Osteolysis
Osteolysis is the softening, absorption and destruction of bony tissue.
Osteolysis causes
Common causes of osteolysis:
- Acroosteolysis
- Adamantinoma
- Aging
- Bone tumors
- Chondroblastoma
- Chondromyxoid fibroma
- Chronic inflammation
- Cleidocranial dysplasia
- Craniomandibular dermatodysostosis
- Desmoplastic fibroma
- Distal clavicle osteolysis
- Enchondroma
- Enchondromatosis
- Epithelioid hemangioendothelioma
- Ewing sarcoma
- Gorham vanishing bone disease
- Hairy cell leukemia
- Hajdu-Cheney syndrome
- Idiopathic multicentric osteolysis
- Myeloma
- Neuroblastoma
- Osteosarcoma
- Periprosthetic osteolysis (osteolysis hip replacement)
- Polyostotic fibrous dysplasia
- Polyostotic osteolytic expansile dysplasia
- Pyknodysostosis
- Reiter syndrome
- Subchondral stress fracture
- Torg-Winchester syndrome
- Van bogaert-hozay syndrome
Distal clavicle osteolysis
Distal clavicle osteolysis is a pathologic process involving resorption of subchondral bone in the distal clavicle 1. Distal clavicle osteolysis usually presents as pain localized to the acromioclavicular joint 2.
Distal clavicle osteolysis was first described in 1936 as a condition secondary to acute shoulder trauma 1. Today, it is described as a complication of trauma associated with contact sports, falls, and motor vehicle accidents. In 1959, distal clavicle osteolysis was reported in an air-hammer operator without evidence of acute trauma. In 1982, Cahill 3 reported on 45 male athletes with distal clavicle osteolysis, confirming repetitive microtrauma as an etiology. Of Cahill’s 45 patients, 44 were weightlifters 3.
Although more than 100 cases have been reported in the US literature, distal clavicle osteolysis may be an underdiagnosed disorder. Its incidence has increased with the growth in popularity of weight training in the past few decades 4.
As more women are participating in competitive and recreational weightlifting and sports that involve overhead throwing, more women are presenting with distal clavicle osteolysis 5. In a retrospective review of 1432 consecutive magnetic resonance imaging (MRI) shoulder reports in patients aged 13-19 years, atraumatic distal clavicle osteolysis was identified in 93 patients (6.5%), of whom 24% were female 6. Patients had varying symptoms; 89% of those with atraumatic distal clavicle osteolysis had pain at the acromioclavicular joint or distal clavicle, and 60% had pain with participation in overhead sports 6.
Most patients with distal clavicle osteolysis respond to conservative management, though symptoms often return with resumption of previous activity. Patients in whom conservative treatment fails or who refuse to limit their activities are candidates for surgical treatment (distal clavicle resection). The only contraindications noted for surgical treatment of distal clavicle osteolysis are those general to surgery.
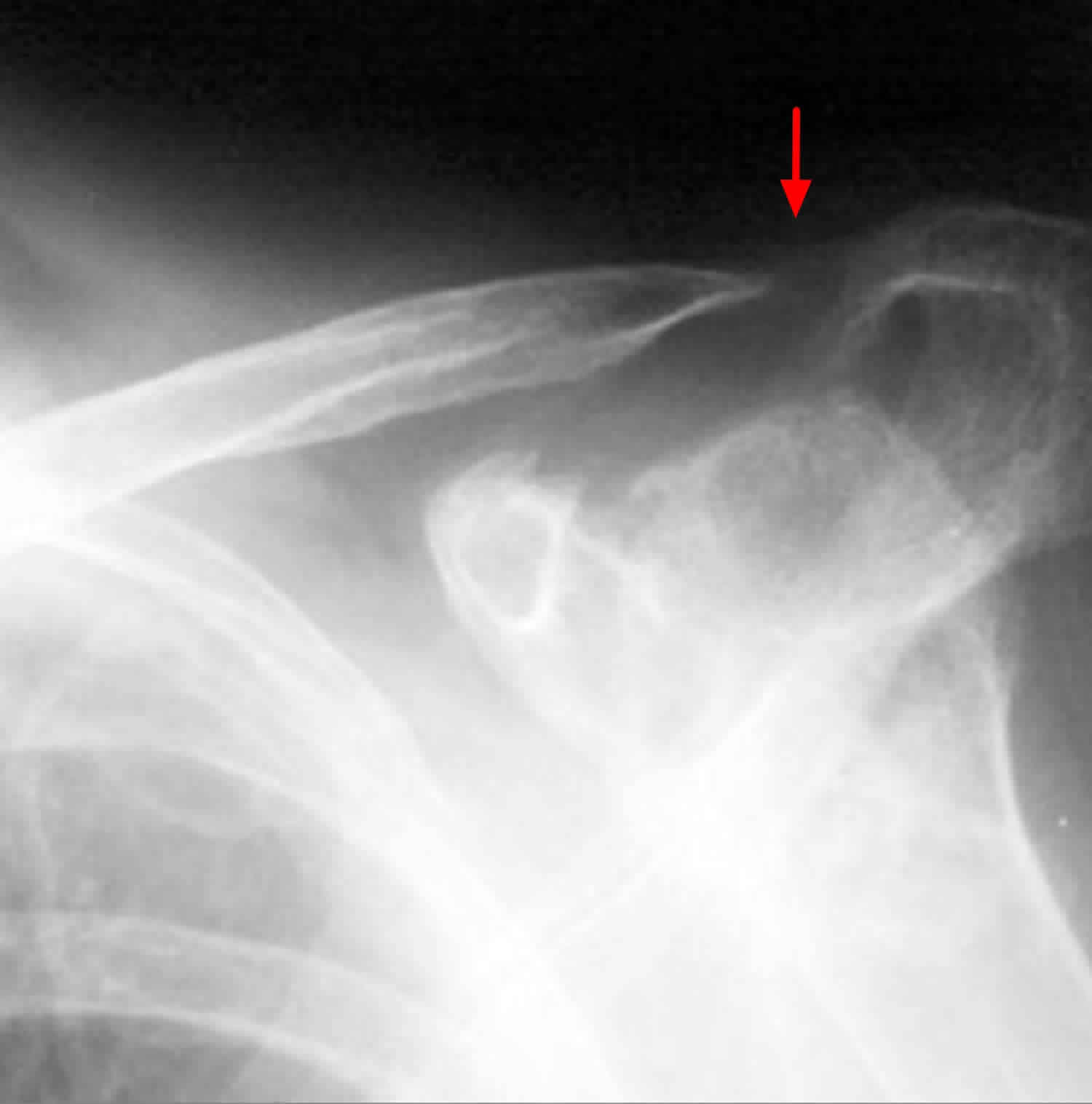
Figure 1. Distal clavicle osteolysis
Distal clavicle osteolysis causes
Different theories concerning the etiology of distal clavicle osteolysis have been suggested:
- The first theory proposed an autonomic neurovascular origin; one author noted the presence of ipsilateral anisocoria in four of eight patients
- A theory set forth in another report proposed synovial invasion of the subchondral bone
- Cahill 3, noting the presence of microfractures in subchondral bone in 50% of his cases, proposed that repetitive microtrauma caused subchondral stress fractures and remodeling; this theory is currently the most widely accepted one.
Distal clavicle osteolysis symptoms
Most patients with distal clavicle osteolysis present with pain over the distal end of the clavicle and acromioclavicular (AC) joint, which is usually described as a dull ache.
Patients with an etiology of trauma report a specific event as the start of their symptoms. In patients with repetitive/overuse injuries, pain is exacerbated by athletic or work activity. In weightlifters, most symptoms occur with the bench press and related exercises 7. A study by Nevalainen et al 8 found that high-intensity bench pressing was a risk factor for distal clavicle osteolysis but that low-intensity bench pressing was not.
On physical examination, patients have point tenderness over the affected acromioclavicular joint, and cross-chest maneuvers elicit pain. Usually, the acromioclavicular joint is not unstable; however, crepitation may be present. Range of motion (ROM) of the glenohumeral joint should be full.
Distal clavicle osteolysis diagnosis
Plain radiography
Obtain anteroposterior (AP) and 10-15° cephalic tilt views. Radiographs often appear normal in the early clinical course. With time, loss of subchondral bone detail in the distal clavicle, microcystic changes in the subchondral area, and widening of the acromioclavicular (AC) joint may be seen. The acromion is spared from lytic changes.
It is important to distinguish distal clavicle osteolysis (distal clavicle osteolysis ) from AC joint arthritis, and outcomes after operative injury can differ substantially between the two conditions. distal clavicle osteolysis can be diagnosed when pathologic changes such as sclerosis, reactive bone formation, and subchondral cysts are restricted to the distal clavicle. The distinction between distal clavicle osteolysis and AC joint arthritis is important because patients with traumatic AC joint arthritis and degenerative arthritis tend to do worse than patients with distal clavicle osteolysis do 9.
The presence of panarticular disease should lead to the consideration of other diagnoses (eg, inflammatory disease).
Bone scanning
If plain radiography is nondiagnostic, technetium-labeled bone scanning may help confirm the diagnosis of distal clavicle osteolysis. Increased radiotracer uptake is seen in the distal clavicle.
Magnetic resonance imaging
Some authors have recommended the use of magnetic resonance imaging (MRI) to rule out additional shoulder pathology 10. MRI will commonly demonstrate increased signal intensity on fat-suppressed T2-weighted and short-tau inversion recovery (STIR) images. Bone marrow edema at the distal clavicle also is a common finding and has been shown to correlate with the severity of symptoms 9.
Procedures
Because of a moderate incidence of concomitant shoulder pathology (eg, rotator cuff pathology, labral pathology, subacromial impingement, glenohumeral instability), a lidocaine injection into the acromioclavicular (AC) joint may help achieve a more accurate diagnosis 11.
Distal clavicle osteolysis treatment
Distal clavicle osteolysis is a self-limiting disorder that typically resolves within 1-2 years with activity modification. Conservative management consists of rest and avoidance of symptomatic activity. Nonsteroidal anti-inflammatory drugs (NSAIDs) can also help alleviate symptoms. Corticosteroid injections are often given; however, they provide little long-term relief. Although most patients respond to conservative management, symptoms often return with resumption of previous activity.
Patients with distal clavicle osteolysis in whom conservative treatment fails or who refuse to limit their activities are candidates for surgical treatment.
The only contraindications noted for surgical treatment of distal clavicle osteolysis are those general to surgery. Most surgical approaches, however, can be performed without general anesthesia (for instance, with intravenous [IV] sedation and interscalene block). Patients who are at particularly high risk with surgical treatment as a consequence of medical comorbidities should consult further with their primary care physician and their institution’s anesthesia department for proper preoperative risk assessment.
Surgical therapy
The classic surgical treatment for distal clavicle osteolysis is distal clavicle resection, a reliable procedure with good-to-excellent results. Excellent results have been reported with arthroscopic distal clavicle resection 12. This approach affords a more cosmetically appealing result, allows earlier return to activity, and provides a means of addressing concomitant intra-articular pathology. Arthroscopic resection can be performed through standard portals from the subacromial space, as well as via a direct superior portal.
A randomized, controlled trial of 38 athletes with distal clavicle osteolysis or isolated posttraumatic arthrosis of the acromioclavicular (AC) joint addressed the question of whether the direct superior approach or the indirect subacromial approach was the better procedure for arthroscopic distal clavicle resection 13. The authors found that both procedures had successful clinical outcomes, with insignificant differences at follow-up, but that the direct approach provided faster improvement and return to activity 13.
The necessary extent of distal clavicle resection has been a subject of debate in the literature. Although Cahill reported excellent results with an open approach resecting 1-2 cm of bone, subsequent arthroscopic studies showed that resection of as little as 4 mm is effective 14. The distal clavicle should be resected enough to prevent AC impingement through a full range of shoulder motion. Careful attention should be taken to avoid excessive bony resection and to avoid violating the posterior superior AC joint capsule, in that this can lead to horizontal instability of the AC joint 15.
Surgical complications
Few complications from surgical treatment of distal clavicle osteolysis have been reported. One theoretical concern with aggressive distal clavicle resection is damage to the underlying neurovascular structures. A risk of infection always exists, though the risk is low in this setting. Potential development of frozen shoulder as a consequence of limited motion is a concern during the postoperative course. As noted, there is a risk of horizontal instability of the clavicle in the event of an overly surgical aggressive resection.
Postoperative care
Early passive range of motion (ROM), including pendulum exercises, is important to prevent loss of shoulder motion. Because the open procedure requires partial detachment of the deltoid, active ROM is usually restricted in the early postoperative course. After arthroscopic treatment, activity is comparatively accelerated, with active ROM started within the first week.
Routine postoperative follow-up at 1-2 weeks is recommended.
Distal clavicle osteolysis prognosis
Although the outcome with conservative treatment is good, many patients are unable to limit their activities. These patients, as well as those in whom conservative treatment is ineffective, can expect good-to-excellent results from surgical intervention. Patients with an etiology of trauma may have an increased risk of unfavorable results. Patients can also develop symptoms in the contralateral extremity.
With regard to surgical treatment, Pensak et al investigated the difference in outcomes between open and arthroscopic resection of the distal clavicle 16. Specifically, arthroscopic resection had a 90% success rate, with the direct approach resulting in quicker returns to work and sport. Poor outcomes were reported for worker’s compensation patients and patients who had posttraumatic distal clavicle osteolysis .
Robertson et al reviewed 49 distal clavicle osteolysis patients, of whom 32 were treated arthroscopically and 17 were treated via an open approach 17. At a mean follow-up of 5.3 years for the open group and 4.2 for the arthroscopic group, the arthroscopic group had a significantly lower Visual Analogue Scale (VAS) score for pain (0.61 ± 1.02 vs 1.59 ± 2.15). In the open group, 100% of patients reported that they would undergo the procedure again, whereas in the arthroscopic group, 97% reported that they would repeat the procedure.
Osteolysis hip replacement
In osteolysis hip replacement also called periprosthetic osteolysis or aseptic loosening, the bone around the implant deteriorates, making the implant loose or unstable 18. Osteolysis following hip replacement was identified as the most significant long‐term adverse effect associated with total hip replacement at the National Institutes of Health consensus conference on total hip joint replacements 19. The incidence of periprosthetic osteolysis in many studies is greater than the sum of all the rest of the complications 18. In the Swedish Total Hip Replacement Register, osteolysis accounted for over 75% of the patients undergoing revision hip surgery 20.
Both the acetabular and femoral components may be affected. Prevalence of aseptic loosening, in most series beyond 10‐years, is reported to be between 32–62%, depending on the type of prosthesis used 21. Fortunately, plastics have improved greatly over the years, so plastic wear and osteolysis occur less frequently today than they did with earlier generations of implants.
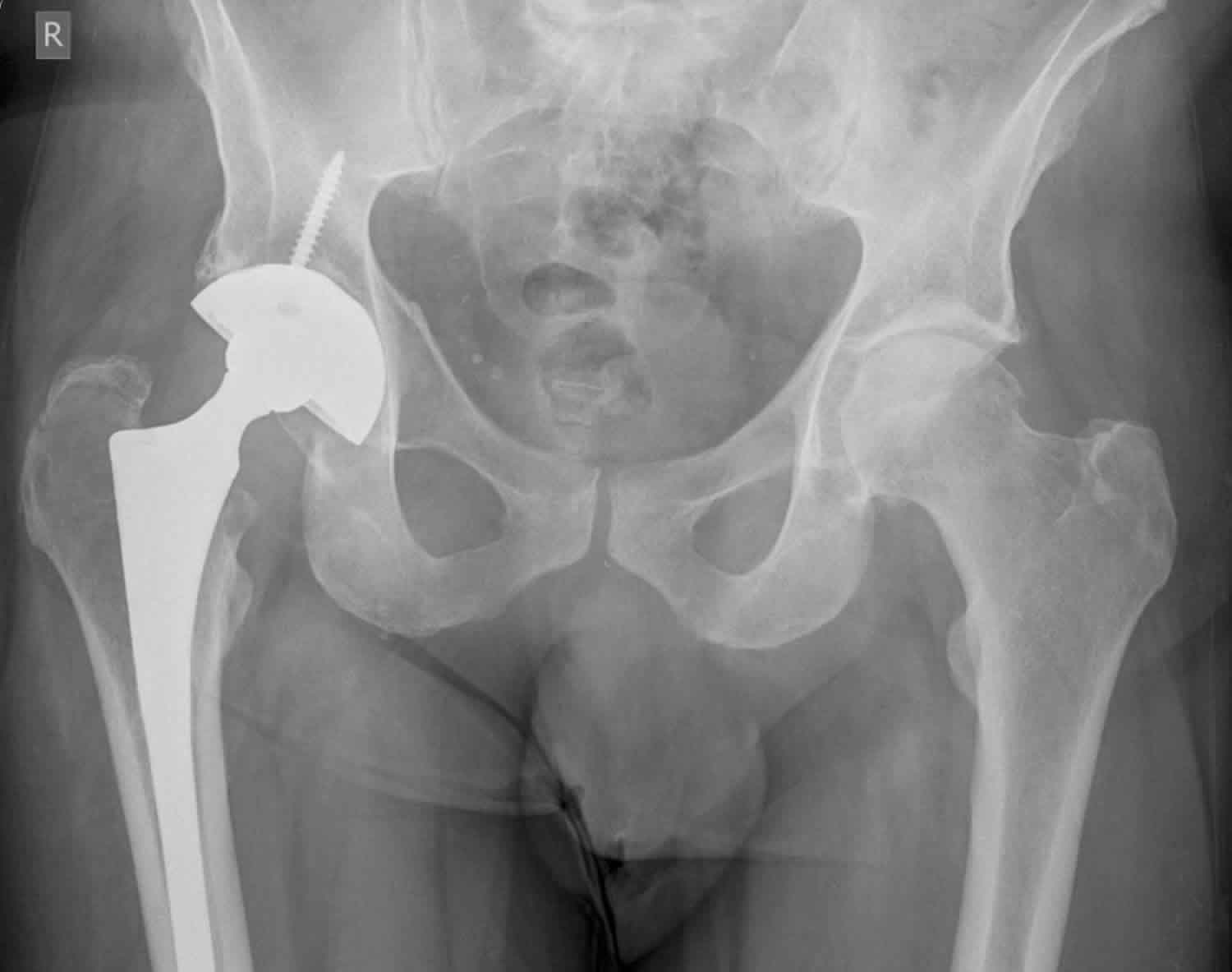
Figure 2. Osteolysis hip replacement
Footnote: Total hip replacement surveillance 70 year old male. X-ray showing lytic area medial wall right acetabulum adjacent to the acetabular cup of a total hip replacement. ?osteolysis ?neoplasia. CT confirms the radiographic finding or a periacetabular lytic lesion, with lysis of the inner cortex and a small bulging soft tissue component.
[Surce 22 ]Osteolysis hip replacement causes
Normal bone maintenance depends on the balance of bone formation and bone resorption that mainly involves the coordinated function of osteoblasts and osteoclasts. There are several mechanisms by which bone loss after a joint replacement may occur.
Ageing
Bone loss may occur as a result of natural ageing. Women can lose up to one third of their cortical bone and half of their trabecular bone throughout their lifetime, while men lose about 60% of that amount 23. However, bone loss secondary to the ageing process has not proved to represent a major threat to the mechanical stability of prosthetic components 24.
Adaptive bone remodelling or stress shielding
Adaptive bone remodelling or stress shielding can occur in response to an altered mechanical environment following a hip replacement. This occurs because there is a redistribution of load and therefore stress, when the femoral head is replaced by the femoral component of a total hip replacement. Consequently, stress on the proximal femoral cortex is lessened, as most of the load bypasses this area and is transmitted in the metal stem to the distal femur. Cemented stems are associated with less stress shielding than uncemented stems 24. Studies have shown that hydroxyapatite fully coated stems are associated with an increased cortical bone stress shielding compared with proximally coated porous stems 25. The amount of coating on most prosthetic stems available today is still greater than that necessary to lower the stress‐shielding effect on the proximal femur 19. However, reducing porous coating to lower stress shielding must be balanced against providing adequate coating to ensure fixation. Long‐term effects of stress shielding on stability of components and further revision surgery are not known 23.
Mechanical factors
Migration of prosthesis is defined as a change in position of prosthesis, cement mantle or both and is thought to indicate implant failure and represent loosening 26. Once migration has begun, stability is lost and periprosthetic particles may modulate latter stages of loosening 26. Mechanisms by which migration occurs are not fully understood. It could be due to fatigue failure of cancellous bone surrounding the prosthesis 27 leading to loss of osteo‐integration of a stable prosthesis, or it could be attributed to surgical techniques—for example, reaming which disturbs capillary circulation of periprosthetic bone, leading to necrosis. The initial use of cement with first generation cementing technique allowed defects and stresses to occur within the cement which resulted in a weaker bone–cement interface and permitted the ingress of polyethylene particles. thus resulting in loosening 28. With improved cementing techniques which include the use of a medullary plug, a cement gun, lavage of the canal, pressurisation, centralisation of the stem, and reduction in porosity in the cement, the incidence of femoral lysis has been reduced 28.
Fluid pressure
Once a synovial‐like membrane has formed, synovial fluid pressure within the joint may cause osteolysis 29. With loading on the prosthesis, pressure on fluid within the membrane may rise significantly. Sustained elevated pressure can ultimately disturb normal perfusion and oxygenation of bone and, when transmitted to the membrane–bone interface, results in osteocyte destruction and bone necrosis.
Particulate debris
Bone loss can occur secondary to a biological reaction to particulate debris from implants. It is now widely accepted that this is the principal mechanism responsible for periprosthetic osteolysis.10 Particulate polyethylene is considered to be the substance causing the most tissue reaction, forming up to 90% of the debris volume 30. Other particles that have been implicated in development of osteolysis include submicron‐sized ultra‐high‐molecular‐weight polyethylene, polymethylmethacrylate (cement), and metallic debris such as cobalt and titanium alloys, silicates and stainless steel 31. These particles probably exert their effects by either promoting third body wear of polyethylene, with ultra‐high‐molecular‐weight polyethylene triggering the cellular response; or they instigate the release of inflammatory mediators which results in chronic inflammation and tissue damage that erodes the supporting bone with subsequent implant loosening.
Migration of particles
Particulate matter and cement are dispersed in joint fluid. The concept of “effective joint space”, which includes all periprosthetic regions that are accessible to joint fluid and thus particulate debris, has been proposed as a mechanism for migration of particles 32. Presence of particulate matter in joint fluid will initiate a localised macrophage‐induced phagocytosis and result in bone resorption. As bone is resorbed, a pool is formed, promoting more flow (preferential flow) into that region and thus delivering more particles and causing more localised bone resorption 32. This cycle continues and eventually a significant quantity of bone is resorbed which becomes evident as an osteolytic area on a radiograph. As fluid pressure propels joint fluid and thus particulate debris through the effective joint space, it will result in progressive bone loss 32.
Small particles (0.5–10 μm) are the most active and when generated will follow a route of least resistance and become interposed between the bone–cement interface or between the bone–implant interface in uncemented prostheses 33. In cemented femoral components, the path of least resistance is along the cement–metal or cement–bone interface. For example, particles may be driven along the interface through defects between stem and cement and provide a route through which joint cavity contents may reach the endosteal surface of the femur, leading to localised bone lysis 34.
The coating of implants with ceramics such as hydroxyapatite has been shown to reduce wear particle migration along the implant interface by creating a seal between the bone–implant interface 35. In extensively coated stems, osteolytic lesions are more likely to occur proximally and do not always result in loosening of the implant.4,11,12,25 This is in contrast to patch‐coated stems, which allow the joint fluid to reach the diaphysis, thus resulting in osteolysis more distally 20.
Cellular response to particles
The cellular response to particles is complex and not fully understood 33. The presence of particulate debris initiates phagocytosis by macrophages and macrophage‐derived foreign body giant cells. As a consequence, macrophages and possibly other cells including fibroblasts release cytokines such as tumour necrosis factor‐α, interleukins (IL‐1, IL‐6, IL‐10), proteolytic enzymes and prostaglandins (PGE2) 23. Osteoblasts may also cause secretion of specific cytokines by activated macrophages 23. These intracellular mediators induce a complex cellular response, which initiates a focal bone resorptive process mediated primarily by osteoclasts and to a lesser degree by monocytes 23. This in turn results in loosening of components.
Biological response to wear debris
Presence of wear debris does not always result in osteolysis. For osteolysis to occur, rate of production of wear particles must exceed an individual’s capacity to remove the debris such that a threshold is reached above which development of osteolysis is more likely 36. Furthermore, normal repair mechanisms that are responsible for preventing formation of osteolytic lesions must become unable to halt the disease progression 36. Therefore, an individual’s biological response to presence of wear debris must play an important role in development of osteolysis. This explains why in some cases of osteolysis the entity is self‐limiting while in others the biological process is progressive 18. The rate of progression seems to be higher in patients with prosthetic loosening 18.
Wear and osteolysis
Wear is defined as the loss of material from a surface due to motion. It is thought that the main types of wear in a metal on polyethylene bearing surfaces are adhesive wear (when two bearing surfaces bond together on loading and the weaker of the bearing surfaces is transferred onto the harder one on relative motion) and abrasive wear (due to asperities on the harder of the bearing surfaces producing grooves onto the surface of the softer material, resulting in removal of material). Third body wear refers to motion between two primary bearing surfaces with third body particles (cement, bone, metal or polyethylene) trapped between them. Linear wear rate is defined as penetration of the metallic head into the plastic cup. The incidence of osteolysis rises significantly as linear wear rate rises above 0.1 mm/year, while osteolysis is rare at a wear rate of less than this 36.
Osteolysis hip replacement symptoms
Clinically, most patients are asymptomatic and diagnosed only following an incidental finding on late postoperative radiographs 18. In a minority of cases, patients are symptomatic and present with thigh pain (usually indicates femoral component loosening), groin pain (usually indicates acetabular loosening) or fractures of the femur or acetabulum.
Osteolysis hip replacement diagnosis
Radiolucent lines are seen around loose prosthesis on radiographs, most commonly in lateral and anterior aspects of the femur 37. Radioisotope scans may reveal areas of increased activity in areas of loosening. In the majority of cases, radiographic evidence of the disease process only manifests five years or more after insertion of the prosthesis 18.
Osteolysis hip replacement treatment
Several considerations must be taken into account when managing the patient with osteolysis. In the asymptomatic patient, factors such as patient age, past medical history, degree and type of bone loss, rate of progression of osteolysis, properties of the implant to be revised, and patient’s activity level must be borne in mind 37. Surgery is usually indicated if bone loss is extensive or progressive 38. Curettage and grafting of the defects, stem retention with exchange of the femoral head is a viable option in the asymptomatic patient with a well‐fixed stem. In the medically unfit patient or in the elderly, a conservative approach with regular follow‐up is a reasonable approach.
In the symptomatic patient, surgery is usually warranted and is guided by host factors mentioned above and the extent of femoral bone loss. The main principles in the management of osteolysis are to identify and remove the source of the wear particles, remove the loose components and fill in any cavitary defects. In cases where osteolysis is minimal, any primary cemented or non‐cemented prosthesis can be used. In patients with moderate and moderate to severe osteolysis, the treatment options include a cemented prosthesis and a proximal or extensively porous coated un‐cemented implant. In cases of severe osteolysis or in the multiply revised patient, a long stem prosthesis cemented into a proximal or distal femoral allograft is often used.
Various methods have been attempted to reduce the incidence of osteolysis and thus extend the life of artificial joints. For example, changes have been made in device designs, implants, materials and fixation methods. Despite this, revision rates remain at around 10% after 10 years, with cemented prostheses having a lower revision rate than cementless prostheses 39.
Most patients with aseptic loosening will, therefore, need to undergo revision surgery. Furthermore, at a time when average life expectancy is continuing to rise, joint replacements are being performed on ever younger patients. It can therefore be expected that the need for revision hip arthroplasty will continue to rise for the foreseeable future. Currently, revision hip replacements account for around 15% of all total hip arthroplasties performed in the UK 40.
Considerations in revision hip surgery
The two main aims of a revision procedure are to achieve immediate fixation and long‐ term stability. However, the reduction of bone stock available for subsequent implant fixation probably accounts for inferior results attained in revision surgery compared with the primary procedure 41. This is partly due to an inadequate amount of bone being available into which new prosthetic components can be fixed, and partly due to the fact that the existing bone is often not strong enough to support loads that are placed on the prosthetic components 42. Furthermore, bone loss that accompanies aseptic loosening is often extensive and involves many areas in combination 42. For this reason bone grafts and bone graft substitutes are increasingly been used to replenish bone loss that occurs with loosening.
Bone grafts
Bone has three unique properties that is essential for successful healing and incorporation of bone grafts 43. These include osteogenesis (ability of bone to self‐generate new bone formation), osteoinduction (ability to recruit mesenchymal stem cells, from the surrounding host, which then differentiates into new bone), and osteoconduction (process of ingrowth of capillaries, perivascular tissue and osteoprogenitor cells from host bed into graft structure; the graft functions as a scaffold for ingrowth of new bone).
Autografts
Autografts are considered the gold standard of bone transplantation because they posses all three unique properties described above 43. They are usually obtained from the iliac crests, femoral heads or fibula of the patient. There are, however, several limitations in use of autografts: (1) only a restricted amount of bone can be acquired by this technique and this is usually insufficient to fill large defects that are associated with loosening; (2) harvesting the graft from patient’s own skeleton can compromise normal skeletal architecture and mechanical integrity of donor sites; (3) donor site complications and morbidity can result in increased patient recovery time, disability and chronic pain 44; (4) acquisition of bone graft increases operative time and blood loss; (5) viable cells harvested from the donor site may not survive when they are detached from their vascular supply 44.
Allografts
Due to the previously mentioned limitations of the use of autografts, allografts have become an attractive alternative. These are most often obtained from femoral heads of other patients undergoing total hip replacements, or from femurs or tibias harvested from fresh cadavers. Although use of allografts in the form of morselised chips to fill cavitary defects have had good clinical success,36,37 their use in revision surgery has shown inconsistent results 45. Unlike autografts, they only possess osteoconductive and limited osteoinductive properties but lack osteogenesis and remodel at a much slower rate 44.
There are also several other disadvantages of using allografts. For example, up to a fifth of all donated femoral heads have shown to be contaminated with bacteria.39 Furthermore, grafts have been known to transmit pathogens such as hepatitis B, hepatitis C and HIV to patients, and they may contain prions 46. For this reason, allografts are intensively treated before preservation for storage. However, the preservation process can affect mechanical and biological properties of the graft 47 and may not inactivate prions 46.
In contrast to autografts, allografts in general have a higher incidence of delayed incorporation, non‐unions, delayed unions and failure rates 48. Finally, due to increased use of allografts, demands for cancellous allografts may outstrip supply in the future 49.
Bone graft substitutes
A variety of bone graft substitutes including titanium fibremetals, collagen, bioactive glasses and ceramics composed of hydroxyapatite (HA), tricalcium phosphate or both have also been used to overcome problems that have arisen with bone grafts 50. Hydroxyapatite and calcium phosphates have been shown to evoke a biological response similar to bone and show great potential as bone graft substitutes, particularly in contained bone defects 51. Furthermore, they can be readily moulded into desired sizes and shapes and the porous nature of these materials facilitates bony ingrowth. In addition, these materials are osteoconductive, biocompatible, easily sterilised, not immunogenic and can be used when large amounts of bone grafts are not available. However, their disadvantages include a lack of osteogenic and osteoinductive properties and their limited ability to offer immediate structural support 51.
Impaction grafting
Another method that has been employed to treat cavitary defects, in the proximal femur, has been the use of impaction allografting, whereby a morsellised cancellous allograft is impacted into the proximal femur to provide immediate mechanical function. While in structural grafts bone ingrowth does not usually exceed 2–3 mm, in impacted morsellised allografts the bone growth distance has been shown to be greater than this distance, suggesting that the impacted graft may be superior with bone growth distance 52. Furthermore, to date, this is the only technique that has been shown to reverse the loss of bone stock caused by osteolysis 52. However, early subsidence of the femoral component, prosthetic dislocations and a high incidence of intra‐ and postoperative femoral fractures have been reported with this technique 52.
Excision arthroplasty (Girdlestone’s procedure)
This procedure involves removal of the femoral head and allowing a fibrous union to occur between the proximal femur and acetabulum. It converts a painful but stable joint into one that is unstable but less painful. Most patients require walking‐aids and mobilise only for short distances postoperatively. In modern times, this procedure is only undertaken as a salvage procedure and is not suitable for young patients.
Acetabular revision options
The goals of acetabular revision surgery are to restore the biomechanics of the hip and to restore structural integrity and continuity. The results of cemented acetabular revisions have been disappointing 42. Cemented reconstructions using allograft has also produced discouraging results 24. Uncemented porous‐coated sockets can be used successfully to reconstruct most acetabular defects encountered during revision surgery 53. Screws or cages may need to be used to secure the acetabular component into the pelvis. Where major segmental defects are present and prosthetic stability is not possible in host bone, structural allografts are often used.
References- Distal clavicle osteolysis. https://emedicine.medscape.com/article/1262297-overview
- Patel DR, Breisach S. Evaluation and management of shoulder pain in skeletally immature athletes. Transl Pediatr. 2017 Jul. 6 (3):181-189.
- Cahill BR. Osteolysis of the distal part of the clavicle in male athletes. J Bone Joint Surg Am. 1982 Sep. 64 (7):1053-8.
- Scavenius M, Iversen BF. Nontraumatic clavicular osteolysis in weight lifters. Am J Sports Med. 1992 Jul-Aug. 20 (4):463-7.
- Matthews LS, Simonson BG, Wolock BS. Osteolysis of the distal clavicle in a female body builder. A case report. Am J Sports Med. 1993 Jan-Feb. 21 (1):150-2.
- Roedl JB, Nevalainen M, Gonzalez FM, Dodson CC, Morrison WB, Zoga AC. Frequency, imaging findings, risk factors, and long-term sequelae of distal clavicular osteolysis in young patients. Skeletal Radiol. 2015 May. 44 (5):659-66.
- Tao HM, Chen J, Ji YY, Yang DS. Post-traumatic osteolysis of the distal clavicle, pubis and ischium in 7 patients. Chin J Traumatol. 2004 Aug. 7 (4):247-52.
- Nevalainen MT, Ciccotti MG, Morrison WB, Zoga AC, Roedl JB. Distal clavicular osteolysis in adults: association with bench pressing intensity. Skeletal Radiol. 2016 Nov. 45 (11):1473-9.
- DeFroda SF, Nacca C, Waryasz GR, Owens BD. Diagnosis and Management of Distal Clavicle Osteolysis. Orthopedics. 2017 Mar 1. 40 (2):119-124.
- Kassarjian A, Llopis E, Palmer WE. Distal clavicular osteolysis: MR evidence for subchondral fracture. Skeletal Radiol. 2007 Jan. 36 (1):17-22.
- Shaffer BS. Painful conditions of the acromioclavicular joint. J Am Acad Orthop Surg. 1999 May-Jun. 7 (3):176-88.
- Freedman BA, Javernick MA, O’Brien FP, Ross AE, Doukas WC. Arthroscopic versus open distal clavicle excision: comparative results at six months and one year from a randomized, prospective clinical trial. J Shoulder Elbow Surg. 2007 Jul-Aug. 16 (4):413-8.
- Charron KM, Schepsis AA, Voloshin I. Arthroscopic distal clavicle resection in athletes: a prospective comparison of the direct and indirect approach. Am J Sports Med. 2007 Jan. 35 (1):53-8.
- Nuber GW, Bowen MK. Arthroscopic treatment of acromioclavicular joint injuries and results. Clin Sports Med. 2003 Apr. 22 (2):301-17.
- Eskola A, Santavirta S, Viljakka HT, Wirta J, Partio TE, Hoikka V. The results of operative resection of the lateral end of the clavicle. J Bone Joint Surg Am. 1996 Apr. 78 (4):584-7.
- Pensak M, Grumet RC, Slabaugh MA, Bach BR Jr. Open versus arthroscopic distal clavicle resection. Arthroscopy. 2010 May. 26 (5):697-704.
- Robertson WJ, Griffith MH, Carroll K, O’Donnell T, Gill TJ. Arthroscopic versus open distal clavicle excision: a comparative assessment at intermediate-term follow-up. Am J Sports Med. 2011 Nov. 39 (11):2415-20.
- Harris W H. Wear and periprosthetic osteolysis: the problem. Clin Orthop 200139366–70.
- NIH Consensus Development Panel on Total Hip Replacement NIH consensus conference: total hip replacement. JAMA 19952731950–1956.
- Malchau H, Herberts P, Eisler T. et al The Swedish Total Hip Replacement Register. J Bone Joint Surg Am 200284‐A(Suppl 2)2–20.
- Clohisy J C, Harris W H. The Harris‐Galante uncemented femoral component in primary total hip replacement at 10 years. J Arthroplasty 1999;14,915–917.
- Hip replacement periacetabular osteolysis. https://radiopaedia.org/cases/hip-replacement-periacetabular-osteolysis-1?lang=us
- Rubash H E, Sinha R K, Shanbhag A S. et al Pathogenesis of bone loss after total hip arthroplasty. Orthop Clin North Am 199829173–186.
- Jasty M, Maloney W J, Bragdon C R. et al Histomorphological studies of the long‐term skeletal responses to well fixed cemented femoral components. J Bone Joint Surg Am 1990721220–1229.
- Weinans, Huiskes R, Grootenboer H J. Effects of fit and bonding characteristics of femoral stems on adaptive bone remodeling. J Biomech Eng 1994116393–400.
- Harris W H, McCarthy J C, Jr, O’Neill D A. Femoral component loosening using contemporary techniques of femoral cement fixation. J Bone Joint Surg Am 1982641063–1067.
- Taylor M, Tanner K E. Fatigue failure of cancellous bone: a possible cause of implant migration and loosening. J Bone Joint Surg Br 199779181–182.
- SchuLte K R, Callaghan J J, Kelley S S. et al The outcome of Charnley total hip arthroplasty with cement after a minimum twenty‐year follow‐up. J Bone Joint Surg Am 199375961–975.
- Aspenberg P, Van d V. Fluid pressure may cause periprosthetic osteolysis. Particles are not the only thing. Acta Orthop Scand 1998, 691–4.
- Campbell P, Ma S, Yeom B. et al Isolation of predominantly submicron‐sized UHMWPE wear particles from periprosthetic tissues. J Biomed Mater Res 1995; 29(1)127–131.
- Jacobs J J, Roebuck K A, Archibeck M. et al Osteolysis: basic science. Clin Orthop 2001;393, 71–77.
- Schmalzried T P, Jasty M, Harris W H. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am 199274849–863.
- Jacobs J J, Roebuck K A, Archibeck M. et al Osteolysis: basic science. Clin Orthop 200139371–77.
- Anthony P P, Gie G A, Howie C R. et al Localised endosteal bone lysis in relation to the femoral components of cemented total hip arthroplasties. J Bone Joint Surg Br 199072971–979.
- Zicat B, Engh C A, Gokcen E. Patterns of osteolysis around total hip components inserted with and without cement. J Bone Joint Surg Am 199577432–439.
- Dumbleton J H, Manley M T, Edidin A A. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty 200217649–661.
- Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83(979):312–316. doi:10.1136/pgmj.2006.053215 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2600070
- Stauffer R N. Ten‐year follow‐up study of total hip replacement. J Bone Joint Surg Am 198264983–990.
- National Institute for Clinical Excellence The effectiveness and cost effectiveness of different prostheses for primary total hip replacement. London: NICE, 2000
- National Audit Office Hip replacements: an update‐ report by the comptroller and auditor general; HC 956 Session 2002– 2003
- Pellicci P M, Wilson P D, Jr, Sledge C B. et al Long‐term results of revision total hip replacement. A follow‐up report. J Bone Joint Surg Am 198567513–516.
- Amstutz H C, Ma S M, Jinnah R H. et al Revision of aseptic loose total hip arthroplasties. Clin Orthop 198217021–33.
- Behairy Y, Jasty M. Bone grafts and bone substitutes in hip and knee surgery. Orthop Clin North Am 199930661–671.
- Vaccaro A R. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics 200225(5 Suppl)s571–s578.
- Hozack W J, Bicalho P S, Eng K. Treatment of femoral osteolysis with cementless total hip revision. J Arthroplasty 199611668–672.
- Betz R R. Limitations of autograft and allograft: new synthetic solutions. Orthopedics 200225(5 Suppl)s561–s570.
- Boyce T, Edwards J, Scarborough N. Allograft bone. The influence of processing on safety and performance. Orthop Clin North Am 199930571–581.
- Gross A E, McKee N H, Pritzker K P. et al Reconstruction of skeletal deficits at the knee. A comprehensive osteochondral transplant program. Clin Orthop 198317496–106.
- Galea G, Kopman D, Graham B J. Supply and demand of bone allograft for revision hip surgery in Scotland. J Bone Joint Surg Br 199880595–599.
- Bucholz R W, Carlton A, Holmes R E. Hydroxyapatite and tricalcium phosphate bone graft substitutes. Orthop Clin North Am 198718323–334.
- Delloye C, Cnockaert N, Cornu O. Bone substitutes in 2003: an overview. Acta Orthop Belg 2003691–8.
- Toms A D, Barker R L, Jones R S. et al Impaction bone‐grafting in revision joint surgery. J Bone Joint Surg Am 2004862050–2060.
- Dorr L D, Wan Z. Ten years of experience with porous acetabular components for revision surgery. Clin Orthop Relat Res 1995319191–200.