Metallosis
Metallosis is defined as aseptic fibrosis, local necrosis, or loosening of a prosthetic device secondary to metal corrosion and release of metallic wear debris in periprosthetic soft and bony tissues 1. Metallosis occurs due to metallic erosion and release of metallic debris ions, which induce massive local cytokines liberation from inflammatory cells 2. The systemic absorption of metallic particles can cause several clinical presentations depending on the predominant affected system. Metallosis may present locally with pain, instability, and metallic debris staining the local tissue. Systemically, metallosis has been linked to neurologic complications (visual, hearing, and cognitive deficits), cardiac failure, and hypothyroidism. Importantly, metallic debris can also have superficial manifestations of periarticular skin tattooing, due to an adverse local tissue reaction. Although the structural component of failed joint arthroplasty can be revised to improve pain, stability, and functioning, any skin tattooing secondary to metallosis presents the treating physician with clinical challenge.
Although the real incidence of metallosis is unknown, metallosis is described as a rare diagnosis and it seems to be more frequent in hip than in knee arthroplasty with a 5% estimated incidence in the hip prosthetic replacements 3. In knee arthroplasty, metallosis generally occurs as a result of polyethylene wear of the tibial or metal-back patellar component 4.
Metallosis is caused by the infiltration and accumulation of metallic debris into the peri-prosthetic structures, deriving from friction between metallic prosthetic components 4. However, metallosis also occurs in non-metallic prostheses 5. Metallosis usually happens as a result of metal-on-metal implants in which the articulating surfaces of the implant are made from metal, in patients with metal-on-metal arthroplasty or in patients with polyethylene wear or fracture with subsequent abrasion of the underlying metal surfaces 6. Typically, metallosis occurs after some years of the arthroplasty operation 7.
Metallosis is most commonly associated with failed metal-on-metal hip prostheses and is characterized locally by heavy staining of surrounding soft tissue, metallic synovitis, joint effusion, and gradual loosening of the prosthesis 8. Additionally, metallic debris can also lead to periarticular superficial skin manifestations. The release of metal ions has further been known to lead to systemic upsets including neurologic deficit (declining vision, hearing, or cognition; headaches), cardiac failure, and hypothyroidism 8. As the number of patients seeking major orthopedic interventions grows, the incidence of metallosis-related skin tattooing will also increase. The structural components of a failed joint replacement can be revised (improving patients’ pain and functioning). However, any skin tattooing secondary to metallosis presents the treating dermatologist with clinical challenge, due to lack of research regarding treatment of this condition.
There are differing opinions internationally about whether follow-up of patients with metal-on-metal implants should include regular blood testing for cobalt and chromium ions. There is no consensus on what might be the significance of particular levels of these ions in a patient’s blood. However, there is some evidence that consistently rising blood levels of cobalt or chromium ions over time could provide an indication that a metal-on-metal hip implant is starting to fail. On balance, the Therapeutic Goods Administration recommends that patients with metal-on-metal implants be followed up regularly and that, in addition to soft tissue imaging such as ultrasound and/or MRI, the follow-up include blood tests for cobalt and chromium. Patients who have concerns about their hip or knee replacement should seek advice from their orthopedic surgeon and/or family physician.
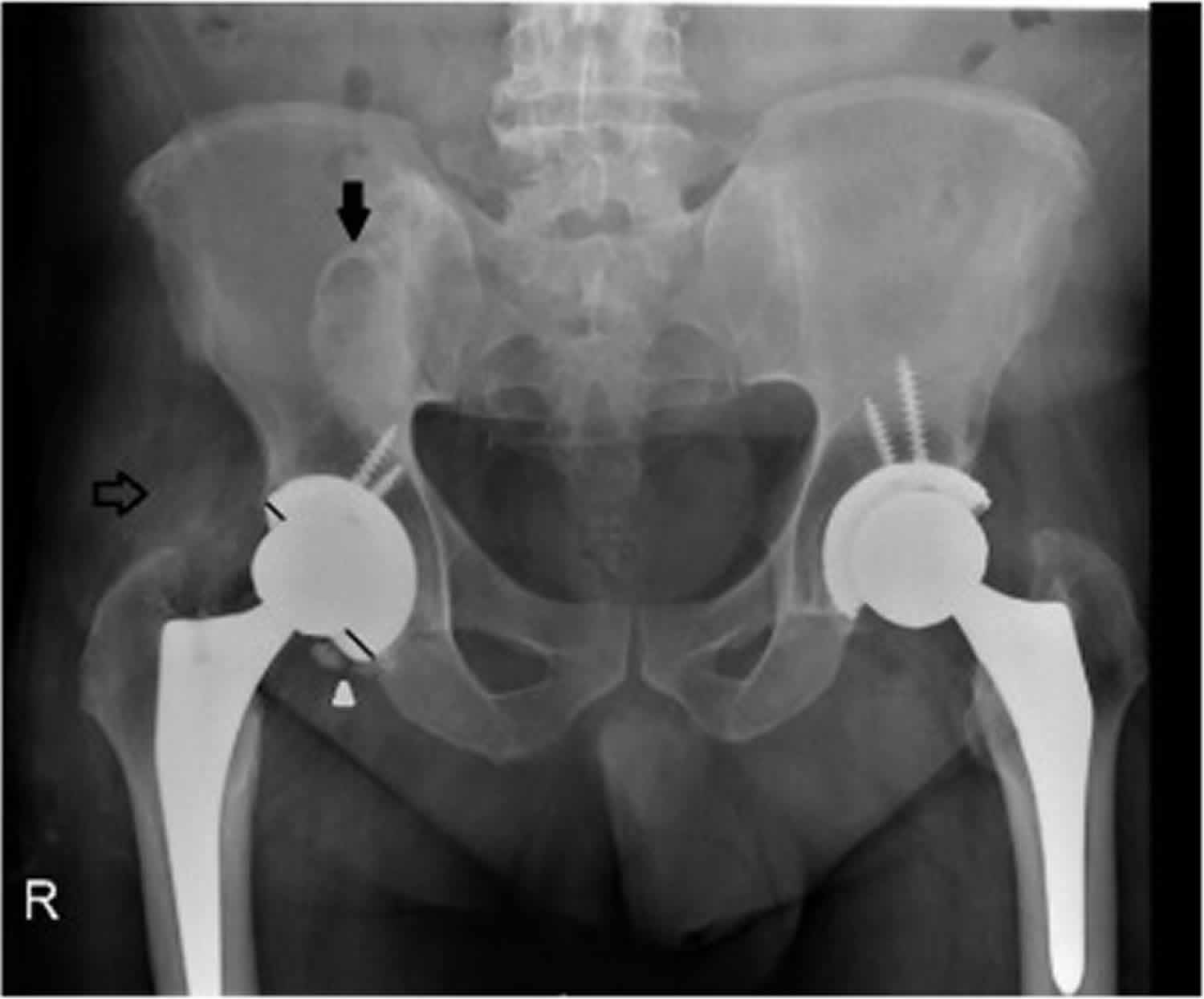
Figure 1. Metallosis hip replacement
Footnote: A 55-years-old man with metallosis. Plain film of pelvis, revealing two round high-density images, one denser projected on the right iliac bone and theother above the right femoral neck (arrows). High density fragments adjacentto the inferior acetabular border were also noted (arrowhead). There is also aneccentric position of the femoral head in the acetabular dome (non equal distancebetween the femoral head margin and the acetabular border – trace lines).
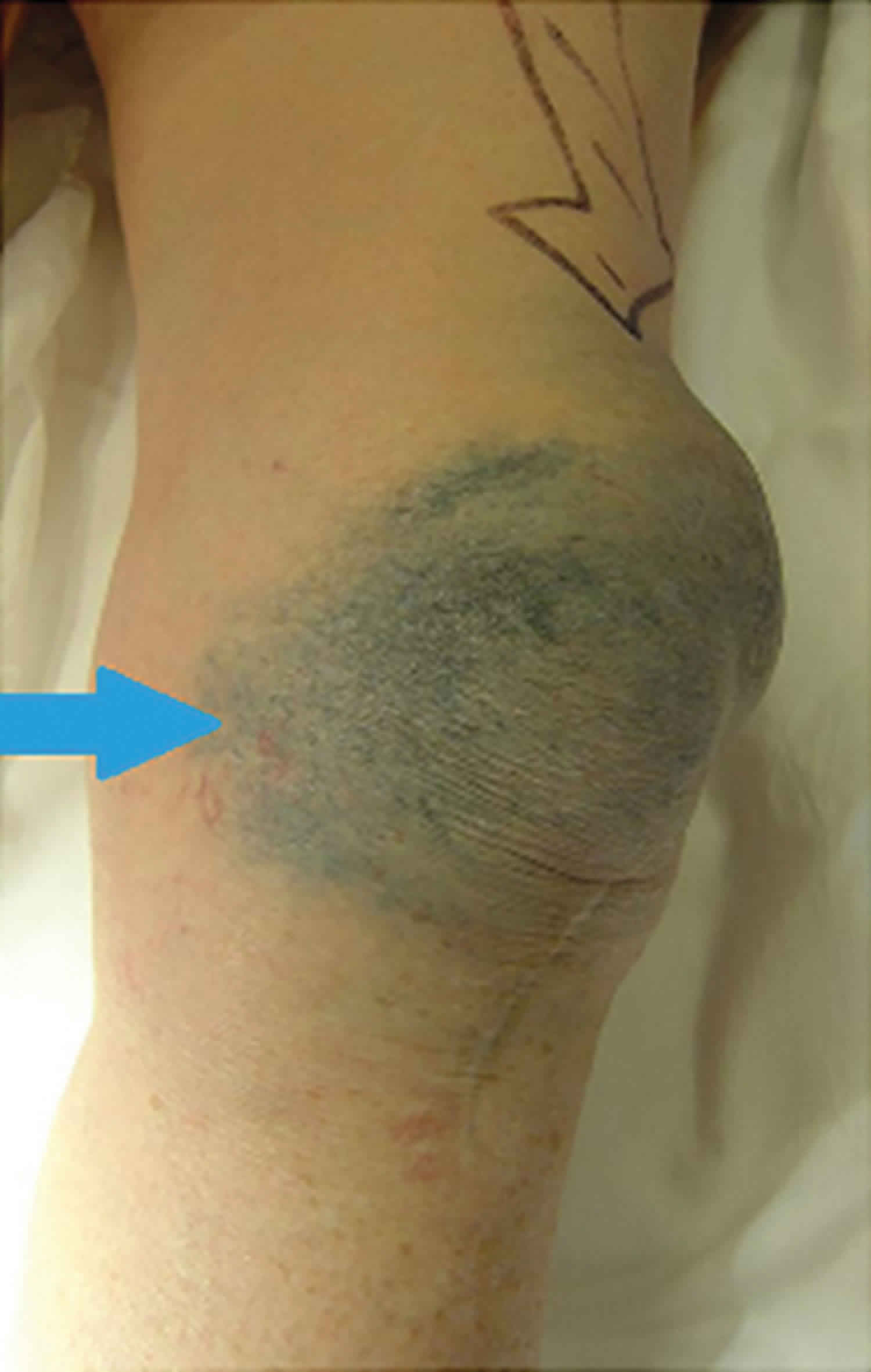
[Source 5 ]Figure 2. Metallosis knee replacement
Footnote:74-year-old woman who presented to her general practitioner after experiencing 2 years of worsening metallic skin tattooing, pain, and swelling to her left knee. Skin discoloration and joint effusion (arrow) after knee arthroplasty failure, prior to revision surgery. Six years earlier, the patient had left total knee replacement, with a metal-backed implant to resurface her patella, and an anterior knee midline scar. The patient underwent joint revision, where periarticular stained soft tissues of the knee were debrided (primarily joint capsule/synovium), and the patella component of the prosthesis was replaced with an alternative. The patient remained pain-free with good function at 5-year follow-up, but had ongoing concern about cosmesis of her persistent skin tattooing, which remained untreated.
[Source 9]What is a metal-on-metal hip replacement implant?
Hip replacement implants are designed to replace hip joints that have degenerated and become painful or (less commonly) were deformed at birth or damaged during an accident. The implants replace the painful, deformed or damaged ‘ball and socket’ joint of the hip with components made of metal, plastic or ceramic. The ‘ball’ part of the joint is called the femoral head, and the ‘socket’ part of the joint is called the acetabulum, or acetabular cup.
Some hip implants comprise a femoral head component made from a metal alloy containing cobalt and chromium that moves inside a metal acetabular cup or cup liner component. Such implants are commonly known as “metal-on-metal” hip replacements.
There are several types of hip replacement, but the two types of hip replacement surgery in which metal-on-metal components have been widely used are conventional total hip replacement where all the bone of the femoral head is replaced by the metal implant, and resurfacing total hip replacement (often just called resurfacing hip replacement) where much of the femoral head is retained and a hollow metal cap is placed over it, while a matching metal cup (similar to that used with a conventional total hip replacement) is placed in the acetabulum. Resurfacing hip replacement surgery replaces the surfaces of the patient’s hip joint and removes very little femoral bone compared to a conventional total hip replacement. It is important to note that resurfacing total hip replacements are of the metal-on-metal type in almost all cases.
Whether used in conventional or resurfacing hip replacement procedures, metal-on-metal implants are used because the articulating (moving) surfaces of these implants can be precisely engineered to be extremely smooth and hard. metal-on-metal hip replacements are thought to provide advantages over metal-on-plastic hip replacements including lower wear rates and a lower incidence of dislocation. Dislocation is when the femoral head comes out of the acetabular cup and this is very painful and debilitating. In some cases further surgery is required to repair the hip joint. metal-on-metal components allow the design to accommodate larger head sizes, which in turn experience lower dislocation rates. A lower wear rate prolongs the life of the hip implant and delays the need for replacement (known as revision) surgery.
Metal-on-Metal Hip Implants Information for Orthopaedic Surgeons
General recommendations for orthopaedic surgeons AFTER Metal-on-Metal Hip Replacement and Resurfacing Surgery (Follow-up) 10
- At the time of hospital discharge, schedule the patient for routine office follow-up.
- At the time of hospital discharge, review with the patient or caregiver the signs/symptoms of adverse events.
- The U.S. Food and Drug Administration (FDA) recommends routine long-term follow-up of patients with metal-on-metal hip implants, typically to occur every 1 to 2 years.
- If the patient experiences any pain or decreased hip function approximately 3 or more months after surgery, instruct the patient to contact you for a follow-up exam.
- Patient follow-up visits should include:
- Physical exam with functional assessment, including careful assessment for hip abduction strength to rule out abductor damage
- Check for asymptomatic local swelling or masses; and
- Assessment of organs and systems for changes including systemic adverse events in cardiovascular, nervous, endocrine (especially thyroid) and renal systems.
- Appropriate radiographs to check for implant positioning and osteolysis
- Schedule certain patients with increased risk of device wear or adverse reaction to metal debris for closer follow-up. These may include:
- Patients with bilateral implants
- Patients with resurfacing systems with small femoral heads (less than or equal to 44 mm)
- Female patients
- Patients receiving high doses of corticosteroids
- Patients with evidence of renal insufficiency
- Patients with suppressed immune systems
- Patients with suboptimal alignment of device components
- Patients with suspected metal sensitivity (e.g. cobalt, chromium, nickel)
- Patients who are severely overweight (BMI >40)
- Patients with high levels of physical activity
- Pay close attention to signs of the following local and systemic symptoms or complications associated with metal-on-metal (metal-on-metal) hip implants:
- Local symptoms or complications:
- Hypersensitivity (allergic type reaction)
- Loosening
- Infection
- Osteolysis (bone loss)
- Soft tissue mass or pseudotumor (fluid-filled or solid soft tissue mass around the replaced joint that is diagnosed radiologically)
- Femoral neck fracture (for resurfacing systems)
- Systemic symptoms or complications:
- General hypersensitivity reaction (skin rash)
- Cardiomyopathy
- Neurological changes including sensory changes (auditory, or visual impairments)
- Psychological status change (including depression or cognitive impairment)
- Renal function impairment
- Thyroid dysfunction (including neck discomfort, fatigue, weight gain or feeling cold
- Local symptoms or complications:
Follow-up for asymptomatic patients clinical evaluation
- If a patient with a metal-on-metal hip implant is asymptomatic and has a well-functioning hip, follow-up should occur periodically (typically 1 to 2 years), as with any hip arthroplasty.
- Be aware the following patients may experience increased wear of the implant and/or adverse reaction to metal debris and require closer monitoring. They include:
- Patients with bilateral implants
- Patients with resurfacing systems with small femoral heads (less than or equal to 44mm)
- Female patients
- Patients receiving high doses of corticosteroids
- Patients with evidence of renal insufficiency
- Patients with suppressed immune systems
- Patients with suboptimal alignment of device components
- Patients with suspected metal sensitivity (e.g. cobalt, chromium, nickel)
- Patients who are severely overweight (BMI >40)
- Patients with high levels of physical activity
- Patient follow-up visits should include:
- Physical exam with functional assessment; with attention to abductor strength and Trendelenburg test to rule out abductor damage
- Checking for asymptomatic local swelling or masses; and
- Assessment for possible systemic adverse events in cardiovascular, nervous, endocrine (especially thyroid) and renal systems.
- Appropriate radiographs to evaluate for osteolysis or loosening.
Additional testing for asymptomatic patients
- If clinical and imaging data support that the hip prosthesis is functioning properly and the patient is asymptomatic, the FDA is not aware of data which supports the need to routinely check blood metal ion levels or perform soft tissue imaging.
- Findings of lesions on soft tissue imaging, or of elevated blood metal ion levels in the absence of symptoms have been reported in a limited number of research studies for some metal-on-metal hip implant patients. These studies are difficult to interpret because:
- The exact incidence or prevalence of asymptomatic lesions and their natural history is not known.
- The correlation between elevated blood metal ion levels in isolation and development of potential local or systemic adverse reactions is not well established
- If the orthopaedic surgeon determines it is in the best interest of the patient to conduct soft tissue imaging, please review the FDA’s recommendations.
- If the orthopaedic surgeon determines it is in the best interest of the patient to test metal ion levels, please review the FDA’s recommendations.
- Findings of lesions on soft tissue imaging, or of elevated blood metal ion levels in the absence of symptoms have been reported in a limited number of research studies for some metal-on-metal hip implant patients. These studies are difficult to interpret because:
Follow-up for symptomatic patients
Clinical evaluation
- If a patient experiences local symptoms (i.e. pain or swelling at or near the hip, a change in walking ability such as development of Trendelenburg gait, or a noise from the hip joint) more than three months after metal-on-metal (metal-on-metal) hip implant surgery, conduct a thorough evaluation, with attention to abductor weakness or Trendelenburg sign to identify abductor damage.
- Follow-up of symptomatic patients with metal-on-metal hip implants should occur at least every six months.
- Guide your clinical evaluation by the symptoms and physical findings, including an assessment for well-known and more common emergent complications including joint infection, implant loosening, peri-prosthetic facture and dislocation.
- Recognize that localized lesions associated with reactions to metal debris may also present with pain or a variety signs and symptoms including:
- Local nerve palsy
- Palpable mass
- Local swelling
- Joint dislocation or subluxation
- Abductor weakness
Additional testing for symptomatic patients
- In some patients with symptoms, plain radiograph findings (e.g. osteolysis, femoral neck narrowing, component suboptimal positioning, fracture), in conjunction with other non-imaging information, are sufficient to indicate a need for revision surgery.
- In other symptomatic patients, cross-sectional imaging should be considered to diagnose and assess soft tissue findings surrounding an implant.
- Patients with metal-on-metal hip implants who develop any symptoms or physical findings that indicate their device may not be functioning properly, should be considered for metal ion testing. It is important to note that at the current time, there is insufficient evidence in the U.S. demonstrating a correlation between a metal ion level in isolation and the presence of localized lesions, clinical outcomes and/or the need for revision surgery.
Assessment for systemic effects
- Evaluate patients with new systemic symptoms in collaboration with their primary medical physicians or specialists to determine the cause of their symptoms or findings.
- Patients with evidence of excessive device wear or a localized adverse local tissue reaction should be assessed for potential systemic effects of exposure to metal ions.
- A thorough physical examination should be performed by the patient’s health care team, which include primary medical physicians and/or specialists, with particular focus on cardiovascular, neurological, endocrinological (especially thyroid), and renal systems.
- Because metal ions are cleared through the kidneys, a patient who has renal insufficiency may be at higher risk for systemic adverse events. Blood urea nitrogen (BUN) and creatinine levels may be measured to evaluate a patient’s renal function.
Device revisions
- At this time, there is not enough evidence to provide a science-based recommendation for a threshold value of metal ion levels in the blood that would serve as a trigger for revision.
- The decision to revise a patient’s metal-on-metal hip implant should be made in response to the overall clinical scenario and results of diagnostic testing.
- In extreme cases, adverse local tissue reactions may significantly damage periprosthetic bone, muscle, and nerves. Therefore patients with progressing adverse local tissue reactions, may be considered for earlier revision to prevent extensive damage.
- If you recommend revision, advise patients of the surgical risks of revision as well as the expected outcomes, including:
- Revision procedures are complicated and may not fully resolve a patient’s pain nor restore complete function;
- In case of adverse local tissue reactions, revision of a metal-on-metal hip may have a worse prognosis than revision of other types of bearing surfaces especially for subsequent hip dislocation.
- Possible need for an additional revision procedure in the future; and
- Metal ion levels should be expected to decrease after revision.
- In selecting components for revision:
- Check the specific device labeling for compatibility of device components.
- Consider the benefits and risks of all bearing surfaces for each patient.
- If a patient is suspected to have developed metal sensitivity, carefully select the materials of the revision components (potentially avoiding materials with nickel or chromium).
- In order to better understand the performance of these devices and causes of failure, a retrieval analysis is recommended, especially in cases where there is no obvious cause for implant failure such as suboptimal positioning.
- Talk to the patient about returning the implant for retrieval analysis. A retrieval analysis is a vital part of a manufacturer’s quality system allowing them to assess manufacturing and quality processes through their Corrective and Preventative Action system.
- If the patient agrees, contact the manufacturer prior to revision surgery for instructions on returning the implant for analysis.
Along with the implant, provide non-identifying information about the patient, the date of procedure, observations from the revision surgery and the histopathology report from any tissue sent to the hospital pathologist. - Report any adverse events believed to be related to the metal-on-metal system to FDA through MedWatch, the FDA Safety Information and Adverse Event Reporting Program (https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program/reporting-serious-problems-fda).
Metallosis causes
Metallosis is caused by the release of metallic debris, secondary to hardware failure 8. The metallic debris induces a massive release of cytokines from inflammatory cells, making a revision necessary whenever osteolysis and loosening of the prosthesis occur. T lymphocytes were activated by metal particles present in periprosthetic membranes 4.
Metallosis was first described in association with setting of the fixation of fractures with metal implants 11. The adoption of articular components made of other materials such as polyethylene or ceramic has dramatically reduced its incidence in patients with articular prostheses and nowadays it is a rare complication (5.3% of total hip arthroplasty complications) 12. Although less frequent, even with polyethylene or ceramic articular components metallosis can occur if there is abnormal metal-on-metal contact due to wear or fracture of the articular component 13. Wear-through and dislodgement of the acetabular liner may be influenced by several factors, including use of thin polyethylene insert and the method of sterilization treatment of polyethylene liners 14. Chronic abrasion between the metal components induces the release and infiltration of metallic particles, activating a local chronic inflammatory reaction and systemic absorption of metallic particles. This gives rise to a variable range of local and systemic alterations, depending on metal type, particle size, volume and time of exposure 14.
Metallosis post–joint arthroplasty
Babis et al 15 reported a 70-year-old woman with a right total hip replacement, with a history of developmental dysplasia of the hip from childhood, who presented with severe pain and periarticular skin metallic staining. On joint revision, the porous tantalum acetabular component of the hip replacement had failed. Serum levels of tantalum had increased 2,000 fold and subsequently dropped by 25% 6 months after re-revision (tantalum implants were reused) 15.
Munro-Ashman and Miller 16 patch-tested 35 patients who suffered metal-to-metal prosthesis failures. A total of 35 patients in this unsatisfactory group were patch-tested; 16 were positive to metals, 13 to cobalt, 4 to nickel, and 2 to chromate. Only two patients showed any skin lesions—one a localized dermatitis around the knee joint from nickel sensitivity, and one with widespread scattered circular erythematous lesions suggestive of a generalized allergic vasculitis from cobalt exposure 16.
The authors recognized that roughly half of arthroplasty failures may be related to metal sensitivities (particularly cobalt) and suggested taking a careful history for metal sensitivity and patch-testing high-risk patients with the metals. Titanium 318 was suggested as a satisfactory substitute for cobalt chrome alloy if reactions were encountered or anticipated.
Bradberry et al 17 systematically reviewed toxicities attributed to metals released from hip implants, surmising that high circulating concentrations of cobalt from failed hip replacements could cause neurologic damage, hypothyroidism, and/or cardiomyopathy, which may fail to reverse after joint revision or removal.
Metallosis can lead to osteolysis and subsequent implant failure, which may be seen in noninvasive plain radiography. An often nonspecific radiographic sign observed is periprosthetic osteolysis; more specific signs that have been reported are the “bubble,” “cloud,” and “metal-line” signs 18. Serologic testing for metallic ions reveals elevated levels. A normal serum cobalt level is around 0.19 μg/L, with levels greater than 5 μg/L posing risk of neurologic and cardiac abnormalities 19.
Histopathology of metallosis
Asahina et al 20 reported a 59-year-old woman with a background of rheumatoid arthritis who experienced metallosis tracking to the distal end of the forearm, several centimeters beyond the prosthesis location, several months after elbow arthroplasty. The joint remained fully functional; she was pain-free and had no lymphadenopathy. Histopathology showed fine brown-black particles dispersed in the dermis, phagocytosed by macrophages, or else accumulating around eccrine glands and capillaries, indicating an adverse reaction to metal debris. X-ray analysis of the tissue showed high levels of iron, nickel, and chromium.
The authors theorized that problematic metallic particles in metallosis follow a random diffusion throughout soft tissue. Macrophages then attempt to phagocytose these foreign bodies following failure of the mechanism to remove them from dermal tissue. It was also suggested that the origin of the metallic staining was secondary to precipitation of the metallic particles from the instruments used during the arthroplasty procedure.
Matziolis et al 21 noted a case of metallosis following a total hip replacement, where histopathology showed numerous histiocytes and foreign body giant cells containing fine metal (cobalt-chromium) particles and moderately dense lymphoplasmacytic infiltrates. The metal particles appear as extracellular metal deposits or as debris in the cytoplasm of histiocytes and foreign body giant cells (hematoxylin and eosin staining, ×400 focus).
Akimoto et al 22 noted an 80-year-old woman, who presented with metallosis mimicking a malignant skin tumor 6 years after right total hip arthroplasty. Histology showed diffuse granulomatous inflammation, large lymphatic spaces, and fine brown-black granules within the histiocytes (likely originating from the titanium acetabular cup and screws). The authors considered that in this case, the granulomatous tumorlike reaction was possibly a more severe immunologic reaction to the inflammatory metallic debris products.
Metallosis symptoms
Patients may be asymptomatic with isolated imaging findings suggesting wear-through, fracture or dislodgment of the liner; an eccentric femoral head will be evident in all cases 14. Metallosis may present locally with pain, instability, audible crepitus or squeaking on weight bearing and metallic debris staining the local tissue. Pain, pseudotumoral mass formation and osteolysis are the commonest local changes 23:848–857.)). The spread of metallosis or infection along the psoas muscle has already been described and it may be associated to either direct spread through the bursa, or acetabular fissures arising at the time of surgery which allowed the initial process to extend 24. Importantly, metallic debris can also have superficial manifestations of periarticular skin tattooing, due to an adverse local tissue reaction. Although the structural component of failed joint arthroplasty can be revised to improve pain, stability, and functioning, any skin tattooing secondary to metallosis presents the treating physician with clinical challenge.
Systemically, metallosis has been linked to neurologic complications (visual, hearing, and cognitive deficits), cardiac failure, hypothyroidism 8 and hemolytic anemia 5. The systemic effects are mainly caused by an immunologic response due to metal sensitivity. High levels of chromium and cobalt components are related to headache and cognitive changes, hematological abnormalities and neuromuscular changes 25. Titanium alloy components absorption effects (titanium, aluminum and vanadium) are less known, but have been recently described in the literature 26. Although titanium has been regarded as inert and biocompatible, titanium particles and ions can also induce the release of potentially osteolytic cytokines and cause necrosis, fibrosis and other structural changes in regional lymph nodes, liver and spleen 27. Hemolytic anemia process will probably be also related to an immunological process, induced by metal sensitivity, but the real mechanism has not yet been described.
Metallosis diagnosis
Metallosis diagnosis may be made at joint aspiration only, when dense black fluid is obtained, so fluid analysis is not essential 28.
Diagnostic testing (such as soft tissue imaging and metal ion testing) may provide important clinical information for assessing the risks of metallosis in patients with metal-on-metal implants. While the results of these diagnostic tests can be indicative of adverse tissue reactions, they cannot be relied upon as the only sign that a hip is failing.
When conducting soft tissue imaging or metal ion testing in metal-on-metal patients, consider the following 29:
Soft Tissue Imaging
- For some patients, cross-sectional imaging is required to assess and diagnose potential changes in the soft tissues surrounding an implant.
- The benefits and risks of using different types of diagnostic imaging procedures should be considered when determining the most appropriate imaging modality for each patient.
metal-on-metal hip implants can create metal artifacts (distortions of the image due to the presence of metal in the imaging field) in some imaging modalities. - The most utilized methods of imaging soft tissue surrounding metal-on-metal hip implants include:
- Magnetic Resonance Imaging (MRI) with metal artifact reduction sequences (MARS)
- Strengths: MRI uses non-ionizing radiation and offers the best visualization of soft tissues surrounding a metal-on-metal hip implant and detection of potential adverse local tissue reactions.
- Weaknesses: Most metal-on-metal hip implants in the U.S. have not been evaluated for safety in an MR environment so the likelihood of adverse events, such as heating of the tissue near the implant, may not be known.
- Computed Tomography Scan (CT)
- Strength: CT offers the best visualization of implant positioning and bony tissue
- Weaknesses: CT uses ionizing radiation and provides lower soft tissue visualization. Image artifacts from the implant may distort the image.
- Ultrasound
- Strengths: Ultrasound allows soft tissue visualization without metal artifacts and uses non-ionizing radiation.
- Weaknesses: Image quality in ultrasound is very operator dependent. Ultrasound provides a lower resolution soft tissue image than MRI and has a limited depth penetration.
- Magnetic Resonance Imaging (MRI) with metal artifact reduction sequences (MARS)
- If it is determined that MRI imaging of a metal-on-metal hip implant patient is appropriate, the FDA recommends that the orthopaedic surgeon:
- Consult with the radiologist to evaluate the benefits and risks of utilizing MRI with metal artifact reduction;
- Use the available device-specific labeling to help determine appropriate scan sequences; and
- Inform the MRI site that the patient has a metal-on-metal hip implant.
Metal Ion Testing
Some patients with a metal-on-metal hip implant may have elevated metal ion levels in their bloodstream. These elevated metal ions are predominately cobalt and chromium, but can also include other metals such as molybdenum and titanium. Depending on the clinical scenario, orthopaedic surgeons may recommend measuring metal ion levels.
Who should be tested?
As part of their overall clinical evaluation, orthopaedic surgeons should consider measurements of metal ion levels in symptomatic patients with metal-on-metal hip implants. Serial measurements of metal levels in conjunction with clinical dynamics can help optimizing individual patient management.
- The orthopaedic surgeon should consider testing patients with metal-on-metal hip implants who develop any symptoms that may indicate that their device may not be functioning properly.
- At the current time, there is insufficient evidence to recommend metal ion testing in patients with metal-on-metal hip implants that have none of the metallosis signs or symptoms described above and the orthopaedic surgeon feels the hip is functioning properly and has normal radiographs.
What should be tested?
Test Method:
- Measure cobalt using an inductively coupled plasma mass spectrometer (ICP-MS), equipped with either a collision cell or dynamic reaction cell technology, or use a sector field inductively coupled plasma mass spectrometer (SF-ICP-MS). The FDA recommends testing cobalt in EDTA anti-coagulated whole blood using a validated method that meets the Clinical Laboratory Improvement Amendments (CLIA) validation criteria. The method should accurately and precisely measure concentrations as low as 1 µg/L cobalt.
- If chromium is measured, use an ICP-MS, equipped with either a collision cell or dynamic reaction cell technology, or use SF-ICP-MS (in medium or high resolution mode) to resolve potential polyatomic interferences. The FDA recommends testing chromium in EDTA anti-coagulated whole blood using a validated method that meets the Clinical Laboratory Improvement Amendments validation criteria. The method should accurately and precisely measure concentrations as low as 1 µg/L chromium.
Selecting a Testing Lab:
- Ensure the lab is Clinical Laboratory Improvement Amendments (CLIA)-certified and is using a validated method that meets the CLIA validation criteria for EDTA anti-coagulated whole blood;
- Ensure the lab has an ongoing quality assurance and quality control program for the measurand in EDTA anti-coagulated whole blood;
- Ensure the lab provides collection kits (including all components necessary for sample collection and processing) that are free of significant metal contamination and fit for purpose (i.e., measuring trace and ultra-trace cobalt and chromium). The lab is responsible for validating that all components within the collection kit are not contaminated;
- Ensure the lab participates in a Centers for Medicare and Medicaid Services (CMS) approved trace element proficiency testing program (e.g. New York State Department of Health, Wadsworth Center) for that particular measurand. A lab’s results for that measurand should be within the defined acceptance criteria of the proficiency testing program.
- Ensure that the lab has performed an appropriate study, or references appropriate data, to define valid reference range values.
Interpreting Test Results:
- The metal ion concentration values should be considered in addition to the overall clinical scenario including symptoms, physical findings, and other diagnostic results when determining further actions.
- When assessing metal ion concentration values, it is important to determine if the patient has any other reasons to have metal ion concentration values above reference range values including:
- Other implanted metal hardware
- Occupational exposure
- Renal insufficiency
- Dietary supplements
- Metal ion concentration values should be interpreted in conjunction with blood urea nitrogen (BUN) and creatinine to concurrently evaluate the patient’s renal function.
- Test results should be interpreted considering the measurement error of the method and the lab’s reference range. The measurement error can be estimated by reviewing the lab’s proficiency testing results and the imprecision of the method.
- Ideally, follow test results serially in a patient, ensuring that the same sample type, measurement method and the same laboratory are utilized for each measurement. If a different laboratory was utilized, the ordering physician needs to consider the test results in light of potentially differing laboratory methods that may not have values that are interchangeable. The ordering physician should interpret the test results considering the total error of the method(s) to determine if metal ion levels are changing.
International regulatory agencies developed specific follow-up recommendations for patients with metal-on-metal (metal-on-metal) hip implants, including recommendations for blood tests including specific metal ion threshold values. As noted earlier, there are differences between the usage and availability of metal-on-metal hip implants in the U.S. and those outside the U.S., as well as differences in laboratory practices. For this reason, these recommendations may not be appropriate for patients in the U.S. As noted above, several factors can impact the accuracy, reproducibility, and clinical interpretation of the test results.
At the current time, there is not enough evidence in the U.S. demonstrating a correlation between a metal ion level in isolation and the presence of localized lesions, clinical outcomes and/or the need for revision surgery 29.
Metallosis treatment
Metallosis treatment consists of surgical revision with replacement of the prosthesis components, complete surgical debridement of osteolytic lesions and bone grafting with allograft chips. Complete removal of all metal debris is difficult and may result in extensive tissue damage 14. In the reported case, drainage of the large pelvic collection was also required. CT imaging allowed not only the diagnosis of the initially missed pelvic collection, but also contributed to the correct surgical planning. A successful collection drainage through a transacetabular approach during the prosthesis revision was performed.
For successful management of skin metallosis, the orthopedic surgeon must revise the arthroplasty, addressing all relevant factors, and seek referral to the dermatologist as appropriate.
Histologically, metallosis involves metallic particles migrating from the deeper periarticular tissues superficially to the dermis, with varying immunologic reactions. There is little in the current literature to recommend an optimal laser treatment; current research looks at treatment of tattoo particles introduced superficially and less likely to be concentrated in deeper tissues as in metallosis 8.
Adequate treatments with the fractionated CO 2 ablative laser, or the Q-switched Nd:YAG 1,064-nm laser, capable of either allowing the particles to be released from the dermis (transepidermal elimination) or breaking up the particles at least in the dermis, are likely to be successful 8. Although particles deeper than the dermis would fail to be treated with laser. Because no current research into laser or other treatment of metallosis has been conducted, clinical trials would be of benefit. This would help guide the physician toward optimal therapy when addressing this condition.
References- Chang JD, Lee SS, Hur M, Seo EM, Chung YK, Lee CJ. Revision total hip arthroplasty in hip joints with metallosis: a single-center experience with 31 cases. J Arthroplasty. 2005;20(5):568–573. doi: 10.1016/j.arth.2005.04.001
- Pesce V, Maccagnano G, Vicenti G, Notarnicola A, Lovreglio P,Soleo L, et al. First case report of vanadium metallosis after ceramic-on-ceramic total hip arthroplasty. J Biol Regul Homeost Agents2013;27, October–December 4:1063–8.
- Metallosis: A diagnosis not only in patients with metal-on-metal prostheses. European Journal of Radiology Open 2 (2015) 3–6 https://doi.org/10.1016/j.ejro.2014.11.001
- Panni, A. S., Vasso, M., Cerciello, S., & Maccauro, G. (2011). Metallosis following Knee Arthroplasty: A Histological and Immunohistochemical Study. International Journal of Immunopathology and Pharmacology, 711–719. https://doi.org/10.1177/039463201102400317
- Oliveira CA, Candelária IS, Oliveira PB, Figueiredo A, Caseiro-Alves F. Metallosis: A diagnosis not only in patients with metal-on-metal prostheses. Eur J Radiol Open. 2014;2:3–6. Published 2014 Nov 28. doi:10.1016/j.ejro.2014.11.001 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4750564
- Berry DJ, Barnes CL, Scott RD, Cabanela ME, Poss R. Catastrophic failure of the polyethylene liner of uncemented acetabular components. J Bone Joint Surg Br. 1994;76(4):575–578.
- Khan RJ, Wimhurst J, Foroughi S, Toms A. The natural history of metallosis from catastrophic failure of a polyethylene liner in a total hip. J Arthroplasty. 2009 Oct;24(7):1144.e1-4. doi: 10.1016/j.arth.2008.09.002. Epub 2008 Oct 9. https://doi.org/10.1016/j.arth.2008.09.002
- Thomas S, Gouk C, Jayasakeera N, Freeman M. The Sequelae of Metallosis Resulting in Skin Pigmentation and Tattooing: A Case Presentation and Literature Review. Surg J (N Y). 2016;2(4):e143–e146. Published 2016 Dec 14. doi:10.1055/s-0036-1596060 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5553500
- Jayasekera N, Gouk C, Patel A, Eyres K. Apparent skin discoloration about the knee joint: a rare sequela of metallosis after total knee replacement. Case Rep Orthop. 2015;2015:891904
- Metal-on-Metal Hip Implants Information for Orthopaedic Surgeons. https://www.fda.gov/medical-devices/metal-metal-hip-implants/information-orthopaedic-surgeons
- von Lüdinghausen M., Meister P., Probst J. Metallosis after osteosynthesis. Pathol Eur. 1970;5(3):307–314.
- Roth T.D., Maertz N., Parr J., Buckwalter K., Choplin R. CT of the hip prosthesis: appearance of components, fixation, and complications. Radiographics. 2012;32:1089–1107.
- Willis-Owen CA1, Keene G.C., Oakeshott R.D. Early metallosis-related failure after total knee replacement: a report of 15 cases. J Bone Joint Surg Br. 2011;93, February 2:205–209.
- Cipriano CA1, Issack P.S., Beksac B., Della Valle A.G., Sculco T.P., Salvati E.A. Metallosis after metal-on-polyethylene total hip arthroplasty. Am J Orthop (Belle Mead NJ) 2008;37, February 2:E18–E25.
- Babis G C, Stavropoulos N A, Sasalos G, Ochsenkuehn-Petropoulou M, Megas P. Metallosis and elevated serum levels of tantalum following failed revision hip arthroplasty—a case report. Acta Orthop. 2014;85(06):677–680.
- Munro-Ashman D, Miller A J. Rejection of metal to metal prosthesis and skin sensitivity to cobalt. Contact Dermat. 1976;2(02):65–67.
- Bradberry S M, Wilkinson J M, Ferner R E. Systemic toxicity related to metal hip prostheses. Clin Toxicol (Phila) 2014;52(08):837–847.
- Sharareh B, Phan D L, Goreal W, Schwarzkopf R. Metallosis presenting as knee pain 26 years after primary total knee arthroplasty. J Orthop Case Rep. 2015;5(02):62–65.
- Tower S S. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847–2851.
- Asahina A, Fujita H, Fukuda S et al.Extensive skin pigmentation caused by deposits of metallic particles following total elbow arthroplasty: metallosis or not? Br J Dermatol. 2007;157(05):1074–1076.
- Matziolis G, Perka C, Disch A. Massive metallosis after revision of a fractured ceramic head onto a metal head. Arch Orthop Trauma Surg. 2003;123(01):48–50.
- Akimoto M, Hara H, Suzuki H. Metallosis of the skin mimicking malignant skin tumour. Br J Dermatol. 2003;149(03):653.
- Chang E.Y., McAnally J.L., Van Horne J.R., Statum S., Wolfso T., Gams A. Metal-on-metal total hip arthroplasty: do symptoms correlate with MR imaging findings? Radiology. 2012;265(December (3
- Rymaruk S., Razak A., McGivney R. Metallosis, psoas abscess and infected hip prosthesis in a patient with bilateral metal on metal total hip replacement. J Surg Case Rep. 2012;2012, May 5:11.
- Campbell P., Shimmin A., Walter L., Solomon M. Metal sensitivity as a cause of groin pain in metal-on-metal hip resurfacing. Arthroplast J. 2008;23, October 7):1080–1085.
- Pesce V., Maccagnano G., Vicenti G., Notarnicola A., Lovreglio P., Soleo L. First case report of vanadium metallosis after ceramic-on-ceramic total hip arthroplasty. J Biol Regul Homeost Agents. 2013;27, October–December 4:1063–1068.
- Miloŝev L., Antoliĉ V., Minoviĉ A., Cör A., Herman S., Pavlovcic V.P. Campbell extensive metallosis and necrosis in failed prostheses with cemented titanium-alloy stems and ceramic heads. J Bone Joint Surg Br. 2000;82, April 3:352–357.
- Cipriano CA1, Issack P.S., Beksac B., Della Valle A.G., Sculco T.P., Salvati E.A. Metallosis after metal-on-polyethylene total hip arthroplasty. Am J Orthop (Belle Mead NJ) 2008;37,February 2:E18–E25.
- Information about Soft Tissue Imaging and Metal Ion Testing. https://www.fda.gov/medical-devices/metal-metal-hip-implants/information-about-soft-tissue-imaging-and-metal-ion-testing