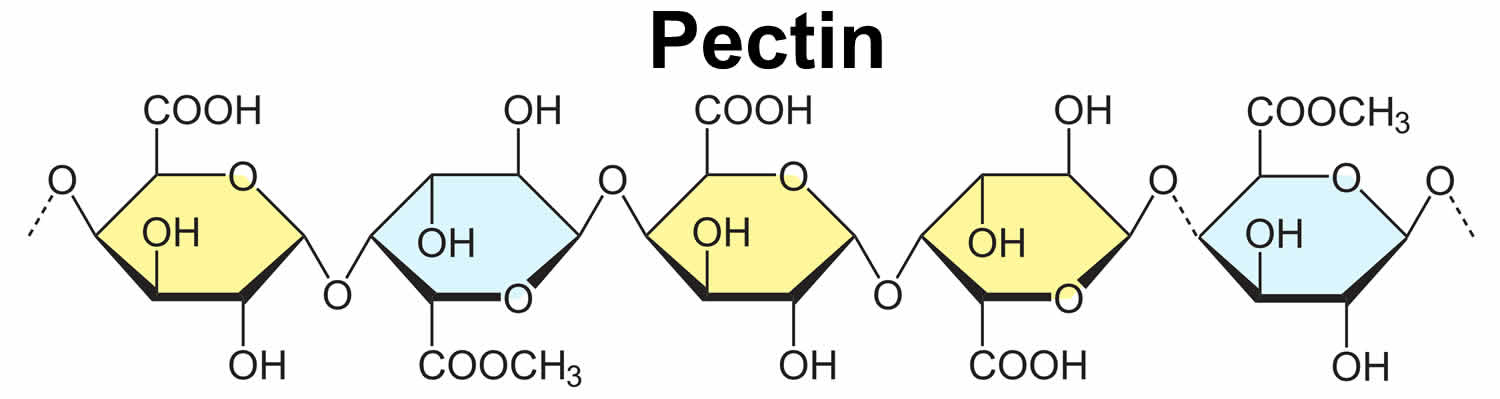
What is pectin
Pectin is a high-molecular-weight carbohydrate polymer derived from the cell wall of plants which is present in virtually all plants where it contributes to the cell structure. The term pectin covers a number of polymers which vary according to their molecular weight, chemical configuration, and content of neutral sugars, and different plant types produce pectin with different functional properties 1. The word ‘pectin’ comes from the Greek word pektos which means firm and hard, reflecting pectin’s ability to form gels 1. The gelling properties of pectin have been known for centuries, but the isolation of commercial pectin only started at the beginning of the twentieth century. Depending on the molecular composition there are different types of pectin characterizing themselves particularly by different gelling mechanisms. Pectin is produced commercially as a white to light brown powder, mainly extracted from citrus fruits and is used in food (food additives pectin E 440i and amidated pectin E 440ii) as a gelling agent, particularly in jams and jellies. In the United States, pectin is generally recognized as safe (GRAS) for human consumption 2. Pectin is also used in dessert fillings, medicines, sweets, as a stabilizer in fruit juices and milk drinks, and as a source of dietary fiber. Pectins vary in their chain lengths, complexity and the order of each of the monosaccharide units. The characteristic structure of pectin is a linear chain of alpha(1-4)linked D-galacturonic acid that forms the pectin-backbone, a homogalacturonan.
Pectin (E 440i) and amidated pectin (E 440ii) are authorized as food additives in the European Union and specific purity criteria have been defined in Commission Regulation (EU) No 231/2012 3. E 440 includes both pectin (E 440i) and amidated pectin (E 440ii).
Pectin (E 440i) consists of the partial methyl esters of polygalacturonic acid, while amidated pectin (E 440ii) consists of both partial methyl esters and amides of polygalacturonic acid. The average molecular weight and the degree of methylation of pectins are highly variable, being their physicochemical properties, such as viscosity and gel strength, largely dependent on these parameters.
An evaluation of pectin E 440i and amidated pectin E 440ii is also available from the Nordic Council of Ministers 4. It was stated that pectin has been a natural component of human diet throughout evolution and there is no indication of toxic effects induced by pectin or amidated pectin.
The European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies established a cause and effect relationship between the consumption of pectins and a reduction of post‐prandial glycemic responses and maintenance of normal blood cholesterol concentrations 5. In order to obtain these physiological effects, 10 g of pectins per meal or 6 g of pectins per day, in one or more servings, respectively, are required.
An acceptable daily intake (ADI) ‘not specified’ was allocated by the Scientific Committee for Food for pectin (E 440i) and amidated pectin (E 440ii) 6. Pectin (E 440i) and amidated pectin (E 440ii) would not be absorbed intact, but extensively fermented by intestinal microbiota in humans; products formed from pectins in the gastrointestinal tract are similar to manufactured pectin‐derived acidic oligosaccharides. There is no indication of genotoxicity for pectin (E 440i) and amidated pectin (E 440ii), although the available data were limited 3. No adverse effects were reported in a chronic toxicity study in rats at levels up to 5,000 mg pectin/kg body weight per day, the highest dose tested 3. No treatment‐related effects were observed in a dietary one‐generation reproductive toxicity study with pectin‐derived acidic oligosaccharides in rats at up to 6,200 mg/kg body weight per day, the highest dose tested. The European Food Safety Authority Panel did not consider pectin (E 440i) and amidated pectin (E 440ii) as having allergenic potential 3. A dose of 36 g/day (equivalent to 515 mg/kg body weight per day) for 6 weeks in humans was without adverse effects 3. Exposure to pectins from their use as food additives ranged up to 442 mg/kg body weight per day for toddlers at the 95th percentile 3. The European Food Safety Authority Panel concluded that there is no safety concern for the use of pectin (E 440i) and amidated pectin (E 440ii) as food additives for the general population and that there is no need for a numerical acceptable daily intake (ADI) 3.
Pectin benefits
Reduces the postprandial levels of glucose
In 2012, the European Food Safety Authority Panel took into account the consistency of a post-prandial blood glucose-lowering effect of pectins consumed in foods or meals across the studies considered, and that the mechanism by which pectins could exert the claimed effect is well known. On the basis of the data presented, the European Food Safety Authority Panel concludes that a cause and effect relationship has been established between the consumption of pectins and a reduction of post-prandial glycemic responses 7. The European Food Safety Authority Panel considers that, in order to bear the claim, foods should provide at least 10 g of pectins per meal 7. The target population is adults willing to reduce their post-prandial glycemic responses.
Maintenance of normal blood cholesterol concentrations
In weighing the evidence, the European Food Safety Authority Panel took into account the consistency of the total and LDL “bad” cholesterol lowering effect of pectins across the studies considered, and that the mechanism by which pectins could exert the claimed effect is well known. On the basis of the data presented, the European Food Safety Authority Panel concludes that a cause and effect relationship has been established between the consumption of pectins and maintenance of normal blood cholesterol concentrations 7. The European Food Safety Authority Panel considers that, in order to bear the claim, foods should provide at least 6 g per day of pectins in one or more servings 7. The target population is adults.
Sources of pectin
Pears, apples, guavas, quince, plums, gooseberries, and oranges and other citrus fruits contain large amounts of pectin, while soft fruits, like cherries, grapes, and strawberries, contain small amounts of pectin.
Typical levels of pectin in fresh plants are:
- apples, 1–1.5%
- apricots, 1%
- cherries, 0.4%
- oranges, 0.5–3.5%
- carrots 1.4%
- citrus peels, 30%
The main raw materials for pectin production are dried citrus peels or apple pomace, both by-products of juice production. Pomace from sugar beets is also used to a small extent.
From these materials, pectin is extracted by adding hot dilute acid at pH-values from 1.5 – 3.5. During several hours of extraction, the protopectin loses some of its branching and chain length and goes into solution. After filtering, the extract is concentrated in a vacuum and the pectin is then precipitated by adding ethanol or isopropanol. An old technique of precipitating pectin with aluminium salts is no longer used (apart from alcohols and polyvalent cations, pectin also precipitates with proteins and detergents).
Alcohol-precipitated pectin is then separated, washed and dried. Treating the initial pectin with dilute acid leads to low-esterified pectins. When this process includes ammonium hydroxide (NH3(aq)), amidated pectins (E 440ii) are obtained.
What is pectin used for?
The main use for pectin is as a gelling agent, thickening agent and stabilizer in food. The classical application is giving the jelly-like consistency to jams or marmalades, which would otherwise be sweet juices. Pectin also reduces syneresis in jams and marmalades and increases the gel strength of low-calorie jams. For household use, pectin is an ingredient in gelling sugar (also known as “jam sugar”) where it is diluted to the right concentration with sugar and some citric acid to adjust pH. In some countries, pectin is also available as a solution or an extract, or as a blended powder, for home jam making. For conventional jams and marmalades that contain above 60% sugar and soluble fruit solids, high-ester pectins are used. With low-ester pectins and amidated pectins, less sugar is needed, so that diet products can be made.
Pectin is used in confectionery jellies to give a good gel structure, a clean bite and to confer a good flavor release. Pectin can also be used to stabilize acidic protein drinks, such as drinking yogurt, to improve the mouth-feel and the pulp stability in juice based drinks and as a fat substitute in baked goods 8. Typical levels of pectin used as a food additive are between 0.5 and 1.0% – this is about the same amount of pectin as in fresh fruit 9.
In medicine, pectin increases viscosity and volume of stool so that it is used against constipation and diarrhea. Until 2002, it was one of the main ingredients used in Kaopectate a medication to combat diarrhea, along with kaolinite. It has been used in gentle heavy metal removal from biological systems 10. Pectin is also used in throat lozenges as a demulcent.
In cosmetic products, pectin acts as a stabilizer. Pectin is also used in wound healing preparations and specialty medical adhesives, such as colostomy devices.
Sriamornsak 11 revealed that pectin could be used in various oral drug delivery platforms, e.g., controlled release systems, gastro-retentive systems, colon-specific delivery systems and mucoadhesive delivery systems, according to its intoxicity and low cost. It was found that pectin from different sources provides different gelling abilities, due to variations in molecular size and chemical composition. Like other natural polymers, a major problem with pectin is inconsistency in reproducibility between samples, which may result in poor reproducibility in drug delivery characteristics.
In ruminant nutrition, depending on the extent of lignification of the cell wall, pectin is up to 90% digestible by bacterial enzymes. Ruminant nutritionists recommend that the digestibility and energy concentration in forages be improved by increasing pectin concentration in the forage.
In cigars, pectin is considered an excellent substitute for vegetable glue and many cigar smokers and collectors use pectin for repairing damaged tobacco leaves on their cigars.
Is pectin bad for you?
The present opinion deals with the re‐evaluation of the safety of pectin (E 440i) and amidated pectin (E 440ii) when used as food additives, including food supplements and does not include a safety assessment of other uses of pectins. Pectin (E 440i) and amidated pectin (E 440ii) are authorized food additives in the European Union Regulation (EC) No 1333/20081 3. E 440 includes both pectin (E 440i) and amidated pectin (E 440ii). Regulation No 1333/2008 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re‐evaluated by European Food Safety Authority.
The European Food Safety Authority Panel Findings Summary:
- based on these reported use levels, a refined exposure of up to 442 mg/kg body weight per day for toddlers in these categories (brand‐loyal scenario) was estimated;
- pectin and amidated pectin are not absorbed intact, but extensively fermented by intestinal microbiota in animals and humans;
- adequate toxicity data were available;
- in oral subchronic studies with pectins in rats, no adverse effects were observed at doses ranging from 3,366 mg/kg body weight per day to 13,500 mg/kg body weight per day, the highest doses tested. In subchronic studies with pectin‐derived acidic oligosaccharides in the diet in rats, a no‐observed‐adverse‐effect level (NOAEL) of 1,700 mg/kg body weight per day was identified;
- no effects on body weight and food intake were observed in male neonatal pigs exposed to 0.3% pectin in formula (equal to 1,049 mg pectin/kg body weight per day) for 28 days, while in the same study such effects were observed at 1% pectin in formula (equal to 4,015 mg pectin/kg body weight per day);
- no adverse effects were reported in a chronic study in rats at up to 5,000 mg pectin/kg body weight per day, the highest dose tested;
- there is no concern with respect to genotoxicity for pectin and amidated pectin;
- a daily dose of 36,000 mg pectin (equivalent to 515 mg/kg body weight per day) for 6 weeks in humans was associated with abdominal distension and increasing flatus in some individuals, effects which were considered by the European Food Safety Authority Panel as undesirable, but not adverse,
The European Food Safety Authority Panel concluded that there is no need for a numerical acceptable daily intake (ADI) for pectin (E 440i) and amidated pectin (E 440ii) and that there is no safety concern for the general population at the refined exposure assessment for the reported uses and use levels of pectins (E 440) as food additives 3.
Concerning the use of pectins (E 440) in ‘dietary foods for special medical purposes and special formulae for infants’ and in ‘dietary foods for babies and young children for special medical purposes:
- for populations consuming foods for special medical purposes and special formulae, the 95th percentile of maximum exposure assessments calculated based on the maximum reported data from food additive producers were up to 1,349 mg/kg body weight per day for infants;
- infants and young children consuming these foods may be exposed to a greater extent to pectins (E 440) than their healthy counterparts because the permitted levels of pectins (E 440) in formulae for special medical purposes are 2‐fold higher (1%) than in formulae for healthy individuals;
- infants and young children consuming foods belonging to these food categories may show a higher susceptibility to the gastrointestinal effects of pectins than their healthy counterparts due to their underlying medical condition;
- no effects on body weight and food intake were observed in male neonatal pigs exposed to 0.3% pectin in formula (equal to 1,049 mg pectin/kg body weight per day) for 28 days, while in the same study, such effects were observed at 1% pectin in formula (equal to 4,015 mg pectin/kg body weight per day);
- in infants and young children (< 18 months of age), a formula containing 0.5% pectin (E 440) given for 3 months was well tolerated without adverse effects;
- no human studies investigating the adverse effects of formulae containing pectins (E 440) at the maximum permitted level of 1% for the relevant age group were available.
The European Food Safety Authority Panel concluded, that the available data do not allow for an adequate assessment of the safety of use of pectins (E 440) in infants and young children consuming these foods for special medical purposes at the presently authorized maximum use levels of 1% 3.
The European Food Safety Authority Panel recommended that:
- the European Commission considers lowering the maximum limits for the impurities of toxic elements arsenic, lead, mercury and cadmium in the EU specifications for pectin (E 440i) and amidated pectin (E 440ii) in order to ensure that pectin (E 440i) and amidated pectin (E 440ii) as food additives will not be a significant source of exposure to those toxic elements in food; special requirements might be defined in the specifications for pectin (E 440i) and amidated pectin (E 440ii) to be used in formulae or food for infants, toddlers and other young children;
- limits for aluminium should be considered for inclusion in the EU specifications, as aluminium can be used in the manufacturing process;
- the European Commission considers harmonizing the microbiological specifications for polysaccharidic thickening agents, such as pectins, and including criteria for the absence of Salmonella spp. and Escherichia coli, for total aerobic microbial count and for total combined yeasts and molds count in the EU specifications for pectin (E 440i) and amidated pectin (E 440ii);
- additional clinical data should be generated to assess the safety of pectins (E 440) when used in ‘dietary foods for special medical purposes and special formulae for infants’ and in ‘dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC’;
- due to the discrepancies observed between the data reported from industry and the Mintel database, where pectins are labelled in more products than in food categories for which data were reported from industry, the European Food Safety Authority Panel recommended collection of data on usage and use levels of pectins (E 440) in order to perform a more realistic exposure assessment.
In 2014, Joint Food and Agriculture Organization (FAO)/World Health Organisation (WHO) Expert Committee on Food Additives (JECFA) evaluated the safety of using non‐amidated pectin (E 440i) in infant formula and formula for special medical purposes intended for infants 12. In this document, it was stated that ‘the Committee was made aware that a further pectin product is available on the market. This product, known as pectin‐derived acidic oligosaccharides, is produced by enzymatic hydrolysis of pectin. Pectin‐derived acidic oligosaccharides has not been evaluated by the Committee and is not covered by the existing specifications for pectins.’ The Joint FAO/WHO Expert Committee concluded that the use of non‐amidated pectin in infant formulas at the maximum proposed use levels (0.5%) is of concern 12. This conclusion was based on the decreased food intake and body weight gain in neonatal pigs 12.
In the updated 2016 safety evaluation, the Joint FAO/WHO Expert Committee concluded that at the new maximum proposed use level of pectin in infant formula (0.2%), the estimated exposure of infants aged 0–12 weeks would be up to 360 and 440 mg pectin/kg body weight per day at mean and high consumption, respectively 13. The margins of exposure for average and high consumers were 2.9 and 2.4, respectively, when compared with the no‐observed‐adverse-effect level (NOAEL) of 1,049 mg/kg body weight per day, identified in the piglet study 13. On the basis of a number of considerations (low toxicity of pectin, no‐observed‐adverse-effect level (NOAEL) derived from a relevant study in neonatal piglets, relation of adverse effects in piglet study to viscosity, support of clinical studies for tolerance of infants to pectin up to the concentration of 0.2% and conservative exposure estimates), the Joint FAO/WHO Expert Committee concluded that the margins of exposure calculated for the use of pectin at a concentration of 0.2% in infant formula indicate a low risk for the health of infants aged 0–12 weeks and is not of concern 13. The Joint FAO/WHO Expert Committee further stated that ‘there is variability in medical conditions among infants requiring formula for special medical purposes and that these infants would be normally under medical supervision’.
The European Food Safety Authority Panel noted that according to the EU specifications for pectin (E 440i) and amidated pectin (E 440ii), impurities of the toxic elements arsenic, lead, mercury and cadmium are accepted up to concentrations of 3, 5, 1 and 1 mg/kg, respectively 3. Contamination at such levels could have a significant impact on the exposure to these metals, for which the exposure is already close to the health‐based guidance values or benchmark doses (lower confidence limits) established by the European Food Safety Authority. The European Food Safety Authority Panel noted that, in June 2016, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) lowered the limit for lead to 2 mg/kg for general use and 0.5 mg/kg for use in infant formula.
Data on in vitro degradation of pectins and amidated pectins indicated that their digestibility was low in the upper parts of the digestive tract, but they would be fermented during their passage through the large intestine. These in vitro data are in agreement with in vivo studies demonstrating the absence of degradation of pectins in germ‐free rats by comparison to conventional animals. As demonstrated in ileostomy patients, the main end products of this colonic anaerobic digestive process are short‐chain fatty acids (SCFA), such as acetic, propionic and butyric acids, which are absorbed from the colon and considered of no safety concern by the European Food Safety Authority Panel. These data indicated that pectins and amidated pectins would not be absorbed intact but extensively fermented by intestinal microbiota in animals and humans.
As products formed from pectins in the gastrointestinal tract are similar to manufactured pectin‐derived acidic oligosaccharides, in 2015, JECFA concluded that studies using pectin‐derived acidic oligosaccharides were relevant for the evaluation of pectins in infant formulae. The European Food Safety Authority Panel agreed with this conclusion.
The acute oral toxicity of pectin is low. Data on amidated pectin were not available, but acute oral toxicity was expected to be low, based on the structural similarity to pectin 3.
The oral exposure to non‐amidated (E 440i) and amidated pectin (E 440ii) at a dose level up to 13,500 mg/kg body weight per day did not result in effects of toxicological relevance in a subchronic feeding study in rats 3. No adverse effects were detected in a subchronic drinking water study in rats at a concentration of 5% in the water, equal to 3,366 mg/kg body weight per day in males and 3,916 mg/kg bw per day in females, the highest dose tested 3. The reduction of protein digestibility and calcium absorption was shown in a subacute feeding study in rats at a dose of 12,000 mg/kg body weight per day. In a 13‐week dietary study with pectin‐derived acidic oligosaccharides in F1 rats, an increased level of urinary calcium and a minimal degree of urothelial hyperplasia was observed in the 5% group (3,400 mg/kg body weight per day). In an additional 13‐week study in rats with pectin‐derived acidic oligosaccharides, a no‐observed‐adverse‐effect level (NOAEL) of 1,700 mg/kg body weight per day was identified 3.
The European Food Safety Authority Panel noted that, although the available test tube and animal study data were limited, no genotoxic activity has been observed for pectin 3. This conclusion was also supported by the negative results obtained with manufactured pectin‐derived acidic oligosaccharides. Data on amidated pectin were not available, but considering its chemical structure and its negligible absorption, the European Food Safety Authority Panel considered that there is no concern with respect to genotoxicity for amidated pectin E 440ii 3.
A chronic dietary toxicity study in rats with pectin or amidated pectin showed no adverse effects at 10% in the diet, equivalent to 5,000 mg/kg body weight per day, the highest dose tested 3.
Two reproductive toxicity studies and one developmental toxicity study with pectin, which were considered inadequate for risk assessment, were available. In a dietary one‐generation reproductive toxicity study with pectin‐derived acidic oligosaccharides in rats, a no‐observed‐adverse‐effect level (NOAEL) of 6,200 mg/kg body weight per day, the highest dose tested, was identified.
The European Food Safety Authority Panel considered that there is no indication that the reported immune‐modulatory properties of pectin may lead to an adverse response, the data being rather indicative of an effect which would limit the hypersensitivity response. Therefore, the European Food Safety Authority Panel did not consider pectin (E 440i) and amidated pectin (E 440ii) as having an allergenic potential.
A daily dose of 36,000 mg pectin (equivalent to 515 mg/kg body weight per day) for 6 weeks in humans was associated with abdominal distension and increasing flatus in some individuals, effects which were considered by the European Food Safety Authority Panel as undesirable, but not adverse 3.
No adverse effects were noted in four studies in infants with infant formulae containing pectin and pectin‐derived acidic oligosaccharides. Pectin (4 mg/kg body weight per day) improved the permeability of the small intestine in young infant boys (5–12 months) with persistent diarrhea.
The safety of pectin (high‐ester pectin extracted from citrus peel and standardized by the addition of sucrose) in a milk replacer was tested in neonatal pigs (Yorkshire‐bred, age 2 days). The NOAEL for this study was 1,049 mg/kg body weight per day. Based on the data available, the European Food Safety Authority Panel considered that this conclusion appeared appropriate. JECFA 13 concluded from the data of a new piglet study that the reduced milk replacer consumption observed in both studies at a dose level of 1% pectin in milk replacer was likely due to delayed gastric emptying and/or prolonged gut transit resulting from consumption of the highly viscous 1% pectin diet. The data available from this study were not sufficient for the European Food Safety Authority Panel to confirm the conclusion of JECFA.
To assess the dietary exposure to pectins (E 440) from their use as food additives, the exposure was calculated based on (1) maximum use levels provided to EFSA (defined as the maximum level exposure assessment scenario), and (2) reported use levels or analytical data (defined as the refined exposure assessment scenario, brand‐loyal and non‐brand‐loyal).
Pectins (E 440) are authorized in a wide range of foods. The European Food Safety Authority Panel did identify brand loyalty to specific food categories in toddlers (e.g. flavored fermented milk products and flavored drinks). Further, the European Food Safety Authority Panel considered that the non‐brand‐loyal scenario covering other population groups was the most appropriate and realistic scenario for risk characterization, because it is assumed that the population would probably be exposed long‐term to the food additive present at the mean reported use level in processed food.
A maximum estimated exposure assessment scenario taking into account the food for special medical purposes for infants and young children. Dietary foods for infants for special medical purposes and special formulae for infant. Dietary foods for babies and young children for special medical purposes as defined by Commission Directive 1999/21/EC was also performed using food additive producers data to estimate exposure for infants and toddlers who may be on a specific diet. This exposure scenario considered products belonging to food categories, excluding infant formulae, where pectins (E 440), according to the EU regulation, are not authorized. Considering that this diet is required due to specific needs, it is assumed that consumers are loyal to the food brand, therefore only the maximum brand‐loyal estimated exposure scenario was performed.
A refined estimated exposure assessment scenario taking into account the consumption of food supplements was also performed to estimate exposure for children, adolescents, adults and the elderly, for consumers only, as exposure via food supplements may deviate largely from that via food, and the number of food supplement consumers may be low depending on the population and survey.
The refined estimates were based on 23 out of 81 food categories in which pectins (E 440) are authorized. Overall, the European Food Safety Authority Panel considered that the uncertainties identified would, in general, result in an overestimation of the real exposure to pectins (E 440) as food additives in European countries for the maximum level exposure scenario and for the refined exposure assessment scenarios when considering only food additive uses for which data have been provided.
However, the European Food Safety Authority Panel noted that, it may be assumed that pectins (E 440) are used in several food categories (n = 9) for which no data have been provided by food industry. The European Food Safety Authority Panel noted that out of these nine food categories, fruit juices and fruit nectars are products highly consumed. If this would be confirmed, it would therefore result in an underestimation of the exposure.
A possible additional exposure to pectins (E 440) from their use directly by the consumer (adding pectins to thicken foods, e.g. jams, marmalades) and from their use as food additives authorized in accordance with Annex III to Regulation (EC) No 1333/2008 were not considered in any of the above exposure assessment scenarios.
The European Food Safety Authority Panel also noted that the refined exposure estimates were based on information reported on the use levels of pectins (E 440) by food industry. If actual practices change, these refined estimates may no longer be representative and should be updated.
Conclusions
General population
- the data received for the 23 food categories were adequate for a combined exposure assessment for these categories;
- based on these reported use levels, a refined exposure of up to 442 mg/kg body weight per day for toddlers in these categories (brand‐loyal scenario) was estimated;
- pectin and amidated pectin are not absorbed intact, but extensively fermented by intestinal microbiota in animals and humans;
- adequate toxicity data were available;
- in oral subchronic studies with pectins in rats, no adverse effects were observed at doses ranging from 3,366 mg/kg body weight per day to 13,500 mg/kg body weight per day, the highest doses tested. In subchronic studies with pectin‐derived acidic oligosaccharides in the diet in rats, a no‐observed‐adverse‐effect level (NOAEL) of 1,700 mg/kg body weight per day was identified;
- no effects on body weight and food intake were observed in male neonatal pigs exposed to 0.3% pectin in formula (equal to 1,049 mg pectin/kg body weight per day) for 28 days, while in the same study, such effects were observed at 1% pectin in formula (equal to 4,015 mg pectin/kg body weight per day);
- no adverse effects were reported in a chronic study in rats at up to 5,000 mg pectin/kg body weight per day, the highest dose tested;
- there is no concern with respect to genotoxicity for pectin and amidated pectin;
- a daily dose of 36,000 mg pectin (equivalent to 515 mg/kg body weight per day) for 6 weeks in humans was associated with abdominal distension and increasing flatus in some individuals, effects which were considered by the European Food Safety Authority Panel as undesirable, but not adverse,
The European Food Safety Authority Panel concluded that there is no need for a numerical acceptable daily intake (ADI) for pectin (E 440i) and amidated pectin (E 440ii) and that there is no safety concern for the general population at the refined exposure assessment for the reported uses and use levels of pectins (E 440) as food additives.
Infants and young children consuming foods for special medical purposes and special formulae
Concerning the use of pectins (E 440) in ‘dietary foods for special medical purposes and special formulae for infants’ and in ‘dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC’ and given that:
- for populations consuming foods for special medical purposes and special formulae, the 95th percentile of maximum exposure assessments calculated based on the maximum reported data from food additive producers was up to 1,349 mg/kg body weight per day for infants;
- infants and young children consuming these foods may be exposed to a greater extent to pectins (E 440) than their healthy counterparts because the permitted levels of pectins (E 440) in formulae for special medical purposes are twofold higher (1%) than in formulae for healthy individuals;
- infants and young children consuming foods belonging to these food categories may show a higher susceptibility to the gastrointestinal effects of pectins than their healthy counterparts due to their underlying medical condition;
- no effects on body weight and food intake were observed in male neonatal pigs exposed to 0.3% pectin in formula (equal to 1,049 mg pectin/kg body weight per day) for 28 days, while in the same study, such effects were observed at 1% pectin in formula (equal to 4,015 mg pectin/kg body weight per day);
- in infants and young children (< 18 months of age), a formula containing 0.5% pectin (E 440) given for 3 months was well tolerated without adverse effects;
no human studies investigating the adverse effects of formulae containing pectins (E 440) at the maximum permitted level of 1% for the relevant age group were available.
The European Food Safety Authority Panel concluded that the available data do not allow for an adequate assessment of the safety of use of pectins (E 440) in infants and young children consuming these foods for special medical purposes at the presently authorized maximum use levels of 1%.
The European Food Safety Authority Panel recommended that:
- the European Commission considers lowering the maximum limits for the impurities of toxic elements arsenic, lead, mercury and cadmium in the EU specifications for pectin (E 440i) and amidated pectin (E 440ii) in order to ensure that pectin (E 440i) and amidated pectin (E 440ii) as food additives will not be a significant source of exposure to those toxic elements in food; special requirements might be defined in the specifications for pectin (E 440i) and amidated pectin (E 440ii) to be used in formulae or food for infants, toddlers and other young children;
- limits for aluminium should be considered for inclusion in the EU specifications, as aluminium can be used in the manufacturing process;
- the European Commission considers harmonising the microbiological specifications for polysaccharidic thickening agents, such as pectins, and including criteria for the absence of Salmonella spp. and Escherichia coli, for total aerobic microbial count and for total combined yeast and mould count in the EU specifications for pectin (E 440i) and amidated pectin (E 440ii);
- additional clinical data should be generated to assess the safety of pectins (E 440) when used in ‘dietary foods for special medical purposes and special formulae for infants’ (FC 13.1.5.1) and in ‘dietary foods for babies and young children for special medical purposes as defined in Directive 1999/21/EC’;
- due to the discrepancies observed between the data reported from industry and the Mintel database, where pectins are labelled in more products than in food categories for which data were reported from industry, the European Food Safety Authority Panel recommended collection of data on usage and use levels of pectins (E 440) in order to perform a more realistic exposure assessment.
- Pectin Properties and Determination. Encyclopedia of Food Sciences and Nutrition (Second Edition), 2003
- U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS). https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices
- Re‐evaluation of pectin (E 440i) and amidated pectin (E 440ii) as food additives. EFSA Journal 6 July 2017. https://doi.org/10.2903/j.efsa.2017.4866
- TemaNord (Nordic Council of Ministers), 2002. Food Additives in Europe 2000 − Status of safety assessments of food additives presently permitted in the EU. Pectin and amidated pectin.
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2010. Scientific Opinion on the substantiation of health claims related to pectins and reduction of post‐prandial glycaemic responses (ID 786), maintenance of normal blood cholesterol concentrations (ID 818) and increase in satiety leading to a reduction in energy intake (ID 4692) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2010;8(10):1747, 17 pp. https://doi.org/10.2903/j.efsa.2010.1747
- SCF (Scientific Committee for Food), 1978. Report of the Scientific Committee for Food. 7th Series, 44 pp.
- Scientific Opinion on the substantiation of health claims related to pectins and reduction of post-prandial glycaemic responses (ID 786), maintenance of normal blood cholesterol concentrations (ID 818) and increase in satiety leading to a reduction in energy intake (ID 4692) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. EFSA Journal 2010;8(10):1747 https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2010.1747
- Industrial pectins: Sources, production and applications. Carbohydrate Polymers Volume 12, Issue 1, 1990, Pages 79-99. https://doi.org/10.1016/0144-8617(90)90105-2
- Chemistry and uses of pectin — A review. Critical Reviews in Food Science and Nutrition Volume 37, 1997 – Issue 1. https://doi.org/10.1080/10408399709527767
- Zy Z, Liang L, Fan X, Yu Z, Hotchkiss AT, Wilk BJ, Eliaz I.”The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels”, Altern Ther Health Med. 2008 Jul-Aug;14(4):34-8.
- Sriamornsak, P. (2011). “Application of pectin in oral drug delivery”. Expert Opinion on Drug Delivery. 8 (8): 1009–1023. https://doi.org/10.1517/17425247.2011.584867
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2015. Safety evaluation of certain food additives. Pectins (addendum). Seventy‐ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series, No. 70, 139 pp. http://apps.who.int/iris/bitstream/handle/10665/171781/9789240693982_eng.pdf;jsessionid=94E488D53FC1AADB16BBA3B52297AD21?sequence=3#page=147
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2016a. Summary report of the 82nd Joint FAO/WHO Expert Committee on Food Additives (JECFA) meeting – Food additives. JECFA/82/SC. http://www.who.int/foodsafety/areas_work/chemical-risks/JECFA82_SummaryReport.pdf?ua=1