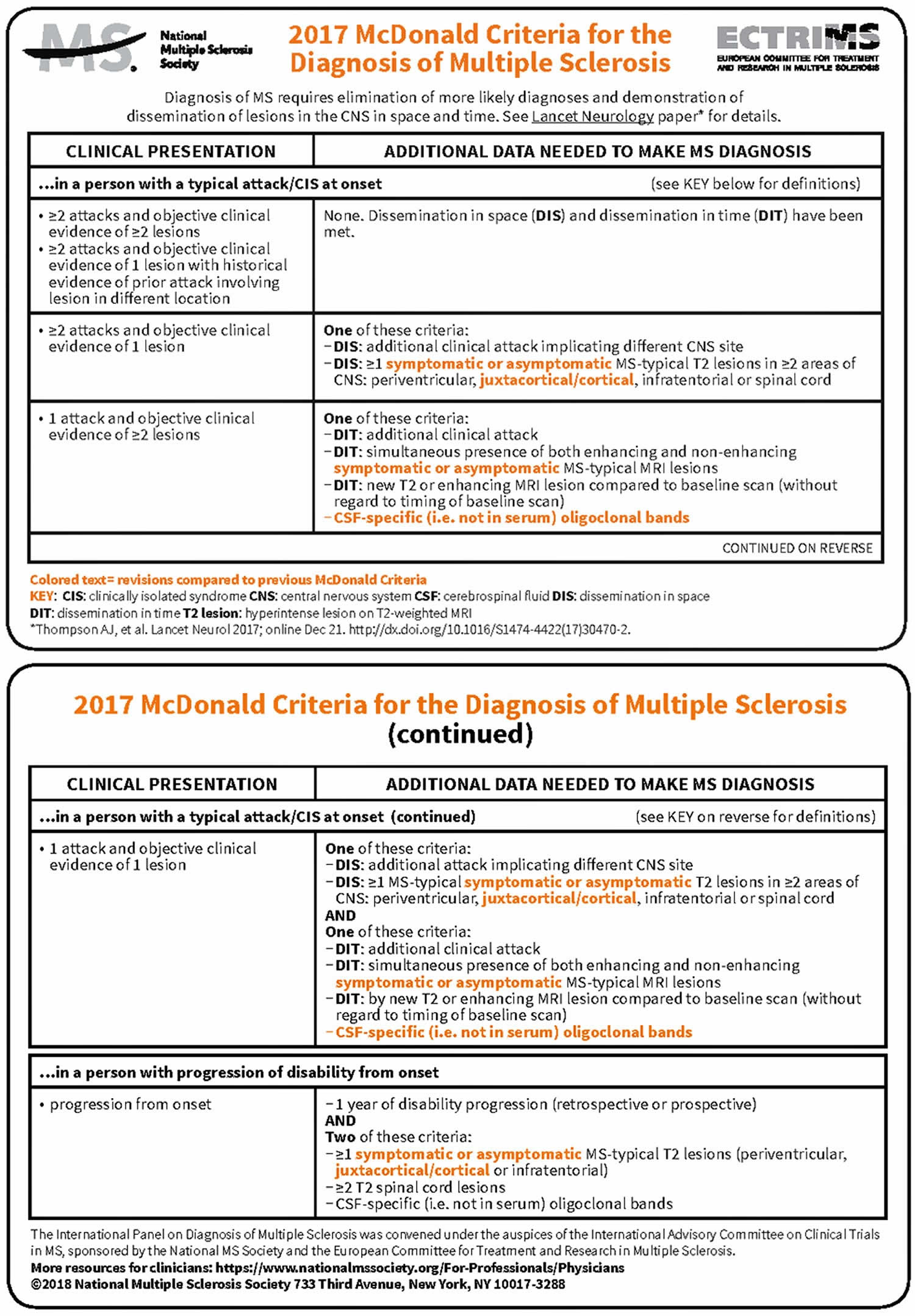
McDonald criteria
McDonald criteria are diagnostic criteria for diagnosis of multiple sclerosis (MS). In 2017 an international panel in association with the National Multiple Sclerosis Society of America recommended revised diagnostic criteria for multiple sclerosis (see Figure 1 below) 1. The expert taskforce included representatives of the American Academy of Neurology, the Radiological Society of North America, the American Society of Neuroradiology, the National Institutes of Health, the Magnetic Resonance Imaging in MS (MAGNIMS), and the North American Imaging in Multiple Sclerosis Cooperative (NAIMS). They make use of advances in MRI imaging techniques and are intended to replace the Poser criteria and the older Schumacher criteria. The new criteria facilitate the diagnosis of MS in patients who present with signs and symptoms suggestive of multiple sclerosis (MS). These include monosymptomatic disease, disease with a typical relapsing-remitting course or insidious progression but no clear attacks and remissions. The update reviewed the four imaging protocols: routine brain, progressive multifocal leukoencephalopathy (PML) surveillance, spinal cord, and orbits.
Figure 1. McDonald criteria for diagnosis of multiple sclerosis
Role of MRI in diagnosis of multiple sclerosis
MRI has been increasingly used to support the diagnosis of multiple sclerosis and to look for atypical radiological features arguing against this diagnosis. The Magnetic Resonance Imaging in MS (MAGNIMS) and the Consortium of Multiple Sclerosis Centers recently proposed standardized MRI protocols for the diagnostic process, to determine prognosis, and for follow-up 2. Brain and spinal cord MRI remain the most useful paraclinical tests to aid the diagnosis of multiple sclerosis and can substitute for clinical findings in the determination of dissemination in space (DIS) or dissemination in time (DIT) in patients with a typical clinically isolated syndrome.
The Panel recommended that brain MRI be obtained in all patients being considered for a diagnosis of multiple sclerosis, recognising that it might at times not be possible because of availability, cost, or contraindication. There was general agreement that, although spinal MRI is not mandatory in all cases, it is advisable when the presentation suggests a spinal cord localisation, when there is a primary progressive course, when considering multiple sclerosis in a population in which the disease is less common (eg, older individuals or non-white populations), or when additional data are needed to increase diagnostic confidence (eg, when brain MRI findings only just fulfill the criteria for dissemination in space [DIS]) 2. Spinal MRI seems to be less useful in the diagnosis of multiple sclerosis in children than in adults 3.
Role of CSF examination in diagnosis of multiple sclerosis
Although CSF examination has been de-emphasised in successive iterations of the McDonald criteria, it remains a valuable diagnostic test 4. In the appropriate clinical setting, evidence of intrathecal antibody synthesis, although not specific for multiple sclerosis, supports the diagnosis 5. Conversely, CSF findings atypical of multiple sclerosis (eg, an elevated protein concentration of >100 mg/dL, pleocytosis with >50 cells per mm³, or the presence of neutrophils, eosinophils, or atypical cells) suggest other diseases 6.
The Panel’s discussion of CSF recognized the importance of using appropriate and standardised technology 5. The qualitative demonstration of two or more CSF-specific oligoclonal bands more reliably indicates intrathecal antibody synthesis than do other tests, such as the IgG index 5. Positive results on these other tests should be interpreted with caution when testing for oligoclonal bands is negative or not done. The sensitivity of oligoclonal band testing depends on the method used; agarose gel electrophoresis with isoelectric focusing and immunoblotting or immunofixation for IgG is the most sensitive approach at present 5. Importantly, analysis of paired CSF and serum samples is essential to confirm that the oligoclonal bands are unique to CSF.
Although CSF examination is not mandatory in some cases (eg, a patient with a typical clinically isolated syndrome supported by characteristic MRI findings, unequivocal demonstration of dissemination in space (DIS) and dissemination in time (DIT), and an absence of atypical clinical or imaging features), the threshold for CSF examination should be low to increase diagnostic confidence. CSF examination is strongly recommended in the following situations: when clinical and MRI evidence is insufficient to support a diagnosis of multiple sclerosis, particularly if initiation of disease-modifying therapies is being considered; when there is a presentation other than a typical clinically isolated syndrome, including a progressive course at onset (primary progressive multiple sclerosis); when clinical, imaging, or laboratory features are atypical of multiple sclerosis; and in populations in which multiple sclerosis is less common (eg, children, older individuals, or non-white populations). Although the absence of CSF oligoclonal bands does not rule out multiple sclerosis, particularly early in the condition and in children 5, caution should be exercised in making this diagnosis when CSF oligoclonal bands are not detected and, certainly, in the presence of atypical clinical, imaging, or CSF findings.
Glossary
Attack
Attack, relapse, exacerbation, and (when it is the first episode) clinically isolated syndrome are synonyms. See clinically isolated syndrome and relapse for descriptions.
Clinically isolated syndrome
A monophasic clinical episode with patient-reported symptoms and objective findings reflecting a focal or multifocal inflammatory demyelinating event in the CNS, developing acutely or subacutely, with a duration of at least 24 hour, with or without recovery, and in the absence of fever or infection; similar to a typical multiple sclerosis relapse (attack and exacerbation) but in a patient not known to have multiple sclerosis 7. Thus, if the patient is subsequently diagnosed with multiple sclerosis (by fulfilling dissemination in space and time, and ruling out other diagnoses), the clinically isolated syndrome was that patient’s first attack. A clinically isolated syndrome can be monofocal (reflecting pathology in a single location) or multifocal; the specific manifestations of a clinically isolated syndrome depend on the anatomical location (or locations) of the pathology. Typical presentations include unilateral optic neuritis, focal supratentorial syndrome, focal brainstem or cerebellar syndrome, or partial myelopathy; examples of atypical presentations include bilateral optic neuritis, complete ophthalmoplegia, complete myelopathy, encephalopathy, headache, alteration of consciousness, meningismus, or isolated fatigue 8.
Cortical MRI lesions
Lesions within the cerebral cortex. Typically, special MRI techniques such as double inversion recovery, phase-sensitive inversion recovery, and magnetisation-prepared rapid acquisition with gradient echo sequences are required to visualize these lesions 9. The lesions detected by these techniques are primarily of the leukocortical type; subpial lesions are rarely detected. Care is needed to distinguish potential cortical lesions from neuroimaging artefacts 9.
Dissemination in space
The development of lesions in distinct anatomical locations within the CNS—ie, indicating a multifocal CNS process.
Dissemination in time
The development or appearance of new CNS lesions over time.
Exacerbation
Attack, relapse, exacerbation, and (when it is the first episode) clinically isolated syndrome are synonyms. See clinically isolated syndrome and relapse for descriptions.
Infratentorial MRI lesion
A T2-hyperintense lesion in the brainstem (typically near the surface), cerebellar peduncles, or cerebellum 8.
Juxtacortical MRI lesion
A T2-hyperintense cerebral white matter lesion abutting the cortex, and not separated from it by white matter 8.
Lesion
An area of hyperintensity on a T2-weighted or proton-density-weighted MRI scan that is at least 3 mm in long axis 2.
Objective clinical or paraclinical evidence (as it relates to a current or historical attack)
An abnormality on neurological examination, imaging (MRI or optical coherence tomography), or neurophysiological testing (visual evoked potentials) that corresponds to the anatomical location suggested by the symptoms of the clinically isolated syndrome—eg, optic disc pallor or a relative afferent pupillary defect, optic nerve T2 hyperintensity on MRI, retinal nerve fiber layer thinning on optical coherence tomography, or P100 latency prolongation on visual evoked potentials in a patient reporting a previous episode of self-limited, painful, monocular visual impairment. Caution should be exercised in accepting symptoms accompanied only by patient-reported subjective alteration as evidence of a current or previous attack.
Periventricular MRI lesion
A T2-hyperintense cerebral white matter lesion abutting the lateral ventricles without white matter in between, including lesions in the corpus callosum but excluding lesions in deep grey matter structures 8.
Progressive course
A multiple sclerosis course characterized by steadily increasing objectively documented neurological disability independent of relapses. Fluctuations, periods of stability, and superimposed relapses might occur. Primary progressive multiple sclerosis (a progressive course from disease onset) and secondary progressive multiple sclerosis (a progressive course following an initial relapsing-remitting course) are distinguished 7.
Radiologically isolated syndrome
MRI findings strongly suggestive of multiple sclerosis in a patient with no neurological manifestations or other clear-cut explanation.
Relapse
A monophasic clinical episode with patient-reported symptoms and objective findings typical of multiple sclerosis, reflecting a focal or multifocal inflammatory demyelinating event in the CNS, developing acutely or subacutely, with a duration of at least 24 hour, with or without recovery, and in the absence of fever or infection. Attack, relapse, exacerbation, and (when it is the first episode) clinically isolated syndrome are synonyms.
Relapsing-remitting course
A multiple sclerosis course characterised by relapses with stable neurological disability between episodes 7.
Spinal cord MRI lesion
A hyperintense lesion in the cervical, thoracic, or lumbar spinal cord seen on T2 plus short tau inversion recovery, proton-density images, or other appropriate sequences, or in two planes on T2 images 8.
References- Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–73 https://doi.org/10.1016/S1474-4422(17)30470-2
- Rovira A Wattjes MP Tintore M et al. MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—clinical implementation in the diagnostic process. Nat Rev Neurol. 2015; 11: 471-482
- Hummel H-M Bruck W Dreha-Kulaczewski S Gartner J Wuerfel J. Pediatric onset multiple sclerosis: McDonald criteria 2010 and the contribution of spinal cord MRI. Mult Scler J. 2013; 19: 1330-1335
- Arrambide G Tintore M. CSF examination still has value in the diagnosis of MS—commentary. Mult Scler J. 2016; 22: 997-998
- Andersson M Alvarez-Cermeno J Bernardi G et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry. 1994; 57: 897-902
- Stangel M Fredrikson S Meinl E Petzold A Stuve O Tumani H. The utility of cerebrospinal fluid analysis in patients with multiple sclerois. Nat Rev Neurol. 2013; 9: 267-276
- Lublin FD Reingold SC Cohen JA et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014; 83: 278-286
- Brownlee WJ Hardy TA Fazekas F Miller DH. Multiple Sclerosis 1. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2016; 389: 1336-1346
- Filippi M Rocca MA Ciccarelli O et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016; 15: 292-303