What is myeloperoxidase
Myeloperoxidase is a hemoprotein enzyme expressed in azurophilic granules of neutrophils and in the lysosomes of monocytes 1. Myeloperoxidase generates numerous reactive oxidant species and has the unique ability to produce reactive chlorinating species which are particularly potent against invading viruses and bacteria 2. Myeloperoxidase has strong antibacterial properties and is unique in its ability to generate potent bactericidal compounds such as hypochlorous acid from hydrogen peroxide and the halide, chloride 3.
Neutrophils are considered as the first line of defense against pathogens, and during phagocytosis, they undergo a process termed the respiratory burst in which most bacteria are killed and digested in the phagosomes. The myeloperoxidase-enriched azurophilic granules in neutrophils fuse with the phagosome and are released into the phagosomes when the common membrane is ruptured. These neutrophils, after encountering pathogens, generate a respiratory burst via activation of NADPH oxidase that leads to the production of superoxide, hydrogen peroxide, and other reactive oxygen derivatives such as hypochlorous acid, a primary product of myeloperoxidase activity. The antimicrobial activities of myeloperoxidase-hypochlorous acid not only restricted to killing bacteria but also fungi, viruses, erythrocytes, tumor cells, natural killer (NK) cells and platelets indicates its role in the innate immune response. Reports have also shown that myeloperoxidase is involved in terminating the respiratory burst since individuals with myeloperoxidase-deficient neutrophils have a prolonged respiratory burst, and an increase in hydrogen peroxide production.
Myeloperoxidase-derived hypochlorous acid has been shown to be the required source of reactive oxygen species (ROS) for neutrophil extracellular traps (NETs), that are DNA structures released due to de-condensation of chromatin formation, that not only trap bacteria, but also regulates both the innate and adaptive immune response in many ways.
myeloperoxidase is believed to play a role in suppressing of the adaptive immune response as demonstrated by two models of enhanced T cell-mediated skin delayed-type hypersensitivity and antigen-induced arthritis in Mpo−/− mice. Mechanistically, myeloperoxidase released from neutrophils inhibits LPS-induced DC activation as measured by decreased IL-12 production and CD86 expression consequently, limiting T cell proliferation and proinflammatory cytokine production. In contrast, a pathogenic role for myeloperoxidase in driving autoimmune inflammation was demonstrated using myeloperoxidase-deficient mice in the K/BxN arthritis and collagen-induced arthritis (CIA) models exhibiting reduced disease severity. Also, increased myeloperoxidase levels and activity have been observed in many inflammatory conditions and autoimmune diseases including multiple sclerosis (MS) and rheumatoid arthritis.
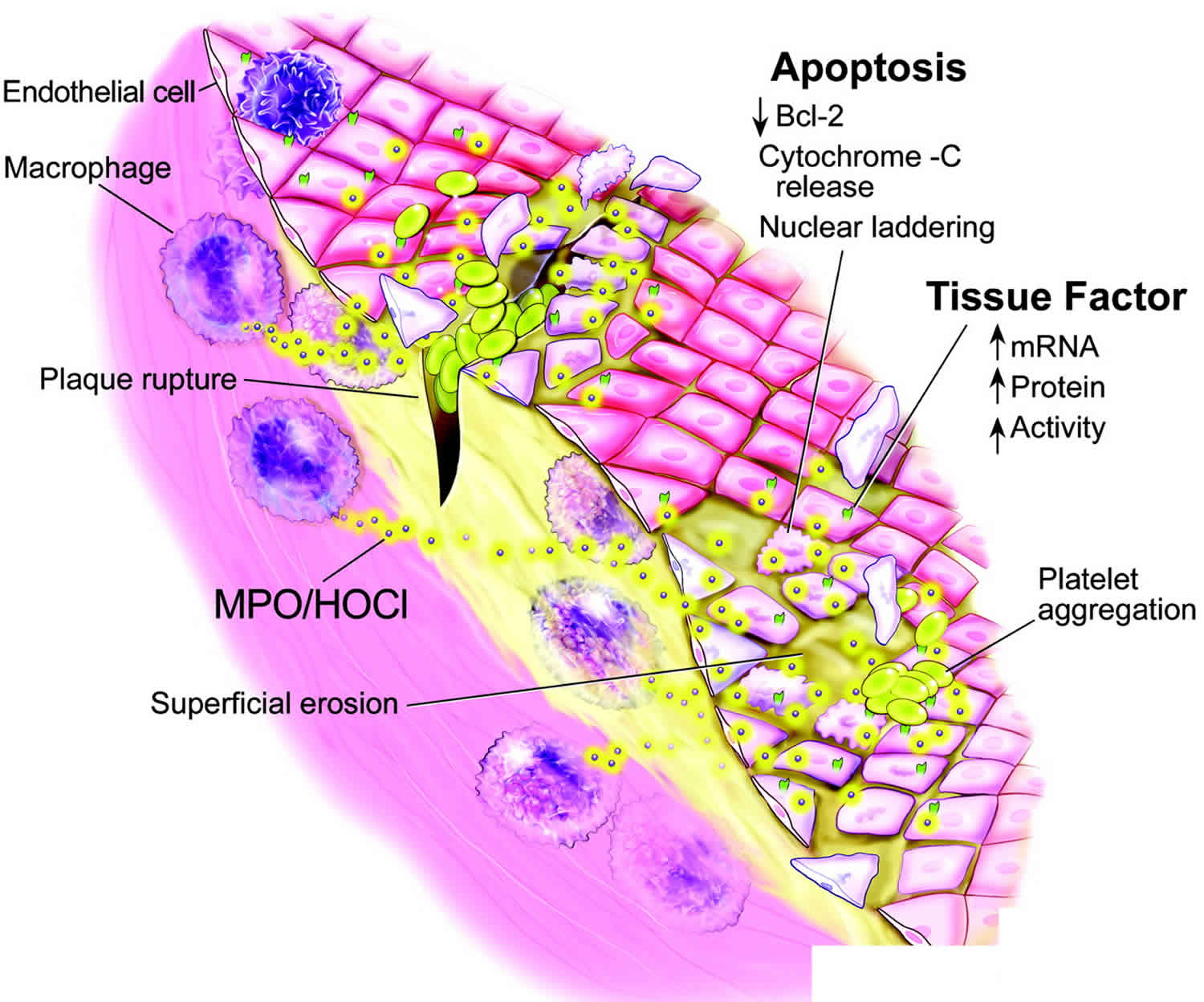
myeloperoxidase plays a role in modulation of vasculature functioning, associated with chronic vascular diseases such as atherosclerosis. In the extracellular matrix (ECM), myeloperoxidase works as a nitric oxide (NO)-scavenger consuming nitric oxide (NO) that leads to impaired endothelial relaxation. myeloperoxidase and its oxidative species present in the atherothrombotic tissue, promotes lipid peroxidation, conversion of LDL cholesterol to a highly-uptake atherogenic form, selectively modulates Apolipoprotein A-I (apoA-I) generating dysfunctional HDL particles more susceptible to degradation and impairs the ability of apoA-I to promote cholesterol efflux. Moreover, elevated systemic levels of myeloperoxidase and its oxidation products are associated with increased cardiovascular risk. However, the measurement of myeloperoxidase as a cardiovascular risk marker has not generally been accepted like the more standardized assay for high sensitive C-reactive protein 4.
Myeloperoxidase is encoded by a single gene located on band 17q22-23 is a tetramer of 150 kDa consisting of two heavy chains, two light chain subunits, and two iron atoms 1. The myeloperoxidase gene encodes for a single translational product that after glycosylation and proteolytic processing is packaged as mature myeloperoxidase in the azurophilic granules. myeloperoxidase production is determined by various genetic mutations and deletions. myeloperoxidase, once released by activated leukocytes, utilizes the oxidative potential of its co-substrate hydrogen peroxide in the peroxidation and halogenation cycles to generate the cytotoxic oxidant species and potent bactericidal compound hypochlorous acid along with other toxic oxidants that are capable of not only initiating lipid peroxidation but also promoting a series of post-translational modifications, such as halogenation, nitration, and oxidative cross-linking to target proteins at sites of inflammation. The toxicity and bactericidal activities of hypochlorous acid occur due to its potential to modify lipids, DNA, amines, and tyrosine into halohydrins, 5-chlorouracil, chloramines and 3-chlorotyrosine respectively 5.
Myeloperoxidase test
The presence of myeloperoxidase can be determined using numerous techniques, including histochemical staining, immunocytochemistry, and flow cytometry. Depending on the assay used, one must ensure that eosinophilic peroxidase from eosinophils does not cause false-positive results 6.
The easiest technique is to perform direct visualization of neutrophils on a peripheral blood smear that has been stained for peroxidase. The clinician can ask the pathologist to examine the neutrophils for peroxidase when a peripheral smear is requested 7.
Dihydrorhodamine 123 (DHR) assay, a flow cytometric assay, is often used to measure the presence of reactive oxygen intermediates in the work-up of a patient with suspected immunodeficiency. This assay is easier, more reliable, and more sensitive than nitroblue tetrazolium dye reduction assay in the diagnosis of chronic granulomatous disease. At this time, a dihydrorhodamine 123 assay should not be used as a screen for myeloperoxidase deficiency because of variable results and poor sensitivity in detecting partial myeloperoxidase deficiency. If a dihydrorhodamine 123 assay is consistent with a diagnosis of chronic granulomatous disease but the clinical history is more consistent with myeloperoxidase deficiency, further laboratory testing should be performed (eg, genetic sequencing or intracellular staining with anti-myeloperoxidase antibody) 8.
Myeloperoxidase as a prognostic tool and therapeutic target
In the early to mid-2000s, there was great interest in myeloperoxidase as an inflammatory biomarker of adverse outcome in patients with acute chest pain and for diagnosis of heart attack (myocardial infarction). A landmark paper in 2003 suggested that a single serum measurement of myeloperoxidase on presentation in patients with chest pain could identify patients at risk for subsequent cardiac events even in the absence of heart attack (myocardial infarction) and elevated troponin 9. In patients with acute ST-elevation myocardial infarction, myeloperoxidase risk stratified these patients in terms of death and repeat myocardial infarction and was shown to be a better predictor of cardiac death and myocardial infarction than high-sensitivity C-reactive protein or troponin 10. Additionally, myeloperoxidase predicted adverse event rates in systolic and diastolic heart failure, predicted progression of heart failure, and more accurately predicted the risk of endothelial dysfunction compared to high-sensitivity C-reactive protein 11. Indeed, the cumulative literature suggested that myeloperoxidase could be used to predict vulnerable plaque and risk stratify all things cardiac. However, in the years that followed these initial observations, numerous papers contradicted these findings or at best failed to corroborate them 12. Following this, interest in myeloperoxidase as a major mechanism of coronary artery disease and heart failure declined, aside from a few scattered reports.
There are likely several reasons. First, when considering therapy for any aspect of a human multigenetic and multifactorial disease process, it is important to consider the number of contributing factors that may affect the development and progression of disease. When factors contributing to plaque vulnerability, heart attack (myocardial infarction), and subsequent heart failure are taken into consideration, the possibilities are almost endless. The more contributing factors, the less likely affecting 1 of them alone will have a significant effect on disease. This multifactorial contribution is well demonstrated by studies in which outcomes are better predicted by a biomarker panel that integrates the multiple biologic pathways involved in the process 13.
Second, targeting myeloperoxidase itself may be fraught with difficulty. The neutrophil burst is critical to fighting infection and there is concern that innate immune responses will suffer if off-target effects predominate. Inhibitors of myeloperoxidase may have crossover effects that negatively affect other peroxidases, for example, thyroid peroxidase.
Myeloperoxidase deficiency
Some patients with myeloperoxidase deficiency have impaired microbial killing, but most are asymptomatic 14. Myeloperoxidase deficiency, first described in 1954 is an autosomal recessive disorder caused by mutations in the MPO gene on chromosome 17 1. Myeloperoxidase deficiency is the commonest inherited defect of phagocytes. Patients with myeloperoxidase deficiency have impaired microbial killing, but the majority are asymptomatic clinically except if they are also diabetic 15.
Most individuals with partial or total myeloperoxidase deficiency have no increased frequency of infections, probably because myeloperoxidase-independent mechanisms in the polymorphonuclear leukocytes can take over 14. In general, it is considered a relatively benign immunodeficiency and was removed from the Classification of Primary Immunodeficiency Disease by the Primary Immunodeficiency Disease Classification Committee of the International Union of the Immunologic Societies in 2005 14.
Severe infections are uncommon, occurring in fewer than 5% of patients with myeloperoxidase deficiency. If infectious disease occurs, it is usually a fungal infection (particularly candidal, such as C albicans or C tropicalis) that occurs in a patient who also has diabetes mellitus. Patients without diabetes mellitus rarely have problems, although the reason for this is unknown. Possibly, myeloperoxidase deficiency becomes clinically significant only in the presence of an additional defect in the host defense, or perhaps the myeloperoxidase-independent system is defective in some patients with diabetes mellitus.
Myeloperoxidase deficiency was initially believed to be very rare with only 17 cases had been reported up to 1979. In the United States and Europe, an estimated frequency is 1 per 2,00 to 4,000 individuals, and in the Japanese population, it is 1 per 55,000 people. However, modern diagnostic techniques have provided a better approach to the diagnosis of myeloperoxidase deficiency, and it is a more common condition than initially believed 14.
Physicians should entertain the diagnosis of myeloperoxidase deficiency in cases of invasive fungal infection in a patient with no known predisposing immune defects (eg, chemotherapy, corticosteroid treatment) or in a patient with concomitant diabetes mellitus. Some consider peroxidase staining of the peripheral blood smear to be part of the complete evaluation of a patient with a suspected immunodeficiency.
What causes myeloperoxidase deficiency?
Primary myeloperoxidase deficiency is inherited as an autosomal recessive disorder and is generally present with varying degree of severity and clinical features. A number of point germline mutations resulting in myeloperoxidase deficiency have been detected in primary form. Some of these mutations are associated with the defective posttranslational processing of the myeloperoxidase precursor protein and others with pre-translational defects caused by mutations in the regulatory portion of the myeloperoxidase gene. Most of the mutations associated with hereditary form are R569W (the commonest), Y173C, M251T, G501S, and R499C and a deletion of 14 bases (D14) within exon 9. It is noteworthy that eosinophils are never involved in myeloperoxidase deficiency since eosinophil peroxidase is encoded by another gene than myeloperoxidase.
Secondary myeloperoxidase deficiency is less common than the hereditary form but may develop due to somatic mutations of the myeloperoxidase gene. In most cases, the deficiency is partial and affects only a proportion of neutrophils. The acquired form is usually transient and generally resolves once the underlying condition improves 16.
The various disorders that can lead to acquired or secondary myeloperoxidase deficiency include diabetes mellitus, pregnancy, iron deficiency, renal transplantation, thrombotic diseases, lead poisoning, obstructive jaundice, disseminated cancers, hematologic disorders and neoplasms such as acute and chronic myeloid leukemia, myelodysplastic syndrome, polycythemia vera, Hodgkin lymphoma, severe infections, cytotoxic agents, and some anti-inflammatory drugs like sulfapyridine 16.
Myeloperoxidase deficiency symptoms
The majority of patients with myeloperoxidase deficiency are asymptomatic with no increase in infections. However recurrent severe infections with Candida Albicans have been observed only in patients who also suffered from other conditions such as diabetes mellitus. Thus, in these patients, it is not clear if the infections were a mere outcome of myeloperoxidase deficiency or if other myeloperoxidase independent mechanisms were also responsible. In a study conducted by European researchers, only 50% of the complete myeloperoxidase deficient patients had infectious complications, the rest were asymptomatic, and only 10% of the patients suffered from life-threatening infectious complications. The lack of serious infections in myeloperoxidase deficiency compared to chronic granulomatous disease emphasizes the importance of the reactive oxygen species such as amplified hydrogen peroxide generation serving as a potent bactericidal agent that protects patients with myeloperoxidase deficiency. However, the absence of myeloperoxidase-mediated species such as hypochlorous acid appears to be crucial in the failure to abort fungal infections such as Candidiasis.
Myeloperoxidase deficiency diagnosis
The first step in diagnosing the myeloperoxidase deficiency is determination of peroxidase activity by histochemical staining of leukocytes, immunocytochemistry, or, more commonly, flow cytometry which allows assessment of functional myeloperoxidase within neutrophils. Immunoblotting of isolated leukocytes for myeloperoxidase protein also provides additional information into the molecular basis of the observed absence of functional enzyme independent of its enzymatic activity.
Differential diagnosis of myeloperoxidase deficiency from disorders presenting with similar clinical signs and symptoms includes Chediak-Higashi syndrome, Hyperimmunoglobulinemia E, leukocyte adhesion deficiency, neutropenia, neutrophil actin dysfunction, lazy leukocyte syndrome and any condition that can cause secondary myeloperoxidase deficiency. In any patient with disseminated fungal infections, myeloperoxidase deficiency should be considered as a differential.
Myeloperoxidase deficiency treatment
Generally, since most individuals with myeloperoxidase deficiency do not suffer from infections and are typically asymptomatic, prophylactic antibiotics are discouraged and not indicated. However, caution should be taken in patients afflicted with concomitant diabetes mellitus, with a high incidence of localized and systemic infections, where prompt and aggressive treatment with antimicrobials is usually necessary to control infections. It is prudent and cautious to avoid treatments that can increase the propensity of fungal infections such as prolonged use of antibiotics or steroids.
Myeloperoxidase deficiency prognosis
A group from Europe who studied patients with complete myeloperoxidase deficiency found that about half had infectious complications, while the other half were asymptomatic. Infectious complications were life threatening in about 10% of cases.
Others have reported severe infections occurring in fewer than 5% of patients with myeloperoxidase deficiency (this frequency may be based on the inclusion of both complete and partial deficiencies). Infections generally occur only in patients who have concomitant diabetes mellitus.
Increased incidence of malignancy
A strong association between total myeloperoxidase deficiency and malignancies has been reported by several independent investigators. In vitro, myeloperoxidase-deficient neutrophils have decreased destruction of malignant cells demonstrating that the myeloperoxidase system plays a central role in tumor surveillance 17.
myeloperoxidase is released from neutrophils in lung tissue in response to pulmonary insult including damage secondary to tobacco smoke exposure. myeloperoxidase has been shown to convert the metabolites of benzopyrene from tobacco smoke into a highly reactive carcinogen. Researchers have demonstrated that decreased myeloperoxidase can decrease lung cancer risk 18.
References- Pahwa R, Jialal I. Myeloperoxidase Deficiency. [Updated 2019 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470278
- Zhang R., Shen Z., Nauseef W.M., et al. (2002) Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem 277:46116–2.
- Xiao X, Saha P, Yeoh BS, Hipp JA, Singh V, Vijay-Kumar M. Myeloperoxidase deficiency attenuates systemic and dietary iron-induced adverse effects. J. Nutr. Biochem. 2018 Dec;62:28-34.
- Shiohara M, Komiyama A. [Myeloperoxidase deficiency]. Ryoikibetsu Shokogun Shirizu. 2000;(32):183-5.
- Nauseef WM. Diagnostic assays for myeloperoxidase and myeloperoxidase deficiency. Methods Mol. Biol. 2014;1124:537-46.
- Nauseef WM. Diagnostic assays for myeloperoxidase and myeloperoxidase deficiency. Methods Mol Biol. 2014. 1124:537-46.
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007 Oct. 219:88-102.
- Mauch L, Lun A, O’Gorman MRG, et al. Chronic granulomatous disease (CGD) and complete myeloperoxidase deficiency both yield strongly reduced dihydrorhodamine 123 test signals but can be easily discerned in routine testing for CGD. Clin Chem. Mar 2007. 53:890-896.
- Brennan M.L., Penn M.S., Van Lente F., et al. (2003) Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med 349:1595–1604.
- Khan S.Q., Kelly D., Quinn P., et al. (2007) Myeloperoxidase aide prognostication together with N-terminal pro-B-type natriuretic peptide in high-risk patients with acute ST elevation myocardial infarction. Heart 93:826–831.
- Tang W.H., Tong W., Troughton R.W., et al. (2007) Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol 49:2364–2370.
- Rudolph V., Keller T., Schultz A., et al. (2012) Diagnostic and prognostic performance of myeloperoxidase plasma levels compared with sensitive troponins in patients admitted with acute onset chest pain. Circ Cardiovasc Genet 5:561–568.
- Ky B., French B., Levy W.C., et al. (2012) Multiple biomarkers for risk prediction in chronic heart failure. Circ Heart Fail 5:183–190.
- Myeloperoxidase deficiency. https://emedicine.medscape.com/article/887599-overview
- Milligan KL, Mann D, Rump A, Anderson VL, Hsu AP, Kuhns DB, Zerbe CS, Holland SM. Complete Myeloperoxidase Deficiency: Beware the “False-Positive” Dihydrorhodamine Oxidation. J. Pediatr. 2016 Sep;176:204-6.
- Aratani Y, Miura N, Ohno N, Suzuki K. [Role of neutrophil-derived reactive oxygen species in host defense and inflammation]. Med Mycol J. 2012;53(2):123-8.
- Lanza F. Clinical manifestation of myeloperoxidase deficiency. J Mol Med. 1998 Sep. 76(10):676-81.
- Taioli E, Benhamou S, Bouchardy C, et al. Myeloperoxidase G463A polymorphism and lung cancer: a HuGE genetic susceptibility to environmental carcinogens pooled analysis. Genet Med. Feb 2007. 9:67-73.