Midline shift brain
Midline shift is a finding described on transverse (axial) slices from CT and MRI studies 1. Midline shift describes the situation where the midline of the intracranial anatomy is no longer in the midline and is the result of pushing or pulling forces within either side of the intracranial compartment (see Figure 1). Before the advent of cross sectional imaging, midline shift was assessed by displacement of the calcified pineal gland on a frontal radiograph of the skull 2. Midline shift is measured in millimeters, as the perpendicular distance between a midline structure usually the septum pellucidum and a line designated the midline. The midline is assumed to be coplanar with falx cerebri, and is best represented as a line drawn between the anterior and posterior attachments of the falx to the inner table of the skull. Care must be taken if there is existing asymmetry of the ventricles or the falx. If the falx is not straight, a line between the free edges of the anterior and posterior falx can be used instead. The superior sagittal sinus can also be used to indicate the posterior falcine attachment provided it is truly midline and not coursing to one side as is seen sometimes with a dominant transverse sinus.
Midline shift can be caused by conditions including traumatic brain injury 3, stroke, hematoma, brain edema or birth deformity that leads to a raised intracranial pressure. Midline shift typically results from unilateral frontal, parietal, or temporal lobe mass effect displacing the cingulate gyrus beneath the free edge of the falx cerebri and is associated with a number of neurological complications.
Midline shift sign is considered ominous because it is commonly associated with a distortion of the brain stem that can cause serious dysfunction evidenced by abnormal posturing and failure of the pupils to constrict in response to light 3. Midline shift is often associated with high intracranial pressure (ICP), which can be life-threatening 3. In fact, midline shift is a measure of intracranial pressure (ICP); presence of midline shift is an indication of raised intracranial pressure 4. Presence of midline shift is an indication for neurosurgeons to take measures to monitor and control ICP 3. Significant midline shift (> 5 mm) is a key indication for surgical management in a number of traumatic brain lesions, including extra- and subdural hematomas and traumatic parenchymal lesions 5.
The displacement of brain structures described above illustrates the Monro-Kellie doctrine, which states that in an adult the cranial volume is a constant. The cranial contents consist primarily of brain, cerebrospinal fluid (CSF) and blood vessels. If a mass such as a hematoma, tumor or edema develops, these elements must shift to accommodate the mass. Since the cranial volume is a constant, part of the cranial contents will herniate through the tentorial incisura to make room for the mass. The opposite occurs in a patient with loss of brain mass, such as occurs after a stroke, wherein the CSF spaces often enlarge to fill the void.
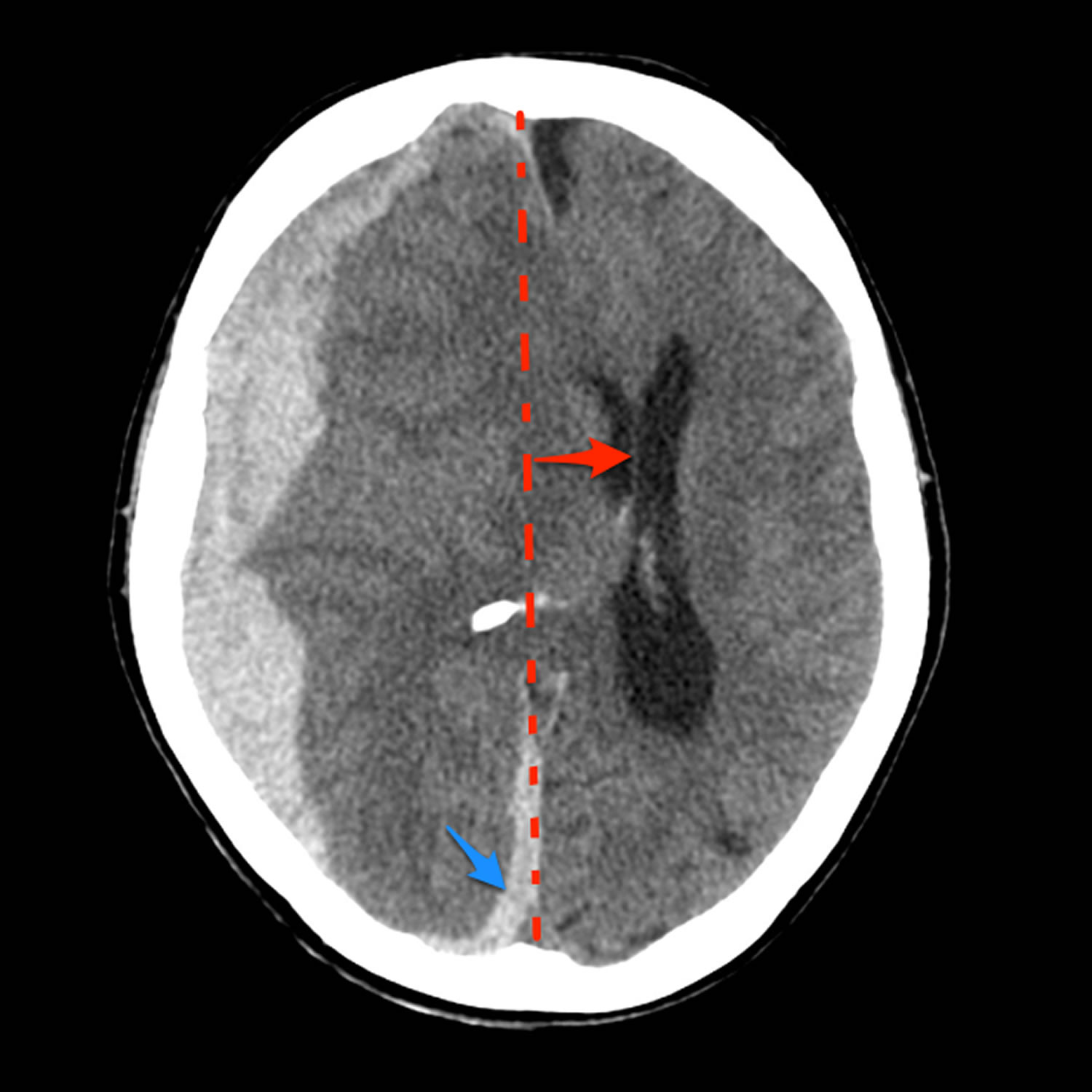
Figure 1. Midline shift
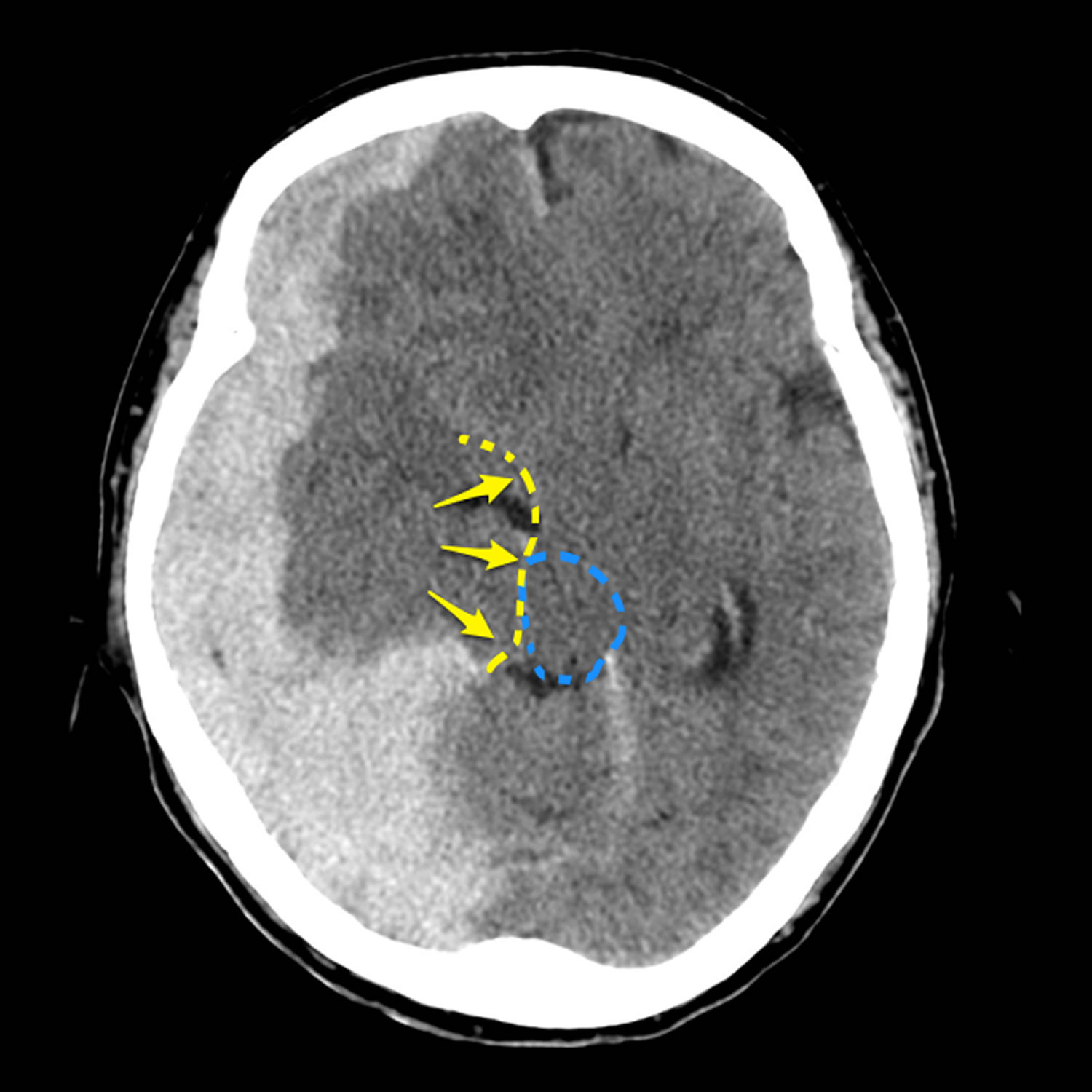
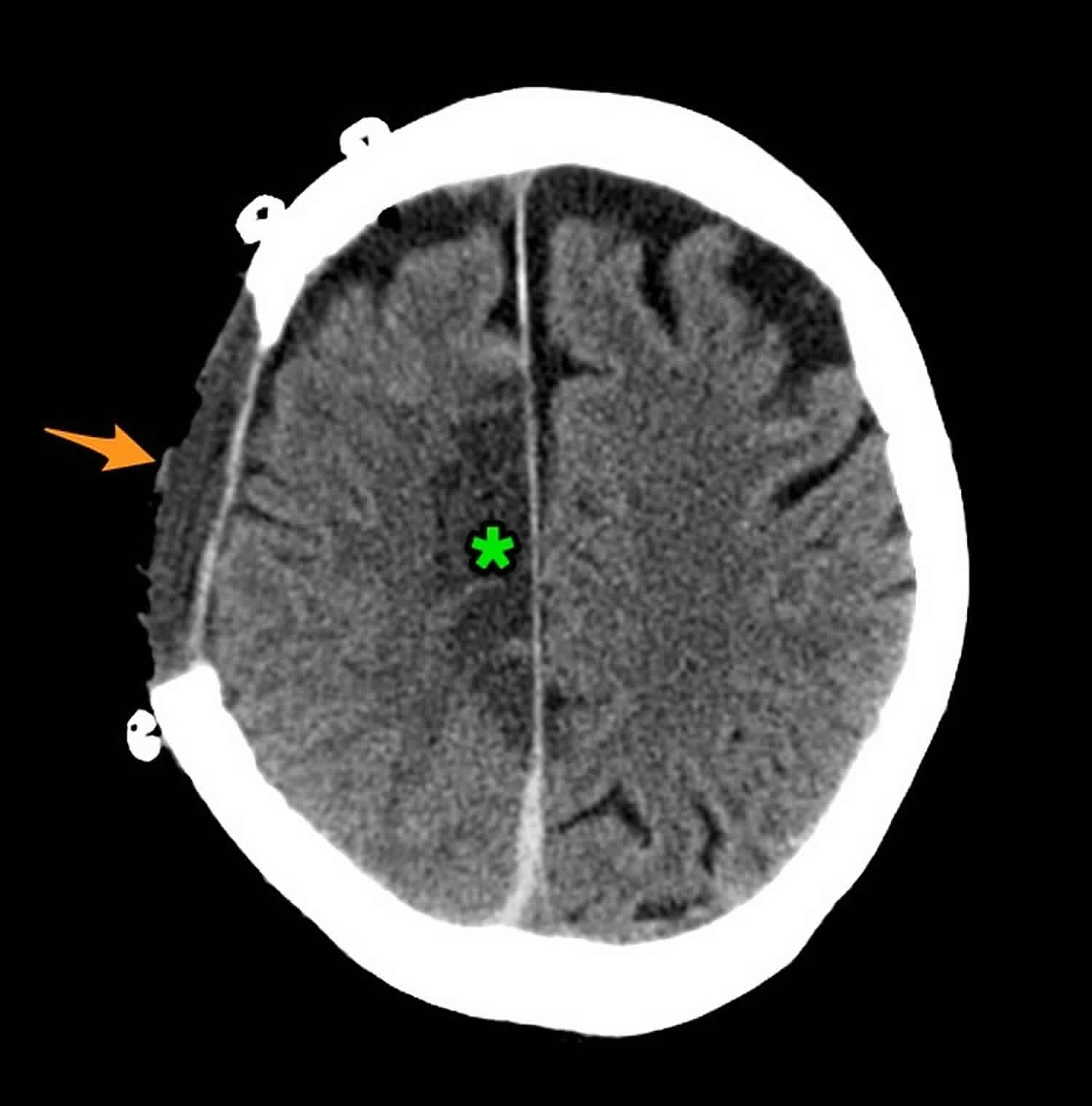
Footnote: 80 year old female with altered conscious state following fall. Patient on anticoagulation (warfarin) for atrial fibrillation. The huge acute subdural overlying the right cerebral hemisphere as well as filling the floor of the middle cranial fossa and tracking along the posterior aspect of the falx (blue arrow), results in marked mass effect and midline shift (red). There is marked uncal herniation (yellow) with significant distortion of the midbrain (blue dotted line). Following the craniectomy (orange arrow) and evacuation of the hematoma, an anterior cerebral artery infarct has developed on the right (green *). This case illustrates just how large acute subdural hemorrhages can be, and that with prompt surgery patients can be saved. In this case the degree of midline shift was such that a right sided anterior cerebral artery infarct developed.
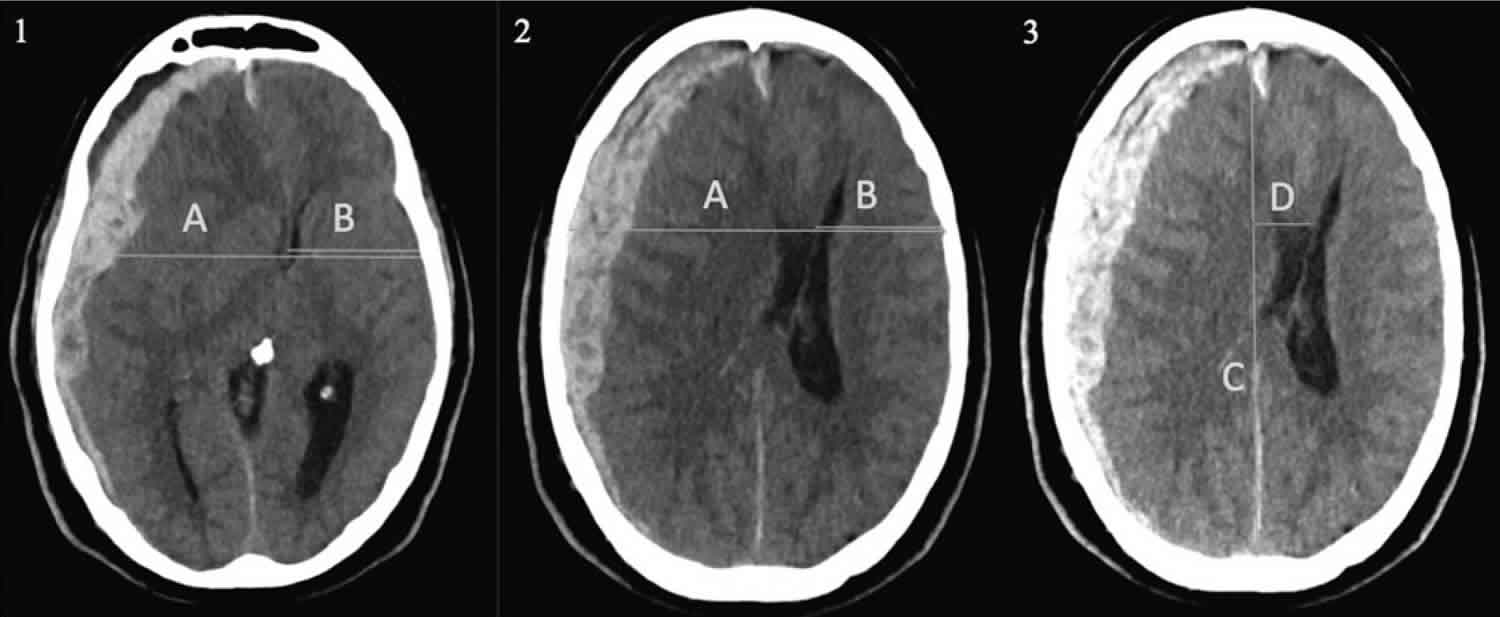
[Source 6 ]Figure 2. Different ways to measure midline shift
Footnote: (1) The (A/2-B) method according to Bullock and colleagues 7, where A is the width of the intracranial space and B is the distance from the tabula interna to the septum pellucidum at the foramen of
Monro (midline shift= 1.44 cm). (2) The (A/2-B) method where shift is measured at the foramen of Monro, or alternatively at the site of largest displacement, according to the Common Data Elements (TBI-CDE) (midline shift= 2.01 cm). (3) A third method, which is commonly used in routine radiological practice. The ideal midline C is determined as the line between the most anterior and posterior part of the falx cerebri. Line D is drawn to the septum pellucidum and is then calculated as shift (midline shift= 1.94 cm).
Midline shift brain causes
Midline shift brain causes include:
- Head trauma (epidural hematoma, subdural hematoma, intracerebral hemorrhage or contusions)
- The leading causes of head trauma are (1) motor vehicle-related injuries, (2) falls, and (3) assaults 9.
- Based on the mechanism, head trauma is classified as (1) blunt (most common mechanism), (2) penetrating (most fatal injuries), (3) blast.
- Most severe traumatic brain injuries result from motor vehicle collisions and falls.
- Brain tumors
- Stroke
- Nontraumatic intracerebral hemorrhage (aneurysm rupture)
- Idiopathic or benign intracranial hypertension
- Hydrocephalus
- Meningitis
Midline shift brain symptoms
Symptoms of elevated intracranial hypertension are primarily derived from neurological irritation, compression, or displacement and papilledema. Non-specific headaches are recorded in almost all cases and are likely mediated via the pain fibers of the trigeminal nerve in the dura and blood vessels of the brain. Pain is generally diffuse and worse in the mornings with exacerbation by the Valsalva maneuver. Nausea and vomiting are common presentations of elevated intracranial pressure. Patients can present with double vision most frequently with horizontal diplopia associated with CN VI palsy from compression. Transient visual abnormalities occur frequently, often described as a gradual dimming of vision in one or both of the eyes. Visual abnormalities worsen with changes in posture. Peripheral visual loss may be reported and most commonly begins in the nasal inferior quadrant with subsequent loss of the central visual field. Alterations in visual acuity with blurring or distortion may occur. Variable degrees of loss of color distinction may occur. In more severe or chronic cases, a sudden visual loss can occur due to intraocular hemorrhage. Tinnitus with a pulsing rhythm exacerbated by supine or bending positions and Valsalva maneuver can occur. Radicular pain, numbness, or paresthesias are possible and most commonly associated with localized compression or possible herniation of the brain. Neurological findings are indications of severe disease. The anatomical locations where herniation is most likely to occur include the subfalcine, central transtentorial, uncal transtentorial, cerebellar tonsillar/foramen magnum, and transcalvarial lobes. These types of changes may lead to decreased consciousness or responsiveness. Focal neurological constellations depend on which region of the brain has herniated. Often this results in a stupor state or more severely with coma due to the local effect of mass lesions or pressure on the reticular formations of the midbrain. It may further lead to respiratory compromise.
Physical exam findings can vary widely depending on etiology. A change in mental status or comatose patient should prompt urgent evaluation. A complete neurological assessment is essential whenever intracranial hypertension is suspected. Cranial nerve assessment is particularly important for identifying lesions. Cranial nerve VI palsy is most common. Blunting of the pupillary reflex with fixed dilation of one pupil is also highly associated with herniation syndromes. Spontaneous periorbital bruising may be present as well. A classic triad of bradycardia, respiratory depression, and hypertension is known as Cushing’s triad and is highly indicative of intracranial hypertension. Fundoscopic examination looking for retinal hemorrhages or papilledema is essential. Alterations in respiratory drive and effort may occur leading to failure of respiration and oxygenation.
Infants can have widening of cranial sutures and bulging fontanelle.
Midline shift brain diagnosis
Complete blood count (CBC) and complete metabolic panel (CMP) are usually checked in all patients with midline shift or suspected raised intracranial pressure to evaluate for infection, anemia, and electrolyte abnormalities. Initial evaluation should include a head CT scan. CT scan findings of cerebral edema such as compressed basal cisterns and midline shift are predictive of elevated ICP. However, the absence of these findings does not rule out intracranial hypertension. A head MRI is more accurate than head CT in evaluating elevated intracranial pressure and to looking for potential etiology. Bedside ultrasonography also can be used to measure the diameter of the optic nerve sheath to determine intracranial hypertension. However, this study is limited by operator skill and not frequently used. A lumbar puncture may sometimes be needed for diagnosis. However, it should be delayed until neuroimaging, especially in those with suspicion of impending herniation. When lumber puncture is performed, in addition to measuring opening pressures, CSF should also be tested for infection and other potential etiology. Invasive measurement of intracranial pressure is definitive for diagnosis and improves the physician’s ability to maintain adequate cerebral perfusion pressure. There are 4 main anatomical sites used for clinical measurement of intracranial pressure: intraventricular, intraparenchymal, subarachnoid, and epidural. Ventriculostomy catheter is preferred device for intracranial pressure monitoring and can be used even for therapeutic CSF drainage to lower intracranial pressure. When ventricles cannot be cannulated, intraparenchymal devices using microsensor and fibreoptic transducer may be used. Subdural and epidural monitors are not as accurate as ventriculostomy and parenchymal monitors 10.
Midline shift brain treatment
A sudden increase in intracranial pressure is a neurosurgical emergency, requiring close monitoring in an intensive care unit (ICU) setting. For acute intracranial hypertension, a patient should first be stabilized with healthcare professionals aiming for hemodynamic stability, and preventing and treating factors that may aggravate or precipitate intracranial hypertension. These patients should have close monitoring of heart rate, blood pressure, body temperature, ventilation and oxygenation, blood glucose, input and output, and ECG. Patients with suspected intracranial hypertension, especially with severe traumatic brain injury, should also have intracranial pressure monitoring 11.
It is vital to prevent and treat factors that may aggravate or precipitate intracranial hypertension 12. These interventions are used to buy time until the underlying etiology is identified and corrected.
- Keep the head elevated to 30 degrees and neutrally positioned to minimize venous outflow resistance and improve cerebral spinal fluid displacement from the intracranial to the spinal compartment.
- Hypoxia and hypercapnia can increase intracranial pressure. Controlling intracranial pressure through optimal respiratory management is crucial. It is essential to control ventilation to maintain a normal PaCO2 and maintain adequate oxygenation without increasing the PEEP.
- Agitation and pain can increase blood pressure and intracranial pressure. Adequate sedation and analgesia is an important adjunctive treatment. Since most sedating medications can have effects on blood pressure, medications with minimal hypotensive effect should be preferred. Hypovolemia can precipitate the hypotensive side effects and should be treated before administering sedative agents. Shorter-acting agents have the advantage of allowing brief interruption of sedation to evaluate neurological status.
- Fever can increase brain metabolic rate and is a potent vasodilator, which in turn, increase the cerebral blood flow and increased intracranial pressure. Fever should be controlled with antipyretics and cooling blankets and infectious causes must be ruled out.
- Elevated blood pressure is commonly seen in patients with intracranial hypertension especially when due to traumatic brain injury. In patients with untreated intracranial mass lesions, cerebral perfusion is maintained by the higher blood pressure, and systemic hypertension should not be treated. The absence of an intracranial mass lesion presents a more individualized, controversial decision when treating systemic hypertension. When antihypertensive are used, the preferred treatment includes beta-blockers like labetalol and esmolol or calcium channel blockers because they reduced blood pressure without affecting intracranial pressure. Agents with short half-lives should be preferred. Avoid vasodilators like sodium nitroprusside, nitroglycerin, and nifedipine.
- Seizures can contribute and complicate elevated intracranial pressure and should be prevented by prophylactic medications, especially in severe traumatic brain injuries.
For patients with sustained intracranial hypertension, additional measures are needed to control the intracranial pressure 12.
- Emergent surgical management should be considered when there is sudden intracranial hypertension, or it is refractory to medical management.
- Nondepolarizing muscle relaxants along with sedatives may be used to treat intracranial hypertension caused by posturing, coughing or agitation. When a neuromuscular blockade is used, EEG should be monitored to rule out convulsive states.
- Hyperosmolar therapy is used for severe, acute intracranial hypertension.
Mannitol is commonly used as a hyperosmolar agent and is usually given as a bolus of 0.25 to 1 g/kg body weight 12. Serum osmolality should be kept less than 320 mOsm to avoid side effects of therapy like renal failure, hypokalemia, and hypo-osmolarity.
Hypertonic saline can also create an osmotic shift from the interstitial space of brain parenchyma into the intravascular compartment in the presence of an intact blood-brain barrier. Hypertonic saline has an advantage over mannitol for hypovolemic and hypotensive patients. Adverse effects of hypertonic saline administration include hematological and electrolyte abnormalities. Hyponatremia should be excluded before administering hypertonic saline to reduce the risk of central pontine myelinolysis 12.
- Hyperventilation can be used for rapid reduction in intracranial pressure if there are clinical signs of herniation or with severe intracranial hypertension. Hyperventilation decreases PaCO2 which causes vasoconstriction of cerebral arteries, resulting in reduced cerebral blood flow and reduced intracranial pressure.
- Barbiturate coma should be considered for patients with refractory intracranial hypertension.
- Routine induction of hypothermia is not indicated; however, moderate hypothermia may be an effective adjunctive treatment for increased intracranial pressure refractory to other medical management.
- Steroids are commonly used for primary and metastatic brain tumors to decrease vasogenic cerebral edema. For other neurosurgical disorders like traumatic brain injury or spontaneous intracerebral hemorrhage, steroids have not been shown to have a benefit, and sometimes may even be detrimental.
Surgical interventions
- Resection of intracranial mass lesions producing elevated intracranial pressure should be done as soon as possible.
- CSF drainage lowers intracranial pressure immediately by reducing intracranial volume. This modality can be an important adjunct treatment for lowering intracranial pressure. However, it has limited utility when the brain is diffusely swollen and the ventricles are collapsed.
- Decompressive craniectomy is used to treat severe uncontrolled intracranial hypertension. It involves surgical removal of part of the calvaria to create a window in the skull, allowing for herniation of swollen brain through the bone window to relieve pressure.
Epidural hematoma
Epidural hematoma is a neurosurgical emergency. It, therefore, requires urgent surgical evacuation to prevent irreversible neurological injury and death secondary to hematoma expansion and herniation. A neurosurgical consultation should be the urgently obtained as it is important to intervene within 1 to 2 hours of presentation 13.
The priority is to stabilize the patient, including the ABCs (airway, breathing, circulation), and these should be addressed urgently.
Surgical intervention is recommended in patients with:
- Acute epidural hematoma
- Hematoma volume greater than 30 ml regardless of Glasgow coma score (GCS)
- Glasgow coma score less than 9 with pupillary abnormalities like anisocoria
Operative management
In patients with acute and symptomatic epidural hematomas, the treatment is craniotomy and hematoma evacuation. Based on the available literature, “trephination” (or burr hole evacuation) is often a crucial form of intervention if more advanced surgical expertise is unavailable; it may even decrease mortality. However, the performance of a craniotomy, if feasible, can provide a more thorough evacuation of the hematoma.
Non-operative management
There is a scarcity of literature comparing conservative management with surgical intervention in patients with epidural hematoma. However, a non-surgical approach may be considered in a patient with acute epidural hematoma who has mild symptoms and meets all of the criteria listed below:
- Epidural hematoma volume of less than 30 ml
- Clot diameter of less than 15 mm
- Midline shift of less than 5 mm
- Glasgow coma score greater than 8 and on physical examination, shows no focal neurological symptoms.
If the decision is made to manage acute epidural hematoma non-surgically, close observation with repeated neurological examinations and continuous surveillance with brain imaging is required, as the risk for hematoma expansion and clinical deterioration is present. The recommendation is to obtain a follow-up head CT scan within 6 to 8 hours following brain injury.
Midline shift brain prognosis
Midline shift brain prognosis is highly variable depending on cause and varies from benign to lethal. Children usually can tolerate higher intracranial pressure (ICP) for a longer period.
References- Midline shift (summary). https://radiopaedia.org/articles/midline-shift-summary?lang=us
- Midline shift. https://radiopaedia.org/articles/midline-shift?lang=us
- Surgical management of head trauma. Neuroimaging Clin N Am. 2002 May;12(2):339-43. https://doi.org/10.1016/S1052-5149(02)00013-8
- Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008 Aug;7(8):728-41. doi: 10.1016/S1474-4422(08)70164-9. https://doi.org/10.1016/S1474-4422(08)70164-9
- Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW. Guidelines for the surgical management of traumatic brain injury author group: acknowledgments. Neurosurgery. 2006;58(suppl_3):S2-vi. doi: 10.1093/neurosurgery/58.suppl_3.S2-vi
- Subdural hemorrhage – on warfarin. https://radiopaedia.org/cases/subdural-haemorrhage-on-warfarin-1
- Bullock, M.R., Chesnut, R., Ghajar, J., Gordon, D., Hartl, R., Newell, D.W., Servadei, F., Walters, B.C., and Wilberger, J.E. (2006). Introduction. Neurosurgery 58, S2-1–S2-3.
- Vande Vyvere, Thijs & Wilms, Guido & Claes, Lene & Leon, Francisco & Nieboer, Daan & Verheyden, Jan & van den Hauwe, Luc & Pullens, Pim & Maas, Andrew & Golubović, Jagoš & Parizel, Paul & Younsi, Alexander. (2018). Central versus Local Radiological Reading of Acute CT Characteristics in Multicentre Traumatic Brain Injury Research. Journal of Neurotrauma. 36. 10.1089/neu.2018.6061.
- Portaro S, Naro A, Cimino V, Maresca G, Corallo F, Morabito R, Calabrò RS. Risk factors of transient global amnesia: Three case reports. Medicine (Baltimore). 2018 Oct;97(41):e12723.
- Sun S, Li Y, Zhang H, Wang X, She L, Yan Z, Lu G. The effect of mannitol in the early stage of supratentorial hypertensive intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. 2018 Dec 18
- Jha RM, Kochanek PM. A Precision Medicine Approach to Cerebral Edema and Intracranial Hypertension after Severe Traumatic Brain Injury: Quo Vadis? Curr Neurol Neurosci Rep. 2018 Nov 07;18(12):105.
- Sharma S, Hashmi MF, Kumar A. Intracranial Hypertension. [Updated 2019 Sep 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507811
- Gutowski P, Meier U, Rohde V, Lemcke J, von der Brelie C. Clinical Outcome of Epidural Hematoma Treated Surgically in the Era of Modern Resuscitation and Trauma Care. World Neurosurg. 2018 Oct;118:e166-e174