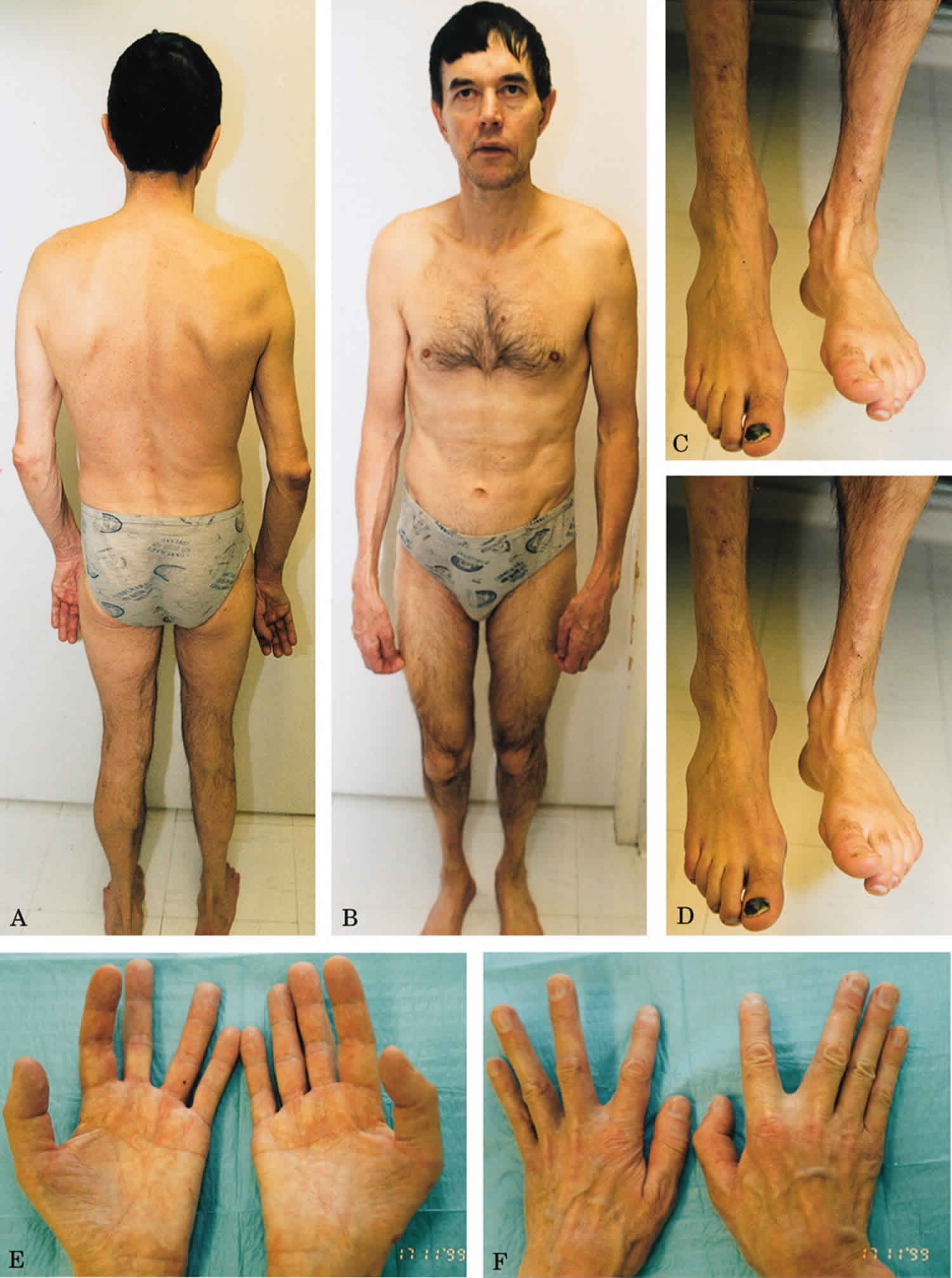
What is myopathy
Myopathy means “disease of muscle.” More specifically, myopathies are diseases that cause problems with the tone and contraction of skeletal muscles (muscles that control voluntary movements). Myopathy can cause pain, stiffness (called myotonia) to weakness even paralysis, with different degrees of severity. Some myopathies, especially when they’re present from birth (congenital myopathy), have life-threatening complications. But, with time and physical therapy, some people born with myopathies can gain muscle strength. Others often can manage their symptoms through medication, lifestyle modifications, or use of orthopedic and respiratory equipment.
Myopathy types
Alcoholic myopathy
Alcoholic myopathy occurs in 40 to 60 percent of chronic alcoholics 1. Although alcohol-related muscle disease is nearly 5 times more common than liver cirrhosis (which is present in 10 to 15 percent of people with alcohol use disorder), data are lacking on its contribution to long-term health and disease in patients with alcohol use disorder 2.
Acute alcoholic myopathy is present in 0.5 to 2.0 percent of alcoholics, with an estimated overall prevalence of 20 cases per 100,000 people in the Western Hemisphere 3. Chronic alcoholic myopathy is one of the most common types of myopathy, with an overall prevalence of 2,000 cases per 100,000 people. Based on these prevalence estimates, chronic alcohol-related myopathy is 10 times more common than the most common inherited myopathy (i.e., nemaline myopathy), which has a prevalence of 200 cases per 100,000 individuals, and 67 to 1,000 times more common than Duchenne’s muscular dystrophy with an estimated prevalence of 2 to 30 per 100,000 people 3. However, it is difficult to ascertain the exact prevalence, because the spectrum of clinical disease in alcohol-related myopathy varies 2. In a study of alcoholics without a known diagnosis of myopathy, up to 46 percent exhibited myopathic changes on muscle biopsies and presented with demonstrable reductions in strength compared with healthy control subjects 4. The role of this subclinical disease in the development of future clinically evident symptoms remains poorly understood.
The presence of liver cirrhosis also may influence the development of myopathy in people with alcohol use disorder, because patients with cirrhosis secondary to chronic alcohol consumption commonly manifest muscle wasting 5. In a study of chronic alcoholic men, lean muscle mass was significantly lower in those with cirrhosis than in those without cirrhosis 4. Lifetime ethanol consumption was an independent predictor of greater muscle loss among this population 6. Recent studies also suggest that the loss of muscle mass and strength associated with aging (i.e., sarcopenia) is more prevalent with advancing stages of cirrhosis and frequently occurs even in the absence of concomitant alcohol use 7.
Alcoholic myopathy signs and symptoms
Clinically, acute alcoholic myopathy is characterized by weakness, pain, tenderness, and swelling of affected muscles 5. Alcoholic myopathy often occurs after an alcohol binge characterized by consumption of 4 to 5 alcoholic drinks during a single episode, resulting in blood alcohol levels of 0.08 g/dL or above, and resolves within 1 to 2 weeks of abstinence from alcohol 8. A common manifestation of acute alcoholic myopathy is a breakdown of muscle tissue and release of muscle-fiber content into the blood (i.e., rhabdomyolysis). It most severely affects muscles close to the body’s midline (i.e., proximal muscles), primarily the pelvic and shoulder girdles, in a focal and asymmetric fashion. Clinical evidence of this type of myopathy may be associated with laboratory evidence of muscle injury, accompanied by elevations in the enzyme creatinine kinase and the protein myoglobin that is found in heart and skeletal muscle. This so-called rhabdomyolytic variant of acute alcoholic myopathy, which in severe cases may precipitate acute renal failure, represents the most common nontraumatic cause of rhabdomyolysis in hospitalized patients 9.
Conversely, chronic alcoholic myopathy—the most frequent presentation of alcohol-related myopathy—presents with progressive proximal muscle weakness over weeks to months. Infrequently, patients experience pain, local muscle atrophy, muscle twitching, and/or muscle tightness (i.e., myotonia) 9. Chronic alcoholic myopathy is uncommon in patients under the age of 30 9. Evidence of myopathy is associated with cumulative lifetime consumption of alcohol, with changes most evident with long-term, high-dose consumption (>10 kg of pure alcohol/kg of body weight) 9. Thus, chronic alcohol-related myopathy occurs most commonly among people ages 40 to 60, with equal distribution between men and women. Chronic alcoholic myopathy has a higher incidence in patients with evidence of other alcohol-related organ dysfunction, occurring in 50 percent of patients with liver cirrhosis and 82 percent of those with alcohol-related heart muscle disease (i.e., cardiomyopathy) 9. Clinical series also indicate that patients with chronic alcoholic myopathy may be predisposed to presenting with episodes of acute alcoholic skeletal myopathy. Up to 30 to 46 percent of patients with a history of chronic alcohol abuse report episodic muscle pain (i.e., myalgia), weakness, and darkening of urine following an alcohol binge 9.
Human studies have demonstrated significant reductions in muscle mass associated with chronic alcohol consumption. Computerized tomography imaging of a region of the lower back (i.e., the L4 vertebrae) in a small cohort of chronic alcoholic subjects demonstrated a significantly reduced muscle area compared with healthy control subjects 10. Similar results were found by Kvist and colleagues 11 who noted significantly reduced femoral and gluteal muscle areas in chronic alcoholics, even though total lean body mass as determined from total potassium content did not differ significantly.
In contrast to the findings on muscle-mass changes in chronic alcoholics, analyses of the impact of alcohol consumption and abuse on exercise capacity have yielded conflicting results. Exposure to low doses of alcohol produced no effect on peak exercise capacity in healthy participants undergoing cycle ergometry or treadmill testing 12. Similarly, reductions in peak strength measured by dynamometry were evident with moderate alcohol consumption, but not in individuals reporting lower alcohol consumption. However, consumption of higher doses of alcohol before exercise resulted in prolonged exercise times and failure to reach maximum oxygen consumption in healthy subjects 13. Thus, alcohol may have a dose-dependent effect on exercise-induced muscle changes 14. Finally, studies of postexercise muscle function suggest that alcohol consumption may impair normal muscle remodeling after exercise-induced injury 14.
Although an increasing number of studies are investigating the effects of alcohol use in healthy athletes, few studies have evaluated the long-term impact of chronic heavy alcohol consumption on muscle function. In survey studies, up to 15 percent of patients with alcohol use disorder reported significant mobility impairments that occurred more frequently with greater alcohol disease severity and with the presence of alcohol-related comorbidities 15. Studies of male patients enrolled in alcohol treatment programs also showed alterations in exercise capacity. For example, compared with age-adjusted control subjects, detoxified alcoholics demonstrated significant reductions in isokinetic torque, work, and power as well as isometric and isotonic muscle loading 16. Reductions in maximal isometric voluntary force measured by knee extension also were more pronounced in older recovering alcoholics 17.
Alcoholic myopathy treatment
Currently, the only known effective treatment for alcoholic myopathy is complete abstinence from alcohol 5. Fortunately, up to 85 percent of patients with biopsy-proven alcoholic myopathy demonstrate objective functional improvement in muscle strength within the first year of alcohol-drinking cessation and complete normalization of strength by the fifth year of abstinence 18. Even for patients unable to completely abstain from alcohol, reduced cumulative alcohol consumption results in improvements in muscle strength over time 18. Acute alcoholic myopathy usually reverses within days or weeks of abstinence, whereas chronic myopathic changes usually resolve within 2 to 12 months 19. Moreover, nutritional optimization, including correction of vitamin and electrolyte deficiencies, is associated with greater improvement of muscle health 9.
Physiotherapy often is recommended in patients with acute or chronic alcoholic myopathy, although its benefit on myopathy resolution has not been studied rigorously. Use of physical-activity interventions, primarily aerobic exercise and/or resistance-training activities, has been shown to improve exercise capacity in alcohol use disorder patients 20. Although previous randomized controlled trials of exercise were not limited to patients with alcohol-related myopathy, they demonstrated significant improvements in maximal oxygen consumption and baseline heart rate in individuals with alcohol use disorder subjected to standardized exercise interventions compared with control subjects 20. Additional studies are needed to understand whether exercise interventions are particularly beneficial to patients with alcohol use disorder and alcohol-related myopathy and to further elucidate the effects of exercise on alcohol-related muscle changes, particularly at the cellular and molecular levels.
Novel therapeutic agents increasingly are being explored for treatment of myopathic disease. Although to date many of these have not been studied in alcohol-related myopathy, they present exciting targets for potentially ameliorating the substantial burden of alcoholic myopathy. Agents targeting hormonal pathways, muscle-injury pathways, and vitamin deficiencies related to muscle disease are being actively investigated. Studies stimulating the growth hormone axis using injection of IGF-1 and IGF-1 binding complex in alcohol-fed rats have achieved restoration of muscle protein synthesis to basal control values 21. Other studies found that oral or intravenous administration of ghrelin, an upstream regulator of the growth hormone/mTOR axis, can help maintain lean muscle mass in patients with wasting (i.e., cachexia) related to cancer or chronic lung disease 22. Treatment with agents that can inhibit myostatin function (i.e., myostatin antagonists) in heart failure and sarcopenia models resulted in a reduction in Smad signaling, preventing loss of muscle mass 23. Active trials of pharmacologic myostatin antagonists are in early-stage clinical investigations. Finally, there are conflicting data regarding the use of selective androgen receptor modulators to maintain muscle mass. Early (i.e., Phase 1 and 2) clinical studies of these agents in patients with cancer and age-related sarcopenia showed increased lean muscle mass; however, these findings could not be replicated in larger Phase 3 trials 24. Additional studies are needed to understand the role of selective androgen receptor modulators as single-drug or combination therapy for muscle wasting.
Inflammatory myopathy
Inflammatory myopathies are a group of diseases that involve chronic (long-standing) muscle inflammation, muscle weakness, and, in some cases, muscle pain. Myopathy is a general medical term used to describe a number of conditions affecting the muscles. All myopathies cause muscle weakness.
The inflammatory myopathies are rare and can affect both adults and children. Dermatomyositis is the most common chronic form in children. Polymyositis and dermatomyositis are more common in females while inclusion body myositis affects more men. Inclusion body myositis usually affects individuals over age 50.
The four main types of chronic, or long-term, inflammatory myopathies are:
- Polymyositis
- Dermatomyositis
- Inclusion body myositis
- Necrotizing autoimmune myopathy
Inflammatory myopathy causes
General muscle inflammation or myositis, may be caused by:
- autoimmune disorders in which the immune system attacks muscle
- an allergic reaction following exposure to a toxic substance or medicine
- a virus or other infectious organism such as bacteria or fungi
Although the cause of many inflammatory myopathies is unknown, the majority are considered to be autoimmune disorders, in which the body’s immune response system that normally defends against infection and disease attacks its own muscle fibers, blood vessels, connective tissue, organs, or joints.
Inflammatory myopathy signs and symptoms
General symptoms of chronic inflammatory myopathy include slow but progressive muscle weakness. Inflammation damages the muscle fibers, which causes weakness, and may affect the arteries and blood vessels that pass through muscle. Other symptoms include fatigue after walking or standing, frequent episodes of tripping or falling, and difficulty swallowing or breathing. Some individuals may have muscle pain or muscles that are tender to touch.
- Polymyositis affects skeletal muscles (the type involved in body movement) on both sides of the body. It is rarely seen in persons younger than age 20. Generally, the onset occurs between age 30 and 60. Signs and symptoms of polymyositis vary considerably from person to person, which can make it difficult to diagnose. Untreated progressive muscle weakness may lead to difficulty swallowing, speaking, rising from a sitting position, climbing stairs, lifting objects, or reaching overhead. Some people with polymyositis may also develop arthritis, shortness of breath, heart arrhythmias (irregular heartbeats), or congestive heart failure (when the heart is no longer able to pump out enough oxygen-rich blood).
- Dermatomyositis is characterized by a skin rash that precedes or accompanies progressive muscle weakness. The rash appears patchy, with purple or red discolorations, and characteristically develops on the eyelids and on muscles used to extend or straighten joints, including knuckles, elbows, knees, and toes. Red rashes may also occur on the face, neck, shoulders, upper chest, back, and other locations. There may be swelling in the affected areas. The rash sometimes occurs without obvious muscle involvement and often becomes more evident with sun exposure. Adults with dermatomyositis may experience weight loss or a low-grade fever, have inflamed lungs, and be sensitive to light. Adult dermatomyositis, unlike polymyositis, may accompany tumors of the breast, lung, female genitalia, or bowel. Children and adults with dermatomyositis may develop calcium deposits, which appear as hard bumps under the skin or in the muscle (called calcinosis). Calcinosis most often occurs one to three years after disease onset but may occur many years later. These deposits are seen more often in childhood dermatomyositis than in dermatomyositis that begins in adulthood.
In some cases of polymyositis and dermatomyositis, distal muscles, which are the muscles away from the center of the body, such as those in the forearms and around the ankles and wrists), may be affected as the disease progresses. Polymyositis and dermatomyositis may be associated with collagen-vascular or autoimmune diseases such as lupus. Polymyositis may also be associated with infectious disorders such as HIV, which causes AIDS.
- Inclusion body myositis is the most common form of inflammatory myopathy in people age 50 years and older and is characterized by slow, progressive muscle weakness and wasting over the course of months or years. Inclusion body myositis affects both proximal and distal muscles, typically in the thighs and forearms, and is often occurs on both sides of the body, although muscle weakness may affect only one side of the body. It also includes features of muscle degeneration with multi-protein aggregates (clumps) in the muscle that can contain toxins seen in Alzheimer’s disease and other neurodegenerative diseases.
Falling and tripping are usually the first noticeable symptoms. The disorder often begins with weakness in the wrists and fingers that causes difficulty with pinching, buttoning, and gripping objects. People may experience weakness in their wrist and finger muscles and atrophy (thinning or loss of muscle bulk) in their forearm muscles and quadriceps muscles in the thighs. Difficulty swallowing occurs in approximately half of inclusion body myositis cases due to involvement of the throat muscles.
Symptoms of the disease usually begin after the age of 50, although the disease can occur earlier. Unlike polymyositis and dermatomyositis, inclusion body myositis occurs more frequently in men than in women.
- Necrotizing autoimmune myopathy is a rare and relatively newly recognized subgroup of inflammatory myopathies. Necrotizing autoimmune myopathy can occur at any age but usually affects adults. Its symptoms are similar to polymyositis and dermatomyositis, with weakness in both the upper and lower body, difficulty rising from low chairs, climbing stairs, or lifting objects. However, the onset of these symptoms can be more severe and sudden, reaching their peak over a period of days or weeks. Other symptoms include fatigue, weight loss, and muscle pain. Necrotizing autoimmune myopathy occurs alone or after viral infections, in association with cancer, in people with connective-tissue disorders such as scleroderma, or, rarely, in people taking cholesterol lowering medications (statins). Muscle weakness and pain may continue to worsen even after individuals stop taking the drugs.
- Childhood inflammatory myopathies have some similarities to adult dermatomyositis and polymyositis. They typically affect children ages 2 to 15 years. Symptoms include proximal muscle weakness and inflammation, edema (an abnormal collection of fluids within body tissues that causes swelling), muscle pain, fatigue, skin rashes, abdominal pain, fever and contractures. Contractures result from shortening of muscles or tendons around joints, are caused by inflammation in the muscle tendons, and prevent the joints from moving freely. Children with inflammatory myopathies may have difficulty swallowing and breathing. The heart may also be affected. Between 20 to 40 percent of children with juvenile dermatomyositis develop calcinosis, which can cause significant muscle weakness and pain, joint contracture, skin ulcers, and decreased muscle bulk.
Inflammatory myopathy diagnosis
Diagnosis is based on medical history, results of a physical examination that includes tests of muscle strength, and blood samples that show elevated levels of various muscle enzymes and autoantibodies. Diagnostic tools include:
- electromyography to record the electrical activity generated by muscles during contraction and at rest
- ultrasound to look for muscle inflammation
- magnetic resonance imaging to reveal abnormal muscle anatomy.
A biopsy sample of muscle tissue should be examined for signs of chronic inflammation, muscle fiber death, vascular deformities, or other changes specific to the diagnosis of a particular type of inflammatory myopathy. A skin biopsy can show changes in the skin associated with dermatomyositis.
Inflammatory myopathy treatment
Chronic inflammatory myopathies cannot be cured in most adults but many of the symptoms can be treated. Options include:
- medication
- physical therapy
- exercise
- heat therapy
- orthotics and assistive devices
- rest
Dermatomyositis, polymyositis, and necrotizing autoimmune myopathy are first treated with high doses of corticosteroid drugs, such as prednisone. This is most often given as an oral medication but can be delivered intravenously.
Immunosuppressant drugs, such as azathioprine and methotrexate, may reduce inflammation in individuals who do not respond well to prednisone. Periodic treatment using intravenous immunoglobulin can increase the chance for recovery in individuals with dermatomyositis, polymyositis, or necrotizing autoimmune myopathy. Other immunosuppressive agents that may treat the inflammation associated with dermatomyositis and polymyositis include cyclosporine A, cyclophosphamide, mycophenolate mofetil, and tacrolimus.
Injections of adrenocorticotropic hormone gel may be another option for people who do not respond to or cannot tolerate other drug treatment options. Biologic therapies such as rituximab or tumor necrosis factor (TNF) inhibitors such as infliximab or etanercept may be used in severe cases where other treatment options have failed. However, there are very few studies that have shown how well these agents treat polymyositis and dermatomyositis.
Physical therapy is usually recommended to prevent muscle atrophy as well as to maintain muscle strength and range of motion. Bed rest for an extended period of time should be avoided, as people may develop muscle atrophy, decreased muscle function, and joint contractures. A low-sodium diet may help to reduce edema (swelling) and cardiovascular (heart and blood vessel) complications. Occupational therapy can include an assessment of daily activities to address tasks such as feeding, bathing, and dressing.
Many individuals with dermatomyositis may need a topical ointment such as corticosteroids, tacrolimus, or pimecrolimus for their skin disorder. They should wear a high-protection sunscreen and protective clothing, particularly those who are light-sensitive. In rare instances, surgery may be required to remove calcium deposits that cause nerve pain and recurrent infections.
There is no standard, evidence-based course of treatment for inclusion body myositis. The disease is generally unresponsive to corticosteroids and immunosuppressive drugs. Some evidence suggests that immunosuppressive medications or intravenous immunoglobulin may have a slight, but short-lasting, beneficial effect in a small number of cases. Physical therapy may be helpful in maintaining mobility. Other therapy is symptomatic and supportive.
Inflammatory myopathy prognosis
In most cases, the symptoms of dermatomyositis resolve with therapy. The disease is usually more severe and resistant to therapy in individuals with heart problems. Approximately one-third of individuals with juvenile-onset dermatomyositis recover from their illness, one-third have a relapsing-remitting course of disease, and the other third have a more chronic course of illness.
The prognosis for polymyositis varies. Most individuals respond fairly well to therapy, but some people have a more severe disease that does not. These individuals may have significant disability. Since polymyositis can cause difficulty swallowing, people can become malnourished. They are also at increased risk for falling, which can lead to hip and other bone fractures, disability, or death. In rare cases people with severe and progressive muscle weakness can develop respiratory failure or pneumonia.
Although necrotizing autoimmune myopathy is more difficult to treat than polymyositis and dermatomyositis, it generally responds well to long-term combination immunosuppressive therapies.
Inclusion body myositis is generally resistant to all therapies and currently available treatments do little to slow its progression.
Metabolic myopathy
Metabolic diseases of muscle are caused by a different genetic defect that impairs the body’s metabolism (the collection of chemical changes that occur within cells during normal functioning).
Metabolic myopathy types
- Acid maltase deficiency (AMD, Pompe disease, glycogenosis type 2, lysosomal storage disease). Acid maltase deficiency is a group of diseases that interferes with the processing of food (in this case, carbohydrates) for energy production. Acid maltase deficiency causes slowly progressive weakness, especially of the respiratory muscles and those of the hips, upper legs, shoulders and upper arms. Enlargement of the tongue and liver impairment occur in the infantile form but rarely in the older-onset forms. Cardiac involvement may occur in the infantile or childhood forms but is less common in adults. The childhood and adult-onset forms are milder than the infantile form, but may cause severe weakness and respiratory insufficiency and without treatment, shortened life span. This disease has its onset anywhere from infancy to adulthood. Acid maltase deficiency is slowly progressive and less severe in its childhood- and adult-onset forms. Prior to the development of treatment, the infantile form was often fatal within the first year of life. In 2006, the U.S. Food and Drug Administration (FDA) granted approval for the use of Myozyme as a treatment for Pompe disease. In 2010, Genzyme announced the availability of Lumizyme, which is similar to Myozyme, for patients with acid maltase deficiency. Both drugs substitute for the enzyme missing in Pompe disease and may keep muscle cells from dying. They have significantly improved the outlook for people with acid maltase deficiency.
- Carnitine deficiency. Carnitine deficiency is one of a group of metabolic muscle diseases that interferes with the processing of food (in this case, fats) for energy production. Carnitine deficiency disease has its onset from infancy to early adulthood and is slowly progressive. If confined to muscles, this disease causes weakness in the hips, shoulders, and upper arms and legs. The neck and jaw muscles also may be weak. Heart muscle weakness may occur. In more severe cases, in which other tissues are affected, symptoms can include low blood sugar, fatigue, vomiting, abdominal pain, growth retardation, low weight, enlarged liver and episodes of brain function abnormalities.
- Carnitine palmityl transferase deficiency (CPT deficiency). Carnitine palmityl transferase deficiency is one of a group of metabolic muscle diseases that interferes with the processing of food (in this case, fats) for energy production. Carnitine palmityl transferase deficiency is caused by a genetic defect in the carnitine palmityl transferase 2 enzyme (CPT2), which normally escorts breakdown products of fats from the main part of the muscle cell into the mitochondria , where they can be further metabolized for energy. Symptoms usually are brought on by prolonged and intense exercise, especially in combination with fasting, but may not appear for several hours after activity stops. Short periods of exercise usually don’t provoke symptoms. Symptoms also can be brought on by illness, cold, stress or menstruation. This disorder causes muscle pain, stiffness and tenderness, while weakness is less common. Breakdown of muscle tissue during an attack can cause myoglobinuria (rust-colored urine). If the CPT2 enzyme is completely lost, this disease has a rapid progression leading to death in infancy. If some enzyme activity remains, there is little or no progression with normal strength between episodes.
- Debrancher enzyme deficiency (Cori or Forbes disease, glycogenosis type 3). Debrancher enzyme deficiency is a metabolic muscle disorder, a group of diseases that interferes with the processing of food (in this case, carbohydrates) for energy production. Debrancher enzyme deficiency is caused by a defect in the debrancher enzyme gene, which interferes with the breakdown of glycogen (stored sugar) in the muscles and liver. Debrancher enzyme deficiency principally affects the liver. It causes swelling of the liver, slowing of growth, low blood sugar levels and, sometimes, seizures. In children, these symptoms often improve around puberty. Muscle weakness may develop later in life, and is most pronounced in the muscles of the forearms, hands, lower legs and feet. Weakness often is accompanied by loss of muscle bulk and exercise intolerance. The heart can be affected as well. Cori or Forbes disease can begin anywhere from infancy to the 50s and is slowly progressive. The infantile-onset form may be life-threatening in childhood.
- Lactate dehydrogenase deficiency (glycogenosis type 11). Lactate dehydrogenase deficiency is a metabolic muscle disorder, a group of diseases that interferes with the processing of food (in this case, carbohydrates) for energy production. Lactate dehydrogenase deficiency is caused by a genetic defect in the lactate dehydrogenase enzyme, which normally recycles byproducts of carbohydrate metabolism. Lactate dehydrogenase deficiency results in exercise intolerance and episodes of myoglobinuria (acute muscle breakdown leading to rust-colored urine). A skin rash is common, probably because skin cells need lactate dehydrogenase. Lactate dehydrogenase deficiency has its onset in early adulthood and does not progress.
- Myoadenylate deaminase deficiency. Myoadenylate deaminase deficiency is a metabolic muscle disease that interferes with the muscle cell’s processing of adenosine triphosphate (ATP), the major energy molecule of the cell. Myoadenylate deaminase deficiency is caused by a genetic defect in the myoadenylate deaminase enzyme, which affects the cell’s ability to process ATP. Myoadenylate deaminase deficiency may cause exercise intolerance, cramps and muscle pain; although, in many cases, people with deficiencies in this enzyme may experience no symptoms. Myoadenylate deaminase deficiency has its onset in adulthood and does not progress.
- Phosphofructokinase deficiency (Tarui disease, glycogenosis type 7). Phosphofructokinase deficiency is caused by a genetic defect in the phosphofructokinase enzyme, which affects the breakdown of glucose (sugar). Phosphofructokinase deficiency results in exercise intolerance, with pain, cramps and, occasionally, myoglobinuria (acute muscle breakdown leading to rust-colored urine). Phosphofructokinase deficiency symptoms are very similar to those of phosphorylase deficiency, but people with this disorder are less likely to experience the “second wind” phenomenon. A carbohydrate meal typically worsens exercise capacity in this condition by lowering blood levels of fats, which are the major muscle energy fuels for those with the disorder. A partial deficiency of phosphofructokinase in the red blood cells results in the breakdown of those cells and an increase in blood levels of bilirubin (a chemical found in red blood cells), although the person usually experiences no symptoms. Phosphofructokinase deficiency can begin anywhere from the teens to the 30s, and is not progressive, although weakness between episodes of exercise intolerance may occur late in the disease.
- Phosphogylcerate kinase deficiency (glycogenosis type 9). Phosphogylcerate kinase deficiency is caused by a genetic defect in the phosphoglycerate kinase enzyme, which normally breaks down glucose (sugar) for energy production. Phosphogylcerate kinase deficiency may cause anemia, enlargement of the spleen, mental retardation and epilepsy. More rarely, it causes weakness, exercise intolerance, muscle cramps and episodes of myoglobinuria (acute muscle breakdown leading to rust-colored urine). Phosphogylcerate kinase deficiency has its onset from infancy to early adulthood, and its muscle symptoms are slowly progressive. As with other metabolic disorders, the earlier the onset, the more severe the symptoms.
- Phosphogylcerate mutase deficiency (glycogenosis type 10). Phosphogylcerate mutase deficiency is caused by a genetic defect in the phosphoglycerate mutase enzyme, which normally helps break down glucose (sugar) for energy production. Phosphogylcerate mutase deficiency causes exercise intolerance, cramps, muscle pain and, sometimes, myoglobinuria (acute muscle breakdown leading to rust-colored urine). Permanent weakness is rare. Phosphogylcerate mutase deficiency has its onset anywhere from childhood to early adulthood and progresses slowly, if at all.
- Phosphorylase deficiency (McArdle disease, myophosphorylase deficiency, glycogenosis type 5). Phosphorylase deficiency (McArdle disease) is caused by a genetic defect in the phosphorylase (also known as myophosphorylase) enzyme, which affects the breakdown of glocogen, the stored form of glucose (sugar). Phosphorylase deficiency (McArdle disease) causes exercise intolerance, such as cramps, muscle pain and weakness, shortly after beginning exercise. A person with McArdle diseaser may tolerate light-to-moderate exercise such as walking on level ground, but strenuous exercise usually will bring on symptoms quickly. Resting may lead to a “second wind,” in which activity is then better tolerated. Isometric exercises that require strength, such as lifting heavy objects, squatting or standing on tiptoe, also may cause muscle damage. Symptoms of McArdle disease vary in severity among people and even within the same person from day to day. Symptoms usually don’t persist between attacks, although fixed weakness later in life is possible. The condition usually begins before age 15 and is generally not progressive, although weakness between episodes of exercise sometimes develops.
Metabolic myopathy causes
Normally, fuel molecules derived from food must be broken down further inside each cell before they can be used by the cells’ mitochondria to make energy. (The mitochondria inside each cell could be called the cell’s “engines.”) Metabolic muscle diseases are caused by problems in the way certain fuel molecules are processed before they enter the mitochondria, or by the inability to get fuel molecules into mitochondria.
Metabolic myopathy signs and symptoms
Muscles require a lot of energy to work properly, and metabolic diseases of muscle interfere with chemical reactions involved in drawing energy from food. When energy levels become too low, muscle weakness and exercise intolerance with muscle pain or cramps may occur.
In a few metabolic muscle disorders, symptoms aren’t caused so much by a lack of energy, but rather by unused fuel molecules that build up inside muscle cells. This buildup may damage the cells, leading to chronic weakness.
Metabolic myopathy prognosis
Metabolic myopathies that have their onset in infancy tend to be the most severe, and some forms can be fatal. Those that begin in childhood or adulthood tend to be less severe, and changes in diet and lifestyle can benefit most people with the milder forms.
Congenital myopathy
Congenital myopathy refers to a group of muscle disorders that appear at birth or in infancy. Typically, an infant with a congenital myopathy will be “floppy,” have difficulty breathing or feeding, and will lag behind other babies in meeting normal developmental milestones such as turning over or sitting up. Other signs and symptoms of some congenital myopathies include feeding and breathing difficulties, as well as skeletal conditions, such as curvature of the spine (scoliosis), weak bones (osteopenia) or hip problems. Signs and symptoms of congenital myopathies may not be apparent until later in infancy or childhood.
Muscle weakness can occur for many reasons, including a problem with the muscle, a problem with the nerve that stimulates the muscle, or a problem with the brain. Therefore, to diagnose a congenital myopathy, a neurologist will perform a detailed physical exam as well as tests to determine the cause of weakness. If a myopathy is suspected, possible tests include a blood test for a muscle enzyme called creatine kinase, an electromyogram (EMG) to evaluate the electrical activity of the muscle, a muscle biopsy, and genetic testing.
There are no known cures for congenital myopathies. Supportive treatments include physical, occupational and speech therapies, nutritional support, and assisted breathing, if needed. Genetic counseling may help assess the risk of congenital myopathies in future pregnancies.
Congenital myopathy types
There are currently seven distinct types of congenital myopathy, with some variation in symptoms, complications, treatment options, and outlook.
- Nemaline myopathy is the most common congenital myopathy. Infants usually have problems with breathing and feeding. Later, some skeletal problems may arise, such as scoliosis (curvature of the spine). In general, the weakness does not worsen during life.
- Myotubular myopathy is rare and only affects boys. Weakness and floppiness are so severe that a mother may notice reduced movements of the baby in her womb during pregnancy. There are usually significant breathing and swallowing difficulties; many children do not survive infancy. Osteopenia (weakening of the bones) is also associated with this disorder.
- Centronuclear myopathy is rare and begins in infancy or early childhood with weakness of the arms and legs, droopy eyelids, and problems with eye movements. Weakness often gets worse with time.
- Central core disease varies among children with regard to the severity of problems and the degree of worsening over time. Usually, there is mild floppiness in infancy, delayed milestones, and moderate limb weakness, which do not worsen much over time. Children with central core disease may have life-threatening reactions to general anesthesia. Treatment with the drug salbutamol has been shown to reduce weakness significantly, although it does not cure the disorder.
- Multi-minicore disease has several different subtypes. Common to most is severe weakness of the limbs and scoliosis. Often breathing difficulties occur as well. Some children have weakened eye movements.
- Congenital fiber-type disproportion myopathy is a rare disorder that begins with floppiness, limb and facial weakness, and breathing problems.
- Hyaline body myopathy is a disorder characterized by the specific appearance under the microscope of a sample of muscle tissue. It probably includes several different causes. Because of this, the symptoms are quite variable.
Congenital myopathy causes
Congenital myopathies are caused by one or more genetic abnormalities in genes that control muscle development.
Risk factors for congenital myopathy
The only known risk factor for congenital myopathies is having a blood relative with one of these conditions, or one or both parents who carry a mutated gene that causes them.
Congenital myopathy prevention
There’s no way to prevent congenital myopathies. If you’re at high risk of having a child with a congenital myopathy, you may want to consult a genetic counselor before becoming pregnant.
A genetic counselor can help you understand your chances of having a child with a congenital myopathy. He or she can also explain the prenatal tests that are available and help explain the pros and cons of testing.
Congenital myopathy symptoms
Signs and symptoms vary depending on the type of congenital myopathy. The severity of signs and symptoms also varies, though the conditions are often stable or slowly progressing.
Common signs and symptoms include:
- Lack of muscle tone
- Muscle weakness
- Delayed motor skills
- Noticeable facial weakness
- Drooping eyelids
- Muscle cramps or contractions
Congenital myopathy complications
Congenital myopathies are associated with a number of complications, such as:
- Delays in motor skills
- Scoliosis
- Pneumonia
- Respiratory failure
- Feeding problems
- Death
Congenital myopathy diagnosis
To diagnose the condition, your doctor will review your medical and family history. He or she will conduct a physical and a neurological examination to find the cause of the muscle weakness and rule out other conditions. Your doctor may conduct several tests to diagnose congenital myopathy.
- Blood tests may be ordered to detect an enzyme called creatine kinase.
- Electrocardiogram (ECG). An electrocardiogram may be conducted to observe your heart’s electrical activity.
- Electromyography (EMG). Electromyography measures electrical activity within muscles.
- Genetic testing may be recommended to verify a particular mutation.
- Muscle biopsy. A specialist may remove and examine a small sample of tissue (biopsy) from your muscle.
Prenatal diagnosis
If you have a known family history of congenital myopathies, you can opt for minimally invasive prenatal testing. Chorionic villus sampling can be done after 11 weeks of pregnancy. Amniocentesis can be done after 15 weeks, and cordocentesis can be done shortly after that.
The risk of pregnancy loss associated with these tests is less than 1 percent.
Congenital myopathy treatment
Congenital myopathies can’t be cured, but doctors can help you manage the condition and symptoms. Treatment may include several options.
- Genetic counseling. Genetic counselors may help you understand the genetics of the condition.
- Medications. Medications may help treat symptoms of some myopathies. For example, the drug albuterol (Proair HFA, Ventolin HFA, others) reduces muscle weakness in central core disease, and may be helpful in other congenital myopathies.
- Nutritional and respiratory support. Nutritional or respiratory support may be needed as the condition progresses.
- Orthopedic treatments. Orthopedic support devices or other treatments, such as surgery to correct or improve scoliosis, may be helpful.
- Physical, occupational or speech therapy. Physical, occupational or speech therapy may help manage symptoms.
Because of advancements in supportive care, more people with congenital myopathies are living into adulthood and beyond.
Congenital myopathy coping and support
When you learn your child has a congenital myopathy, you may experience a range of emotions, including anger, fear, worry, sorrow and guilt. You may not know what to expect, and you may worry about your ability to care for your child. The best antidote for fear and worry is information and support.
Consider these steps to prepare yourself and to care for your child:
- Find a team of trusted professionals. You’ll need to make important decisions about your child’s education and treatment. Build a team of health care providers, teachers and therapists you trust. These professionals can help evaluate the resources in your area and explain state and federal programs for children with disabilities.
- Seek out other families who are dealing with the same issues. Ask your doctor if your community has a support groups for parents of children with congenital myopathies. You can also find internet support groups. Family and friends also can be a source of understanding and support.
Statin myopathy
Statin-induced myopathy encompasses a rare heterogeneous group of muscle manifestations that have not yet been well characterized 25. Statin-induced myopathy most of which are self-limited and improve with discontinuation of the offending agent. In a subgroup, an autoimmune necrotizing myopathy develops that persists after discontinuation of statins. Specific autoantibody testing can help identify these patients in clinical practice and determine the need for immunosuppressive therapy. Among patients using statins, the estimated immune-mediated necrotizing myopathy incidence rate is 2-3 per 100,000 patients, with increased risk among patients over 50 years of age 26. All patients with statin-induced myopathy showed moderate to severe proximal and trunk weakness and myalgia 27. With small number showing occasional dysphagia. The patients exhibited extremely high creatine kinase (CK) levels with peak values between 5900 and 9584 U/L. All patients had a medical history of long-term statin use (3 to 18 years). EMG revealed a myopathic pattern with fibrillation activity and bizarre high frequency discharges in all patients. All patients started steroid treatment for at least 6 months, after the ineffectiveness of this therapy, Villa et al 27 performed three cycles of IVIg one month apart from one another. If patients still had symptoms or signs of myopathy, they had another immunosuppressive therapy 27.
There is some epidemiological evidence that adverse effects of statins on muscle are more common in patients with predisposing factors, such as a history of increased creatine kinase (CK) levels, a family history of myopathy, or a previous diagnosis of neuromuscular diseases or hypothyroidism 28. Moreover, certain genetic factors may increase the risk of experiencing statin-induced muscle toxicity. One of these is a common single-nucleotide polymorphism in the SLCO1B1 gene, which is associated with a higher risk of myopathy in patients taking simvastatin, although it is uncertain whether the risk also applies to other statins (e.g. rosuvastatin or atorvastatin) 29. Mitochondrial dysfunction, oxidative stress, and several mechanisms derived from impaired mevalonate metabolism, such as isoprenylation of small G-proteins, have been implicated in the mechanism of statin toxicity 30.
Genetic susceptibility related to the pharmacokinetics and pharmacodynamics of the drug is also of paramount relevance in statin-related myopathy. Polymorphisms of the SLCO1B1 gene are the best-known pharmacokinetic-related alteration, although polymorphisms of other genes have also been described 29. Regarding pharmacodynamics, certain genetic factors can increase the risk of statin-induced myopathy. Some examples are those associated with plasma membrane calcium transporting ATPase or with mitochondrial energy production regulated by the CoQ2 gene. This gene codes for the coenzyme Q10, known as ubiquinone, which is located in the phospholipid bilayer of mitochondria and is essential for obtaining energy, especially in muscle tissue 28.
Recently, a new etiopathogenetic mechanism has been proposed, in which the immune system also plays a role. This is the case of immune-mediated necrotizing myopathy and antibodies against 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), the enzyme that is usually upregulated by statins. Statins upregulate 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and overexpression of this enzyme likely facilitates presentation of highly immunogenic HMGCR peptides by the HLA DRB1*11:01, thus triggering the autoimmune disease 31.
In susceptible patients who have a characteristic HLA or an appropriate genetic background, 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), the molecular target of statins, may trigger the autoimmune phenomenon after some type of interaction with the drug. Once the autoimmune process is started, HMGCR overexpression by statins is not needed to maintain the disease, as regenerating cells naturally express 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) 32 and will maintain the feed-forward loop of autoimmunity. Anti-HMGCR can be found in muscle cells, and some authors have reported that levels of this antibody correlate with the CK concentration and clinical disease activity, thus suggesting a possible role of these antibodies in the pathogenesis of the disease 33. In addition, experimental studies in muscle biopsies of patients with anti-HMGCR have shown that these autoantibodies may impair muscle regeneration and induce muscle atrophy 34.
Nemaline myopathy
Nemaline myopathy is called rod body disease, is an inherited myopathy, a group of diseases that causes problems with the tone and contraction of skeletal muscles. Nemaline myopathy gets its name from the fact that the muscle cells contain abnormal clumps of threadlike material — probably disorganized filament proteins — called nemaline bodies (nema is Greek for “thread”).
Nemaline myopathy causes
Nemaline myopathy is caused by a variety of genetic defects, each one affecting one of the filament proteins required for muscle tone and contraction. Nemaline myopathy can be inherited in an autosomal recessive or autosomal dominant pattern, meaning it can be produced by defective genes contributed by either one or both parents.
Nemaline myopathy signs and symptoms
Nemaline myopathy causes weakness and poor tone (hypotonia) in the muscles of the face, neck and upper limbs, and often affects the respiratory muscles (those that control breathing).
The infantile-onset cases tend to be the most severe. Usually, infants with the disease lack the muscle strength and tone required for simple postures and movements. They also have serious difficulties with feeding and respiration.
For adults, even noncongenital forms of the disease can cause life-threatening respiratory problems. Adults also might experience swallowing and speech problems, and those with restricted mobility might develop scoliosis.
Nemaline myopathy prognosis
Onset occurs from birth to adulthood. While respiratory failure or lung infections can be life-threatening in infancy, some affected infants survive to adulthood.
Affected children usually attain motor milestones slowly, and at puberty they might experience further weakening. However, even people who have had the disease since birth can lead active lives.
Mitochondrial myopathy
Mitochondrial diseases affect the mitochondria — tiny energy factories found inside almost all our cells. A mitochondrial disease that causes prominent muscular problems is called a mitochondrial myopathy, while a mitochondrial disease that causes both prominent muscular and neurological problems is called a mitochondrial encephalomyopathy (encephalo refers to the brain).
Mitochondrial myopathy types
Kearns-Sayre syndrome
Onset: Before age 20
Symptoms: This disorder is defined by progressive external ophthalmoplegia and pigmentary retinopathy, a “salt-and-pepper” pigmentation in the retina that can affect vision, but often leaves it intact. Other common symptoms include conduction block (in the heart) and ataxia. Less typical symptoms are mental retardation or deterioration, delayed sexual maturation and short stature.
Leigh syndrome (subacute necrotizing encephalomyopathy) and maternally inherited Leigh syndrome
Onset: Infancy
Symptoms: Leigh syndrome causes brain abnormalities that can result in ataxia, seizures, impaired vision and hearing, developmental delays and altered control over breathing. It also causes muscle weakness, with prominent effects on swallowing, speech and eye movements.
Mitochondrial DNA depletion syndrome
Onset: Infancy
Symptoms: Mitochondrial DNA depletion syndrome typically causes muscle weakness and/or liver failure, and more rarely, brain abnormalities. “Floppiness,” feeding difficulties and developmental delays are common symptoms; progressive external ophthalmoplegia and seizures are less common.
Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS)
Onset: Childhood to early adulthood
Symptoms: MELAS causes recurrent stroke-like episodes in the brain, migraine-type headaches, vomiting and seizures, and can lead to permanent brain damage. Other common symptoms include progressive external ophthalmoplegia, general muscle weakness, exercise intolerance, hearing loss, diabetes and short stature.
Mitochondrial neurogastrointestinal encephalomyopathy
Onset: Usually before age 20
Symptoms: Mitochondrial neurogastrointestinal encephalomyopathy causes progressive external ophthalmoplegia, ptosis (droopy eyelids), limb weakness and gastrointestinal (digestive) problems, including chronic diarrhea and abdominal pain. Another common symptom is peripheral neuropathy (a malfunction of the nerves that can lead to sensory impairment and muscle weakness).
Myoclonus epilepsy with ragged red fibers
Onset: Late childhood to adolescence
Symptoms: The most prominent symptoms are myoclonus (muscle jerks), seizures, ataxia and muscle weakness. The disease also can cause hearing impairment and short stature.
Neuropathy, ataxia and retinitis pigmentosa
Onset: Infancy to adulthood
Symptoms: Neuropathy, ataxia and retinitis pigmentosa causes neuropathy (a malfunction of the nerves that can lead to sensory impairment and muscle weakness), ataxia and retinitis pigmentosa (degeneration of the retina in the eye, with resulting loss of vision). It also can cause developmental delay, seizures and dementia.
Pearson syndrome
Onset: Infancy
Symptoms: Pearson syndrome causes severe anemia and malfunction of the pancreas. Children who survive the disease usually go on to develop Kearns-Sayre syndrome.
Progressive external ophthalmoplegia
Onset: Usually in adolescence or early adulthood
Symptoms: Progressive external ophthalmoplegia — the gradual paralysis of eye movements — is often a symptom of mitochondrial disease, but sometimes it stands out as a distinct syndrome. It’s frequently associated with exercise intolerance.
Mitochondrial myopathy signs and symptoms
The main symptoms of mitochondrial myopathy are muscle weakness and atrophy (shrinking), and exercise intolerance. It’s important to remember that these symptoms vary greatly from one person to the next, even in the same family.
In some individuals, weakness is most prominent in muscles that control movements of the eyes and eyelids. Two common consequences are the gradual paralysis of eye movements, progressive external ophthalmoplegia and ptosis.
Mitochondrial myopathies also can cause weakness and atrophy in other muscles of the face and neck, which can lead to slurred speech and difficulty with swallowing. Sometimes, people with mitochondrial myopathies experience loss of muscle strength in the arms or legs.
These diseases also can cause significant weakness in the muscles that support breathing.
Muscular and neurological problems — such as muscle weakness, exercise intolerance, hearing loss, trouble with balance and coordination, seizures and learning deficits — are common features of mitochondrial disease, because muscle cells and nerve cells have especially high energy needs. Other frequent complications include impaired vision, heart defects, diabetes and stunted growth.
Usually, a person with a mitochondrial disease has two or more of these conditions, some of which occur together so regularly that they’re grouped into syndromes.
The main problems associated with mitochondrial disease — low energy, free radical production and lactic acidosis — can result in a variety of symptoms in many different organs of the body.
Most affected people have some, but not necessarily all, of these problems.
Exercise intolerance
Exercise intolerance, also called exertional fatigue, refers to unusual feelings of exhaustion brought on by physical exertion. The degree of exercise intolerance varies greatly among individuals. Some people might only have trouble with athletic activities like jogging, while others might experience problems with everyday activities like walking to the mailbox or lifting a milk carton.
Sometimes, exercise intolerance is associated with painful muscle cramps and/or injury-induced pain. The cramps are actually sharp contractions that can seem to temporarily lock the muscles. Injury-induced pain is caused by a process of acute muscle breakdown called rhabdomyolysis, leading to leakage of myoglobin from the muscles into the urine (myoglobinuria). Cramps or myoglobinuria usually occur when someone with exercise intolerance “overdoes it,” and can happen during the overexertion or several hours afterward.
Breathing issues
Sometimes these diseases can cause significant weakness in the muscles that support breathing. Mitochondrial encephalomyopathies also may cause brain abnormalities that alter the brain’s control over breathing.
Cardiac care
Sometimes, mitochondrial diseases directly affect the heart. In these cases, the usual cause is an interruption in the rhythmic beating of the heart, called conduction block. Cardiac muscle damage also may occur.
Other potential health issues
Some people with mitochondrial disease experience serious kidney problems, gastrointestinal problems and/or diabetes. Some of these problems are direct effects of mitochondrial defects in the kidneys, digestive system or pancreas (in diabetes), and others are indirect effects of mitochondrial defects in other tissues.
For example, rhabdomyolysis (acute muscle breakdown) can lead to kidney problems by causing a protein called myoglobin to leak from ruptured muscle cells into the urine. This condition, myoglobinuria, stresses the kidneys’ ability to filter waste from the blood and can cause kidney damage.
Special issues in children
Progressive external ophthalmoplegia and drooping of the upper eyelids, called ptosis, typically cause only mild visual impairment in adults, they’re potentially more harmful in children with mitochondrial myopathies.
Because the development of the brain is sensitive to childhood experiences, progressive external ophthalmoplegia or ptosis during childhood can sometimes cause permanent damage to the brain’s visual system.
Due to muscle weakness, brain abnormalities or a combination of both, children with mitochondrial diseases may have developmental delays. For example, they might take an unusually long time to reach motor milestones such as sitting, crawling and walking. As they get older, they may be unable to get around as easily as other children their age, and may have speech problems and/or learning disabilities.
Symptoms of encephalomyopathies
A mitochondrial encephalomyopathy typically includes some of the above-mentioned symptoms of myopathy (muscle disease) plus one or more neurological symptoms. Again, these symptoms show a great deal of individual variability in both type and severity.
Hearing impairment, migraine-like headaches and seizures are among the most common symptoms of mitochondrial encephalomyopathy. In at least one syndrome, headaches and seizures often are accompanied by stroke-like episodes.
In addition to affecting the musculature of the eye, a mitochondrial encephalomyopathy can affect the eye itself and parts of the brain involved in vision. For instance, vision loss due to optic atrophy (shrinkage of the optic nerve) or retinopathy (degeneration of some of the cells that line the back of the eye) is a common symptom of mitochondrial encephalomyopathy. These effects are more likely to cause serious visual impairment.
Often, mitochondrial encephalomyopathy causes ataxia, or trouble with balance and coordination. People with ataxia are usually prone to falls.
Mitochondrial myopathy causes
Mitochondrial myopathies are caused by mutations, or changes, in genes — the cells’ blueprint for making proteins. Mitochondrial myopathies are inheritable, although they can occur with no family history, and they often affect members of the same family in different ways.
The genes involved in mitochondrial disease normally make proteins that work inside the mitochondria. Within each mitochondrion (singular of mitochondria), these proteins make up part of an assembly line that uses fuel molecules derived from food to manufacture the energy molecule adenosine triphosphate (ATP). This highly efficient manufacturing process requires oxygen; outside the mitochondrion, there are less efficient ways of producing ATP without oxygen.
Proteins at the beginning of the mitochondrial assembly line act like cargo handlers, importing the fuel molecules — sugars and fats — into the mitochondrion. Next, other proteins break down the sugars and fats, extracting energy in the form of charged particles called electrons.
Proteins toward the end of the line — organized into five groups called complexes I, II, III, IV and V — harness the energy from those electrons to make ATP. Complexes I through IV shuttle the electrons down the line and are therefore called the electron transport chain, and complex V actually churns out ATP, so it’s also called ATP synthase.
A deficiency in one or more of these complexes is the typical cause of a mitochondrial disease. In fact, mitochondrial diseases are sometimes named for a specific deficiency, such as complex I deficiency.
When a cell is filled with defective mitochondria, not only does it become deprived of ATP, it also can accumulate a backlog of unused fuel molecules and oxygen, with potentially disastrous effects.
In such cases, excess fuel molecules are used to make ATP by inefficient means, which can generate potentially harmful byproducts such as lactic acid. This also occurs when a cell has an inadequate oxygen supply, which can happen to muscle cells during strenuous exercise. The buildup of lactic acid in the blood — called lactic acidosis — is associated with muscle fatigue, and might actually damage muscle and nerve tissue.
Meanwhile, unused oxygen in the cell can be converted into destructive compounds called reactive oxygen species, including so-called free radicals. (These are the targets of antioxidant drugs and vitamins.)
ATP derived from mitochondria provides the main source of power for muscle cell contraction and nerve cell firing. So, muscle cells and nerve cells are especially sensitive to mitochondrial defects. The combined effects of energy deprivation and toxin accumulation in these cells probably give rise to the main symptoms of mitochondrial myopathies and encephalomyopathies.
Mitochondrial myopathy inheritance patterns
Mitochondrial genetics are complex, and often, a mitochondrial disease can be difficult to trace through a family tree. But since they’re caused by defective genes, mitochondrial diseases do run in families.
To understand how mitochondrial diseases are passed on through families, it’s important to know that there are two types of genes essential to mitochondria. The first type is housed within the nucleus — the part of our cells that contains most of our genetic material, or DNA. The second type resides exclusively within DNA contained inside the mitochondria themselves.
Mutations in either nuclear DNA (nDNA) or mitochondrial DNA (mtDNA) can cause mitochondrial disease.
Most nDNA (along with any mutations it has) is inherited in a Mendelian pattern, loosely meaning that one copy of each gene comes from each parent. Also, most mitochondrial diseases caused by nDNA mutations (including Leigh syndrome, MNGIE and even MDS) are autosomal recessive, meaning that it takes mutations in both copies of a gene to cause disease.
Unlike nDNA, mtDNA passes only from mother to child. That’s because during conception, when the sperm fuses with the egg, the sperm’s mitochondria — and its mtDNA — are destroyed. Thus, mitochondrial diseases caused by mtDNA mutations are unique because they’re inherited in a maternal pattern.
Another unique feature of mtDNA diseases arises from the fact that a typical human cell — including the egg cell — contains only one nucleus but hundreds of mitochondria. A single cell can contain both mutant mitochondria and normal mitochondria, and the balance between the two will determine the cell’s health.
This helps explain why the symptoms of mitochondrial disease can vary so much from person to person, even within the same family.
Imagine that a woman’s egg cells (and other cells in her body) contain both normal and mutant mitochondria, and that some have just a few mutant mitochondria, while others have many. A child conceived from a “mostly healthy” egg cell probably won’t develop disease, and a child conceived from a “mostly mutant” egg cell probably will.
Also, the woman may or may not have symptoms of mitochondrial disease herself.
These diseases also can arise in a sporadic fashion, meaning they may occur with no family history.
The risk of passing on a mitochondrial disease to your children depends on many factors, including whether the disease is caused by mutations in nDNA or mtDNA.
Mitochondrial myopathy prognosis
The age of onset and progression of mitochondrial myopathy varies greatly from type to type.
Myopathy causes
Causes of myopathy include
- Injury or overuse, such as sprains or strains, cramps or tendinitis
- A genetic disorder, such as muscular dystrophy
- Some cancers
- Inflammation, such as myositis
- Diseases of nerves that affect muscles
- Infections
- Certain medicines
Sometimes the cause is not known.
Myopathy symptoms
The main symptom is weakness.
Other symptoms include cramps and stiffness.
Myopathy diagnosis
Blood tests sometimes show abnormally high muscle enzymes. If a muscle disorder might also affect other family members, genetic testing may be done.
When someone has symptoms and signs of a muscle disorder, tests such as an electromyogram, muscle biopsy, or both can confirm whether it is a myopathy. A muscle biopsy examines a tissue sample under a microscope to confirm disease. Sometimes, a blood test to check for a genetic disorder is all that is needed based on someone’s symptoms and family history.
Myopathy treatment
Treatments for the myopathies depend on the disease or condition and specific causes. Supportive and symptomatic treatment may be the only treatment available or necessary for some disorders. Treatment for other disorders may include drug therapy, such as immunosuppressives, physical therapy, bracing to support weakened muscles, and surgery.
Treatment depends on the cause. It usually includes:
- Bracing
- Medication
- Physical therapy
- Preventing the condition from getting worse by treating the underlying condition causing the muscle weakness
- Surgery (sometimes)
Your health care provider can tell you more about your condition and treatment options.
Myopathy prognosis
The prognosis for individuals with a myopathy varies. Some individuals have a normal life span and little or no disability. For others, however, the disorder may be progressive, severely disabling, life-threatening, or fatal.
References- Molecular and cellular events in alcohol-induced muscle disease. Fernandez-Solà J, Preedy VR, Lang CH, Gonzalez-Reimers E, Arno M, Lin JC, Wiseman H, Zhou S, Emery PW, Nakahara T, Hashimoto K, Hirano M, Santolaria-Fernández F, González-Hernández T, Fatjó F, Sacanella E, Estruch R, Nicolás JM, Urbano-Márquez A. Alcohol Clin Exp Res. 2007 Dec; 31(12):1953-62.
- Relationship between ethanol-related diseases and nutritional status in chronically alcoholic men. Estruch R, Nicolás JM, Villegas E, Junqué A, Urbano-Márquez A. Alcohol Alcohol. 1993 Sep; 28(5):543-50.
- The importance of alcohol-induced muscle disease. Preedy VR, Ohlendieck K, Adachi J, Koll M, Sneddon A, Hunter R, Rajendram R, Mantle D, Peters TJ. J Muscle Res Cell Motil. 2003; 24(1):55-63.
- The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. Urbano-Márquez A, Estruch R, Fernández-Solá J, Nicolás JM, Paré JC, Rubin E. JAMA. 1995 Jul 12; 274(2):149-54.
- Simon L, Jolley SE, Molina PE. Alcoholic Myopathy: Pathophysiologic Mechanisms and Clinical Implications. Alcohol Res. 2017;38(2):207–217. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513686
- Nutritional status in chronically alcoholic men from the middle socioeconomic class and its relation to ethanol intake. Nicolás JM, Estruch R, Antunez E, Sacanella E, Urbano-Márquez A. Alcohol Alcohol. 1993 Sep; 28(5):551-8.
- Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hanai T, Shiraki M, Ohnishi S, Miyazaki T, Ideta T, Kochi T, Imai K, Suetsugu A, Takai K, Moriwaki H, Shimizu M. Hepatol Res. 2016 Jul; 46(8):743-51.
- Alcoholic myopathy. Perkoff GT. Annu Rev Med. 1971; 22():125-32.
- Effects of alcohol on skeletal and cardiac muscle. Urbano-Márquez A, Fernández-Solà J. Muscle Nerve. 2004 Dec; 30(6):689-707.
- Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Thapaliya S, Runkana A, McMullen MR, Nagy LE, McDonald C, Naga Prasad SV, Dasarathy S. Autophagy. 2014 Apr; 10(4):677-90.
- Distribution of adipose tissue and muscle mass in alcoholic men. Kvist H, Hallgren P, Jönsson L, Pettersson P, Sjöberg C, Sjöström L, Björntorp P. Metabolism. 1993 May; 42(5):569-73.
- Effects of the acute ingestion of small amounts of alcohol upon 5-mile run times. Houmard JA, Langenfeld ME, Wiley RL, Siefert J. J Sports Med Phys Fitness. 1987 Jun; 27(2):253-7.
- Effect of a small dose of alcohol on the endurance performance of trained cyclists. Lecoultre V, Schutz Y. Alcohol Alcohol. 2009 May-Jun; 44(3):278-83.
- Post-exercise alcohol ingestion exacerbates eccentric-exercise induced losses in performance. Barnes MJ, Mündel T, Stannard SR. Eur J Appl Physiol. 2010 Mar; 108(5):1009-14.
- The association between alcohol consumption patterns and health-related quality of life in a nationally representative sample of South Korean adults. Kim K, Kim JS. PLoS One. 2015; 10(3):e0119245.
- Muscle performance in detoxified alcoholics. York JL, Hirsch JA, Pendergast DR, Glavy JS. J Stud Alcohol. 1999 May; 60(3):413-21.
- Muscle performance in detoxified alcoholics. York JL, Hirsch JA, Pendergast DR, Glavy JS. J Stud Alcohol. 1999 May; 60(3):413-21
- Low-dose ethanol consumption allows strength recovery in chronic alcoholic myopathy. Fernández-Solà J, Nicolás JM, Sacanella E, Robert J, Cofan M, Estruch R, Urbano-Márquez A. QJM. 2000 Jan; 93(1):35-40.
- Chronic alcoholic skeletal myopathy–common and reversible. Peters TJ, Martin F, Ward K. Alcohol. 1985 May-Jun; 2(3):485-9.
- A preliminary, randomized trial of aerobic exercise for alcohol dependence. Brown RA, Abrantes AM, Minami H, Read JP, Marcus BH, Jakicic JM, Strong DR, Dubreuil ME, Gordon AA, Ramsey SE, Kahler CW, Stuart GL. J Subst Abuse Treat. 2014 Jul; 47(1):1-9.
- IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Lang CH, Frost RA, Svanberg E, Vary TC. Am J Physiol Endocrinol Metab. 2004 Jun; 286(6):E916-26.
- Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Garcia JM, Friend J, Allen S. Support Care Cancer. 2013 Jan; 21(1):129-37.
- Acute antibody-directed myostatin inhibition attenuates disuse muscle atrophy and weakness in mice. Murphy KT, Cobani V, Ryall JG, Ibebunjo C, Lynch GS. J Appl Physiol (1985). 2011 Apr; 110(4):1065-72.
- Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, Hancock ML, Johnston MA, Steiner MS. Lancet Oncol. 2013 Apr; 14(4):335-45.
- Selva-O’Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14(3):215–224. doi:10.1080/1744666X.2018.1440206 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6019601
- Absence of anti-HMG-CoA reductase autoantibodies in severe self-limited statin-related myopathy. Floyd JS, Brody JA, Tiniakou E, Psaty BM, Mammen A. Muscle Nerve. 2016 Jun; 54(1):142-4.
- Villa L, Lerario A, Calloni S, et al. Immune-mediated necrotizing myopathy due to statins exposure. Acta Myol. 2018;37(4):257–262. Published 2018 Dec 1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6416701
- Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, De Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, März W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN, European Atherosclerosis Society Consensus Panel. Eur Heart J. 2015 May 1; 36(17):1012-22.
- SLCO1B1 variants and statin-induced myopathy–a genomewide study. SEARCH Collaborative Group., Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. N Engl J Med. 2008 Aug 21; 359(8):789-99.
- Mechanism of statin-induced rhabdomyolysis. Sakamoto K, Kimura J. J Pharmacol Sci. 2013; 123(4):289-94.
- Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Mammen AL, Gaudet D, Brisson D, Christopher-Stine L, Lloyd TE, Leffell MS, Zachary AA. Arthritis Care Res (Hoboken). 2012 Aug; 64(8):1233-7.
- 3-hydroxy 3-methylglutaryl coenzyme A reductase inhibition impairs muscle regeneration. Trapani L, Segatto M, La Rosa P, Fanelli F, Moreno S, Marino M, Pallottini V. J Cell Biochem. 2012 Jun; 113(6):2057-63.
- Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, Casciola-Rosen LA. Arthritis Rheum. 2011 Mar; 63(3):713-21.
- Pathogenic role of anti-signal recognition protein and anti-3-Hydroxy-3-methylglutaryl-CoA reductase antibodies in necrotizing myopathies: Myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Arouche-Delaperche L, Allenbach Y, Amelin D, Preusse C, Mouly V, Mauhin W, Tchoupou GD, Drouot L, Boyer O, Stenzel W, Butler-Browne G, Benveniste O. Ann Neurol. 2017 Apr; 81(4):538-548.