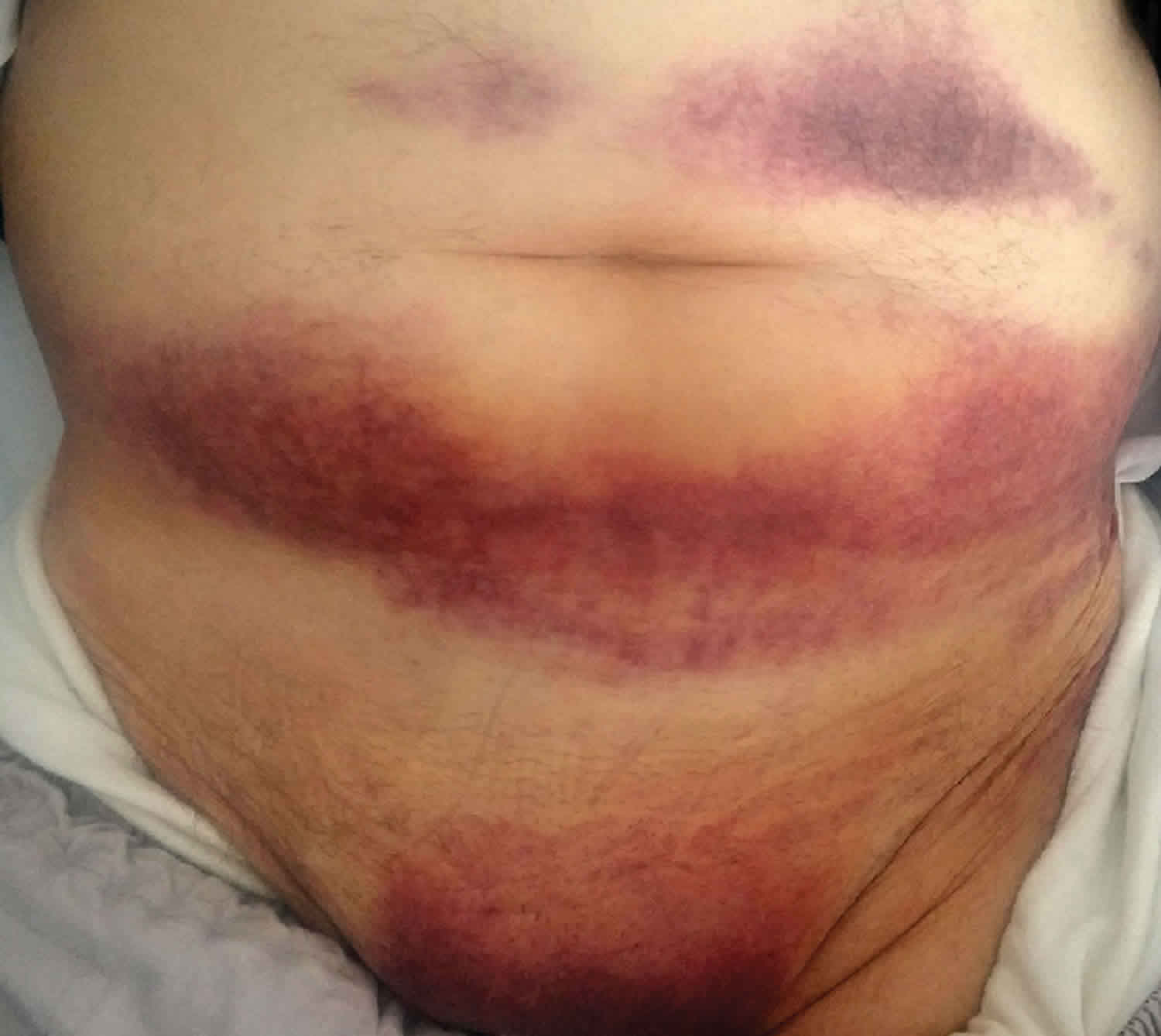
Rectus sheath hematoma
Rectus sheath hematoma is an accumulation of blood in the sheath of the rectus abdominis muscle. Rectus sheath hematoma is the result of bleeding into the rectus sheath from damage to the superior or inferior epigastric arteries or their branches or from a direct tear of the rectus muscle 1. Rectus sheath hematoma can mimic almost any abdominal condition. Rectus sheath hematoma is an entity, accounting for less than 2% of patients complaining of acute abdominal pain and often clinically misdiagnosed cause of abdominal pain 2. Rectus sheath hematoma may mimic several more common conditions, such as intestinal obstruction, perforated peptic ulcer, pancreatitis, diverticulitis, tumors or a ruptured aortic aneurysm 3. Thus, a high index of suspicion and a careful diagnostic workup are mandatory to reach an accurate diagnosis, so as to focus on the correct treatment. While usually a self-limiting entity, rectus sheath hematoma can cause hypovolemic shock following sufficient expansion, with associated mortality.
Known risk factors predisposing to rectus sheath hematoma are anticoagulation (nearly invariably present); older age; female sex; pregnancy; trauma; iatrogenic/surgery; chronic medical conditions, such as hypertension, atherosclerosis or hematologic diseases; coughing and forceful rectus muscle contractions 4.
The overall mortality rate of rectus sheath hematoma is around 4% and rises up to 25% in anticoagulated patients due to increased hemorrhage volume 3; an early recognition of rectus sheath hematoma may be associated with improved chances of survival 5.
In a 2016 review by Sheth et al. 6 evaluating a series of patients with rectus sheath hematoma, women were more likely than men to develop a rectus sheath hematoma, with a ratio of 1.7 to 1. This is consistent with epidemiologic data demonstrated in other studies. The mean age of patients in Sheth’s review was 67 years. The overall mortality rate associated with rectus sheath hematoma is less than 2% in the most recent publications 6.
A few well-documented risk factors are associated with rectus sheath hematoma development. The greatest risk is in those who are therapeutically anticoagulated. In Sheth’s review, almost 70% of patients were on some form of anticoagulation 6. Naturally, as the prevalence of chemical anticoagulation increases, one may reason that the incidence of rectus sheath hematoma also rises. However, there is a paucity of data in the modern literature to reflect this. In the same series, nearly 60% of patients with rectus sheath hematoma also had chronic kidney disease stage III or greater. Other risk factors, in order of descending prevalence, include abdominal wall injections, steroid or immunosuppressant therapy, cough, femoral puncture, and antiplatelet therapy 6.
Rectus sheath hematoma causes
Rectus sheath hematoma occurs as a result of injury to an epigastric artery or its perforating branches within the rectus muscle. Recall that the blood supply to the rectus abdominis muscles originates from the superior and inferior epigastric arteries. The superior epigastric artery arises from the internal thoracic artery and travels caudally within the rectus sheath to anastomose with the inferior epigastric artery. The inferior epigastric artery, deriving from the external iliac, travels cephalad along the posterior surface of the rectus muscle where it lacks the protection of a posterior rectus sheath until it reaches the arcuate line. The arcuate line is located a third the distance between the umbilicus and the pubic symphisis 7.
Epigastric artery rupture can occur when there is direct abdominal trauma or excessively forceful contraction of the abdominal wall. The resulting hematoma may present differently depending on whether it arises in the upper or lower abdomen. When the superior epigastric artery is injured, the resulting hematoma is typically smaller and quickly tamponaded within the rectus sheath. On the other hand, rupture of the inferior epigastric artery is not as easily controlled, owing to the lack of posterior sheath. This allows the development of a larger and generally more clinically significant hematoma 7.
Several risk factors of rectus sheath hematoma can be obtained in the history. In most cases of rectus sheath hematoma, one or more precipitating factors can be found. Reports of spontaneous rectus sheath hematoma exist, but more likely, in these cases, the precipitating factor was not appreciated. Anticoagulation is the most frequent predisposing factor, and severe coughing is the most important inciting factor.
Anticoagulation
Rectus sheath hematoma is a well-recognized complication of anticoagulant therapy. Anticoagulation can be a predisposing factor, or it can directly cause rectus sheath hematoma by accidental intramuscular injection of low-molecular-weight heparin (LMWH). Heparin-induced immune microangiopathy has been proposed as a mechanism of the pathogenetic process. Rectus sheath hematoma secondary to anticoagulation may have greater morbidity and mortality because of increased hemorrhage volume. Even when coagulation factors are within the therapeutic range, a substantial risk of hemorrhage still exists 8.
Coughing
Rectus sheath hematoma can occur after bouts of severe coughing, explaining its association with asthma, tuberculosis, influenza, pertussis, and other respiratory infections 8.
Pregnancy
Rectus sheath hematoma is associated with pregnancy in the gravid state, during labor, and in the early postpartum period.
Previous abdominal surgery
Abdominal operations predispose to rectus sheath hematoma because surgical scars redirect the shearing forces on muscle contraction, placing more stress on the epigastric vessels.
Recent abdominal surgery
Excessive retraction or inadequate hemostasis can cause rectus sheath hematoma that may become evident up to 4 weeks after the procedure.
Chronic kidney disease
In a study by Sheth et al 8 involving 115 hospitalized patients with a confirmed diagnosis of rectus sheath hematoma, 58.3% of them had chronic kidney disease of stage 3 or higher.
Steroid or immunosuppressive therapy
In the above-mentioned study by Sheth et al, 41.7% of the patients were undergoing steroid/immunosuppressive treatment 8.
External trauma
The nature of the trauma can be trivial. Tight contraction of the recti in anticipation of a blow predisposes to rectus sheath hematoma formation 8.
Vigorous uncoordinated rectus muscle contraction
Rectus sheath hematoma has been observed in a healthy man leaping over a ditch and in a woman rising from a chair to adjust a curtain rod. In a similar manner, sports activities and exercises, such as golf, tennis, skiing, and weight lifting, have caused rectus sheath hematoma. Activities with significant Valsalva effort, such as coughing, sneezing, straining from constipation, urination, and sexual intercourse, have been implicated in rectus sheath hematoma.
General medical conditions
General medical conditions that predispose to rectus sheath hematoma can be categorized as those causing damage to blood vessels; those causing failure of coagulation; or as anomalous conditions, such as endometriosis in the rectus sheath. Vascular conditions of hypertension, arteriosclerosis, and collagen vascular disease are associated with rectus sheath hematoma. Disorders of coagulation associated with rectus sheath hematoma include leukemia, myeloproliferative disorders, hemophilia, and blood dyscrasias.
Unusual
Case reports have also described rectus sheath hematoma related to acupuncture and follicle aspiration for in vitro fertilization. Minor surgical procedures such as diagnostic or therapeutic paracentesis have also been shown to cause rectus sheath hematoma 9. In addition to low-molecular-weight heparin (LMWH) injections, rectus sheath hematoma has also been seen in any abdominal wall medication injections (eg, insulin) 10. These unusual causes underscore the importance in obtaining a thorough history from the patient.
Rectus sheath hematoma symptoms
In a large case series of 126 patients with rectus sheath hematoma, Cherry et al. 11 report that the most common presenting symptoms are abdominal pain (84%) and a palpable abdominal wall mass (63%). The character of the abdominal pain varies but is often described as acute in onset but more often, it develops over a period of several hours. The abdominal pain is typically sharp and severe , with an associated palpable abdominal mass. Pain is usually worse with movement and is often unilateral. Constant pain with episodic abdominal cramping is also a frequent symptom. In atypical cases, the pain may develop insidiously, making the abdominal mass difficult to differentiate from an abdominal wall neoplasm.
Anorexia, nausea, vomiting, diarrhea, constipation, tenesmus, and bladder irritability are all compatible with the diagnosis of rectus sheath hematoma. The severity of symptoms is related to the degree of peritoneal irritation.
The Carnett sign, first described in the 1920s, is positive if there is an exacerbation of the pain with flexion of the abdominal wall muscles. This indicates the origin of the pain likely is the abdominal wall rather than an intra-abdominal source. The Fothergill sign refers to a palpable abdominal mass that does not cross the midline and remains palpable with abdominal wall flexion, indicating rectus sheath hematoma 7. Approximately 55% of patients demonstrate a decrease in hemoglobin of at least 0.4 g/dL from their baseline. Other signs and symptoms are less common but include abdominal wall ecchymosis (17%), tachycardia (13%), hypotension (7.9%), syncope (1.6%) and fever and chills. As mentioned above, the patient may even be peritoneal (9.5%) if the hematoma extends posteriorly and encroaches upon the peritoneum below the arcuate line 11.
Symptoms of hypovolemic shock with weakness, confusion, pallor, and diaphoresis can develop in patients with a large rectus sheath hematoma. According to the widely accepted classification of hemorrhagic shock, patients generally do not manifest any hemodynamic changes associated with hemorrhage until at least 15% to 30% of blood volume is lost. While this certainly is a possibility in the setting of rectus sheath hematoma, it is the case in only about 1% to 13% of patients 11. Thus the absence of these changes, such as tachycardia, hypotension, or orthostasis, should not lessen the clinician’s suspicion for the diagnosis.
Rectus sheath hematoma diagnosis
A low-grade fever is common in rectus sheath hematoma. The rectus sheath hematoma can be large enough to compromise intravascular volume, with resultant signs of hypovolemic shock including hypotension, tachycardia, and tachypnea.
Abdominal examination
Typically, the abdominal examination reveals a palpable, painful, firm, nonpulsatile abdominal mass corresponding to the rectus sheath. The mass may be bilobar with a central groove. The mass does not move with respiration. Because the hematoma is deep to the subcutaneous tissue and rectus muscles, the mass is not always palpable, particularly in obese patients. In 2000, Berna et al’s case series reported a palpable mass detected in 8 of 12 patients 12.
Hyperesthesia of the overlying skin is not uncommon. Bowel sounds may be absent. Signs of local peritoneal irritation with rebound tenderness and involuntary guarding may be present. This finding is most often seen in infra-umbilical hematomas due to the thin transverse fascialis serving as the only barrier between a hematoma and the peritoneum. Rarely, a hematoma may cause extraperitoneal compression of the abdominal cavity and cause abdominal compartment syndrome, or even rupture into the peritoneum, causing a chemical peritonitis.
The Fothergill sign is useful in determining whether an abdominal mass is part of the abdominal wall or whether it is in the abdomen. It is elicited by voluntary contraction of the rectus muscles by the patient lifting either his or her head or legs while in the supine position. With this action, rectus sheath hematomas become fixed, more painful, and more tender, while intra-abdominal masses become less distinct and less tender. The Fothergill sign may be inconclusive in patients who are obese or pregnant. As described by Fothergill in 1926 13:
- This patient complains of pain and the medical man finds the swelling. The trouble is that he seldom knows how long the swelling has been present. The main point is the recognition that these swellings are part and parcel of the abdominal wall. This is generally made by noting that they can still be felt when the recti are in action, and that they become fixed as the muscles contract
The Carnett sign is an additional test to assist in differentiating between abdominal wall and intra-abdominal pathology. It is performed by having the patient lie supine and tensing the abdominal musculature by raising either the head or the shoulder off the table. A positive sign is elicited if abdominal tenderness is increased or unchanged while tensing the abdomen. This indicates an abdominal wall process. A negative sign, or decreased abdominal tenderness while tensing the abdomen, suggests intra-abdominal pathology. Previous studies have demonstrated this sign to be fairly sensitive but not specific for abdominal wall pathology.
The Cullen sign of periumbilical ecchymosis is associated with retroperitoneal or abdominal wall hemorrhage. In rectus sheath hematoma, ecchymosis appears after 2-5 days. The ecchymosis uncommonly extends into the flanks.
The Grey-Turner sign is another manifestation of retroperitoneal hemorrhage. This finding of flank ecchymosis was initially described in hemorrhagic pancreatitis, and along with the Cullen sign, it is not specific for retroperitoneal or abdominal wall hemorrhage.
Pelvic examination
The pelvic examination may reveal a mass anterior to the vagina and above the pubis. The pelvic examination may be misleading, particularly in those cases that demonstrate unilateral adnexal tenderness and mass.
Laboratory studies
The most valuable laboratory studies for the evaluation of rectus sheath hematoma are hemoglobin/hematocrit and coagulation studies. As previously mentioned, over half of patients would be expected to demonstrate a decline in hemoglobin of greater than or equal to 0.4 g/dL. This is neither sensitive nor specific and thus cannot reliably indicate the presence or severity of a hematoma. However, following the trend of serial hemoglobin values can help establish a trajectory for the patient’s course.
All patients with a suspected rectus sheath hematoma should have baseline coagulation studies at the time of their initial evaluation. Again, a large majority of patients with rectus sheath hematoma are taking some form of therapeutic anticoagulation. For those taking warfarin, the INR can help steer the decision to administer a reversal agent. With the increasing use of antiplatelet therapies and novel oral anticoagulants, the INR may be less informative but should still be obtained as part of a coagulation panel.
Complete blood cell count
The hematocrit may be normal for small rectus sheath hematomas or significantly depressed with a large hematoma. Serial blood counts may be useful in an expanding hematoma to assess the need for blood transfusion or more aggressive therapeutic measures. The reported white blood cell count ranges from 6.6 X 103 to 29 X 103. As in other acute abdominal disorders, a normal white blood cell count does not rule out rectus sheath hematoma.
Coagulation factors
Although coagulation factors are not helpful for patients on LMWHs, they are useful for patients on oral anticoagulation drugs or for those with a pathologic failure of coagulation. Rectus sheath hematomas are more likely with supratherapeutic anticoagulation, but they can occur in the therapeutic range. Patients undergoing reversal of anticoagulation benefit from serial coagulation factors to assess the response to therapy.
Arterial or venous blood gas
Knowledge of the base deficit from a blood gas level is useful in patients with hypovolemic shock due to rectus sheath hematoma. Serial blood gas levels can be used to guide fluid resuscitation.
Imaging studies
When the history and physical examination findings raise suspicion for rectus sheath hematoma, ultrasonography and CT scanning are commonly used to help confirm the diagnosis. Before the advent of ultrasonography and CT scanning, the correct clinical diagnosis was only made in 17-40% of cases prior to exploratory laparotomy or death.
Ultrasonography
Ultrasonography can be used as a first-line diagnostic test for rectus sheath hematoma, or it can be used to monitor the evolution of a known hematoma. Ultrasonography provides rapid accurate information about the size, the location, and the physical characteristics of the mass. It is safe and well tolerated. It does not expose the patient to radiation or intravenous contrast material. The typical ultrasonographic findings are sufficient to establish the diagnosis.
Case series have shown it to be around 80% sensitive and specific. However, its reliability is limited by technician skill, body habitus, and patient tolerance. Typical ultrasound findings include a homogeneous fluid collection within the abdominal wall. It may appear heterogeneous, depending on its chronicity and evolution of the hematoma. Ultrasound also allows for serial examination of the size of the hematoma, though this is operator dependent. Doppler color flow does not reliably detect the presence of active extravasation.
CT scanning
CT may be used as a first-line diagnostic procedure in the evaluation for rectus sheath hematoma, or it may follow nondiagnostic ultrasonographic findings. CT permits a precise determination of the location, the size, and the extension of the hematoma. Information is also obtained about the rectus abdominis muscle and the perimuscular tissue. CT may be more appropriate than ultrasonography as a first-line test because it simultaneously aids in the diagnosis of rectus sheath hematoma and rules out other abdominal pathology.
CT is the diagnostic test of choice for rectus sheath hematoma and is superior to ultrasonography in sensitivity and specificity. Patients who are pediatric, pregnant, or have renal insufficiency may benefit from ultrasonography as a first-line test to avoid radiation and intravenous contrast material. In patients with renal insufficiency, a noncontrast CT scan can be used and will still show the typical findings of rectus sheath hematoma, although the ability to find active extravasation or to rule out other abdominal pathology is limited.
CT imaging has been reported to be nearly 100% sensitive and specific for rectus sheath hematoma. Typical CT findings include an often spindle-shaped mass posterior to the rectus abdominis muscle. Its density depends on its chronicity and the use of intravenous contrast. A contrasted study is preferred to detect the presence of active extravasation, though this is not always possible, owing to the prevalence of kindney disease in this patient population. Active extravasation appears as a hyper-dense contrast blush within the mass. There is often accompanying soft tissue edema of the surrounding structures. Though this method of imaging is highly accurate, ultrasound may still be the preferred test in certain circumstances, such as in patients who are pregnant or have kidney disease 7.
Types of rectus sheath hematoma on CT scanning:
In 1996, Berna used the appearance of rectus sheath hematomas on CT scans to differentiate 3 levels of severity with disposition and therapeutic implications 14.
- Type 1: The hematoma is intramuscular, and an increase in the size of the muscle is observed, with an ovoid or fusiform aspect and hyperdense foci or a diffusely increased density. The hematoma is unilateral and does not dissect along the fascial planes.
- Type 2: The hematoma is intramuscular (mimicking type I) but with blood between the muscle and the transversalis fascia. It may be unilateral or bilateral, and no blood is observed occupying the prevesical space. A fall in hematocrit may be observed.
- Type 3: The hematoma may or may not affect the muscle, and blood is observed between the transversalis fascia and the muscle, in the peritoneum, and in the prevesical space. A hematocrit effect can be observed, and on occasion, hemoperitoneum is produced.
MRI
MRI is useful in differentiating chronic rectus sheath hematoma from other anterior abdominal wall masses when CT findings are not specific. Chronic rectus sheath hematoma is demonstrated as high signal intensity on both T1- and T2-weighted images up to 10 months following the onset of the hematoma. In acute rectus sheath hematoma of less than 48 hours’ duration, the MRI of rectus sheath hematoma does not reveal high signal intensity and is not useful in the diagnosis 15. Many clinicians will be limited by the availability of MRI in their practices, particularly when other types of imaging studies are readily available.
Rectus sheath hematoma treatment
Once rectus sheath hematoma is diagnosed, the patient’s clinical condition determines appropriate treatment and disposition. Treatment may be either conservative or invasive.
Fortunately, the majority of cases of rectus sheath hematoma can be successfully managed nonoperatively. Multiple case series have demonstrated that around 80% of patients may be managed with no invasive intervention, including rest, ice, compression, and analgesia 11. In patients with coagulopathy, cessation of anticoagulation therapy or if needed reversal of the coagulopathy is sufficient to allow the bleeding to tamponade within the sheath. In patients with significant anemia or hemodynamic instability, transfusion of blood products is indicated. If despite these measures, the patient has persistent evidence of bleeding, the most appropriate initial intervention is angioembolization, which has been reported to control ongoing hemorrhage in virtually 100% of cases successfully 16. It is rare for rectus sheath hematoma to require surgical intervention, and in fact, laparotomy in many cases would pose a bigger risk of bleeding to the patient than is necessary, especially in patients with coagulopathy.
Anticoagulation reversal
Patients who are undergoing invasive procedures and those with hemodynamic instability, expanding hematomas, or symptomatic anemia should be considered for anticoagulation reversal. For patients taking oral anticoagulation, reversal can be achieved with phytonadione plus fresh frozen plasma (FFP). The patient’s clinical condition determines the aggressiveness of anticoagulation reversal.
Intravenous phytonadione can be administered at a dose of 1-10 mg. Intravenous phytonadione is associated with rare but well-documented cases of anaphylactoid reactions; thus, it must be administered with care at a rate no greater than 1 mg/min, with frequent (every 15 min, 30 min, and 1 h) vital sign measurements. A patient given a dose of 10 mg of intravenous phytonadione may be refractory to Coumadin for several weeks, making 2.5 mg or 5 mg a better dose in all but the most severe rectus sheath hematomas.
Subcutaneous phytonadione is associated with an unpredictable therapeutic response and is not recommended.
Oral phytonadione has a time to onset that is too slow for a patient who is actively bleeding.
FFP is administered in a volume of 15 mL/kg and provides coagulation factors for as long as 8 hours. FFP may have to be administered with diuretics if volume overload is a concern.
Patients on heparin can have coagulation reversed by protamine at a dose of 1 mg per 100 U of heparin. Heparin has a half-life of 60 minutes. A dose of protamine reverses all of the heparin administered in the past hour, one half of the heparin of the previous hour, and one fourth of the heparin given 2 hours previously, assuming that no recent bolus has been administered.
Patients on LMWHs can have their anticoagulation partially reversed by protamine, although refractory heparinoid fractions are present.
Transfusion
The decision to transfuse is made depending on the patient’s need for fluid resuscitation; the presence of comorbid conditions, such as active coronary ischemia; the degree of anemia; and the need for an operative procedure for control of bleeding. In 1988, Zainea 17. reported the transfusion of blood in 4 of 8 patients in a case series, although none of the patients were hemodynamically unstable. In Berna’s case series of 12 anticoagulated patients in 2000, all 5 patients with type III hematomas required transfusion and had alterations in hemodynamic variables 12. Transfusion requirements are generally 2-6 U of packed red blood cells.
Two main modalities exist for invasive control of active bleeding in rectus sheath hematoma: (1) therapeutic angiography with embolization of the bleeding vessel and (2) operative therapy with clot evacuation, ligation of bleeding vessels, and closed-suction drainage. Invasive treatment should be considered in patients with enlarging hematomas, hemodynamic instability unresponsive to fluid resuscitation, peritoneal signs, pain not well controlled with analgesics, and persistent gastrointestinal or urinary symptoms. Patients with significant comorbidities may not be candidates for invasive therapy.
Arterial embolization
In 1980, Levy first described the transcatheter Gelfoam embolization technique in the treatment of rectus sheath hematoma 18. This invasive therapy can produce hemostasis, reduce the size of the hematoma, decrease the need for blood product transfusion, and prevent rupture into the abdomen. Embolization with thrombin, Gelfoam, or coil is an alternative to surgery for conditions not responding to conservative management.
Operative exploration
Surgical treatment includes evacuation of the hematoma, ligation of bleeding vessels, repair of the rectus sheath, drainage (when indicated), and closure of the abdominal wall. Recurrences following surgical therapy have not been reported.
The decision to admit a patient with rectus sheath hematoma depends on the clinical data regarding hemodynamic status and comorbid conditions as well as the size of the hematoma. Patients on anticoagulation therapy should be admitted to ensure that the hematoma is not expanding and to plan restarting anticoagulation as appropriate. In general, patients with type 1 hematomas do not require hospitalization. Patients with type 2 and type 3 hematomas usually do require hospitalization. Patients with type 2 hematomas can be admitted to the floor during the first 24-48 hours to evaluate evolution of the hematoma. Patients with type 3 hematomas often present with hemodynamic instability requiring fluid resuscitation and blood transfusion that is best managed in the intensive care unit setting.
Postdischarge care includes rest, analgesics, hematoma compression, ice packs, and treatment of predisposing conditions. Type 1 hematomas resolve after approximately 1 month. Type 2 hematomas require 2-4 months, and type 3 hematomas require more than 3 months and as long as a year for complete resolution.
Rectus sheath hematoma prognosis
The majority of patients recover well with no complications as the hematoma is reabsorbed in 2 to 3 months 19. In those with an indication for therapeutic anticoagulation, patients should be counseled on the risk for recurrence with the resumption of therapy.
References- Rectus Sheath Hematoma. https://reference.medscape.com/article/776871-overview
- Klingler PJ, Wetscher G, Glaser K, Tschmelitsch J, Schmid T, Hinder RA. The use of ultrasound to differentiate rectus sheath hematoma from other acute abdominal disorders. Surg Endosc. 1999;13(11):1129–1134. doi: 10.1007/s004649901188
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg. 2015;13:267–271. doi: 10.1016/j.ijsu.2014.12.015
- Salemis NS, Gourgiotis S, Karalis G. Diagnostic evaluation and management of patients with rectus sheath hematoma. A retrospective study. Int J Surg. 2010;8(4):290–293. doi: 10.1016/j.ijsu.2010.02.011
- Isik A, Peker K, Soyturk M, Firat D, Yoruker U, Yilmaz A. Diagnostic evaluation and treatment of patients with rectus abdominis hematoma. Cir Esp. 2015;93(9):580–588. doi: 10.1016/j.ciresp.2015.02.014
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of Risk Factors for Rectus Sheath Hematoma. Clin. Appl. Thromb. Hemost. 2016 Apr;22(3):292-6.
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg. 2015 Jan;13:267-271.
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of Risk Factors for Rectus Sheath Hematoma. Clin Appl Thromb Hemost. 2014 Oct 7.
- Ko SB, Choi HA, Malhotra R, Lee K. Giant rectus sheath hematoma after therapeutic paracentesis resulting in hemodynamic instability in the intensive care unit. Hosp Pract (Minneap). Jun 2010. 38(3):52-5.
- Auten JD, Schofer JM, Banks SL, Rooney TB. Exercise-induced bilateral rectus sheath hematomas presenting as acute abdominal pain with scrotal swelling and pressure: case report and review. J Emerg Med. Apr 2010. 38(3):e9-12.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore). 2006 Mar;85(2):105-10.
- Berna JD, Zuazu I, Madrigal M, et al. Conservative treatment of large rectus sheath hematoma in patients undergoing anticoagulant therapy. Abdom Imaging. 2000 May-Jun. 25(3):230-4.
- Fothergill WE. Hematoma in the abdominal wall simulating pelvic new growth. Br Med J. 1926. 1:941-2.
- Berna JD, Garcia-Medina V, Guirao J, Garcia-Medina J. Rectus sheath hematoma: diagnostic classification by CT. Abdom Imaging. 1996 Jan-Feb. 21(1):62-4.
- Unger EC, Glazer HS, Lee JK, Ling D. MRI of extracranial hematomas: preliminary observations. AJR Am J Roentgenol. 1986 Feb. 146(2):403-7.
- Rimola J, Perendreu J, Falcó J, Fortuño JR, Massuet A, Branera J. Percutaneous arterial embolization in the management of rectus sheath hematoma. AJR Am J Roentgenol. 2007 Jun;188(6):W497-502.
- Zainea GG, Jordan F. Rectus sheath hematomas: their pathogenesis, diagnosis, and management. Am Surg. 1988 Oct. 54(10):630-3.
- Levy JM, Gordon HW, Pitha NR, Nykamp PW. Gelfoam embolization for control of bleeding from rectus sheath hematoma. AJR Am J Roentgenol. 1980 Dec. 135(6):1283-4.
- Buffone A, Basile G, Costanzo M, Veroux M, Terranova L, Basile A, Okatyeva V, Cannizzaro MT. Management of patients with rectus sheath hematoma: Personal experience. J. Formos. Med. Assoc. 2015 Jul;114(7):647-51.