Mucinous cystic neoplasm
Mucinous cystic neoplasms are defined as mucin-producing and septated cyst-forming epithelial precancerous conditions of the pancreas with a distinctive ovarian-type stroma 1. Usually solitary, mucinous cystic neoplasms size ranges between 5 and 35 cm with a thick fibrotic wall and without communication with the ductal system 2. Mucinous cystic neoplasms are rare and in most series, less common than intraductal papillary mucinous neoplasms and serous cystic neoplasms 3. Mucinous cystic neoplasms show a female to male ratio of 20 to 1 and a mean age at diagnosis of between 40 and 50 years (range 14-95 years) 1. The site of the mucinous cystic neoplasm is in the body and tail of the pancreas in 95%-98% of cases 4. When localized in the pancreatic head, mucinous cystoadenocarcinoma is more prevalent 5.
Invasive carcinoma incidence in mucinous cystic neoplasm varies between 6% and 36% 6. The Ulm series reported on 39 patients with mucinous cystic neoplasms and a malignant histology in 51%, including carcinoma in situ and advanced cancer 2. The explanation of this wide range may be the difficulty in interpreting the data on the prevalence of carcinoma because the majority of series have only indicated the advanced form.
Macroscopically, mucinous cystic neoplasms usually appear as solitary, multilocular or unilocular lesions with a mean size of 7-8 cm (range 0.5-35 cm) with a thick fibrotic wall and containing mucin, even when hemorrhagic, watery or necrotic content is observed 6.
In 2004, the consensus conference of the International Association of Pancreatology in Sendai (Japan) 7 established that the histological presence of unique ovarian-type stroma was mandatory to diagnose mucinous cystic neoplasm and that this was not found in other pancreatic neoplasms 8. Mucinous cystic neoplasms display no communication with the pancreatic ductal system, although some studies suggested that a small proportion of mucinous cystic neoplasms may show microscopic communication with the pancreatic ducts 9.
Under light microscopy, the cysts are lined by a columnar mucin-producing epithelium with different grade of dysplasia: mild (mucinous cystic neoplasm adenoma), moderate (mucinous cystic neoplasm borderline), and severe (mucinous cystic neoplasm carcinoma in situ) 10. The epithelial lining is positive for CKs (CK7, CK8, CK18, CK19), EMA and, less frequently, CK20, CEA, DUPAN-2 and CA 19-9 11. An invasive adenocarcinoma of the tubular or ductal type is associated in about one-third of cases 12. The immunophenotype of ovarian-type stroma is similar to the normal ovarian one with positivity for vimentin, calretinin, tyrosine hydroxylase, SMA, α-inhibin, Melan-A, CD99 and Bcl-2 and frequently for PR and ER. The origin of ovarian stroma of the pancreas is still being debated 13. A stimulation of endodermal immature stroma by female hormones or primary yolk cell implantation in the pancreas has been suggested in literature 14 because buds of the genital tract and dorsal pancreas are adjacent to each other during embryogenesis. Moreover, dorsal pancreatic enlargement mainly gives rise to the pancreatic body and tail, and this could explain the predilection of mucinous cystic neoplasms for the distal pancreas 15.
Although the pathologic diagnosis of malignancy is based on invasion of the pancreatic parenchyma or metastases 16, mucinous cystic neoplasms that do not have conclusive evidence of carcinoma are considered premalignant 5.
A thickened wall with peripheral calcification and papillary proliferations, vascular involvement and hypervascular pattern should be considered as suggestive of mucinous cystic neoplasm with malignant changes 17. Although the invasive mucinous cystic neoplasm (mucinouscystoadenocarcinoma or mucinous cystic neoplasm with associated invasive carcinoma) is generally a tubular/ductal carcinoma 6, rare histological variants are represented by undifferentiated carcinoma with osteoclast-like giant cells 18, adenosquamous or colloid cells 19, or sarcomatoid carcinoma 13, carcinosarcoma and choriocarcinoma 20.
The increasing degree of dysplasia and tendency for invasion have been correlated with activating point mutations in the k-ras gene and mutations in the TP5 gene 21; moreover, the discovery that the inactivation of SMAD4/DPC4 in the epithelium of the invasive mucinous cystic neoplasms, but not in the ovarian-like stroma, could suggest that the ovarian-type stroma is not neoplastic 22.
Mucinous cystic neoplasm symptoms
The signs and symptoms of mucinous cystic neoplasm of the pancreas include:
- vague abdominal pain or discomfort
- a firm, but not tender, lump in the abdomen
- yellowing of the skin and the whites of the eyes (called jaundice)
- weight loss
- diabetic symptoms, which include unusual thirst, frequent urination, extreme fatigue or lack of energy, nervousness and sweating
The majority of mucinous cystic neoplasms are slow growing and asymptomatic 23. In a series of 212 consecutive patients with cystic pancreatic lesions, 36.7% were asymptomatic and among them 28% had mucinous cystic neoplasms; in the symptomatic group, 16% had mucinous cystic neoplasm 24. In spite of these lesions being occasionally discovered in patients scanned for other indications 25, the typical clinical appearance is characterized by epigastric heaviness and fullness (60%-90%) or by an abdominal mass (30%-60%) 26. Nausea, vomiting (20%-30%) and back pain (7%-40%) can also be present.
No specific symptom was significantly associated with a likelihood of malignancy 27 although increasing anorexia and weight loss (10%-40%) may be associated with malignant changes 27.
Mucinous cystic neoplasm diagnosis
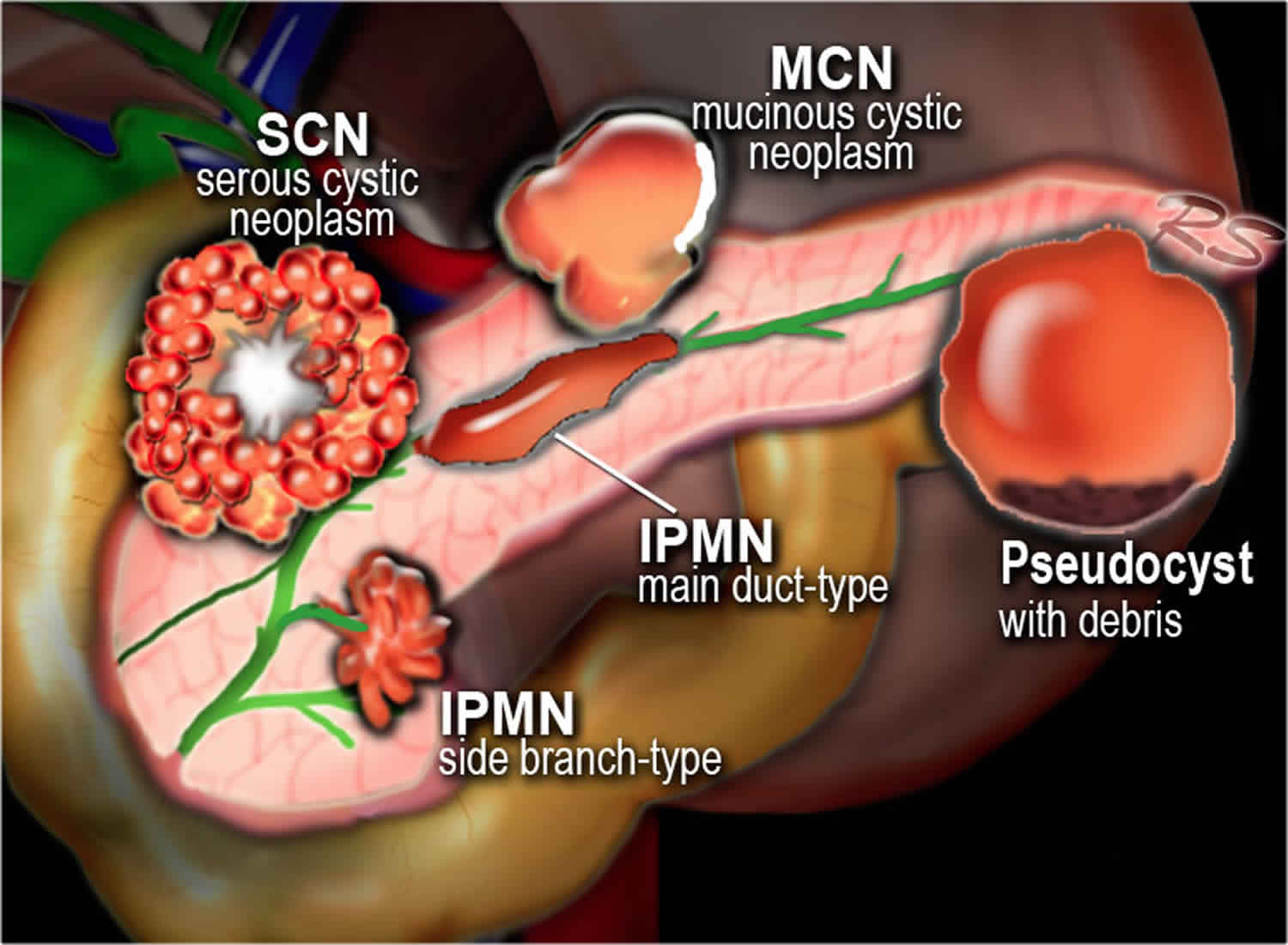
Mucinous cystic neoplasms main differential diagnosis includes other neoplastic cystic lesions (serous cystic neoplasm and the intraductal papillary mucinous neoplasms) and non-neoplastic cystic lesions (pancreatic pseudocysts). There is no single discriminating test, but preoperative diagnosis depends on a combination of modes, including clinical features, tumor markers, CT and MRI, endoscopic ultrasound with cyst fluid analysis, and positron emission tomography (PET).
High values of CEA and CA 19-9 show a high positive predictive value for pancreatic malignancy or pre-malignancy in the preoperative assessment of pancreatic cystic mass (70%-100%) 23. A CEA level of more than 400 ng/mL is a good predictor of malignancy in mucinous cystic neoplasms (sensitivity 45%-50%, specificity 95%-100%, accuracy 75%-80%) 28.
Trans-abdominal ultrasound examination has a low accuracy (50%) for cystic neoplasms of the pancreas 29.
Endoscopic ultrasound improves that accuracy and allows better evaluation of the wall as it may show separation or nodules within the cyst. Furthermore, endoscopic ultrasound can be used to obtain aspiration of the cyst contents and to perform a biopsy of the wall. Cyst fluid amylase concentration of < 250 U/L has been considered capable of excluding pseudocysts of the pancreas (sensitivity 40%-45%, specificity 95%-100%, accuracy 60%-65%), while CEA < 5 ng/mL could suggest a benign etiology (sensitivity 45%-50%, specificity 95%-100%, accuracy 65%-70%) 30. Endoscopic ultrasound-fine needle aspiration (FNA) cytology and cyst fluid CEA greater than 192 ng/mL show the highest accuracy (79%) for differentiating mucinous cystic from non-mucinous cystic neoplasms 31. On the contrary endoscopic ultrasound morphology alone cannot distinguish between the two groups 31.
In any case, the main differential diagnosis of mucinous cystic neoplasms is with serous cystic neoplasms which have a low CEA in the fluid and an equal distribution throughout the pancreas, with pancreatic pseudocysts that usually show necrotic debris within the cyst cavity, and with branch duct intraductal papillary mucinous neoplasms communicating with the ductal pancreatic system and consequently showing elevated cystic fluid amylase 4.
Although pancreatitis may be present in the history of patients with pancreatic cystic neoplasms, when a cyst arises in a patient with chronic pancreatitis, the most frequent diagnosis is pancreatic pseudocyst 32. On the other hand, when pancreatitis is unexpected and occurs for the first time, the cyst could be a tumor, determining the development of pancreatitis due to compression of the pancreatic duct 33. This is a crucial problem, because the risk of managing cystic mucinous neoplasms in patients with a prior history of pancreatitis, like pseudocysts by a pseudocyst-jejunal anastomosis or pseudocyst-gastrostomy, is higher than usual, with disastrous long-term prognosis 34. Proper sampling of pseudocysts is essential and should consist of sampling of the cyst wall during surgery or analysis of cyst content during minimal access drainage procedures. Although the clinical context, radiological imaging and biochemical findings may help differentiate pancreatic pseudocyst from cystic neoplasms, small lesions may be problematic.
The image based classification system proposed by Sahani et al 25, in which cystic pancreatic lesions are classified in four subtypes, is reported in Table 1.
The demonstration of a solid component, invasion outside the confines of the pancreas, or pancreatic duct obstruction through endoscopic ultrasound is highly indicative of malignancy with sensitivity, specificity and accuracy of 70%, 100% and 60%, respectively 25. However, in the absence of these findings the ability of endoscopic ultrasound to diagnose malignancy is limited with an overall sensitivity, specificity and accuracy of 56%, 45% and 51%, respectively 31. The added advantage of endoscopic ultrasound in performing aspiration of cyst content and sampling of the cyst wall and septa or mural nodules is that it allows small lesions as well as suspicious areas to be analysed. Laparoscopic and intraoperative ultrasounds are highly operator dependent with an accuracy ranging from 40% to 90% 35.
Multidetector computed tomography and magnetic resonance cholangiopancreaticography (MRCP) play a critical role in assessment, defining size, septation, calcifications, nodules of the wall, and communication with the ductal system of the pancreatic cyst.
At cross-sectional imaging, the mucinous cystic neoplasm appears as a unilocular or multilocular single macrocyst with a solid component, with no communication with the main duct 25. The internal architecture of the cyst, including septa and internal wall, is best appreciated with MR imaging 36.
Recently, Kim et al 37 defined some significant CT features for differentiating mucinous cystic neoplasms from serous cystic neoplasms and intraductal papillary mucinous neoplasms: the shape is smooth in mucinous cystic neoplasms, multicystic and lobulated in serous cystic neoplasms, and pleomorphic and clubbed finger-like in intraductal papillary mucinous neoplasms; the main pancreatic duct is not dilated or proximally only in serous cystic neoplasms, and if dilated, whole in intraductal papillary mucinous neoplasm.
In spite of the improvement in pancreatic tumor visualization resulting from CT and MRI, the ability to perform diagnosis of these techniques individually – as well as endoscopic ultrasound – remains poor (25%-30%) 38. In a multivariate analysis by Visser et al 39 in 2008, the combination of CT and MRI data showed an accuracy ranging from 44% to 83%.
Cross-sectional imaging generally shows peripheral calcification, a thickened wall, papillary proliferations, vascular involvement and hypervascular pattern in the cases of malignant mucinous cystic neoplasms 40. Although peripheral eggshell calcification is not easily detected by CT, this is a specific feature of the mucinous cystic neoplasms and is highly predictive of malignancy 41.
The clinical value of MRCP is similar to endoscopic retrograde cholangiopancreatography or percutaneous transhepatic cholangiography 42 but an MR multi-imaging protocol, which includes MR cross-sectional imaging, magnetic resonance cholangiopancreaticography and dynamic contrast-enhanced MR angiography, integrates the advantages of multiple imaging techniques without morbidity 43.
The role of PET in managing pancreatic cystic lesions is currently limited but recent studies report detection of malignant pancreatic cysts with sensitivity and positive predictive values above 90% 44.
In spite of a complete diagnostic assessment, the surgeon’s preoperative diagnosis is correct in one-third of cases, incorrect in another third, and non-specific in the remainder 39.
Table 1. Imaging-based classification system of cystic pancreatic lesions
| Type of lesions | Morphologic features | Pancreatic cystic lesions |
| Unilocular cyst | Without internal septation and solid component or wall calcification | Serous cystic neoplasm, intraductal papillary mucinous neoplasm1, pancreatic pseudocyst |
| Microcystic lesion | Six or more cysts with diameter 0.2 mm-2 cm, external lobulation, fibrous central scar with or without stellate calcifications | Serous cystic neoplasm |
| Macrocystic lesions | Diameter > 2 cm, with internal septation and solid component or wall calcification | Intraductal papillary mucinous neoplasm1, mucinous cystic neoplasm2 |
| Cyst with a solid component | Unilocular or multilocular | Intraductal papillary mucinous neoplasm1, mucinous cystic neoplasm2 |
Footnote: 1 With or 2 without communication with main duct, respectively.
[Source 1 ]Mucinous cystic neoplasm treatment
Surgical excision is indicated for all mucinous cystic neoplasms considered pre-malignant. Factors influencing treatment include tumor histological features, the patient’s age and surgical risk, and tumor size and location.
Left pancreatectomy
Because mucinous cystic adenoma of the pancreas are usually localized at the level of the body and tail of the pancreas, the most common operation performed to cure these neoplasms is distal pancreatectomy, which is a safe procedure in high volume centres (overall postoperative morbidity ranging from 5% to 50% and a mortality rate of 0%) 45. The main complication, pancreatic fistula, occurs in 15%-20% of cases 46.
The distal pancreatectomy technique was first described in 1913 by Mayo 47 and the spleen-preserving distal pancreatectomy was outlined in 1943 by Mallet-Guy et al 48. Preservation of the spleen can be performed with or without preservation of the splenic artery and vein. In 1988, Warshaw described a technique without the preservation of the splenic artery and vein, ligating the splenic vessels at the hilum 49. Although this method appears technically less difficult and can be performed in a shorter operating time, it has been associated with a higher incidence of spleen vascular insufficiency 50. However, this procedure should be considered in the event of an inflamed or fibrosed splenic artery and vein 49. Spleen-preserving techniques must be avoided when in the presence of the largest tumors or risk factors for invasive malignancy, such as the size of the lesion, eggshell calcifications and mural nodules, in order to perform the complete oncological lymph node dissection 30. However, these techniques are preferred in all other cases to avoid long term infectious and haematological complications 49.
Studies comparing patients undergoing distal pancreatectomy with or without splenectomy show no significant differences compared to perioperative complications, mean operating time, pancreatic fistula rate, length of hospital stay and mortality 51.
Mucinous cystic neoplasms affecting the pancreatic neck or the proximal body could be managed either by an extended right or, more frequently, by an extended left pancreatectomy. These extended resections of normal pancreatic tissue may induce endocrine and exocrine insufficiency respectively in 30%-35% and 15%-20%, which in a benign or premalignant disease could be discussable 52.
Middle pancreatectomy
Middle pancreatectomy can be considered in the surgical management of mucinous cystic neoplasms located at the level of the pancreatic proximal body or neck, preserving endocrine and exocrine function with respect to extended left pancreatectomy or pancreaticoduodenectomy, and also preserving the spleen.
The main pitfalls of this technique are the technical difficulty, the higher incidence of postoperative complications and the risk of recurrence from potentially residual neoplasm 53.
Different techniques have been proposed for gastrointestinal reconstruction, including jejunal anastomosis of the stump or the distal stump, with pancreaticoduodenal or pancreaticogastric anastomosis 53.
In the literature, mortality after middle pancreatectomy was none and the overall morbidity was 25%-35% 54. The incidence of overall pancreatic fistula was 22%-45% and the type of reconstruction through Roux-en-Y pancreatojejunostomy or pancreaticogastrostomy did not affect the rate of any complication 54. Moreover, the incidence of endocrine and exocrine insufficiency after middle pancreatectomy was 4%-7% and 5%-8%, respectively 54.
Enucleation
Because the probability of malignancy in patients with mucinous cystic neoplasms smaller than 2 cm without nodules is very low, enucleation could be performed to avoid post-operative pancreatic insufficiency 27. This procedure is proposed for patients with mucinous cystic neoplasms smaller than 2 cm with benign features and superficially located 55. Enucleation can be performed without risk of recurrence but has been associated with a higher incidence of pancreatic fistula (30%-50%) 56.
Whipple procedure
A major oncologic resection, applying a Kausch-Whipple or pylorus-preserving technique, is recommended for mucinous cystic neoplasms that are localized monocentrically in the head.
The operative mortality ranges from 0% to 5% and is generally related to pancreatic anastomosis complications 46. The most common complications following the Whipple procedure are delayed gastric emptying and the pancreatic fistula occurring in 5%-10% and 6%-20% of operations, respectively 46.
When an enucleation is impossible or contraindicated, mucinous cystic neoplasms localized monocentrically in the pancreatic head that do not have an association with an invasive pancreatic cancer could be treated by duodenum-preserving total pancreatic head resection 57.
This procedure shows significant advantages when compared to Traverso-Longmire or Whipple pancreaticoduodenectomy, as regards the postoperative rate of morbidity and mortality, glucose metabolism, hospitalization and costs 58.
Lymphadenectomy
Pancreatectomy with lymph node dissection is necessary when an invasive carcinoma is suspected. Although the preoperative and intraoperative assessment of the grade of invasiveness is often difficult, whenever any doubt exists typical resection with lymph node dissection must be pursued 7. There is no evidence in literature of invasive mucinous cystic adenocarcinoma with distant lymph node metastases, so only a loco-regional lymphadenectomy is justified 27. Because the probability of malignancy is very low in the cases of small mucinous cystic neoplasms without nodules, lymphadenectomy can be avoided 52.
Laparoscopy
In the cases of benign-appearing and small malignant lesions (< 5 cm), a minimally invasive approach may be considered 59. Recent experiences from high-volume centers demonstrate that the laparoscopic approach for distal pancreatectomy for mucinous cystic neoplasms of the body and tail of the pancreas is feasible and safe 60. The complication rate of laparoscopic distal splenopancreatectomy (Lap SDP) ranges between 15% and 20% 61 with a mortality rate of 0%. In spleen-preserving laparoscopic pancreatic (Lap SPDP) resection, the overall morbidity ranges from 25% to 40% with a mortality rate of 0% 62. The overall reported pancreatic fistula was 5%-8% and 10%-15% after Lap SPDP and Lap SDP, respectively 62. This laparoscopic approach decreases the hospital stay and minimizes the cosmetic impact of the surgical wound 60.
Chemotherapy
Gemcitabine (GEM) is the standard therapy for advanced pancreatic cancer 63. Its effectiveness against advanced mucinous cystic neoplasms has been reported 64.
Recently, some combinations have been reported to be superior to gemcitabine alone 65. Gemcitabine-oxaliplatin treatment has been proposed to be more effective in terms of clinical progression-free survival 64.
Discordant results on survival were reported by phase II and III trials combining gemcitabine and inhibitors of epidermal growth factor receptor (cetuximab) and vascular endothelial growth factor (bevacizumab) 66.
Other modest but interesting advances have been provided by combinations such as gemcitabine-capecitabine and gemcitabine plus a platinum salt 67. In spite of this, survival results remain disappointing.
Conservative treatment
A conservative management with regular follow-up has been proposed in the presence of asymptomatic cystic lesions of the pancreas smaller than 3 cm without mural nodules, because the reported risk of malignancy in these cysts was found to be 3% 2. The suggested follow-up consisting of cross-sectional imaging and fine needle aspiration (FNA) cytology should be performed every 6 month for a period of 2 years and yearly after that. This should be continued for at least 4 years and then the interval of follow-up can be lengthened after 6 years of no change 30. When the cyst enlarges or when symptoms occur (in up to 20% of patients after follow-up), surgery is mandatory. The reported incidence of the subsequent resection due to change of the clinical, radiological and biochemical features of the lesions after initial conservative treatment was 4%-10% and malignancy rate in these cases was 3% 68.
Mucinous cystic neoplasm prognosis
After resection, in the absence of invasive carcinoma, prognosis of mucinous cystic neoplasms is excellent, with an overall survival rate of 100% 27 and patients do not need follow-up, since several studies have shown that the risk of recurrence following resection is 0% 69. Patients with invasive mucinous cystadenocarcinoma, show a 5-year survival rate of 20%-60%, which is much better than that for non-mucinous cystic neoplasm-associated ductal adenocarcinoma 16. When an anaplastic carcinoma of the pancreas associated with mucinous cystic neoplasm is reported, the prognosis is obviously extremely poor, with a 3-year survival rate lower than 3% 6.
References- Testini M, Gurrado A, Lissidini G, Venezia P, Greco L, Piccinni G. Management of mucinous cystic neoplasms of the pancreas. World J Gastroenterol. 2010;16(45):5682–5692. doi:10.3748/wjg.v16.i45.5682 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2997983
- Reddy RP, Smyrk TC, Zapiach M, Levy MJ, Pearson RK, Clain JE, Farnell MB, Sarr MG, Chari ST. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol. 2004;2:1026–1031.
- Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B, Klöppel G. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–178.
- Fernández-del Castillo C. Mucinous cystic neoplasms. J Gastrointest Surg. 2008;12:411–413.
- Sarr MG, Carpenter HA, Prabhakar LP, Orchard TF, Hughes S, van Heerden JA, DiMagno EP. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231:205–212.
- Campbell F, Azadeh B. Cystic neoplasms of the exocrine pancreas. Histopathology. 2008;52:539–551.
- Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32.
- Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am. 2007;36:831–849, vi.
- Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol. 2007;20 Suppl 1:S71–S93.
- Zamboni G, Capelli P, Pesci A, Brighenti A. Pathology of cystic tumor. In: Procacci C, Megibow AJ, eds , editors. Imaging of the pancreas: cystic and rare tumors. Berlin: Springer-Verlag; 2003. pp. 9–30.
- Erdogan D, Lamers WH, Offerhaus GJ, Busch OR, Gouma DJ, van Gulik TM. Cystadenomas with ovarian stroma in liver and pancreas: an evolving concept. Dig Surg. 2006;23:186–191.
- Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152–161.
- Wenig BM, Albores-Saavedra J, Buetow PC, Heffess CS. Pancreatic mucinous cystic neoplasm with sarcomatous stroma: a report of three cases. Am J Surg Pathol. 1997;21:70–80.
- Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, Sessa F, Capella C, Solcia E, Rickaert F, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–422.
- Longnecker DS, Adler G, Hruban RH, Klöppel G. Intraductal papillary-mucinous neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, eds , editors. Pathology and Genetics of Tumours of the Digestive System. WHO Classification of Tumours. Lyon: IARC Press; 2000. pp. 237–240.
- Wilentz RE, Albores-Saavedra J, Zahurak M, Talamini MA, Yeo CJ, Cameron JL, Hruban RH. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 1999;23:1320–1327.
- Goh BK, Tan YM, Yap WM, Cheow PC, Chow PK, Chung YF, Wong WK, Ooi LL. Pancreatic serous oligocystic adenomas: clinicopathologic features and a comparison with serous microcystic adenomas and mucinous cystic neoplasms. World J Surg. 2006;30:1553–1559.
- Posen JA. Giant cell tumor of the pancreas of the osteoclastic type associated with a mucous secreting cystadenocarcinoma. Hum Pathol. 1981;12:944–947.
- Lüttges J, Feyerabend B, Buchelt T, Pacena M, Klöppel G. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 2002;26:466–471.
- Bloomston M, Chanona-Vilchis J, Ellison EC, Ramirez NC, Frankel WL. Carcinosarcoma of the pancreas arising in a mucinous cystic neoplasm. Am Surg. 2006;72:351–355.
- Yoshizawa K, Nagai H, Sakurai S, Hironaka M, Morinaga S, Saitoh K, Fukayama M. Clonality and K-ras mutation analyses of epithelia in intraductal papillary mucinous tumor and mucinous cystic tumor of the pancreas. Virchows Arch. 2002;441:437–443.
- Iacobuzio-Donahue CA, Wilentz RE, Argani P, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Dpc4 protein in mucinous cystic neoplasms of the pancreas: frequent loss of expression in invasive carcinomas suggests a role in genetic progression. Am J Surg Pathol. 2000;24:1544–1548.
- Garcea G, Ong SL, Rajesh A, Neal CP, Pollard CA, Berry DP, Dennison AR. Cystic lesions of the pancreas. A diagnostic and management dilemma. Pancreatology. 2008;8:236–251.
- Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–433; discussion 433-434.
- Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471–1484.
- Goh BK, Tan YM, Chung YF, Cheow PC, Ong HS, Chan WH, Chow PK, Soo KC, Wong WK, Ooi LL. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg. 2008;143:956–965.
- Crippa S, Salvia R, Warshaw AL, Domínguez I, Bassi C, Falconi M, Thayer SP, Zamboni G, Lauwers GY, Mino-Kenudson M, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571–579.
- van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383–389.
- Shyr YM, Su CH, Tsay SH, Lui WY. Mucin-producing neoplasms of the pancreas. Intraductal papillary and mucinous cystic neoplasms. Ann Surg. 1996;223:141–146.
- Edirimanne S, Connor SJ. Incidental pancreatic cystic lesions. World J Surg. 2008;32:2028–2037.
- Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336.
- Brambs HJ, Juchems M. [Cystic tumors of the pancreas] Radiologe. 2008;48:740–751.
- Simeone DM. SSAT/AGA/ASGE state of the art conference on cystic neoplasms of the pancreas. J Gastrointest Surg. 2008;12:1475–1477.
- Scott J, Martin I, Redhead D, Hammond P, Garden OJ. Mucinous cystic neoplasms of the pancreas: imaging features and diagnostic difficulties. Clin Radiol. 2000;55:187–192.
- Fernández-del Castillo C, Alsfasser G, Targarona J, Brugge WR, Warshaw AL. Serum CA 19-9 in the management of cystic lesions of the pancreas. Pancreas. 2006;32:220.
- Sarr MG, Murr M, Smyrk TC, Yeo CJ, Fernandez-del-Castillo C, Hawes RH, Freeny PC. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg. 2003;7:417–428.
- Kim SY, Lee JM, Kim SH, Shin KS, Kim YJ, An SK, Han CJ, Han JK, Choi BI. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006;187:1192–1198.
- Planner AC, Anderson EM, Slater A, Phillips-Hughes J, Bungay HK, Betts M. An evidence-based review for the management of cystic pancreatic lesions. Clin Radiol. 2007;62:930–937.
- Visser BC, Muthusamy VR, Yeh BM, Coakley FV, Way LW. Diagnostic evaluation of cystic pancreatic lesions. HPB (Oxford) 2008;10:63–69.
- Goh BK, Tan YM, Cheow PC, Chung YF, Chow PK, Wong WK, Ooi LL. Outcome of distal pancreatectomy for pancreatic adenocarcinoma. Dig Surg. 2008;25:32–38.
- Loftus EV Jr, Olivares-Pakzad BA, Batts KP, Adkins MC, Stephens DH, Sarr MG, DiMagno EP. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Members of the Pancreas Clinic, and Pancreatic Surgeons of Mayo Clinic. Gastroenterology. 1996;110:1909–1918.
- Zhong L. Magnetic resonance imaging in the detection of pancreatic neoplasms. J Dig Dis. 2007;8:128–132.
- Kim MJ, Mitchell DG, Ito K, Outwater EK. Biliary dilatation: differentiation of benign from malignant causes–value of adding conventional MR imaging to MR cholangiopancreatography. Radiology. 2000;214:173–181.
- Sperti C, Pasquali C, Decet G, Chierichetti F, Liessi G, Pedrazzoli S. F-18-fluorodeoxyglucose positron emission tomography in differentiating malignant from benign pancreatic cysts: a prospective study. J Gastrointest Surg. 2005;9:22–28; discussion 28-29.
- Rodríguez JR, Germes SS, Pandharipande PV, Gazelle GS, Thayer SP, Warshaw AL, Fernández-del Castillo C. Implications and cost of pancreatic leak following distal pancreatic resection. Arch Surg. 2006;141:361–365; discussion 366.
- Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N, Mantovani W, Pederzoli P. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg. 2005;242:767–771, discussion 771-773.
- Mayo WJ. I. The Surgery of the Pancreas: I. Injuries to the Pancreas in the Course of Operations on the Stomach. II. Injuries to the Pancreas in the Course of Operations on the Spleen. III. Resection of Half the Pancreas for Tumor. Ann Surg. 1913;58:145–150.
- Mallet-Guy P, Vachon A. Pancreatites Chroniques Gauches. Paris: Masson; 1943.
- Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg. 1988;123:550–553.
- Miura F, Takada T, Asano T, Kenmochi T, Ochiai T, Amano H, Yoshida M. Hemodynamic changes of splenogastric circulation after spleen-preserving pancreatectomy with excision of splenic artery and vein. Surgery. 2005;138:518–522.
- Lee SY, Goh BK, Tan YM, Chung YF, Cheow PC, Chow PK, Wong WK, Ooi LL. Spleen-preserving distal pancreatectomy. Singapore Med J. 2008;49:883–885.
- Crippa S, Bassi C, Warshaw AL, Falconi M, Partelli S, Thayer SP, Pederzoli P, Fernández-del Castillo C. Middle pancreatectomy: indications, short- and long-term operative outcomes. Ann Surg. 2007;246:69–76.
- Bassi C. Middle segment pancreatectomy: a useful tool in the management of pancreatic neoplasms. J Gastrointest Surg. 2007;11:421–424.
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13.
- Sperti C, Pasquali C, Ferronato A, Pedrazzoli S. Median pancreatectomy for tumors of the neck and body of the pancreas. J Am Coll Surg. 2000;190:711–716.
- Crippa S, Bassi C, Salvia R, Falconi M, Butturini G, Pederzoli P. Enucleation of pancreatic neoplasms. Br J Surg. 2007;94:1254–1259.
- Nakao A, Fernández-Cruz L. Pancreatic head resection with segmental duodenectomy: safety and long-term results. Ann Surg. 2007;246:923–928; discussion 929-931.
- Pan ZG, Wang B. Anaplastic carcinoma of the pancreas associated with a mucinous cystic adenocarcinoma. A case report and review of the literature. JOP. 2007;8:775–782.
- Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693–698; discussion 698-700.
- Melotti G, Butturini G, Piccoli M, Casetti L, Bassi C, Mullineris B, Lazzaretti MG, Pederzoli P. Laparoscopic distal pancreatectomy: results on a consecutive series of 58 patients. Ann Surg. 2007;246:77–82.
- Takaori K, Tanigawa N. Laparoscopic pancreatic resection: the past, present, and future. Surg Today. 2007;37:535–545.
- Fabre JM, Dulucq JL, Vacher C, Lemoine MC, Wintringer P, Nocca D, Burgel JS, Domergue J. Is laparoscopic left pancreatic resection justified? Surg Endosc. 2002;16:1358–1361.
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413.
- Shimada K, Iwase K, Aono T, Takeda S, Yoshida H, Koma M, Nomura M, Nishikawa K, Tamagawa H, Matsuda C, et al. A case of advanced mucinous cystadenocarcinoma of the pancreas with peritoneal dissemination responding to gemcitabine. Gan To Kagaku Ryoho. 2009;36:995–998.
- Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–1438.
- Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237.
- Rivera F, López-Tarruella S, Vega-Villegas ME, Salcedo M. Treatment of advanced pancreatic cancer: from gemcitabine single agent to combinations and targeted therapy. Cancer Treat Rev. 2009;35:335–339.
- Walsh RM, Vogt DP, Henderson JM, Hirose K, Mason T, Bencsath K, Hammel J, Brown N. Management of suspected pancreatic cystic neoplasms based on cyst size. Surgery. 2008;144:677–684; discussion 684-685.
- Garcea G, Gouda M, Hebbes C, Ong SL, Neal CP, Dennison AR, Berry DP. Predictors of severity and survival in acute pancreatitis: validation of the efficacy of early warning scores. Pancreas. 2008;37:e54–e61