Merkel cell carcinoma
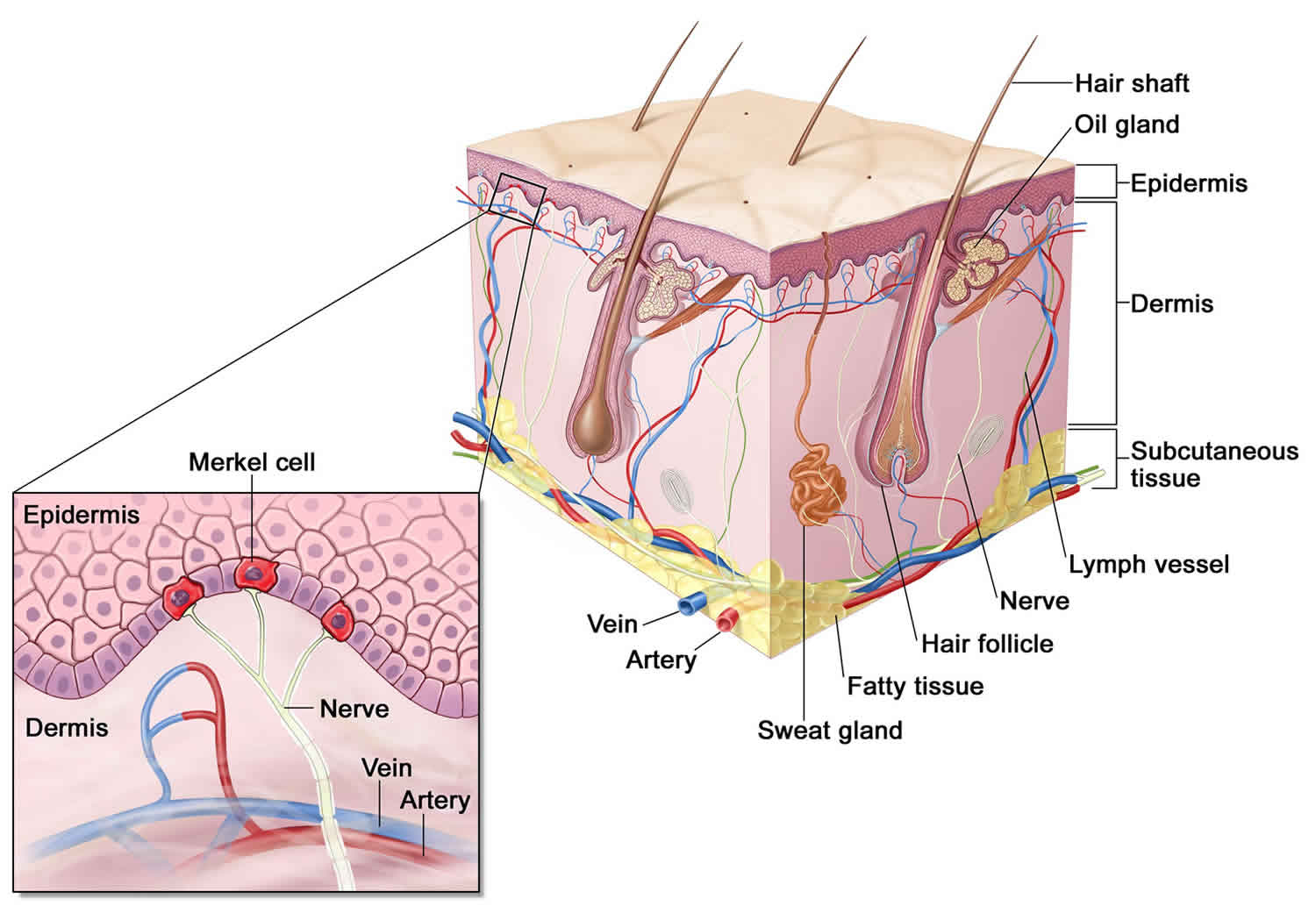
Merkel cell carcinoma also called Merkel cell cancer, neuroendocrine carcinoma of the skin or trabecular cancer of the skin, is a rare, highly aggressive neuroendocrine skin cancer with a high risk for returning (recurring) and spreading (metastasizing), often within two to three years after initial diagnosis 1, 2, 3, 4. Merkel cells are thought to be a type of skin neuroendocrine cell because they share some features with nerve cells and hormone-making cells. Merkel cells are found mainly at the base of the top layer of the skin (the epidermis) (see Figure 2). Merkel cells are very close to nerve endings in the skin. They help you sense light touch, which lets you do things like feel the fine details on an object’s surface. While rare, Merkel cell carcinomas are often aggressive and can advance rapidly which is why early detection and removal are especially important. Merkel cell carcinoma is 40 times more rare than melanoma, with an estimated one case per 130,000 people in the U.S. 5. About 2,000 cases of Merkel cell carcinoma are diagnosed in the United States each year 6. Experts expect that this will increase to 3,250 cases diagnosed annually by 2025, with similar increases expected in Australia and many European countries 7. Merkel cell carcinoma incidence increases progressively with age. There are few cases in patients younger than 50 years, and the median age at diagnosis is about 65 years 8. More than 4 out of 5 Americans diagnosed with Merkel cell carcinoma are older than age 70 6. And men are nearly 2-times more likely to have it compared with women, with women showing improved clinical outcomes 6, 9. Merkel cell carcinoma incidence is considerably greater in whites than blacks (more than 9 out of 10 cases of Merkel cell carcinoma in the United States are diagnosed in whites) and slightly greater in males than females 10, 11, 6.
Specific risk factors for developing Merkel cell carcinoma include ultraviolet (UV) exposure, advancing age, immunosuppression, and the Merkel cell polyomavirus (MCPyV) infection 12, 13, 14. Merkel cell carcinoma occurs most frequently in sun-exposed areas of skin, particularly the head and neck, followed by the extremities, and then the trunk 15, 16. Incidence has been reported to be greater in geographic regions with higher levels of ultraviolet B sunlight 17.
Merkel cell carcinoma is rare and dangerous but treatable, especially when found at an early stage 18. Be watchful for any new or changing lesions on your skin and look out for these warning signs.
If you’ve been treated for a previous Merkel cell carcinoma, pay close attention to the site and the surrounding region. Contact your medical team immediately if you see any suspicious changes.
Because Merkel cell carcinoma is such an uncommon form of skin cancer, it is best to seek treatment at an academic center with physicians who have specialized expertise in caring for people with this particular disease. A multi-disciplinary team experienced in the care of Merkel cell carcinoma is recommended. Dermatologists, surgeons, medical oncologists and radiation oncologists need to confer to determine the best plan for a given case.
While treatment options for Merkel cell carcinoma depend on the stage of the disease and the overall health of the patient, treatment includes surgical removal of the primary tumor along with:
- Radiation therapy
- Immunotherapy
- Chemotherapy
Merkel cell carcinoma key points:
- Increasing numbers of Merkel cell carcinomas have been reported by some centers in recent years.
- Merkel cell carcinoma mainly affects older people, with most cases occurring after the age of 50.
- Merkel cell carcinoma is slightly more common in men.
- Merkel cell carcinoma occurs on parts of the body commonly exposed to sunlight, most often the head and neck.
- Merkel cell carcinoma is also more common and more serious in those that are immune suppressed, such as patients with solid organ transplants, human immunodeficiency virus (HIV) infection, hematological malignancy or on drugs such as azathioprine.
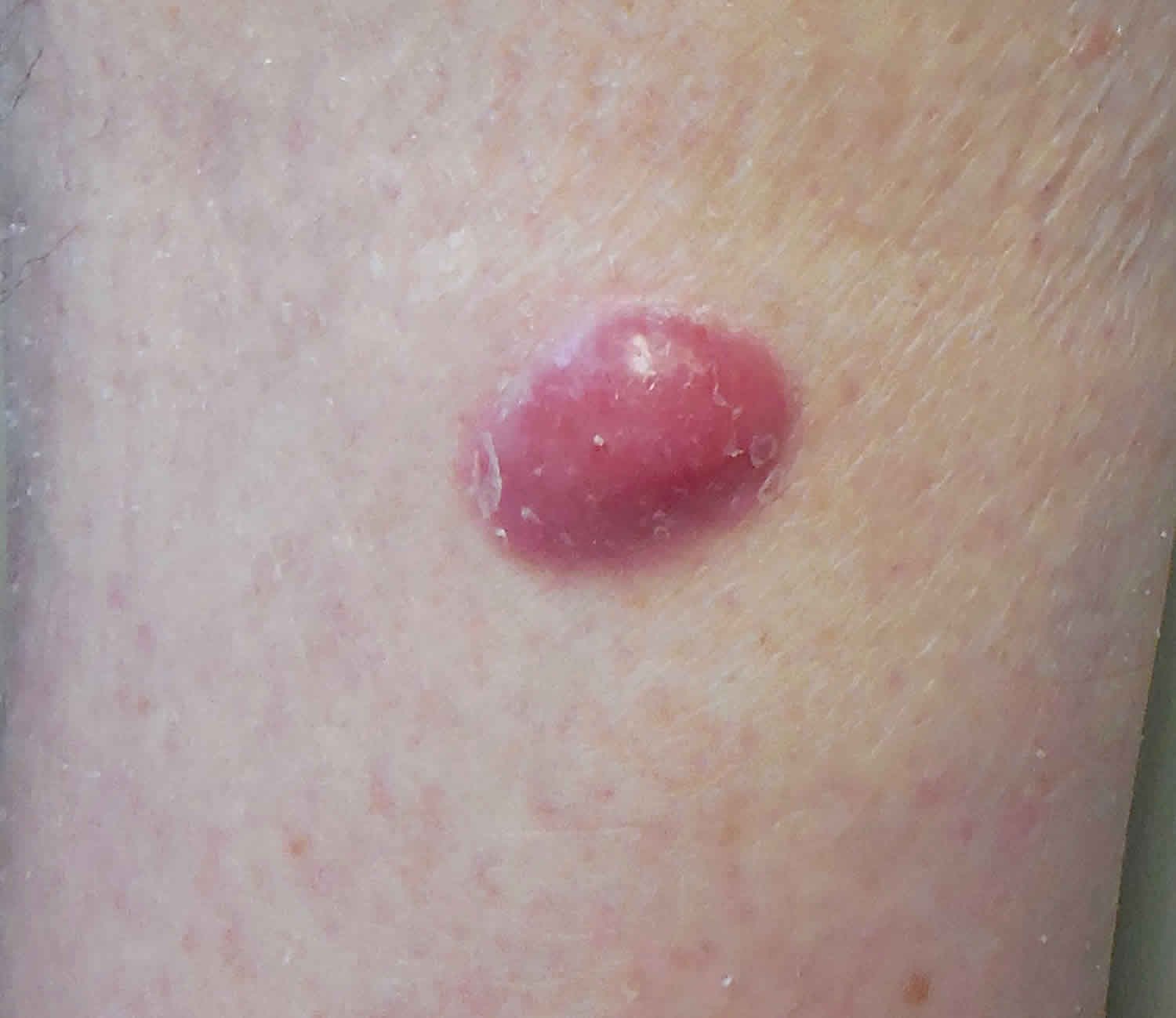
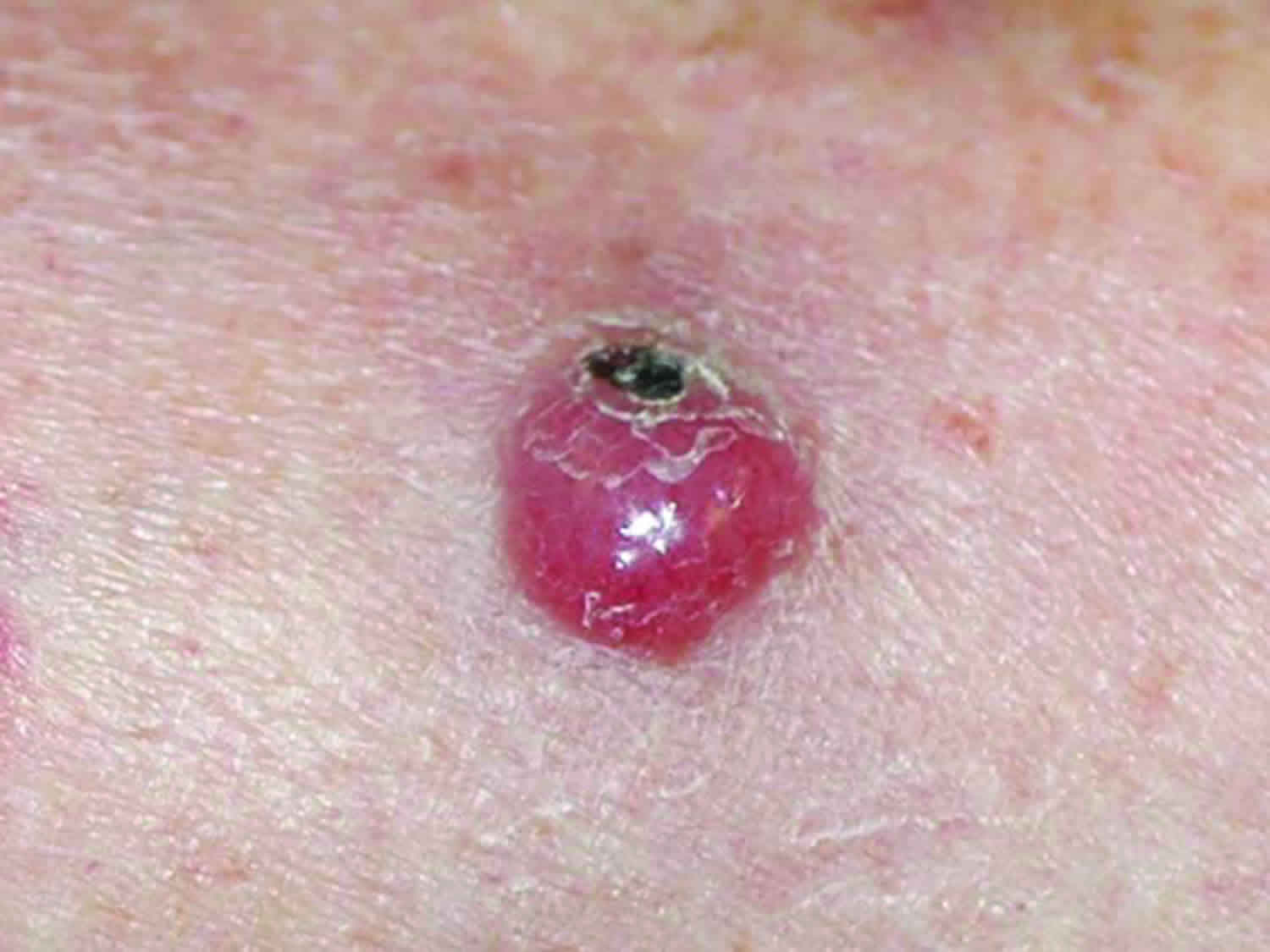
Figure 1. Merkel cell carcinoma
Figure 2. Merkel Cell anatomy
Footnote: Merkel cells are located deep in the top layer of skin. Merkel cells are connected to nerves, signaling touch sensation as “touch receptors.” Merkel cell carcinoma was named after Merkel cells due to the similar microscopic features; however, recent research suggests that it is unlikely that Merkel cell carcinoma originates directly from normal Merkel cells with recent investigation pointing to their origin being early B-cells (lymphocytes) based on cellular morphology, the expression of early B-cell markers and clonal immunoglobulin chain rearrangement.
How dangerous is Merkel cell carcinoma?
While Merkel cell carcinoma is about three to five times more likely to be deadly than melanoma, with early detection, Merkel cell carcinoma can be treated successfully. Five-year survival rates are overall 30–50% 18. Survival rates are better for patients initially diagnosed with Stage 1 Merkel cell carcinoma and in younger patients than when it has already metastasized or patients are over 80 years of age.
If you think you might have Merkel cell carcinoma, see your doctor. Treatment becomes increasingly difficult once the disease has spread, but new options are now available. Thanks to advances in the field of immunotherapy, Merkel cell carcinoma survival rates are improving.
What you can do:
- Examine your skin head-to-toe every month.
- See your dermatologist every year for a professional skin exam. If you are newly diagnosed with Merkel cell carcinoma, seek a consultation with a multidisciplinary expert. Find a specialist here.
- If you’ve had an Merkel cell carcinoma, follow up regularly with your dermatologist once treatment is complete. Follow the exam schedule your doctor recommends — it’s the best way to quickly detect a recurrence. Consider getting the AMERK biomarker blood test that can help detect recurrences early.
- Safeguard your skin and avoid unprotected UV exposure. It is the single most effective way to reduce your risk of developing Merkel cell carcinoma.
- Practice sun-safe habits, such as seeking shade and wearing protective clothing, a wide-brimmed hat and UV-blocking sunglasses. Use a broad-spectrum sunscreen every day. Sunscreen is a good defense against premature aging and skin cancer. It’s never too late to start wearing it.
Merkel cell carcinoma causes
Increased incidence of Merkel cell carcinoma has also been seen in people treated heavily with methoxsalen (psoralen) and ultraviolet A (PUVA) for psoriasis (3 of 1,380 patients, 0.2%), and those with chronic immune suppression, especially from chronic lymphocytic leukemia, human immunodeficiency virus, and previous solid organ transplant 19.
The immune system is believed to play a critical role in Merkel cell carcinoma pathogenesis with both Merkel cell polyoma virus (MCPyV) and ultraviolet (UV) radiation identified as risk factors for oncogenesis 2. In 2008, a novel polyomavirus called Merkel cell polyomavirus (MCPyV) was first reported in Merkel cell carcinoma tumor specimens 20, a finding subsequently confirmed in other laboratories 21. High levels of viral DNA and clonal integration of the Merkel cell polyomavirus in Merkel cell carcinoma tumors have also been reported 22 along with expression of certain viral antigens in Merkel cell carcinoma cells and the presence of antiviral antibodies. Merkel cell polyomavirus has been detected in about 80% Merkel cell carcinomas tested. However, not all cases of Merkel cell carcinoma appear to be associated with Merkel cell polyomavirus infection 23.
Merkel cell polyomavirus has been detected at very low levels in normal skin distant from the Merkel cell carcinoma primary, in a significant percentage of patients with non-Merkel cell carcinoma cutaneous disorders, in normal-appearing skin in healthy individuals, and in nonmelanoma skin cancers in immune-suppressed individuals 24. Various methods have been used to identify and quantify the presence of Merkel cell polyomavirus in Merkel cell carcinoma tumor specimens, other non-Merkel cell carcinoma tumors, blood, urine, and other tissues 25.
The significance of the new Merkel cell polyomavirus findings remains uncertain. The prognostic significance of viral load, antibody titer levels, and the role of underlying immunosuppression in hosts (from disease and medications) are under investigation.
Prevalence of Merkel cell polyomavirus appears to differ between Merkel cell carcinoma patients in the United States and Europe versus Australia. There may be two independent pathways for the development of Merkel cell carcinoma: one driven by the presence of Merkel cell polyomavirus, and the other driven primarily by sun damage, especially as noted in patient series from Australia 26.
Immunosuppression is an important factor for the development of Merkel cell carcinomas. The immune system is believed to play an important role in both the development of Merkel cell carcinoma and subsequent therapeutic response 2. Increased risk of Merkel cell carcinoma development has been shown in immunosuppressed patient populations including, but not limited to, patients with HIV/AIDS 27, patients undergoing solid organ transplant 28, 29 and patients with chronic lymphocytic leukemia 30 and non-Hodgkin lymphoma 31, 32. Furthermore, Merkel cell carcinoma in immunosuppressed patients has been observed to sometimes regress with improvement in immune function 33, 34, emphasizing the role immune surveillance in Merkel cell carcinoma development 35. Immunosuppression has been shown in multiple studies to be a negative independent prognostic factor for patients with Merkel cell carcinoma 36, 37, 38, 39, 40. With regard to differential impact on clinical outcomes of immunosuppression in Merkel cell carcinoma, Cook et al. 41 performed a retrospective analysis of 89 patients with non-metastatic Merkel cell carcinoma and found increased mortality risk for immunosuppressed patients with HIV/AIDS and organ transplant relative to patients with autoimmune disease (reference group). Yusuf et al. 36 found immune status to be an independent predictor of overall survival for patients with Merkel cell carcinoma and found immunosuppression to be associated with overall survival, with the lowest 3-year overall survival rates for immunosuppressed patients with solid organ transplants.
Merkel cell carcinoma was previously believed to arise from Merkel cells, which are pressure receptors in the skin. A recent investigation is pointing to their origin being early B-cells (lymphocytes) based on cellular morphology, the expression of early B-cell markers and clonal immunoglobulin chain rearrangement.
Although no unique marker for Merkel cell carcinoma has been identified, a variety of molecular and cytogenetic markers of Merkel cell carcinoma have been reported 42.
Risk factors for developing Merkel cell carcinoma
Anything that increases your likelihood of developing a disease like Merkel cell carcinoma (Merkel cell skin cancer) is called a risk factor.
These factors put you at increased risk for the disease.
- History of unprotected exposure to ultraviolet (UV) radiation from the sun or indoor tanning. UV exposure creates a double threat for Merkel cell carcinoma. Not only does UV exposure cause damage that increases your skin cancer risk; it also suppresses your immune system, reducing its ability to repair damage and fight skin cancers and other diseases. No matter the source of UV radiation, there is a clear and dangerous correlation between exposure and Merkel cell carcinoma.
- Weakened immune system, due to a medical condition or medications. If your immune system is persistently weakened or suppressed, you are about 15 times more likely to develop Merkel cell carcinoma than people with healthy immune systems. However, more than 90 percent of Merkel cell carcinoma cases arise in people with no known immune problems. Immunosuppression can be caused by:
- Diseases such as HIV and certain cancers including chronic lymphocytic leukemia and lymphomas.
- Immunosuppressant medications used for transplant recipients and for autoimmune diseases including lupus, psoriasis, Crohn’s disease and rheumatoid arthritis.
- Immunosuppressed Merkel cell carcinoma patients often develop the disease at a far younger age; about half of Merkel cell carcinomas in these patients occur before age 50.
- If your immune system is suppressed, talk to your doctors about your chances of developing Merkel cell carcinoma, and be sure to protect yourself against other risk factors, especially UV exposure.
- History of skin cancer, If you’ve had melanoma, squamous cell carcinoma (SCC), basal cell carcinoma (BCC) or Bowen’s disease (an early form of SCC) — skin cancers frequently caused by UV exposure — you are more likely to develop Merkel cell carcinoma.
- Age: Most people who develop Merkel cell carcinoma are over 50 years old.
- Gender: Men are more likely to get the disease. This might be because they tend to get more sun exposure.
- Fair skin: People with fair skin are at greater risk for Merkel cell carcinoma, but it can affect anyone.
- Merkel cell polyomavirus. Evidence of the Merkel cell polyomavirus can be found in most, but not all, Merkel cell tumors. The virus, discovered in 2008, lives in the skin of most people, without signs and symptoms, and without ever developing into Merkel cell carcinoma. Since it’s a very common virus, and since Merkel cell carcinoma is an extremely rare disease, scientists are still not certain about exactly how or why the virus causes Merkel cell carcinoma in some people. What is clear is that other factors such as UV exposure and immunosuppression also play a role in initiating the growth of Merkel cell carcinoma tumors.
Merkel cell carcinoma prevention
While your risk of getting Merkel cell carcinoma (Merkel cell cancer) is low, and some risk factors for Merkel cell carcinoma, such as your age, sex, and skin color can’t be controlled, there are things you can do that might help lower your risk. These might also lower your risk of getting more common types of skin cancer, as well as some other types of cancer.
- Limit your exposure to ultraviolet (UV) rays. The most important way to lower your risk of skin cancers (including Merkel cell carcinoma) is to limit your exposure to ultraviolet (UV) rays. The main types of UV rays that can affect your skin include UVA rays and UVB rays. UVB rays have more energy and are a more potent cause of at least some skin cancers, but both UVA and UVB rays can damage skin and cause skin cancer. There are no safe UV rays. Practice sun safety when you are outdoors. The US National Weather Service and the Environmental Protection Agency (EPA) have developed the UV Index, which gives you an idea of how strong the UV light is in your area on any given day, on a scale from 1 to 11+. A higher number means greater risk of exposure to UV rays and a higher chance of sunburn and skin damage that could ultimately lead to skin cancer. Further information about the UV Index, as well as your local UV Index forecast, can be found on the EPA’s website at https://www.epa.gov/sunsafety/uv-index-1
- Simply staying in the shade is one of the best ways to limit your UV exposure. If you are going to be in the sun, “Slip! Slop! Slap! and Wrap” is a catchphrase that can help you remember some of the key steps you can take to protect yourself from UV rays:
- Slip on a shirt. Many companies now make clothing that’s lightweight, comfortable, and protects against UV rays even when wet. It tends to be more tightly woven, and some have special coatings to help absorb UV rays. These sun-protective clothes may have a label listing the UV protection factor (UPF) value (the level of protection the garment provides from the sun’s UV rays, on a scale from 15 to 50+). The higher the UPF, the higher the protection from UV rays.
- Slop on sunscreen (use a “broad spectrum SPF50+” sunscreen that filters out about 98% of the UV rays). Broad spectrum means it protect against both UVA and UVB rays. Sunscreens labeled with SPFs as high as 100+ are available. Only “broad spectrum” sunscreen products with an SPF of 15 or higher can state that they help protect against skin cancer and early skin aging if used as directed with other sun protection measures. However, no sunscreen protects you completely.
- Slap on a hat. A hat with at least a 2- to 3-inch brim all around is ideal because it protects areas that are often exposed to intense sun, such as the ears, eyes, forehead, nose, and scalp. A dark, non-reflective underside to the brim can also help lower the amount of UV rays reaching the face from reflective surfaces such as water. A shade cap (which looks like a baseball cap with about 7 inches of fabric draping down the sides and back) also is good, and will provide more protection for the neck. If you don’t have a shade cap (or another good hat) available, you can make one by wearing a large handkerchief or bandana under a baseball cap. A baseball cap protects the front and top of the head but not the neck or the ears, where skin cancers commonly develop. Straw hats are not as protective as hats made of tightly woven fabric.
- Wrap on sunglasses to protect the eyes and skin around them. The ideal sunglasses should block 99% to 100% of UVA and UVB rays. Before you buy, check the label to make sure they do. Labels that say “UV absorption up to 400 nm” or “Meets ANSI UV Requirements” mean the glasses block at least 99% of UV rays. Those labeled “cosmetic” block about 70% of UV rays. If there is no label, don’t assume the sunglasses provide any UV protection.
- Slip on a shirt. Many companies now make clothing that’s lightweight, comfortable, and protects against UV rays even when wet. It tends to be more tightly woven, and some have special coatings to help absorb UV rays. These sun-protective clothes may have a label listing the UV protection factor (UPF) value (the level of protection the garment provides from the sun’s UV rays, on a scale from 15 to 50+). The higher the UPF, the higher the protection from UV rays.
- Simply staying in the shade is one of the best ways to limit your UV exposure. If you are going to be in the sun, “Slip! Slop! Slap! and Wrap” is a catchphrase that can help you remember some of the key steps you can take to protect yourself from UV rays:
- Don’t use tanning beds or sunlamps. Many people believe the UV rays of tanning beds are harmless. This is not true. Tanning lamps give off UV rays, which can cause long-term skin damage and can contribute to skin cancer. Most skin doctors and health organizations recommend not using tanning beds and sun lamps.
- Protect children from the sun. Children need special care, since they tend to spend more time outdoors and can burn more easily. Parents and other caregivers should protect children from excess sun exposure by using the steps above. Children need to be taught about the dangers of too much sun exposure as they become more independent.
- Keep your immune system strong. Having a weakened immune system greatly increases the risk of getting Merkel cell carcinoma, as well as other types of skin cancer. In some cases, such as organ transplant, you can’t control the things that must be done that weaken (suppress) your immune system. But something you can control is being infected with HIV. Infection with HIV, the virus that causes AIDS, weakens the immune system. Avoiding known risk factors for HIV infection, such as intravenous (IV) drug use and having unprotected sex with many partners, can also lower your risk of immune system problems. This, in turn, might help keep you from getting Merkel cell carcinoma and many other types of cancer.
Merkel cell carcinoma symptoms
Merkel cell carcinoma (MCC) tumors often, but not always, appear on sun-exposed areas of your body, especially your face, neck, arms, and legs, but it can occur anywhere on your body. The tumors are not nearly as distinctive as other more common skin cancers such as basal cell carcinoma and can appear as a pearly pimple-like lump, sometimes skin-colored, pink, red, purple or bluish-red, though they are rarely tender to the touch. Sometimes the skin on the top of the tumor breaks open and bleeds. The rapid speed at which they grow is what often causes patients and doctors to take notice. Because of its nonspecific clinical appearance, Merkel cell carcinoma is rarely suspected before a biopsy is performed 43.
Merkel cell cancers spread through the lymphatic system and multiple metastases can develop around the main tumor (local recurrence). Merkel cell carcinoma may also spread to lymph nodes in the neck, armpits and groin. This is more likely in thicker tumors. Most recurrences occur within the first two years after diagnosis.
Merkel cell carcinoma is rare, and it can look like many other, more common types of skin cancer or other skin problems when it first appears. It’s very important to have any new, growing, or changing lumps, bumps, or spots on your skin checked by a doctor as soon as possible so that the cause can be found and treated, if needed. The earlier any type of skin cancer is found, the easier it might be to treat.
Can Merkel cell carcinoma be found early?
While the American Cancer Society doesn’t have guidelines for the early detection of skin cancer, knowing your own skin is important in finding skin cancer early. Learn the patterns of moles, blemishes, freckles, and other marks on your skin so that you’ll notice any changes. Many doctors recommend checking your own skin once a month. Self-exams are best done in a well-lit room in front of a full-length mirror. Use a hand-held mirror for areas that are hard to see, such as the backs of your thighs.
Examine all of your skin, including your palms and soles, scalp, ears, nails, and your back. A friend or family member can also help you with these exams, especially for those hard-to-see places, like your scalp and back.
Be sure to show your doctor any skin changes that concern you and have them look at areas that may be hard for you to see. Any spots on your skin that are new or changing in size, shape, or color should be seen by a doctor right away. If you can’t see your doctor soon, you might want to take good close-up photos of the area so your doctor can see if it’s changing when you do get an appointment.
Any unusual sore, lump, blemish, marking, or change in the way an area of the skin looks or feels may be a sign of skin cancer or a warning that it might occur. The area might become red, swollen, scaly, crusty, or start oozing or bleeding. It may feel itchy, tender, or painful.
Merkel cell tumors usually look like firm, pink, red, or purple lumps or bumps on sun-exposed areas of the skin. They usually don’t hurt, but they can grow quickly and can sometimes open up as ulcers or sores.
Merkel cell carcinoma diagnosis
Merkel cell carcinoma should be considered in any tumor with “AEIOU” clinical features, which are present in about 90% of patients with Merkel cell skin cancer.
- Asymptomatic or non-tender
- Expanding rapidly
- Immune suppressed
- Older than 50
- UV-exposed fair skin
Not all patients have every element in the “AEIOU” mnemonic; however, in this study 44, 89% of patients met three or more criteria, 52% met four or more criteria, and 7% met all five criteria.
The main test is a biopsy of the tumor. This shows characteristic Merkel cell carcinoma pathology. Immunohistochemistry can be helpful as cytokeratin-20 (CK20) is positive in up to 95% of tumors and thyroid transcription factor (TTF1) is usually negative.
A general examination, including evaluation of local lymph nodes, and staging investigations may be arranged to determine whether the tumor has spread to other sites.
Staging investigations may include:
- Sentinel node biopsy. A sentinel lymph node biopsy can be used to find the lymph nodes that are likely to be the first place the Merkel cell carcinoma would go if it has spread. These lymph nodes are called sentinel nodes.
- Lymph node ultrasound scan
- Imaging using X-rays, computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) scans. They can be used to see if Merkel cell carcinoma has spread to lymph nodes or to other organs in the body. Imaging tests can also be done to help see how well treatment is working or to look for possible signs of cancer coming back (recurring) after treatment.
If an imaging work-up is performed, it may include a computed tomography (CT) scan of the chest and abdomen to rule out primary small cell lung cancer as well as distant and regional metastases. Imaging studies designed to evaluate suspicious signs and symptoms may also be recommended. In one series, CT scans had an 80% false-negative rate for regional metastases 45. Head and neck presentations may require additional imaging. Magnetic resonance imaging has been used to evaluate Merkel cell carcinoma but has not been studied systematically 46. Fluorine F 18-fludeoxyglucose positron emission tomography results have been reported only in selected cases 47. Routine blood work as a baseline has been recommended but has not been studied systematically. There are no known circulating tumor markers specifically for Merkel cell carcinoma.
A specific Merkel cell carcinoma staging system is published by the American Joint Committee (AJCC) on Cancer.
Longterm survival is likely if the lymph nodes do not contain tumor cells.
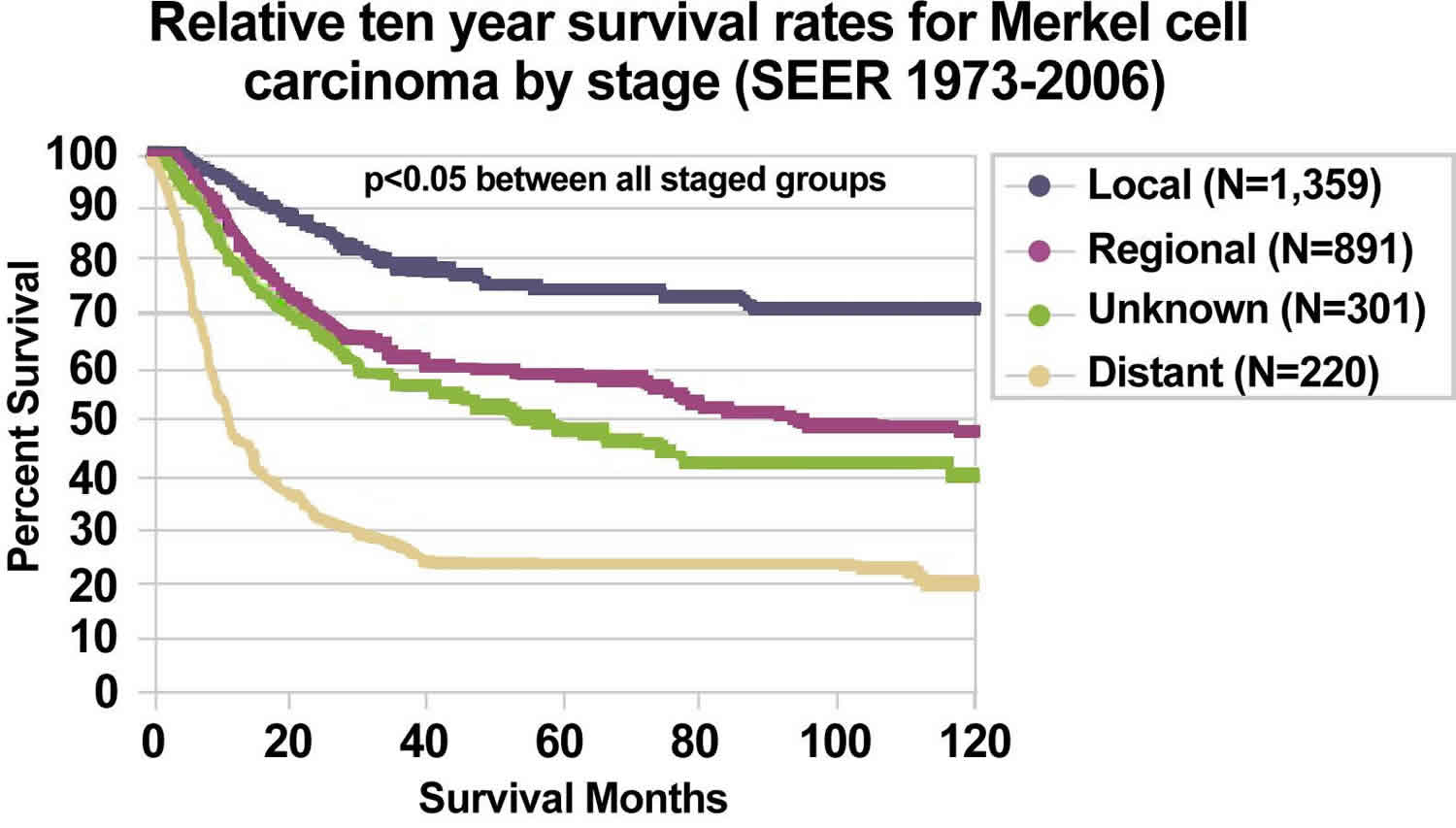
The results of initial clinical staging of Merkel cell carcinoma vary widely in the literature, based on retrospective case series reported over decades. For invasive cancers, 48.6% were localized, 31.1% were regional, and 8.2% were distant 48.
Merkel cell carcinoma that presents in regional nodes without an identifiable primary lesion is found in a minority of patients, with the percent of these cases varying among the reported series. Tumors without an identifiable primary lesion have been attributed to either spontaneous regression of the primary or metastatic neuroendocrine carcinoma from a clinically occult site 49.
Challenges in diagnosis
Diagnosis and management of Merkel cell carcinoma presents distinct challenges, including:
- Misdiagnosis: 56 percent of Merkel cell carcinomas are believed to be benign when initially examined by physicians, who may mistake the tumors for cysts or infected hair follicles.
- Delayed medical attention: Medical care is often not sought early as these lesions do not appear concerning. About 5 percent of Merkel cell carcinomas occur in mucosal sites such as the mouth, nasal cavity and throat, where these painless lesions are hard to spot before the disease has advanced.
- Undetectable: In one study, 14 percent of Merkel cell carcinomas were initially discovered in the lymph nodes already, without any tumor being identified.
Since Merkel cell carcinoma is a rare and aggressive cancer, delayed detection, diagnosis and treatment are especially concerning, because as the disease progresses, treatment becomes more difficult and the risk of recurrence increases.
Initial Clinical Evaluation
Because local-regional spread is common, newly diagnosed Merkel cell carcinoma patients require a careful clinical examination that includes looking for satellite lesions and regional nodal involvement and tests are done to find out if cancer cells have spread to other parts of the body.
Tailoring an imaging work-up to the clinical presentation and any relevant signs and symptoms should be considered. There has been no systematic study of the optimal imaging work-up for newly diagnosed patients, and it is not clear if all newly diagnosed patients, especially those with the smallest primaries, benefit from a detailed imaging work-up.
The process used to find out if cancer has spread to other parts of the body is called staging. The information gathered from the staging process determines the stage of the disease. It is important to know the stage in order to plan treatment.
The following tests and procedures may be used in the staging process:
- CT scan (CAT scan): A procedure that makes a series of detailed pictures of areas inside the body, taken from different angles. The pictures are made by a computer linked to an x-ray machine. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. A CT scan of the chest and abdomen may be used to check for primary small cell lung cancer, or to find Merkel cell carcinoma that has spread. A CT scan of the head and neck may also be used to find Merkel cell carcinoma that has spread to the lymph nodes. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
- PET scan (positron emission tomography scan): A procedure to find malignant tumor cells in the body. A small amount of radioactive glucose (sugar) is injected into a vein. The PET scanner rotates around the body and makes a picture of where glucose is being used in the body. Malignant tumor cells show up brighter in the picture because they are more active and take up more glucose than normal cells do.
- Lymph node biopsy: There are several types of lymph node biopsy used to stage Merkel cell carcinoma.
- Sentinel lymph node biopsy: The removal of the sentinel lymph node during surgery. The sentinel lymph node is the first lymph node in a group of lymph nodes to receive lymphatic drainage from the primary tumor. It is the first lymph node the cancer is likely to spread to from the primary tumor. A radioactive substance and/or blue dye is injected near the tumor. The substance or dye flows through the lymph ducts to the lymph nodes. The first lymph node to receive the substance or dye is removed. A pathologist views the tissue under a microscope to look for cancer cells. If cancer cells are not found, it may not be necessary to remove more lymph nodes. Sometimes, a sentinel lymph node is found in more than one group of nodes.
- Lymph node dissection: A surgical procedure in which the lymph nodes are removed and a sample of tissue is checked under a microscope for signs of cancer. For a regional lymph node dissection, some of the lymph nodes in the tumor area are removed. For a radical lymph node dissection, most or all of the lymph nodes in the tumor area are removed. This procedure is also called lymphadenectomy.
- Core needle biopsy: A procedure to remove a sample of tissue using a wide needle. A pathologist views the tissue under a microscope to look for cancer cells.
- Fine-needle aspiration biopsy: A procedure to remove a sample of tissue using a thin needle. A pathologist views the tissue under a microscope to look for cancer cells.
- Immunohistochemistry: A laboratory test that uses antibodies to check for certain antigens (markers) in a sample of a patient’s tissue. The antibodies are usually linked to an enzyme or a fluorescent dye. After the antibodies bind to a specific antigen in the tissue sample, the enzyme or dye is activated, and the antigen can then be seen under a microscope. This type of test is used to help diagnose cancer and to help tell one type of cancer from another type of cancer.
Merkel cell carcinoma staging
After someone is diagnosed with Merkel cell cancer, your medical team will try to figure out if it has spread, and if so, how far. This process is called staging. Staging is a way to understand how much the cancer has grown and how far it has spread. It helps determine how serious the cancer is, how best to treat it and the risk of it coming back. Doctors also use a cancer’s stage when talking about survival statistics.
American Joint Committee on Cancer (AJCC) Stage Groupings and the TNM system is frequently used to stage Merkel cell carcinoma. It’s a classification based on three factors:
- T represents the size of the original tumor, its growth rate and other factors.
- N indicates whether the cancer has spread to the local lymph nodes and to what extent.
- M stands for the spread or metastasis to distant lymph nodes and organs.
Numbers or letters after T, N, and M provide more details about each of these factors. Higher numbers mean the cancer is more advanced. Once the patient’s TNM categories have been established, the overall stage number is assigned. As a rule, the lower the stage number, the less the disease has progressed. For example, the earliest stage Merkel cell cancers are called stage 0 (or carcinoma in situ), and then range from stages I (1) through IV (4). Cancer staging can be complex, so ask your doctor to explain it to you in a way you understand.
The staging system in the Table 1 below uses the pathologic stage also called the surgical stage. This is the staging system most often used for Merkel cell cancer. It’s decided after testing the tissue taken out during an operation.
Sometimes, if surgery can’t be done right away or at all, the cancer will be given a clinical stage instead. This is based on the results of a physical exam, biopsy, and imaging tests. The clinical stage will be used to help plan treatment. In some cases, though, the cancer has spread further than the clinical stage estimates, and may not predict the patient’s outlook as well as a pathologic stage. Clinical staging for Merkel cell carcinoma follows a separate staging system that’s not covered in the table below. If your cancer has been clinically staged, ask your doctor for information about your specific stage.
The following stages are used for Merkel cell carcinoma:
Table 1. Merkel cell carcinoma stages
| AJCC Stage | Stage grouping | Stage description* |
|---|---|---|
| 0 | Tis N0 M0 | The cancer is only in the epidermis, the outermost skin layer (Tis). It has not spread to nearby lymph nodes (N0) or distant sites (M0). This stage is also known as carcinoma in situ (Tis). |
| I | T1 N0 M0 | The cancer is no more than 2 centimeters (cm) across (about 4/5 inch). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| IIA | T2 or T3 N0 M0 | The cancer is more than 2, but less than 5 cm (about 2 inches) across (T2) OR the tumor is more than 5 cm across (T3). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| IIB | T4 N0 M0 | The cancer has grown into nearby tissues such as muscles, bones, or cartilage (T4). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| IIIA | T1, T2, T3, or T4 N1a(sn) or N1a M0 | The cancer can be any size or may have grown into nearby tissues (T1, T2, T3, or T4) AND the cancer has spread to nearby lymph nodes, but this was found during a lymph node biopsy or surgery and was not seen on exams or imaging tests (N1a[sn] or N1a). It has not spread to distant sites (M0). |
| OR | ||
| T0 N1b M0 | There’s no sign of a primary cancer (T0) AND the cancer has spread to nearby lymph nodes, which was seen on exams or imaging tests and then confirmed by biopsy or surgery (N1b). It has not spread to distant sites (M0). | |
| IIIB | T1, T2, T3, or T4 N1b, N2 or N3 M0 | The cancer can be any size or may have grown into nearby tissues (T1, T2, T3, or T4) AND any of the following: It has spread to nearby lymph nodes, which was seen on exams or imaging tests and then confirmed by biopsy or surgery (N1b). It has spread toward a nearby lymph node area without reaching the lymph nodes (N2). This is called in transit metastasis. It has spread toward a nearby lymph node area (called in transit metastasis) and has reached the lymph nodes (N3). It has not spread to distant sites (M0). |
| IV | T0, T1, T2, T3 or T4 Any N M1 | The cancer can be any size or may have grown into nearby tissues (T0, T1, T2, T3, or T4) AND might or might not have spread to nearby lymph nodes (Any N). It has spread to distant lymph nodes or organs, such as the lungs or skin (M1). |
Footnotes: * The following categories are not listed on the table above:
- TX: Main tumor cannot be assessed due to lack of information.
- T0: No evidence of a primary tumor.
- NX: Regional lymph nodes cannot be assessed due to lack of information.
Stage 0 (carcinoma in situ) Merkel cell carcinoma
In stage 0, abnormal Merkel cells are found in the top layer of skin. These abnormal cells may become cancer and spread into nearby normal tissue.
Stage 1 Merkel cell carcinoma
In stage 1, the tumor is 2 centimeters or smaller.
Stage 2 Merkel cell carcinoma
Stage 2 Merkel cell carcinoma is divided into stages 2A and 2B.
- In stage 2A, the tumor is larger than 2 centimeters.
- In stage 2B, the tumor has spread to nearby connective tissue, muscle, cartilage, or bone.
Stage 3 Merkel cell carcinoma
Stage 3 Merkel cell carcinoma is divided into stages 3A and 3B.
In stage 3A, either of the following is found:
- the tumor may be any size and may have spread to nearby connective tissue, muscle, cartilage, or bone. A lymph node cannot be felt during a physical exam but cancer is found in the lymph node by sentinel lymph node biopsy or after the lymph node is removed and checked under a microscope for signs of cancer; or
- a swollen lymph node is felt during a physical exam and/or seen on an imaging test. When the lymph node is removed and checked under a microscope for signs of cancer, cancer is found in the lymph node. The place where the cancer began is not known.
In stage 3B, the tumor may be any size and:
- may have spread to nearby connective tissue, muscle, cartilage, or bone. A swollen lymph node is felt during a physical exam and/or seen on an imaging test. When the lymph node is removed and checked under a microscope for signs of cancer, cancer is found in the lymph node; or
- cancer is in a lymph vessel between the primary tumor and lymph nodes that are near or far away. Cancer may have spread to lymph nodes.
Stage 4 Merkel cell carcinoma
In stage 4, the tumor has spread to skin that is not close to the primary tumor or to other parts of the body, such as the liver, lung, bone, or brain.
Recurrent Merkel Cell Carcinoma
Recurrent Merkel cell carcinoma is cancer that has recurred (come back) after it has been treated. The cancer may come back in the skin, lymph nodes, or other parts of the body. It is common for Merkel cell carcinoma to recur.
Merkel cell carcinoma treatment
Once Merkel cell carcinoma is diagnosed, multidisciplinary consultation is usual. As 5-year survival rates for Merkel cell carcinoma are only around 50%, early aggressive treatment is required, most often with a combination of surgery and radiation therapy.
Two competing philosophies underlie many of the controversies about the most appropriate method of treating Merkel cell carcinoma. In the first philosophy, Merkel cell carcinoma is treated like other nonmelanoma skin cancers, with an emphasis on treating local-regional disease with surgery and radiation as appropriate. In the second philosophy, Merkel cell carcinoma is treated according to its biologic features. This approach makes it analogous to small cell lung cancer, which is assumed to be a systemic disease, and leads to a more routine recommendation of systematic adjuvant chemotherapy 51.
Localized Merkel cell carcinoma
Surgical excision is the main treatment of primary Merkel cell carcinoma. Primary surgical resection with the intent of obtaining histologically negative margins may be achieved with wide local excision, narrow margin excision, or with Mohs micrographic surgery with sentinel lymph node biopsy (localized disease). The National Comprehensive Cancer Network (NCCN) and the European Association of Dermato-Oncology (EADO)–European Organisation for Research and Treatment of Cancer (EORTC) guidelines recommend a 1–2 cm excision margin down to the muscle fascia or the pericranium (the membrane that externally covers the skull), regardless of tumor size 52, 53, 54. However, there is limited clinical data with regard to the optimal resection margin for Merkel cell carcinoma. Furthermore, the European Association of Dermato-Oncology (EADO)–European Organisation for Research and Treatment of Cancer (EORTC) guidelines recommend complete histologic inspection of the margins of excised tissue using Mohs microscopically controlled surgery, but experience is limited in Merkel cell carcinoma 55, 56, 57. Of note, the safety margin is intended to remove microscopic satellite metastases rather than to ensure clear resection margins of the primary tumor 52, 53. Any reconstruction involving tissue displacement should be postponed until negative margins have been confirmed and sentinel lymph node biopsy is performed, if applicable. Surgical techniques for reconstruction of the skin defect should take further adjuvant radiotherapy into account.
With regard to optimal surgical resection technique for patients with early stage Merkel cell carcinoma, the available literature is limited to retrospective single institution or database analyses in the absence of level 1 evidence. Shaikh et al. 58 performed an analysis of the SEER database of patients with microscopically confirmed Merkel cell carcinoma (Merkel cell cancer) with 2093 patients (92.3%) treated with wide local excision and 174 patients (7.7%) treated with Mohs micrographic surgery. No significant differences in overall survival or Merkel cell carcinoma-specific survival were demonstrated in their cohort. Mohs micrographic surgery was more likely to be used for Merkel cell carcinoma of the head and neck. Singh et al. 59 performed an analysis of the National Cancer Database, including 1795 patients with localized (stage 1 or 2) Merkel cell carcinoma treated with wide local excision (n = 1685), and did not demonstrate a significant difference in overall survival between the two groups. In the absence of prospective data, either wide local excision or Mohs micrographic surgery appear to be efficacious for tumor ablation for well-selected tumors. Mohs micrographic surgery in particular may be beneficial for Merkel cell carcinoma of the head and neck where tissue preservation may be paramount.
Allen and colleagues performed a retrospective analysis of 251 patients with Merkel cell carcinoma treated at Memorial Sloan Kettering Cancer Center and did not find a surgical margin of ≥1 cm to be associated with decreased local recurrence in comparison to margins <1 cm 60. Perez et al. 61 performed a retrospective examination of 240 patients with Merkel cell carcinoma treated at the Moffitt Cancer Center and did not find local recurrence to be significantly different between patients with 1 cm resection margins (2.9%), 1.1 to 1.9 cm margins (2.8%), or ≥2 cm margins (5.2%). Of note, 69.2% of patients in their cohort received adjuvant radiation therapy. In contrast, Andruska and colleagues 62 performed a retrospective analysis of 79 patients with stage 1 or 2 Merkel cell carcinoma treated with wide local excision at Washington University in St. Louis and demonstrated higher disease-specific survival for patients at 1 year with ≥2 cm margins (87.8%) relative to patients with 1 to 1.9 cm margins (71.4%) and margins < 1 cm (57.7%). The majority of patients (68%) did not receive adjuvant radiotherapy in their cohort. Additional considerations include the ability to undergo primary wound closure, which may be associated with superior cosmesis and decreased postoperative care/costs relative to graft or flap closure, and may be difficult to perform with larger resection margins (56.5% of patients in the cohort presented by Perez et al. with 1 cm margins underwent primary closure compared to 34.1% of patients with margins ≥ 2 cm). When considering the available literature in total, 1 to 2 cm resection margins when clinically feasible appear appropriate with consideration for adjuvant radiotherapy in the setting of close (2 cm or less) resection margins along with additional clinical risk factors.
The primary site may be treated with radiotherapy postoperatively, especially for large lesions (> 2 cm). Radiation treatment leads to increased local and regional disease control and higher long-term survival rates.
The relevant lymph nodes may also be surgically removed or irradiated as a prophylactic measure.
In a review of 18 case series, 279 of 926 patients (30.1%) developed local recurrence during follow-up, excluding those presenting with distant metastatic disease at presentation. These recurrences have been typically attributed to inadequate surgical margins or possibly a lack of adjuvant radiation therapy 63.
Given the propensity of Merkel cell carcinoma to recur locally (sometimes with satellite lesions and/or in-transit metastases), wide local excision to reduce the risk of local recurrence has been recommended for patients with clinical stage 1 or stage 2 disease.
Recommendations about the optimal minimum width and depth of normal tissue margin to be excised around the primary tumor differ among the various retrospective case series, but this question has not been studied systematically 64. No definitive data suggest that extremely wide margins improve overall survival, although some reports suggest that wider margins appear to improve local control 63. Frozen-section evaluation of margins may be useful, especially when the tumor is in an anatomical site that is not amenable to wide margins.
Some authors have advocated the use of Mohs micrographic surgery as a tissue-sparing technique. The relapse rate has been reported to be similar to or better than that of wide excision, but comparatively few cases have been treated in this manner and none in randomized, controlled trials 65.
Radiation therapy
Because of the aggressive nature of Merkel cell carcinoma, its apparent radiosensitivity, and the high incidence of local and regional recurrences (including in-transit metastases after surgery alone to the primary tumor bed), some clinicians have recommended adjuvant radiation therapy to the primary site and nodal basin. The National Comprehensive Cancer Network suggests consideration of adjuvant radiation therapy targeting the primary tumor bed for patients with clinically node-negative localized Merkel cell carcinoma and no baseline risk factors (primary tumor > 2 cm, lymphovascular invasion, head and neck primary site, immunosuppression) in the setting of known clinical risk factors, including positive or close resection margins or lymphovascular invasion 52. Adjuvant radiation therapy consideration of the tumor bed is recommended for patients with one or more of the aforementioned baseline risk factors in the setting of a narrow resection margin (<1 cm). Nodal basin radiation in contiguity with radiation to the primary site has been considered, especially for patients with larger tumors, locally unresectable tumors, close or positive excision margins that cannot be improved by additional surgery, and those with positive regional lymph nodes, especially after sentinel lymph node dissection (stage 2) 66. Similar recommendations are given regarding primary tumor bed resection for clinically node-positive patients (without nonregional or distant disease). Immunosuppressed patients are at higher risk for recurrence and radiation therapy should be strongly considered for these patients 37, 67. Similarly, recommendations from the European Association of Dermato-Oncology (EADO)–European Organisation for Research and Treatment of Cancer (EORTC) support consideration of adjuvant radiation therapy for most patients with cN0 (nodes not clinically detectable but no pathologic examination) Merkel cell carcinoma 68.
With regard to the draining lymph node radiation therapy, observation is recommended for most patients with localized Merkel cell carcinoma and negative sentinel lymph node biopsy with radiation therapy consideration recommended for patients at higher risk for sentinel lymph node biopsy failure (prior surgery/resection, suboptimal sentinel lymph node biopsy such as failure to perform IHC, profound immunosuppression, or with head and neck primary tumors given potential multiple draining LN basins. Of note, elective nodal radiation therapy for head and neck tumors must be carefully weighed with the potential treatment effects of irradiating multiple nodal basins). The European Association of Dermato-Oncology (EADO)–European Organisation for Research and Treatment of Cancer (EORTC) in general does not recommend adjuvant radiation therapy of the draining nodal basin after therapeutic nodal dissection, but supports consideration of nodal radiation therapy at the discretion of a multidisciplinary tumor board, particularly in the case of involved lymph nodes with extracapsular extension 68.
With regard to time to radiation therapy initiation, the National Comprehensive Cancer Network recommends expeditious initiation of radiation therapy after appropriate postsurgical healing 52. Tsang et al. 69 found relatively high rates of disease progression after surgery but prior to radiation therapy initiation (five of 11 patients waiting for adjuvant radiation therapy). Two recent analyses of the National Cancer Database did not demonstrate time to radiation therapy initiation to be associated with overall survival for patients with localized Merkel cell carcinoma 70, 71, and may offer reassurance for patients requiring additional time for optimal postsurgical healing. Given the limitations of the National Cancer Database, including lack of cancer-specific outcomes, including locoregional control or disease progression prior to radiation therapy, attempts should be made to limit any unnecessary delays to radiation therapy initiation after wound healing.
Gillenwater et al. 72 performed a retrospective analysis of 66 patients with Merkel cell carcinoma of the head and neck and found the use of postoperative radiotherapy to be associated with improved local and regional control, though no difference in disease-specific survival was noted. Clark et al. 73 found adjuvant radiotherapy to be associated with improved locoregional control and disease-free survival in a retrospective analysis of 110 patients with Merkel cell carcinoma of the head and neck. Chen and colleagues 74 queried the National Cancer Database and found both postoperative radiation therapy and chemoradiation to be associated with improved overall survival for patients with Merkel cell carcinoma of the head and neck.
When recommended, the radiation dose given has been at least 50 Gy to the surgical bed with margins and to the draining regional lymphatics, delivered in 2 Gy fractions. For patients with unresected tumors or tumors with microscopic evidence of spread beyond resected margins, higher doses of 56 Gy to 65 Gy to the primary site have been recommended 75. Guidelines from the National Comprehensive Cancer Network recommend adjuvant radiation therapy doses (conventionally fractionated at 2 Gy/fraction) of 50–56 Gy in the setting of R0 resection, 56–60 Gy in the setting of R1 resection (microscopically positive margins), and 60 to 66 Gy in the setting of R2 resection (grossly positive margins) unamenable to further resection. radiation therapy doses of 60–66 Gy are recommended for patients unamenable to surgical resection 52. The National Comprehensive Cancer Network guidelines acknowledge limited evidence supporting dosing recommendations and mention that dose recommendations are provided based on clinical practice of National Comprehensive Cancer Network member institutions and evidence from other cutaneous malignancies. Similar radiation therapy doses are recommended by the European Association of Dermato-Oncology (EADO)–European Organisation for Research and Treatment of Cancer (EORTC) (50 Gy with a 10 Gy boost to tumor bed) 68. With regard to optimal adjuvant radiation therapy dose, Patel et al. 76 performed an analysis of the National Cancer Database of patients with Merkel cell carcinoma of the head and neck and found adjuvant radiation doses of 50 to 55 Gy to be associated with optimal survival. A subsequent analysis of the National Cancer Database of patients with stages 1–3 Merkel cell carcinoma suggested that conventionally fractionated (1.8 to 2 Gy per fraction) adjuvant radiation therapy doses of 50 to 57 Gy may be associated with optimal survival for these patients 77. Limitations inherent to analyses of the National Cancer Database, including lack of granularity regarding radiation target/portals, and cancer-specific endpoints, including Merkel cell carcinoma-specific death and local, regional, and distant control, are present and should be considered when evaluating such investigations. Furthermore, it is unclear if optimal adjuvant radiation therapy doses vary according to Merkel cell polyomavirus (MCPyV) status, which merits future investigation.
Both National Comprehensive Cancer Network and European Association of Dermato-Oncology (EADO)–European Organisation for Research and Treatment of Cancer (EORTC) guidelines recommend consideration of radiation therapy for palliation of symptomatic Merkel cell carcinoma unamenable to resection/radiation therapy as definitive treatment. Palliative dose fractionation schema include 30 Gy in 10 fractions, 20 Gy in 4 or 5 fractions, and 8 Gy in 1 fraction which can be considered for symptomatic primary, regional, and distant sites of disease. In particular, single fraction radiotherapy (8 Gy) has been demonstrated with excellent target control and favorable treatment effect profiles for patients with metastatic Merkel cell carcinoma 78, and intriguingly has been associated with durable local control and limited treatment effects in a retrospective analysis of 12 patients with localized (stage 1/2) Merkel cell carcinoma of the head and neck treated with surgical resection followed by single fraction radiation therapy 79. Such hypofractionated regimens merit further prospective study with larger patient cohorts, and currently may be reasonable to consider for patients with symptomatic metastases, or for adjuvant therapy for patients unable to receive conventionally fractionated radiation therapy.
With regard to optimal radiotherapy targeting of the resected primary tumor, the National Comprehensive Cancer Network recommends generous (~5 cm) margins around the resected tumor bed if clinically feasible 80. Such generous margins may be difficult to incorporate in Merkel cell carcinoma of the head and neck secondary to proximity of vital normal anatomy, and ultimate selection of radiotherapy margins must balance coverage of satellite/local in-transit disease and clinical risk factors for local recurrence (tumor size, lymph-vascular space invasion, immune status, etc.) with treatment morbidity. Elective targeting of the in-transit lymphatics or draining regional nodes is recommended in general only when the nodal bed is in close proximity to the primary tumor. If no sentinel lymph node biopsy or nodal dissection is performed, or if conditions exist which may increase the potential for false-negative sentinel lymph node biopsy (previous wide local excision, operator error, failure to perform appropriate immunohistochemistry on sentinel lymph nodes), elective radiotherapy of draining nodal beds can be considered. Elective nodal irradiation is also recommended to be considered in cases of unsuccessful sentinel lymph node biopsy for Merkel cell carcinoma of the head and neck, and for patients with Merkel cell carcinoma and profound immunosuppression who are at higher risk of presenting with nodal disease 36 and at higher risk for regional recurrence 81. In a randomized trial of patients with stage 1 Merkel cell carcinoma treated with wide local excision and adjuvant radiotherapy of the tumor bed plus or minus elective nodal irradiation (ENI, 50 Gy in 25 fractions) which prematurely closed after enrolling 83 patients, elective nodal irradiation was associated with significantly reduced risk of regional relapse, though no significant benefit in overall survial was demonstrated 82. In the setting of clinically evident adenopathy in the absence of sentinel lymph node biopsy for confirmation or therapeutic nodal dissection, radiotherapy targeting the involved nodal bed to radiotherapy doses is suggested for gross disease as noted above. Regional radiotherapy is also suggested for patients with positive sentinel lymph node biopsy or after lymph node dissection with multiple involved lymph nodes or extracapsular extension. Subsites of the head and neck may have complex nodal drainage, with potential multiple draining nodal basins. Malar cheek Merkel cell carcinoma tumors may drain to ipsilateral facial nodes, the ipsilateral submandibular (IB) basin, the ipsilateral parotid nodes, or preauricular nodes, amongst other draining basins. Merkel cell carcinoma of the ear may drain to the preauricular, postauricular, or upper jugulodigastric nodes (level II), amongst other basins. Primary tumors involving the pinna or posterior to the external auditory canal may additionally drain to the posterior cervical triangle nodes (level VA/VB) 83.
Recommendations from the European Association of Dermato-Oncology (EADO)–European Organisation for Research and Treatment of Cancer (EORTC) are similar with certain differences. sentinel lymph node biopsy is recommended whenever feasible for clinically node-negative disease, with recommendations for therapeutic nodal dissection for positive sentinel lymph node biopsy. In part due to the lack of overall survival benefit demonstrated in the aforementioned trial of elective nodal irradiation for patients with stage 1 Merkel cell carcinoma by Jouary et al. 82, and a lack of overall survival benefit demonstrated with adjuvant radiotherapy for patients with stage 3 (lymph node positive) Merkel cell carcinoma in an analysis of the National Cancer Database by Bhatia et al. 84, adjuvant radiotherapy of the draining lymph nodes is not recommended in general by the European multi-society guidelines 68. Given the paucity of level 1 evidence, and the differing therapeutic ratios with adjuvant radiotherapy pending anatomic subsite and other abovementioned clinical risk factors, both the National Comprehensive Cancer Network and European society guidelines recommend discussion of adjuvant therapy by an Merkel cell carcinoma-specific multidisciplinary tumor board for individualized recommendations. Such tumor boards may also be optimally situated with regard to definitive radiotherapy for patients unwilling or unable to undergo primary surgical resection.
As immunotherapy becomes increasingly integrated into the treatment of metastatic, locally advanced, and localized Merkel cell carcinoma, questions remain regarding the optimal integration of radiotherapy with regard to the target (should elective nodal irradiation using traditional dose/fractionation schema be omitted given the potential for abrogation of the host immune response due to treatment related lymphopenia and radiation-stimulated cytokine signaling/pathways which may have a net immunosuppressive effect), as well as fractionation (is there a benefit to hypofractionated radiotherapy regimens which preclinically have been associated with differing effects regarding the tumor microenvironment, including immune response in other tumor subtypes 85, 86, 87, 88, 89 and are being studied in ongoing prospective clinical trials for patients with Merkel cell carcinoma). Questions also remain regarding optimal fractionation/targets for patients with profound immunosuppression who may have suboptimal clinical outcomes with conventionally fractionated radiotherapy relative to immunocompetent patients 81.
Disease involving regional lymph nodes
If cancer has spread to involve the lymph nodes, these may be surgically removed or treated with radiotherapy. In some cases, systemic chemotherapy may also be administered.
The role of elective lymph node dissection in the absence of clinically positive lymph nodes has not been studied in formal clinical trials. In small case series, elective lymph node dissection has been recommended for larger primary tumors, tumors with more than ten mitoses per high-power field, lymphatic or vascular invasion, and the small-cell histologic subtypes 90.
Sentinel lymph node biopsy has been suggested as a preferred initial alternative to complete elective lymph node dissection for the proper staging of Merkel cell carcinoma. Sentinel lymph node biopsy has less morbidity than complete nodal dissection. Furthermore, for Merkel cell carcinoma sites with indeterminate lymphatic drainage, such as those on the back, sentinel lymph node biopsy techniques can be used to identify the pertinent lymph node bed(s). If performed, sentinel lymph node biopsy is done at the time of the wide resection when the local lymphatic channels are still intact.
Several reports have found the use of sentinel lymph node biopsy techniques in Merkel cell carcinoma to be reliable and reproducible 91. However, the significance of sentinel lymph node positivity remains unclear.
- One meta-analysis of ten case series found that sentinel lymph node positivity strongly predicted a high short-term risk of recurrence and that subsequent therapeutic lymph node dissection was effective in preventing short-term regional nodal recurrence 92.
- Another meta-analysis of 12 retrospective case series (only partially overlapping the collection of case series in the previous meta-analysis) found that 45:
- Sentinel lymph node biopsy detected Merkel cell carcinoma spread in one-third of patients whose tumors would have otherwise been clinically and radiologically understaged.
- The recurrence rate was three times higher in patients with a positive sentinel lymph node biopsy than in those with a negative sentinel lymph node biopsy.
- Between 2006 and 2010, a large, retrospective, single-institutional series of 95 patients (with a total of 97 primary tumors) identified a sentinel lymph node in 93 instances, and nodal tumor was seen in 42 patients. Immunohistochemical techniques were used to assess node positivity. Various models of tumor and patient characteristics were studied to predict node positivity. There was no subgroup of patients predicted to have lower than 15% to 20% likelihood of sentinel lymph node positivity, suggesting that sentinel lymph node biopsy may be considered for all curative patients with clinically negative lymph nodes and no distant metastases 93.
- From 1996 to 2010, another retrospective, single-institutional study of 153 patients with localized Merkel cell carcinoma who underwent sentinel lymph node biopsy analyzed factors associated with sentinel lymph node positivity. The best predictors of sentinel lymph node biopsy positivity were tumor size and lymphovascular invasion 94.
In the absence of adequately powered, prospective, randomized clinical trials, the following questions remain:
- Should every positive sentinel lymph node biopsy be followed routinely by completion nodal surgery and/or radiation therapy?
- Are outcomes demonstrably improved by routinely adding radiation if lymph node surgery reveals tumor in multiple nodes and/or extracapsular extension and/or lymphovascular invasion?
- Should patients with Merkel cell carcinomas smaller than 1 cm routinely undergo sentinel lymph node dissection?
- Should patients with negative or omitted nodal work-up routinely undergo local or local-regional radiation therapy?
- Should immunohistochemical staining techniques be used to identify micrometastases in lymph nodes, and is micrometastatic disease in nodes clinically relevant?
At present, the primary role of lymph node surgery is for staging and guiding additional treatment.
Based on a small number of retrospective studies, therapeutic dissection of the regional lymph nodes after a positive sentinel lymph nodeD appears to minimize but not totally eliminate the risk of subsequent regional lymph node recurrence and in-transit metastases 95. There are no data from prospective randomized trials demonstrating that definitive regional nodal treatment with surgery improves survival.
Distant metastatic Merkel cell carcinoma
Any distant metastatic Merkel cell carcinoma is very serious and has a very poor prognosis. If time permits it’s often a good idea to get a second opinion from a team of experts. Treatment of the metastatic disease is aimed at improving quality of life. Treatment options might include surgery, radiation therapy, chemotherapy, immunotherapy, or some combination of these. The benefits of each treatment need to be weighed against the side effects they might cause. Be sure you understand the goal of each treatment and its possible downsides before starting treatment.
A positive response has been reported in up to 50% of patients with advanced Merkel cell carcinoma when treated with the immune checkpoint inhibitors avelumab (Bavencio), pembrolizumab (Keytruda), retifanlimab (Zynyz), and nivolumab (Opdivo). Pembrolizumab, nivolumab, retifanlimab and avelumab are anti-PD-1 and PD-L1 antibodies that function to restore active T cell response against the tumor. Response to PD-1 blockade can occur in virus-positive and virus-negative subtypes 96. These drugs have been shown to shrink or slow the growth of some advanced Merkel cell carcinoma tumors, sometimes even after other treatments have been tried 97, 98, 99, 100. Clinical trials are on-going. Note that new Merkel cell carcinoma has been reported during checkpoint inhibitor treatment of other forms of cancer.
In March 2017, the US the Food and Drug Administration granted accelerated approval to another PD-1/PD-L1 agent, avelumab (Bavencio®) to treat metastatic Merkel cell carcinoma. Approval was based on a trial of avelumab that showed a response in one-third of patients.
Experimental treatment with other agents is being explored.
Consensus treatment guidelines are published by the National Comprehensive Cancer Network. Guidelines from the National Comprehensive Cancer Network currently recommend consideration of immunotherapy for patients with metastatic Merkel cell carcinoma (pembrolizumab, nivolumab, or avelumab), or recurrent locally advanced Merkel cell carcinoma unamenable to definitive resection or radiation therapy (pembrolizumab) who do not have contraindications for receiving immunotherapy such as vital solid organ transplantation requiring immunosuppression or severe auto-immune conditions. Neoadjuvant or adjuvant immunotherapy off protocol is not currently recommended.
Chemotherapy
A variety of chemotherapy regimens have been used for patients with Merkel cell carcinoma in the settings of adjuvant, advanced, and recurrent therapy 101. Even though no phase 3 clinical trials have been conducted to demonstrate that adjuvant chemotherapy produces improvements in overall survival, some clinicians recommend its use in most cases because of the following:
- A biologic analogy is made between Merkel cell carcinoma and the histologically similar small cell carcinoma of the lung, which is considered a systemic disease.
- The risk of metastases and progression with Merkel cell carcinoma is high.
- Good initial clinical response rates have been noted with some chemotherapy regimens.
When possible, patients encouraging to participate in clinical trials should be considered.
From 1997 to 2001, the Trans-Tasman Radiation Oncology Group performed a phase 2 evaluation of 53 Merkel cell carcinoma patients with high-risk, local-regional disease. High risk was defined as recurrence after initial therapy, involved lymph nodes, primary tumor larger than 1 cm, gross residual disease after surgery, or occult primary with positive lymph nodes. Therapy included local-regional radiation (50 Gy in 25 fractions), synchronous carboplatin (area under the curve = 4.5), and IV etoposide (89 mg/m² on days 1–3 in weeks 1, 4, 7, and 10). Surgery was not standardized for either the primary tumor or the lymph nodes, and 12 patients had close margins, positive margins, or gross residual disease. Twenty-eight patients had undissected nodal beds, and the remainder had a variety of nodal surgeries. With a median follow-up of 48 months, 3-year overall survival was 76%, local-regional control was 75%, and distant control was 76%. Radiation reactions in the skin and febrile neutropenia were significant clinical acute toxicities. Because of the heterogeneity of the population and the nonstandardized surgery, it is difficult to infer a clear treatment benefit from the chemotherapy 102.
In a subsequent report, the same investigators evaluated a subset of these protocol patients (n = 40, after excluding patients with unknown primaries) and compared them with 61 historical controls who received no chemotherapy, were treated at the same institutions, were diagnosed before 1997, and had no routine imaging staging studies. Radiation was given to 50 patients. There was no significant survival benefit seen for chemotherapy patients 103.
In a subsequent, pilot clinical trial of 18 patients from 2004 to 2006, the same investigators attempted to reduce the skin and hematological toxicity seen in Study 96-07. The drug schedule was changed to carboplatin (area under the curve = 2) administered weekly during radiation beginning day 1 for a maximum of five doses, followed by three cycles of carboplatin (area under the curve 4.5, and IV etoposide 80 mg/m² on days 1–3 beginning 3 weeks after radiation and repeated every 3 weeks for three cycles). The radiation was similar to the earlier trial 102. Early results suggest less toxicity, but other clinical outcomes have not yet been reported 104.
Use of chemotherapy has also been reported in selected patients with locally advanced and metastatic disease. In one retrospective study of 107 patients, 57% of patients with metastatic disease and 69% with locally advanced disease responded to initial chemotherapy. Median overall survival was 9 months for patients with metastatic disease and 24 months for patients with locally advanced disease. At 3 years, overall survival was projected to be 17% for those with metastatic disease and 35% for those with locally advanced disease. Toxicity was significant, however, and without clear benefit, particularly in older patients 105.
Treatment options for recurrent Merkel Cell carcinoma
If Merkel cell carcinoma comes back after treatment, further treatment depends on where it comes back and what types of treatment were used before.
Treatment of recurrent Merkel cell carcinoma may include the following:
- Wide local excision to remove a larger area of tissue than was removed in earlier surgery. A lymph node dissection may also be done.
- Radiation therapy after surgery.
- Chemotherapy.
- Radiation therapy and/or surgery as palliative treatment to relieve symptoms and improve quality of life.
If Merkel cell cancer comes back on the skin where it first started, surgery (with wider margins) can often be done to try to remove it. This might be followed by radiation therapy to the area if it hasn’t been given before. If the nearby lymph nodes haven’t been treated, they might be removed and/or treated with radiation. Some doctors might consider giving chemotherapy as well, but it’s not clear how helpful this might be.
If Merkel cell cancer comes back in the nearby lymph nodes and they have not been treated before, they might be removed and/or treated with radiation. Some doctors might consider giving chemotherapy too, but, again, it’s not clear how helpful this is.
Cancers that come back in distant parts of the body can be hard to treat. Surgery and/or radiation therapy might be used, but the goal is usually to ease symptoms rather than try to cure the cancer. Chemotherapy can often shrink or slow the growth of the cancer for a time and can help relieve symptoms. But chemotherapy can also cause side effects that need to be taken into account. Treatment with an immunotherapy drug might be another option. These drugs have been shown to be helpful against some advanced Merkel cell carcinomas.
The benefits of each treatment need to be weighed against the side effects they might cause. Be sure you understand the goal of each treatment and its possible downsides before starting treatment.
Follow-up
The most appropriate follow-up techniques and frequency for patients treated for Merkel cell carcinoma have not been prospectively studied. Because of the propensity for local and regional recurrence, clinicians should perform at least a thorough physical examination of the site of initial disease and the regional lymph nodes. Imaging studies may be ordered to evaluate signs and symptoms of concern, or they may be performed to identify distant metastases early; but, there are no data suggesting that early detection and treatment of new distant metastases results in improved survival.
Guidelines from the National Comprehensive Cancer Network recommend physical examination, including complete skin and nodal evaluation every 3 to 6 months for the first 3 years, and every 6–12 months thereafter 80. Imaging is recommended for patients deemed to be at increased risk of recurrence. Clinical examination, including consideration of ultrasound examination of at-risk nodal basins, is recommended every 4 months for the first 3 years, then every 6 months for 5 years 68. Imaging with either diagnostic CT or PET/CT is suggested yearly for the first five years. As mentioned in the section above regarding serology, serial measurement of T-antigen oncoprotein titers should be strongly considered for patients with baseline oncoprotein levels 106. Patients with negative baseline oncoprotein titers may also warrant consideration of increased imaging and physical examination frequency.
In one series of 237 patients presenting with local or regional disease, the median time-to-recurrence was 9 months (range, 2–70 months). Ninety-one percent of recurrences occurred within 2 years of diagnosis 107. It has been suggested that the intensity of follow-up can be gradually diminished after 2 to 3 years as the majority of recurrences are likely to have already occurred 107.
Merkel cell carcinoma prognosis
In a review of patients from 18 case series, 279 of 926 patients (30.1%) developed local recurrence during follow-up, excluding those presenting with distant metastatic disease. These events have been typically attributed to inadequate surgical margins and/or a lack of adjuvant radiation therapy. In addition, 545 of 982 patients (55.5%) had lymph node metastases at diagnosis or during follow-up 108.
In the same review of 18 case series, the most common sites of distant metastases were distant lymph nodes (60.1%), distant skin (30.3%), lung (23.4%), central nervous system (18.4%), and bone (15.2%) 108. Many other sites of disease have also been reported, and the distribution of metastatic sites varies among case series.
In one series of 237 patients presenting with local or regional disease, the median time-to-recurrence was 9 months (range, 2–70 months). Ninety-one percent of recurrences occurred within 2 years of diagnosis 107.
Potential prognostic factors
The extent of disease at presentation appears to provide the most useful estimate of prognosis 109.
Diagnostic procedures, such as sentinel lymph node biopsy, may help distinguish between local and regional disease at presentation. One-third of patients who lack clinically palpable or radiologically visible nodes will have microscopically evident regional disease 45. The likelihood is that nodal positivity may be substantially lower among patients with small tumors (e.g., ≤1.0 cm) 110.
Many retrospective studies have evaluated the relationship of a wide variety of biological and histological factors to survival and local-regional control. Many of these reports are confounded by small numbers, potential selection bias, referral bias, short follow-up, no uniform clinical protocol for both staging and treatment, and are underpowered to detect modest differences.
A large, single-institution, retrospective study of 156 Merkel cell carcinoma patients, with a median follow-up of 51 months (range, 2–224 months), evaluated histologic factors potentially associated with prognosis 111. Although this report is subject to potential selection and referral bias, both univariate and multivariate analyses demonstrated a relationship between improved cause-specific survival and circumscribed growth pattern versus infiltrative pattern, shallow-tumor depth versus deep-tumor depth, and absence of lymphovascular invasion versus presence of lymphovascular invasion. Adoption of these findings into a global prognostic algorithm awaits independent confirmation by adequately powered studies.
A 2009 study 112 investigated whether the presence of newly identified Merkel cell polyomavirus in Merkel cell carcinoma tumor specimens influenced clinical outcome among 114 Finnish patients with Merkel cell carcinoma. In this small study, patients whose tumors were Merkel cell polyomavirus-positive appeared to have better survival than patients whose tumors were Merkel cell polyomavirus-negative. Standardization of procedures to identify and quantify Merkel cell polyomavirus and relevant antibodies is needed to improve understanding of both prognostic and epidemiologic questions 113.
The most significant prognostic parameters for Merkel cell carcinoma include tumor size and the presence of locoregional or distant metastases. These factors form the basis of the American Joint Committee on Cancer (AJCC) staging system for Merkel cell carcinoma 10. Although an increasing primary tumor size correlates with an increased risk of metastatic disease, Merkel cell carcinoma tumors of any size have significant risk of occult metastasis, supporting the use of sentinel lymph node biopsy for all cases 93. Additional features of the primary tumor, such as lymphovascular invasion and tumor growth pattern, may also have prognostic significance. Clinically detectable nodal disease is associated with worse outcome than microscopic metastases 10. Other findings associated with worse prognosis include sheet-like involvement in lymph node metastases and an increasing number of metastatic lymph nodes 114.
The bulk of Merkel cell carcinoma literature is from small case series, which are subject to many confounding factors. For this reason, the relapse and survival rates reported by stage vary widely in the literature. In general, lower-stage disease is associated with better overall survival 115.
Outcomes from patients presenting with small volume local disease and pathologically confirmed cancer-negative lymph nodes report a cause-specific 5-year survival exceeding 90% in one report 111.
A tabular summary of treatment results of Merkel cell carcinoma from 12 series illustrates the difficulty in comparing outcome data among series 109.
Using the SEER (Surveillance, Epidemiology, and End Results) Program registry Merkel cell carcinoma staging system adopted in 1973, Merkel cell carcinoma survival data (1973–2006) by stage is summarized below 48.
.Figure 3. Merkel cell carcinoma survival rate
Merkel cell carcinoma survival rates
Survival rates can give you an idea of what percentage of people with the same type and stage of cancer are still alive a certain amount of time (usually 5 years) after they were diagnosed. Survival rates can’t tell you how long you will live, but they may help give you a better understanding of how likely it is that your treatment will be successful. Keep in mind that survival rates are estimates and are often based on previous outcomes of large numbers of people who had a specific cancer, but they can’t predict what will happen in any particular person’s case. A relative survival rate compares people with the same type and stage of cancer to people in the overall population. For example, if the 5-year relative survival rate for a localized stage of Merkel cell carcinoma (MCC) is 75%, it means that people who have that cancer are, on average, about 75% as likely as people who don’t have that cancer to live for at least 5 years after being diagnosed. These statistics can be confusing and may lead you to have more questions. Your doctor is familiar with your situation; ask how these numbers may apply to you.
The SEER database (Surveillance, Epidemiology, and End Results database, maintained by the National Cancer Institute) tracks 5-year relative survival rates for Merkel cell carcinoma in the United States, based on how far the cancer has spread. The SEER database, however, does not group cancers by the American Joint Committee on Cancer (AJCC) TNM stages (stage 1, stage 2, stage 3, etc.). Instead, the SEER database groups cancers into localized, regional, and distant stages:
- Localized: There is no sign that the cancer has spread outside of the skin where it started.
- Regional: The cancer has spread outside the skin where it started to nearby structures or lymph nodes.
- Distant: The cancer has spread to distant parts of the body, such as the lungs, liver, or distant parts of the skin.
Table 2. Merkel cell carcinoma 5 year relative survival rates
| SEER stage | 5-year relative survival rate |
|---|---|
| Localized | 75% |
| Regional | 61% |
| Distant | 24% |
| All SEER stages combined | 65% |
Footnotes: These numbers are based on people diagnosed with Merkel cell carcinoma between 2012 and 2018.
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread. But other factors, such as your age and overall health, where on your body the cancer starts, and how well the cancer responds to treatment, can also affect your outlook.
- People now being diagnosed with Merkel cell carcinoma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least 5 years earlier.
Abbreviation: SEER = Surveillance, Epidemiology, and End Results database, maintained by the National Cancer Institute
[Source 116 ] References- Villani A, Fabbrocini G, Costa C, Carmela Annunziata M, Scalvenzi M. Merkel Cell Carcinoma: Therapeutic Update and Emerging Therapies. Dermatol Ther (Heidelb). 2019;9(2):209–222. doi:10.1007/s13555-019-0288-z https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6522614
- Yusuf MB, McKenzie G, Rattani A, Tennant P, Bumpous J, Miller D, Dunlap N. Merkel Cell Carcinoma of the Head and Neck: Epidemiology, Pathogenesis, Current State of Treatment and Future Directions. Cancers (Basel). 2021 Jul 13;13(14):3506. doi: 10.3390/cancers13143506
- Brady M, Spiker AM. Merkel Cell Carcinoma of the Skin. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482329
- Paulson K., Park S.Y., Vandeven N.A., Lachance K., Thomas H., Chapuis A.G., Harms K.L., Thompson J.A., Bhatia S., Stang A., et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018;78:457–463. doi: 10.1016/j.jaad.2017.10.028
- Merkel Cell Carcinoma Overview. https://www.skincancer.org/skin-cancer-information/merkel-cell-carcinoma
- Key Statistics for Merkel Cell Carcinoma. https://www.cancer.org/cancer/types/merkel-cell-skin-cancer/about/key-statistics.html
- Zaar O, Gillstedt M, Lindelöf B, Wennberg-Larkö AM, Paoli J. Merkel cell carcinoma incidence is increasing in Sweden. J Eur Acad Dermatol Venereol. 2016;30(10):1708–1713.
- Agelli M, Clegg LX: Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol 49 (5): 832-41, 2003.
- Tam M., Luu M., Barker C.A., Gharavi N.M., Hamid O., Shiao S.L., Nguyen A., Lu D.J., Ho A.S., Zumsteg Z.S. Improved survival in women versus men with merkel cell carcinoma. J. Am. Acad. Dermatol. 2021;84:321–329. doi: 10.1016/j.jaad.2020.02.034
- Harms K.L., Healy M.A., Nghiem P., Sober A.J., Johnson T.M., Bichakjian C.K., Wong S.L. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann. Surg. Oncol. 2016;23:3564–3571. doi: 10.1245/s10434-016-5266-4
- Young JL, Ward KC, Ries LAG: Cancer of rare sites. In: Ries LAG, Young JL, Keel GE, et al., eds.: SEER Survival Monograph: Cancer Survival Among Adults: U. S. SEER Program, 1988-2001, Patient and Tumor Characteristics. Bethesda, MD: National Cancer Institute, 2007. NIH Pub. No. 07-6215, pp 251-61.
- Giroulet F, Tabotta F, Pomoni A, Prior J. Primary parotid Merkel cell carcinoma: a first imagery and treatment response assessment by 18F-FDG PET. BMJ Case Rep. 2019 Mar 9;12(3):e226511. doi: 10.1136/bcr-2018-226511
- Villani A, Fabbrocini G, Costa C, Carmela Annunziata M, Scalvenzi M. Merkel Cell Carcinoma: Therapeutic Update and Emerging Therapies. Dermatol Ther (Heidelb). 2019 Jun;9(2):209-222. doi: 10.1007/s13555-019-0288-z
- Aung PP, Parra ER, Barua S, Sui D, Ning J, Mino B, Ledesma DA, Curry JL, Nagarajan P, Torres-Cabala CA, Efstathiou E, Hoang AG, Wong MK, Wargo JA, Lazar AJ, Rao A, Prieto VG, Wistuba I, Tetzlaff MT. B7-H3 Expression in Merkel Cell Carcinoma-Associated Endothelial Cells Correlates with Locally Aggressive Primary Tumor Features and Increased Vascular Density. Clin Cancer Res. 2019 Jun 1;25(11):3455-3467. doi: 10.1158/1078-0432.CCR-18-2355
- Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Peñas PF, Nghiem P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008 Mar;58(3):375-81. doi: 10.1016/j.jaad.2007.11.020
- Uitentuis SE, Louwman MWJ, van Akkooi ACJ, Bekkenk MW. Treatment and survival of Merkel cell carcinoma since 1993: A population-based cohort study in The Netherlands. J Am Acad Dermatol. 2019 Oct;81(4):977-983. doi: 10.1016/j.jaad.2019.01.042
- Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomarkers Prev. 1999 Feb;8(2):153-8. Erratum in: Cancer Epidemiol Biomarkers Prev 1999 May;8(5):485.
- Farley C.R., Perez M.C., Soelling S.J., Delman K.A., Harit A., Wuthrick E.J., Messina J.L., Sondak V.K., Zager J.S., Lowe M.C. Merkel Cell Carcinoma Outcomes: Does AJCC8 Underestimate Survival? Ann. Surg. Oncol. 2020;27:1978–1985. doi: 10.1245/s10434-019-08187-w
- Lunder EJ, Stern RS: Merkel-cell carcinomas in patients treated with methoxsalen and ultraviolet A radiation. N Engl J Med 339 (17): 1247-8, 1998.
- Feng H., Shuda M., Chang Y., Moore P.S. Clonal Integration of a Polyomavirus in Human Merkel Cell Carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586
- Garneski KM, Warcola AH, Feng Q, Kiviat NB, Leonard JH, Nghiem P. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol. 2009 Jan;129(1):246-8. doi: 10.1038/jid.2008.229
- Houben R, Schrama D, Becker JC. Molecular pathogenesis of Merkel cell carcinoma. Exp Dermatol. 2009 Mar;18(3):193-8. doi: 10.1111/j.1600-0625.2009.00853.x
- Paik JY, Hall G, Clarkson A, Lee L, Toon C, Colebatch A, Chou A, Gill AJ. Immunohistochemistry for Merkel cell polyomavirus is highly specific but not sensitive for the diagnosis of Merkel cell carcinoma in the Australian population. Hum Pathol. 2011 Oct;42(10):1385-90. doi: 10.1016/j.humpath.2010.12.013
- Andres C, Belloni B, Puchta U, Sander CA, Flaig MJ. Prevalence of MCPyV in Merkel cell carcinoma and non-MCC tumors. J Cutan Pathol. 2010 Jan;37(1):28-34. doi: 10.1111/j.1600-0560.2009.01352.x
- Laude HC, Jonchère B, Maubec E, Carlotti A, Marinho E, Couturaud B, Peter M, Sastre-Garau X, Avril MF, Dupin N, Rozenberg F. Distinct merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with merkel cell carcinoma. PLoS Pathog. 2010 Aug 26;6(8):e1001076. doi: 10.1371/journal.ppat.1001076
- Buck CB, Lowy DR. Immune readouts may have prognostic value for the course of merkel cell carcinoma, a virally associated disease. J Clin Oncol. 2011 Apr 20;29(12):1506-8. doi: 10.1200/JCO.2010.34.0745
- Engels E.A., Frisch M., Goedert J.J., Biggar R.J., Miller R.W. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–498. doi: 10.1016/S0140-6736(02)07668-7
- Clarke C.A., Robbins H.A., Tatalovich Z., Lynch C.F., Pawlish K.S., Finch J.L., Hernandez B.Y., Fraumeni J.F., Jr., Madeleine M.M., Engels E.A. Risk of Merkel Cell Carcinoma after Solid Organ Transplantation. J. Natl. Cancer Inst. 2015;107:dju382. doi: 10.1093/jnci/dju382
- Garrett G.L., Blanc P.D., Boscardin J., Lloyd A.A., Ahmed R.L., Anthony T., Bibee K., Breithaupt A., Cannon J., Chen A., et al. Incidence of and Risk Factors for Skin Cancer in Organ Transplant Recipients in the United States. JAMA Dermatol. 2017;153:296–303. doi: 10.1001/jamadermatol.2016.4920
- Koljonen V., Kukko H., Pukkala E., Sankila R., Böhling T., Tukiainen E., Sihto H., Joensuu H. Chronic lymphocytic leukaemia patients have a high risk of Merkel-cell polyomavirus DNA-positive Merkel-cell carcinoma. Br. J. Cancer. 2009;101:1444–1447. doi: 10.1038/sj.bjc.6605306
- Koljonen V., Rantanen M., Sahi H., Mellemkjær L., Hansen B.T., Chen T., Hemminki K., Pukkala E. Joint occurrence of Merkel cell carcinoma and non-Hodgkin lymphomas in four Nordic countries. Leuk. Lymphoma. 2015;56:3315–3319. doi: 10.3109/10428194.2015.1040010
- Bloom R., Amber K.T., Nouri K. An increased risk of non-Hodgkin lymphoma and chronic lymphocytic leukemia in US patients with Merkel cell carcinoma versus Australian patients: A clinical clue to a different mechanism of pathogenesis? Australas. J. Dermatol. 2016;57:e114–e116. doi: 10.1111/ajd.12325
- Burack J., Altschuler E.L. Sustained remission of metastatic Merkel cell carcinoma with treatment of HIV infection. J. R. Soc. Med. 2003;96:238–239. doi: 10.1177/014107680309600512
- Muirhead R., Ritchie D.M. Partial Regression of Merkel Cell Carcinoma in Response to Withdrawal of Azathioprine in an Immunosuppression-induced Case of Metastatic Merkel Cell Carcinoma. Clin. Oncol. 2007;19:96. doi: 10.1016/j.clon.2006.10.001
- Bhatia S., Afanasiev O., Nghiem P. Immunobiology of Merkel Cell Carcinoma: Implications for Immunotherapy of a Polyomavirus-Associated Cancer. Curr. Oncol. Rep. 2011;13:488–497. doi: 10.1007/s11912-011-0197-5
- Yusuf M.B., Gaskins J., Rattani A., McKenzie G., Mandish S., Wall W., Farley A., Tennant P., Bumpous J., Dunlap N. Immune Status in Merkel Cell Carcinoma: Relationships with Clinical Factors and Independent Prognostic Value. Ann. Surg. Oncol. 2021:1–12. doi: 10.1245/s10434-021-09944-6
- Asgari M.M., Sokil M.M., Warton E.M., Iyer J., Paulson K.G., Nghiem P. Effect of Host, Tumor, Diagnostic, and Treatment Variables on Outcomes in a Large Cohort with Merkel Cell Carcinoma. JAMA Dermatol. 2014;150:716. doi: 10.1001/jamadermatol.2013.8116
- Paulson K.G., Iyer J.G., Blom A., Warton E.M., Sokil M., Yelistratova L., Schuman L., Nagase K., Bhatia S., Asgari M.M., et al. Systemic Immune Suppression Predicts Diminished Merkel Cell Carcinoma–Specific Survival Independent of Stage. J. Investig. Dermatol. 2013;133:642–646. doi: 10.1038/jid.2012.388
- Yusuf M.B., Gaskins J., Wall W., Tennant P., Bumpous J., Dunlap N. Immune status and the efficacy of radiotherapy on overall survival for patients with localized Merkel cell carcinoma: An analysis of the National Cancer Database. J. Med. Imaging Radiat. Oncol. 2020;64:435–443. doi: 10.1111/1754-9485.13039
- Yusuf M., Gaskins J., May M.E., Mandish S., Wall W., Fisher W., Tennant P., Jorgensen J., Bumpous J., Dunlap N. Immune status and the efficacy of adjuvant radiotherapy for patients with localized Merkel cell carcinoma of the head and neck. Clin. Transl. Oncol. 2020;22:2009–2016. doi: 10.1007/s12094-020-02338-2
- Cook M., Baker K., Redman M., Lachance K., Nguyen M.H., Parvathaneni U., Bhatia S., Nghiem P., Tseng Y.D. Differential Outcomes Among Immunosuppressed Patients with Merkel Cell Carcinoma: Impact of Immunosuppression Type on Cancer-specific and Overall Survival. Am. J. Clin. Oncol. 2019;42:82–88. doi: 10.1097/COC.0000000000000482
- Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol. 2009 Aug 20;27(24):4021-6. doi: 10.1200/JCO.2009.22.6605
- Nghiem P, McKee PH, Haynes HA: Merkel cell (cutaneous neuroendocrine) carcinoma. In: Sober AJ, Haluska FG, eds.: Skin Cancer. Hamilton, Ontario: BC Decker Inc., 2001, pp 127-141.
- Heath M, Jaimes N, Lemos B, et al.: Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol 58 (3): 375-81, 2008.
- Gupta SG, Wang LC, Peñas PF, et al.: Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch Dermatol 142 (6): 685-90, 2006.
- Anderson SE, Beer KT, Banic A, et al.: MRI of merkel cell carcinoma: histologic correlation and review of the literature. AJR Am J Roentgenol 185 (6): 1441-8, 2005.
- Iagaru A, Quon A, McDougall IR, et al.: Merkel cell carcinoma: Is there a role for 2-deoxy-2-[f-18]fluoro-D-glucose-positron emission tomography/computed tomography? Mol Imaging Biol 8 (4): 212-7, 2006 Jul-Aug.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al.: Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol 37 (1): 20-7, 2010.
- Missotten GS, de Wolff-Rouendaal D, de Keizer RJ: Merkel cell carcinoma of the eyelid review of the literature and report of patients with Merkel cell carcinoma showing spontaneous regression. Ophthalmology 115 (1): 195-201, 2008.
- American Joint Committee on Cancer. Merkel Cell Carcinoma. In: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017:549.
- Busse PM, Clark JR, Muse VV, Liu V. Case records of the Massachusetts General Hospital. Case 19-2008. A 63-year-old HIV-positive man with cutaneous Merkel-cell carcinoma. N Engl J Med. 2008 Jun 19;358(25):2717-23. doi: 10.1056/NEJMcpc0803063
- Bichakjian CK, Olencki T, Aasi SZ, Alam M, Andersen JS, Blitzblau R, Bowen GM, Contreras CM, Daniels GA, Decker R, Farma JM, Fisher K, Gastman B, Ghosh K, Grekin RC, Grossman K, Ho AL, Lewis KD, Loss M, Lydiatt DD, Messina J, Nehal KS, Nghiem P, Puzanov I, Schmults CD, Shaha AR, Thomas V, Xu YG, Zic JA, Hoffmann KG, Engh AM. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018 Jun;16(6):742-774. doi: 10.6004/jnccn.2018.0055
- Lebbe C, Becker JC, Grob JJ, Malvehy J, Del Marmol V, Pehamberger H, Peris K, Saiag P, Middleton MR, Bastholt L, Testori A, Stratigos A, Garbe C; European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of Merkel Cell Carcinoma. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015 Nov;51(16):2396-403. doi: 10.1016/j.ejca.2015.06.131
- Tai P. A practical update of surgical management of merkel cell carcinoma of the skin. ISRN Surg. 2013;2013:850797. doi: 10.1155/2013/850797
- Ellis DL, Davis RS. Evidence-based management of primary and localized Merkel cell carcinoma: a review. Int J Dermatol. 2013 Oct;52(10):1248-58. doi: 10.1111/ijd.12091
- Kline L, Coldiron B. Mohs Micrographic Surgery for the Treatment of Merkel Cell Carcinoma. Dermatol Surg. 2016 Aug;42(8):945-51. doi: 10.1097/DSS.0000000000000801
- O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma. Comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997 Oct;23(10):929-33. Erratum in: Dermatol Surg 1998 FEb;24(2)299.
- Shaikh W.R., Sobanko J.F., Etzkorn J.R., Shin T.M., Miller C.J. Utilization patterns and survival outcomes after wide local excision or Mohs micrographic surgery for Merkel cell carcinoma in the United States, 2004–2009. J. Am. Acad. Dermatol. 2018;78:175–177. doi: 10.1016/j.jaad.2017.09.049
- Singh B., Qureshi M.M., Truong M.T., Sahni D. Optimal Surgical Modality for Early Merkel Cell Carcinoma—Results from the National Cancer Data Base. J. Am. Acad. Dermatol. 2018 doi: 10.1016/j.jaad.2018.05.019
- Allen P.J., Bowne W.B., Jaques D.P., Brennan M.F., Busam K., Coit D.G. Merkel Cell Carcinoma: Prognosis and Treatment of Patients from a Single Institution. J. Clin. Oncol. 2005;23:2300–2309. doi: 10.1200/JCO.2005.02.329
- Perez M.C., De Pinho F.R., Holstein A., Oliver D.E., Naqvi S.M.H., Kim Y., Messina J.L., Burke E., Gonzalez R.J., Sarnaik A.A., et al. Resection Margins in Merkel Cell Carcinoma: Is a 1-cm Margin Wide Enough? Ann. Surg. Oncol. 2018;25:3334–3340. doi: 10.1245/s10434-018-6688-y
- Andruska N., Mahapatra L., Brenneman R.J., Rich J.T., Baumann B.C., Compton L., Thorstad W.L., Daly M.D. Reduced Wide Local Excision Margins are Associated with Increased Risk of Relapse and Death from Merkel Cell Carcinoma. Ann. Surg. Oncol. 2021;28:3312–3319. doi: 10.1245/s10434-020-09145-7
- Nghiem P, James N: Merkel cell carcinoma. In: Wolff K, Goldsmith LA, Katz SI, et al., eds.: Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill , 2008, pp 1087-94.
- Senchenkov A, Barnes SA, Moran SL. Predictors of survival and recurrence in the surgical treatment of merkel cell carcinoma of the extremities. J Surg Oncol. 2007 Mar 1;95(3):229-34. doi: 10.1002/jso.20647
- Wilson LD, Gruber SB. Merkel cell carcinoma and the controversial role of adjuvant radiation therapy: clinical choices in the absence of statistical evidence. J Am Acad Dermatol. 2004 Mar;50(3):435-7; discussion 437-8. doi: 10.1016/j.jaad.2003.09.011
- Eng TY, Boersma MG, Fuller CD, Goytia V, Jones WE 3rd, Joyner M, Nguyen DD. A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol. 2007 Dec;30(6):624-36. doi: 10.1097/COC.0b013e318142c882
- Bryant M.K., Ward C., Gaber C.E., Strassle P.D., Ollila D.W., Laks S. Decreased survival and increased recurrence in Merkel cell carcinoma significantly linked with immunosuppression. J. Surg. Oncol. 2020;122:653–659. doi: 10.1002/jso.26048
- Lebbe C., Becker J.C., Grob J.J., Malvehy J., del Marmol V., Pehamberger H., Peris K., Saiag P., Middleton M.R., Bastholt L., et al. Diagnosis and treatment of Merkel Cell Carcinoma. European consensus-based interdisciplinary guideline. Eur. J. Cancer. 2015;51:2396–2403. doi: 10.1016/j.ejca.2015.06.131
- Tsang G., O’Brien P., Robertson R., Hamilton C., Wratten C., Denham J. All delays before radiotherapy risk progression of Merkel cell carcinoma. Australas. Radiol. 2004;48:371–375. doi: 10.1111/j.0004-8461.2004.01321.x
- Yusuf M., Gaskins J., Tennant P., Bumpous J., Dunlap N. Survival Impact of Time to Initiation of Adjuvant Radiation for Merkel Cell Carcinoma: An Analysis of the National Cancer Database. Pract. Radiat. Oncol. 2019;9:e372–e385. doi: 10.1016/j.prro.2019.03.004
- Shinde A., Verma V., Jones B.L., Li R., Glaser S., Freeman M., Melstrom L., Kang R., Parvathaneni U., Modi B., et al. The Effect of Time to Postoperative Radiation Therapy on Survival in Resected Merkel Cell Carcinoma. Am. J. Clin. Oncol. 2019;42:636–642. doi: 10.1097/COC.0000000000000565
- Gillenwater A.M., Hessel A.C., Morrison W.H., Burgess M.A., Silva E.G., Roberts D., Goepfert H. Merkel cell carcinoma of the head and neck: Effect of surgical excision and radiation on recurrence and survival. Arch. Otolaryngol. Head Neck Surg. 2001;127:149–154. doi: 10.1001/archotol.127.2.149
- Clark J.R., Veness M.J., Gilbert R., O’Brien C.J., Gullane P.J. Merkel cell carcinoma of the head and neck: Is adjuvant radiotherapy necessary? Head Neck. 2007;29:249–257. doi: 10.1002/hed.20510
- Chen M.M., Roman S.A., Sosa J.A., Judson B.L. The Role of Adjuvant Therapy in the Management of Head and Neck Merkel Cell Carcinoma: An analysis of 4815 patients. JAMA Otolaryngol. Head Neck Surg. 2015;141:137–141. doi: 10.1001/jamaoto.2014.3052
- Goessling W, McKee PH, Mayer RJ. Merkel cell carcinoma. J Clin Oncol. 2002 Jan 15;20(2):588-98. doi: 10.1200/JCO.2002.20.2.588
- Patel S.A., Qureshi M.M., Mak K.S., Sahni D., Giacalone N.J., Ezzat W., Jalisi S., Truong M.T. Impact of total radiotherapy dose on survival for head and neck Merkel cell carcinoma after resection. Head Neck. 2017;39:1371–1377. doi: 10.1002/hed.24776
- Yusuf M., Gaskins J., Wall W., Tennant P., Bumpous J., Dunlap N. Optimal adjuvant radiotherapy dose for stage I, II or III Merkel cell carcinoma: An analysis of the National Cancer Database. Jpn. J. Clin. Oncol. 2019;50:175–184. doi: 10.1093/jjco/hyz153
- Iyer J.G., Parvathaneni U., Gooley T., Miller N.J., Markowitz E., Blom A., Lewis C.W., Doumani R.F., Parvathaneni K., Anderson A., et al. Single-fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer Med. 2015;4:1161–1170. doi: 10.1002/cam4.458
- Cook M.M., Schaub S.K., Goff P.H., Fu A., Park S.Y., Hippe D.S., Liao J.J., Apisarnthanarax S., Bhatia S., Tseng Y.D., et al. Postoperative, Single-Fraction Radiation Therapy in Merkel Cell Carcinoma of the Head and Neck. Adv. Radiat. Oncol. 2020;5:1248–1254. doi: 10.1016/j.adro.2020.07.003
- Bichakjian C.K., Olencki T., Aasi S.Z., Alam M., Andersen J.S., Blitzblau R., Bowen G.M., Contreras C.M., Daniels G.A., Decker R., et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018;16:742–774. doi: 10.6004/jnccn.2018.0055
- Tseng Y.D., Nguyen M.H., Baker K., Cook M., Redman M., Lachance K., Bhatia S., Liao J.J., Apisarnthanarax S., Nghiem P.T., et al. Effect of Patient Immune Status on the Efficacy of Radiation Therapy and Recurrence-Free Survival Among 805 Patients with Merkel Cell Carcinoma. Int. J. Radiat. Oncol. 2018;102:330–339. doi: 10.1016/j.ijrobp.2018.05.075
- Jouary T., Leyral C., Dreno B., Doussau A., Sassolas B., Beylot-Barry M., Renaud-Vilmer C., Guillot B., Bernard P., Lok C., et al. Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: A multicentric prospective randomized study. Ann. Oncol. 2012;23:1074–1080. doi: 10.1093/annonc/mdr318
- Creighton F., Bergmark R., Emerick K. Drainage Patterns to Nontraditional Nodal Regions and Level IIB in Cutaneous Head and Neck Malignancy. Otolaryngol. Head Neck Surgery. 2016;155:1005–1011. doi: 10.1177/0194599816662864
- Bhatia S., Storer B.E., Iyer J.G., Moshiri A., Parvathaneni U., Byrd D., Sober A.J., Sondak V.K., Gershenwald J.E., Nghiem P. Adjuvant Radiation Therapy and Chemotherapy in Merkel Cell Carcinoma: Survival Analyses of 6908 Cases from the National Cancer Data Base. J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djw042
- Lee Y., Auh S.L., Wang Y., Burnette B., Meng Y., Beckett M., Sharma R., Chin R., Tu T., Weichselbaum R.R., et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870
- Hennel R., Brix N., Seidl K., Ernst A., Scheithauer H., Belka C., Lauber K. Release of monocyte migration signals by breast cancer cell lines after ablative and fractionated γ-irradiation. Radiat. Oncol. 2014;9:85. doi: 10.1186/1748-717X-9-85
- Popp I., Grosu A.L., Niedermann G., Duda D.G. Immune modulation by hypofractionated stereotactic radiation therapy: Therapeutic implications. Radiother. Oncol. 2016;120:185–194. doi: 10.1016/j.radonc.2016.07.013
- Crocenzi T., Cottam B., Newell P., Wolf R.F., Hansen P.D., Hammill C., Solhjem M.C., To Y.-Y., Greathouse A., Tormoen G., et al. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J. Immunother. Cancer. 2016;4:45. doi: 10.1186/s40425-016-0149-6
- Frey B., Rückert M., Weber J., Mayr X., Derer A., Lotter M., Bert C., Rödel F., Fietkau R., Gaipl U.S. Hypofractionated Irradiation Has Immune Stimulatory Potential and Induces a Timely Restricted Infiltration of Immune Cells in Colon Cancer Tumors. Front. Immunol. 2017;8:231. doi: 10.3389/fimmu.2017.00231
- Haag ML, Glass LF, Fenske NA: Merkel cell carcinoma. Diagnosis and treatment. Dermatol Surg 21 (8): 669-83, 1995.
- Rodrigues LK, Leong SP, Kashani-Sabet M, et al.: Early experience with sentinel lymph node mapping for Merkel cell carcinoma. J Am Acad Dermatol 45 (2): 303-8, 2001.
- Mehrany K, Otley CC, Weenig RH, et al.: A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatol Surg 28 (2): 113-7; discussion 117, 2002.
- Schwartz JL, Griffith KA, Lowe L, et al.: Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol 29 (8): 1036-41, 2011.
- Fields RC, Busam KJ, Chou JF, et al.: Recurrence and survival in patients undergoing sentinel lymph node biopsy for merkel cell carcinoma: analysis of 153 patients from a single institution. Ann Surg Oncol 18 (9): 2529-37, 2011.
- Maza S, Trefzer U, Hofmann M, et al.: Impact of sentinel lymph node biopsy in patients with Merkel cell carcinoma: results of a prospective study and review of the literature. Eur J Nucl Med Mol Imaging 33 (4): 433-40, 2006.
- Nghiem P., Bhatia S., Lipson E.J., Sharfman W.H., Kudchadkar R.R., Brohl A.S., Friedlander P.A., Daud A., Kluger H.M., Reddy S.A., et al. Durable Tumor Regression and Overall Survival in Patients with Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. J. Clin. Oncol. 2019;37:693–702. doi: 10.1200/JCO.18.01896
- D’Angelo S.P., Bhatia S., Brohl A.S., Hamid O., Mehnert J.M., Terheyden P., Shih K.C., Brownell I., Lebbé C., Lewis K.D., et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: Long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J. Immunother. Cancer. 2019;8:e000674. doi: 10.1136/jitc-2020-000674
- D’Angelo S.P., Russell J., Lebbé C., Chmielowski B., Gambichler T., Grob J.-J., Kiecker F., Rabinowits G., Terheyden P., Zwiener I., et al. Efficacy and Safety of First-line Avelumab Treatment in Patients with Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol. 2018;4:e180077. doi: 10.1001/jamaoncol.2018.0077
- Lambert J., Marrel A., D’Angelo S.P., Burgess M.A., Chmielowski B., Fazio N., Gambichler T., Grob J.-J., Lebbé C., Robert C., et al. Patient Experiences with Avelumab in Treatment-Naïve Metastatic Merkel Cell Carcinoma: Longitudinal Qualitative Interview Findings from JAVELIN Merkel 200, a Registrational Clinical Trial. Patient-Patient-Cent. Outcomes Res. 2020;13:457–467. doi: 10.1007/s40271-020-00428-5
- Nghiem P.T., Bhatia S., Lipson E.J., Kudchadkar R.R., Miller N.J., Annamalai L., Berry S., Chartash E.K., Daud A., Fling S.P., et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702
- Henness S, Vereecken P: Management of Merkel tumours: an evidence-based review. Curr Opin Oncol 20 (3): 280-6, 2008.
- Poulsen M, Rischin D, Walpole E, et al.: High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study–TROG 96:07. J Clin Oncol 21 (23): 4371-6, 2003.
- Poulsen MG, Rischin D, Porter I, et al.: Does chemotherapy improve survival in high-risk stage I and II Merkel cell carcinoma of the skin? Int J Radiat Oncol Biol Phys 64 (1): 114-9, 2006.
- Poulsen M, Walpole E, Harvey J, et al.: Weekly carboplatin reduces toxicity during synchronous chemoradiotherapy for Merkel cell carcinoma of skin. Int J Radiat Oncol Biol Phys 72 (4): 1070-4, 2008.
- Voog E, Biron P, Martin JP, et al.: Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer 85 (12): 2589-95, 1999.
- Paulson K.G., Lewis C.W., Redman M.W., Simonson W.T., Lisberg A., Ritter D., Morishima C., Hutchinson K., Mudgistratova L., Blom A., et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: A prospective validation study. Cancer. 2017;123:1464–1474. doi: 10.1002/cncr.30475
- Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005 Apr 1;23(10):2300-9. doi: 10.1200/JCO.2005.02.329
- Medina-Franco H, Urist MM, Fiveash J, et al.: Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol 8 (3): 204-8, 2001.
- Eng TY, Boersma MG, Fuller CD, et al.: A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol 30 (6): 624-36, 2007.
- Stokes JB, Graw KS, Dengel LT, et al.: Patients with Merkel cell carcinoma tumors < or = 1.0 cm in diameter are unlikely to harbor regional lymph node metastasis. J Clin Oncol 27 (23): 3772-7, 2009.
- Andea AA, Coit DG, Amin B, et al.: Merkel cell carcinoma: histologic features and prognosis. Cancer 113 (9): 2549-58, 2008.
- Sihto H, Kukko H, Koljonen V, et al.: Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst 101 (13): 938-45, 2009.
- Rockville Merkel Cell Carcinoma Group: Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol 27 (24): 4021-6, 2009.
- Ko JS, Prieto VG, Elson PJ, et al.: Histological pattern of Merkel cell carcinoma sentinel lymph node metastasis improves stratification of Stage III patients. Mod Pathol 29 (2): 122-30, 2016.
- Eng TY, Boersma MG, Fuller CD, et al.: Treatment of merkel cell carcinoma. Am J Clin Oncol 27 (5): 510-5, 2004.
- Survival Rates for Merkel Cell Carcinoma. https://www.cancer.org/cancer/types/merkel-cell-skin-cancer/detection-diagnosis-staging/survival-rates.html