Nephrotoxicity
Nephrotoxicity is a poisonous effect of some substances, both toxic chemicals and medications, on the kidney. Risk factors of drug-induced nephrotoxicity include drug overdose, drug-drug interactions and drug-related adverse effects. Nephrotoxins are chemicals displaying nephrotoxicity. Drugs remain a relatively common cause of acute and chronic kidney injury. A combination of factors including the innate nephrotoxicity of drugs, underlying patient characteristics that increase their risk for kidney injury, and the metabolism and pathway of excretion by the kidneys of the various agents administered enhance risk for drug-induced nephrotoxicity 1. The nephrotoxic effect of most drugs is more profound in patients who already suffer from renal impairment. Some drugs may affect renal function in more than one way. There are various forms of nephrotoxicity. Nephrotoxicity should not be confused with the fact that some medications have a predominantly renal excretion and need their dose adjusted for the decreased renal function (e.g. heparin).
Nephrotoxicity is usually monitored through a simple blood test. An elevated level of creatinine indicates poor renal function. Normal creatinine levels are between 80 – 120 mm/l. In interventional radiology, a patients’ creatinine levels are all checked prior to a procedure. Should an elevated creatinine level be found, a special contrast medium or radiocontrast is used which is less harmful for the patient.
Creatinine clearance is another measure of renal function, which may be more useful clinically when dealing with patients with early kidney disease.
Medications are a relatively common cause of kidney injury 2. The epidemiology of drug-induced nephrotoxicity is currently based on literature focusing on acute kidney injury (sudden episode of kidney failure or kidney damage that happens within a few hours or a few days). Drug-induced nephrotoxicity in adults is approximately 14%–26% in prospective cohort studies of acute kidney injury, whereas 16% of hospitalized acute kidney injury is due to drugs in the pediatric population 3. Drug-induced nephrotoxicity is more common in hospitalized patients, in particular intensive care unit patients 4.
Importantly, the general population is exposed to a large number of prescribed and over-the-counter drugs as well as a variety of substances available at health food stores (natural products, supplements, herbal remedies) 5. Various imaging agents used for diagnostic purposes are also associated with nephrotoxicity 6. However, not all patients exposed to the various potential nephrotoxins develop kidney disease. Thus, the nephrotoxicity of medications, drugs, and other ingested substances is a complicated process that involves a combination of factors. These include the inherent nephrotoxic potential of the drug, underlying patient characteristics that enhance their risk for kidney injury, and the metabolism and excretion of the potential offending agent by the kidney 7.
Medications causing nephrotoxicity:
- Adefovir
- Kanamycin
- Cyclophosphamide
- Cisplatin: Cisplatin is one of the most widely used and most potent chemotherapy drugs. Cisplatin, while highly toxic, is one of the most heavily utilized chemotherapeutic agents for hematologic and solid tumor malignancies. It can be used as a single-agent or in combination therapy for induction and neoadjuvant therapy. Cisplatin is U.S. Food and Drug Administration (FDA) approved for the treatment of advanced ovarian cancer, testicular cancer, and bladder carcinoma 8. However, cisplatin is frequently used in an off-label fashion when the benefits may outweigh the risks of adverse drug effects. Severe renal toxicity, including acute renal failure, may occur with Cisplatin administration. These effects are cumulative and dose-related. Pretreatment hydration plays a significant role in preventing renal toxicity 9. The dose of cisplatin may have to be adjusted based on renal function with close monitoring of the glomerular filtration rate (GFR) 10.
- Vancomycin: Vancomycin is a tricyclic glycopeptide antibiotic originally derived from the organism Streptococcus orientalis. Vancomycin is used for the treatment and prevention of various bacterial infections caused by gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). It is also effective for streptococci, enterococci, and methicillin-susceptible Staphylococcus aureus (MSSA) infections. Vancomycin has numerous FDA-approved and off-label clinical uses 11.
- Tacrolimus: Tacrolimus is an immunosuppressive agent used for prophylaxis of organ rejection post-transplant 12. Tacrolimus use is in combination with one or, most commonly, two other immunosuppressive medications. It has an application as an agent for as prevention or treatment for certain autoimmune diseases. In solid organ transplantation, it serves as the treatment of organ rejection in kidney, liver, and heart allogeneic transplants. There is also an off-label indication for the prevention of rejection in lung transplant patients. Tacrolimus indications also include topical use in moderate to severe atopic dermatitis, as well as other off-label dermatologic disease states 13. Since tacrolimus use is typically in combination with other immunosuppressants, target levels usually decrease as post-transplant time increases to minimize Calcineurin Inhibitor mediated nephrotoxicity and adverse effects 14.
- Aminoglycosides antibiotics (e.g., gentamicin, tobramycin, amikacin, neomycin, plazomicin, and streptomycin). Nephrotoxicity due to aminoglycosides may appear in up to 10 to 25% of patients. In patients receiving aminoglycoside therapy, renal tubular toxicity decreased blood flow to the kidneys, and reduced GFR most commonly causes the nephrotoxicity seen. Renal effects with aminoglycosides generally are reversible. Furthermore, there are risk factors associated with the development of aminoglycoside-induced nephrotoxicity, including dehydration, pregnancy, and hepatic dysfunction. Taking other medications concurrently with aminoglycosides that can cause nephrotoxicity, such as NSAIDs, cyclosporine, and diuretics, also put a patient at risk for renal problems. It is important to monitor patient renal function when taking aminoglycosides 15.
Nephrotoxic drugs
The initial step in the development of kidney injury involves exposure to a potentially toxic offending agent. The general population is exposed to a variety of potential nephrotoxic substances including prescribed therapeutic agents, over-the-counter products, diagnostic agents, and environmental substances 1. Examples of potentially nephrotoxic drugs that are utilized to treat various disease processes include antimicrobial agents, anticancer drugs, analgesics, and immunosuppressive agents 16. Furthermore, a large number of new medications with unknown nephrotoxic potential make it through clinical trials and are subsequently released into clinical practice where they cause kidney injury. This is likely related to exposure of these new drugs in patients who have comorbidities or other characteristics that increase nephrotoxic risk that were not included in clinical trials. Although clinicians prescribe the vast majority of potentially nephrotoxic medications, many are also available as over-the-counter preparations. Radiocontrast agents, in particular those delivered intra-arterially at high dose, are another potential cause of acute kidney injury 6.
In addition to rge U.S. Food and Drug Administration (FDA)–approved medications, unregulated sources of potentially nephrotoxic substances are the alternative/complementary products, which are widely available at most health food stores 17. Included are items described as herbal remedies, natural products, and nutritional supplements 5. Another concern is that these products often contain a number of harmful chemicals and/or contaminants that are not listed on the label 5. Not uncommonly, the substances listed on the package label are present in varying amounts ranging from large, to small, to even nonexistent. In addition to direct nephrotoxicity, herbal products may interact with conventional drugs producing another potential avenue of nephrotoxicity. Examples of such unlisted contents include Ephedra species and aristolochic acid as well herbal products adulterated with phenylbutazone and other nonsteroidal anti-inflammatory drugs (NSAIDs), cadmium, and dichromate 5.
Nephrotoxic drugs and intoxicants list
Therapeutic medications
- Antimicrobial
- Aminoglycosides
- Antiviral agents
- Amphotericin B
- Colistin
- Polymixin B
- Sulfadiazine
- Quinolones
- Vancomycin
- Chemotherapy
- Platins
- Ifosfamide
- Mitomycin
- Gemcitabine
- Methotrexate
- Pentostatin
- Interleukin-2 (high dose)
- Antiangiogenesis agents
- Immunotherapies (immune checkpoint inhibitors, chimeric antigen receptor T cells)
- Analgesics
- Nonsteroidal anti-inflammatory drugs (NSAIDs)
- Selective Cyclo-oxygenase-2 inhibitors
- Phenacetin
- Analgesic combinations
- Immunosuppressives
- Calcineurin inhibitors
- Sirolimus, everolimus
- Other
- Angiotensin-converting enzyme inhibitors (ACE inhibitors) / angiotensin-receptor blockers(ARBs) / renin inhibitors
- Sodium glucose transporter-2 (SGLT-2) inhibitors (canagloflozin, dapagliflozin)
- Methoxyflurane
- Sucrose (IVIg excipient), hydroxyethyl starch, mannitol, dextran
- Pamidronate, Zolendronate
- Topiramate, Zonisamide
- Orlistat
- Statins
- Mesalamine
Alternative medicine/supplement products
- Herbal remedies
- Aristolochic acid
- Ephedra sp.
- Glycyrrhiza sp.
- Datura sp.
- Taxus celebica
- Uno degatta
- Cape aloes
- Adulterants
- Mefenamic acid
- Dichromate
- Cadmium
- Phenylbutazone
- Melamine
Diagnostic agents
- Radiocontrast
- High osmolar
- Low osmolar
- Iso-osmolar
- Other agents
- Gadolinium (in high dose)
- Oral sodium phosphate solution (colonoscopy prep)
Environmental intoxicants
- Heavy metals
- Lead
- Mercury
- Cadmium
- Uranium
- Copper
- Bismuth
- Solvents
- Hydrocarbons
- Other toxins
- Silicon
- Germanium
Types of nephrotoxicity
Cardiovascular
- General: diuretics, β-blockers, vasodilator agents
- Local: ACE inhibitors, ciclosporin.
Direct tubular effect
- Proximal convoluted tubule: Aminoglycoside (Amikacin sulfate) antibiotics (e.g. gentamicin), amphotericin B, cisplatin, radiocontrast media, immunoglobulins, mannitol
- Distal tubule: Nonsteroidal anti-inflammatory drugs (NSAIDs) (e.g. aspirin, ibuprofen, diclofenac), ACE inhibitors, ciclosporin, lithium salts, cyclophosphamide, amphotericin B
- Tubular obstruction: sulphonamides, methotrexate, aciclovir, polyethylene glycol.
Acute interstitial nephritis
- β-lactam antibiotics, vancomycin, rifampicin, sulphonamides, ciprofloxacin, NSAIDs, ranitidine, cimetidine, furosemide, thiazides, phenytoin.
Acute glomerulonephritis
- Penicillamine.
Causes of diabetes insipidus
- Lithium salts
- Amphotericin B
- Fluoride
- Demeclocycline
- Foscarnet.
Other nephrotoxins
- Heavy metals interfere with enzymes of energy metabolism.
- Aristolochic acid, found in some plants and, more dangerously, in some herbal supplements derived from those plants, has been shown to have nephrotoxic effects on humans.
Nephrotoxicity signs and symptoms
Symptoms of nephrotoxicity may begin so slowly that you don’t notice them right away.
Healthy kidneys prevent the buildup of wastes and extra fluid in your body and balance the salts and minerals in your blood—such as calcium, phosphorus, sodium, and potassium. Your kidneys also make hormones that help control blood pressure, make red blood cells, and keep your bones strong.
Nephrotoxicity means your kidneys no longer work well enough to do these jobs and, as a result, other health problems develop. As your kidney function goes down, you may:
- have swelling, usually in your legs, feet, or ankles
- get headaches
- feel itchy
- feel tired during the day and have sleep problems at night
- feel sick to your stomach, lose your sense of taste, not feel hungry, or lose weight
- make little or no urine
- have muscle cramps, weakness, or numbness
- have pain, stiffness, or fluid in your joints
- feel confused, have trouble focusing, or have memory problems
Kidney disease can lead to other health problems. Your health care team will work with you to help you avoid or manage:
- High blood pressure. High blood pressure can be both a cause and a result of kidney disease. High blood pressure damages your kidneys, and damaged kidneys don’t work as well to help control your blood pressure. With kidney failure, your kidneys can’t get rid of extra water. Taking in too much water can cause swelling, raise your blood pressure, and make your heart work harder. Blood pressure-lowering medicines, limiting sodium and fluids in your diet, staying physically active, managing stress, and quitting smoking can help you control your blood pressure.
- Heart disease. Kidney disease and heart disease share two of the same main causes: diabetes and high blood pressure. People with kidney disease are at high risk for heart disease, and people with heart disease are at high risk for kidney disease. The steps that you take to manage your kidney disease, blood pressure, cholesterol, and blood glucose (if you have diabetes) will also help you prevent heart attacks or strokes.
- Anemia. When kidneys are damaged, they don’t make enough erythropoietin (EPO), a hormone that helps make red blood cells. Red blood cells carry oxygen from your lungs to other parts of your body. When you have anemia, some organs—such as your brain and heart—may get less oxygen than they need and may not function as well as they should. Anemia can make you feel weak and lack energy. Your health care provider may prescribe iron supplements. In some cases, your provider may prescribe medicines to help your body make more red blood cells.
- Malnutrition. As your kidney disease gets worse, it can be a challenge to keep yourself well fed. You may not feel hungry, food may taste different, or you may lose interest in food. Infections and other stresses on your body can make it hard for your body to use the food you do eat. Working closely with a dietitian to be sure you’re eating enough of the right foods can have long-term benefits for people with kidney disease.
- Feeling itchy. Itching is common and happens for different reasons. You may feel itchy because you have dry skin. Using a moisturizer may help. Or, you may feel itchy because you have too much phosphorus in your blood. Eating less phosphorus may help stop the itching. Your health care provider may prescribe a medicine called a phosphate binder for you to take with meals. These medicines keep the phosphorus in your food from entering your bloodstream. UV light from sunlight or a light box helps some people find relief
- Mineral and Bone Disorder. Healthy kidneys balance the levels of calcium and phosphorus in your blood and make hormones that help keep your bones strong. As kidney function drops, your kidneys
- make less of the hormone that helps your body absorb calcium. Like one domino knocking over another, the low level of calcium in your blood triggers the release of parathyroid hormone (PTH). Parathyroid hormone (PTH) moves calcium from your bones into your blood. Too much parathyroid hormone (PTH) can also make you feel itchy.
- don’t remove as much phosphorus. Extra phosphorus in your blood also pulls calcium from your bones.
- Without treatment, bones may become thin and weak. You may feel bone or joint pain. Changes to your eating plan, medicines, supplements, and dialysis may help.
Following your treatment plan can help you avoid or address most of these symptoms. Your treatment plan may include regular dialysis treatments or a kidney transplant, a special eating plan, physical activity, and medicines.
Factors associated with drug-induced nephrotoxicity
The development of drug-induced nephrotoxicity can be best understood by examining the factors that contribute to nephrotoxicity 16. Exposure to a potentially nephrotoxic medication is an obvious requirement. Drugs may be modestly nephrotoxic or maintain high risk to cause kidney injury on the basis of their structure, dose, metabolic handling, excretory pathway through the kidney, and other characteristics 4. Underlying patient characteristics, such as comorbid conditions, genetic determinants of drug metabolism and transport, and immune response genes, are also important in drug nephrotoxicity 4. As the kidney metabolizes and excretes (through filtration and tubular secretion) many ingested drugs, the interaction of these substances with various parts of the nephron may be associated with nephrotoxicity 4. For kidney injury to occur, some combination of these three risk factors is generally present. More often than not, more than one is present. It is the differences in structure of the ingested drug, underlying patient characteristics, and alterations in kidney handling of the ingested substance that likely explain the variability and heterogeneity observed with drug-induced nephrotoxicity.
Risk factors for drug nephrotoxicity
Drug factors
- Prolonged dosing periods and nephrotoxic drug exposure
- Potent direct nephrotoxic drug effects
- Combinations of toxins/drugs promoting enhanced nephrotoxicity
- Competition between endogenous and exogenous toxins for transporters, increasing drug accumulation within the tubular cell
- Insoluble drug and/or metabolite with intratubular crystal precipitation
- Drug that accumulates in lysosome due to lack of enzymes to metabolize the drug
Patient factors
- Female sex
- Old age (>65 yr of age)
- Nephrotic syndrome
- Cirrhosis/obstructive jaundice (nephrotoxic bile acids)
- Acute kidney injury
- Chronic kidney disease
- True or effective volume depletion (kidney hypoperfusion)
- Decreased GFR
- Enhanced proximal tubular toxin reabsorption
- Sluggish distal tubular urine flow rates
- Metabolic perturbations
- Hypokalemia, hypomagnesemia, hypercalcemia
- Alkaline or acid urine pH
- Immune response genes increasing allergic drug response
- Pharmacogenetics favoring drug toxicity
- Gene mutations in hepatic and kidney P450 system
- Gene mutations in kidney transporters and transport proteins
Kidney factors
- High rate of blood delivery to the kidneys (approximately 25% of cardiac output)
- Increased drug concentrations within the kidney medulla and interstitium
- Biotransformation of drugs to nephrotoxic metabolites and reactive oxygen species
- High metabolic rate of tubular cells (i.e., loop of Henle) within a hypoxic environment
- Proximal tubular uptake of drugs
- Apical drug uptake via endocytosis or pinocytosis with drug accumulation
- Basolateral drug transport via human organic anion transporters or human organic cation transporters with drug accumulation
- Reduced drug efflux via apical transporters with drug accumulation
Drug dose and duration of therapy
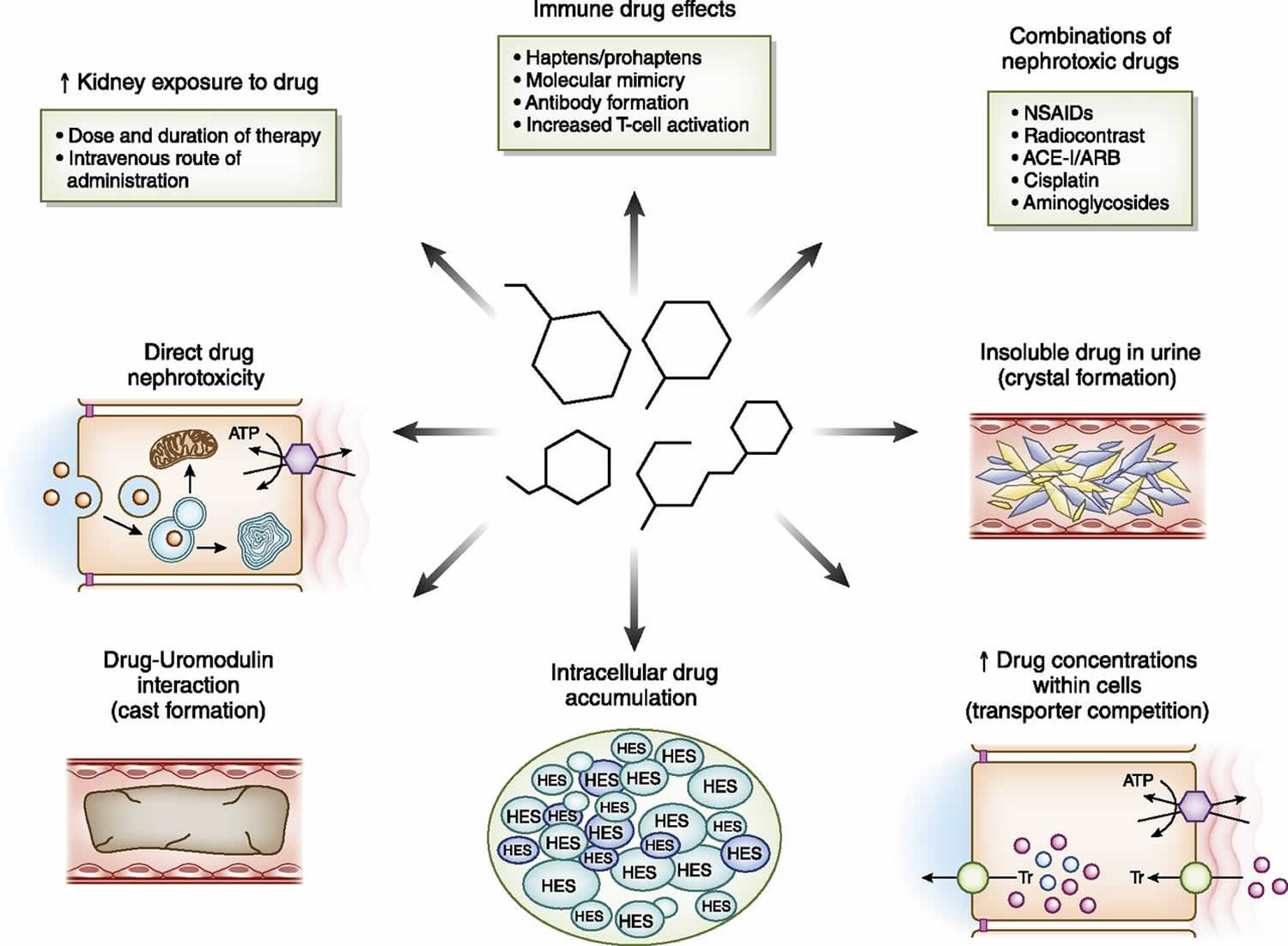
One of the most important parts of drug-induced nephrotoxicity is the innate kidney toxicity of the offending agent. A number of drug characteristics and their varied mechanisms of action play a role in causing kidney injury (Figure 1). High doses and prolonged courses of certain nephrotoxins will enhance risk for kidney injury via excessive exposure of the kidney, even in patients with minimal or no underlying risk. Several drugs such as the aminoglycosides, platinums, amphotericin B, and colistin fall into this category 18.
Figure 1. Drug-induced nephrotoxicity
Footnote: Drug factors associated with increased risk for nephrotoxicity. Medications cause kidney injury through various mechanisms. Increased exposure of the kidney on the basis of route, dose, and duration of drug exposure; drug-related immune effects (such as B-lactams, proton pump inhibitors, NSAIDs, and immune checkpoint inhibitors); combined nephrotoxic drug exposure; and drug and metabolite insolubility in the urine (such as methotrexate, acyclovir, and sulfadiazine) lead to kidney injury. In addition, increased drug concentrations within tubular cells are due to transport effects (such as tenofovir and cisplatin), intracellular accumulation of certain drugs due to lack of metabolizing enzymes (such as sucrose and hydroxyethyl starch), innate direct cell toxicity (such as aminoglycosides, colistin, and amphotericin B), and intratubular cast formation from drugs interacting with uromodulin (vancomycin).
Abbreviations: ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; HES = hydroxyethyl starch; NSAIDs = nonsteroidal anti-inflammatory drugs; PPI = proton pump inhibitor; Tr = transporter.
[Source 1 ]Drug characteristics (solubility, structure, and charge)
Drugs and metabolites that are insoluble in the urine may cause acute crystalline nephropathy by precipitating in distal tubular lumens 19. This process is enhanced further by reduced urinary flow rates, urine pH (depending on drug pKa), excessive drug dosing, and rapid infusion rates. In addition to obstructing urinary flow, precipitated crystals induce inflammation in the surrounding interstitium. Medications associated with development of crystalline nephropathy include methotrexate, acyclovir, indinavir/atazanavir, sulfadiazine, vitamin C, foscarnet, oral sodium-phosphate, and triamterene.
A number of medications used for intravascular volume repletion (dextran, hydroxyethyl starch) or as carrier molecules (sucrose with intravenous immunoglobuling) are associated with osmotic nephropathy 20. These drugs accumulate within phagolysosomes of proximal tubular cells. Because of their structure, these molecules cannot be metabolized and ultimately cause lysosomal dysfunction and cell swelling.
An interesting drug characteristic that enhances nephrotoxicity is the positive charge of polycationic aminoglycosides, which are attracted to the negatively charged proximal tubular membrane phospholipids 21. This facilitates drug binding to the megalin/cubilin receptor complex. For example, aminoglycoside nephrotoxicity is in part related to their cationic charge—neomycin has higher cationic charge and is more nephrotoxic than amikacin, which has a lower cationic charge.
Drug combinations
Combinations of potential nephrotoxic drugs can increase risk for kidney injury with examples including vancomycin+piperacillin/tazobactam, aminoglycosides+cephalothin, NSAIDs+radiocontrast, and cisplatin+aminoglycosides 22. The pathway of excretion by the kidney represents another risk for drug nephrotoxicity. Medications compete with endogenously produced substances (and other drugs) for transport proteins and influx/efflux transporters, which can increase intracellular drug concentration and risk for kidney injury 4. These drug-drug interactions increase kidney injury and overall drug toxicity.
Innate drug nephrotoxicity
A number of medications maintain higher potential for causing kidney injury on the basis of their more significant innate nephrotoxicity. These drugs, which include the aminoglycosides, amphotericin B, the polymyxins, and cisplatin, may cause kidney injury with therapeutic doses and brief durations of exposure 4. Accumulation of high concentrations of the polycationic aminoglycosides within intracellular lysosomes causes lysosomal injury, which is associated with phospholipid membrane injury, oxidative stress, and mitochondrial dysfunction. This promotes proximal tubular cell apoptosis and necrosis with clinical manifestations such as an isolated proximal tubulopathy or acute kidney injury 4.
Amphotericin B, and the lipid/liposomal formulations to a lesser degree, cause kidney injury by disrupting tubular cell membranes and increasing permeability to cations, which result in tubular dysfunction due to cell swelling/dysfunction 23. In general, the lipid/liposomal formulations are less nephrotoxic. The polymixin antimicrobial agents, colistin and polymyxin B, are highly nephrotoxic with a very narrow therapeutic window. Nephrotoxicity is related to their D-amino content and fatty acid component, which increases cellular membrane permeability and allows cation influx 24. This effect leads to tubular cell swelling and lysis with acute kidney injury development.
The acyclic nucleotide phosphonates (adefovir, cidofovir, tenofovir) enter the cell via basolateral human organic anion transporter–1 (hOAT-1) and promote cellular injury primarily through disturbing mitochondrial function. Mitochondrial injury is manifested by mitochondrial enlargement, clumped cristae, and convoluted contours that impair cellular energetics 25. Tenofovir, which is employed widely to treat hepatitis B virus and HIV infection, is associated with proximal tubulopathy and acute kidney injury 25.
Antiangiogenesis therapy with monoclonal antibodies against vascular endothelial growth factor (VEGF), circulating soluble VEGF receptors, and small molecule tyrosine kinase inhibitors that impair intracellular VEGF signaling pathways are associated with various forms of kidney injury 26. In the kidney, VEGF is produced by podocytes and binds glomerular and peritubular capillary endothelial cell VEGF receptors. Glomerular endothelial VEGF receptor binding maintains normal fenestrated endothelial health and is important for normal functioning of the glomerular basement membrane 26. Reduction in VEGF levels or signaling pathways by antiangiogenic drugs promotes loss of the healthy fenestrated endothelial phenotype and promotes microvascular injury and thrombotic microangiopathy, causing proteinuria and acute kidney injury. Reduced nephrin expression in the slit diaphragms may also contribute to the development of proteinuria. Although other kidney lesions occur with these drugs, endothelial injury and thrombotic microangiopathy are most common 26. By interfering with local alternative complement pathway regulators, these drugs may also activate complement and increase risk for thrombotic microangiopathy 27.
Drug-induced inflammation
Another pathway of drug-induced nephrotoxicity is through induction of an inflammatory response by the host, which can target the kidney 28. Through multiple mechanisms (hapten/prohapten, molecular mimicry, immune-complex formation), medications can promote the development of acute interstitial nephritis leading to acute kidney injury and/or various urinary abnormalities such as tubular proteinuria, pyuria, and hematuria 28. Classic drugs associated with acute interstitial nephritis include antimicrobial agents (in particular B-lactams and sulfonamides), NSAIDs, proton pump inhibitors, and aminosalicylates 29. Newer agents such as the immune checkpoint inhibitors (ipilimumab, nivolumab, pembrolizumab) cause acute interstitial nephritis via activation of T cells and perhaps reducing tolerance to exogenous drugs 30. As will be discussed, the patient’s genetic makeup may enhance immunogenicity to exogenous agents.
Drug-induced cast nephropathy
Another intriguing drug-related kidney injury is vancomycin-related obstructive tubular cast formation. Using immunohistologic staining techniques to detect vancomycin in kidney tissue, casts composed of noncrystal nanospheric vancomycin aggregates entangled with uromodulin have been observed in patients with acute kidney injury 31. In these patients, high vancomycin trough plasma levels were observed. These same vancomycin casts were reproduced experimentally in mice using in vivo imaging techniques. Thus, the interaction of uromodulin with nanospheric vancomycin aggregates represents a new mode of tubular injury with development of vancomycin-associated cast nephropathy 31.
The Patient
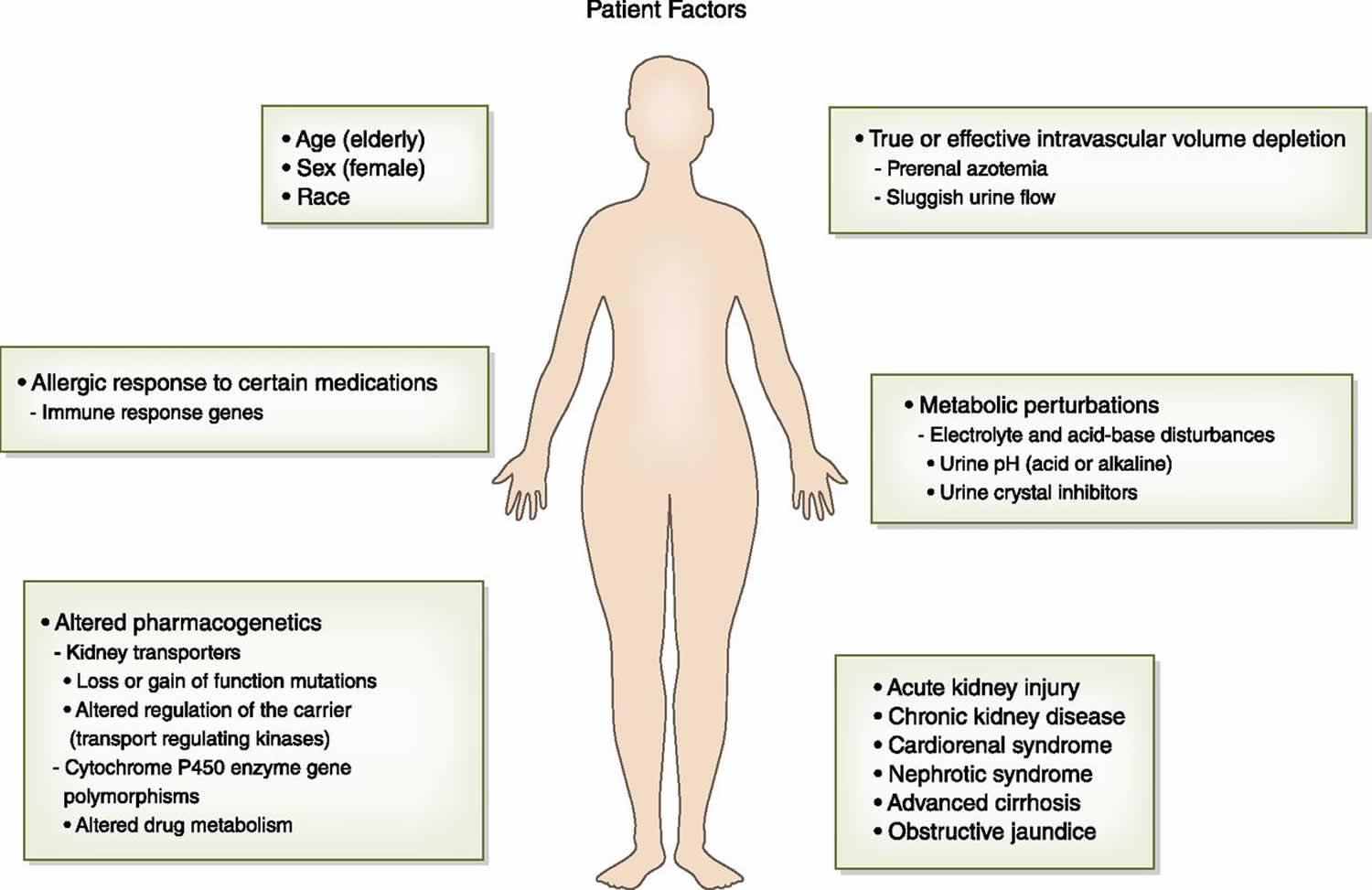
There are a number of patient-specific factors that increase risk for medication-induced nephrotoxicity (Figure 2). Underlying risk factors for nephrotoxicity may be nonmodifiable, such as older age and female sex, which are associated with decreased lean body mass and reduced total body water that can lead to excess drug dosing (6–9). A “normal serum creatinine” in these patients may actually be a lower GFR. Women and the elderly have lower serum albumin concentrations—hypoalbuminemia results in reduced drug binding and increased free drug concentrations that can be nephrotoxic (6–9,35–38). In addition to these factors, the elderly have an increased propensity to vasoconstriction from excessive circulating angiotensin II and endothelin levels and have higher levels of oxidatively modified biomarkers (58). These factors combine to increase patient exposure to excess drug concentrations and nephrotoxicity risk.
Figure 2. Patient factors that increase risk for drug-induced nephrotoxicity
Footnote: Patient factors that increase risk for drug-induced nephrotoxicity. Patients have risk factors from nonmodifiable characteristics such as age, sex, race, and the genetic makeup of immune response genes and drug metabolizing enzymes and transport pathways that enhance the nephrotoxicity of drugs. Comorbid conditions such as liver disease, heart disease, and chronic kidney disease and acutely developed diseases such as intravascular volume depletion, metabolic perturbations, and acute kidney injury are also important risk factors for drug-induced nephrotoxicity.
[Source 1 ]Genetic makeup
Along the lines of nonmodifiable risk factors is the patient’s underlying genetic makeup. In fact, the role of pharmacogenetics as an explanation for the heterogeneous patient response to drugs (underdosing, therapeutic dosing, and overdosing) reflects genetic makeup and supports the need for “personalized” or “precision” medicine. As such, underlying host genetic makeup can enhance vulnerability of the kidney to potential nephrotoxins 32. There are data that suggest that metabolic pathways, transport proteins, and drug transporters vary between patient populations due to the effect of genetic composition. Several enzymes that comprise the hepatic cytochrome P450 (CYP450) enzyme system have gene polymorphisms that are associated with reduced drug metabolism and subsequent end organ toxicity. Because the kidney also possesses CYP450 enzymes that participate in drug metabolism 32, it is not surprising that gene polymorphisms favoring reduced drug metabolism could increase nephrotoxic risk.
Polymorphisms of genes encoding proteins involved in the metabolism and subsequent elimination of drugs by the kidney as well as the repair pathways after drug injury are correlated with various levels of drug sensitivity. Polymorphisms in genes encoding ERCC1, a key enzyme in the DNA repair pathway by which cells repair platinum-induced DNA damage, may be associated with increased nephrotoxicity 33. Polymorphisms in cytosolic glutathione-S-transferase enzymes, which normally function to detoxify reactive molecules such as cisplatin, increase risk for nephrotoxicity with exposure to this drug 34.
Loss-of-function mutations in apical secretory transporters that reduce drug efflux from the cell into the urine, and mutations in kinases that regulate drug carrier proteins, can impair drug elimination and promote nephrotoxicity by elevating intracellular drug concentrations 32. It is probable that patients differ in the function and regulation of receptors, channels, carriers, and transporters that regulate the metabolism and elimination of drugs by the kidneys. Tenofovir-induced Fanconi syndrome represents one such example 34. Patients with HIV receiving tenofovir who developed Fanconi syndrome were noted to have a single nucleotide polymorphism: 1249 G→A single nucleotide polymorphism in the gene coding the multidrug-resistant protein-2 efflux transporter, which transports tenofovir out of the cell into the urine. In contrast, treated patients with HIV who did not develop Fanconi syndrome did not have the gene polymorphism 34.
Genetic alterations in a patient’s immune system may also enhance risk for drug nephrotoxicity via inflammatory injury. The administered drug or its metabolite may form adducts that modify their physical structure, which enhances their immunogenicity 35. Heterogeneity in patient response to drugs and exogenous agents exists, with one example being the heightened allergic response of some individuals as compared with others. As such, differences in innate host immune response genes can predispose some patients to developing an allergic reaction to a medication 35. In fact, the variability of immune responses has been demonstrated in patients who develop drug-induced acute interstitial nephritis, which appears to be a T cell–driven process 36. Thus, enhanced vulnerability to an allergic response in the kidney and the associated development of acute interstitial nephritis reflect yet another form of drug nephrotoxicity.
Comorbid diseases
Underlying acute kidney injury and chronic kidney disease are also important risk factors for increasing vulnerability to nephrotoxic injury 37. The decline in GFR and increase in tubular secretion of endogenous substances (and medications) increase risk for adverse drug-related kidney effects. GFR reduction can also result in excessive drug dosing for medications excreted by the kidneys, increased drug exposure in a reduced number of functioning nephrons and ischemia preconditioned tubular cells, and more robust oxidative injury response to various medications by the kidney. In addition, increased tubular secretion of drugs that are cleared by both glomerular filtration and tubular secretion may enhance kidney tubular toxicity 37.
Other types of systemic and kidney disease may also increase the nephrotoxic effects of drugs. Nephrotic syndrome and cirrhosis enhance nephrotoxic risk through multiple mechanisms that include altered kidney perfusion from reduced effective circulating blood volume, hypoalbuminemia with increased free circulating drug levels, and unrecognized kidney impairment 37. Obstructive jaundice also enhances toxicity to certain drugs, such as the aminoglycosides, through altered hemodynamics such as decreased renal blood flow and direct toxic effects of bile salts on tubular epithelia 38. True volume depletion from vomiting, diarrhea, and diuretics as well as effective volume depletion associated with congestive heart failure, ascites, and sepsis increase risk for drug nephrotoxicity. Induction of kidney hypoperfusion and prerenal physiology by these comorbidities increases the nephrotoxicity of many drugs 37. Ultimately, reduced kidney perfusion enhances nephrotoxicity in drugs excreted through the kidneys by fostering drug overdosing, increasing drug concentrations within tubular cells in drugs reabsorbed by the proximal tubule, and enhancing drug/metabolite crystal precipitation within distal tubular lumens in the setting of sluggish urinary flow rates of insoluble drugs 37.
Metabolic disturbances
A number of metabolic abnormalities can also increase risk for adverse kidney effects with certain drugs. For example, electrolyte disorders such as hypokalemia, hypomagnesemia, and hypocalcemia increase the nephrotoxicity associated with the aminoglycosides 37. Severe hypercalcemia leads to afferent arteriolar vasoconstriction and tubular sodium and water wasting, which induces prerenal physiology, which enhances nephrotoxic drug injury. Metabolic disorders that alter urinary pH also increase risk for intratubular crystal deposition with certain drugs 37. Systemic metabolic acidosis or alkalosis may decrease or increase urine pH, whereas proximal and distal renal tubular acidoses are associated with alkaline urine due to impaired ability of the kidney to excrete H+ ion. Acidic urinary pH (<5.5) increases intratubular crystal deposition with drugs such as sulfadiazine, methotrexate, and triamterene that have limited solubility in a low-pH environment 39. Alkaline urine (pH>6.0) increases crystal precipitation within tubular lumens from drugs such as indinavir, atazanavir, oral sodium phosphate solution, and ciprofloxacin 39. In addition, drugs such as topiramate, zonisamide, and acetazolamide induce the formation of an alkaline urine by inhibiting carbonic anhydrase thereby promoting precipitation of calcium-phosphate within tubules and enhancing risk for nephrolithiasis 40.
The Kidney
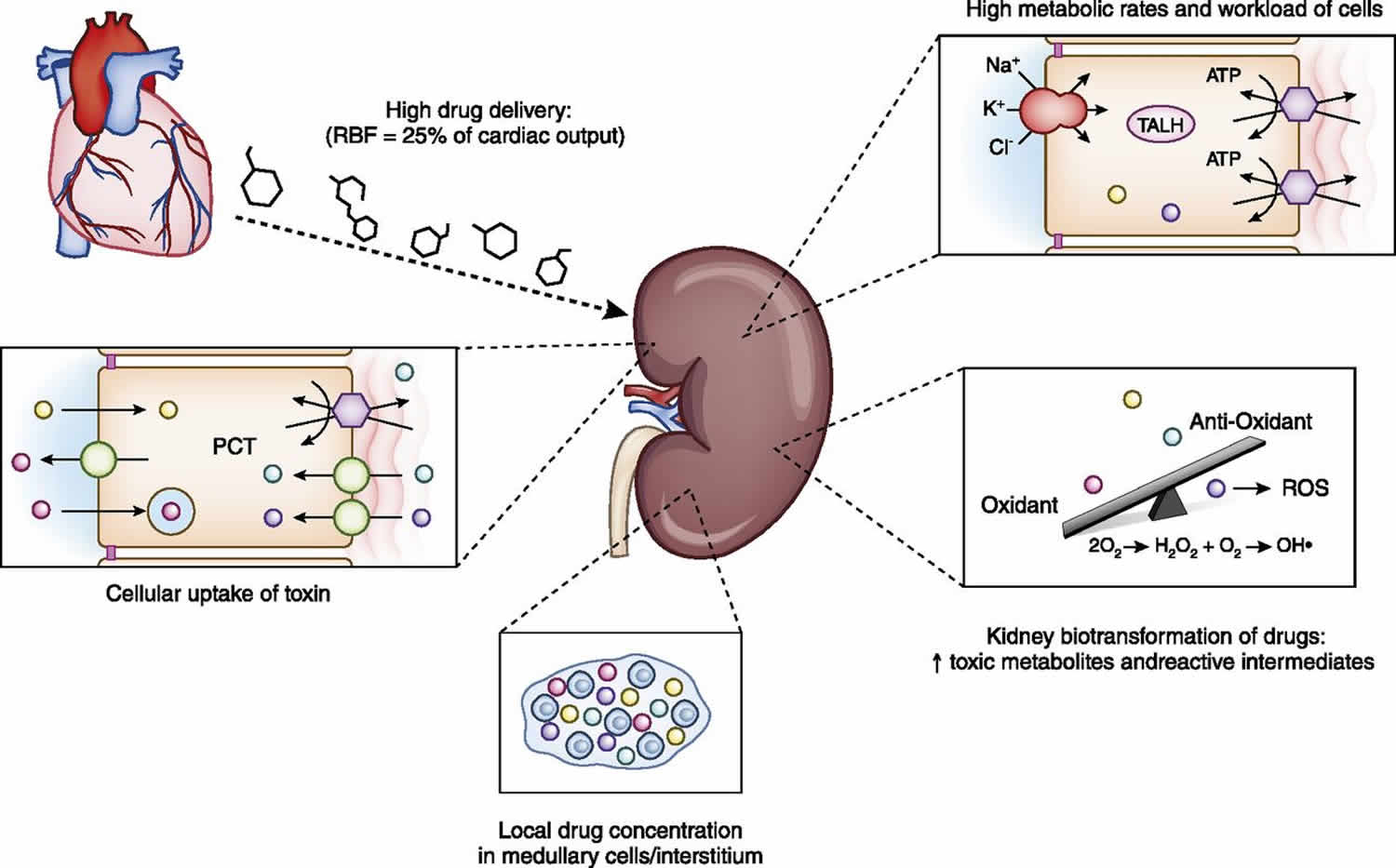
The mechanism by which the kidney metabolizes and excretes various drugs and toxins importantly contributes to drug nephrotoxicity (Figure 3). The high rate of drug and toxin delivery to the kidney, a result of high renal blood flow, which approximates 25% of cardiac output, exposes the kidney to significant drug concentrations 37. In addition, many tubular cells, particularly those in the loop of Henle, reside in a relatively hypoxic environment due to the high metabolic requirements associated with active solute transport by Na+-K+-ATPase–driven transport 37. Excessive cellular workload of these cells in this relatively hypoxic environment enhances risk for a nephrotoxic-related injury. High concentrations of certain medications and their metabolites develop in the kidney medulla and interstitium from the enormous concentrating ability of the kidney, which can induce kidney injury through direct toxicity as well as ischemic damage from reduced prostaglandin and increased thromboxane production 37.
Figure 3. Kidney factors that enhance risk for drug-induced nephrotoxicity
Footnote: High renal blood flow increases drug delivery and exposure to the kidney. High metabolic rates of thick ascending loop of Henle tubular cells increase risk for drug nephrotoxicity. Kidney metabolism of drugs to toxic metabolites and reactive oxygen species overwhelms local antioxidants and promotes tubular injury. Increased concentrations of potentially nephrotoxic drugs in the medulla and interstitium increase kidney injury. Apical uptake of certain drugs (aminoglycosides, hydroxyethyl starch) and basolateral transport of drugs through the organic anion transporter (tenofovir) and organic cation transporter (cisplatin) increase kidney toxicity.
Abbreviations: PCT = proximal convoluted tubule; RBF = renal blood flow; ROS = reactive oxygen species; TALH = thick ascending loop of Henle.
[Source 1 ]Drug metabolism
In addition to hepatic metabolism, a number of drugs undergo biotransformation by kidney enzyme systems, including the CYP450 and flavin-containing monooxygenases 37. This leads to the potential formation of nephrotoxic metabolites and reactive oxygen species as seen with the aminoglycosides, platinums, and several other medications 37. These byproducts of biotransformation may swing the balance in favor of oxidative stress, which outstrips natural antioxidants and increases kidney injury via DNA strand breaks, nucleic acid alkylation or oxidation, lipid peroxidation, and protein damage 37.
Drug excretory pathway
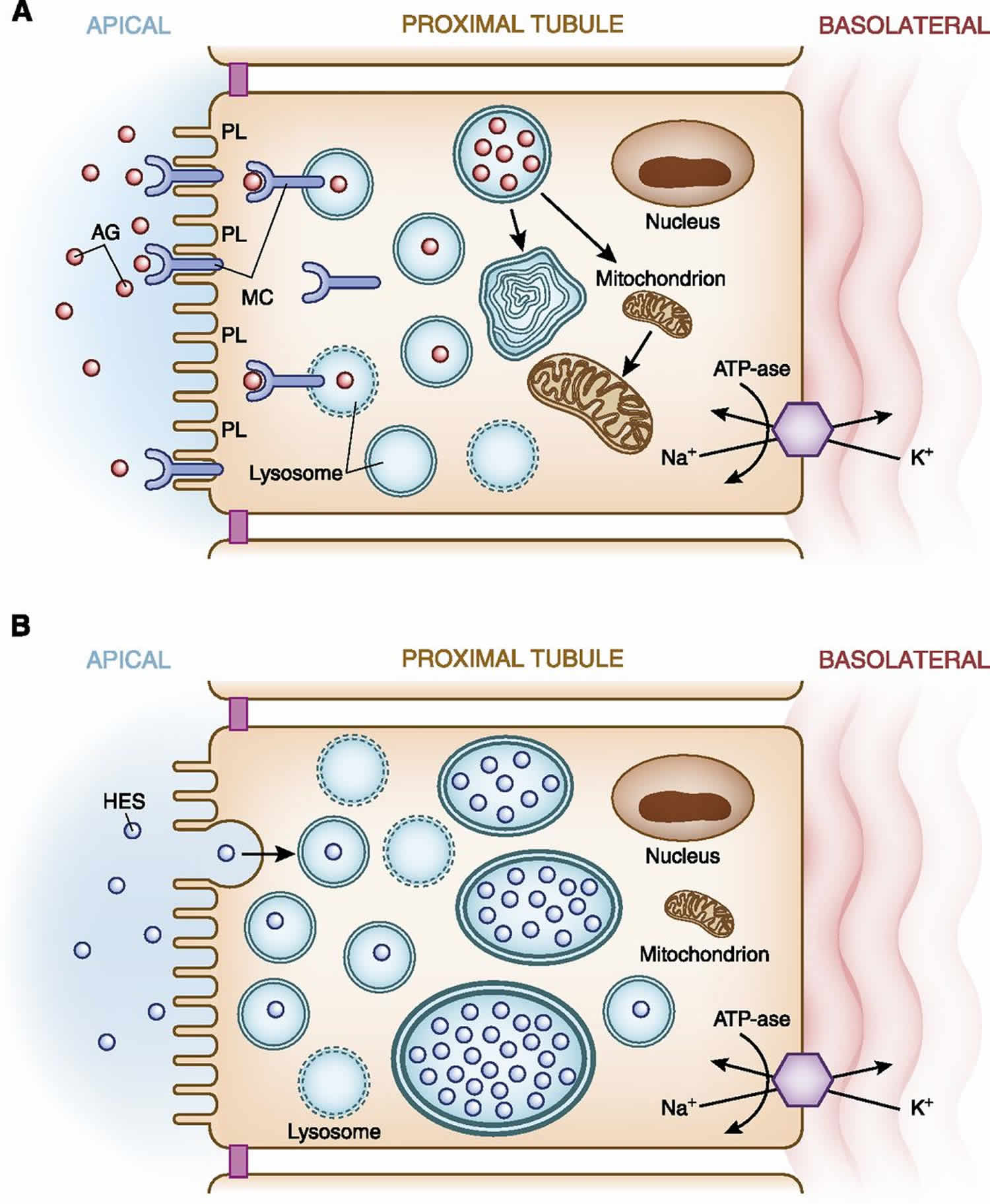
Drugs are excreted from the body by both glomerular filtration and tubular secretion. An important avenue of kidney injury occurs with excretion of drugs via the active transporters in proximal tubular cells 37. Extensive tubular cell uptake of potential nephrotoxic drugs via both apical and basolateral transport systems underlies development of kidney injury. From the urinary space, apical uptake of drugs occurs via endocytosis/pinocytosis and other active/passive transport pathways 37. Medications taken up via this pathway include polycationic aminoglycosides (Figure 4A), heavy metals, and various complex sugars and starches. In the case of aminoglycosides, after endocytic receptor (megalin/cubilin) binding and uptake of these cationic ligands, these drugs are translocated into the lysosomal compartment where they accumulate and subsequently form myeloid bodies 37. Myeloid bodies are membrane fragments and damaged organelles formed as a consequence of aminoglycoside inhibition of lysosomal enzymes. This apical pathway of uptake leads to accumulation of a critical concentration of aminoglycoside within cells, which triggers an injury cascade leading to cell injury and death, which present clinically as a proximal tubulopathy and/or acute kidney injury. Filtered dextran, sucrose, and hydroxyethyl starch may cause tubular injury when they undergo pinocytosis by proximal tubular cells (6,9,34,35). Similar to the aminoglycosides, after pinocytosis these substances are taken up by and collect in lysosomes (Figure 4B). The absence of cellular enzymes capable of metabolizing these substances allows them to build up within the cytoplasm and cause tubular cell injury and acute kidney injury 37.
Figure 4. Apical transport of drugs in the proximal tubule
Footnote: (A) Aminoglycosides Apical membrane handling of substances, in this example aminoglycosides, by proximal tubular cells increases cellular uptake of this nephrotoxic drug. Polycationic aminoglycosides are attracted to the anionic phospholipid membranes where they interact with megalin-cubilin receptor on the apical surface. The aminoglycosides are endocytosed and enter the cell where they are translocated into lysosomes. Lysosomal injury and rupture along with mitochondrial injury result in tubular cell injury. (B) Hydroxyethyl starch. Apical membrane handling of hydroxyethyl starch by proximal tubular cells increases cellular uptake of this potentially nephrotoxic drug. Hydroxyethyl starch as well as sucrose (carrier for intravenous immunoglobulin), dextran, and mannitol undergo pinocytosis and enter the cell where they are translocated into lysosomes. The lack of enzymes necessary to metabolize these substances allows accumulation within lysosomes, which causes cell swelling (occluding tubular lumens) and eventual lysosomal rupture resulting in tubular cell injury.
Abbreviations: AG = aminoglycosides; HES = hydroxyethyl starch; IVIg = intravenous immunoglobulin; K+ = potassium; MC = megalin-cubilin; Na+ = sodium; PL = anionic phospholipids.
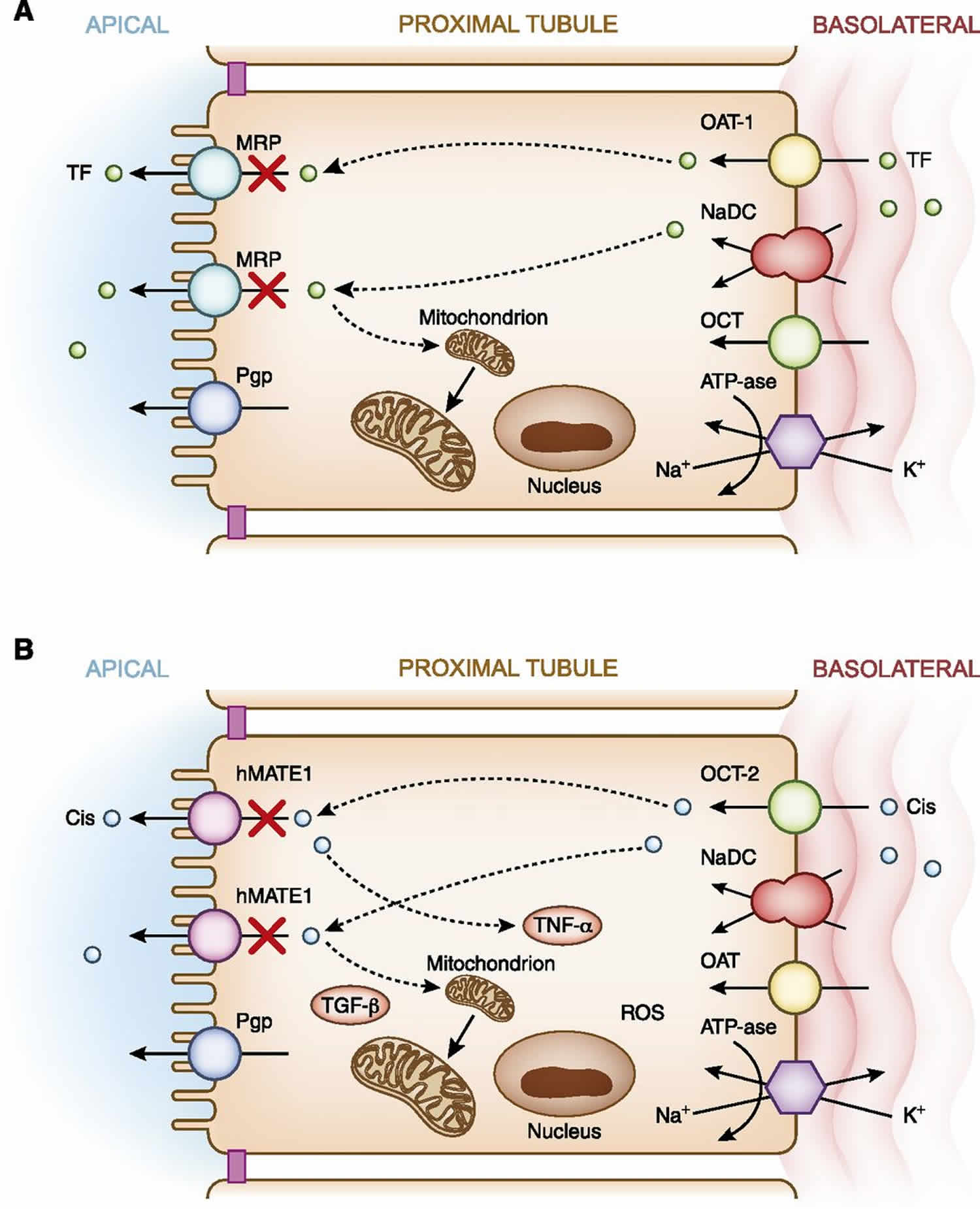
[Source 1 ]In addition to apical uptake of drugs, another pathway of proximal tubular cell drug exposure occurs via basolateral delivery via the peritubular capillaries 41. After delivery of potentially nephrotoxic drugs by the peritubular capillaries, uptake into proximal tubular cells occurs via a family of active transporters 41. These include the human organic anion transporter for negatively charged drugs and the human organic cation transporters for positively charged drugs 41. Endogenously produced anionic and cationic substances, as well as exogenously administered drugs, compete for transport via these pathways. Classic examples of potentially nephrotoxic drugs utilizing these transport pathways are the acyclic nucleotide phosphonates such as tenofovir (Figure 5A), which are transported via hOAT-1 (6,26,43), and cisplatin, which is transported via human organic cation transporter-2 (hOCT-2) (Figure 5B) 41. Upon transport of drugs into proximal tubular cell cytoplasm, they move through the intracellular space by various regulated carrier proteins, and subsequently exit from cells via apical transport proteins 41. Transport of drugs through proximal tubular cells, as well as the buildup of drug concentrations when transport out of cells is blunted (or transport into the cell is increased), enhances risk for nephrotoxicity 41. Examples of the former are loss-of-function mutations in and competition for apical secretory transporters 41. This reduces nephrotoxin efflux from cell into urine, which may promote accumulation of toxic substances within proximal tubular cells and cause cellular injury via apoptosis or necrosis (Figure 5). An example of the latter is reduced glomerular filtration of drug, which increases proximal tubular drug secretion and increases tubular cell drug exposure 37. Ultimately, this extensive trafficking of drugs increases tubular exposure and risk for elevated concentration of potentially nephrotoxic drugs when other risk factors supervene.
Figure 5. Basolateral transport of drugs in the proximal tubule
Footnote: (A) Tenofovir. Basolateral handling of certain drugs, in this example tenofovir, by proximal tubular cells may lead to cellular injury. Tenofovir is delivered to the basolateral membrane, transported into the cell via the human organic anion transporter-1, and excreted by various apical transporters into the urinary space. In this example, transport by the multidrug-resistance protein transporters is inhibited or dysfunctional, causing intracellular accumulation of drug and nephrotoxicity via mitochondrial toxicity. (B) Cisplatin. Basolateral handling of certain drugs such as cisplatin by proximal tubular cells may lead to cellular injury. Cisplatin is delivered to the basolateral membrane, transported into the cell via the human organic cation transporter-2, and excreted by various apical transporters into the urinary space. Intracellular accumulation of cisplatin due to increased basolateral uptake or deficient efflux by the human multidrug and toxin extrusion protein transporter1 ( hMATE1) transporters into the urine leads nephrotoxicity via production of a number of substances (TNF-α, TGF-β, and reactive oxygen species), which promote mitochondrial toxicity.
Abbreviations: Cis = cisplatin; hMATE1 = human multidrug and toxin extrusion protein transporter; K+ = potassium; MRP = multidrug resistance protein transporter; Na+ = sodium; NaDC = sodium dicarboxylate transporter; OAT-1 = organic anion transporter-1; OCT-1= organic cation transporter-1; Pgp = P-glycoprotein transporter; ROS = reactive oxygen species; TF = tenofovir; TGF-β= transforming growth factor β; TNF-α = tumor necrosis factor α.
[Source 1 ] References- Pharmacology behind Common Drug Nephrotoxicities. Mark A. Perazella. CJASN Dec 2018, 13 (12) 1897-1908; DOI: 10.2215/CJN.00150118
- Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015
- Moffett BS, Goldstein SL: Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol 6: 856–863, 2011
- Perazella MA: Drug use and nephrotoxicity in the intensive care unit. Kidney Int 81: 1172–1178, 2012
- Luciano RL, Perazella MA: Aristolochic acid nephropathy: Epidemiology, clinical presentation, and treatment. Drug Saf 38: 55–64, 2015
- Perazella MA, Reilly RF: Imaging patients with kidney disease: How do we approach contrast-related toxicity? Am J Med Sci 341: 215–221, 2011
- Markowitz GS, Perazella MA: Drug-induced renal failure: A focus on tubulointerstitial disease. Clin Chim Acta 351: 31–47, 2005
- Gold JM, Raja A. Cisplatin (Cisplatinum) [Updated 2019 Sep 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547695
- Crona DJ, Faso A, Nishijima TF, McGraw KA, Galsky MD, Milowsky MI. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist. 2017 May;22(5):609-619.
- Otani IM, Wong J, Banerji A. Platinum Chemotherapy Hypersensitivity: Prevalence and Management. Immunol Allergy Clin North Am. 2017 Nov;37(4):663-677.
- Ishii H, Hirai K, Sugiyama K, Nakatani E, Kimura M, Itoh K. Validation of a Nomogram for Achieving Target Trough Concentration of Vancomycin: Accuracy in Patients With Augmented Renal Function. Ther Drug Monit. 2018 Dec;40(6):693-698.
- Araya AA, Tasnif Y. Tacrolimus. [Updated 2019 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544318
- Ruzicka T, Assmann T, Lebwohl M. Potential future dermatological indications for tacrolimus ointment. Eur J Dermatol. 2003 Jul-Aug;13(4):331-42.
- Nankivell BJ, PʼNg CH, OʼConnell PJ, Chapman JR. Calcineurin Inhibitor Nephrotoxicity Through the Lens of Longitudinal Histology: Comparison of Cyclosporine and Tacrolimus Eras. Transplantation. 2016 Aug;100(8):1723-31.
- Wargo KA, Edwards JD. Aminoglycoside-induced nephrotoxicity. J Pharm Pract. 2014 Dec;27(6):573-7.
- Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015
- Luciano RL, Perazella MA: Nephrotoxic effects of designer drugs: Synthetic is not better! Nat Rev Nephrol 10: 314–324, 2014
- Perazella MA, Izzedine H: New drug toxicities in the onco-nephrology world. Kidney Int 87: 909–917, 2015
- Stratta P, Lazzarich E, Canavese C, Bozzola C, Monga G: Ciprofloxacin crystal nephropathy. Am J Kidney Dis 50: 330–335, 2007
- Dickenmann M, Oettl T, Mihatsch MJ: Osmotic nephrosis: Acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis 51: 491–503, 2008
- Nagai J, Takano M: Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet 19: 159–170, 2004
- Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL: Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: A systematic review and meta-analysis. Crit Care Med 46: 12–20, 2018
- Alexander BD, Wingard JR: Study of renal safety in amphotericin B lipid complex-treated patients. Clin Infect Dis 40[Suppl 6]: S414–S421, 2005
- Falagas ME, Kasiakou SF. Nephrotoxicity of intravenous colistin: A prospective evaluation. Crit Care 10: R27 1–13, 2006
- Izzedine H, Harris M, Perazella MA: The nephrotoxic effects of HAART. Nat Rev Nephrol 5: 563–573, 2009
- Izzedine H, Escudier B, Lhomme C, Pautier P, Rouvier P, Gueutin V, Baumelou A, Derosa L, Bahleda R, Hollebecque A, Sahali D, Soria JC: Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): An 8-year observational study at a single center. Medicine (Baltimore) 93: 333–339, 2014
- Keir LS, Firth R, Aponik L, Feitelberg D, Sakimoto S, Aguilar E, Welsh GI, Richards A, Usui Y, Satchell SC, Kuzmuk V, Coward RJ, Goult J, Bull KR, Sharma R, Bharti K, Westenskow PD, Michael IP, Saleem MA, Friedlander M: VEGF regulates local inhibitory complement proteins in the eye and kidney. J Clin Invest 127: 199–214, 2017
- Moledina DG, Perazella MA: Drug-induced acute interstitial nephritis. Clin J Am Soc Nephrol 12: 2046–2049, 2017
- Moledina DG, Perazella MA: Proton pump inhibitors and CKD. J Am Soc Nephrol 27: 2926–2928, 2016
- Shirali AC, Perazella MA, Gettinger S: Association of acute interstitial nephritis with Programmed Cell Death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 68: 287–291, 2016
- Luque Y, Louis K, Jouanneau C, Placier S, Esteve E, Bazin D, Rondeau E, Letavernier E, Wolfromm A, Gosset C, Boueilh A, Burbach M, Frère P, Verpont MC, Vandermeersch S, Langui D, Daudon M, Frochot V, Mesnard L: Vancomycin-associated cast nephropathy. J Am Soc Nephrol 28: 1723–1728, 2017
- Awdishu L, Nievergelt CM, Davenport A, Murray PT, Macedo E, Cerda J, Chakaravarthi R, Ramachandra Rao SP, Holden A, Goldstein SL, Mehta RL: Rationale and Design of the Genetic Contribution to Drug Induced Renal Injury (DIRECT) Study. Kidney Int Rep 1: 288–298, 2016
- Suk R, Gurubhagavatula S, Park S, Zhou W, Su L, Lynch TJ, Wain JC, Neuberg D, Liu G, Christiani DC: Polymorphisms in ERCC1 and grade 3 or 4 toxicity in non-small cell lung cancer patients. Clin Cancer Res 11: 1534–1538, 2005
- Petros WP, Hopkins PJ, Spruill S, Broadwater G, Vredenburgh JJ, Colvin OM, Peters WP, Jones RB, Hall J, Marks JR: Associations between drug metabolism genotype, chemotherapy pharmacokinetics, and overall survival in patients with breast cancer. J Clin Oncol 23: 6117–6125, 2005
- Krishnan N, Perazella MA: Drug-induced acute interstitial nephritis: Pathology, pathogenesis, and treatment. Iran J Kidney Dis 9: 3–13, 2015
- Spanou Z, Keller M, Britschgi M, Yawalkar N, Fehr T, Neuweiler J, Gugger M, Mohaupt M, Pichler WJ: Involvement of drug-specific T cells in acute drug-induced interstitial nephritis. J Am Soc Nephrol 17: 2919–2927, 2006
- Perazella MA: Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol 4: 1275–1283, 2009
- Schrier RW, Kaloyanides GJ, Bosmans J-L, DeBroe ME: Antibiotic and Immunosuppression-related renal failure. In: Diseases of the Kidney and Urogenital Tract, edited by Schrier RW, Philadelphia, PA, Lippincott Williams & Wilkinson, 2001, pp 1137–1174
- Perazella MA: Onco-nephrology: Renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol 7: 1713–1721, 2012
- Vega D, Maalouf NM, Sakhaee K: Increased propensity for calcium phosphate kidney stones with topiramate use. Expert Opin Drug Saf 6: 547–557, 2007
- Hucke A, Ciarimboli G: The role of transporters in the toxicity of chemotherapeutic drugs: Focus on transporters for organic cations. J Clin Pharmacol 56[Suppl 7]: S157–S172, 2016