Pasteurellosis
Human pasteurellosis is symptomatic human infection (zoonotic infection) mainly transmitted by wild and domestic cats and dogs caused by Pasteurella species. According to data in the literature, two thirds of human Pasteurella infections are zoonotic in origin. Humans acquire Pasteurella infection primarily through contact with animals, most usually through animal bites, scratches, licks on skin abrasions, or contact with mucous secretions derived from pets 1. The prevalence of antisera to Pasteurella multocida was 2-fold higher in healthy individuals with occupational or pet exposure than in a control group with no reported exposure 2, indicating that animal exposure increases the likelihood of subclinical carriage or infection. Roughly 300,000 (1%) annual visits to the emergency rooms in the United States are due to animal bite or scratch wounds 3. Pasteurella species are isolated from infections resulting from 50% of dog bites and 75% of cat bites 4. Other contact with animals, such as kissing or licking of skin abrasions or mucosal surfaces (eyes, nose, and mouth), can also result in infection with Pasteurella multocida 5. In nearly all reported cases of Pasteurella multocida infection, evidence of prior animal exposure or contact was indicated.
Human pasteurellosis is divided into invasive and localized infections. Invasive pasteurellosis includes bacteremia, septic shock, large-joint arthritis, neurological infections, abdominal infections, and pulmonary pasteurellosis 6. Other severe invasive infections (meningitis, endocarditis, and peritonitis) caused by various Pasteurella spp. have also been described; however, their incidences are rare 7. Localized pasteurellosis caused by Pasteurella spp. are characterized by cutaneous inflammation that usually develops shortly after animal bites or scratches. Later, local complications including osteomyelitis, septic arthritis, and abscess formation or systemic infection such as infection of large articulated joints, meningitis, intraabdominal infection, sepsis, and pneumonia may develop 8. In immunocompromised patients, pasteurella infection may manifest as severe pneumonia, sepsis, or a fatal form of pasteurellosis. In patients with underlying pulmonary disease, pneumonia, empyema, and lung abscess caused by Pasteurella spp. can be detected, whereas in patients with liver dysfunction, sepsis has been described 9. Human pasteurellosis is not a common cause of death in humans because of commonly used prophylactic treatment after animal bites or scratches; however, deaths caused by Pasteurella sp. infection have been increasing in the United States 9.
A survey of the literature over the past 30 years suggests that 20 to 30 human deaths due to pasteurellosis occur annually worldwide, but this rate appears to be rising and in nearly all cases death appears to result as a complication from infection acquired through animal exposure 1. Among the Pasteurella species, Pasteurella multocida is the predominant human pathogen encountered, especially in severe disease cases 10, although Pasteurella canis may be more prevalent with dog bites 11.
The mortality rate was 4 %, including 11 % and 1.4 % of patients with invasive or localized disease respectively 6. Risk factors statistically associated with the invasive pasteurellosis vs the local pasteurellosis were as follows: (1) average age, 63 years (22-93 years) vs 51 years (2-89 years), (2) alcohol consumption, 77.8 % vs 25 %, (3) tobacco use, 64 % vs 25 % (p = 0.006), and (4) chronic liver disease, 21 % vs 1.5 %. Age was the only significant risk factor identified using multivariate analysis. Overall, 27 % of patients had an invasive pasteurellosis and experienced significant mortality (11 %). Advanced age, chronic liver disease were the main risk factors associated with invasive pasteurellosis. Healthcare providers should be aware of these risk factors when patients are exposed to cats or dogs.
In the literature, dog bite is the most common cause of bite injury, followed by cat bite. Most bite injuries are minor wounds; thus, patients generally do not seek medical attention. Dog bites may be associated with fractures because of their high energy, whereas cat bites frequently cause puncture wounds, leading to the development of tenosynovitis 12. Wounds caused by dog bites are less frequently infected than wounds caused by cat bites, because patients with cat bites or scratches have pasteurellosis more frequently than do patients with dog bites. Literature data show that cat bites or scratches are more dangerous than dog bites because the injuries tend to be deep and are therefore difficult to clean properly 13. When Körmöndi et al 14 studied the complications following dog or cat bites, they observed that after dog bites, the rates of functional disability, osteomyelitis, and amputation were slightly higher than for those after cat bites. Lymphangitis could not be detected after dog bites.
Most hospitalized patients with animal bites were admitted because of cat bites. Patients without animal bites were more frequently hospitalized, and their length of stay was longer, compared with patients who had animal bites. Patients treated in the ICU did not have animal bites, and most of them had no animal contact, as well. In a study, among patients with invasive pasteurellosis, the death rate was 27.1% 14. Similarly, the death rate was 21% in Giordano et al.’s observational study 15; in Nollet’s study, the death rate from invasive infections was 11% and the death rate for localized infections was 1.4% 8.
Patients with invasive pasteurellosis were more frequently hospitalized than patients with localized Pasteurella infections and the average length of stay among patients with invasive pasteurellosis was also longer. Hospital admission was almost the same in patients with dog bites (68.3%) and in patients with cat-induced injury (64.2%). The average length of hospital stay was longer (11 [range 1–60] days) in cases of dog bites than in cases of cat bites or scratches (8.3 [range 3–41] days) 14. In patients with localized pasteurellosis, injuries affected mostly the upper extremities (75 patients, 65.8%); in 23 patients, lower extremities were affected, mainly shins; in 6 patients, the face; in 1 patient, the eye; and in 1 patient, the genitals 14.
The genus Pasteurella is a member of the Pasteurellaceae family, which includes a large and diverse group of Gram-negative Gammaproteobacteria, whose members are not only human or animal commensals and/or opportunistic pathogens but also outright pathogens 16.
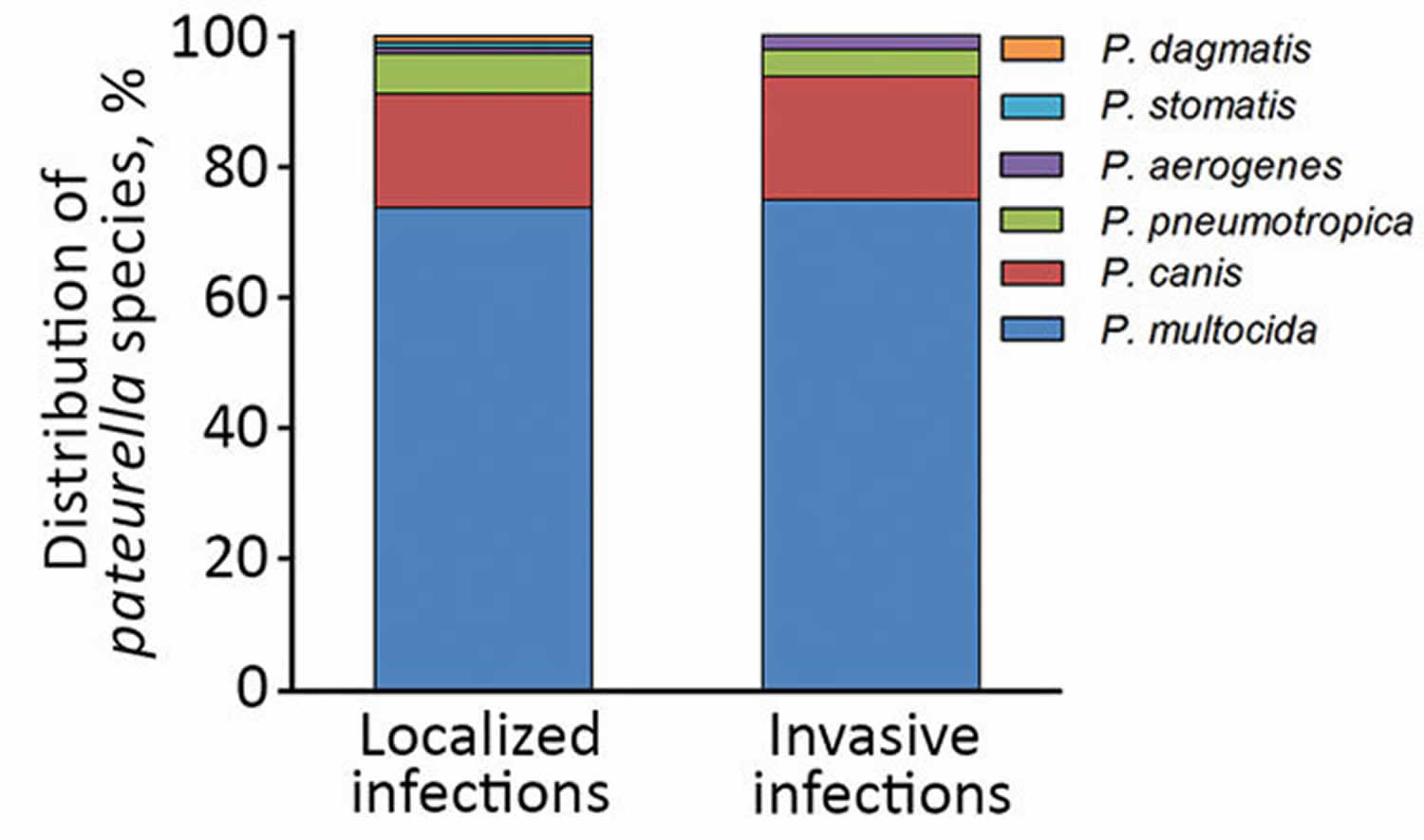
Human pasteurellosis have been reported from Pasteurella multocida, the most common pathogen and type species for the Pasteurella genus, which includes Pasteurella multocida subspecies multocida, Pasteurella multocida subspecies septica, and Pasteurella multocida subspecies gallicida, Pasteurella canis, Pasteurella dagmatis, and Pasteurella stomatis 17. All are associated with dogs and cats. Pasteurella multocida subspecies multocida is the most frequent clinical Pasteurella isolate in humans, ahead of Pasteurella canis and Pasteurella multocida subspecies septica 18. This fact was also confirmed by investigators; 68.7% of Pasteurella strains were Pasteurella multocida, the second most common species was Pasteurella canis (17.1%), and third was Pasteurella pneumotropica (5.2%). Differences between species isolated from localized and invasive Pasteurella infections could be detected, a finding also confirmed by Nollet et al. 8.
Pasteurella multocida isolates are classified based on a combination of capsular polysaccharide serotyping, which distinguishes isolates into one of five capsular serogroups (ie, A, B, D, E, and F); serotypes A and D account for most human pasteurellosis 1. Pasteurella multocida isolates are classified based on a combination of capsular polysaccharide serotyping, which distinguishes isolates into one of the five capsular serogroups A (hyaluronic acid) 19, B (arabinose, mannose, and galactose) 20, D (heparin) 21, E (uncharacterized), or F (chondroitin) 22. Isolates are also subtyped based on their lipopolysaccharide (LPS), which separates isolates further into 16 serovars 23. Isolate designations usually consist of a capsular serogroup letter followed by a somatic serovar number (e.g., A:1, A:2, A:3, B:2, etc.). The polysaccharide structure and biosynthetic genes have been determined for three of the capsular serotypes 24, as well as for the lipopolysaccharides from a number of isolates 25. Related species include Pasteurella aerogenes, Pasteurella bettyae, Pasteurella caballi, and Pasteurella pneumotropica 26. Polymerase chain reaction (PCR) plus sequence-based ribotyping analysis using universal primers for the 16sRNA gene or rpoB gene sequencing have now superseded phenotypic methods for the identification, characterization, and differentiation of Pasteurella multocida and other Pasteurella spp 17. Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry can also be used for accurate identification 17. The complete genome sequence of an avian clone of Pasteurella multocida and the type strain Pasteurella multocida subsp multocida ATCC 43137 have been determined 27.
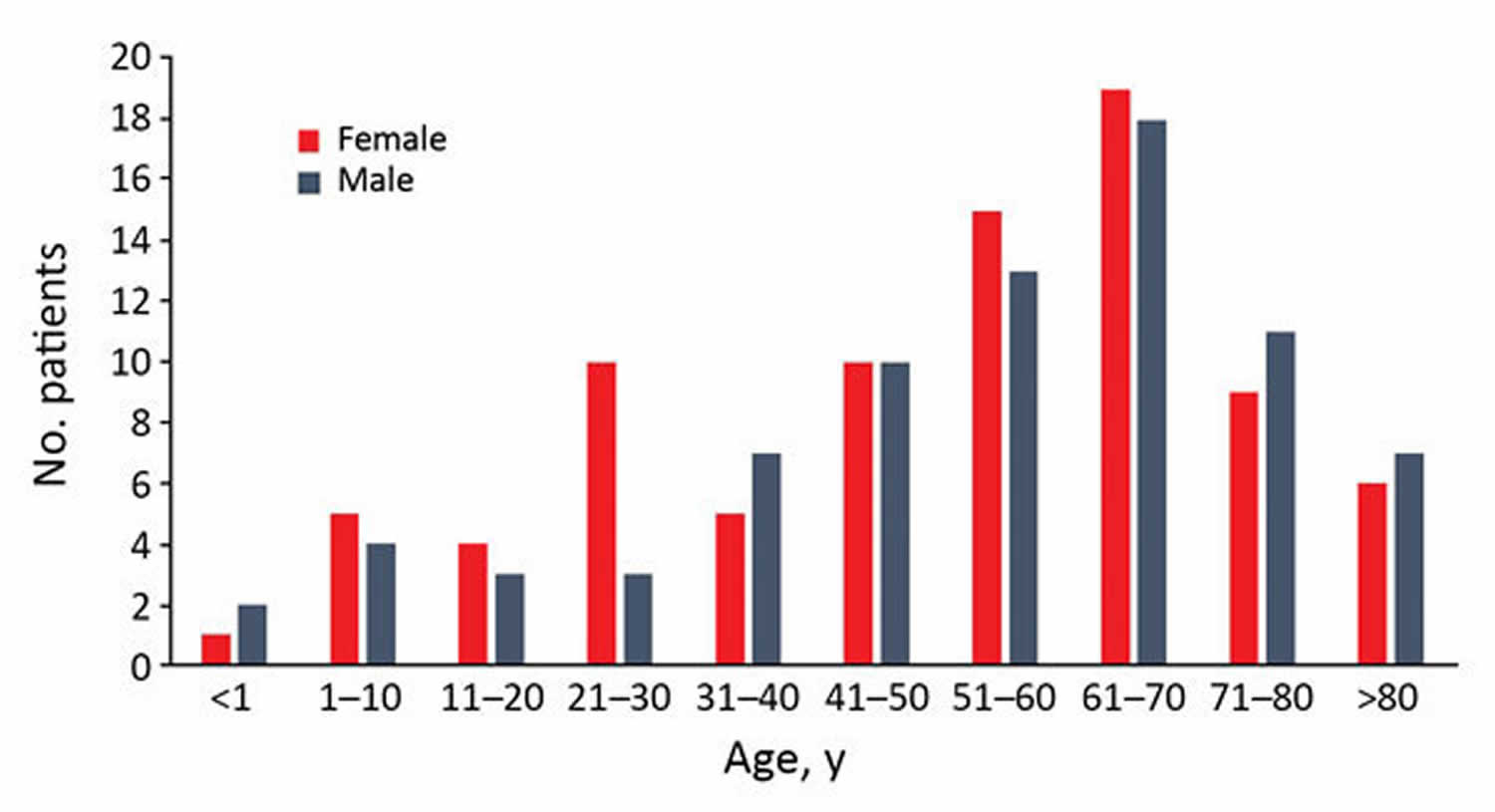
Figure 1. Distribution of pasteurellosis cases according to age group and sex, Szeged, Hungary, 2002–2015.
[Source 14 ]Figure 2. Distribution of various Pasteurella species in localized and invasive pasteurellosis, Szeged, Hungary, 2002–2015.
[Source 14 ]Pasteurella transmission
Infections with Pasteurella requiring medical intervention commonly arise as a result of bite or scratch wounds from pets, predominantly cats and dogs 1, but also from other domestic animals 28. Bite wound infections with Pasteurella tend to be highly aggressive with skin or soft tissue inflammation, erythema, local lymphadenopathy, fever, pain, and swelling often manifesting within 24 hours, but they can present as early as 8 to 12 hours 29. Pasteurellosis has an overall mortality rate of 25 to 30% among reported human cases of animal bite wounds 30, with bacteremia found in 40 to 63% of all pasteurellosis patients and meningitis plus neurological complications found in 17 to 29% of patients.
Pasteurella infections that do not result from bite wounds are likewise most often associated with Pasteurella multocida strains 30. and usually involve contact of skin lesions or naso-oropharynx or other upper respiratory mucosa with animals or animal secretions, particularly in young children, the elderly, or pregnant or immunocompromised individuals 31.
Neonatal meningitis (usually with septicemia) has been reported 32, but in nearly all cases the most likely route of transmission was attributed to direct exposure to pets or other domestic animals. Vertical transmission from mother to child was reported rarely 33. Only three instances of human-to-human horizontal transmission have been reported. In two cases transmission was likely from the father, who had exposure to chickens 34 or sheep 35, and in the third case the mother tested negative for Pasteurella multocida colonization but the grandmother tested positive, as did her pet dog 36.
Patients with underlying diseases that contribute to an immunocompromised condition, such as cirrhosis (liver dysfunction) 37, renal failure (kidney dysfunction requiring dialysis or indwelling catheters) 38, or HIV-positive status (especially if taking immunosuppressive drugs or experiencing other disease conditions) 39, have an increased risk of peritonitis, endocarditis, and/or septicemia caused by Pasteurella multocida. This is particularly the case if there is a history of exposure to pets. Indeed, in almost all of the above-mentioned reports, the authors caution patients with these conditions about the risks associated with exposure to pets and/or alert clinicians to consider possible complications with Pasteurella multocida infection for cases with pet ownership or a history of animal exposure.
It is noteworthy that in human infection cases the subspecies or serotype of the Pasteurella multocida clinical isolate is rarely reported 40. However, there are a few studies where this has been examined retrospectively. In one study, 143 isolates collected from human patients over a 12-year period (1983 to 1994) were biochemically characterized for distribution at the species and subspecies levels as well as capsular groups 41. Most of the isolates were determined to be Pasteurella multocida subsp. multocida, with the remaining being Pasteurella multocida subsp. septica, Pasteurella multocida subsp. gallicida, Pasteurella canis, Pasteurella dogmatis, and Pasteurella stomatis. While Pasteurella multocida strains were associated with cat and dog bites, Pasteurella canis, Pasteurella dogmatis, and Pasteurella stomatis strains were recovered only from dog bites, and Pasteurella multocida subsp. multocida and Pasteurella multocida subsp. septica were most frequently associated with cat bites. Most of the animal bite isolates were non-group A capsular strains (serogroup D) and were associated more with disseminated disease. Capsular serogroup A strains were associated more with respiratory infections. Similar findings were observed for isolates recovered from infected patients in four other studies involving 159 strains 42, 107 strains 43, 54 strains 44, and 20 strains 45.
Rare cases of zoonotic transmission through wild animals
Although zoonotic transmission from wild animals is relatively rare, Pasteurella infection is a serious concern in cases of bite wounds from wild animals. Similar to that for bites from their smaller domestic relatives, Pasteurella infection is a high risk for bites from large cats, including lions, tigers, cougars, and others (159, 162, 164, 166, 293, 371–375). Cases of Pasteurella infections have also been reported for bites or exposure to mucous secretions from other wild and domestic animals, including rats, opossums, horses, and rabbits (166, 259, 287, 295, 376). Although these incidents of severe outcome from zoonotic exposure appear to be relatively uncommon, it has been proposed based on historical precedence that there is a potential threat for any pathogen that exclusively infects animals to evolve into a pathogen that more readily transmits among animals and humans and then converts into a bona fide human-specific pathogen (377). It is not hard to speculate that there is potential for a zoonotic pathogen such as Pasteurella, which is highly prevalent in animals and can transmit to humans, to convert into a human pathogen upon acquisition of additional virulence traits.
Pasteurella symptoms
Common symptoms of pasteurellosis in humans from animal bite wounds are swelling (edema), cellulitis (diffuse, localized inflammation with redness and pain), and bloody or suppurative/purulent exudate (drainage) at the wound site 46. Leukocyte and neutrophil counts are typically high at the infection site, and inflammation develops very rapidly. In more severe cases, pasteurellosis can rapidly progress to bacteremia (fulminant sepsis) 47 and other complications such as osteomyelitis (inflammation of the bone) 48, endocarditis (inflammation of the heart) 49 and meningitis (inflammation of the meninges) 50. Respiratory infection in humans is relatively uncommon but can occur in patients with chronic pulmonary disease 51. In these instances, pasteurellosis can present as severe bilateral consolidating pneumonia and also can cause lymphadenopathy (swelling of the lymph nodes), epiglottitis, and abscess formation 52.
Localized Pasteurella infections secondary to bites, scratches, or licking were the most prevalent type 14. Most patients with localized pasteurellosis were female (59.6%). For invasive Pasteurella infections, however, male predominance (66.7%) was observed, and that difference was statistically significant 14. The difference in the age distribution among those with localized (median age 53.5 [range 0–97] years) and invasive (median age 63 [range 0–87] years) infection was also statistically significant 14. In the invasive-Pasteurella infection group, the largest number of patients (25%) had various abscesses (e.g., liver abscess, pulmonary abscess, abscesses after sigmoid resection); 16.7% patients had pneumonia, and 16.7% had bacteremia. Respiratory failure was diagnosed in 12.5% patients, osteomyelitis was in 8.3% patients, pleurisy in 4.2% patients, and arthritis in 4.2% patients. Central nervous system infection, adnexitis associated with pelvic inflammatory disease, peritonitis, pacemaker infection, cirrhosis, and gangrene were also recorded 14.
In 7% patients with localized Pasteurella infections and 77.1% patients with invasive Pasteurella infections, underlying diseases were recorded in the medical history (Table 1); the difference between the 2 groups was statistically significant. Some patients with invasive infections had multiple underlying disorders. Cardiovascular disease, diabetes, and malignancy were the most frequent underlying diseases.
Of 114 patients with localized Pasteurella infection, 94 (82.5%) had contact with a dog (43.6%) or cat (56.4%) 14. Ten patients had no animal contact, and no data were available for 10 patients 14. Animal contacts were recorded in 20.8% of 48 cases of invasive Pasteurella infection; no information about animal contacts was available in 33.3% cases. Twenty-two (45.8%) of 48 patients with invasive pasteurellosis had no animal contact.
Most injuries (74.6%) in the localized-Pasteurella infection group were attributed to animal bites. Scratch wounds caused by cats were recorded in 12 patients; however, some of them had simultaneous bite injuries. Six cases of cat-associated injury were observed in women 21–30 years of age; in the same age group of male patients (3 patients), none had animal contact (see Figure 1 above). Differences in the distribution of various Pasteurella spp. between localized and invasive infections could not be detected (see Figure 2).
The French Pasteurella National Center has reported that the prevalence of central nervous system infection caused by Pasteurella spp. is <1% of all Pasteurella infections 53. In adults, meningitis caused by Pasteurella infection is usually associated with cranial trauma or surgery or chronic otitis 54.
Table 1. Presence of underlying disease in cases of local and invasive Pasteurella spp. infections among patients in Szeged, Hungary, 2002–2015
| Underlying disease | No. (%) patients | |
|---|---|---|
| Local infection | Invasive infection | |
| Malignancy | 0 | 10 (20.8) |
| Cardiac | 0 | 6 (12.5) |
| Diabetes mellitus | 4 (3.5) | 6 (12.5) |
| >Pulmonary | 0 | 2 (4.2) |
| Hepatic (cirrhosis) | 0 | 2 (4.2) |
| Prosthesis | 0 | 2 (4.2) |
| Psychiatric | 1 (0.9) | 1 (2.1) |
| Genetic (Down syndrome) | 0 | 1 (2.1) |
| Multiple | 1 (0.9) | 7 (14.6) |
| No data | 106 (93) | 11 (22.9) |
| Total | 114 (100) | 48 (100) |
Human diseases caused by other pasteurellaceae
In contrast to Pasteurella, most of the other members of the Pasteurellaceae family are primarily animal commensals or enzootic or epizootic pathogens, with a few notable exceptions which are primarily human pathogens. These will be discussed in this section.
Haemophilus
Haemophilus influenzae is frequently found as a commensal in healthy adult humans but can cause invasive infections in humans, which present as cellulitis, arthritis, pneumonia, sepsis, or meningitis and can often become life threatening. There are six identifiable types and some nontypeable strains of H. influenzae associated with human disease. H. influenzae type b (Hib) is the most prominent form 55. Before introduction of the Hib vaccine, Hib was responsible for about 20,000 cases of and about 1,000 deaths from severe disease in children annually, but invasive Hib disease has nearly been eradicated since the introduction of the Hib conjugate vaccine 56. Other strains of H. influenzae, particularly noncapsular (nontypeable) strains, remain important pathogens in humans worldwide 57.
Haemophilus influenzae biogroup aegyptius is responsible for recurring outbreaks of seasonal acute, purulent conjunctivitis, more commonly known as pink eye 58. In 1984, a new, highly virulent strain emerged in Brazil that caused a highly lethal disseminated disease in young children, called Brazilian purpuric fever (BPF) 58. The infection presented as an acute, purulent conjunctivitis before rapid onset of bacteremia and progression to septic shock, with mortality rates as high as 70% 59. A pan-genomic analysis of the invasive BPF isolate with other noninvasive H. influenzae isolates responsible for conjunctivitis identified significant differences in the repertoire of autotransporter adhesins as well as new fimbrial proteins, which were suggested to contribute to virulence through altered host-pathogen interactions 58.
Haemophilus haemolyticus is closely related to H. influenzae but is generally considered to be a nonpathogenic human commensal found in the pharynges of some individuals 60. However, H. haemolyticus can be mistaken as nontypeable H. influenzae due to its lack of a capsule and its variable hemolytic properties on blood agar 60. Cases associated with invasive clinical disease in postsurgical patients have been identified using 16S rRNA sequencing 61.
Haemophilus ducreyi is a strict human pathogen that naturally infects genital and nongenital skin 62, causing genital ulcerative disease known as chancroid 63. Interestingly, Haemophilus ducreyi is more closely related to the animal pathogens M. haemolytica and Actinobacillus pleuropneumoniae than to other human pathogens of the Pasteurellaceae family 64. Identification of virulence genes and elucidation of the molecular basis of pathogenesis in H. ducreyi may provide insight into how a pathogen could adapt to occupy a unique niche in a human host 63.
Actinobacillus
Most Actinobacillus species are enzootic or epizootic pathogens; Actinobacillus hominis and Actinobacillus ureae are the only known exceptions, being highly adapted to humans. Both are relatively uncommon commensals of the human respiratory tract but can cause infections. Actinobacillus hominis can cause lower respiratory tract infections that can progress to bacteremia, sepsis, or meningitis and in severe cases can result in death, particularly in immunocompromised individuals 65. Most cases of Actinobacillus ureae infections are associated with predisposing factors, such as head trauma, a neurosurgical procedure, liver cirrhosis, alcoholism, diabetes, malnutrition, or immunosuppression 66. For example, Actinobacillus ureae meningitis was found in a number of immunocompromised patients 66. Actinobacillus ureae has also been associated with bone marrow infection and septic arthritis in a patient with rheumatoid arthritis taking a tumor necrosis factor alpha (TNF-α) inhibitor 67.
Aggregatibacter
Aggregatibacter actinomycetemcomitans is a common periodontal pathogen responsible for periodontitis, a chronic inflammatory disease that manifests as loss of supporting connective tissue and alveolar bone around teeth with symptoms of malodor, gingival bleeding, pain, and swelling 68. It is also a major pathogen causing endocarditis 69 and brain abscesses 70. A. actinomycetemcomitans produces two toxins as major virulence factors responsible for pathogenesis: a pore-forming leukotoxin (LtxA) that kills white blood cells in gingiva and thereby helps evade the host immune response during infection 71 and a cytolethal distending toxin (Cdt) that enters host cells to cause cell cycle arrest or apoptosis through its DNase activity and thereby causes extensive damage to gingival tissue 72.
Aggregatibacter aphrophilus is also an oral commensal occasionally found in humans that causes bone and joint infections and endocarditis in some cases 73. Its genome contains genes that encode a type VI secretion system (T6SS) as well as several putative T6SS effector proteins that may contribute to virulence 74.
Pasteurellosis complications
Ileostomy, hysterectomy, skin transplantation, and amputation were documented in 4 cases of invasive pasteurellosis 14. Ileostomy was performed because of sudden onset of perforation; during the surgical procedure, a sample from the abdominal cavity was collected for microbiological culture, and Pasteurella multocida was isolated. Total abdominal hysterectomy and bilateral salpingo-oophorectomy were performed in a young woman with bilateral tubo-ovarian abscess due to Pasteurella multocida infection. A pacemaker electrode, a prosthesis, and a catheter were removed in 3 different cases because of endocarditis, recurrent inflammations around knee prosthesis, and purulent drainage around the dialysis catheter.
After localized Pasteurella infection, severe complications were registered in 23 cases (20.2%); functional disability, mainly in fingers, developed in 10 patients (6 cases associated with dog bites, 4 cases with cat bites and scratches); and lymphangitis was observed in 7 of 53 (13.2%) patients. Following bites and scratches, 3 patients had amputations (2 from dog bites, 1 from a cat bite), affecting mainly the fingers; 3 patients had osteomyelitis (associated with dog bites) as a consequence of pasteurellosis. The high number of complications emphasizes the importance of localized and invasive pasteurella infections.
Of the 162 patients with pasteurellosis, 143 (88.2%) patients recovered; in 6 cases, no data were available about the outcome of pasteurellosis (in these cases, patients were transferred to other hospitals). Thirteen of the 162 patients died; all these patients had invasive pasteurellosis infections. Seven of these 13 patients had pneumonia and respiratory failure, 5 patients had bacteremia, and 1 patient had panencephalitis after traumatic brain injury. The median age of patients who died was 69.5 (range 39–84) years; most of them had multiple underlying diseases. Laboratory investigations revealed elevated leukocytes, C-reactive protein levels, and liver enzymes in all patients who died from pasteurellosis. In 4 cases, animal contacts, including dogs, cats, and other animals, were recorded in the patient’s medical history. No information about animal contact was available in 5 cases, and in 4 cases, no animal contact could be found. In cases of animal contacts, no bite or scratch was mentioned by the patients or relatives.
From clinical specimens (respiratory tract specimens, cerebrospinal fluid, and blood), Pasteurella multocida was isolated from 12 patients, and Pasteurella canis from 1 patient. All 13 of these patients were hospitalized; the average of length of hospital stay was 10.3 (range 1–30) days. Pasteurella sp. was isolated from respiratory specimens or pleural fluid from 6 patients, from blood culture from 4 patients, from 2 wound specimens, and from 1 cerebrospinal fluid specimen.
Pasteurellosis treatment
Broad-spectrum antibiotics that target Pasteurella, as well as other Gram-negative and Gram-positive bacteria, are the preferred prophylaxis for animal bites, which tend to be polymicrobial in nature 75. Pasteurella species are not very susceptible to erythromycin, lincosamides (such as clindamycin), or certain β-lactams (such as dicloxacillin or cephalexin), so these antibiotics are not recommended as monovalent treatments for animal bites. Instead, a combination of amoxicillin and the β-lactamase inhibitor clavulanic acid (Augmentin), doxycycline plus metronidazole for patients with penicillin allergies, or clindamycin plus a fluoroquinolone (ciprofloxacin, or trimethoprim-sulfamethoxazole combination for children or ceftriaxone for pregnant women) is the recommended treatment regimen 47.
References- Wilson BA, Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev. 2013;26(3):631–655. doi:10.1128/CMR.00024-13 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3719492
- Choudat D, Le Goff C, Delemotte B, Paul G, Mady V, Fages J, Conso F. 1987. Occupational exposure to animals and antibodies against Pasteurella multocida. Br. J. Ind. Med. 44:829–833
- Weiss HB, Friedman DI, Coben JH. 1998. Incidence of dog bite injuries treated in emergency departments. JAMA 279:51–53
- Rempe B, Aloi M, Iskyan K. 2009. Evidence-based management of mammalian bite wounds. Pediatr. Emerg. Med. Pract. 6:1–22
- Souza MJ. 2011. One health: zoonoses in the exotic animal practice. Vet. Clin. North Am. Exot. Anim. Pract. 14:421–426
- Nollet V, Souply L, Rosolen B, Mohseni-Zadeh M, Martinot M. Risk factors for invasive pasteurellosis: a retrospective case study. Eur J Clin Microbiol Infect Dis. 2016;35(12):1975–1981. doi:10.1007/s10096-016-2749-y
- Branch J, Kakutani T, Kuroda S, Shiba Y, Kitagawa I. Pasteurella multocida infective endocarditis: a possible link with primary upper respiratory tract infection. Intern Med. 2015;54:3225–31. 10.2169/internalmedicine.54.4973
- Nollet V, Souply L, Rosolen B, Mohseni-Zadeh M, Martinot M. Risk factors for invasive pasteurellosis: a retrospective case study. Eur J Clin Microbiol Infec Dis. 2016;35:1975–81.
- Wilson BA, Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev. 2013;26:631–55. 10.1128/CMR.00024-13
- Felix M, Tallon P, Salavert M, Navarro V, Breton JR, Perez-Belles C, Gobernado M. 2003. Bacteremia due to Pasteurella spp.: a rare process in our hospital over the last 8 years. Enferm. Infecc. Microbiol. Clin. 21:334–339
- Mohan K, Kelly PJ, Hill FW, Muvavarirwa P, Pawandiwa A. 1997. Phenotype and serotype of Pasteurella multocida isolates from diseases of dogs and cats in Zimbabwe. Comp. Immunol. Microbiol. Infect. Dis. 20:29–34
- Raval P, Khan W, Haddad B, Mahapatra AN. Bite injuries to the hand – review of the literature. Open Orthop J. 2014;8(Suppl 1):204–8. 10.2174/1874325001408010204
- Christenson ES, Ahmed HM, Durand CM. Pasteurella multocida infection in solid organ transplantation. Lancet Infect Dis. 2015;15:235–40. 10.1016/S1473-3099(14)70895-3
- Körmöndi S, Terhes G, Pál Z, et al. Human Pasteurellosis Health Risk for Elderly Persons Living with Companion Animals. Emerg Infect Dis. 2019;25(2):229–235. doi:10.3201/eid2502.180641 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6346445
- Giordano A, Dincman T, Clyburn BE, Steed LL, Rockey DC. Clinical features and outcomes of Pasteurella multocida infection. Medicine (Baltimore). 2015;94:e1285. 10.1097/MD.0000000000001285
- Harper M, Boyce JD, Adler B. 2006. Pasteurella multocida pathogenesis: 125 years after Pasteur FEMS Microbiol. Lett. 265:1–10
- Zbinden R. Aggregatibacter, Capnocytophaga, Eikenella, Kingella, Pasteurella, and other fastidious or rarely encountered Gram-negative rods. In: Manual of Clinical Microbiology, 11th Ed, Jorgenesen JH, Pfaller MA (Eds), American Society for Microbiology, Washington, DC 2015. p.652.
- Holst E, Rollof J, Larsson L, Nielsen JP. Characterization and distribution of Pasteurella species recovered from infected humans. J Clin Microbiol. 1992;30:2984–7
- Rosner H, Grimmecke HD, Knirel YA, Shashkov AS. 1992. Hyaluronic acid and a (1-4)-beta-d-xylan, extracellular polysaccharides of Pasteurella multocida (Carter type A) strain 880. Carbohydr. Res. 223:329–333
- Boyce JD, Chung JY, Adler B. 2000. Genetic organisation of the capsule biosynthetic locus of Pasteurella multocida M1404 (B:2). Vet. Microbiol. 72:121–134
- DeAngelis PL, White CL. 2002. Identification and molecular cloning of a heparosan synthase from Pasteurella multocida type D. The J. Biol. Chem. 277:7209–7213
- DeAngelis PL, Gunay NS, Toida T, Mao WJ, Linhardt RJ. 2002. Identification of the capsular polysaccharides of Type D and F Pasteurella multocida as unmodified heparin and chondroitin, respectively. Carbohydr. Res. 337:1547–1552
- Harper M, Boyce JD, Adler B. 2012. The key surface components of Pasteurella multocida: capsule and lipopolysaccharide. Curr. Top. Microbiol. Immunol. 361:39–51
- Watt JM, Swiatlo E, Wade MM, Champlin FR. 2003. Regulation of capsule biosynthesis in serotype A strains of Pasteurella multocida. FEMS Microbiol. Lett. 225:9–14
- Harper M, St Michael F, Vinogradov E, John M, Boyce JD, Adler B, Cox AD. 2012. Characterization of the lipopolysaccharide from Pasteurella multocida Heddleston serovar 9: identification of a proposed bi-functional dTDP-3-acetamido-3,6-dideoxy-alpha-d-glucose biosynthesis enzyme. Glycobiology 22:332–344
- von Graevenitz A, Zbinden R, Mutters R. Actinobacillus, Capnocytophaga, Eikenella, Kingella, Pasteurella, and other fastidious or rarely encountered Gram-negative rods. In: Manual of Clinical Microbiology, 9th Ed, Murray PR (Ed), American Society for Microbiology, Washington, DC 2007. p.621.
- May BJ, Zhang Q, Li LL, et al. Complete genomic sequence of Pasteurella multocida, Pm70. Proc Natl Acad Sci U S A 2001; 98:3460.
- Yefet E, Abozaid S, Nasser W, Peretz A, Zarfin Y. 2011. Unusual infection-Pasteurella canis bacteremia in a child after exposure to rabbit secretions. Harefuah 150:13–70
- Fleisher GR. 1999. The management of bite wounds. N. Engl. J. Med. 340:138–140
- Koch CA, Robyn JA. 1998. Risk of animal contact in immunocompromised hosts. Arch. Intern. Med. 158:1036
- Heydemann J, Heydemann JS, Antony S. 2010. Acute infection of a total knee arthroplasty caused by Pasteurella multocida: a case report and a comprehensive review of the literature in the last 10 years. Int. J. Infect. Dis. 14:e242–e245
- Koranyi KI, Barson WJ, Studley JK. 2005. Meningitis in a young infant. Pediatr. Infect. Dis. J. 24:387.
- Hillery S, Reiss-Levy EA, Browne C, Au T, Lemmon J. 1993. Pasteurella multocida meningitis in a two-day old neonate. Scand. J. Infect. Dis. 25:655–658
- Spadafora R, Pomero G, Delogu A, Gozzoli L, Gancia P. 2011. A rare case of neonatal sepsis/meningitis caused by Pasteurella multocida complicated with status epilepticus and focal cerebritis. Pediatr. Med. Chir. 33:199–202
- Guillet C, Join-Lambert O, Carbonnelle E, Ferroni A, Vachee A. 2007. Pasteurella multocida sepsis and meningitis in 2-month-old twin infants after household exposure to a slaughtered sheep. Clin. Infect. Dis. 45:e80–e81
- Siahanidou T, Gika G, Skiathitou AV, Oikonomopoulos T, Alexandrou-Athanassoulis H, Koutouzis EI, Syriopoulou VP. 2012. Pasteurella multocida infection in a neonate: evidence for a human-to-human horizontal transmission. Pediat. Infect. Dis. J. 31:536–537
- Koch CA, Mabee CL, Robyn JA, Koletar SL, Metz EN. 1996. Exposure to domestic cats: risk factor for Pasteurella multocida peritonitis in liver cirrhosis? Am. J. Gastroenterol. 91:1447–1449
- Cooke FJ, Kodjo A, Clutterbuck EJ, Bamford KB. 2004. A case of Pasteurella multocida peritoneal dialysis-associated peritonitis and review of the literature. Int. J. Infect. Dis. 8:171–174
- Guerin JM, Mofredj A, Raskine L, Leibinger F. 1994. Septicemia and purulent meningitis caused by Pasteurella multocida in a HIV positive patient. Presse Med. 23:631
- Tay L, Tey BH, Lim YS, Chew LS, Seng ST. 1992. Pasteurella multocida infection in Singapore. Trop. Geogr. Med. 44:359–361
- Donnio PY, Lerestif-Gautier AL, Avril JL. 2004. Characterization of Pasteurella spp. strains isolated from human infections. J. Comp. Pathol. 130:137–142
- Holst E, Rollof J, Larsson L, Nielsen JP. 1992. Characterization and distribution of Pasteurella species recovered from infected humans. J. Clin. Microbiol. 30:2984–2987
- Talan DA, Citron DM, Abrahamian FM, Moran GJ, Goldstein EJ. 1999. Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N. Engl. J. Med. 340:85–92
- Avril JL, Donnio PY. 1987. Characterization of Pasteurella species isolated from man. Pathol. Biol. (Paris) 35:169–172
- Chen HI, Hulten K, Clarridge JE., III 2002. Taxonomic subgroups of Pasteurella multocida correlate with clinical presentation. J. Clin. Microbiol. 40:3438–3441
- Adler AC, Cestero C, Brown RB. 2011. Septic shock from Pasturella multocida following a cat bite: case report and review of literature. Conn. Med. 75:603–605
- Oehler RL, Velez AP, Mizrachi M, Lamarche J, Gompf S. 2009. Bite-related and septic syndromes caused by cats and dogs. Lancet Infect. Dis. 9:439–447
- Desai SS, Groves RJ, Glew R. 1990. Subacute Pasteurella osteomyelitis of the hand following dog bite. Orthopedics 13:653–656
- Khan MF, Movahed MR, Jung J. 2012. Pasteurella multocida endocarditis. J. Heart Valve Dis. 21:260–262
- Kawashima S, Matsukawa N, Ueki Y, Hattori M, Ojika K. 2010. Pasteurella multocida meningitis caused by kissing animals: a case report and review of the literature. J. Neurol. 257:653–654
- Klein NC, Cunha BA. 1997. Pasteurella multocida pneumonia. Semin. Respir. Infect. 12:54–56
- Myers EM, Ward SL, Myers JP. 2012. Life-threatening respiratory pasteurellosis associated with palliative pet care. Clin. Infect. Dis. 54:e55–e57
- Escande F, Lion C. Epidemiology of human infections by Pasteurella and related groups in France. Zentralbl Bakteriol. 1993;279:131–9. 10.1016/S0934-8840(11)80499-8
- Kumar A, Devlin HR, Vellend H. Pasteurella multocida meningitis in an adult: case report and review. Rev Infect Dis. 1990;12:440–8. 10.1093/clinids/12.3.440
- Broome CV. 1987. Epidemiology of Haemophilus influenzae type b infections in the United States. Pediatr. Infect. Dis. J. 6:779–782
- Agrawal A, Murphy TF. 2011. Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J. Clin. Microbiol. 49:3728–3732
- Livorsi DJ, Macneil JR, Cohn AC, Bareta J, Zansky S, Petit S, Gershman K, Harrison LH, Lynfield R, Reingold A, Schaffner W, Thomas A, Farley MM. 2012. Invasive Haemophilus influenzae in the United States, 1999-2008: epidemiology and outcomes. J. Infect. 65:496–504
- Strouts FR, Power P, Croucher NJ, Corton N, van Tonder A, Quail MA, Langford PR, Hudson MJ, Parkhill J, Kroll JS, Bentley SD. 2012. Lineage-specific virulence determinants of Haemophilus influenzae biogroup aegyptius. Emerg. Infect. Dis. 18:449–457
- Harrison LH, Simonsen V, Waldman EA. 2008. Emergence and disappearance of a virulent clone of Haemophilus influenzae biogroup aegyptius, cause of Brazilian purpuric fever. Clin. Microbiol. Rev. 21:594–605
- McCrea KW, Xie J, LaCross N, Patel M, Mukundan D, Murphy TF, Marrs CF, Gilsdorf JR. 2008. Relationships of nontypeable Haemophilus influenzae strains to hemolytic and nonhemolytic Haemophilus haemolyticus strains. J. Clin. Microbiol. 46:406–416
- Anderson R, Wang X, Briere EC, Katz LS, Cohn AC, Clark TA, Messonnier NE, Mayer LW. 2012. Haemophilus haemolyticus isolates causing clinical disease. J. Clin. Microbiol. 50:2462–2465
- Bong CT, Bauer ME, Spinola SM. 2002. Haemophilus ducreyi: clinical features, epidemiology, and prospects for disease control. Microbes Infect. 4:1141–1148
- Janowicz DM, Ofner S, Katz BP, Spinola SM. 2009. Experimental infection of human volunteers with Haemophilus ducreyi: fifteen years of clinical data and experience. J. Infect. Dis. 199:1671–1679
- Gioia J, Qin X, Jiang H, Clinkenbeard K, Lo R, Liu Y, Fox GE, Yerrapragada S, McLeod MP, McNeill TZ, Hemphill L, Sodergren E, Wang Q, Muzny DM, Homsi FJ, Weinstock GM, Highlander SK. 2006. The genome sequence of Mannheimia haemolytica A1: insights into virulence, natural competence, and Pasteurellaceae phylogeny. J. Bacteriol. 188:7257–7266
- Friis-Moller A, Christensen JJ, Fussing V, Hesselbjerg A, Christiansen J, Bruun B. 2001. Clinical significance and taxonomy of Actinobacillus hominis. J. Clin. Microbiol. 39:930–935
- de Castro N, Pavie J, Lagrange-Xelot M, Bouvry D, Delisle F, Parrot A, Molina JM. 2007. Severe Actinobacillus ureae meningitis in an immunocompromised patient: report of one case and review of the literature. Scand. J. Infect. Dis. 39:1076–1079
- Kaur PP, Derk CT, Chatterji M, Dehoratius RJ. 2004. Septic arthritis caused by Actinobacillus ureae in a patient with rheumatoid arthritis receiving anti-tumor necrosis factor-alpha therapy. J. Rheumatol. 31:1663–1665
- Henderson B, Ward JM, Ready D. 2010. Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen? Periodontology 2000 54:78–105
- Paturel L, Casalta JP, Habib G, Nezri M, Raoult D. 2004. Actinobacillus actinomycetemcomitans endocarditis. Clin. Microbiol. Infect. 10:98–118
- Rahamat-Langendoen JC, van Vonderen MG, Engstrom LJ, Manson WL, van Winkelhoff AJ, Mooi-Kokenberg EA. 2011. Brain abscess associated with Aggregatibacter actinomycetemcomitans: case report and review of literature. J. Clin. Periodontol. 38:702–706
- Kachlany SC. 2010. Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. J. Dent. Res. 89:561–570
- Damek-Poprawa M, Korostoff J, Gill R, Dirienzo JM. 2013. Cell junction remodeling in gingival tissue exposed to a microbial toxin. J. Dent. Res. 92:518–523
- Huang ST, Lee HC, Lee NY, Liu KH, Ko WC. 2005. Clinical characteristics of invasive Haemophilus aphrophilus infections. J. Microbiol. Immunol. Infect. 38:271–276
- Di Bonaventura MP, DeSalle R, Pop M, Nagarajan N, Figurski DH, Fine DH, Kaplan JB, Planet PJ. 2009. Complete genome sequence of Aggregatibacter (Haemophilus) aphrophilus NJ8700. J. Bacteriol. 191:4693–4694
- Goldstein EJ, Citron DM, Merriam CV, Tyrrell KL. 2012. Ceftaroline versus isolates from animal bite wounds: comparative in vitro activities against 243 isolates, including 156 Pasteurella species isolates. Antimicrob. Agents Chemother. 56:6319–6323