Mediastinal lymph nodes
Mediastinal lymph nodes drain the thoracic viscera, including the lungs, heart, thymus, and thoracic esophagus. Because mediastinal lymph nodes are not directly demonstrable upon physical examination, their enlargement must be indirectly assessed. Supraclavicular adenopathy is often associated with mediastinal adenopathy. Mediastinal lymph nodes may cause cough, wheezing, dysphagia, airway erosion with hemoptysis, atelectasis, and the obstruction of the great vessels, which constitutes superior vena cava syndrome. Airway compromise may be life threatening.
The mediastinal compartment contains multiple critical organs and vessels and serves as the central hub for lymphatic drainage 1. The mediastinum is classically subdivided into three functional divisions: anterior (pre-vascular), middle (visceral), and posterior (paravertebral) mediastinum (see Figures 3 and 4 below). These subdivisions are used to describe the locations of lesions, thereby helping to facilitate a differential diagnosis and communication between healthcare providers. Lymph nodes are present in all three functional compartments of the mediastinum, though most lymphatic tissue is found in the anterior and middle compartments, and the etiology of lymphatic pathology varies by subdivision. Dividing the mediastinum helps to narrow down the lengthy differential diagnoses which can present in the thorax (including, but not limited to: infections like tuberculosis, nodal spread of lung cancer, sarcoidosis, lymphoma, silicosis, and asbestosis). In contrast to the functional subdivisions, intrathoracic lymph node locations have been traditionally mapped into 14 stations according to their relationship to landmarks encountered during mediastinoscopy and thoracotomy for lung cancer. Stations 1–9 correspond to mediastinal nodal groups, while stations 10–14 represent hilar and other more peripheral extra mediastinal nodal groups.
The most current map of intrathoracic lymph nodes is the International Association for the Study of Lung Cancer (IASLC) map 2. The IASLC atlas supersedes all previous schema and reconciles discrepancies among older popular systems such as the Naruke lymph node classification and the Mountain-Dresler modified version of the American Thoracic Society lymph node map 3.
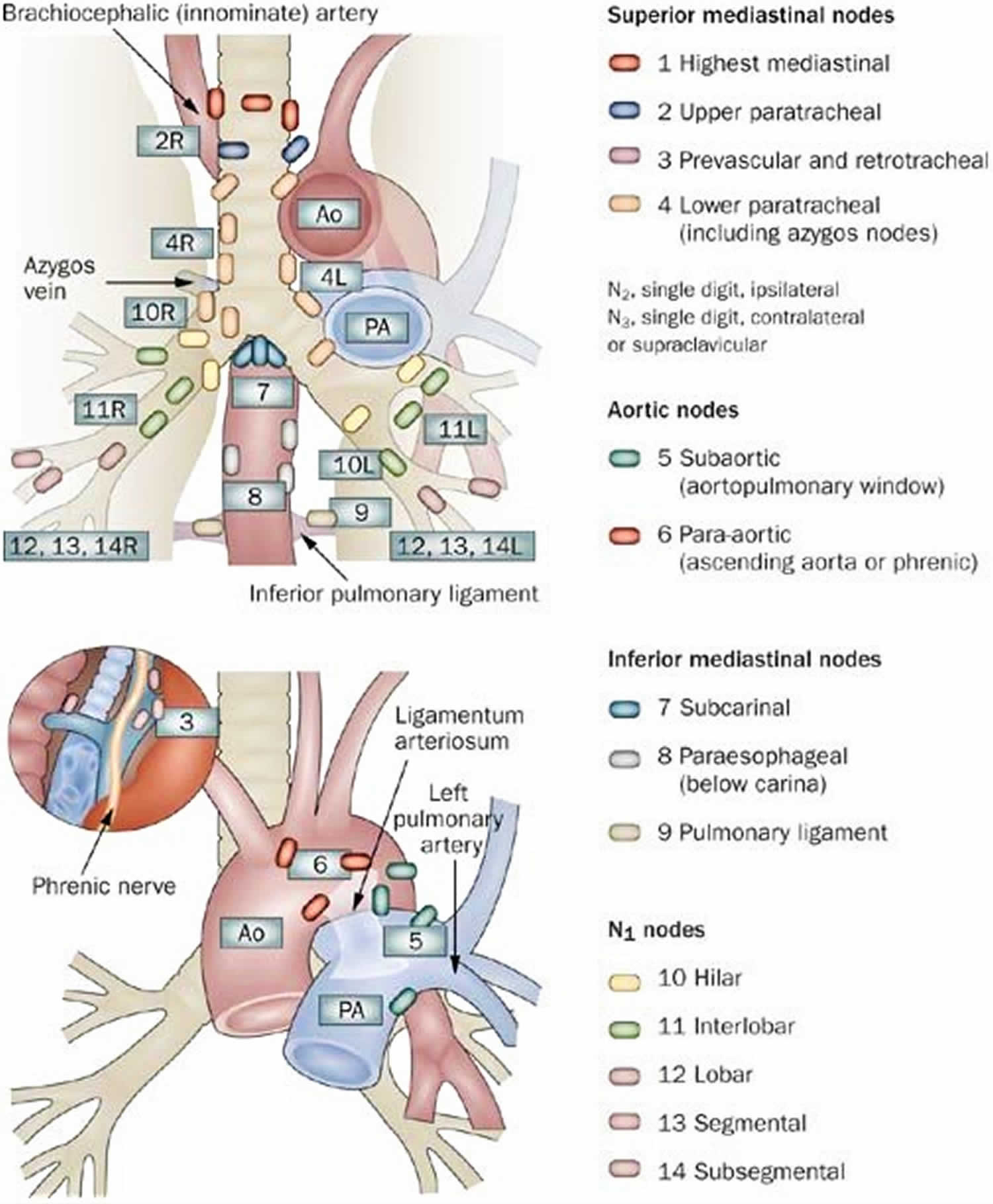
Figure 1. Mediastinal lymph nodes
Footnote: Mediastinal lymph node stations, according to American Thoracic Society Regional Lymph Node Station criteria.
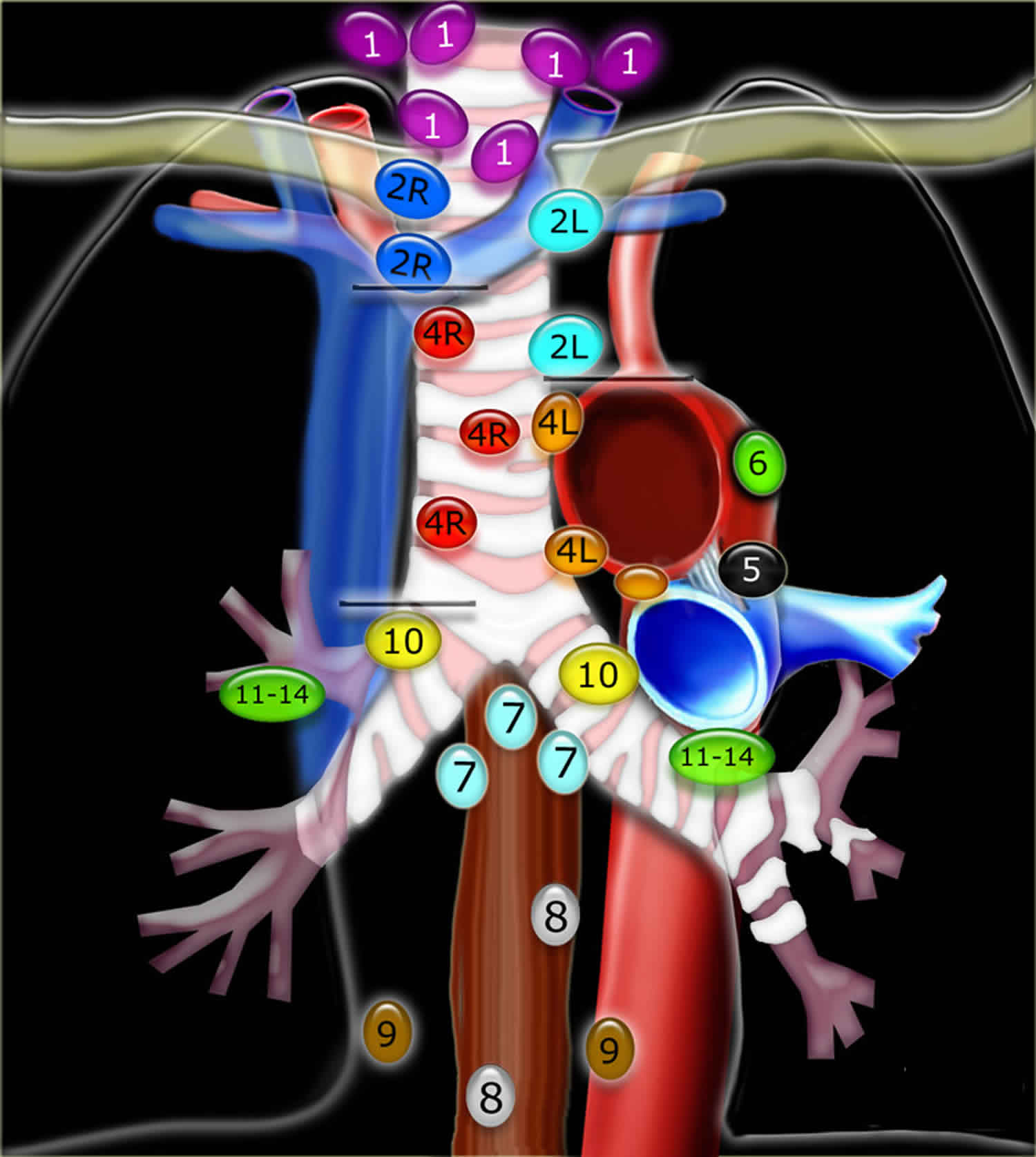
[Source 4 ]Figure 2. Mediastinal lymph nodes
Footnotes: International Association for the Study of Lung Cancer (IASLC) lymph node map 2009.
Supraclavicular nodes
1. Low cervical, supraclavicular and sternal notch nodes
- From the lower margin of the cricoid to the clavicles and the upper border of the manubrium.
- Upper border: lower margin of cricoid.
- Lower border: clavicles and upper border of manubrium.
- The midline of the trachea serves as border between 1R and 1L.
Superior Mediastinal Nodes 2-4
- 2R.Upper Paratracheal
- From upper border of manubrium to the intersection of caudal margin of innominate (left brachiocephalic) vein with the trachea.
- 2R nodes extend to the left lateral border of the trachea.
- 2L.Upper Paratracheal
- From the upper border of manubrium to the superior border of aortic arch.
- 2L nodes are located to the left of the left lateral border of the trachea.
- 3A.Pre-vascular
- These nodes are not adjacent to the trachea like the nodes in station 2, but they are anterior to the vessels.
- 3P.Pre-vertebral
- Nodes not adjacent to the trachea like the nodes in station 2, but behind the esophagus, which is prevertebral.
- 4R.Lower Paratracheal
- From the intersection of the caudal margin of innominate (left brachiocephalic) vein with the trachea to the lower border of the azygos vein.
- 4R nodes extend from the right to the left lateral border of the trachea.
- 4L.Lower Paratracheal
- From the upper margin of the aortic arch to the upper rim of the left main pulmonary artery.
Aortic Nodes 5-6
- 5. Subaortic
- These nodes are located in the AP window lateral to the ligamentum arteriosum.
- These nodes are not located between the aorta and the pulmonary trunk but lateral to these vessels.
- 6. Para-aortic
- These are ascending aorta or phrenic nodes lying anterior and lateral to the ascending aorta and the aortic arch.
Inferior Mediastinal Nodes 7-9
- 7.Subcarinal
- 8. Paraesophageal
- Nodes below carina.
- 9. Pulmonary Ligament
- Nodes lying within the pulmonary ligaments.
Hilar, Lobar and (sub)segmental Nodes 10-14
These are all N1-nodes.
- 10. Hilar nodes
- These include nodes adjacent to the main stem bronchus and hilar vessels.
- On the right they extend from the lower rim of the azygos vein to the interlobar region.
- On the left from the upper rim of the pulmonary artery to the interlobar region.
In the setting of primary intrathoracic tumors, it is critical to accurately identify the station from which lymph nodes are excised/sampled, since information regarding which lymph nodes the tumor has spread to is essential to the surgical staging of a patient’s regional disease. In the case of lung cancer, tumor involvement of ipsilateral station 10-14 lymph nodes, all of which are extrapleural, is considered N1 disease while mediastinal lymph nodes from stations 2-9 that are ipsilateral to the malignancy (to include subcarinal lymph nodes for either side of disease) are considered N2 disease. Contralateral mediastinal or hilar lymph nodes, as well as station 1 lymph nodes that have tumor cells present, are considered N3 disease. In contrast, with esophageal cancer, the N stage of the disease is determined by the number of regional lymph nodes involved by tumor with N1 being 1-2 positive (+) lymph nodes, N2 employed for 3-6 +lymph nodes and N3 designating cases with >=7 +lymph nodes. Additionally, not all nodal stations used by the International Association for the Study of Lung Cancer (IASLC) for lung cancer are considered regional lymph nodes for esophageal cancer staging, and some stations are named differently, such as the designation of upper, middle, and lower paraesophageal lymph node stations
In additional to metastases, malignant mediastinal lymph nodes can be due to lymphomas, a group of cancers that arise from white blood cells. This group of diseases is the most frequent cause of nodal pathology of the anterior compartment but is also a common cause of adenopathy in the middle compartment.
Mediastinal adenopathy may be due to non-neoplastic entities. Primary acute or chronic infectious and inflammatory processes occur in all compartments of the mediastinum and may result in lymph node enlargement. Likewise, pulmonary and systemic infections such as pneumonia and human immunodeficiency virus can result in pathologically enlarged mediastinal lymph nodes. If mediastinal lymph nodes are calcified, it helps narrow the differential diagnosis to entities such as granulomatous diseases (histoplasmosis, tuberculosis), silicosis, sarcoidosis, and treated lymphoma. When lymphatic vessels of the mediastinum are damaged, the resulting chylothorax can interfere with normal immunologic, nutritional, and respiratory function.
Enlarged mediastinal lymph nodes may be found incidentally, and guidelines for surveillance and evaluation can be found in the Journal of the American College of Radiology. For example, nodes may be particularly worrisome if they are large, asymmetric, or demonstrate FDG avidity on PET/CT scans. Nodes that are larger than 15 mm may warrant special attention, especially if there is no known explanation, such as a history of sarcoidosis. The American College of Radiology guidelines also recognize the value of categorizing lymph nodes by the aforementioned divisions, identifying abnormal lymph node contours, and whether the masses are solid or cystic 5.
What is the mediastinum
The mediastinum is a broad central chest cavity that separates the lungs from the other structures in the chest. Mediastinum extends from the sternum to the bodies of the vertebrae, and from the superior thoracic aperture to the diaphragm. Generally, mediastinum is further divided into three main parts: anterior mediastinum, posterior mediastinum, and middle mediastinum. The borders of the mediastinum include the thoracic inlet superiorly, the diaphragm inferiorly, the spine posteriorly, the sternum anteriorly, and the pleural spaces laterally. The mediastinum contains the thymus gland, the pericardia! sac, the heart, the aorta, esophagus, trachea (windpipe) and the major arteries and veins. Additionally, the mediastinum serves as a passageway for structures such as the esophagus, thoracic duct, and various components of the nervous system as they traverse the thorax on their way to the abdomen.
The thoracic cavity has three parts: (a) two lateral parts, each containing a lung surrounded by a pleural cavity and (b) a central band of organs called the mediastinum (“in the middle”). The medial wall of each pleural cavity is the mediastinum. Two pleural cavities, one on either side of the mediastinum, surround the lungs.
The medial surface of the right lung lies adjacent to a number of important structures in the mediastinum and the root of the neck. These include the:
- heart,
- inferior vena cava,
- superior vena cava,
- azygos vein, and
- esophagus.
For organizational purposes, the mediastinum is subdivided into several smaller regions. A transverse plane extending from the sternal angle (the junction between the manubrium and the body of the sternum) to the intervertebral disc between vertebrae T4 and T5 separates the mediastinum into the:
- Superior mediastinum, and
- Inferior mediastinum, which is further partitioned into:
- anterior, middle, and posterior mediastinum by the pericardia! sac.
Figure 3. Mediastinum
Figure 4. Mediastinum cavity
Mediastinal lymph zones and stations
As mentioned, based upon lung cancer staging guidelines, the intrathoracic lymph nodes are divided into 14 stations which are grouped into 7 zones. Stations 1–9 located in the mediastinal pleural reflection, while stations 10–14 are distal to the mediastinal pleural reflection and within the visceral pleura. Stations 1, 2, 4, and 10-14 additionally have R and L designators for right and left while station 3 has A and P designators for anterior and posterior. Because the intrathoracic are frequently considered in conjunction with the mediastinal lymph nodes, but extra mediastinal lymph nodes (stations 10-14), they will be discussed briefly in this article for completeness
Supraclavicular Zone
Station 1 (Supraclavicular): Station 1 comprises the most cranial station of mediastinal nodes. It includes lymph nodes in the sternal notch, supraclavicular and lower cervical regions (thus overlapping with some cervical lymph node maps used in head and neck cancers). The cricoid cartilage serves as the upper border of station 1. Station 1 extends inferiorly to the upper margin of the manubrium and tops of the clavicles. The midline of the trachea is used to designate which lymph nodes are 1R and 1L.
Upper Zone (Superior Mediastinal lymph nodes)
Station 2 (Upper Paratracheal): The station 2 lymph nodes all abut the trachea and, in contrast to station 1, the left lateral wall of the trachea instead of the midline, is used as the boundary to differentiate between 2R versus 2L. The upper border of station 2 is the apex of the ipsilateral lungs and pleural spaces, and in the midline, the upper border of the manubrium. The lower border of station 2 on the right (2R) is where the inferior margin of the left brachiocephalic vein crosses the trachea, while the lower border of station 2 on the left (2L) is the superior border of the aortic arch.
Station 3 (Prevascular and Retrotracheal): The prevascular lymph nodes (3A) are all located behind the sternum and anterior to the superior vena cava and left carotid artery. The superior border is the apex of the chest (like station 2), but extends further caudal, to the level of the carina. Retrotracheal lymph nodes (3P), as their name implies, are those located in the area posterior to the trachea, likewise extending from the apex of the chest to the carina.
Station 4 (Lower Paratracheal): Lower paratracheal nodes are along the distal trachea, bordered superiorly by station 2 and extending to the level of the carina. They lie posterior to the aortic vasculature, and like station 2, the left lateral wall of the trachea instead of the midline, is used as the boundary to differentiate between 4R and 4L.
Aortopulmonary Zone
Station 5 (Subarotic): These lymph nodes are also known commonly as aortopulmonary (AP) window lymph nodes and are located lateral to the ligamentum arteriosum, the remnant of the ductus arteriosus. The lower margin of the aortic arch serves as the upper border of station 5 while the superior margin of the left pulmonary artery demarcates the lower extension.
Station 6 (Paraaortic): The para-aortic lymph nodes lie on the anterior and lateral aspect of the ascending aorta and aortic arch, anterior and/or above the subaortic (AP window) lymph nodes. The phrenic nerve may be used as a landmark for identifying lymph nodes that are classified as paraaortic.
Subcarinal Zone
Station 7 (Subcarinal): Subcarinal nodes lie directly below the carina and between the mainstem bronchi. To differentiate them from the paraesophageal lymph nodes that are found more caudal, the distal aspect of the bronchus intermedius and origin of the left lower lobe bronchus are used to demarcate the right and left inferior extensions of station 7. In most patients, this results in an inferior margin that is canted from horizontal given that the termination of the bronchus intermedius is usually lower than the origin of the left lower lobe bronchus).
Lower Zone (Inferior Mediastinal lymph nodes)
Station 8 (Paraesophageal): Paraesophageal nodes are those mediastinal lymph nodes found inferior to the subcarinal lymph nodes, along the anterior or lateral aspects of the esophagus, down to the esophageal hiatus of the diaphragm.
Station 9 (Pulmonary Ligament): Pulmonary ligament nodes associated with the pulmonary ligaments. These “ligaments” are not actually ligaments but represent the mediastinal parietal pleural reflections that occur below the right and left pulmonary roots (9R and 9L).
Hilar Zone + Interlobar and Peripheral Zone (Extra-Mediastinal lymph nodes)
These lymph nodes are all outside the pleural reflection of the mediastinum but within the pulmonary visceral pleura.
Station 10 (Hilar): These lymph nodes are found along the right and left mainstem bronchi, before they bifurcate, and are designated 10R and 10L, respectively.
Station 11 (Interlobar): Station 11 is made up of lymph nodes located between the lobar bronchi, just beyond the bifurcation of each mainstem bronchi.
Stations 12-14 (Peripheral): These are also known as lobar, segmental and subsegmental lymph nodes, depending on whether they are located along the lobar, segmental or subsegmental bronchi. These lymph nodes are infrequently seen and difficult to accurately categorize on imaging, hence many use the broad term of peripheral lymph nodes for stations 12-14.
It should be noted that while the above classification system is the most widely utilized mapping scheme and often employed when describing mediastinal lymph nodes outside the setting of lung cancer, other disease-specific maps are appropriate in certain instances such as esophageal cancer staging 6.
In addition to mediastinal lymph nodes, the thoracic duct is an important component of the intrathoracic lymphatic system. It is the largest single lymphatic vessel in the chest, beginning at the superior aspect of the cisterna chyli, at the level of the L2 vertebra. From there, it courses cranially between the posterior margin of the aorta and anterior margin of the spine until approximately the region of T5 vertebra where it drains into the venous system near the junction of the left subclavian and internal jugular veins. Approximately 75% of the body’s lymph fluid drains via the thoracic duct into the venous system, accounting for lymphoid drainage from the entire body except for the right arm and right side of the head (the nodes of which drain into the junction of the right subclavian and internal jugular veins) 7.
Mediastinal lymph nodes function
The network of lymphatic vessels and lymph nodes extends throughout the entire body to support the proper function of its immune system along with absorption of dietary fats and fluid homeostasis. Unlike arteries and veins, the lymphatics are blind-ending vessels that transport interstitial fluid, immune cells, and macromolecules, collectively called lymph, through lymph nodes and back to the chest for return into the blood supply via the subclavian veins. Lymph originates when blood plasma leaks out of capillaries and into the interstitial space. Along with the normal macromolecules and lymphocytes within plasma, dead cells and material that may be harmful to the body such as bacteria, viruses, and tumor cells also leak out and in turn enter the lymph. The fluid and cells are transported back to the chest by the lymphatic vessels. Along the way, nodes, like those found in the mediastinum, sample the lymph, filtering out potential threats and unwanted cellular debris before it re-enters the blood supply. Macrophages within the lymph nodes phagocytize these unwanted substances and then break them down for recycling by the body. T-lymphocytes sample antigens from pathogens and interact with B cells to initiate clonal expansion for antibody production. With immune cell activation, lymph nodes grown as cells within divide. Rapid growth can cause pain while slow mitotic activity (often seen with cancer) results in painless lymphadenopathy 8.
Mediastinal lymph node sampling is critical to the initial diagnosis and staging of lung and esophageal cancers and lymphoma. Additionally, it is essential for detecting primary or recurrent nodal metastases in extrathoracic malignancies. This can be done by a variety of techniques employed by a variety of specialists with the technique usually guided by the location of nodal disease. Cardiothoracic surgeons may employ mediastinoscopy for the sampling of station 1, 2R/L, 3, 4R/L, and 7. An extended mediastinoscopy or video-assisted thoracotomy (VATS) can extend their range to include biopsy of stations 5 and 6. Gastroenterologists may use esophageal ultrasound (EUS) which allows for fine needle aspiration (FNA) of mediastinal lymph node stations 2L, 4L, 7, 8, and 9. Pulmonologists can use transbronchial needle aspiration (TBNA) as well as endobronchial ultrasound (EBUS) FNA which allows for tissue sampling of the bilateral station 10-12 lymph nodes (hilar and peripheral stations) in addition to 3P, station 7 and bilateral station 2 and 4 lymph nodes. Similarly, these techniques can be used for the diagnosis of other disorders that may involve the mediastinal lymph nodes such as sarcoidosis, Castleman disease, or tuberculosis.
In addition to cancer staging, mediastinal lymphatic disorders can have other surgical implications. Chylous pleural effusions can occur when the intrathoracic lymphatic tissue is damaged, either by a result of infection/inflammation or trauma/surgery, and lymph leaks into the pleural spaces. Less commonly, a chylopericardium can result if the insulting event results in a fistulous connection between the lymphatics and pericardium. If embolization of the lymphatic system is indicated after a chylothorax develops, magnetic resonance ductography has the best visualization to guide surgical intervention, but CT scans, lymphoscintigraphy, and lymphangiography can be used alternatively 9.
Mediastinal lymphadenopathy
Mediastinal lymphadenopathy or mediastinal adenopathy is an enlargement of the mediastinal lymph nodes. Lymphadenopathy refers to lymph nodes that are abnormal in size (e.g., greater than 1 cm) or consistency. Mediastinal lymph nodes drain the thoracic viscera, including the lungs, heart, thymus, and thoracic esophagus. Because mediastinal lymph nodes are not directly demonstrable upon physical examination, their enlargement must be indirectly assessed. Supraclavicular adenopathy is often associated with mediastinal adenopathy. Mediastinal lymph nodes may cause cough, wheezing, dysphagia, airway erosion with hemoptysis, atelectasis, and the obstruction of the great vessels, which constitutes superior vena cava syndrome. Airway compromise may be life threatening.
Mediastinal lymphadenopathy causes
Mediastinal lymphadenopathy is usually a sign of serious underlying disease 10. More than 95% of mediastinal masses are caused by tumors or cysts 10. Lymphomas and acute lymphoblastic leukemia are the most common causes and usually involve the anterior mediastinum. These malignancies are associated with a high risk of superior vena cava syndrome and are associated with several potentially life-threatening complications, as follows:
- The danger of sedation of patients, especially in the supine position for scans and procedures (The prone position actually may be safer.)
- The risk during intubation of these patients, usually at the time of biopsy or placement of a central venous catheter
- The risk of cardiovascular collapse during general anesthesia because of compression of venous return or because of previously undiagnosed pleural effusions
- The risk of losing the ability to establish a pathologic diagnosis because of the use of steroids or radiation therapy
Unlike most other adenopathies, mediastinal lymphadenopathy is less frequently a result of infection. Infections frequently involve the hilar region and include histoplasmosis, coccidioidomycosis, and tuberculosis.
Nonlymphoid mediastinal tumors may be confused with adenopathy. These include neurogenic tumors (usually found in the posterior mediastinum), germ cell tumors, and teratomas.
Nonneoplastic conditions may also be confused with mediastinal adenopathy. These include the typically large thymus of a child, substernal thyroid glands, bronchogenic cysts, and abnormalities of the great vessels.
References- Burlew JT, Banks KP. Anatomy, Thorax, Mediastinal Lymph Nodes. [Updated 2019 Feb 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532863
- Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P., Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009 May;4(5):568-77. doi: 10.1097/JTO.0b013e3181a0d82e. https://doi.org/10.1097/JTO.0b013e3181a0d82e
- Carter BW, Tomiyama N, Bhora FY, Rosado de Christenson ML, Nakajima J, Boiselle PM, Detterbeck FC, Marom EM. A modern definition of mediastinal compartments. J Thorac Oncol. 2014 Sep;9(9 Suppl 2):S97-101.
- Navani, Neal & Spiro, Stephen & Janes, Sam. (2009). Mediastinal staging of NSCLC with endoscopic and endobronchial ultrasound. Nature reviews. Clinical oncology. 6. 278-86. 10.1038/nrclinonc.2009.39
- Munden RF, Carter BW, Chiles C, MacMahon H, Black WC, Ko JP, McAdams HP, Rossi SE, Leung AN, Boiselle PM, Kent MS, Brown K, Dyer DS, Hartman TE, Goodman EM, Naidich DP, Kazerooni EA, Berland LL, Pandharipande PV. Managing Incidental Findings on Thoracic CT: Mediastinal and Cardiovascular Findings. A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2018 Aug;15(8):1087-1096.
- Liao S, von der Weid PY. Lymphatic system: an active pathway for immune protection. Semin. Cell Dev. Biol. 2015 Feb;38:83-9.
- Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol. 2017 Jan;12(1):36-42.
- Aspelund A, Robciuc MR, Karaman S, Makinen T, Alitalo K. Lymphatic System in Cardiovascular Medicine. Circ. Res. 2016 Feb 05;118(3):515-30.
- Johnson OW, Chick JF, Chauhan NR, Fairchild AH, Fan CM, Stecker MS, Killoran TP, Suzuki-Han A. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol. 2016 Aug;26(8):2482-93.
- Lymphadenopathy Clinical Presentation. https://emedicine.medscape.com/article/956340-clinical