that all three components of the malaria life cycle are present:
- Anopheles mosquitoes, which able to feed on humans humans, and in which the parasites can complete the “invertebrate host” half of their life cycle
- Humans. who can be bitten by Anopheles mosquitoes, and in whom the parasites can complete the “vertebrate host” half of their life cycle
- Malaria parasites.
Climate
Climate is a key determinant of both the geographic distribution and the seasonality of malaria. Without sufficient rainfall, mosquitoes cannot survive and if not sufficiently warm, parasites cannot survive in the mosquito.
Anopheles lay their eggs in a variety of fresh or brackish bodies of water, with different species having different preferences. Eggs hatch within a few days, with resulting larvae spending 9-12 days to develop into adults in tropical areas. If larval habitats dry up before the process is completed, the larvae die; if rains are excessive, they may be flushed and destroyed. Life is precarious for mosquito larvae, with most perishing before becoming adults.
Life is usually short for adult mosquitoes as well, with temperature and humidity affecting longevity. Only older females can transmit malaria, as they must live long enough for sporozoites to develop and move to the salivary glands. This process takes a minimum of nine days when temperatures are warm (30°C or 86°F) and will take much longer at cooler temperatures. If temperatures are too cool (15°C or 59°F for Plasmodium vivax, 20°C or 68°F for Plasmodium falciparum), development cannot be completed and malaria cannot be transmitted. Thus, malaria transmission is much more intense in warm and humid areas, with transmission possible in temperate areas only during summer months.
In warm climates people are more likely to sleep unprotected outdoors, thereby increasing exposure to night-biting Anopheles mosquitoes. During harvest seasons, agricultural workers might sleep in the fields or nearby locales, without protection against mosquito bites.
Anopheles mosquitoes
Malaria is transmitted to humans by female mosquitoes of the genus Anopheles. Anophelines are found worldwide except Antarctica. Malaria is transmitted by different Anopheles species in different geographic regions. Within geographic regions, different environments support a different species. The types (species) of Anopheles present in an area at a given time will influence the intensity of malaria transmission. Not all Anopheles are equally efficient vectors for transmitting malaria from one person to another. Those species that are most prone to bite humans are the most dangerous, as bites inflicted on animals that cannot be infected with human malaria break the chain of transmission. If the mosquito regularly bites humans, the chain of transmission is unbroken and more people will become infected. Some species are biologically unable to sustain development of human malaria parasites, while others are readily infected and produce large numbers of sporozoites (the parasite stage that is infective to humans).
Anophelines that can transmit malaria are found not only in malaria-endemic areas, but also in areas where malaria has been eliminated. These areas are thus at risk of re-introduction of the disease.
Many of the most dangerous species bite human indoors. For these species insecticide treated mosquito nets and indoor residual spray (whereby the inner walls of dwellings are coated with a long-lasting insecticide) are effective interventions. Both of these interventions require attention to insecticide resistance, which will evolve if the same insecticide is used continuously in the same area.
Anopheles mosquito life stages
Like all mosquitoes, anopheles mosquitoes go through four stages in their life cycle: egg, larva, pupa, and adult. The first three stages are aquatic and last 7-14 days, depending on the species and the ambient temperature. The biting female Anopheles mosquito may carry malaria. Male mosquitoes do not bite so cannot transmit malaria or other diseases. The adult females are generally short-lived, with only a small proportion living long enough (more than 10 days in tropical regions) to transmit malaria.
- Eggs
- Larvae
- Pupae
- Adults
- Eggs
Adult females lay 50-200 eggs per oviposition. Eggs are laid singly directly on water and are unique in having floats on either side. Eggs are not resistant to drying and hatch within 2-3 days, although hatching may take up to 2-3 weeks in colder climates.
- Larvae
The larvae occur in a wide range of habitats but most species prefer clean, unpolluted water. Larvae of Anopheles mosquitoes have been found in fresh- or salt-water marshes, mangrove swamps, rice fields, grassy ditches, the edges of streams and rivers, and small, temporary rain pools. Many species prefer habitats with vegetation. Others prefer habitats that have none. Some breed in open, sun-lit pools while others are found only in shaded breeding sites in forests. A few species breed in tree holes or the leaf axils of some plants.
Mosquito larvae have a well-developed head with mouth brushes used for feeding, a large thorax, and a segmented abdomen. They have no legs. In contrast to other mosquitoes, Anopheles larvae lack a respiratory siphon and for this reason position themselves so that their body is parallel to the surface of the water.
Larvae breathe through spiracles located on the 8th abdominal segment and therefore must come to the surface frequently.
The larvae spend most of their time feeding on algae, bacteria, and other microorganisms in the surface microlayer. They do so by rotating their head 180 degrees and feeding from below the microlayer. Larvae dive below the surface only when disturbed. Larvae swim either by jerky movements of the entire body or through propulsion with the mouth brushes.
Larvae develop through 4 stages or instars, after which they metamorphose into pupae. At the end of each instar, the larvae molt, shedding their exoskeleton, or skin, to allow for further growth.
- Pupae
The pupa is comma-shaped when viewed from the side. This is a transitional stage between larva and adult. The pupae does not feed, but undergoes radical metamorphosis. The head and thorax are merged into a cephalothorax with the abdomen curving around underneath. As with the larvae, pupae must come to the surface frequently to breathe, which they do through a pair of respiratory trumpets on the cephalothorax. After a few days as a pupa, the dorsal surface of the cephalothorax splits and the adult mosquito emerges onto the surface of the water.
The duration from egg to adult varies considerably among species and is strongly influenced by ambient temperature. Mosquitoes can develop from egg to adult in as little as 7 days but usually take 10-14 days in tropical conditions.
- Adults
Like all mosquitoes, adult anopheles have slender bodies with 3 sections: head, thorax and abdomen.
The head is specialized for acquiring sensory information and for feeding. The head contains the eyes and a pair of long, many-segmented antennae. The antennae are important for detecting host odors as well as odors of aquatic larval habitats where females lay eggs. The head also has an elongate, forward-projecting proboscis used for feeding, and two sensory palps.
The thorax is specialized for locomotion. Three pairs of legs and a single pair of wings are attached to the thorax.
The abdomen is specialized for food digestion and egg development. This segmented body part expands considerably when a female takes a blood meal. The blood is digested over time serving as a source of protein for the production of eggs, which gradually fill the abdomen.
Anopheles mosquitoes can be distinguished from other mosquitoes by the palps, which are as long as the proboscis, and by the presence of discrete blocks of black and white scales on the wings. Adult Anopheles can also be identified by their typical resting position: males and females rest with their abdomens sticking up in the air rather than parallel to the surface on which they are resting.
Adult mosquitoes usually mate within a few days after emerging from the pupal stage. In some species, the males form large swarms, usually around dusk, and the females fly into the swarms to mate. The mating habitats of many species remain unknown.
Males live for about a week, feeding on nectar and other sources of sugar. Females will also feed on sugar sources for energy but usually require a blood meal for the development of eggs. After obtaining a full blood meal, the female will rest for a few days while the blood is digested and eggs are developed. This process depends on the temperature but usually takes 2-3 days in tropical conditions. Once the eggs are fully developed, the female lays them then seeks blood to sustain another batch of eggs.
The cycle repeats itself until the female dies. Females can survive up to a month (or longer in captivity) but most do not live longer than 1-2 weeks in nature. Their chances of survival depend on temperature and humidity, but also upon their ability to successfully obtain a blood meal while avoiding host defenses.
Human factors
Biologic characteristics (inborn and acquired) and behavioral traits can influence an individual’s malaria risk and, on a larger scale, the overall malaria ecology.
Genetic factors
Biologic characteristics present from birth can protect against certain types of malaria. Two genetic factors, both associated with human red blood cells, have been shown to be epidemiologically important. Persons who have the sickle cell trait (heterozygotes for the abnormal hemoglobin gene HbS) are relatively protected against Plasmodium falciparum malaria and thus enjoy a biologic advantage. Because Plasmodium falciparum malaria has been a leading cause of death in Africa since remote times, the sickle cell trait is now more frequently found in Africa and in persons of African ancestry than in other population groups. In general, the prevalence of hemoglobin-related disorders and other blood cell dyscrasias, such as Hemoglobin C, the thalassemias and G6PD deficiency, are more prevalent in malaria endemic areas and are thought to provide protection from malarial disease.
Persons who are negative for the Duffy blood group have red blood cells that are resistant to infection by Plasmodium vivax. Since the majority of Africans are Duffy negative, Plasmodium vivax is rare in Africa south of the Sahara, especially West Africa. In that area, the niche of Plasmodium vivax has been taken over by Plasmodium ovale, a very similar parasite that does infect Duffy-negative persons.
Other genetic factors related to red blood cells also influence malaria, but to a lesser extent. Various genetic determinants (such as the “HLA complex,” which plays a role in control of immune responses) may equally influence an individual’s risk of developing severe malaria.
Acquired immunity
Acquired immunity greatly influences how malaria affects an individual and a community. After repeated attacks of malaria a person may develop a partially protective immunity. Such “semi-immune” persons often can still be infected by malaria parasites but may not develop severe disease, and, in fact, frequently lack any typical malaria symptoms.
In areas with high Plasmodium falciparum transmission (most of Africa south of the Sahara), newborns will be protected during the first few months of life presumably by maternal antibodies transferred to them through the placenta. As these antibodies decrease with time, these young children become vulnerable to disease and death by malaria. If they survive repeated infections to an older age (2-5 years) they will have reached a protective semi-immune status. Thus in high transmission areas, young children are a major risk group and are targeted preferentially by malaria control interventions.
In areas with lower transmission (such as Asia and Latin America), infections are less frequent and a larger proportion of the older children and adults have no protective immunity. In such areas, malaria disease can be found in all age groups, and epidemics can occur.
Behavioral factors
Human behavior, often dictated by social and economic reasons, can influence the risk of malaria for individuals and communities. For example:
- Poor rural populations in malaria-endemic areas often cannot afford the housing and bed nets that would protect them from exposure to mosquitoes. These persons often lack the knowledge to recognize malaria and to treat it promptly and correctly. Often, cultural beliefs result in use of traditional, ineffective methods of treatment.
- Travelers from non-endemic areas may choose not to use insect repellent or medicines to prevent malaria. Reasons may include cost, inconvenience, or a lack of knowledge.
- Human activities can create breeding sites for larvae (standing water in irrigation ditches, burrow pits)
- Agricultural work such as harvesting (also influenced by climate) may force increased nighttime exposure to mosquito bites
- Raising domestic animals near the household may provide alternate sources of blood meals for Anopheles mosquitoes and thus decrease human exposure
- War, migrations (voluntary or forced) and tourism may expose non-immune individuals to an environment with high malaria transmission.
Human behavior in endemic countries also determines in part how successful malaria control activities will be in their efforts to decrease transmission. The governments of malaria-endemic countries often lack financial resources. As a consequence, health workers in the public sector are often underpaid and overworked. They lack equipment, drugs, training, and supervision. The local populations are aware of such situations when they occur, and cease relying on the public sector health facilities. Conversely, the private sector suffers from its own problems. Regulatory measures often do not exist or are not enforced. This encourages private consultations by unlicensed, costly health providers, and the anarchic prescription and sale of drugs (some of which are counterfeit products). Correcting this situation is a tremendous challenge that must be addressed if malaria control and ultimately elimination is to be successful.
Malaria transmission in the United States
Outbreaks of locally transmitted cases of malaria in the United States have been small and relatively isolated, but the potential risk for the disease to re-emerge is present due to the abundance of competent vectors, especially in the southern states.
“Airport” malaria
“Airport” malaria refers to malaria caused by infected mosquitoes that are transported rapidly by aircraft from a malaria-endemic country to a non-endemic country. If the local conditions allow their survival, they can bite local residents who can thus acquire malaria without having traveled abroad.
Congenital malaria
In congenital malaria, infected mothers transmit parasites to their child during pregnancy before or during delivery. Therefore, though congenital transmission is rare, health-care providers should be alert to the diagnosis of malaria in ill neonates and young infants, particularly those with fever.
During evaluation, health-care providers should obtain a complete and accurate travel and residency history on the patient and close relatives. Patients should be asked about transfusion of blood products.
The absence of recent foreign travel or a long interval between immigration of the mother and the birth of the infant being examined should not discourage clinicians from obtaining blood films on the patient to rule out a potentially life-threatening but treatable infection.
Transfusion-transmitted malaria
Transfusion-transmitted malaria is rare in the United States, but it is a potential severe complication in blood recipients. On average, only one case of transfusion-transmitted malaria occurs in the United States every 2 years 8. Because no approved tests are available in the United States to screen donated blood for malaria, prevention of transfusion-transmitted malaria requires careful questioning of prospective donors 9.
Summary of guidelines of the Food and Drug Administration (FDA) and American Association of Blood Banks for deferral of blood donors at increased risk for malaria 10, 9:
- Defer blood donation for 1 year: Travelers who are residents of nonmalarious areas who have been in a malarious area may be accepted as donors 1 year after their return to the nonmalarious area (irrespective of the use of chemoprophylaxis) if they have been free of malaria symptoms.
- Defer blood donation for 3 years: Immigrants or visitors from malarious areas may be accepted 3 years after departure from the area if they have been asymptomatic. Former residents of malarious areas who now live in the United States but who return to visit a malarious area may be accepted as donoros 3 years after their most recent visit. Persons who have had a diagnosis of malaria should be deferred for 3 years after becoming asymptomatic.
Malaria signs and symptoms
There are 4 types of malaria parasites (i.e., Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae) that can infect people. The symptoms of each kind are generally the same. Infection with malaria parasites may result in a wide variety of symptoms, ranging from absent or very mild symptoms to severe disease and even death. Malaria disease can be categorized as uncomplicated or severe (complicated).
Signs and symptoms of malaria may include:
- high fever (can often be 104° F [40° C] and higher)
- chills
- shaking
- extreme sweating
- fatigue
- general feeling of discomfort (called malaise) and body aches
- headache
- nausea, vomiting, and diarrhea
- abdominal pain
- anemia
- jaundice (yellowing of the skin and eyes)
- muscle or joint pain
- rapid breathing
- rapid heart rate
- cough
Some people who have malaria experience cycles of malaria “attacks.” An attack usually starts with shivering and chills, followed by a high fever, followed by sweating and a return to normal temperature.
Most people experience symptoms 10 days to 4 weeks after after being bitten by an infected mosquito. It’s possible to not have symptoms for up to 1 year after you’re infected (some types of malaria parasites can lie dormant in your body for up to a year). Two types of malaria can occur again. The parasites can go dormant in the liver for several months up to 4 years after infection. When they become active again, the person gets sick again.
All the clinical symptoms associated with malaria are caused by the asexual erythrocytic or blood stage parasites. When the parasite develops in the erythrocyte, numerous known and unknown waste substances such as hemozoin pigment and other toxic factors accumulate in the infected red blood cell. These are dumped into the bloodstream when the infected cells lyse and release invasive merozoites. The hemozoin and other toxic factors such as glucose phosphate isomerase (GPI) stimulate macrophages and other cells to produce cytokines and other soluble factors which act to produce fever and rigors and probably influence other severe pathophysiology associated with malaria.
Plasmodium falciparum-infected erythrocytes, particularly those with mature trophozoites, adhere to the vascular endothelium of venular blood vessel walls and do not freely circulate in the blood. When this sequestration of infected erythrocytes occurs in the vessels of the brain it is believed to be a factor in causing the severe disease syndrome known as cerebral malaria, which is associated with high mortality.
Talk to your doctor if you experience a fever while living in or after traveling to a high-risk malaria region. If you have severe symptoms, seek emergency medical attention.
Malaria in pregnancy
Pregnancy decreases immunity against many infectious diseases. Women who have developed protective immunity against Plasmodium falciparum tend to lose this protection when they become pregnant (especially during the first and second pregnancies). Malaria during pregnancy is harmful not only to the mothers but also to the unborn children. The latter are at greater risk of being delivered prematurely or with low birth weight, with consequently decreased chances of survival during the early months of life. For this reason pregnant women are also targeted (in addition to young children) for protection by malaria control programs in endemic countries.
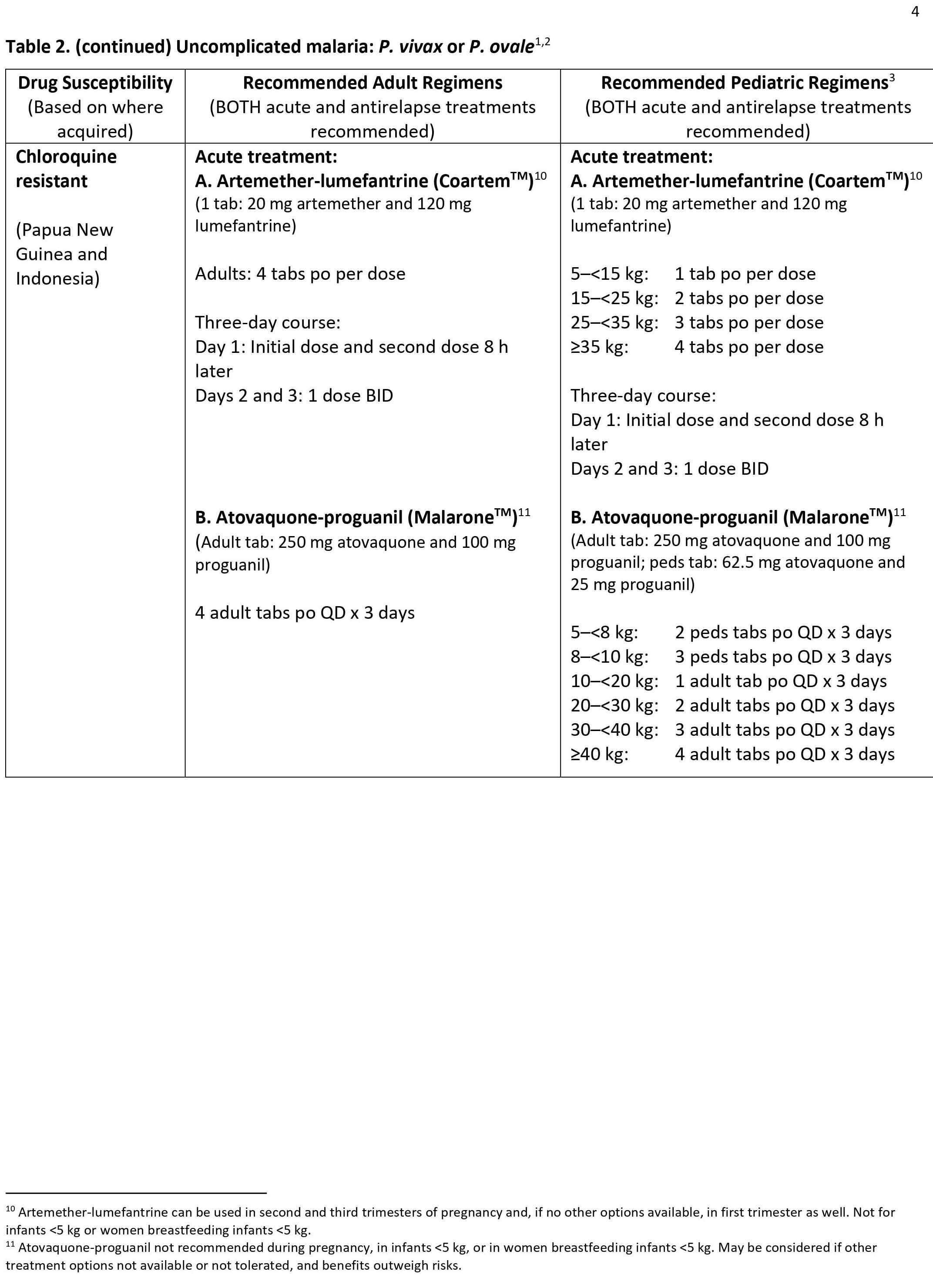
Types of malaria
Uncomplicated malaria
More commonly, the patient presents with a combination of the following symptoms:
- Fever
- Chills
- Sweats
- Headaches
- Nausea and vomiting
- Body aches
- General malaise
In countries where cases of malaria are infrequent, these symptoms may be attributed to influenza, a cold, or other common infections, especially if malaria is not suspected. Conversely, in countries where malaria is frequent, residents often recognize the symptoms as malaria and treat themselves without seeking diagnostic confirmation (“presumptive treatment”).
The classical (but rarely observed) malaria attack lasts 6–10 hours. It consists of:
- A cold stage (sensation of cold, shivering)
- A hot stage (fever, headaches, vomiting; seizures in young children); and
- Finally a sweating stage (sweats, return to normal temperature, tiredness).
Classically (but infrequently observed) the attacks occur every second day with the “tertian” parasites (Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale) and every third day with the “quartan” parasite (Plasmodium malariae).
Physical findings may include the following:
- Elevated temperatures
- Perspiration
- Weakness
- Enlarged spleen
- Mild jaundice
- Enlargement of the liver
- Increased respiratory rate
Severe malaria
Severe malaria is a medical emergency and should be treated urgently and aggressively. Severe malaria occurs when infections are complicated by serious organ failures or abnormalities in the patient’s blood or metabolism. The manifestations of severe malaria include the following 11:
- Cerebral malaria, with abnormal behavior, impairment of consciousness, seizures, coma, or other neurologic abnormalities
- Severe anemia (hemoglobin [Hb] <7g/dL) due to hemolysis (destruction of the red blood cells)
- Hemoglobinuria (hemoglobin in the urine) due to hemolysis
- Acute respiratory distress syndrome (ARDS), an inflammatory reaction in the lungs that inhibits oxygen exchange, which may occur even after the parasite counts have decreased in response to treatment
- Abnormalities in blood coagulation
- Low blood pressure caused by cardiovascular collapse
- Acute kidney injury
- Hyperparasitemia, where more than 5% of the red blood cells are infected by malaria parasites
- Metabolic acidosis (excessive acidity in the blood and tissue fluids), often in association with hypoglycemia
- Hypoglycemia (low blood glucose). Hypoglycemia may also occur in pregnant women with uncomplicated malaria, or after treatment with quinine.
Malaria causes
Malaria is caused by a parasite called Plasmodium that is carried by female Anopheles mosquitos. Five Plasmodium species possess the ability to infect humans: Plasmodium falciparum, Plasmodium ovale, Plasmodium vivax, Plasmodium malariae, and Plasmodium knowlesi 4. If an infected female Anopheles mosquito carrying Plasmodium parasite bites you, the parasite can get into your blood. The parasite lays eggs, which develop into more parasites. They feed on your red blood cells until you get very sick. Because the parasites live in your blood, malaria can also be spread through other ways. These include blood transfusions, organ transplants, shared use of contaminated needles, and from mother to fetus. Malaria not a contagious disease, so it can’t be spread from person to person like a cold or other illness.
Mosquito transmission cycle
- Uninfected mosquito. A mosquito becomes infected by feeding on a person who has malaria.
- Transmission of parasite. If this mosquito bites you in the future, it can transmit malaria parasites to you.
- In the liver. Once the parasites enter your body, they travel to your liver — where some types can lie dormant for as long as a year.
- Into the bloodstream. When the parasites mature, they leave the liver and infect your red blood cells. This is when people typically develop malaria symptoms.
- On to the next person. If an uninfected mosquito bites you at this point in the cycle, it will become infected with your malaria parasites and can spread them to the other people it bites.
The female Anopheles mosquito ingests gametes during a blood meal, which form sporozoites that replicate in the gut 3. During subsequent bloodmeals, saliva containing sporozoites gets released into a human host’s bloodstream 3. Within 60 minutes, sporozoites reach the liver, invade hepatocytes, and then rapidly divide, forming merozoites. In an active infection, organisms reenter the bloodstream and invade red blood cells (erythrocytes) 12. Within erythrocytes, Plasmodia consume hemoglobin and develop from immature trophozoites (ring stage) to either mature trophozoites or gametocytes 13. Mature trophozoites replicate, forming schizonts, disrupting erythrocyte cell membrane integrity, and leading to capillary endothelial adherence and cell lysis 3. Untreated malaria lasts 2 to 24 months 3. Plasmodium vivax and Plasmodium ovale infections may display “dormant schizogony,” where inactive intrahepatic parasites (hypnozoites) remain until reactivation months to years in the future 3.
Other modes of transmission
Because the parasites that cause malaria affect red blood cells, people can also catch malaria from exposure to infected blood, including:
- From mother to unborn child
- Through blood transfusions
- By sharing needles used to inject drugs
Risk factors for developing malaria
The greatest risk factor for developing malaria is to live in or to visit areas where malaria is common. These include the tropical and subtropical regions of:
- Sub-Saharan Africa
- South and Southeast Asia
- Pacific Islands
- Central America and northern South America
- Hispaniola (Haiti and the Dominican Republic)
- Eastern Europe
The degree of risk depends on local malaria control, seasonal changes in malaria rates and the precautions you take to prevent mosquito bites.
Risks of more-severe disease
People at increased risk of serious disease include:
- Young children and infants
- Older adults
- Travelers coming from areas with no malaria
- Pregnant women and their unborn children
In many countries with high malaria rates, the problem is worsened by lack of access to preventive measures, medical care and information.
Immunity can wane
Residents of a malaria region may be exposed to the disease enough to acquire a partial immunity, which can lessen the severity of malaria symptoms. However, this partial immunity can disappear if you move to a place where you’re no longer frequently exposed to the parasite.
Malaria may recur
Some varieties of the malaria parasite, which typically cause milder forms of the disease, can persist for years and cause relapses.
Prevention and control of malaria
To protect yourself from getting malaria, you should do whatever you can to keep from getting mosquito bites. If you live in or are traveling to an area where malaria is common, take steps to avoid mosquito bites. Mosquitoes are most active between dusk and dawn. To protect yourself from mosquito bites, you should:
- Sleep in a room with screens on the windows and doors.
- Sleep under mosquito netting. Bed nets, particularly those treated with insecticides, such as permethrin (a spray that repels mosquitoes), help prevent mosquito bites while you are sleeping.
- Avoid going outdoors without protection in the evening, when mosquitoes are typically more active.
- Cover your skin. Wear pants and long-sleeved shirts. Tuck in your shirt, and tuck pant legs into socks.
- Apply insect repellent to skin. Use an insect repellent registered with the Environmental Protection Agency (EPA) on any exposed skin. These include repellents that contain DEET, picaridin, IR3535, oil of lemon eucalyptus (OLE), para-menthane-3,8-diol (PMD) or 2-undecanone.
- DO NOT use a spray directly on your face.
- DO NOT use products with oil of lemon eucalyptus (OLE) or para-menthane-3,8-diol (PMD) on children under age 3.
- Apply repellent to clothing. Sprays containing permethrin are safe to apply to clothing.
- If you’re pregnant, the CDC advises that you not travel to areas where you could get malaria. Symptoms of malaria are more severe in pregnant women. It can also cause problems with the pregnancy. These include miscarriage, preterm birth, or stillbirth. If you must travel to one of these places, you need to take the preventive medicine.
- See your doctor for medicines that protect you (malaria prophylaxis)
If you’ll be traveling to a location where malaria is common, talk to your doctor a few months ahead of time about whether you should take drugs before, during and after your trip to help protect you from malaria parasites. In general, the drugs taken to prevent malaria are the same drugs used to treat the disease. What drug you take depends on where and how long you are traveling and your own health.
Malaria prophylaxis
Malaria could be fatal even when treated, which is why it is always preferable to prevent malaria cases rather than rely on treating infections after they occur. Several medications are available for malaria prophylaxis. Prophylactic means it’s used to prevent disease. But remember that no medicine can protect you 100%. You should still take other precautions to prevent being bitten by mosquitos.
See your doctor well before your trip. You will need to start taking the medicine a few days or weeks before you leave the country. You take the medicine during your trip and for 1 to 4 weeks after. How long you take it after your trip depends on which medicine you’re taking. It’s important to keep taking the medicine after your trip. The malaria parasites could still be in your blood. If you stop taking the medicine too soon, it could give the parasites a chance to grow and make you sick.
Malaria medicines have some side effects, and not everyone can take them. Your doctor can tell you which medicine is right for you. What type of medicine you take also depends on where you’ll be traveling.
Considerations when choosing a drug for malaria prophylaxis 14:
- Recommendations for drugs to prevent malaria differ by country of travel and can be found in Malaria Information by Country. Recommended drugs for each country are listed in alphabetical order and have comparable efficacy in that country.
- No antimalarial drug is 100% protective and must be combined with the use of personal protective measures, (i.e., insect repellent, long sleeves, long pants, sleeping in a mosquito-free setting or using an insecticide-treated bednet).
- For all medicines, also consider the possibility of drug-drug interactions with other medicines that the person might be taking as well as other medical contraindications, such as drug allergies.
- When several different drugs are recommended for an area, the following table might help in the decision process.
Table 1. Malaria prophylaxis
| Drug | Reasons that might make you consider using this drug | Reasons that might make you avoid using this drug |
Atovaquone/Proguanil (Malarone)
|
|
|
Chloroquine
|
|
|
Doxycycline
|
|
|
Mefloquine
|
|
|
Primaquine
|
|
|
Tafenoquine (Arakoda)
|
|
|
Malaria vaccine
On October 6, 2021, the World Health Organization (WHO) release a recommendation for widespread use of the RTS,S/AS01 (RTS,S) malaria vaccine among children living in sub-Saharan Africa and other regions with moderate to high Plasmodium falciparum malaria transmission 15. The GlaxoSmithKline Biologicals’ RTS,S/AS01, completed Phase III clinical testing 16 and while the pilot studies are still on-going until 2023, sufficient data on safety and efficacy have been collected to allow for a broader recommendation for the use of the vaccine to take place.
The RTS,S malaria vaccine reduced clinical and severe cases of malaria by about one-third in 5–17-month-old children over four years who received the three-dose vaccine series plus a booster dose. The malaria vaccine was less effective in children in the young infant group. The malaria vaccine was generally found to be safe, but there were a few safety signals that warranted further study, including febrile convulsions, meningitis, and cerebral malaria.
Notably, the malaria vaccine provided this protection in settings with ongoing use of other effective malaria prevention and treatment interventions: bed nets, antimalarial drugs for disease treatment, indoor residual insecticide spraying to prevent mosquito-borne transmission, and drugs to protect pregnant women and their newborns from malaria’s adverse effects.
In July 2015, the European Medicines Agency (EMA) gave a positive regulatory assessment of the RTS,S/AS01 malaria vaccine for 5–17-month-olds, but WHO recommended in October 2015 that the vaccine be further evaluated in large-scale pilot studies before recommending it. Large-scale pilots of the vaccine began in Ghana, Kenya, and Malawi in 2019, including several hundreds of thousands of infants. The Centers for Disease Control and Prevention (CDC), in collaboration with Kenya Medical Research Institute (KEMRI) and several other organizations, is leading the evaluation of the large-scale RTS,S/AS01 pilot in western Kenya. The goal of these pilot evaluations is to assess the feasibility of delivering the three-dose vaccine series plus booster through routine health systems, carefully examine the relationship of the vaccine to specific adverse events (febrile seizures, meningitis, cerebral malaria), and also evaluate its impact on all-cause mortality.
Key findings from the malaria vaccine pilots include 17:
- Feasible to deliver: Vaccine introduction is feasible, improves health and saves lives, with good and equitable coverage of RTS,S/AS01 malaria vaccine seen through routine immunization systems.
- Reaching the unreached: RTS,S/AS01 malaria vaccine increases equity in access to malaria prevention.
- Data from the pilot programme showed that more than two-thirds of children in the 3 countries who are not sleeping under a bednet are benefitting from the RTS,S vaccine.
- Layering the tools results in over 90% of children benefitting from at least one preventive intervention (insecticide treated bednets or the malaria vaccine).
- Strong safety profile: To date, more than 2.3 million doses of the vaccine have been administered in 3 African countries – the vaccine has a favorable safety profile.
- No negative impact on uptake of bednets, other childhood vaccinations, or health seeking behavior for febrile illness. In areas where the vaccine has been introduced, there has been no decrease in the use of insecticide-treated nets, uptake of other childhood vaccinations or health seeking behavior for febrile illness.
- High impact in real-life childhood vaccination settings: Significant reduction (30%) in deadly severe malaria, even when introduced in areas where insecticide-treated nets are widely used and there is good access to diagnosis and treatment.
- Highly cost-effective: Modelling estimates that the vaccine is cost effective in areas of moderate to high malaria transmission.
Next steps include funding decisions from the global health community for broader rollout, and country decision-making on whether to adopt the vaccine as part of national malaria control strategies.Further research into ways to maximize efficacy of the RTS,S/AS01 vaccine, including assessing alternative dosing schedules such as a fractionated third dose, is underway.
Whole sporozoite malaria vaccines
Another promising malaria vaccine candidate includes whole sporozoites, the sexual form of the Plasmodium parasite extracted from mosquito salivary glands, which have either been made non-infectious through irradiation or are administered along with chemoprophylaxis. Recent trials have shown that the irradiated whole sporozoite PfSPZ Vaccine made by Sanaria® is safe and well tolerated and had promising protection against malaria when administered intravenously. A trial in western Kenya has shown that the PfSPZ Vaccine is safe and well tolerated in infants and young children. Unfortunately, the vaccine did not provide significant protection against Plasmodium falciparum infection at 6 months, precluding further evaluation in this age groupexternal icon, although other studies are currently evaluating the efficacy of the PfSPZ Vaccine in different populations in Mali, Gabon, Tanzania, and Equatorial Guinea. This study did, however, provide important information on the immune response to the vaccine; this information will aid researchers in developing a more effective vaccine for young children.
Malaria control
Understanding the biology and behavior of Anopheles mosquitoes can aid in designing appropriate control strategies. Factors that affect a mosquito’s ability to transmit malaria include its innate susceptibility to Plasmodium, its host choice, and its longevity. Long-lived species that prefer human blood and support parasite development are the most dangerous. Factors that should be taken into consideration when designing a control program include the susceptibility of malaria mosquitoes to insecticides and the preferred feeding and resting location of adult mosquitoes.
Malaria control is carried out through the following recommended malaria treatment and prevention interventions. The choice of interventions depends on the malaria transmission level in the area (e.g., in areas of low transmission level, intermittent preventive treatment for pregnant women is usually not recommended).
- Case management (diagnosis and treatment) of patients with malaria
- Prevention
- Insecticide-treated nets
- Intermittent preventive treatment of malaria in pregnant women
- Intermittent preventive treatment of malaria in infancy
- Indoor residual spraying
In most malaria-endemic countries, four interventions—case management (diagnosis and treatment), insecticide-treated nets, intermittent preventive treatment of malaria in pregnant women and indoor residual spraying—make up the essential package of malaria interventions.
Occasionally, other interventions are used:
- Larval control and other vector control interventions
- Mass drug administration and Mass fever treatment
In addition, several companies and groups are at work on developing a malaria vaccine, but there is currently no effective malaria vaccine on the market.
Anopheles mosquitoes sources for blood meals
One important behavioral factor is the degree to which an Anopheles species prefers to feed on humans (anthropophily) or animals such as cattle (zoophily). Anthrophilic Anopheles are more likely to transmit the malaria parasites from one person to another. Most Anopheles mosquitoes are not exclusively anthropophilic or zoophilic; many are opportunistic and feed upon whatever host is available. However, the primary malaria vectors in Africa, An. gambiae and An. funestus, are strongly anthropophilic and, consequently, are two of the most efficient malaria vectors in the world.
Anopheles mosquitoes life span
Once ingested by a mosquito, malaria parasites must undergo development within the mosquito before they are infectious to humans. The time required for development in the mosquito (the extrinsic incubation period) takes 9 days or longer, depending on the parasite species and the temperature. If a mosquito does not survive longer than the extrinsic incubation period, then she will not be able to transmit any malaria parasites.
It is not possible to measure directly the life span of mosquitoes in nature, but many studies have indirectly measured longevity by examination of their reproductive status or via marking, releasing, and recapturing adult mosquitoes. The majority of mosquitoes do not live long enough to transmit malaria, but some may live as long as three weeks in nature. Though evidence suggests that mortality rate increases with age, most workers estimate longevity in terms of the probability that a mosquito will live one day. Usually these estimates range from a low of 0.7 to a high of 0.9. If survivorship is 90% daily, then a substantial proportion of the population would live longer than 2 weeks and would be capable of transmitting malaria. Any control measure that reduces the average lifespan of the mosquito population will reduce transmission potential. Insecticides thus need not kill the mosquitoes outright, but may be effective by limiting their lifespan.
Anopheles mosquitoes patterns of feeding and resting
Most Anopheles mosquitoes are crepuscular (active at dusk or dawn) or nocturnal (active at night). Some Anopheles mosquitoes feed indoors (endophagic) while others feed outdoors (exophagic). After blood feeding, some Anopheles mosquitoes prefer to rest indoors (endophilic) while others prefer to rest outdoors (exophilic). Biting by nocturnal, endophagic Anopheles mosquitoes can be markedly reduced through the use of insecticide-treated bed nets or through improved housing construction to prevent mosquito entry (e.g., window screens). Endophilic mosquitoes are readily controlled by indoor spraying of residual insecticides. In contrast, exophagic/exophilic vectors are best controlled through source reduction (destruction of larval habitats).
Anopheles mosquitoes insecticide resistance
Insecticide-based control measures (e.g., indoor spraying with insecticides, insecticide-treated bed nets) are the principal way to kill mosquitoes that bite indoors. However, after prolonged exposure to an insecticide over several generations, mosquitoes, like other insects, may develop resistance, a capacity to survive contact with an insecticide. Since mosquitoes can have many generations per year, high levels of resistance can arise very quickly. Resistance of mosquitoes to some insecticides has been documented within a few years after the insecticides were introduced. There are over 125 mosquito species with documented resistance to one or more insecticides. The development of resistance to insecticides used for indoor residual spraying was a major impediment during the Global Malaria Eradication Campaign. Judicious use of insecticides for mosquito control can limit the development and spread of resistance, particularly via rotation of different classes of insecticides used for control. Monitoring of resistance is essential to alert control programs to switch to more effective insecticides.
Anopheles mosquitoes susceptibility and refractoriness
Some Anopheles species are poor vectors of malaria, as the parasites do not develop well (or at all) within them. There is also variation within species. In the laboratory, it has been possible to select for strains of Anopheles gambiae that are refractory to infection by malaria parasites. These refractory strains have an immune response that encapsulates and kills the parasites after they have invaded the mosquito’s stomach wall. Scientists are studying the genetic mechanism for this response. It is hoped that some day, genetically modified mosquitoes that are refractory to malaria can replace wild mosquitoes, thereby limiting or eliminating malaria transmission.
Malaria complications
Malaria can be fatal, particularly when caused by the plasmodium species common in Africa. The World Health Organization estimates that about 94% of all malaria deaths occur in Africa — most commonly in children under the age of 5. The significant complications of malaria are cerebral malaria, severe malarial anemia, and nephrotic syndrome 13.
Malaria deaths are usually related to one or more serious complications, including:
- Cerebral malaria. If parasite-filled blood cells block small blood vessels to your brain (cerebral malaria), swelling of your brain or brain damage may occur. Cerebral malaria may cause seizures and coma. Cerebral malaria accounts for 80% of fatal malaria cases, most often occurring with Plasmodium falciparum infection 3. Cerebral malaria presents as slow-onset altered mental status, violent behavior, headache, and extremely high fever (up to 107.6 °F [42 degrees Celsius]), followed by coma, metabolic acidosis, hypoglycemia, and possibly seizures and death 12. It most commonly affects children under age 5, with a case fatality rate of 18% 18. Pathogenesis involves malarial rosettes (one infected erythrocyte surrounded by three uninfected erythrocytes), causing cerebral sequestration and vasodilation, as well as excessive oxygen free radicals, IFN-gamma, and TNF-alpha leading to an extreme inflammatory response 18. This leads to congestion, decreased perfusion, endothelial activation, impairment of the blood-brain barrier, and cerebral edema, which increases brain volume 18. Increased brain volume is the major contributor to mortality in cerebral malaria. In a 2015 study of Malawian children with cerebral malaria, 84% of those who died had severely increased brain volume on MRI; children who survived showed lower initial brain volume or a downtrend over time 18.
- Breathing problems. Accumulated fluid in your lungs (pulmonary edema) can make it difficult to breathe.
- Organ failure. Malaria can damage the kidneys or liver or cause the spleen to rupture. Any of these conditions can be life-threatening.
- Nephrotic syndrome occurs secondary to glomerular antigen-antibody complex deposition, and presents similarly to membranoproliferative glomerulonephritis with proteinuria and decreased renal function, which may lead to renal failure. Nephrotic syndrome is common in Plasmodium malariae and Plasmodium knowlesi, possible in Plasmodium vivax, and rare in Plasmodium falciparum and Plasmodium ovale infections 3.
- Anemia. Malaria may result in not having enough red blood cells for an adequate supply of oxygen to your body’s tissues (anemia). Severe malarial anemia stems from TNF-alpha-mediated mechanisms involving both increased destruction and decreased the production of erythrocytes including cell lysis as parasites replicate and exit erythrocytes, splenic removal and autoimmune lysis of immune marked erythrocytes, poor iron incorporation into new heme molecules, and bone marrow suppression during severe infection leading to decreased production 12. Blackwater fever is severe anemia with hemoglobinuria and renal failure in the context of “massive intravascular hemolysis” in the setting of repeat Plasmodium falciparum infections treated with chronic quinine; it is rare and thought to be associated with G6PD deficiency 19.
- Low blood sugar. Severe forms of malaria can cause low blood sugar (hypoglycemia), as can quinine — a common medication used to combat malaria. Very low blood sugar can result in coma or death.
Additional complications include:
- Bilious remittent fever presents with abdominal pain and persistent vomiting that may lead to severe dehydration, jaundice, and dark urine.
- Algid malaria is an adrenal insufficiency due to parasitic congestion and subsequent necrosis of the adrenal glands.
- Acute respiratory distress syndrome, circulatory collapse, disseminated intravascular coagulation, pulmonary edema, coma, and death 3.
Malaria infection during pregnancy may result in low birth weight or fetal demise 3.
Malaria diagnosis
To diagnose malaria, your doctor will likely review your medical history and recent travel, conduct a physical exam, and order blood tests. Blood tests can indicate:
- The presence of the parasite in the blood, to confirm that you have malaria
- Which type of malaria parasite is causing your symptoms
- If your infection is caused by a parasite resistant to certain drugs
- Whether the disease is causing any serious complications
Some blood tests can take several days to complete, while others can produce results in less than 15 minutes. Depending on your symptoms, your doctor may order additional diagnostic tests to assess possible complications.
Diagnosis of malaria depends on the demonstration of parasites in the blood, usually by microscopy. Additional laboratory findings may include mild anemia, mild decrease in blood platelets (thrombocytopenia), elevation of bilirubin, and elevation of aminotransferases.
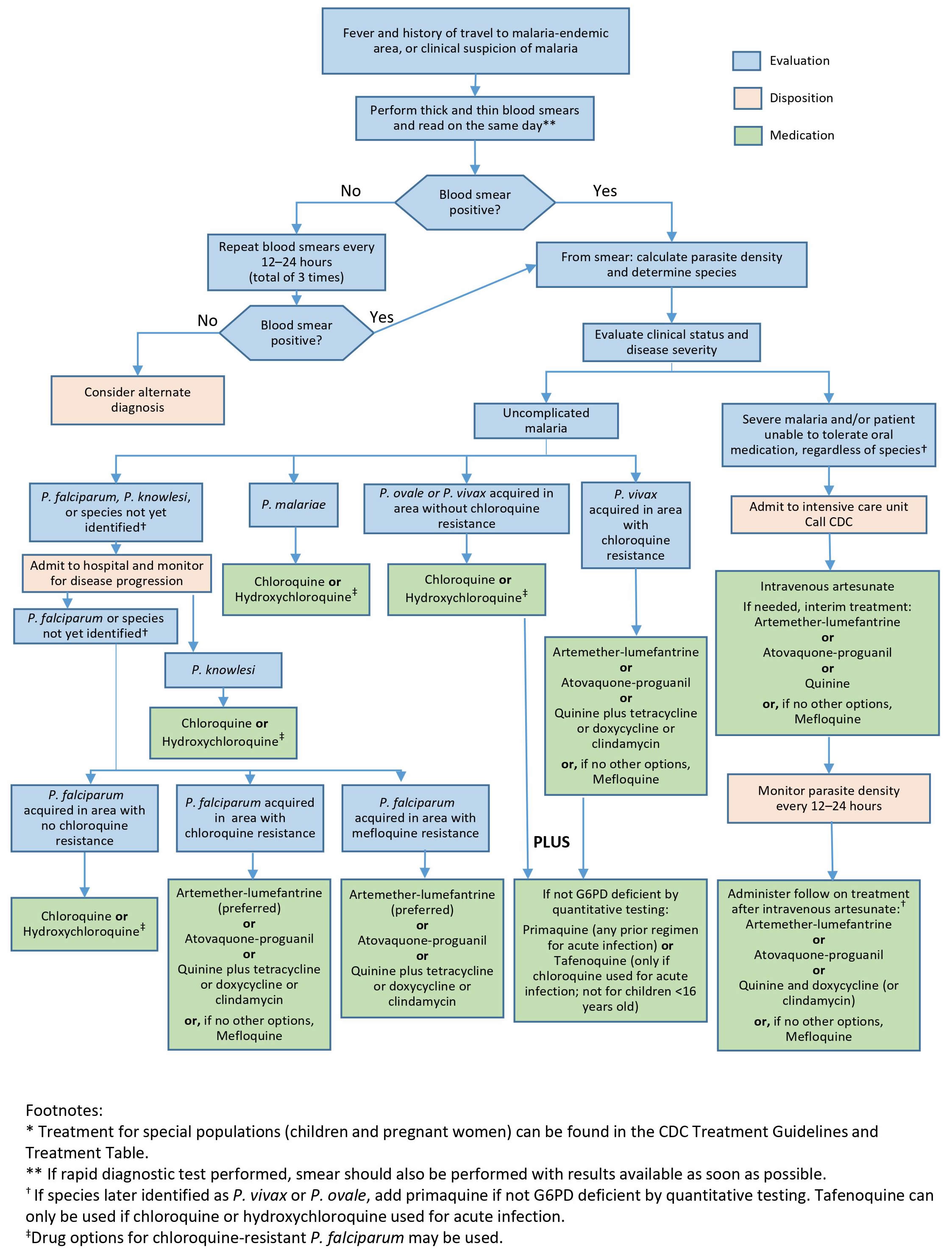
Figure 4. Diagnosis and management algorithm of malaria
[Source 20 ]Microscopic diagnosis
Malaria parasites can be identified by examining under the microscope a drop of the patient’s blood, spread out as a “blood smear” (thick or thin blood smear) on a microscope slide. Prior to examination, the specimen is stained with a Romanovsky stain (most often with the Giemsa stain) to give the parasites a distinctive appearance. Microscopic examination remains the “gold standard” for laboratory confirmation of malaria. However, it depends on the quality of the reagents, of the microscope, and on the experience of the laboratorian. In the United States, there are, on average, 2000 cases of malaria diagnosed and reported each year. Thus, the average laboratorian does not perform this test regularly, and may not be maintaining optimal proficiency.
An initial negative smear does not rule out malaria, as infected erythrocytes may become intravascularly sequestered; if clinical suspicion of malaria is high, smears require repetition in 12 and 24 hours 4. The malarial pigment in monocytes and neutrophils may also manifest on the blood smear, particularly in patients with cerebral malaria 3.
These tests should be performed immediately when ordered by a health-care provider. They should not be saved for the most qualified staff to perform or batched for convenience. In addition, these tests should not be sent out to reference laboratories with results available only days to weeks later. It is vital that health-care providers receive results from these tests within hours in order to appropriately treat their patients infected with malaria.
Varying microscopic appearance of infected erythrocytes guides speciation 21:
- The ring stage in Plasmodium falciparum appears as a “purple spot with a thin ring;” in Plasmodium vivax as a “purple spot with a deformed body;” in Plasmodium ovale as a “ring with a large purple spot;” in Plasmodium malariae as a “purple spot with a thick body;” and in Plasmodium knowlesi as a “purple spot (or spots) with an amorphous thick ring.”
- The trophozoite stage in Plasmodium falciparum appears as “a bigger spot [growing] around a smaller spot;” in Plasmodium vivax as “a misshapen circle which contains an extended spot;” in Plasmodium ovale as “an oval circle (sometimes with small corners) which contains a purple spot with undefined shapes;” in Plasmodium malariae as “basket or band-shaped [without a] spot;” and in Plasmodium knowlesi as a “purple branched spot.”
- The schizont stage in Plasmodium falciparum is not established; in Plasmodium vivax it appears as “not defined purple spots inside a circle;” in Plasmodium ovale as “more than one spot inside an oval circle (sometimes with small corners);” in Plasmodium malariae as “diffuse purple spots around a darker spot;” and in Plasmodium knowlesi as “defined purple spots [that are] easy to count.”
- The gametocyte stage in Plasmodium falciparum appears as “banana [or] sausage-shaped;” in Plasmodium vivax as an “extended, big spot;” in Plasmodium ovale as a “row of accumulated spots;” in Plasmodium malariae as a “big stained spot which almost fill[s] the circle;” and in Plasmodium knowlesi as a “big spot which contains small spots.”
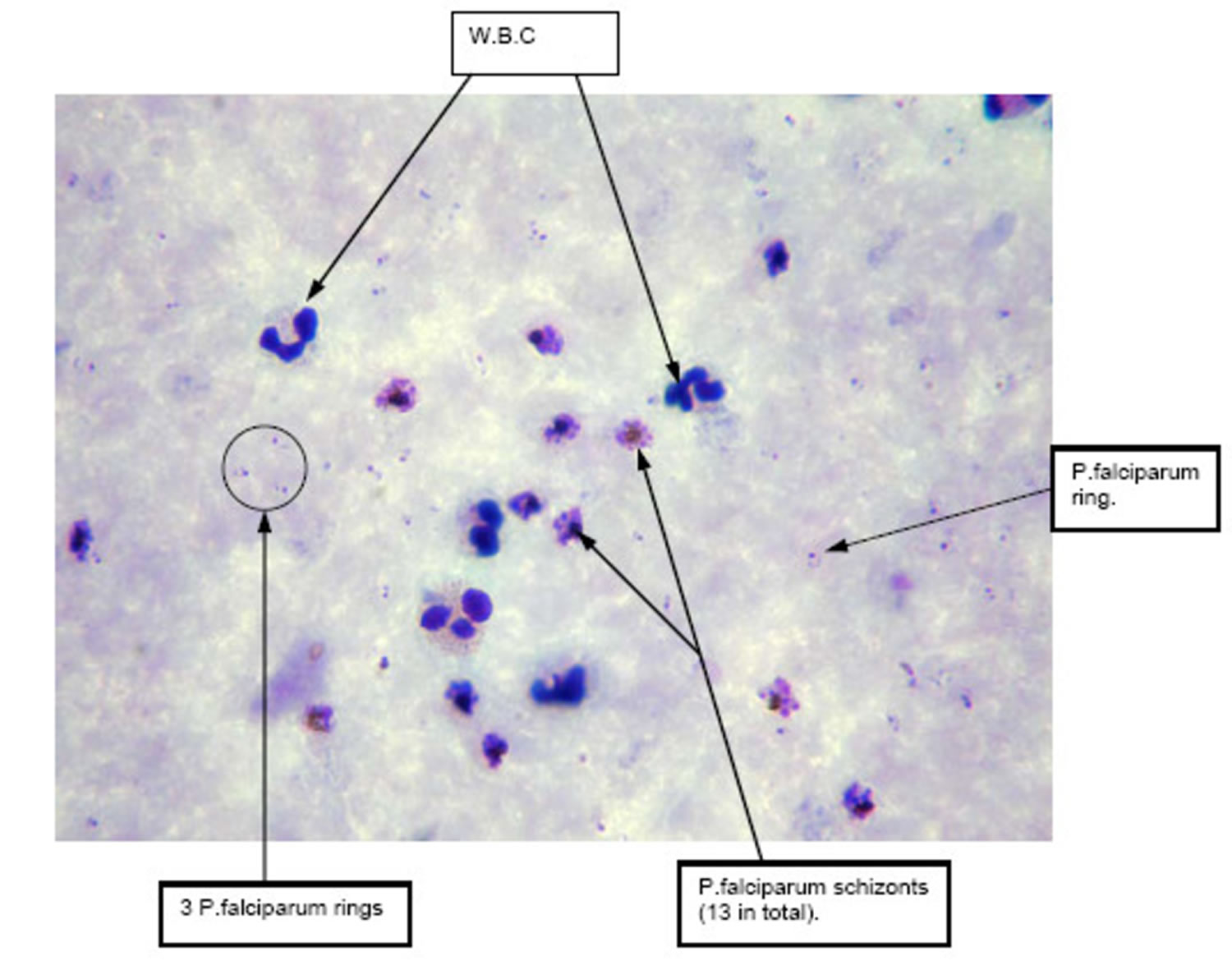
Figure 5. Plasmodium falciparum malaria thick blood smear
Footnote: Thick blood film of patient on admission demonstrating significant presence of schizonts compared with trophozoites (oil immersion × 1000).
[Source 22 ]Antigen detection
Various test kits are available to detect antigens derived from malaria parasites. Such immunologic (“immunochromatographic”) tests most often use a dipstick or cassette format, and provide results in 2-15 minutes. These “Rapid Diagnostic Tests” (RDTs) offer a useful alternative to microscopy in situations where reliable microscopic diagnosis is not available. Malaria Rapid Diagnostic Tests are currently used in some clinical settings and programs. A blood specimen collected from the patient is applied to the sample pad on the test card along with certain reagents. After 15 minutes, the presence of specific bands in the test card window indicate whether the patient is infected with Plasmodium falciparum or one of the other 3 species of human malaria. It is recommended that the laboratory maintain a supply of blood containing Plasmodium falciparum for use as a positive control.
However, the use of the Rapid Diagnostic Test does not eliminate the need for malaria microscopy. The Rapid Diagnostic Test may not be able to detect some infections with lower numbers of malaria parasites circulating in the patient’s bloodstream. Also, there is insufficient data available to determine the ability of this test to detect the 2 less common species of malaria, Plasmodium ovale and Plasmodium malariae. Therefore all negative Rapid Diagnostic Tests must be followed by microscopy to confirm the result.
In addition, all positive Rapid Diagnostic Tests also should be followed by microscopy. The currently approved Rapid Diagnostic Test detects 2 different malaria antigens; one is specific for Plasmodium falciparum and the other is found in all 4 human species of malaria. Thus, microscopy is needed to determine the species of malaria that was detected by the Rapid Diagnostic Test. In addition, microscopy is needed to quantify the proportion of red blood cells that are infected, which is an important prognostic indicator.
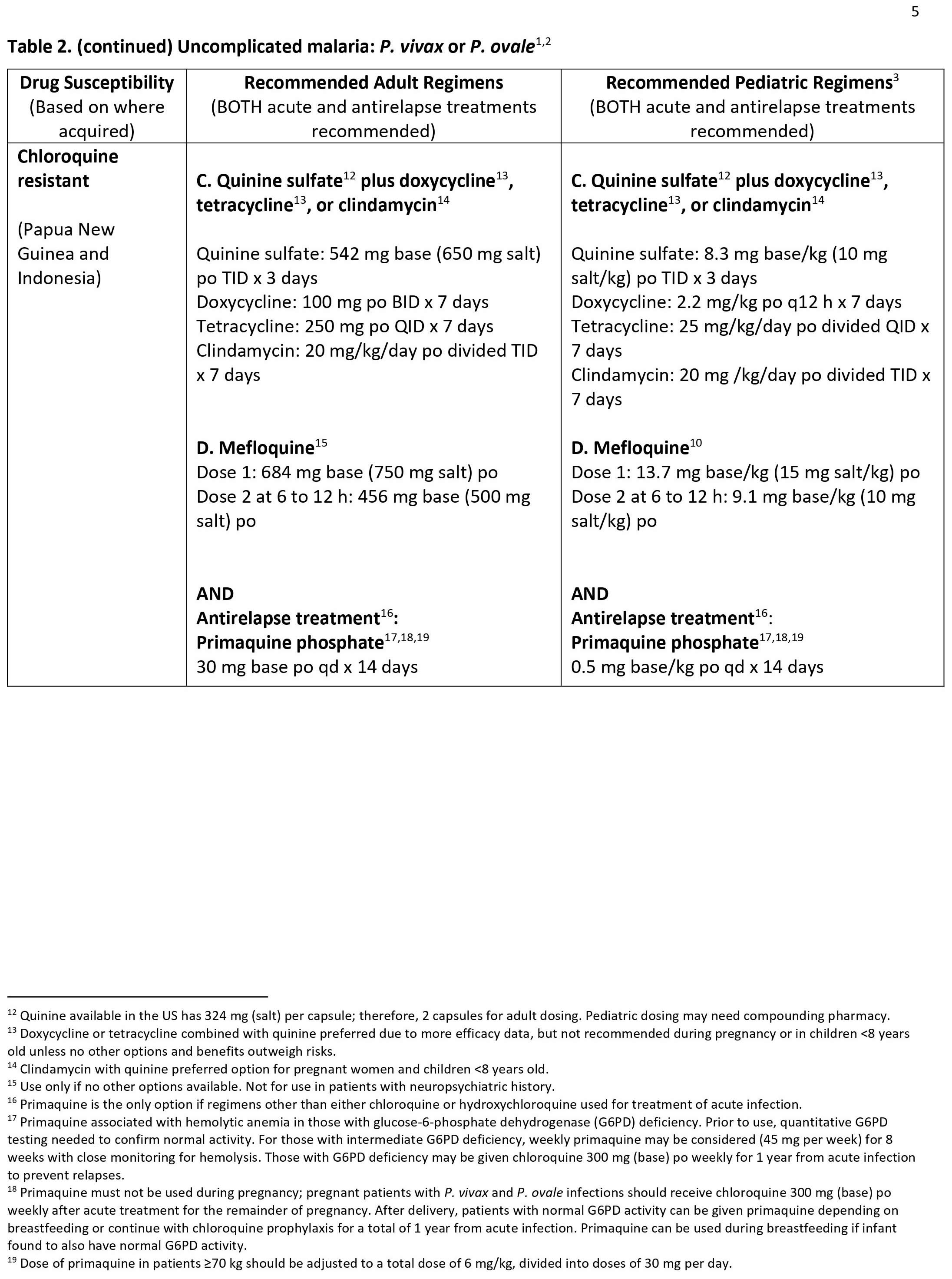
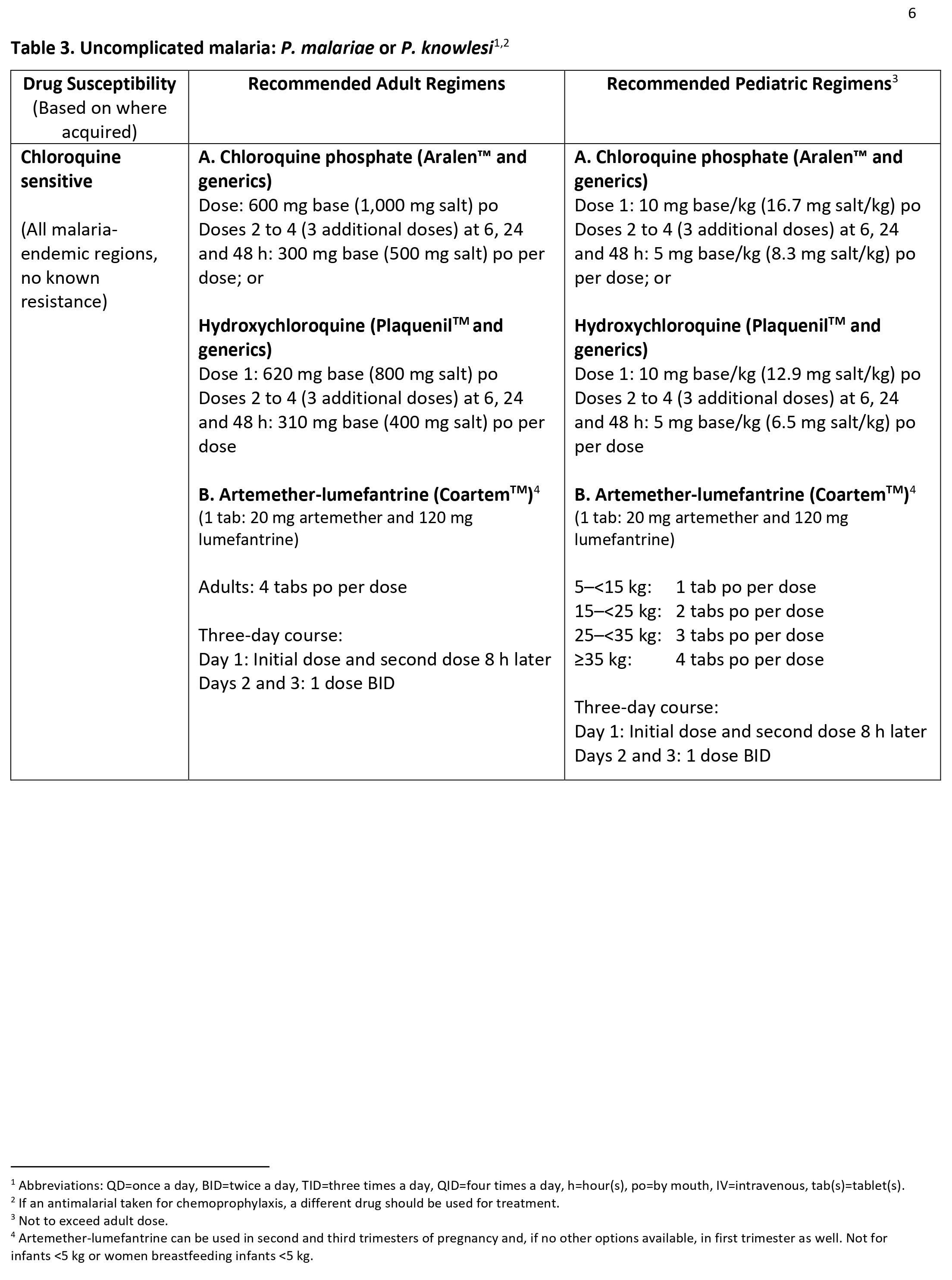
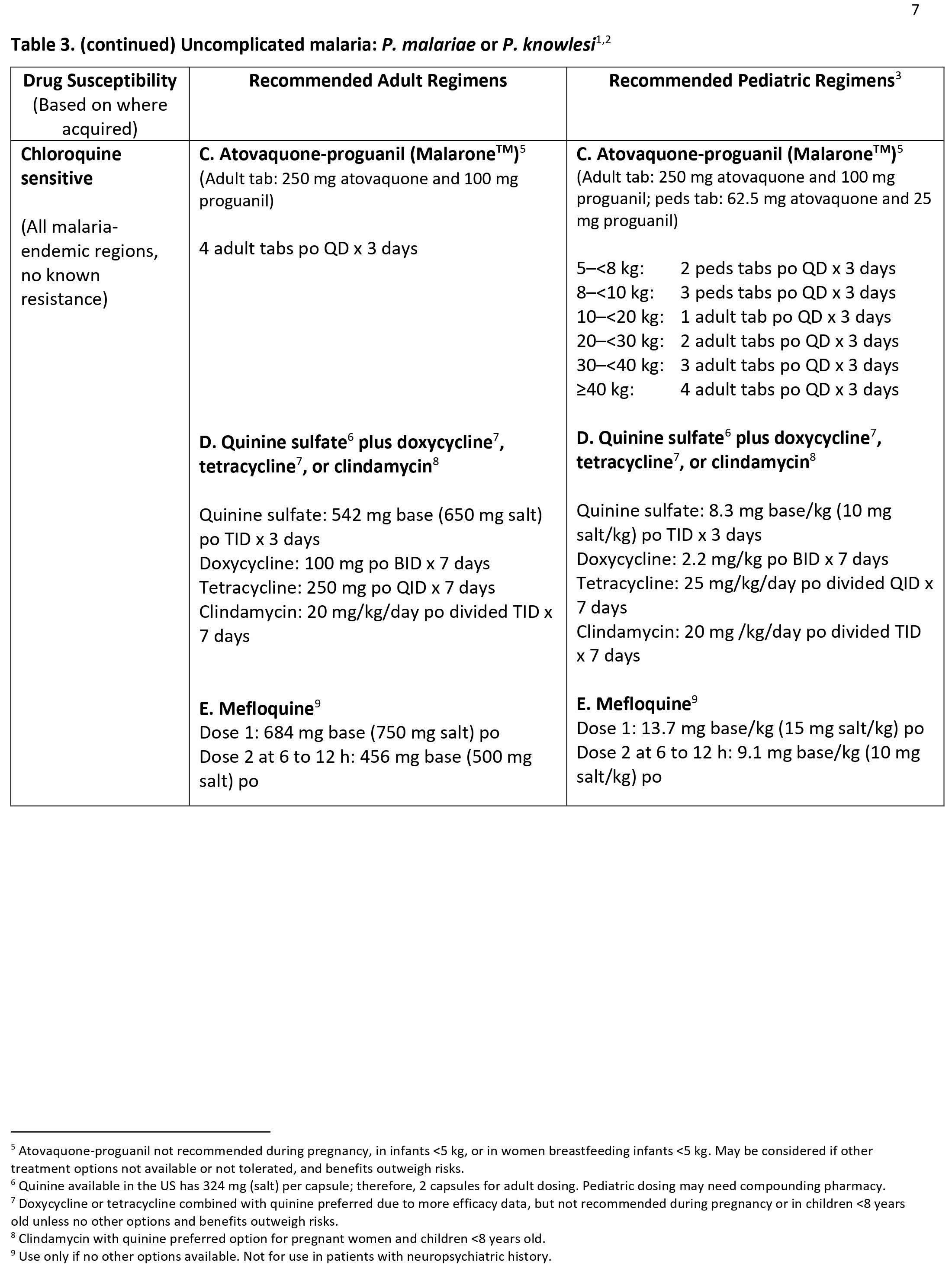
Malaria treatment
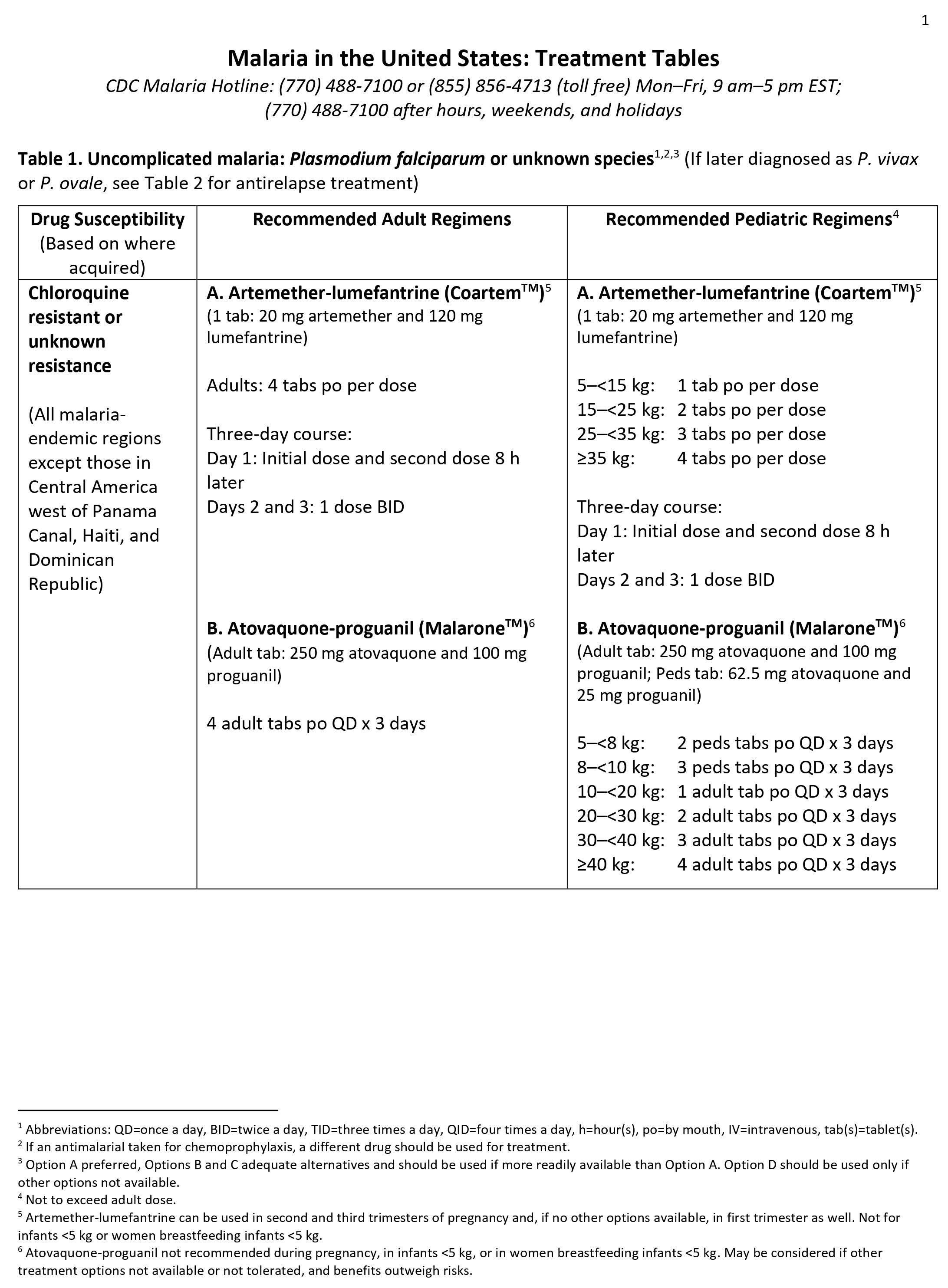
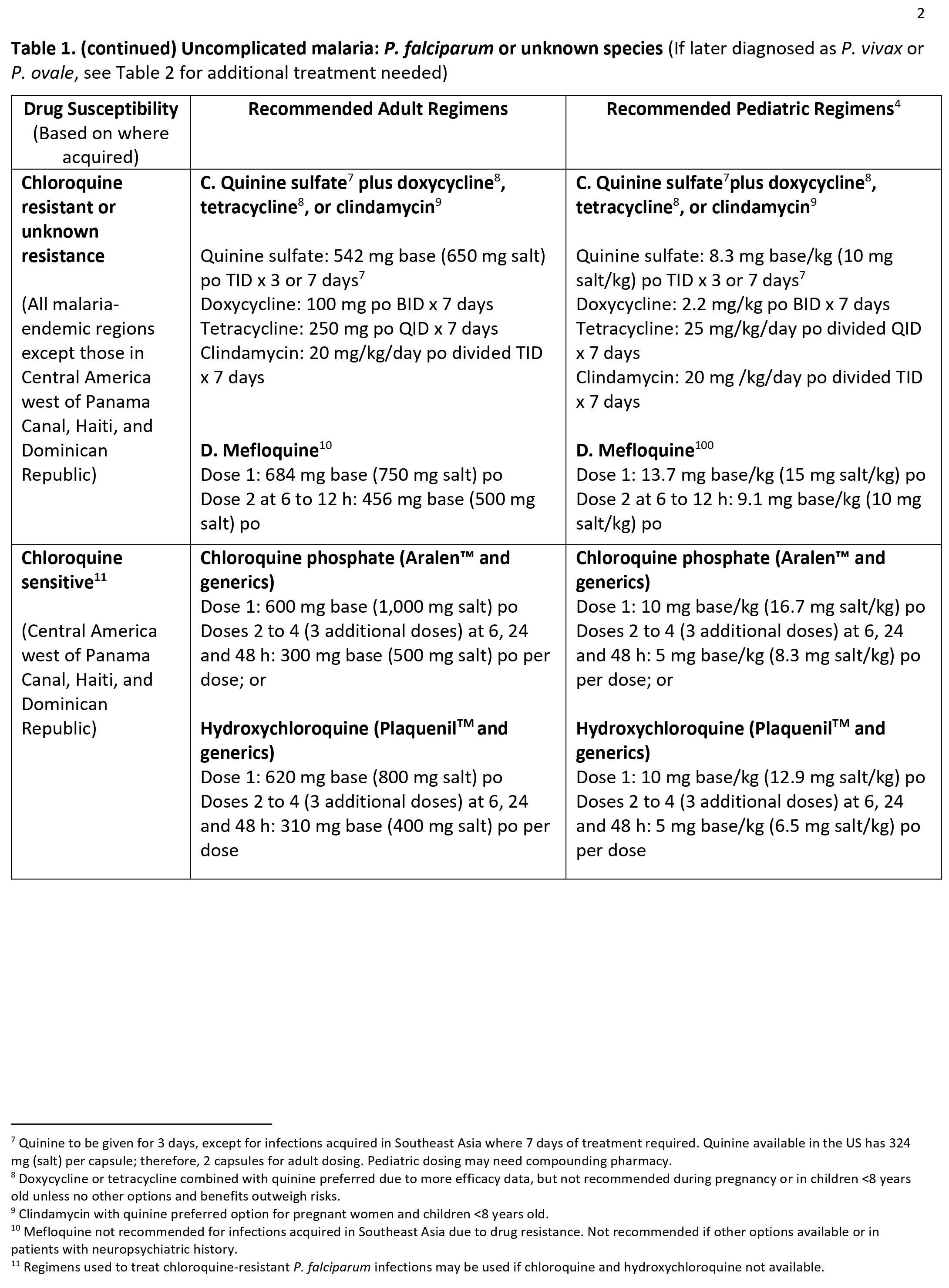
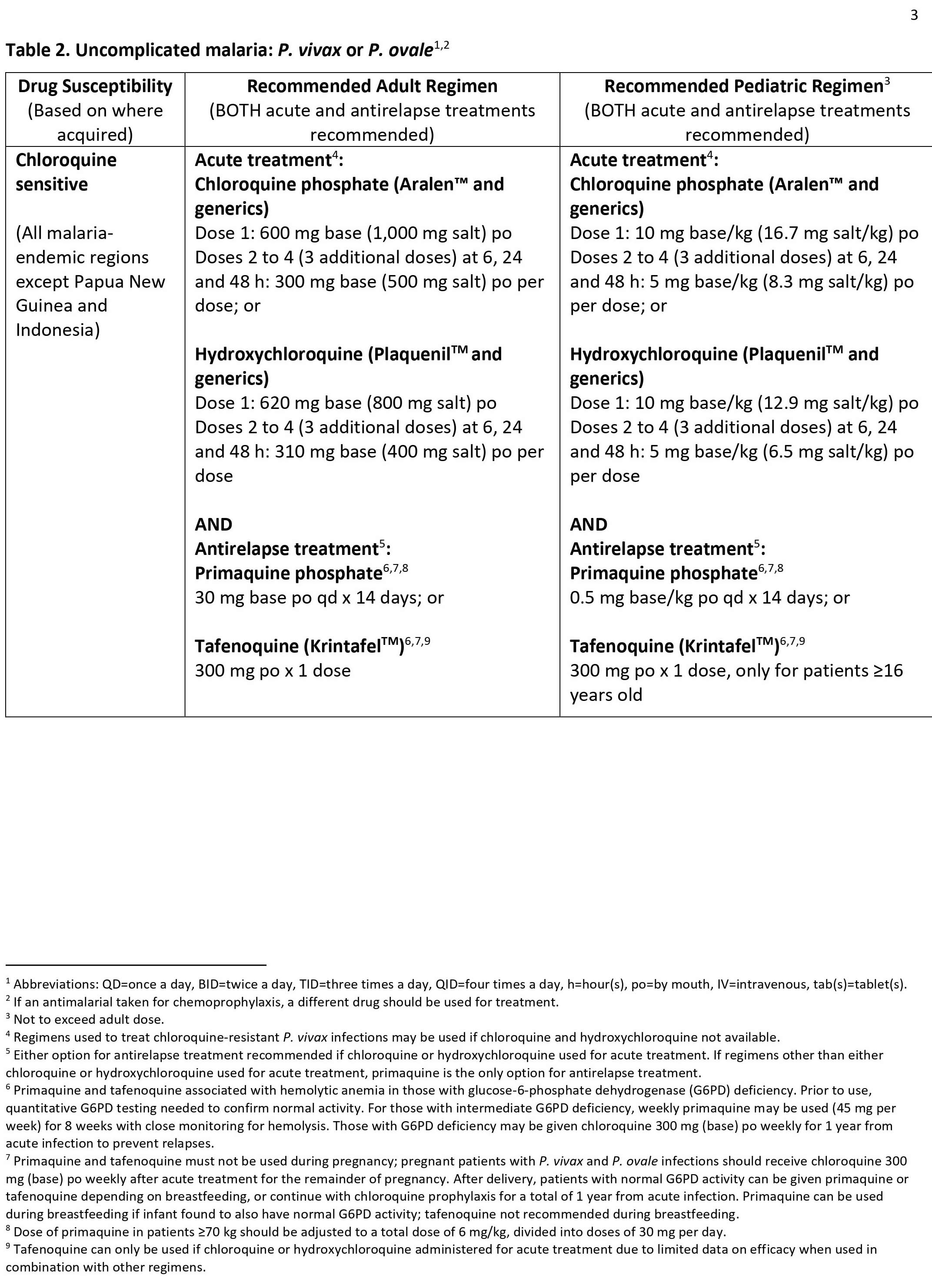
Malaria can become serious quickly. Treatment should start as early as possible. Malaria can be cured with prescription medicines to kill the parasite. What kind of drug you take and how long you take it depend on several factors, including:
- The type of malaria parasite you have. Determination of the infecting Plasmodium species for treatment purposes is important for four main reasons.
- Firstly, Plasmodium falciparum and Plasmodium knowlesi infections can cause rapidly progressive severe illness or death, while the other species, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae, are less likely to cause severe disease.
- Secondly, Plasmodium vivax and Plasmodium ovale infections also require treatment for the hypnozoites, which remain dormant in the liver and can cause relapsing episodes.
- Thirdly, Plasmodium falciparum and Plasmodium vivax species have different drug resistance patterns in different geographic regions of the world.
- Finally, for Plasmodium falciparum and Plasmodium knowlesi infections, the urgent initiation of appropriate therapy is especially critical.
- Where you were infected.
- Knowledge of the geographic area where the infection was acquired provides information on the likelihood of drug resistance of the infecting parasite and enables the treating clinician to choose an appropriate drug or drug combination. If the diagnosis of malaria is suspected and cannot be confirmed or if the diagnosis of malaria is confirmed but species determination is not possible, antimalarial treatment effective against chloroquine-resistant Plasmodium falciparum must be initiated immediately and revisited once confirmatory results become available.
- Your age.
- If you’re pregnant.
- How sick you are when you start treatment (the severity of your symptoms).
- Patients diagnosed with malaria are generally categorized as having either uncomplicated or severe malaria. Patients diagnosed with uncomplicated malaria can be effectively treated with oral antimalarials. However, patients who have one or more of the following clinical criteria (impaired consciousness/coma, severe anemia [hemoglobin <7 g/dL], acute kidney injury, acute respiratory distress syndrome, circulatory collapse/shock, disseminated intravascular coagulation, acidosis, jaundice [along with at least one other sign of severe malaria]) and/or parasite density of ≥5% are considered to have manifestations of severe disease and should be treated aggressively with intravenous antimalarial therapy.
- Previous use of antimalarials, including those taken for malaria chemoprophylaxis. In this case, the treatment regimen should not include the drug or drug combination used for prophylaxis unless no other options are available.
After initiation of treatment, the patient’s clinical and parasitological status should be monitored. In infections with Plasmodium falciparum, Plasmodium knowlesi, or suspected chloroquine-resistant Plasmodium vivax, blood smears should be repeated every 12–24 hours to monitor parasitological response to treatment, i.e., decrease in parasite density. It is recommended to document a negative malaria smear after treatment, but this could be done as an outpatient depending on clinical and parasitological response and the judgement of the treating clinician. Note that gametocytes, the sexual stage of the parasite, are not targeted by most of the antimalarials and should not be counted in assessing parasite density.
Malaria medications
Ideally malaria treatment should not be initiated until the diagnosis has been established by laboratory testing. “Presumptive treatment”, i.e., without prior laboratory confirmation, should be reserved for extreme circumstances, such as strong clinical suspicion of severe disease in a setting where prompt laboratory diagnosis is not available.
Once the diagnosis of malaria has been made, appropriate antimalarial treatment must be initiated immediately. The Malaria Treatment Tables (see the tables below) can be used as a guide for treatment of malaria in the United States. The drug or drug combination recommended for each specific situation is listed, as well as the adult and pediatric doses. It is important to note that the base/salt conversions for antimalarials are a recurrent source of confusion and can contribute to treatment errors. In the treatment table, where appropriate, the antimalarial dose is expressed in base with the salt equivalency noted in parentheses.
The most common antimalarial drugs include:
- Chloroquine phosphate. Chloroquine is the preferred treatment for any parasite that is sensitive to the drug. But in many parts of the world, parasites are resistant to chloroquine, and the drug is no longer an effective treatment.
- Artemisinin-based combination therapies (ACTs). Artemisinin-based combination therapy is a combination of two or more drugs that work against the malaria parasite in different ways. This is usually the preferred treatment for chloroquine-resistant malaria. Examples include artemether-lumefantrine (Coartem) and artesunate-mefloquine.
Other common antimalarial drugs include:
- Atovaquone-proguanil (Malarone)
- Quinine sulfate (Qualaquin) with doxycycline (Oracea, Vibramycin, others)
- Primaquine phosphate
Per the 2019 CDC Guidelines below, appropriate treatment depends on the Plasmodium species, clinical stability, and age of the patient, and regional antimalarial susceptibility:
- Uncomplicated Plasmodium falciparum, Plasmodium malariae or Plasmodium knowlesi infections in chloroquine-sensitive regions are treated with a chloroquine phosphate 600 mg (pediatric: 10 mg/kg) loading dose, followed by 300 mg (pediatric: 5 mg/kg) at 6, 24, 48 hours; or a hydroxychloroquine 620 mg (pediatric: 10 mg/kg) loading dose, followed by 310 mg (pediatric: 5 mg/kg) at 6, 24, and 48 hours.
- Uncomplicated Plasmodium falciparum infections in chloroquine-resistant or unknown regions are treated with atovaquone-proguanil 250 mg/100 mg 4 tabs (pediatric: varied weight-based dosing, 6.5 mg/25 mg tabs) daily for 4 days; or artemether-lumefantrine 20 mg/120 mg 4 tabs (pediatric: varied weight-based tabs) at initial dose, then 8 hours later, then twice daily for 2 days; or quinine sulfate 542 mg (pediatric: 8.3 mg/kg) three times daily for 3 days (7 days if in Southeast Asia) plus either doxycycline 100 mg daily for 7 days (pediatrics 2.2 mg/kg every 12 hours), or tetracycline 250 mg daily for 7 days (pediatric: 25 mg/kg/day divided four times daily for 7 days), or clindamycin 20 mg/kg/day divided three times daily for 7 days (pediatric: same); or mefloquine 684 mg (pediatric: 13.7 mg/kg) loading dose followed by 456 mg (pediatric: 9.1 mg/kg) every 6 to 12 hours for total of 1250 mg (pediatric total: 25 mg/kg).
- Uncomplicated Plasmodium vivax or Plasmodium ovale infections in chloroquine-sensitive regions receive treatment with chloroquine phosphate or hydroxychloroquine as per above, plus either primaquine phosphate 30 mg (pediatric: 0.5 mg/kg) daily for 14 days, or tafenoquine 300 mg once (same in children older than 16 years).
- Uncomplicated Plasmodium vivax infections in chloroquine-resistant regions (Indonesia, Papua New Guinea) get treated with quinine sulfate as per above plus either doxycycline, primaquine, or tafenoquine as per above; or atovaquone-proguanil as per above plus either primaquine or tafenoquine; or mefloquine as per above plus either primaquine or tafenoquine as per above.
- Uncomplicated infections with any species in pregnant women in chloroquine-sensitive regions require treatment with chloroquine or hydroxychloroquine as per above.
- Uncomplicated infections with any species in pregnant women in chloroquine-resistant regions are treated with quinine sulfate as per above plus either clindamycin or mefloquine as per above in the first, second, or third trimesters; or artemether-lumefantrine as per above in only the second and third trimesters.
- Severe malaria infection in unstable, non-pregnant patients in all regions includes IV artesunate 2.4 mg/kg (pediatric: children greater than 20 kg receive 2.4 mg/kg, children less than 20 kg receive 3.0 mg/kg) at 0, 12, 24, and 48 hours and either artemether-lumefantrine, atovaquone-proguanil, doxycycline, or mefloquine as per above.
Table 2. Malaria medications (antimalarial drugs)
Malaria treatment for Pregnant Women
Malaria in pregnant women is associated with high risks of both maternal and perinatal morbidity and mortality. While the mechanism is poorly understood, pregnant women have a reduced immune response and, therefore, less effectively clear malaria infections. In addition, malaria parasites sequester and replicate in the placenta. Pregnant women are three times more likely to develop severe disease than non-pregnant women who acquire malaria in the same geographic area. Malaria infection during pregnancy can lead to miscarriage, premature delivery, low birth weight, congenital infection, and/or perinatal death.
Pregnant women diagnosed with uncomplicated malaria caused by chloroquine-resistant Plasmodium falciparum infection in the second and third trimesters can be treated with artemether-lumefantrine. Artemether-lumefantrine may be used during the first trimester if other treatment options are not available, and if the potential benefit is judged to outweigh the potential risks. In addition, pregnant women of all gestational ages can be treated with mefloquine or a combination of quinine sulfate and clindamycin. Quinine treatment should continue for 7 days for Plasmodium falciparum infections acquired in Southeast Asia and for 3 days for infections acquired elsewhere; clindamycin treatment should continue for 7 days regardless of where the infection was acquired.
For pregnant women diagnosed with uncomplicated malaria caused by Plasmodium malariae, Plasmodium ovale, chloroquine-sensitive Plasmodium vivax, or chloroquine-sensitive Plasmodium falciparum infection, prompt treatment with chloroquine or hydroxychloroquine (treatment schedule as for non-pregnant adult patients) is recommended. For chloroquine-resistant Plasmodium vivax infections, quinine plus clindamycin, or mefloquine should be given instead. For women in their second or third trimesters, artemether-lumefantrine is an additional option.
For Plasmodium vivax or Plasmodium ovale infections, primaquine phosphate and tafenoquine for radical treatment of hypnozoites should not be given during pregnancy. Pregnant patients with Plasmodium vivax or Plasmodium ovale infections should be maintained on chloroquine chemoprophylaxis for the duration of their pregnancy. The chemoprophylactic dose of chloroquine phosphate is 300 mg base (500 mg salt) orally once per week. After delivery, for pregnant patients with normal G6PD activity infected with Plasmodium vivax or Plasmodium ovale infections, subsequent treatment with primaquine phosphate or tafenoquine as described above is needed, but will depend on breastfeeding. If not breastfeeding, either drug can be used depending on the regimen used to treat the acute malaria episode (see section on Plasmodium vivax and Plasmodium ovale above). For women who are breastfeeding, infants should be tested for G6PD deficiency and if found to have normal activity, oral primaquine phosphate can be given to the mother. Tafenoquine is not recommended during breastfeeding. Women who after delivery cannot take primaquine or tafenoquine should be maintained on weekly chloroquine chemoprophylaxis for a total of 1 year after the acute malaria episode.
Pregnant women diagnosed with severe malaria should be treated aggressively with parenteral antimalarial therapy as described below.
Doxycycline and tetracycline are generally not indicated for use in pregnant women. However, in rare instances, doxycycline or tetracycline can be used in combination with quinine if other treatment options are not available or are not being tolerated, and the benefit of adding doxycycline or tetracycline is judged to outweigh the risks.
Atovaquone-proguanil is not indicated for use in pregnant women because of the paucity of data on its safety in pregnant women. However, for pregnant women diagnosed with uncomplicated malaria caused by chloroquine-resistant Plasmodium falciparum infection, atovaquone-proguanil may be used if other treatment options are not available or are not being tolerated, and if the potential benefit is judged to outweigh the potential risks.
Treatment of Severe Malaria
Patients with any manifestations of severe malaria, e.g. impaired consciousness/coma, hemoglobin <7 g/dL, acute kidney injury, acute respiratory distress syndrome, circulatory collapse/shock, acidosis, jaundice (with other signs of severe malaria), disseminated intravascular coagulation (DIC) and/or parasite density of ≥5% should be treated promptly and aggressively with parenteral antimalarial therapy regardless of the species of malaria seen on the blood smear. If severe malaria is strongly suspected but a laboratory diagnosis cannot be made at that time, blood should be collected for diagnostic testing to be done as soon as it becomes available and parenteral antimalarial drugs should be started.
All patients with severe malaria, regardless of infecting species, should be treated with intravenous (IV) artesunate. Clinicians caring for patients with severe malaria at a hospital where commercially-available IV artesunate cannot be obtained within 24 hours, should call CDC to obtain IV artesunate, which is available through CDC as part of an expanded-use investigational new drug (IND) protocol. The CDC Malaria Hotline, (770) 488-7788 or toll free (855) 856-4713, is available Monday–Friday, 9 am–5 pm EST. Outside those hours, providers should call (770) 488-7100 and ask to speak with the CDC Malaria Branch clinician on call.
Severe malaria can progress to a fatal outcome rapidly, so its treatment should be initiated as soon as possible. Clinicians at hospitals where IV artesunate is not in stock should consider interim treatment with an effective oral antimalarial while obtaining IV artesunate from a commercial source or CDC, whichever is fastest. If the patient is unable to tolerate oral medications, clinicians will need to consider alternative ways to administer oral medications while awaiting IV artesunate. For example, for patients with nausea and vomiting, an anti-emetic preceding the antimalarial may help, and, for comatose patients, a nasogastric tube can be considered.
The preferred antimalarial for interim oral treatment is artemether-lumefantrine (Coartem™) because of its fast onset of action. Other oral options include atovaquone-proguanil (Malarone™), quinine, and mefloquine. Intravenous or oral clindamycin and tetracyclines, such as doxycycline, are not adequate for interim treatment. These drugs are slow-acting antimalarials that would not take effect until well after 24 hours, and they are not effective antimalarials for treatment of severe malaria when used alone. As for any malaria treatment, the interim regimen should not include the medication used for chemoprophylaxis if possible.
When IV artesunate arrives, immediately discontinue the oral medication and start parenteral treatment. Each dose of IV artesunate is 2.4 mg/kg. A dose of IV artesunate should be given at 0, 12, and 24 hours.
Note that the weight-based dosing applies to both adults and children. Previously, weight-based dosing was differentiated between children <20kg and those ≥20kg. Current dosing in small children <20kg is based on an unpublished FDA analysis modeling pharmacokinetics in this population using available data. Patients on treatment for severe malaria should have one set of blood smears (thick and thin smear) performed every 12–24 hours until a negative result (no Plasmodium parasites are detected) is reported.
After the initial course of IV artesunate is completed, if parasite density is ≤1% (assessed on a blood smear collected 4 hours after the last dose of IV artesunate) and patient can tolerate oral treatment, a full treatment course with a follow-on regimen must be administered. Artemether-lumefantrine (Coartem™) is the preferred follow-on treatment but adequate alternatives are atovaquone-proguanil (Malarone™), quinine plus doxycycline or clindamycin, or mefloquine. Because of a risk of severe neuropsychiatric adverse events at treatment doses, mefloquine should only be used if other options are not available. If the patient received oral treatment prior to receiving IV artesunate, the same medication can be used as follow-on treatment, but a full regimen is required. As for any malaria treatment, the regimen selection should not include the medication used for chemoprophylaxis.
If, after the 3rd IV artesunate dose, the patient’s parasite density is >1%, IV artesunate treatment should be continued with the recommended dose once a day for a maximum of 7 days until parasite density is ≤1%. Doses given at 0, 12, and 24 hours count as 1 day, which means up to 6 additional days. Clinicians should proceed with full course of oral follow-on treatment as above as soon as parasite density ≤1% and the patient is able to tolerate oral medications. Clinicians can consider placement of nasogastric tube or use of antiemetics to facilitate administration of oral treatment. Call CDC Malaria Hotline if additional artesunate is needed and/or advice on treatment.
For those patients with parasite density ≤1% but who still cannot tolerate oral medications after completing IV artesunate treatment, clinicians can continue IV artesunate, 1 dose daily not to exceed a total course of 7 days. Clinicians should call CDC Malaria Hotline if advice on treatment is needed.
Intravenous artesunate is safe in infants, children, and pregnant women in the second and third trimesters. There are limited clinical data on women taking IV artesunate in the first trimester of pregnancy, but no harmful effects have been observed. Given that severe malaria is especially life threatening for pregnant women and their fetuses, and the lack of other treatment options for severe malaria in the United States, the benefits of treatment with IV artesunate outweigh the risks and IV artesunate should not be withheld. The only formal contraindication to IV artesunate treatment is known allergy to IV artemisinins.
IV artesunate is well tolerated. While rare, delayed post-artemisinin hemolytic anemia has been noted in published case reports following treatment of severe malaria with IV artesunate. Persons with higher parasite density seem to have a higher likelihood of delayed hemolytic anemia after treatment. All persons treated for severe malaria with IV artesunate should be monitored weekly for up to four weeks after treatment initiation for evidence of hemolytic anemia. Weekly laboratory evaluation should include hemoglobin measurement, reticulocyte count, haptoglobin, lactate dehydrogenase (LDH), and total bilirubin. Depending on the intensity of hemolysis and presence of anemia signs and symptoms, blood transfusion may be needed. Cases of delayed post-artemisinin hemolytic anemia in those receiving IV artesunate from CDC should be reported to CDC no longer than 24 hours after diagnosis. Cases of delayed post-artemisnin hemolytic anemia in patients who received Artesunate for InjectionTM should be reported to MedWatch, FDA’s Safety Information and Adverse Event Reporting Program.
Malaria prognosis
Malaria can be cured with prescription drugs. The type of drugs and length of treatment depend on the type of malaria, where the person was infected, their age, whether they are pregnant, and how sick they are at the start of treatment. If the right drugs are used, people who have malaria can be cured and all the malaria parasites can be cleared from their body. However, the disease can continue if it is not treated or if it is treated with the wrong drug. Some drugs are not effective because plasmodium parasite is resistant to them. Some people with malaria may be treated with the right drug, but at the wrong dose or for too short a period of time. The duration of untreated infection and time to relapse vary by location and species.
Two types (species) of parasites, Plasmodium vivax and Plasmodium ovale, have liver stages and can remain in the body for years without causing sickness. If not treated, these liver stages may reactivate and cause malaria attacks (“relapses”) after months or years without symptoms. Plasmodium falciparum and Plasmodium ovale infections last 2 to 3 weeks and may relapse 6 to 18 months later, usually from new primary infection 3. Plasmodium vivax infection lasts 3 to 8 weeks and may relapse months to up to 5 years later 3. People diagnosed with Plasmodium vivax or Plasmodium ovale are often given a second drug to help prevent these relapses. Another type of malaria, Plasmodium malariae infection lasts 3 to 24 weeks, if not treated, has been known to stay in the blood of some people for several decades and may relapse up to 20 years later 3.
Relapse is a case of recurrent symptoms months to years after the resolution of erythrocytic organisms due to reinfection or hypnozoite activation 4. Recrudescence is defined as recurrent symptoms within days to weeks of acute illness due to remaining parasitemia after ineffective or incomplete treatment or failed host immune response, more commonly in Plasmodium falciparum 4. However, in general, if you are correctly treated for malaria, the parasites are eliminated and you are no longer infected with malaria.
References- World malaria report 2020. https://www.who.int/publications/i/item/9789240015791
- About Malaria. https://www.cdc.gov/malaria/about/index.html
- Garcia LS. Malaria. Clin Lab Med. 2010 Mar;30(1):93-129. doi: 10.1016/j.cll.2009.10.001
- Fletcher TE, Beeching NJ. Malaria. J R Army Med Corps. 2013 Sep;159(3):158-66. doi: 10.1136/jramc-2013-000112
- López Del Prado GR, Hernán García C, Moreno Cea L, Fernández Espinilla V, Muñoz Moreno MF, Delgado Márquez A, Polo Polo MJ, Andrés García I. Malaria in developing countries. J Infect Dev Ctries. 2014 Jan 15;8(1):1-4. doi: 10.3855/jidc.4610
- Where Malaria Occurs. https://www.cdc.gov/malaria/about/distribution.html
- Malaria Lifecycle. https://www.cdc.gov/malaria/about/biology/index.html
- Mungai M, Tegtmeier G, Chamberland M, Parise M. Transfusion-transmitted malaria in the United States from 1963 through 1999. N Engl J Med 2001:344:1973-8.
- American Association of Blood Banks. Standards for blood banks and transfusions services, 21st ed. Bethesda, Maryland: American Association of Blood Banks, 2002.
- Zoon K. Recommendations for deferral of donors for malaria risk: letter to all registered blood establishments. Washington, DC: Food and Drug Administration, 1994.
- Management of severe malaria: a practical handbook. https://www.who.int/publications/i/item/9789241548526
- Carlton J. M. (2018). Malaria parasite evolution in a test tube. Science (New York, N.Y.), 359(6372), 159–160. https://doi.org/10.1126/science.aar4189
- Buck E, Finnigan NA. Malaria. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551711
- Choosing a Drug to Prevent Malaria. https://www.cdc.gov/malaria/travelers/drugs.html
- WHO Malaria Vaccine Virtual Press Conference transcript – 6 October 2021. https://www.who.int/publications/m/item/who-malaria-vaccine-virtual-press-conference-transcript—6-october-2021
- Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites. https://doi.org/10.1371/journal.pmed.1001685
- WHO recommends groundbreaking malaria vaccine for children at risk. https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk
- Seydel, K. B., Kampondeni, S. D., Valim, C., Potchen, M. J., Milner, D. A., Muwalo, F. W., Birbeck, G. L., Bradley, W. G., Fox, L. L., Glover, S. J., Hammond, C. A., Heyderman, R. S., Chilingulo, C. A., Molyneux, M. E., & Taylor, T. E. (2015). Brain swelling and death in children with cerebral malaria. The New England journal of medicine, 372(12), 1126–1137. https://doi.org/10.1056/NEJMoa1400116
- Shanks G. D. (2017). The Multifactorial Epidemiology of Blackwater Fever. The American journal of tropical medicine and hygiene, 97(6), 1804–1807. https://doi.org/10.4269/ajtmh.17-0533
- https://www.cdc.gov/malaria/resources/pdf/Malaria_Management_Algorithm.pdf
- Ortiz-Ruiz, A., Postigo, M., Gil-Casanova, S., Cuadrado, D., Bautista, J. M., Rubio, J. M., Luengo-Oroz, M., & Linares, M. (2018). Plasmodium species differentiation by non-expert on-line volunteers for remote malaria field diagnosis. Malaria journal, 17(1), 54. https://doi.org/10.1186/s12936-018-2194-8
- Lwin, Khin & Ashley, Elizabeth & Proux, Stephane & Silamut, Kamolrat & Nosten, Francois & McGready, Rose. (2008). Clinically uncomplicated Plasmodium falciparum malaria with high schizontaemia: A case report. Malaria journal. 7. 57. 10.1186/1475-2875-7-57 https://www.researchgate.net/publication/5449420_Clinically_uncomplicated_Plasmodium_falciparum_malaria_with_high_schizontaemia_A_case_report
- https://www.cdc.gov/malaria/resources/pdf/Malaria_Treatment_Table.pdf