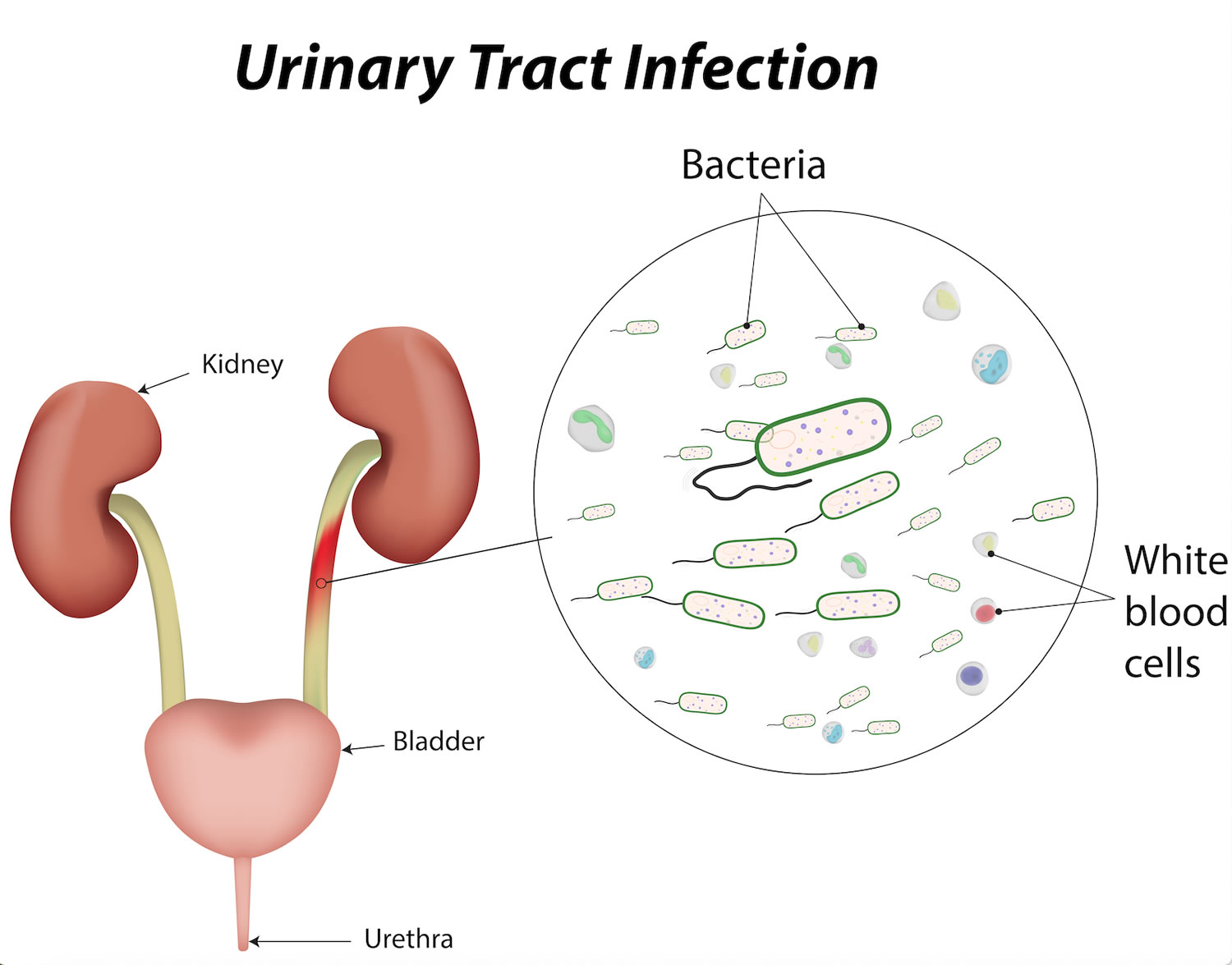
Proteus mirabilis UTI
Proteus mirabilis is a Gram-negative, facultative anaerobe bacilli, part of the Enterobacteriaceae family, with an ability to ferment maltose and inability to ferment lactose 1. Proteus mirabilis is well-known in clinical laboratories and microbiology survey courses as the species that swarms across agar surfaces, overtaking any other species present in the process. Urease production and robust swarming motility are the two hallmarks of this organism. Proteus mirabilis can be found in a wide variety of environments, including soil, water sources, and sewage, but it is predominantly a normal human and animal intestinal flora (along with Klebsiella species, and Escherichia coli) 2. While the Proteus mirabilis bacterium is capable of causing a variety of human infections, including those of wounds, the eye, the gastrointestinal tract, and the urinary tract, it is most noted for infections of the catheterized urinary tract, known as catheter-associated urinary tract infections 3. These infections are common in long-term catheterized patients, such as those who reside in nursing homes and chronic care facilities, and may be of particular danger to spinal cord injury patients 4. Proteus mirabilis is capable of causing symptomatic infections of the urinary tract including cystitis and pyelonephritis and is present in cases of asymptomatic bacteriuria, particularly in the elderly and patients with type 2 diabetes 5. Urinary tract infections (UTIs) and catheter-associated urinary tract infections (CAUTIs) involving Proteus mirabilis are typically complicated by the formation of bladder and kidney stones (urolithiasis) and permanent kidney damage 6, and may progress to bacteremia and potentially life-threatening sepsis 7. Indeed, catheter-associated urinary tract infection is the most common source of bacteremia in nursing homes, bacteremia involving Proteus mirabilis most frequently occurs following UTI or catheter-associated urinary tract infections compared to other sources of infection, and bacteremia and sepsis due to Proteus mirabilis carry a high mortality rate 8. Catheter-associated urinary tract infections (CAUTIs) are also often polymicrobial 9, and Proteus mirabilis is one of the most common organisms present during polymicrobial urine colonization and infection 10.
Proteus mirabilis is often isolated from the gastrointestinal tract, although whether it is a commensal, a pathogen, or a transient organism, is somewhat controversial 11. It is thought that the majority of Proteus mirabilis UTI (urinary tract infections) result from ascension of bacteria from the gastrointestinal tract while others are due to person-to-person transmission, particularly in healthcare settings 12. This is supported by evidence that some patients with Proteus mirabilis UTI have the same strain of Proteus mirabilis in their stool, while others have no Proteus mirabilis in their stools 13. In addition to UTI (urinary tract infection), Proteus mirabilis can also cause infection in the respiratory tract, eye, ear, nose, skin, throat, burns, and wounds and has been implicated in neonatal meningoencephalitis, empyema, and osteomyelitis 14. Several studies have linked Proteus mirabilis to rheumatoid arthritis, although others have failed to find an association 15. It is thought that antibodies against hemolysin and urease enzymes are subsequently able to recognize self antigens targeted in rheumatoid arthritis patients 15.
Proteus mirabilis is an agent of catheter biofilm formation, quickly fouling the surface of a newly inserted urinary catheter. Surface organelles such as fimbriae and other adhesins appear to play a significant role in this process 16. The enzyme urease also contributes dramatically to this process. Urea, your means of eliminating excess nitrogen, is present in high concentrations in urine (~400 mM), is the substrate of urease, and is hydrolyzed to CO2 and NH3. The liberated ammonia raises the pH of the urine and initiates the precipitation of otherwise soluble polyvalent anions and cations present in urine. The result is urolithiasis, the formation of struvite (MgNH3PO4) or apatite (CaPO4) stones. These crystals can form on and within the lumen of catheters, blocking urine flow and necessitating catheter removal and replacement. Stones may also form in the renal tubules or renal pelvis, causing inflammation and often requiring surgical removal. Proteus mirabilis bacterium is capable of invading bladder epithelial cells, and produces a variety of cytotoxins that damage the epithelium, leading to significant histopathology.
Proteus mirabilis causes between 1-10% of all urinary tract infections, varying with the geographic location of the study, the types of samples collected, and the characteristics of the patients examined. In the most recent large North American study, Proteus mirabilis caused 4% of almost 3,000 UTI cases 17. Proteus mirabilis is more common in complicated urinary tract infections (such as patients with spinal cord injury or anatomical abnormality) and especially contributes to catheter-associated UTI (CAUTI), causing 10-44% of long-term catheter-associated urinary tract infections at a cost of $43-256 million in the US annually 4. The wide range of Proteus mirabilis catheter-associated urinary tract infection likely reflects differences in the population surveyed and the types of samples collected. The highest incidence of Proteus mirabilis catheter-associated urinary tract infection occurs in elderly patients during long-term catheterization. Proteus mirabilis is also a common agent of Gram-negative bacteremia, particularly in patients with concurrent UTI; in recent studies, this species was found in 5-20% of these cases and as high as a 50% mortality rate in geriatric patients 18.
Many efforts have been made to design catheters that are resistant to bacterial colonization, with the goals of preventing catheter blockage and reducing UTI in catheterized patients. Catheters made from latex, silicone, polyurethane, and composite biomaterials have been tested, but no single catheter material is sufficient to prevent bacterial colonization 14. Several groups have attempted to prevent biofilm formation on catheters by applying or embedding an antimicrobial solution on the catheter surface or in the catheter material. Antibiotic coatings, such as nitrofurazone or a combination of broad-spectrum antibiotics, have significantly reduced the number of catheter-associated urinary tract infections in small clinical trials 19. However, given the ubiquity of catheterization, this approach will likely result in antibiotic resistance, a difficulty which has not been resolved. A similar approach involving coating catheters with antiseptics, specifically various silver compounds, has been studied extensively. However, the combination of poorly designed clinical trials and conflicting results have made it difficult to determine the efficacy of these treatments 19.
Several promising techniques have been tested in vitro (test tube) but have not yet been tested in clinical trials. These include the use of catheters impregnated with combinations of chlorhexidine, silver sulfadiazine, and triclosan; the use of urease inhibitors to prevent crystalline biofilm formation; and the treatment of catheters with a cocktail of bacteriophages 19. Most of the techniques tested have been chemical, not mechanical, which makes the promising in vitro results using low energy acoustic waves to prevent bacterial attachment and the development of a urinary catheter that uses inflation-generated catheter strain to clear Proteus mirabilis crystalline biofilms intriguing alternative approaches 20.
Although complete prevention of biofilms on catheters is ideal, the most serious complications of catheter-associated urinary tract infection generally occur when the catheter becomes blocked by the presence of crystalline biofilms that are the result of urease activity. A sensor which detects the initial stages of crystalline biofilm formation early enough to allow the catheter to be changed prior to catheter blockage would help mitigate the effects of biofilm formation on catheters. An early prototype of this sensor detected crystalline biofilm formation around 12 days prior to blockage in a clinical trial 21. A more recent sensor, which is amenable to mass production, detected Proteus mirabilis crystalline biofilm formation 17 to 24 hours in advance 22. This technology could significantly decrease the morbidity and costs associated with catheter-associated urinary tract infection.
Proteus mirabilis causes
Ninety percent of Proteus infections occur as a result of Proteus mirabilis, and these are considered community-acquired infections 23.
Though not a common cause of nosocomial (hospital acquired) infections, Proteus species have also been shown to cause infection from the colonized skin and oral mucosa of patients and personnel working in a hospital or long-term care facility.
Patients who acquire an infection in the hospital, have a history of recurrent infections, structural abnormalities of the urinary tract or urethral instrumentation have a greater risk of acquiring an infection by Proteus mirabilis in addition to other organisms such as Klebsiella, Enterobacter, Pseudomonas, Staphylococci, and Enterococci.
Urinary tract infections (UTIs) occur as a result of bacterial migration along the mucosal sheath of the catheter or up the catheter lumen from contaminated urine 24.
The most common clinical manifestations of Proteus infection are urinary tract infections (UTIs). In general, UTIs are more common in individuals aged 20 to 50 years and most common in women of this age group. In otherwise healthy women, Proteus accounts for 1% to 2% of all UTIs (E. coli being the most common), while in hospital-acquired UTIs, Proteus accounts for 5%. Complicated UTIs (i.e., secondary to catheterization) have an even higher association with Proteus infection at 20% to 45%.
Risk factors for UTIs include sexual activity in both men and women, unprotected anal intercourse in men, an uncircumcised penis, or immunodeficiency (e.g., CD4 count less than 200/uL).
Other factors that may increase the risk of infection by Proteus mirabilis include female sex, longer duration of catheterization, improper catheter cleaning or care, underlying illness, and lack of availability of systemic antibiotics.
In the United States, gram-negative bacteremia occurs as a result of genitourinary tract infections in 35% of patients.
Proteus mirabilis UTI symptoms
Individuals with a Proteus infection may present with urethritis, cystitis, prostatitis, or pyelonephritis. A history of frequent renal stones may be indicative of an underlying chronic Proteus infection.
Urethritis typically presents with with pain or burning when urinating (dysuria), pyuria (with or without urethral discharge) and increased urinary frequency. Symptoms are often mild and frequently ignored by patients.
Cystitis, on the other hand, tends to present acutely with dysuria (pain or burning when urinating), increased frequency, and urgency of urination, suprapubic or back pain, small volume urine, dark urine, or hematuria. Patients also may present with a fever which may be indicative of a more severe condition, such as pyelonephritis, bacteremia, or impending sepsis.
Prostatitis occurs more acutely in men than cystitis, with the same set of symptoms, though may also be accompanied by fever and chills. Prostatitis tends to be more common with increased age. If there is associated obstruction, patients also may complain of perianal pain. A diffusely swollen and tender prostate may be noted on palpation during a physical examination.
Pyelonephritis occurs as a complication of either of the conditions mentioned above, and the patient may, therefore, complain of symptoms of urethritis or cystitis. Additional symptoms that are more definitive of pyelonephritis include flank pain, costovertebral angle tenderness, nausea and vomiting, fever, hematuria, and occasionally an enlarged kidney felt on palpation.
Proteus mirabilis UTI diagnosis
The most definitive form of evaluation for an acute Proteus mirabilis infection is a culture. Proteus species are gram-negative, rod-shaped, and facultatively anaerobic. The majority of strains are lactose negative with a characteristic swarming motility that will become evident on agar plates. One must always correlate positive culture results with the clinical presentation of the patient to form an accurate diagnosis.
Additional evaluations include urine sample analysis to evaluate for pyuria and leukocyte esterase. Pyuria is generally present in the case of bacterial urinary tract infection, such that lack of pyuria may indicate an alternate cause of symptoms. Leukocyte esterase dipstick provides a good alternative to microscopy, but is a less sensitive test than microscopic examination. Gram staining of urine may help reveal microscopic bacteriuria which would confirm infection, although the absence of bacteriuria does not exclude it.
A patient with a history of chronically alkaline urine in combination with a positive Proteus culture should be evaluated for renal stones (struvite stones).
If resolution is not seen with antibiotic therapy alone, an ultrasound of the kidneys or a CT abdomen may be warranted to rule out renal stones or a perinephric abscess.
Proteus mirabilis UTI treatment
Empirical treatment for an uncomplicated UTI caused by Proteus mirabilis (much like other uncomplicated UTIs) involves outpatient treatment with either a 3-day course of trimethoprim/sulfamethoxazole or an oral fluoroquinolone (e.g., ciprofloxacin) 25.
An acute, uncomplicated pyelonephritis can be treated on an outpatient basis with fluoroquinolones, although a regimen of 7 to 14 days is recommended. An alternative to this treatment is a one-time dose of ceftriaxone or gentamycin followed by either trimethoprim/sulfamethoxazole, an oral fluoroquinolone, or cephalosporin for 7 to 14 days.
If a patient has a more severe condition or is in an inpatient setting, they may begin antibiotic therapy via intravenous administration of either ceftriaxone, gentamycin, a fluoroquinolone, gentamycin plus ampicillin, or aztreonam until fever resolves. At this point, they may switch to oral therapy with either cephalosporin, an oral fluoroquinolone, or trimethoprim/sulfamethoxazole for up to 14 additional days.
If a patient presents with a complicated UTI (e.g., a man or woman with a history of an underlying condition that may increase the risk of failure of therapy), they may also be treated in an outpatient setting with oral antibiotics for 10 to 21 days as long as they receive adequate follow-up.
Proteus infection can be avoided with proper sanitation and hygiene, such as adequate sterilization of medical equipment and surfaces. Additionally, catheterization should be reserved for patients for whom there is no other option.
Proteus mirabilis antibiotic
Recommended empirical antibiotic treatment includes the following:
- Uncomplicated UTIs in women can be treated on an outpatient basis with an oral quinolone for 3 days or trimethoprim/sulfamethoxazole (TMP/SMZ) for 3 days.
- Acute uncomplicated pyelonephritis in women can be treated with oral quinolones for 7-14 days, single-dose ceftriaxone or gentamicin followed by trimethoprim/sulfamethoxazole, or an oral cephalosporin or quinolone for 14 days as outpatient therapy. For hospitalized patients, therapy consists of parenteral (or oral once the oral route is available) ceftriaxone, quinolone, gentamicin (plus ampicillin), or aztreonam until defervescence. Then, an oral quinolone, cephalosporin, or trimethoprim/sulfamethoxazole for 14 days may be added to complete treatment.
- Complicated UTIs in men and women can be treated with a 10- to 21-day course of oral therapy (in the same manner as for hospitalized patients) as long as the follow-up is adequate.
Proteus mirabilis UTI prognosis
The vast majority of proteus infections are associated with the urinary tract. Most of the infections are sensitive to the currently available antibiotics and the outcomes are good in immunocompetent patients.
Symptoms generally resolve without complications in immunocompetent patients. Immunocompromised patients can be at higher risk for sepsis or prolonged infections.
References- Jamil RT, Foris LA, Snowden J. Proteus Mirabilis Infections. [Updated 2019 Jun 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK442017
- Armbruster CE, Mobley HLT. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Micro. 2012;10:743–754.
- Mobley HLT, Warren JW. Urease-Positive Bacteriuria and Obstruction of Long-Term Urinary Catheters. Journal of Clinical Microbiology. 1987;25:2216–2217.
- Hung EW, Darouiche RO, Trautner BW. Proteus bacteriuria is associated with significant morbidity in spinal cord injury. Spinal Cord. 2007;45:616–620.
- Matthews SJ, Lancaster JW. Urinary tract infections in the elderly population. Am J Geriatr Pharmacother. 2011;9:286–309.
- Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17:227–241.
- Kim BN, Kim NJ, Kim MN, Kim YS, Woo JH, Ryu J. Bacteraemia due to tribe Proteeae: a review of 132 cases during a decade (1991–2000) Scand J Infect Dis. 2003;35:98–103.
- Daniels KR, Lee GC, Frei CR. Trends in catheter-associated urinary tract infections among a national cohort of hospitalized adults, 2001–2010. American Journal of Infection Control. 2014;42:17–22.
- Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE. Diagnosis, Prevention, and Treatment of Catheter-Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clinical Infectious Diseases. 2010;50:625–663.
- Armbruster CE, Prenovost K, Mobley HLT, Mody L. How Often Do Clinically Diagnosed Catheter-Associated Urinary Tract Infections in Nursing Home Residents Meet Standardized Criteria? J Am Geriatr Soc. 2016 doi: 10.1111/jgs.14533.
- Janda JMA, Abbott SL. The Enterobacteria. 2 ed. ASM Press; Washington, D.C.: 2006.
- O’Hara CM, Brenner FW, Miller JM. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin Microbiol Rev. 2000;13:534–546.
- Mathur S, Sabbuba NA, Suller MT, Stickler DJ, Feneley RC. Genotyping of urinary and fecal Proteus mirabilis isolates from individuals with long-term urinary catheters. Eur J Clin Microbiol Infect Dis. 2005;24:643–644.
- Jacobsen SM, Stickler DJ, Mobley HLT, Shirtliff ME. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev. 2008;21:26–59.
- Rashid T, Ebringer A. Rheumatoid arthritis is linked to Proteus–the evidence. Clin Rheumatol. 2007;26:1036–1043.
- Armbruster CE, Mobley HLT, Pearson MM. Pathogenesis of Proteus mirabilis Infection. EcoSal Plus. 2018;8(1):10.1128/ecosalplus.ESP-0009-2017. doi:10.1128/ecosalplus.ESP-0009-2017 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5880328
- Karlowsky JA, Lagacé-Wiens PR, Simner PJ, DeCorby MR, Adam HJ, Walkty A, Hoban DJ, Zhanel GG. Antimicrobial resistance in urinary tract pathogens in Canada from 2007 to 2009: CANWARD surveillance study. Antimicrob Agents Chemother. 2011;55:3169–3175.
- Sader HS, Flamm RK, Jones RN. Frequency of occurrence and antimicrobial susceptibility of Gram-negative bacteremia isolates in patients with urinary tract infection: results from United States and European hospitals (2009-2011). J Chemother. 2014;26:133–138.
- Siddiq DM, Darouiche RO. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urol. 2012;9:305–314.
- Levering V, Wang Q, Shivapooja P, Zhao X, López GP. Soft Robotic Concepts in Catheter Design: an On-Demand Fouling-Release Urinary Catheter. Adv Healthc Mater. 2014 doi:10.1002/adhm.201400035
- Stickler DJ, Jones SM, Adusei GO, Waters MG, Cloete J, Mathur S, Feneley RC. A clinical assessment of the performance of a sensor to detect crystalline biofilm formation on indwelling bladder catheters. BJU Int. 2006;98:1244–1249.
- Malic S, Waters MG, Basil L, Stickler DJ, Williams DW. Development of an “early warning” sensor for encrustation of urinary catheters following Proteus infection. J Biomed Mater Res B Appl Biomater. 2012;100:133–137.
- O’Keefe LC, Koelle P, McGee Z, Dewberry LS, Wright C, Stallings JE, Gates E, Chittur K. Innovations in Worksite Diagnosis of Urinary Tract Infections and the Occupational Health Nurse. Workplace Health Saf. 2019 Jun;67(6):268-274.
- Odoki M, Almustapha Aliero A, Tibyangye J, Nyabayo Maniga J, Wampande E, Drago Kato C, Agwu E, Bazira J. Prevalence of Bacterial Urinary Tract Infections and Associated Factors among Patients Attending Hospitals in Bushenyi District, Uganda. Int J Microbiol. 2019;2019:4246780
- Duarte MJ, Kozin ED, Barshak MB, Reinshagen K, Knoll RM, Abdullah KG, Welling DB, Jung DH. Otogenic brain abscesses: A systematic review. Laryngoscope Investig Otolaryngol. 2018 Jun;3(3):198-208.