What are neural tube defects?
Neural tube defects are serious birth defects that occur in the development of the brain and spinal cord of some babies. The most common neural tube defects are spina bifida (abnormal development of part of the spine and spinal cord) and anencephaly (severely abnormal development of the brain). Spina bifida affects about 1,500 babies a year in the United States. If your baby has spina bifida, the tiny bones of the spine don’t close completely, and part of the spinal cord pokes through the spine. Children with spina bifida may have paralyzed legs (not able to move) and problems controlling their bladder and bowel (going to the bathroom). Milder forms of spina bifida may cause fewer problems for children. Anencephaly is the most severe form of cranial neural tube defect and is characterized by the absence of cortical tissue (although brainstem and cerebellum may be variably present) as well as absence of the cranial vault. Anencephaly affects about 1,000 babies each year in the United States. Anencephaly is caused when the upper part of the neural tube that forms the brain doesn’t close completely. Babies with this condition are missing major parts of the brain, skull and scalp. They do not survive long after birth, usually for just a few hours. Girls are 3 times more likely than boys to have anencephaly.
Neural tube defects develop very early during pregnancy when the neural tube—which forms the early brain and the spinal cord—does not close properly. Neural tube defects are intense birth anomalies that can occur 21 to 28 days after conception 1. Neural tube defects are a significant cause of stillbirth and lifelong handicaps 2. The chance that a pregnancy will be affected by a neural tube defect is less than one in 1,000 3. Neural tube defectss happen in about 3,000 pregnancies each year in the United States. Hispanic women are more likely than non-Hispanic women to have a baby with an neural tube defects. Neural tube defects are a major cause of death and lifelong disability worldwide. Up to 85 percent of neural tube defects can be prevented if women consume enough folic acid before and during early pregnancy. But worldwide only 15 percent of neural tube defects are prevented.
The neural tube comprises of a bundle of nerve sheath which closes to form brain caudally and spinal cord rostrally. The closure should occur at around the 28th day of conception failing which the brain or spinal cord doesn’t form properly.
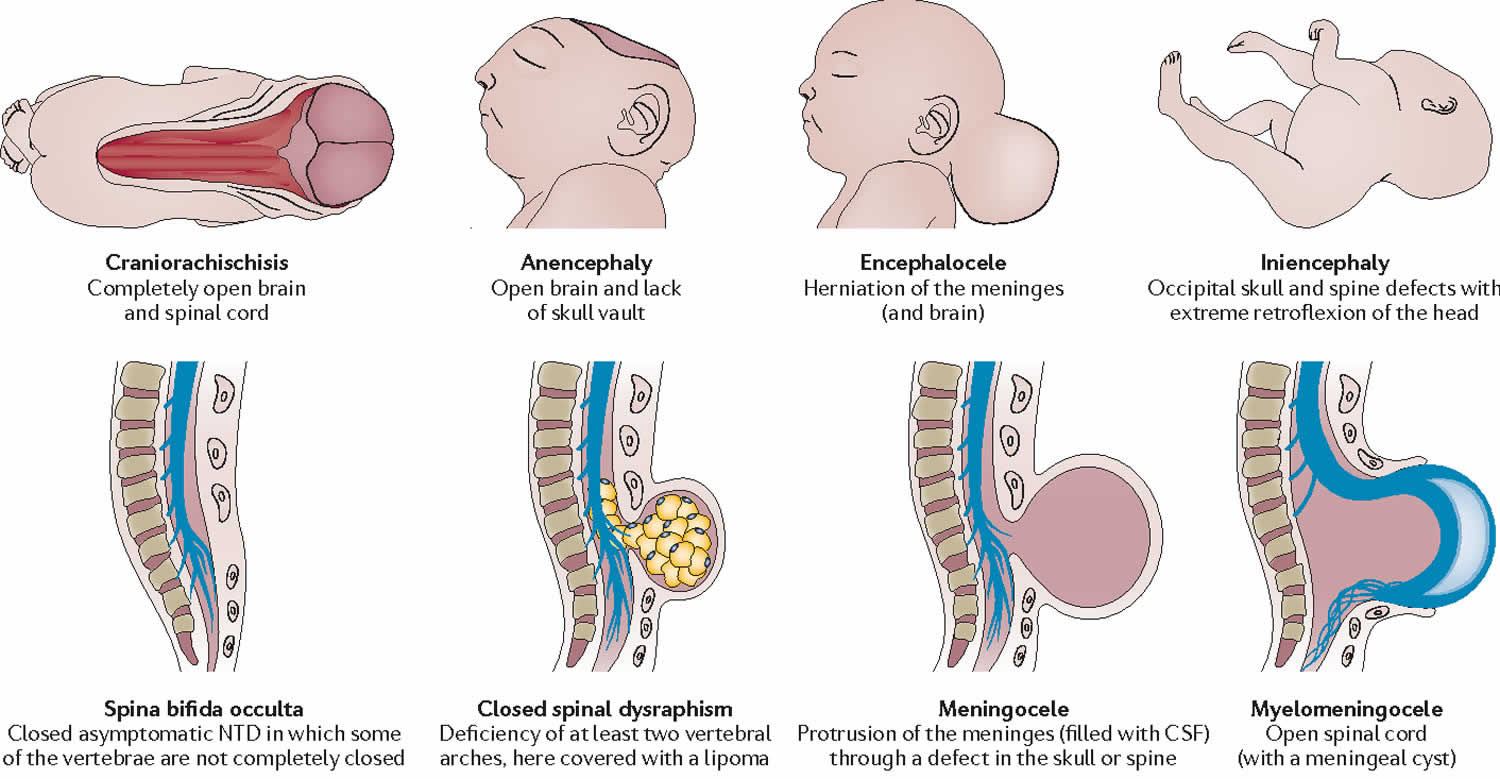
Numerous types of neural tube defect are recognized including 4:
- myelomeningocele (spina bifida) (50%)
- anencephaly (40%)
- encephalocele (5%)
- craniorachischisis
- tethered cord
- exencephaly
- iniencephaly (rare)
- meningoencephalocele
- meningocele
An essential type of neural tube defect is called cranial dysraphism. This anomaly is the failure of cranial neural tube closure and includes anencephaly and encephaloceles. Spinal dysraphism is another type of error that is due to failure of closure of the caudal neuropore and includes spina bifida cystica and occulta. Neural tube defects classify into ventral, dorsal, or midline defects. These defects can further be classified as open where the open neural tube can be exposed to the surrounding environment, or closed where the overlying skin covers the defect. Craniorachischisis is a rare form of neural tube defect in which the neural tube fails to close throughout the entire body axis 5.
The baby’s neural tube closes during the first weeks of pregnancy, often before a woman knows that she is pregnant. If a woman consumes folic acid before and during early pregnancy, it can help increase the chance of her baby’s neural tube closing properly. Waiting until the first prenatal visit (typically, the 6th to 12th week of pregnancy) to start folic acid consumption will not prevent neural tube defects. Therefore, to help prevent neural tube defects, it is important for women to start folic acid consumption before pregnancy begins.
During the first month of life, an embryo (developing baby) grows a primitive tissue structure called the ‘neural tube’. As the embryo develops, the neural tube begins to change into a more complicated structure of bones, tissue and nerves that will eventually form the spine and nervous system.
However, in cases of spina bifida, something goes wrong with the development of the neural tube and the spinal column (the ridge of bone that surrounds and protects the nerves) does not fully close. Spina bifida is a Latin term that means ‘split spine’.
Figure 1. Neural tube development
Figure 2. Neural tube defects
Anencephaly
Anencephaly occurs when the neural tube fails to close at the head. The brain and the skull bones do not develop normally. Infants born with this problem die at, or soon after birth.
Spina bifida
The term spina bifidacomes from the Latin words ‘spina’ meaning spine and ‘bifida’ meaning split or divided. The back-bone (spine) is made up of separate bones called vertebrae, which normally cover and protect the spinal cord.
- When the baby is developing, if the developing neural tube fails to close (usually at the base of the spine), the vertebrae will not completely fuse.
- As a result, the spinal cord and the protective sac that surrounds the cord (meninges)may protrude through the open part of the spine, ie. spina bifida.
Symptoms associated with spina bifida vary depending on the position of the opening along the spine and on how much of the spinal cord, or the protective sac (meninges), protrudes through the vertebrae.
- If only the sac protrudes, the condition is less severe than if the cord itself and the associated nerves protrude and are damaged.
- The condition tends to have a more severe effect when the opening is higher up the spine.
Spina bifida has been grouped into different categories according to the location and severity of the neural tube defect:
- Occulta is where the outer parts of the vertebrae is not completely joined. The spinal cord and covering (meninges)are undamaged. There are often tufts of hair found at the site of the neural tube defect (Figure 3).
- Meningoceleis where the outer parts of the vertebrae are split and the spinal cord is normal. Only the covering of the spinal cord (meninges)is damaged and pushed out through the opening (Figure 3).
- Myelomeningocele where the outer parts of the vertebrae is split and the spinal cord and the meninges are damaged and protruding from the opening (Figure 3).
Some individuals with spina bifida may develop hydrocephalusor ‘water on the brain’. This happens when spinal fluid collects in and around the brain, causing the head to become enlarged. The fluid can be drained through a special tube (called a shunt) that is surgically placed and runs under the skin, down into the chest or abdomen. This treatment helps to reduce the build-up of pressure inside the skull caused by fluid, and to minimise the chance of intellectual impairment occurring.
Figure 3. Spina bifida
What causes neural tube defects
The cause of neural tube defects is not certain but it appears to be due to a combination of genetic and environmental factors.
Multifactorial inheritance refers to the pattern of inheritance of certain conditions due to a combination of both genetic and other factors that may include internal factors such as ageing, and exposure to external environmental factors such as diet, lifestyle, and exposure to chemicals or other toxins.
Multifactorial conditions do not always develop despite the presence of a genetic mutation which increases the person’s risk. For example, not all women who have a mutation in a folate metabolism gene will have a baby with a neural tube defects. The mutation alone is not completely penetrant.
The reason for this incomplete penetranceof the condition is most likely due to the interaction between the information in the gene mutation with the information in one or more other genes and with other ‘environmental’ factors.
Women are at increased risk of having a baby with a neural tube defect if:
- they have already had a baby with a neural tube defect (spina bifida, anencephaly or another neural tube defect)
- they or their partner were born with a neural tube defect
- they or their partner have a close relative born with a neural tube defect
- they have type 1 (insulin dependent) diabetes (not gestational diabetes)
- they have poorly controlled diabetes
- they are obese (BMI>30) 6
- they take certain anti-epileptic medications (antiseizure medicines), especially those containing sodium valproate or valproic acid 7 and other medications (which may affect absorption or metabolism of folate).
- they use opioids in the first 2 months of pregnancy. Opioids are highly addictive drugs. Your provider may prescribe an opioid to you as a painkiller if you’ve been injured or had surgery. Common prescription opioids include codeine, hydrocodone and oxycodone. These often are sold and used illegally. If you take any opioid during pregnancy, it can cause serious problems for your baby, like premature birth and drug withdrawal called neonatal abstinence syndrome (also called NAS). If you’re pregnant and taking any drug or medicine that may be an opioid, tell your health care provider right away.
- they have a high body temperature early in pregnancy. This may be caused by a fever or by spending a lot of time in a hot tub or sauna. If you’re pregnant, stay out of hot tubs and saunas. If you do use them, limit the time to less than 10 minutes.
Getting enough folic acid, vitamin B9, before and during pregnancy prevents most neural tube defects.
How are neural tube defects inherited?
For the majority of neural tube defectss, both genetic factors and environmental factors are involved in the cause of the condition. The combination of genetics and external factors is called multifactorial inheritance. Scientists can see that genetic factors are involved as some women who have a family history of neural tube defectss are more likely to have a baby with a neural tube defects.
- If a woman has had a previous baby with a neural tube defects, the chance of having another affected baby is about 1 in 30. About half of this will be for anencephaly.
- A woman has an equal chance for either spina bifida or anencephaly in future pregnancies, regardless of whichever of these neural tube defects occurred in a previous pregnancy.
- If a woman has a sibling or a parent that had a baby with a neural tube defects, the chance of having an affected baby herself is about 1 in 220.
Neural tube defects prevention
To help prevent neural tube defectss in your baby, before pregnancy take a vitamin supplement with 400 mcg of folic acid every day. About 2 thirds of neural tube defects can be prevented through increasing folate (folic acid) intake at least a month before pregnancy and during the first 3 months of pregnancy. Adequate folate levels are critical during the early days of the developing embryo, particularly the 3rd and 4th week, the period in which neural tube defects occur and when many women won’t know they are pregnant.
You can increase your folate intake by eating folate-rich foods, including folate-fortified foods in your daily diet, or by taking a folic acid supplement. Good sources of folate include green leafy vegetables, fruit (citrus, berries and bananas), legumes and some cereals (bread and many breakfast cereals now have added folate).
Women who take medicines to control epilepsy, seizures or psychiatric disorders should talk to their doctor before taking folate because it can interfere with how their medications work.
Occasionally, some women will take the daily recommended amount of folic acid and still have a baby with a neural tube defect. Although the majority of neural tube defects can be prevented by getting 400 micrograms (mcg) of folic acid every day, some neural tube defects have other causes. If you have had a baby affected by a neural tube defect, be sure to discuss with your doctor or a genetic counselor your risk of having another pregnancy affected with a neural tube defect.
Women at high risk for neural tube defects
If you’re at high risk for having a baby with an neural tube defects, take 4,000 mcg of folic acid each day to help prevent an neural tube defects. Start taking 4,000 mcg 3 months before you get pregnant through 12 weeks of pregnancy. You’re at high risk if:
- You’ve had a pregnancy with an neural tube defects in the past.
- You or your partner has an neural tube defects.
- Your partner has a child with an neural tube defects.
Studies show that taking 4,000 mcg of folic acid before and during early pregnancy can help reduce your risk of having another baby with an neural tube defects by about 70 percent. Ask your provider how to safely get this much folic acid. It’s not safe to take several multivitamins or prenatal vitamins because you can get too much of other nutrients, which may be harmful to your health. Your provider can help you figure out the best and safest way for you to get the right amount of folic acid.
Folic acid and neural tube defects
Folic acid or folate (vitamin B9), is water-soluble B vitamin that is naturally present in some foods, added to others, and available as a dietary supplement. If a woman consumes enough folic acid (400 micrograms (mcg) daily) before and during early pregnancy, it can help prevent her baby from having a neural tube defect. Women can do this by taking a vitamin supplement containing the recommended amount of folic acid or eating enough food that is fortified with folic acid. Fortified foods include enriched breads, pastas, rice, and some breakfast cereals.
The terms “folic acid ” and “folate” often are used interchangeably. However, folate is a general term used to describe the many different forms of vitamin B9: folic acid, dihydrofolate (DHF), tetrahydrofolate (THF), 5, 10-methylenetetrahydrofolate (5, 10-MTHF), and 5-methyltetrahydrofolate (5-MTHF) 8.
Folic acid is the synthetic (that is, not generally occurring naturally) form of folate that is used in supplements and in fortified foods, such as rice, pasta, bread, and some breakfast cereals. In many scientific studies done in countries around the world, folic acid has been shown to be effective in preventing neural tube defects 9.
Natural folate can be found in foods such as leafy green vegetables, citrus fruits, and beans. A woman should eat a balanced diet rich in natural folate from food. However, it is very difficult for most women to get the daily recommended amount of folate through food alone 10.
Food folates are in the tetrahydrofolate form and usually have additional glutamate residues, making them polyglutamates 11. Folic acid is the fully oxidized monoglutamate form of folate (vitamin B9) that is used in fortified foods and most dietary supplements. Some dietary supplements also contain folate in the monoglutamyl form, 5-methyl-tetrahydrofolate (also known as L-5- MTHF, 5-MTHF, L-methylfolate, and methylfolate).
Folate functions as a coenzyme or cosubstrate in single-carbon transfers in the synthesis of nucleic acids (DNA and RNA) and metabolism of amino acids 12. One of the most important folate-dependent reactions is the conversion of homocysteine to methionine in the synthesis of S-adenosyl-methionine, an important methyl donor. Another folate-dependent reaction, the methylation of deoxyuridylate to thymidylate in the formation of DNA, is required for proper cell division. An impairment of this reaction initiates a process that can lead to megaloblastic anemia, one of the hallmarks of folate deficiency 13.
When consumed, food folates are hydrolyzed to the monoglutamate form in the gut prior to absorption by active transport across the intestinal mucosa 14. Passive diffusion also occurs when pharmacological doses of folic acid are consumed. Before entering the bloodstream, the enzyme dihydrofolate reductase reduces the monoglutamate form to tetrahydrofolate and converts it to either methyl or formyl forms 11. The main form of folate in plasma is 5-methyl-tetrahydrofolate.
The activity of dihydrofolate reductase varies greatly among individuals 12. When the capacity of dihydrofolate reductase is exceeded, unmetabolized folic acid can be present in the blood 15. Whether unmetabolized folic acid has any biological activity or can be used as a biomarker of folate status is not known 16. Folate is also synthesized by colonic microbiota and can be absorbed across the colon, although the extent to which colonic folate contributes to folate status is unclear 17. The total body content of folate is estimated to be 15 to 30 mg; about half of this amount is stored in the liver and the remainder in blood and body tissues 11.
Serum folate concentrations are commonly used to assess folate status; a value above 3 ng/mL indicates adequacy 18. This indicator, however, is sensitive to recent dietary intake, so it might not reflect long-term status. Erythrocyte folate concentrations provide a longer-term measure of folate intakes; a concentration above 140 ng/mL indicates adequate folate status 18.
A combination of serum or erythrocyte folate concentration and indicators of metabolic function can also be used to assess folate status. Plasma homocysteine concentration is a commonly used functional indicator of folate status because homocysteine levels rise when the body cannot convert homocysteine to methionine due to a 5-MTHF deficiency 18. Homocysteine levels, however, are not a highly specific indicator of folate status because they can be influenced by other factors, including kidney dysfunction and deficiencies of vitamin B12 and other micronutrients 18. The most commonly used cutoff value for elevated homocysteine levels is 16 micromol/L, although slightly lower values of 12 to 14 micromol/L have also been used 14. A homocysteine cutoff of 10 micromol/L has been proposed for assessing folate status in populations 12.
Can neural tube defects be prevented by taking the vitamin folate?
Every woman has a chance of having a child with a neural tube defects. Most of the time, this chance is small. Studies have shown that 4 to 7 out of 10 (40% to 70%) cases of neural tube defects can be prevented by increasing the mother’s intake of folate before and during early pregnancy.
Many pregnancies are unplanned, so all women should make sure that they have a folate-rich diet, or take a daily folate tablet.
A folate-rich diet with a wide variety of vegetables, fruits, legumes, whole grains and cereals is a healthy way of eating for everyone. Eating this group of foods can help prevent heart disease, some cancers, diabetes and other health problems. It can be difficult to obtain enough folate from your diet so many women choose to take a daily tablet.
In most women, it is possible to reduce the chance of neural tube defectss occurring in a baby by taking folate in a specified dose.
Food sources of folate
Folate is naturally present in a wide variety of foods, including vegetables (especially dark green leafy vegetables), fruits and fruit juices, nuts, beans, peas, seafood, eggs, dairy products, meat, poultry, and grains (Table 1) 19. Spinach, liver, asparagus, and brussels sprouts are among the foods with the highest folate levels.
In January 1998, the U.S. Food and Drug Administration (FDA) began requiring manufacturers to add 140 mcg folic acid/100 g to enriched breads, cereals, flours, cornmeals, pastas, rice, and other grain products 20 to reduce the risk of neural tube defects. Because cereals and grains are widely consumed in the United States, these products have become important contributors of folic acid to the American diet. The fortification program increased mean folic acid intakes in the United States by about 190 mcg/day 21. In April 2016, FDA approved the voluntary addition of up to 154 mcg folic acid/100 g to corn masa flour 22.
Since November 1, 1998, the Canadian government has also required the addition of 150 mcg folic acid/100 g to many grains, including enriched pasta, cornmeal, and white flour 23. Many other countries, including Costa Rica, Chile, and South Africa, have also established mandatory folic acid fortification programs 24.
Table 1. Selected Food Sources of Folate and Folic Acid
| Food | Micrograms (mcg) per serving | Percent DV* |
|---|---|---|
| Beef liver, braised, 3 ounces | 215 | 54 |
| Spinach, boiled, ½ cup | 131 | 33 |
| Black-eyed peas (cowpeas), boiled, ½ cup | 105 | 26 |
| Breakfast cereals, fortified with 25% of the DV† | 100 | 25 |
| Asparagus, boiled, 4 spears | 89 | 22 |
| Brussels sprouts, frozen, boiled, ½ cup | 78 | 20 |
| Lettuce, romaine, shredded, 1 cup | 64 | 16 |
| Avocado, raw, sliced, ½ cup | 59 | 15 |
| Spinach, raw, 1 cup | 58 | 15 |
| Rice, white, medium-grain, cooked, ½ cup† | 54 | 14 |
| Broccoli, chopped, frozen, cooked, ½ cup | 52 | 13 |
| Mustard greens, chopped, frozen, boiled, ½ cup | 52 | 13 |
| Green peas, frozen, boiled, ½ cup | 47 | 12 |
| Kidney beans, canned, ½ cup | 46 | 12 |
| Spaghetti, cooked, enriched, ½ cup† | 45 | 11 |
| Wheat germ, 2 tablespoons | 40 | 10 |
| Tomato juice, canned, ¾ cup | 36 | 9 |
| Crab, Dungeness, 3 ounces | 36 | 9 |
| Orange juice, ¾ cup | 35 | 9 |
| Bread, white, 1 slice† | 32 | 8 |
| Turnip greens, frozen, boiled, ½ cup | 32 | 8 |
| Peanuts, dry roasted, 1 ounce | 27 | 7 |
| Orange, fresh, 1 small | 29 | 7 |
| Papaya, raw, cubed, ½ cup | 27 | 7 |
| Banana, 1 medium | 24 | 6 |
| Yeast, baker’s, ¼ teaspoon | 23 | 6 |
| Egg, whole, hard-boiled, 1 large | 22 | 6 |
| Cantaloupe, raw, cubed, ½ cup | 17 | 4 |
| Vegetarian baked beans, canned, ½ cup | 15 | 4 |
| Fish, halibut, cooked, 3 ounces | 12 | 3 |
| Milk, 1% fat, 1 cup | 12 | 3 |
| Ground beef, 85% lean, cooked, 3 ounces | 7 | 2 |
| Chicken breast, roasted, 3 ounces | 3 | 1 |
Footnote:
* DV = Daily Value. The FDA developed DVs to help consumers compare the nutrient contents of products within the context of a total diet. The Daily Value for folate used for the values in Table 2 is 400 mcg for adults and children age 4 years and older 25. This Daily Value, however, is changing to 400 mcg dietary folate equivalent (DFE) as the updated Nutrition and Supplement Facts labels are implemented 26. Manufacturers will use the following conversion factors: 1 mcg dietary folate equivalent (DFE) = 1 mcg naturally occurring folate = 0.6 mcg folic acid. The updated labels and Daily Values must appear on food products and dietary supplements beginning in January 2020, but they can be used now 27. The FDA does not require food labels to list folate content unless a food has been fortified with this nutrient. Foods providing 20% or more of the Daily Value are considered to be high sources of a nutrient, but foods providing lower percentages of the Daily Value also contribute to a healthful diet.
† Fortified with folic acid as part of the folate fortification program.
Recommended folic acid intakes
Intake recommendations for folate and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by an expert committee of the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine 14. Dietary Reference Intake (DRI) is the general term for a set of reference values used for planning and assessing nutrient intakes of healthy people. These values, which vary by age and sex, include:
- Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an recommended dietary allowance (RDA).
- Estimated Average Requirement (EAR): Average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals.
- Tolerable Upper Intake Level (UL): Maximum daily intake unlikely to cause adverse health effects. The term, tolerable upper intake level (UL), is defined by the Institute of Medicine as “the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population” 28. In 1998, the Institute of Medicine set the upper intake level (UL) at 1,000 micrograms per day (mcg/day) of folic acid (coming from foods fortified with folic acid and from vitamin supplements). When taking supplements, more is not better. Women who can get pregnant (whether planning to or not) need just 400 mcg/day of folic acid, and they can get this amount from vitamins or fortified foods. This is in addition to eating foods rich in folate. But, your doctor might ask you to take more for certain reasons. The Institute of Medicine has not established a separate tolerable upper intake level (UL) for women of reproductive age. It states that, in general, vitamin B12 deficiency among U.S. women of reproductive age is rare, and they are unlikely to have any adverse effects from consuming supplemental folic acid at or above the UL 28. Women who take a 400 mcg folate tablet each day and eat a folate rich diet are not likely to exceed a safe dose of folate. Too much folate can mask vitamin B12 deficiency but this is rare and can be checked by your doctor.
Table 1 lists the current recommended dietary allowances (RDAs) for folate as mcg of dietary folate equivalents (DFEs). The Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine developed dietary folate equivalents (DFEs) to reflect the higher bioavailability of folic acid than that of food folate. At least 85% of folic acid is estimated to be bioavailable when taken with food, whereas only about 50% of folate naturally present in food is bioavailable 11. Based on these values, the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine defined dietary folate equivalent (DFE) as follows:
- 1 mcg DFE = 1 mcg food folate
- 1 mcg DFE = 0.6 mcg folic acid from fortified foods or dietary supplements consumed with foods
- 1 mcg DFE = 0.5 mcg folic acid from dietary supplements taken on an empty stomach
Factors for converting mcg DFE to mcg for supplemental folate in the form of 5-methyl-THF have not been formally established 29.
For infants from birth to 12 months, the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine established an Adequate Intake (AI) for folate that is equivalent to the mean intake of folate in healthy, breastfed infants in the United States (see Table 2).
Table 2. Recommended Dietary Allowances (RDAs) for Folate
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 6 months* | 65 mcg DFE* | 65 mcg DFE* | ||
| 7–12 months* | 80 mcg DFE* | 80 mcg DFE* | ||
| 1–3 years | 150 mcg DFE | 150 mcg DFE | ||
| 4–8 years | 200 mcg DFE | 200 mcg DFE | ||
| 9–13 years | 300 mcg DFE | 300 mcg DFE | ||
| 14–18 years | 400 mcg DFE | 400 mcg DFE | 600 mcg DFE | 500 mcg DFE |
| 19+ years | 400 mcg DFE | 400 mcg DFE | 600 mcg DFE | 500 mcg DFE |
Footnote: Adequate Intake (AI)
Table 3. Tolerable Upper Intake Levels (ULs) for Folate from Supplements or Fortified Foods
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 6 months | Not possible to establish* | Not possible to establish* | ||
| 7–12 months | Not possible to establish* | Not possible to establish* | ||
| 1–3 years | 300 mcg | 300 mcg | ||
| 4–8 years | 400 mcg | 400 mcg | ||
| 9–13 years | 600 mcg | 600 mcg | ||
| 14–18 years | 800 mcg | 800 mcg | 800 mcg | 800 mcg |
| 19+ years | 1,000 mcg | 1,000 mcg | 1,000 mcg | 1,000 mcg |
Footnote: * Breast milk, formula, and food should be the only sources of folate for infants.
Folic acid supplements
Folic acid is available in multivitamins and prenatal vitamins, supplements containing other B-complex vitamins, and supplements containing only folic acid. Common doses range from 400 to 800 mcg in supplements for adults and 200 to 400 mcg in children’s multivitamins 30.
About 85% of supplemental folic acid, when taken with food, is bioavailable 31. When consumed without food, nearly 100% of supplemental folic acid is bioavailable.
Dietary supplements containing 5-methyl-tetrahydrofolate (also called methylfolate), a reduced form of folate, are also available. For some people, supplementation with 5-methyl-tetrahydrofolate might be more beneficial than with folic acid 32. The bioavailability of 5-methyl-tetrahydrofolate in supplements is the same as or greater than that of folic acid 33. However, unlike folic acid, where 0.6 mcg folic acid = 1 mcg DFE, conversion factors for supplemental folate as 5-methyl-tetrahydrofolate have not been formally established 29.
Supplements containing forms of folate other than folic acid (such as 5-MTHF) should not be confused with the natural food folate found in fruits and vegetables. The effectiveness of these supplements in preventing neural tube defects has not been studied.
Should all women have 400 mcg folate each day?
In general, yes. However some women are at higher chance than others of having a baby with a neural tube defect. These women should have more folate in their system and therefore may need to take 5mg of folate every day.Women in this group should talk to their doctor or genetic counsellor before pregnancy for advice about the amount of folate they should take.
Why is folic acid used in food fortification instead of other folate forms?
- Food fortification is the process by which vitamins and minerals are added to foods.
- Folic acid is more heat-stable than natural food folate, which is broken down easily by heat and light; therefore, folic acid is better suited for food fortification because many fortified products, such as bread, are baked 34.
- Folic acid has been shown to be effective in preventing neural tube defects in randomized control trials and food fortification programs 35.
- Folic acid is absorbed easily by the body, and studies have shown that it can increase blood folate concentrations (the amount in the blood) across populations (including those with the MTHFR TT genetic variant) 36.
- No scientific studies exist that show that supplements containing other forms of folate [such as 5-methyltetrahydrofolate (5-MTHF)] can prevent neural tube defects.
Where can I find folic acid in the United States and in what amounts?
- In the United States, folic acid can be found in foods with mandatory or voluntary fortification, or in supplements. All products labeled as “enriched” are required by the U.S. Food and Drug Administration to be fortified (mandatory fortification) with folic acid, in addition to other micronutrients. The dietary labels on these products must specify that folic acid is included as an ingredient 37.
- Researchers currently estimate that in the United States, people consume about 140 micrograms (mcg) of folic acid each day from mandatorily fortified foods 38.
- Voluntarily fortified foods, such as some ready-to-eat cereals, can be fortified with up to 400 mcg of folic acid in each serving.
- In the United States, supplements containing folic acid generally have 400 to 800 mcg of folic acid per dose, but doses up to 1,000 mcg are allowed without a prescription 39.
- The amount of folic acid consumed from mandatorily fortified foods alone (about 140 mcg each day, on average) occurs at much lower levels than the amount consumed from supplements containing folic acid (about 400 to 1,000 mcg from each dose) or from voluntary fortification (400 mcg from each serving) 38.
Is folic acid safe?
- At this time, folic acid taken at or up to the recommended amount of 400 micrograms per day (mcg/day) has not been shown to be harmful. Additional information continues to be assessed as it becomes available.
- The benefits of taking folic acid are well established. Folic acid has been shown to be effective in preventing neural tube defects in randomized control trials and food fortification programs 40.
- Folic acid also prevents a type of anemia called megaloblastic anemia.
- Some vitamins (such as vitamin D and vitamin A) can collect in fat tissues in the body, so they can be toxic if someone consumes too much. Folic acid does not collect in fat, but instead dissolves in water. This means that any amount of folic acid that is not used by the body (also called “unmetabolized folic acid”) goes through the kidneys, into the urine, and out of the body.
Will folic acid be effective for a woman with a variant in the methyltetrahydrofolate reductase (MTHFR) enzyme?
- All women can benefit from getting 400 micrograms per day (mcg/day) of folic acid, especially before and during early pregnancy.
- MTHFR (methyltetrahydrofolate reductase) is an enzyme that plays a role in how all people process folate. A common genetic variant of the MTHFR enzyme (also known as the MTHFR TT or CT genotypes) determines how rapidly some people can process folate. Even though women with the MTHFR TT or CT genotypes process folate more slowly, they can increase their blood folate concentrations enough to help prevent neural tube defects—some serious birth defects of the brain and spine—by consuming the recommended 400 mcg/day of folic acid 41.
- Research studies have shown that among populations in which more people have the MTHFR TT or CT genotypes, getting 400 mcg/day of folic acid before and during early pregnancy can reduce by 85% the risk of having a baby with a neural tube defect 42.
- Many studies have shown that consuming folic acid increases blood folate concentrations 43. For example, a research study among a population at high risk for neural tube defects showed that, after consuming 400 mcg/day of folic acid for three months, average blood folate concentrations increased to levels that would prevent neural tube defects among women with all MTHFR genotypes, including the MTFHR TT genotype 36.
What is unmetabolized folic acid?
- Unmetabolized folic acid is any amount of folic acid that is found in the blood because it has not been converted into other forms of folate or removed from the body through urination.
- Folic acid is absorbed by the intestines into the bloodstream, and then converted to other forms of folate by the liver. The liver is capable of processing only a certain amount of folic acid at one time. Unused folic acid in the blood goes to the kidneys and leaves the body in urine 44.
How much folic acid must someone consume in order to have leftover unmetabolized folic acid?
- Studies dating back to the late 1990s have shown that people taking a single dose of folic acid of more than 200 micrograms (mcg) can have some unmetabolized folic acid circulating in their blood 45.
- Research also has shown that individuals consuming folic acid from fortified foods, ready-to-eat cereals, or vitamin supplements, or any combination thereof, have varying amounts of unmetabolized folic acid in their blood 46. Since the beginning of mandatory folic acid fortification, most people have had some unmetabolized folic acid circulating in their blood 46.
Does unmetabolized folic acid cause health problems?
Although some people have been concerned about unmetabolized folic acid in the blood, no confirmed health risks have been found 20, 33, 34.
A recent review found no evidence of harmful effects of unmetabolized folic acid in the blood of infants 35.
Neural tube defects screening
Most neural tube defects can be diagnosed by one of the following tests:
- Maternal serum alpha-fetoprotein (AFP): a screening test performed in the pregnant woman serum during 16-18 weeks of pregnancy (elevated)
- Amniocentesis: invasive procedure, performed during 15 weeks of pregnancy
- Antenatal ultrasound: allows detection of anencephaly/acrania at 12 weeks of pregnancy
Prenatal ‘triple screen’ maternal blood test looking for an elevated level of alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG) and estriol and testing the amniotic fluid for a high level of alpha-fetoprotein (AFP), acetylcholinesterase, as well as chromosomal abnormalities, are used for screening and detecting neural tube defects 47.
A prenatal anomaly scan also helps in the detection of all types of neural tube defects.
Neural tube defects may be diagnosed during the antenatal ultrasound scan that is carried out around week 12 of the pregnancy or, more likely, during the anomaly scan that is carried out at around weeks 19 to 20.
Ultrasound scans
An ultrasound scan is a safe procedure that uses sound waves to create an image of the inside of your body. Most hospitals will offer women at least 2 ultrasound scans during their pregnancy. The first is usually at around 8 to 14 weeks and is sometimes called the ‘dating scan’ because it can help to determine when the baby is due. This first scan may be able to detect problems with your baby’s spine that could indicate spina bifida if the condition is severe.
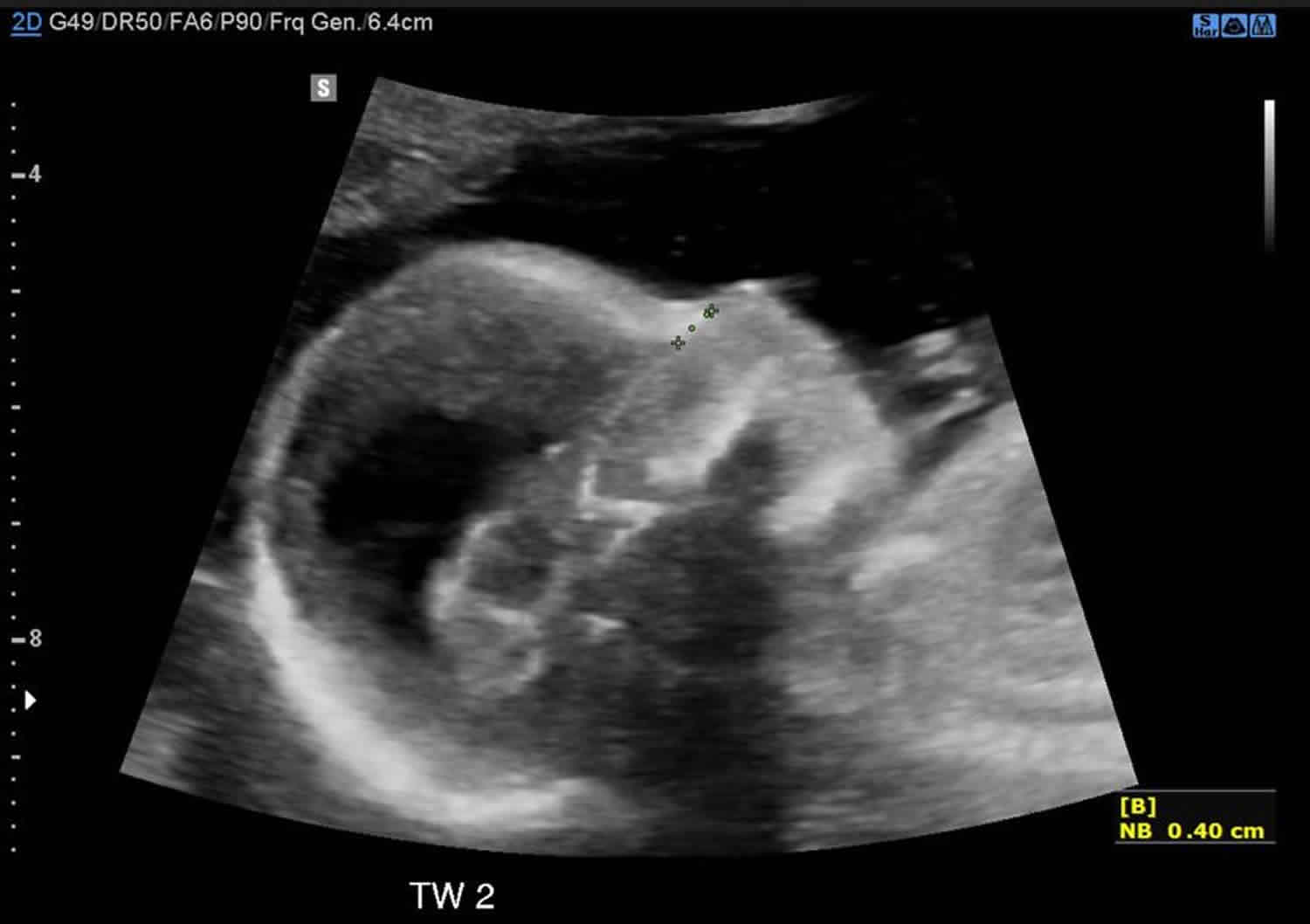
Figure 4. Neural tube defects ultrasound – spina bifida
Footnote: Second trimester morphology ultrasound. Twin 2 breech presentation: Biometry normal for gestational age. The following abnormalities are demonstrated:
- Mild ventriculomegaly with lateral ventricles measuring 11 mm on both sides. CSP and corpus callosum not identified, possibly due to the early gestation and ventriculomegaly.
- The cerebellum is displaced posteriorly consistent with Chiari type 2 malformation.
- There is a neural tube defect extending from L4/L5 down through the sacral spine. The conus appears to extend into the defect.
Bilateral talipes - Deepest pool: 3.8 cm
- Placenta: Posterior, not low lying. Twin 1 centric cord insertion; twin 2 velamentous cord insertion. The cervix is long and closed.
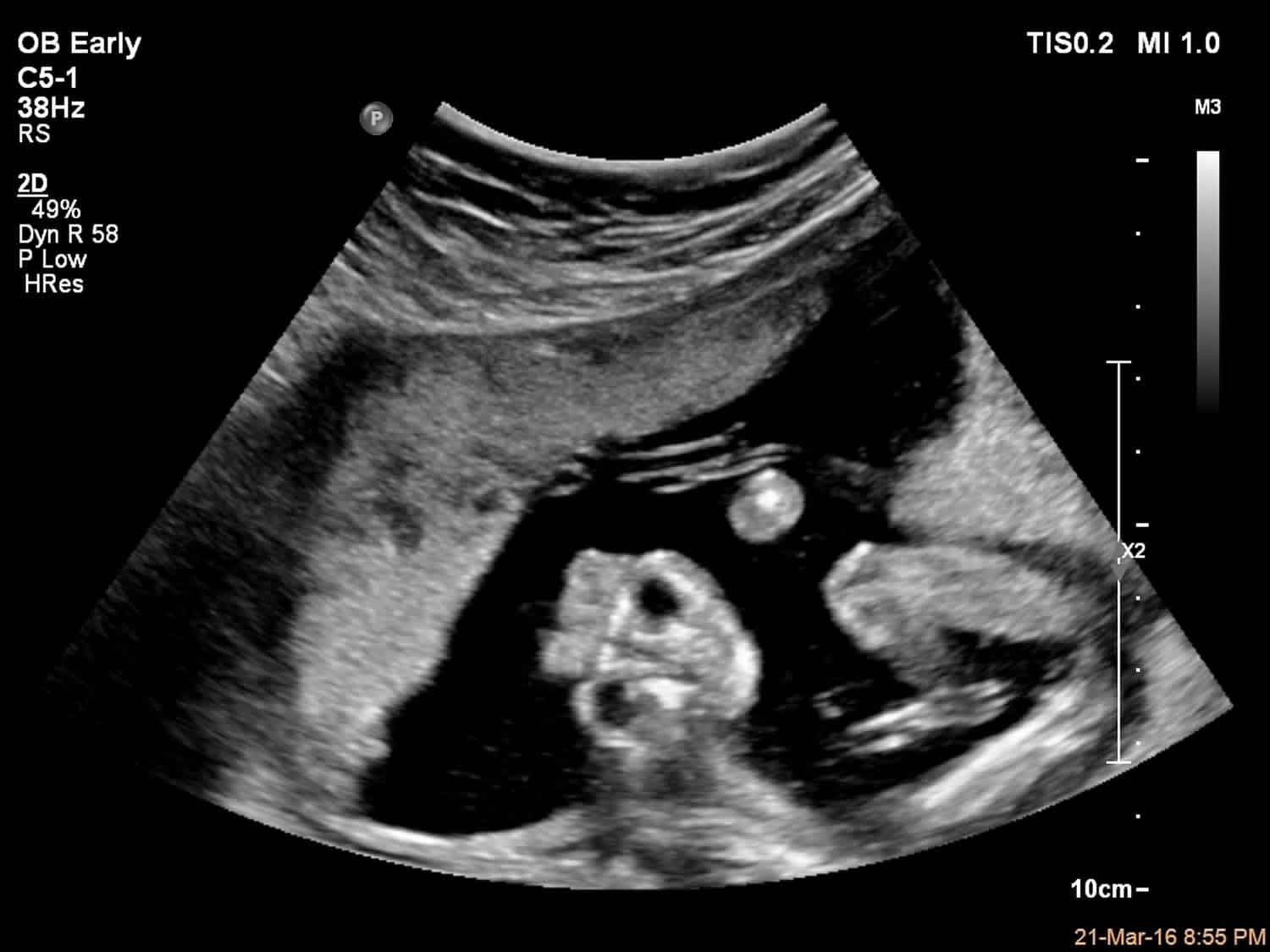
Figure 5. Neural tube defects ultrasound – anencephaly
Footnote: A 21 years old pregnant presenting to the radiology department for antenatal routine ultrasound scan at 17 weeks of gestational age. A single intrauterine fetus at 17 weeks of gestation by AC and FL, with an absence of brain tissue and bony calvarium and a “Frog eye” appearance upon coronal section of the face.
Anomaly scan
The anomaly scan is an ultrasound scan that is carried out around weeks 19 to 20 of your pregnancy. This scan aims to identify any physical problems with your baby. It is usually during this scan that spina bifida is diagnosed.
Coping with the results
If tests confirm that your baby has spina bifida, the implications will be fully discussed with you. You will need to consider your options carefully. Your options are to:
- continue with your pregnancy while getting information and advice so that you are prepared for caring for your baby
- end your pregnancy
If you are considering ending your pregnancy, you should talk to your doctor or midwife. They will be able to provide you with important information and advice.
Your options for ending your pregnancy will depend on how many weeks pregnant you are when you make the decision. If you decide to end your pregnancy, you may wish to talk to a counsellor afterwards. Your doctor or midwife will be able to arrange this for you.
Neural tube defects treatment
Both the management and prognosis is heavily dependent on the type of neural tube defect. The risk for a subsequent pregnancy is thought to be ~5-10%. Surgical correction is the initial treatment in utero or soon after birth with the majority of these surgical patients requiring a ventriculoperitoneal shunt by 12 months of age. Infants with spina bifida even after surgery are still at an elevated risk for nervous system malformations such as skull malformations that press the brain downward toward the spinal canal, and hydrocephalus. Other neurological defects that can occur include lower extremity weakness and paralysis, sensory loss, and bowel and bladder dysfunction. Thus, surgical treatment only provides a partial solution to neural tube defects 48.
References- Singh R, Munakomi S. Embryology, Neural Tube. [Updated 2019 May 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542285
- Salih MA, Murshid WR, Seidahmed MZ. Epidemiology, prenatal management, and prevention of neural tube defects. Saudi Med J. 2014 Dec;35 Suppl 1:S15-28.
- Salih MA, Murshid WR, Seidahmed MZ. Epidemiology, prenatal management, and prevention of neural tube defects. Saudi Med J. 2015;35 Suppl 1: S15-28.
- McDonnell R, Delany V, O’Mahony MT et-al. Neural tube defects in the Republic of Ireland in 2009-11. J Public Health (Oxf). 2015;37 (1): 57-63. doi:10.1093/pubmed/fdu016
- Salih MA, Murshid WR, Seidahmed MZ. Classification, clinical features, and genetics of neural tube defects. Saudi Med J. 2014 Dec;35 Suppl 1:S5-S14
- Huang HY, Chen HL, Feng LP. Maternal obesity and the risk of neural tube defects in offspring: A meta-analysis. (2017) Obesity research & clinical practice. 11 (2): 188-197. doi:10.1016/j.orcp.2016.04.005
- Egen-Lappe V, Hasford J. Drug prescription in pregnancy: analysis of a large statutory sickness fund population. (2004) European journal of clinical pharmacology. 60 (9): 659-66. doi:10.1007/s00228-004-0817-1
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011;3(3):370-84. doi: 10.3390/nu3030370
- De Wals P, Tairou F, Van Allen MI, Uh SH, Lowry RB, Sibbald B, Evans JA, Van den Hof MC, Zimmer P, Crowley M, et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med 2007;357(2):135-42. doi: 10.1056/NEJMoa067103
- General Information About NTDs, Folic Acid, and Folate. https://www.cdc.gov/ncbddd/folicacid/faqs/faqs-general-info.html
- Bailey LB, Caudill MA. Folate. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:321-42.
- Stover PJ. Folic acid. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2012:358-68.
- Carmel R. Folic acid. In: Shils M, Shike M, Ross A, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005:470-81.
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998.
- Paniz C, Bertinato JF, Lucena MR, et al. A daily dose of 5 mg folic acid for 90 days is associated with increased serum unmetabolized folic acid and reduced natural killer cell cytotoxicity in healthy Brazilian adults. J Nutr 2017;147:1677-85.
- Yetley EA, Pfeiffer CM, Phinney KW, et al. Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr 2011;94:303S-12S.
- Lakoff A, Fazili Z, Aufreiter S, et al. Folate is absorbed across the human colon: evidence by using enteric-coated caplets containing 13C-labeled [6S]-5-formyltetrahydrofolate. Am J Clin Nutr 2014;100:1278-86.
- Bailey LB, Stover PJ, McNulty H, et al. Biomarkers of nutrition for development-folate review. J Nutr 2015;145:1636S-80S.
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 28. Nutrient Data Laboratory Home Page, 2016.
- U.S. Food and Drug Administration. Food Standards: Amendment of Standards of Identity For Enriched Grain Products to Require Addition of Folic Acid. Federal Register 1996;61:8781-97.
- Choumenkovitch SF, Selhub J, Wilson PW, et al. Folic acid intake from fortification in United States exceeds predictions. J Nutr 2002;132:2792-8
- U.S. Food and Drug Administration. FDA approves folic acid fortification of corn masa flour 2016. https://www.fda.gov/news-events/press-announcements/fda-approves-folic-acid-fortification-corn-masa-flour
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011;3:370-84.
- Centers for Disease Control and Prevention. CDC grand rounds: Additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep 2010;59:980-4.
- U.S. Food and Drug Administration. Guidance for Industry: A Food Labeling Guide (14. Appendix F: Calculate the Percent Daily Value for the Appropriate Nutrients). 2013.
- U.S. Food and Drug Administration. Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Proposed Extension of Compliance Dates 2017. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels
- U.S. Food and Drug Administration. Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Proposed Extension of Compliance Dates 2017.
- Institute of Medicine. Dietary references intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 1998.
- U.S. Food and Drug Administration. Food Labeling: Revision of the Nutrition and Supplement Facts Labels 2016. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels
- Yeung LF, Cogswell ME, Carriquiry AL, et al. Contributions of enriched cereal-grain products, ready-to-eat cereals, and supplements to folic acid and vitamin B-12 usual intake and folate and vitamin B-12 status in US children: National Health and Nutrition Examination Survey (NHANES), 2003-2006. Am J Clin Nutr 2011;93:172-85.
- Carmel R. Folic acid. In: Shils M, Shike M, Ross A, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005:470-81
- Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2014;44:480-8.
- Henderson AM, Aleliunas RE, Loh SP, et al. l-5-Methyltetrahydrofolate supplementation increases blood folate concentrations to a greater extent than folic acid supplementation in Malaysian women. J Nutr 2018;148:885-90.
- Seyoum E, Selhub J. Properties of food folates determined by stability and susceptibility to intestinal pteroylpolyglutamate hydrolase action. The Journal of nutrition 1998;128(11):1956-60.
- De Wals P, Tairou F, Van Allen MI, Lowry RB, Evans JA, Van den Hof MC, Crowley M, Uh SH, Zimmer P, Sibbald B, et al. Spina bifida before and after folic acid fortification in Canada. Birth Defects Res A Clin Mol Teratol 2008;82(9):622-6. doi: 10.1002/bdra.20485
- Crider KS, Zhu JH, Hao L, Yang QH, Yang TP, Gindler J, Maneval DR, Quinlivan EP, Li Z, Bailey LB, et al. MTHFR 677C->T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. Am J Clin Nutr 2011;93(6):1365-72. doi: 10.3945/ajcn.110.004671
- U.S. Food and Drug Administration. Food Standards: Amendment of Standards of Identity for Enriched Grain Products to Require Addition of Folic Acid: Final Rule. 21 CFR Parts 136, 137, and 139. Federal Register 1996;61(44):8781-97
- Yang Q, Cogswell ME, Hamner HC, Carriquiry A, Bailey LB, Pfeiffer CM, Berry RJ. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003-2006. Am J Clin Nutr 2010;91(1):64-72. doi: ajcn.2009.28401 [pii]10.3945/ajcn.2009.28401
- Hendler SS, Rorvik DR. PDR for Nutritional Supplements. Montvale: Medical Economics Company, Inc, 2001
- De Wals P, Tairou F, Van Allen MI, Lowry RB, Evans JA, Van den Hof MC, Crowley M, Uh SH, Zimmer P, Sibbald B, et al. Spina bifida before and after folic acid fortification in Canada. Birth Defects Res A Clin Mol Teratol 2008;82(9):622-6. doi: 10.1002/bdra.2048
- Crider KS, Devine O, Hao L, Dowling NF, Li S, Molloy AM, Li Z, Zhu J, Berry RJ. Population red blood cell folate concentrations for prevention of neural tube defects: bayesian model. Bmj 2014;349:g4554. doi: 10.1136/bmj.g4554
- Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, Mulinare J, Zhao P, Wong LY, Gindler J, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med 1999;341(20):1485-90.
- Tsang BL, Devine OJ, Cordero AM, Marchetta CM, Mulinare J, Mersereau P, Guo J, Qi YP, Berry RJ, Rosenthal J, et al. Assessing the association between the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism and blood folate concentrations: a systematic review and meta-analysis of trials and observational studies. Am J Clin Nutr 2015;101(6):1286-94. doi: 10.3945/ajcn.114.099994
- Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF, 3rd, Mills JL, Pfeiffer CM, Fazili Z, Zhang M, Ueland PM, et al. Biomarkers of Nutrition for Development-Folate Review. J Nutr 2015;145(7):1636S-80S. doi: 10.3945/jn.114.206599
- Sweeney MR, McPartlin J, Weir DG, Daly L, Scott JM. Postprandial serum folic acid response to multiple doses of folic acid in fortified bread. Br J Nutr 2006;95(1):145-51. doi: S0007114506000183 [pii]
- Pfeiffer CM, Sternberg MR, Fazili Z, Yetley EA, Lacher DA, Bailey RL, Johnson CL. Unmetabolized Folic Acid Is Detected in Nearly All Serum Samples from US Children, Adolescents, and Adults. The Journal of nutrition 2015;145(3):520-31. doi: 10.3945/jn.114.201210
- Rose NC, Mennuti MT. Maternal serum screening for neural tube defects and fetal chromosome abnormalities. West. J. Med. 1993 Sep;159(3):312-7
- Wallingford JB, Niswander LA, Shaw GM, Finnell RH. The continuing challenge of understanding, preventing, and treating neural tube defects. Science. 2013 Mar 01;339(6123):1222002