Microscopic polyangiitis
Microscopic polyangiitis is a vasculitis of small vessels, a rare disorder characterized by inflammation of the small- to medium-sized blood vessels, which can restrict blood flow and damage vital organs and tissues, particularly involving the kidneys, lungs, nerves, skin, and joints 1. Microscopic polyangiitis can worsen rapidly, so early diagnosis and treatment are essential to prevent kidney or respiratory damage, or organ failure 1. Patients frequently present with renal manifestations, but systemic manifestations, arthritis, mononeuritis multiplex, and other signs and symptoms are also common 2.
Microscopic polyangiitis is known as an ANCA (anti-neutrophil cytoplasmic antibody) associated vasculitis, referring to a blood protein that attacks the body’s own cells and tissues. Other forms of ANCA-associated vasculitis include eosinophilic granulomatosis with polyangiitis (Churg-Strauss disease) and granulomatosis with polyangiitis (Wegener’s granulomatosis). People with these types of vasculitis often test positive for ANCA, although the test is not conclusive.
Patients may present with insidious onset fever, arthralgia, weight loss, urinary abnormalities, cough with or without hemoptysis, skin findings consistent with palpable purpura, or non-specific neurological complaints. Some patients may present with acute onset of fulminant disease with frank hemoptysis, hematuria, or even renal failure. Physical examination may show ulceronodular skin lesions of the extremities, fever, tachypnea, and even tachycardia. A pulmonary examination may be significant for rales or bronchial breath sounds in case of pulmonary capillaritis, and Neurological examination may show motor or sensory deficits that are localized to a particular dermatome. Other physical findings will vary depending on the involved capillary bed.
The term microscopic polyarteritis was introduced in the literature by Davson in 1948 to describe the pattern of glomerulonephritis seen in patients of polyarteritis nodosa 3. Microscopic polyangiitis was later described as a pattern of necrotizing vasculitis, without immune complex deposition, affecting small vessels such as the capillaries, venules, and arterioles. Microscopic polyangiitis commonly involves glomerulonephritis, pulmonary capillaritis, and other systemic capillary beds. It shows considerable overlap with Wegener’s granulomatosis. The absence of granulomatous inflammation involving the upper respiratory tract and the presence of pulmonary capillaritis is said to differentiate microscopic polyangiitis from granulomatosis with polyangiitis (Wegener’s granulomatosis) 4. Microscopic polyangiitis is also included in a group of disorders termed as pulmonary-renal syndrome, which includes microscopic polyangiitis, granulomatosis with polyangiitis (Wegener’s granulomatosis), Goodpasture disease, and systemic lupus erythematosus (SLE) 5.
Microscopic polyangiitis can affect people of all ages, but the average age of onset is approximately 50. Microscopic polyangiitis affects both men and women, but men may get microscopic polyangiitis more often. Microscopic polyangiitis is more frequent among Caucasians but can affect people of any race or ethnic background.
Microscopic polyangiitis is rare, with prevalence estimated at 1 to 3 cases per 100,000 people in the United States 6. Internationally, the incidence is approximately 2 cases per 100,000 people in the United Kingdom and an estimated 1 case per 100,000 people in Sweden 7.
Microscopic polyangiitis is a serious but treatable disease. The traditional course of treatment includes corticosteroids such as prednisone used in combination with other medications that suppress the immune system and reduce inflammation. Even with treatment, microscopic polyangiitis is a chronic condition with periods of relapse and remission, so ongoing medical care and monitoring are necessary.
Microscopic polyangiitis causes
The cause of microscopic polyangiitis is not yet fully understood by researchers. Vasculitis is classified as an autoimmune disorder, a disease which occurs when the body’s natural defense system mistakenly attacks healthy tissues. Researchers believe an infection may set the inflammatory process in motion in microscopic polyangiitis. Environmental and some genetic factors may also play a role in vasculitis.
The etiopathogenesis of microscopic polyangiitis and other related vasculitides has largely been attributed to anti-neutrophil-cytoplasmic antibodies (ANCA). These are host-derived auto-antibodies against shielded neutrophilic antigens. These antibodies react against primary granules present in neutrophils and monocytes. The formation of these antibodies has been hypothesized to be a 2-step process 8. The first step involves exposure of neutrophils to inflammatory cytokines leading to surface exposure of cryptogenic antigens like myeloperoxidase. Next, predisposing genetic, environmental and other factors 9 result in the production of myeloperoxidase-ANCA. In the second step, these myeloperoxidase-ANCA cause damage to the host vasculature by reacting and crosslinking neutrophils to the endothelial receptors.
Only 70% of cases of microscopic polyangiitis have ANCA at the time of diagnosis, and most of the cases of limited microscopic polyangiitis do not have ANCA at all. This has lead to the understanding that other factors may also be playing a role in its etiopathogenesis. These include, but are not limited to:
- Infectious causes: There is considerable overlap in the clinical presentation of various infectious processes and microscopic polyangiitis leading to this implication. A possible role of chronic nasal carriage of Staphylococcus aureus has been suggested in relapsing granulomatosis with polyangiitis (Wegener’s granulomatosis) as well 10.
- Drugs: Various drugs like hydralazine, thionamides, sulfasalazine, and minocycline among others have been observed to be associated with the incidence of ANCA-associated vasculitis 11.
- Genetic factors: More recently, A genome-wide study conducted in Europe has implicated genes like HLA-DP, HLA-DR3, and alpha-1 antitrypsin in the pathogenesis of ANCA-associated vasculitides 12.
Microscopic polyangiitis symptoms
The symptoms of microscopic polyangiitis and their severity can vary greatly from person to person, depending on which blood vessels and organs are affected. For some the disease is mild, while for others it may be severe, or even potentially life-threatening if untreated. Microscopic polyangiitis symptoms may come on slowly over a period of months, or develop rapidly in a matter of days.
People with microscopic polyangiitis often feel generally ill, with flu-like symptoms of fatigue, fever, loss of appetite and weight loss. Other symptoms may be related to organ systems affected.
- Kidney inflammation, which may be associated with bloody or dark urine. A patient can have kidney disease without having symptoms; therefore, patients with vasculitis should have regular urine tests.
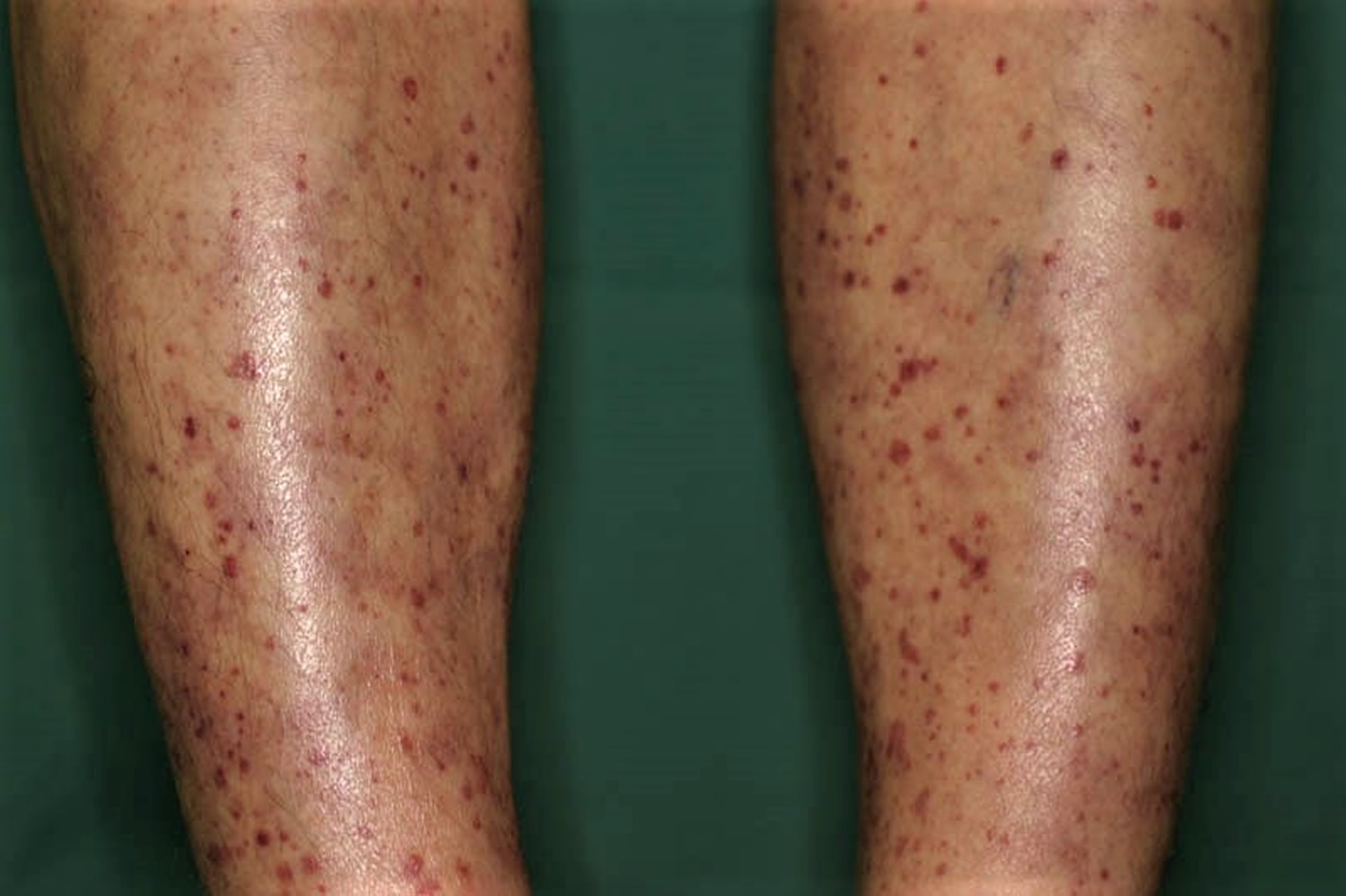
- Skin rashes/lesions, especially on the legs
- Cough (coughing up blood, shortness of breath)
- Nerve problems (tingling, numbness, pain, weakness, “foot drop” or “wrist drop”—inability to lift your foot or wrist)
- Joint and muscle pain
- Abdominal pain with eating
- Eye irritation
Kidney involvement is most common, and up to 80% to 100% of individuals have some form of glomerulonephritis at onset or with disease progression 13. The most common manifestation is a “pauci-immune” form of rapidly progressive glomerulonephritis. Clinical presentation may vary from asymptomatic hematuria (blood in urine), sub-nephrotic proteinuria, a rise in serum creatinine or overt renal failure 14. Lung involvement may be in the form of alveolar hemorrhage, which is sometimes the first presenting symptom of microscopic polyangiitis. Skin involvement include leukocytoclastic-vasculitis, palpable purpura, or nodular and ulcerative lesions that commonly involve the lower extremities. Occasionally eye involvement (conjunctivitis, episcleritis, optic neuropathy) and neurological manifestations (mononeuritis multiplex, cranial neuropathy, among others) may also be seen.
Microscopic polyangiitis complications
Serious, even life-threatening complications, can occur with microscopic polyangiitis, especially with the kidneys and lungs. If you have symptoms that don’t go away, have bloody or dark urine, or are coughing up blood, contact your doctor right away.
Microscopic polyangiitis diagnosis
Because there is no single test for diagnosing microscopic polyangiitis, your doctor will consider a number of factors, including a thorough medical history, physical exam findings, and results of laboratory tests and imaging studies.
A biopsy of the affected tissue is usually obtained to confirm the diagnosis. Histopathological evidence of vasculitis is the gold standard for confirmation of the diagnosis of microscopic polyangiitis and other ANCA-associated vasculitides. The most commonly sampled tissues are renal, skin and lung tissue. Pulmonary findings in microscopic polyangiitis is most commonly a form of diffuse capillaritis (distinguishing it form granulomatosis with polyangiitis that characteristically shows granulomatous lesions). Skin biopsy yields acute or chronic leukocytoclastic vasculitis with neutrophilic infiltrate in the narrow caliber vessels of the superficial dermis. Renal biopsy most commonly varies from mild focal or segmental to a diffuse necrotizing and sclerosing glomerulonephritis that shows minimal to no immune complex deposits on light and immunofluorescent microscopy (“pauci-immune”). The importance of renal biopsy lies in the fact that the severity of renal involvement on histopathological evaluation correlates clinically with disease activity 14. It thus plays an important role in guiding patient management and tapering immunosuppressive therapy. Although this is the mainstay, it has been shown that many patients may have a diffuse interstitial nephritis without involving the glomeruli which may pose a difficulty in diagnosis. Moreover, a few may have immune complex deposits in the glomeruli and experience more severe systemic signs and symptoms 15.
- Urinalysis: The presence of red blood cells may indicate kidney inflammation. Your doctor may use this test to help diagnosis microscopic polyangiitis, and to monitor the kidneys during and after treatment.
- Blood tests: The ANCA test can be helpful when positive. Blood tests that can detect inflammation include the erythrocyte sedimentation rate (ESR) test, commonly called the “sed rate,” and the C-reactive protein (CRP) test. All these tests may support a diagnosis of microscopic polyangiitis, but are not conclusive on their own. A tissue biopsy is typically needed.
- Tissue biopsy: This surgical procedure removes a small tissue sample from an affected organ, which is examined under a microscope for signs of inflammation or tissue damage. Tissues that might be biopsied for microscopic polyangiitis include kidney, lung, skin, nerve and muscle.
- Imaging studies: Chest X-rays may reveal changes in your lungs that are characteristic of microscopic polyangiitis. Computed tomography (CT) and magnetic resonance imaging (MRI) scans provide more detailed images of your internal organs and can show abnormalities.
Microscopic polyangiitis treatment
Microscopic polyangiitis treatment is based on a number of factors, including disease severity and organ involvement. The cornerstone of treatment for microscopic polyangiitis is corticosteroids such as prednisone used in combination with other medications that suppress the immune system and reduce inflammation.
For severe disease, the biologic drug rituximab may be used in combination with prednisone. The U.S. Food and Drug Administration (FDA) in 2011 approved rituximab for the treatment of microscopic polyangiitis and granulomatosis with polyangiitis (Wegener’s granulomatosis). Biologic medications are complex proteins derived from living organisms. They target certain parts of the immune system to control inflammation.
Another option for severe disease is cyclophosphamide, a chemotherapy-type drug that blocks abnormal growth of certain cells in the body, in combination with prednisone. Prednisone is typically started at a high dose and then tapered off slowly. Cyclophosphamide can lower the body’s ability to fight infection, so it is usually limited to a three- to six-month period and replaced with less toxic medications such as mycophenolate mofetil and azathioprine, or methotrexate, a drug commonly used to treat rheumatoid arthritis. Milder forms of microscopic polyangiitis are typically treated with a combination of prednisone and methotrexate.
Some individuals may experience kidney failure, a serious complication that requires dialysis and/or a kidney transplant. Another option for those with very serious microscopic polyangiitis affecting the kidneys or lungs is “plasmapheresis.” Plasmapheresis is a dialysis-like procedure that clears proteins from the plasma of the blood and replaces it with plasma from a donor, or with a plasma substitute.
Once in remission, most patients will likely need to continue taking maintenance medications, such as azathioprine, methotrexate or rituximab, to keep the disease under control 16. The dose of steroids is usually tapered during remission.
Even with effective treatment, microscopic polyangiitis is a chronic disease, and relapses may occur. If your initial symptoms return or you develop new ones, report them to your doctor as soon as possible. Regular doctor visits and ongoing monitoring of lab and imaging tests are important in detecting relapses early.
Side effects microscopic polyangiitis medications
The medications used to treat microscopic polyangiitis have potentially serious side effects, such as lowering your body’s ability to fight infection, and potential bone loss (osteoporosis), among others. Therefore, it’s important to see your doctor for regular checkups. Medications may be prescribed to offset side effects. Infection prevention is also very important. Talk to your doctor about getting a flu shot, pneumonia vaccination, and/or shingles vaccination, which can reduce your risk of infection.
The immunosuppressive agents used in the management of ANCA-associated vasculitides have serious side effects, these may include:
1. Glucocorticoids
- Osteoporosis
- Cataract
- Glaucoma
- Diabetes mellitus
- Electrolyte abnormalities
- Avascular necrosis of bone
2. Cyclophosphamide
- Bone marrow suppression
- Hemorrhagic cystitis
- Bladder carcinoma
- Myelodysplasia
3. Methotrexate
- Hepatotoxicity
- Pneumonitis
- Bone marrow suppression
4. Azathioprine
- Hepatotoxicity
- Bone marrow suppression
5. Rituximab
- Progressive multifocal leukoencephalopathy
- Opportunistic infections
Living with microscopic polyangiitis
Living with a chronic disease such as microscopic polyangiitis can be challenging at times. Fatigue, pain, emotional stress, and medication side effects can take a toll on your sense of well-being, affecting relationships, work and other aspects of your daily life. Sharing your experience with family and friends, connecting with others through a support group, or talking with a mental health professional can help.
Microscopic polyangiitis prognosis
There is no cure for microscopic polyangiitis at this time, but with early diagnosis and proper treatment, many patients can lead full, productive lives. Because relapses can occur with microscopic polyangiitis, follow-up medical care is essential.
With treatment, 90% of patients with microscopic polyangiitis improve and 75% achieve complete remission. The 5-year survival rate is approximately 75%. microscopic polyangiitis carries a worse long-term survival rate than granulomatosis with polyangiitis or Churg-Strauss syndrome, probably because of renal involvement at disease onset.
Of patients with microscopic polyangiitis, 30% relapse in 1-2 years. Oh et al 17 reported that neither myeloperoxidase-ANCA nor proteinase 3 (PR3)-ANCA positivity at diagnosis affected prognosis. However, risk of relapse was significantly higher in patients with chest and renal manifestations, Birmingham vasculitis activity score ≥13.5, or five factor score ≥1.
Long-term damage in a study of 296 patients with microscopic polyangiitis or granulomatosis with polyangiitis, as measured with the Vasculitis Damage Index (VDI), was associated with the severity of initial disease, older age, the number of relapses, and duration of glucocorticoid treatment. Patients were followed for 7 years post-diagnosis. Mean duration of glucocorticoid treatment was 40.4 months 18.
In another study of 151 patients with ANCA-associated vasculitis, patients presenting with pulmonary involvement at baseline had higher damage and disease activity scores at 6, 12 and 24 months follow-up. Patients presenting with lung involvement had an increased risk of developing cardiovascular and renal involvement and were more likely to develop pulmonary fibrosis 19.
References- Microscopic polyangiitis. https://www.vasculitisfoundation.org/education/forms/microscopic-polyangiitis/
- Guillevin L, Durand-Gasselin B, Cevallos R, et al. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999 Mar. 42(3):421-30.
- WAINWRIGHT J, DAVSON J. The renal appearances in the microscopic form of periarteritis nodosa. J Pathol Bacteriol. 1950 Apr;62(2):189-96.
- Jain V, Tiwari V. Microscopic Polyangiitis. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531484
- Chung SA, Seo P. Microscopic polyangiitis. Rheum. Dis. Clin. North Am. 2010 Aug;36(3):545-58.
- Berti A, Cornec D, Crowson CS, Specks U, Matteson EL. The Epidemiology of Antineutrophil Cytoplasmic Autoantibody-Associated Vasculitis in Olmsted County, Minnesota: A Twenty-Year US Population-Based Study. Arthritis & rheumatology (Hoboken, N.J.). 2017 Dec;69(12):2338-2350.
- Lane SE, Watts R, Scott DG. Epidemiology of systemic vasculitis. Curr Rheumatol Rep. 2005 Aug;7(4):270-5.
- Kallenberg CG, Heeringa P, Stegeman CA. Mechanisms of Disease: pathogenesis and treatment of ANCA-associated vasculitides. Nat Clin Pract Rheumatol. 2006 Dec;2(12):661-70.
- de Lind van Wijngaarden RA, van Rijn L, Hagen EC, Watts RA, Gregorini G, Tervaert JW, Mahr AD, Niles JL, de Heer E, Bruijn JA, Bajema IM. Hypotheses on the etiology of antineutrophil cytoplasmic autoantibody associated vasculitis: the cause is hidden, but the result is known. Clin J Am Soc Nephrol. 2008 Jan;3(1):237-52.
- Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann. Intern. Med. 1994 Jan 01;120(1):12-7.
- Pendergraft WF, Niles JL. Trojan horses: drug culprits associated with antineutrophil cytoplasmic autoantibody (ANCA) vasculitis. Curr Opin Rheumatol. 2014 Jan;26(1):42-9.
- Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, Baslund B, Brenchley P, Bruchfeld A, Chaudhry AN, Cohen Tervaert JW, Deloukas P, Feighery C, Gross WL, Guillevin L, Gunnarsson I, Harper L, Hrušková Z, Little MA, Martorana D, Neumann T, Ohlsson S, Padmanabhan S, Pusey CD, Salama AD, Sanders JS, Savage CO, Segelmark M, Stegeman CA, Tesař V, Vaglio A, Wieczorek S, Wilde B, Zwerina J, Rees AJ, Clayton DG, Smith KG. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 2012 Jul 19;367(3):214-23.
- Savage CO, Winearls CG, Evans DJ, Rees AJ, Lockwood CM. Microscopic polyarteritis: presentation, pathology and prognosis. Q. J. Med. 1985 Aug;56(220):467-83.
- Hauer HA, Bajema IM, van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC., European Vasculitis Study Group (EUVAS). Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int. 2002 Jan;61(1):80-9.
- Jennette JC, Falk RJ. The pathology of vasculitis involving the kidney. Am. J. Kidney Dis. 1994 Jul;24(1):130-41.
- Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniené J, Ekstrand A, Gaskin G, Gregorini G, de Groot K, Gross W, Hagen EC, Mirapeix E, Pettersson E, Siegert C, Sinico A, Tesar V, Westman K, Pusey C., European Vasculitis Study Group. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N. Engl. J. Med. 2003 Jul 03;349(1):36-44.
- Oh YJ, Ahn SS, Park ES, Jung SM, Song JJ, Park YB, et al. Chest and renal involvements, Birmingham vascular activity score more than 13.5 and five factor score (1996) more than 1 at diagnosis are significant predictors of relapse of microscopic polyangiitis. Clin Exp Rheumatol. 2017 Jan 19.
- Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Höglund P, et al. Glucocorticoid treatment and damage in the anti-neutrophil cytoplasm antibody-associated vasculitides: long-term data from the European Vasculitis Study Group trials. Rheumatology (Oxford). 2014 Sep 8.
- Hassan TM, Hassan AS, Igoe A, Logan M, Gunaratnam C, McElvaney NG, et al. Lung involvement at presentation predicts disease activity and permanent organ damage at 6, 12 and 24 months follow – up in ANCA – associated vasculitis. BMC Immunol. 2014 May 27. 15:20.