Refeeding syndrome
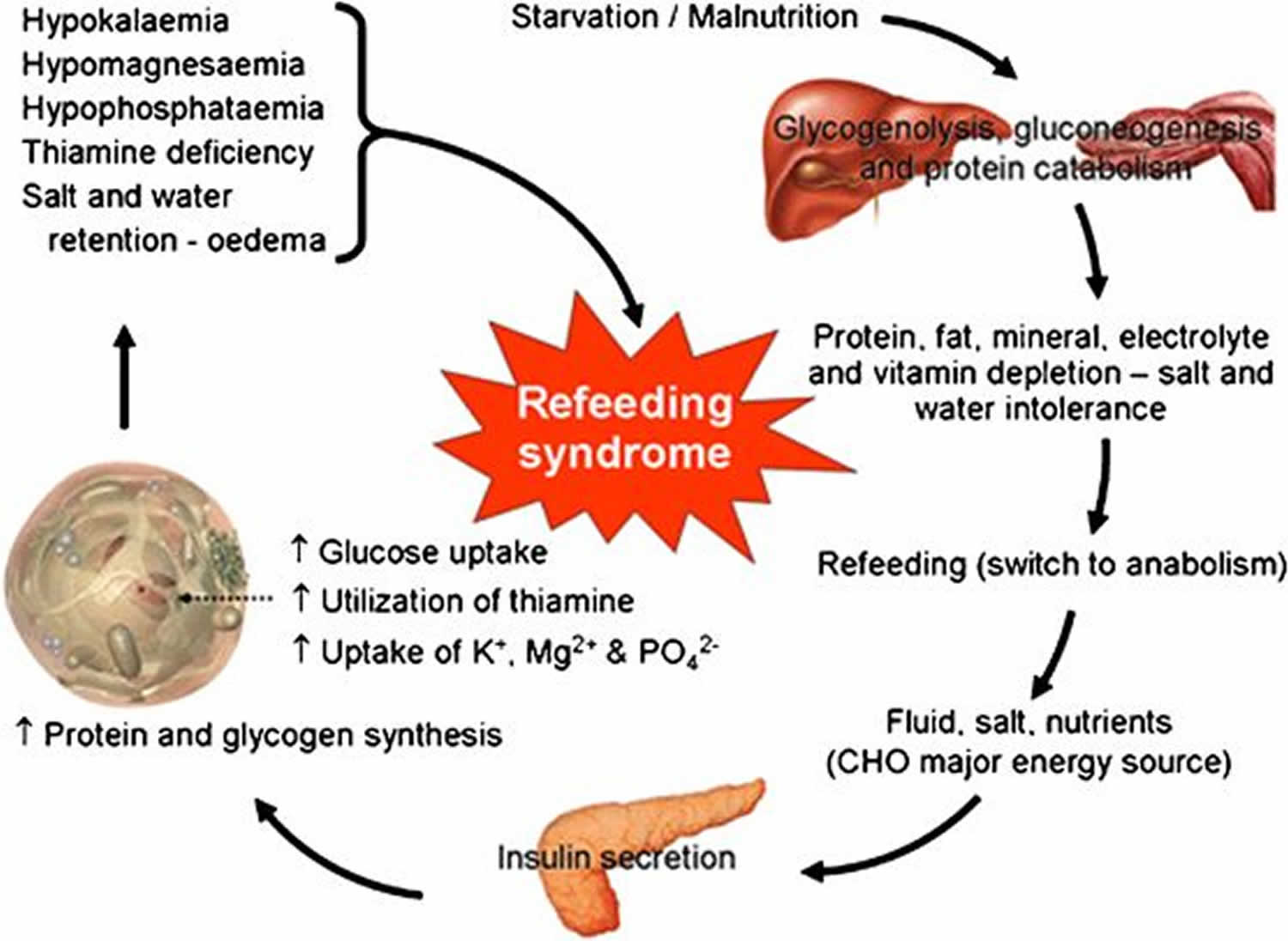
Refeeding syndrome broadly encompasses clinical complications that can occur as result of potentially fatal fluid and electrolyte shifts in malnourished patients during refeeding by oral, enteral, or parenteral routes and can lead to cardiac arrhythmia, muscle weakness and cramping, seizures, delirium, and death 1. These shifts result from hormonal and metabolic changes and may cause serious clinical complications. The hallmark biochemical feature of refeeding syndrome is hypophosphatemia [low serum phosphate concentration < 2.5 mg/dL (0.81 mmol/L)]. However, refeeding syndrome is complex and may also feature abnormal sodium and fluid balance; changes in glucose, protein, and fat metabolism; thiamine deficiency; hypokalemia (low blood level of potassium) and hypomagnesemia [low serum magnesium concentration < 1.8 mg/dL (< 0.70 mmol/L)] 2. Refeeding syndrome was was first described in Far East prisoners during the Second World War 3.
Estimates of the prevalence of refeeding syndrome vary widely from 0.43% to 34% 4. The true incidence of refeeding syndrome is unknown—partly owing to the lack of a universally accepted definition. In a study of 10,197 hospitalised patients the incidence of severe hypophosphatemia was 0.43%, with malnutrition being one of the strongest risk factors 5. Studies report a 100% incidence of hypophosphatemia in patients receiving total parenteral nutrition solutions that do not contain phosphorus. When solutions containing phosphate are used, the incidence can decrease to 18% 6.
Several prospective and retrospective cohort studies of hyperalimentation in intensive care units have documented the occurrence of refeeding syndrome 7. In a well designed prospective cohort study of a heterogeneous group of patients in intensive care units, 34% of patients experienced hypophosphatemia soon after feeding was started (mean (standard deviation) 1.9 (1.1) days) 8. Many case reports have highlighted the potentially fatal nature of the condition 9. However, it is often not recognized or maybe inappropriately treated, especially on general wards 2.
Refeeding syndrome key points
- Refeeding syndrome is a potentially fatal condition, caused by rapid initiation of refeeding after a period of undernutrition
- Refeeding syndrome is characterized by hypophosphatemia, associated with fluid and electrolyte shifts and metabolic and clinical complications
- Awareness of refeeding syndrome and identification of patients at risk is crucial as the condition is preventable and the metabolic complications are avoidable
- Patients at high risk include chronically undernourished patients and those who have had little or no energy intake for more than 10 days
- Refeeding should be started at a low level of energy replacement. Vitamin supplementation should also be started with refeeding and continued for at least 10 days
- Correction of electrolyte and fluid imbalances before feeding is not necessary; it should be done alongside feeding.
Refeeding syndrome causes
The underlying causative factor of refeeding syndrome is the metabolic and hormonal changes caused by rapid refeeding, whether enteral or parenteral. The net result of metabolic and hormonal changes in early starvation is that the body switches from using carbohydrate to using fat and protein as the main source of energy, and the basal metabolic rate decreases by as much as 20-25% 10.
During prolonged fasting, hormonal and metabolic changes are aimed at preventing protein and muscle breakdown. Muscle and other tissues decrease their use of ketone bodies and use fatty acids as the main energy source. This results in an increase in blood levels of ketone bodies, stimulating the brain to switch from glucose to ketone bodies as its main energy source. The liver decreases its rate of gluconeogenesis, thus preserving muscle protein. During the period of prolonged starvation, several intracellular minerals become severely depleted. However, serum concentrations of these minerals (including phosphate) may remain normal. This is because these minerals are mainly in the intracellular compartment, which contracts during starvation. In addition, there is a reduction in renal excretion 11.
Refeeding
During refeeding, glycaemia leads to increased insulin and decreased secretion of glucagon 11. Insulin stimulates glycogen, fat, and protein synthesis. This process requires minerals such as phosphate and magnesium and cofactors such as thiamine. Insulin stimulates the absorption of potassium into the cells through the sodium-potassium ATPase symporter, which also transports glucose into the cells. Magnesium and phosphate are also taken up into the cells. Water follows by osmosis. These processes result in a decrease in the serum levels of phosphate, potassium, and magnesium, all of which are already depleted. The clinical features of the refeeding syndrome occur as a result of the functional deficits of these electrolytes and the rapid change in basal metabolic rate.
Major causes include low phosphorus blood levels following intake of foods high in calories or glucose. Phosphorous depletion causes abnormalities in the cardiorespiratory system, which left untreated can be fatal. Symptoms also develop in response to changes in potassium and magnesium levels. Rapid changes in intake can place excessive strain on the impaired heart which is then unable to maintain adequate circulation.
What electrolytes and minerals are involved in refeeding syndrome?
Phosphorus
Phosphorus is predominantly an intracellular mineral. It is essential for all intracellular processes and for the structural integrity of cell membranes. In addition, many enzymes and second messengers are activated by phosphate binding. Importantly it is also required for energy storage in the form of adenosine triphosphate (ATP). It regulates the affinity of haemoglobin for oxygen and thus regulates oxygen delivery to tissues. It is also important in the renal acid-base buffer system.
In refeeding syndrome, chronic whole body depletion of phosphorus occurs. Also, the insulin surge causes a greatly increased uptake and use of phosphate in the cells. These changes lead to a deficit in intracellular as well as extracellular phosphorus. In this environment, even small decreases in serum phosphorus may lead to widespread dysfunction of cellular processes affecting almost every physiological system 12.
Potassium
Potassium, the major intracellular cation, is also depleted in undernutrition. Again, serum concentration may remain normal. With the change to anabolism on refeeding, potassium is taken up into cells as they increase in volume and number and as a direct result of insulin secretion. This results in severe hypokalaemia. This causes derangements in the electrochemical membrane potential, resulting in, for example, arrhythmias and cardiac arrest.
Magnesium
Magnesium, another predominantly intracellular cation, is an important cofactor in most enzyme systems, including oxidative phosphorylation and ATP production. It is also necessary for the structural integrity of DNA, RNA, and ribosomes. In addition, it affects membrane potential, and deficiency can lead to cardiac dysfunction and neuromuscular complications 13.
Glucose
Glucose intake after a period of starvation suppresses gluconeogenesis through the release of insulin. Excessive administration may therefore lead to hyperglycaemia and its sequelae of osmotic diuresis, dehydration, metabolic acidosis, and ketoacidosis. Excess glucose also leads to lipogenesis (again as a result of insulin stimulation), which may cause fatty liver, increased carbon dioxide production, hypercapnoea, and respiratory failure 14.
Vitamin deficiency
Although all vitamin deficiencies may occur at variable rates with inadequate intake, thiamine is of most importance in complications of refeeding. Thiamine is an essential coenzyme in carbohydrate metabolism. Its deficiency result in Wernicke’s encephalopathy (ocular abnormalities, ataxia, confusional state, hypothermia, coma) or Korsakoff’s syndrome (retrograde and anterograde amnesia, confabulation) 15.
Sodium, nitrogen, and fluid
Changes in carbohydrate metabolism have a profound effect on sodium and water balance. The introduction of carbohydrate to a diet leads to a rapid decrease in renal excretion of sodium and water 16. If fluid repletion is then instituted to maintain a normal urine output, patients may rapidly develop fluid overload. This can lead to congestive cardiac failure, pulmonary edema, and cardiac arrhythmia.
Refeeding syndrome prevention
Identification of high risk patients is crucial 17. Any patient with negligible food intake for more than five days is at risk of developing refeeding problems. Patients may be malnourished as a result of reduced intake (for example, owing to dysphagia, anorexia nervosa, depression, alcoholism); reduced absorption of nutrition (as in, for example, inflammatory bowel disease, celiac disease); or increased metabolic demands (for example, in cancer, surgery). High risk patients include those who have been chronically undernourished, especially those who also have diminished physiological reserve. Patients with dysphagia (for example, as a result of stroke) in particular may be at high risk.
Patients at high risk of refeeding syndrome 17:
- Patients with anorexia nervosa
- Patients with chronic alcoholism
- Oncology patients
- Postoperative patients
- Elderly patients (comorbidities, decreased physiological reserve)
- Patients with uncontrolled diabetes mellitus (electrolyte depletion, diuresis)
- Patients with chronic malnutrition:
- Marasmus
- Prolonged fasting or low energy diet
- Morbid obesity with profound weight loss
- High stress patient unfed for >7 days
- Malabsorptive syndrome (such as inflammatory bowel disease, chronic pancreatitis, cystic fibrosis, short bowel syndrome)
- Long term users of antacids (magnesium and aluminium salts bind phosphate)
- Long term users of diuretics (loss of electrolytes)
Criteria from the guidelines of the National Institute for Health and Clinical Excellence (NICE) for identifying patients at high risk of refeeding problems.
Nutrition support should be considered in people who are malnourished, as defined by any of the following 18:
- Body mass index (BMI) of less than 16 kg/m²
- Unintentional weight loss greater than 15% within the last 3–6 months
- Little or no nutritional intake for more than 10 days
- Low levels of potassium, phosphate or magnesium prior to feeding.
Or patient has two or more of the following 18:
- Body mass index (BMI) less than 18.5 kg/m²
- Unintentional weight loss greater than 10% within the last 3–6 months
- Little or no nutritional intake for more than 5 days
- A history of alcohol abuse or drugs including insulin, chemotherapy, antacids or diuretics.
People at high risk of developing refeeding problems should be cared for by healthcare professionals who are appropriately skilled and trained and have expert knowledge of nutritional requirements and nutrition support. To ensure adequate prevention, the National Institute for Health and Clinical Excellence (NICE) guidelines recommend a thorough nutritional assessment before refeeding is started 18. Recent weight change over time, nutrition, alcohol intake, and social and psychological problems should all be ascertained. Plasma electrolytes (especially phosphate, sodium, potassium, and magnesium) and glucose should be measured at baseline before feeding and any deficiencies corrected during feeding with close monitoring 18.
The prescription for people at high risk of developing refeeding problems should consider 18:
- Starting nutrition support at a maximum of 10 kcal/kg/day, increasing levels slowly to meet or exceed full needs by 4–7 days
- Using only 5 kcal/kg/day in extreme cases (for example, BMI less than 14 kg/m² or negligible intake for more than 15 days) and monitoring cardiac rhythm continually in these people and any others who already have or develop any cardiac arrythmias
- Restoring circulatory volume and monitoring fluid balance and overall clinical status closely
- Providing immediately before and during the first 10 days of feeding: oral thiamine 200–300 mg daily, vitamin B co strong 1 or 2 tablets, three times a day (or full dose daily intravenous vitamin B preparation, if necessary) and a balanced multivitamin/trace element supplement once daily
- Providing oral, enteral or intravenous supplements of potassium (likely requirement 2–4 mmol/kg/day), phosphate (likely requirement 0.3–0.6 mmol/kg/day) and magnesium (likely requirement 0.2 mmol/kg/day intravenous, 0.4 mmol/kg/day oral) unless pre-feeding plasma levels are high. Pre-feeding correction of low plasma levels is unnecessary.
The National Institute for Health and Clinical Excellence (NICE) guidelines recommend that refeeding is started at no more than 50% of energy requirements in “patients who have eaten little or nothing for more than 5 days.” The rate can then be increased if no refeeding problems are detected on clinical and biochemical monitoring 18.
For patients at high risk of developing refeeding syndrome, nutritional repletion of energy should be started slowly (maximum 0.042 MJ/kg/24 hours) and should be tailored to each patient. It can then be increased to meet or exceed full needs over four to seven days. In patients who are very malnourished (body mass index ≤14 or a negligible intake for two weeks or more), the National Institute for Health and Clinical Excellence (NICE) guidelines recommend that refeeding should start at a maximum of 0.021 MJ/kg/24 hours, with cardiac monitoring owing to the risk of cardiac arrhythmias 18. This explicit specification of the rate of refeeding in severely malnourished patients should help avoid complications arising from rapid refeeding and is an improvement on previous guidelines 19. The NICE guidelines also state that correcting electrolyte and fluid imbalances before feeding is not necessary and that this should be done along with feeding. This is a change from previous guidelines4 and potentially avoids prolongation of malnourishment and its effects on patients.
All guidelines recommend that vitamin supplementation should be started immediately, before and for the first 10 days of refeeding. Circulatory volume should also be restored. Oral, enteral, or intravenous supplements of the potassium, phosphate, calcium, and magnesium should be given unless blood levels are high before refeeding. Good quality studies on the exact levels of supplementation are lacking, however, and so the required levels of these supplements cited by NICE 18.
Electrolyte levels should be measured once daily for one week, and at least three times in the following week. Urinary electrolytes could also be checked to help assess body losses and to guide replacement.
Table 1 Protocol for nutritional, anthropometric and clinical monitoring of nutrition support
| Parameter | Frequency | Rationale |
| Nutritional | ||
| Nutrient intake from oral, enteral or parenteral nutrition (including any change in conditions that are affecting food intake) | Daily initially, reducing to twice weekly when stable | To ensure that patient is receiving nutrients to meet requirements and that current method of feeding is still the most appropriate. To allow alteration of intake as indicated |
| Actual volume of feed delivered* | Daily initially, reducing to twice weekly when stable | To ensure that patient is receiving correct volume of feed. To allow troubleshooting |
| Fluid balance charts (enteral and parenteral) | Daily initially, reducing to twice weekly when stable | To ensure patient is not becoming over/under hydrated |
| Anthropometric | ||
| Weight* | Daily if concerns regarding fluid balance, otherwise weekly reducing to monthly | To assess ongoing nutritional status, determine whether nutritional goals are being achieved and take into account both body fat and muscle |
| BMI* | Start of feeding and then monthly | |
| Mid-arm circumference* | Monthly, if weight cannot be obtained or is difficult to interpret | |
| Triceps skinfold thickness | Monthly, if weight cannot be obtained or is difficult to interpret | |
| GI function | ||
| Nausea/vomiting* | Daily initially, reducing to twice weekly | To ensure tolerance of feed |
| Diarrhoea* | Daily initially, reducing to twice weekly | To rule out any other causes of diarrhoea and then assess tolerance of feeds |
| Constipation* | Daily initially, reducing to twice weekly | To rule out other causes of constipation and then assess tolerance of feeds |
| Abdominal distension | As necessary | Assess tolerance of feed |
| Enteral tube – nasally inserted | ||
| Gastric tube position (pH less than or equal to 5.5 using pH paper – or noting position of markers on tube once initial position has been confirmed) | Before each feed begins | To ensure tube in correct position |
| Nasal erosion | Daily | To ensure tolerance of tube |
| Fixation (is it secure?) | Daily | To help prevent tube becoming dislodged |
| Is tube in working order (all pieces intact, tube not blocked/kinked)? | Daily | To ensure tube is in working order |
| Gastrostomy or jejunostomy | ||
| Stoma site | Daily | To ensure site not infected/red, no signs of gastric leakage |
| Tube position (length at external fixation) | Daily | To ensure tube has not migrated from/into stomach and external over granulation |
| Tube insertion and rotation (gastrostomy without jejunal extension only) | Weekly | Prevent internal overgranulation/prevention of buried bumper syndrome |
| Balloon water volume (balloon retained gastrostomies only) | Weekly | To prevent tube falling out |
| Jejunostomy tube position by noting position of external markers | Daily | Confirmation of position |
| Parenteral nutrition | ||
| Catheter entry site* | Daily | Signs of infection/inflammation |
| Skin over position of catheter tip (peripherally fed people)* | Daily | Signs of thrombophlebitis |
| Clinical condition | ||
| General condition* | Daily | To ensure that patient is tolerating feed and that feeding and route continue to be appropriate |
| Temperature/blood pressure | Daily initially, then as needed | Sign of infection/fluid balance |
| Drug therapy* | Daily initially, reducing to monthly when stable | Appropriate preparation of drug (to reduce incidence of tube blockage). To prevent/reduce drug nutrient interactions |
| Long-/short-term goals | ||
| Are goals being met?* | Daily initially, reducing to twice weekly and then progressively to 3–6 monthly, unless clinical condition changes | To ensure that feeding is appropriate to overall care of patient |
| Are goals still appropriate?* | Daily initially, reducing to twice weekly and then progressively to 3–6 monthly, unless clinical condition changes | To ensure that feeding is appropriate to overall care of patient |
| People at home having parenteral nutrition should be monitored using observations marked *. | ||
Table 2 Protocol for laboratory monitoring of nutrition support
| Parameter | Frequency | Rationale | Interpretation |
| Sodium, potassium, urea, creatinine | Baseline Daily until stable Then 1 or 2 times a week | Assessment of renal function, fluid status, and Na and K status | Interpret with knowledge of fluid balance and medication Urinary sodium may be helpful in complex cases with gastrointestinal fluid loss |
| Glucose | Baseline 1 or 2 times a day (or more if needed) until stable Then weekly | Glucose intolerance is common | Good glycaemic control is necessary |
| Magnesium, phosphate | Baseline Daily if risk of refeeding syndrome Three times a week until stable Then weekly | Depletion is common and under recognised | Low concentrations indicate poor status |
| Liver function tests including International Normalised Ratio (INR) | Baseline Twice weekly until stable Then weekly | Abnormalities common during parenteral nutrition | Complex. May be due to sepsis, other disease or nutritional intake |
| Calcium, albumin | Baseline Then weekly | Hypocalcaemia or hypercalcaemia may occur | Correct measured serum calcium concentration for albumin Hypocalcaemia may be secondary to Mg deficiency Low albumin reflects disease not protein status |
| C-reactive protein | Baseline Then 2 or 3 times a week until stable | Assists interpretation of protein, trace element and vitamin results | To assess the presence of an acute phase reaction (APR). The trend of results is important |
| Zinc, copper | Baseline Then every 2–4 weeks, depending on results | Deficiency common, especially when increased losses | People most at risk when anabolic APR causes Zn decrease and Cu increase |
| Seleniuma | Baseline if risk of depletion Further testing dependent on baseline | Se deficiency likely in severe illness and sepsis, or long-term nutrition support | APR causes Se decrease Long-term status better assessed by glutathione peroxidase |
| Full blood count and MCV | Baseline 1 or 2 times a week until stable Then weekly | Anaemia due to iron or folate deficiency is common | Effects of sepsis may be important |
| Iron, ferritin | Baseline Then every 3–6 months | Iron deficiency common in long-term parenteral nutrition | Iron status difficult if APR (Fe decrease, ferritin increase) |
| Folate, B12 | Baseline Then every 2–4 weeks | Iron deficiency is common | Serum folate/B12 sufficient, with full blood count |
| Manganeseb | Every 3–6 months if on home parenteral nutrition | Excess provision to be avoided, more likely if liver disease | Red blood cell or whole blood better measure of excess than plasma |
| 25-OH Vit Db | 6 monthly if on long-term support | Low if housebound | Requires normal kidney function for effect |
| Bone densitometryb | On starting home parenteral nutrition Then every 2 years | Metabolic bone disease diagnosis | Together with lab tests for metabolic bone disease |
| a These tests are needed primarily for people having parenteral nutrition in the community. b These tests are rarely needed for people having enteral tube feeding (in hospital or in the community), unless there is cause for concern. | |||
Refeeding syndrome signs and symptoms
Refeeding syndrome symptoms include:
- development of edema,
- increased liver function tests,
- hypophosphatemia,
- cardiac failure,
- central nervous system depression.
Refeeding syndrome diagnosis
Refeeding syndrome is detected by considering the possibility of its existence and by using the simple biochemical investigations described above. If refeeding syndrome is detected, the rate of feeding should be slowed down and essential electrolytes should be replenished. The hospital specialist dietetics team should be involved.
Refeeding syndrome treatment
The best method for electrolyte repletion has not yet been determined. Hypophosphatemia, hypomagnesemia, and hypokalemia in hospitalized patients are ideally treated with intravenous supplementation (Table 3), but this is not without risks. A prospective comparative cohort study of 27 patients with severe hypophosphatemia showed the safety of administering 15-30 mmol phosphate over three hours via a central venous catheter in an intensive care unit 20. However, the researchers reported the need for repeated doses in most patients. Terlevich et al 21 reported efficacy of 50 mmol phosphate infused into a peripheral vein over 24 hours in 30 patients with no pre-existing renal dysfunction on general wards. Further infusions may be required and so careful monitoring of blood levels is required. Caution is needed in patients with existing renal impairment, hypocalcemia (which may worsen), or hypercalcemia (which may result in metastatic calcification).
Fluid repletion should be carefully controlled to avoid fluid overload as described earlier. Sodium administration should be limited to the replacement of losses. In patients at high risk of cardiac decompensation, central venous pressure and cardiac rhythm monitoring should be considered.
Table 3. Recommendation for phosphate and magnesium supplementation
| Mineral | Dose |
|---|---|
| Phosphate | |
| Maintenance requirement | 0.3-0.6 mmol/kg/day orally |
| Mild hypophosphataemia (0.6-0.85 mmol/l) | 0.3-0.6 mmol/kg/day orally |
| Moderate hypophosphataemia (0.3-0.6 mmol/l) | 9 mmol infused into peripheral vein over 12 hours |
| Severe hypophosphataemia (<0.3 mmol/l) | 18 mmol infused into peripheral vein over 12 hours |
| Magnesium | |
| Maintenance requirement | 0.2 mmol/kg/day intravenously (or 0.4 mmol/kg/day orally ) |
| Mild to moderate hypomagnesaemia (0.5-0.7 mmol/l) | Initially 0.5 mmol/kg/day over 24 hours intravenously, then 0.25 mmol/kg/day for 5 days intravenously |
| Severe hypomagnesaemia (<0.5 mmol/l) | 24 mmol over 6 hours intravenously, then as for mild to moderate hypomagnesaemia (above) |
- Jeon TJ, Lee KJ, Woo HS, et al. Refeeding Syndrome as a Possible Cause of Very Early Mortality in Acute Pancreatitis. Gut Liver. 2019;13(5):576–581. doi:10.5009/gnl18458 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6743809
- Hearing SD. Refeeding syndrome. BMJ 2004;328:908-9.
- SCHNITKER MA, MATTMAN PE, BLISS TL. A clinical study of malnutrition in Japanese prisoners of war. Ann. Intern. Med. 1951 Jul;35(1):69-96.
- Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition. 2001;17:632–637. doi: 10.1016/S0899-9007(01)00542-1
- Camp MA, Allon M. Severe hypophosphatemia in hospitalised patients. Mineral & Electrolyte Metabolism 1990;16:365-8.
- Martinez MJ, Matrinez MA, Montero M, Campelo E, Castro I, Inaraja MT. Hypophosphatemia in postoperative patients on total parenteral nutrition:influence of nutritional support teams. Nutr Hosp 2006;21:657-60.
- Crook MA, Hally V, Pantelli JV. The importance of the refeeding syndrome. Nutrition 2001;17:632-7.
- Marik PE, Bedigan MK. Refeeding hypophosphataemia in an intensive care unit: a prospective study. Arch Surg 1996;131:1043-7.
- Weinsier RL, Krumdieck CL. Death resulting from overzealous total parenteral nutrition: the refeeding syndrome revisited. Am J Clin Nutr 1980;34:393-9.
- McCray S, Walker S, Parrish CR. Much ado about refeeding. Practical Gastroenterology 2004;XXVIII(12):26-44.
- Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336(7659):1495–1498. doi:10.1136/bmj.a301 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2440847
- Knochel JP. The pathophysiology and clinical charactertistics of severe hypophosphatemia. Arch Intern Med 1977;137:203-20.
- Wacker WEC, Parisi AF. Magnesium metabolism. N Engl J Med 1968;278:658-63.
- Klein CJ, Stanek GS, Wiles CE. Overfeeding macronutrients to critically ill adults: metabolic complications. J Am Diet Assoc 1998;98:795-806.
- Reuler JB, Girard DE, Cooney TG. Wernicke’s encephalopathy. N Engl J Med 1985;312:1035-9.
- Veverbrants E, Arky RA. Effects of fasting and refeeding: I. Studies on sodium, potassium and water excretion on a constant electrolyte and fluid intake. J Clin Endocrinol Metab 1969;29:55-62.
- Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition. https://www.nice.org.uk/guidance/cg32
- Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition. https://www.nice.org.uk/guidance/cg32/chapter/1-Guidance
- Dewar H, Horvath R. Refeeding syndrome. In: Todorovic VE, Micklewright A, eds. A pocket guide to clinical nutrition 2nd ed. British Dietetic Association, 2001
- Perrault MM, Ostrop NJ, Tierney MG. Efficacy and safety of intravenous phosphate replacement in critically ill patients. Ann Pharmacother 1997;31:683-8
- Terlevich A, Hearing SD, Woltersdorf WW, Smyth C, Reid D, Mccullagh E, et al. Refeeding syndrome: effective and safe treatment with Phosphates Polyfusor. Aliment Pharmacol Ther 2003;17:1325-9.