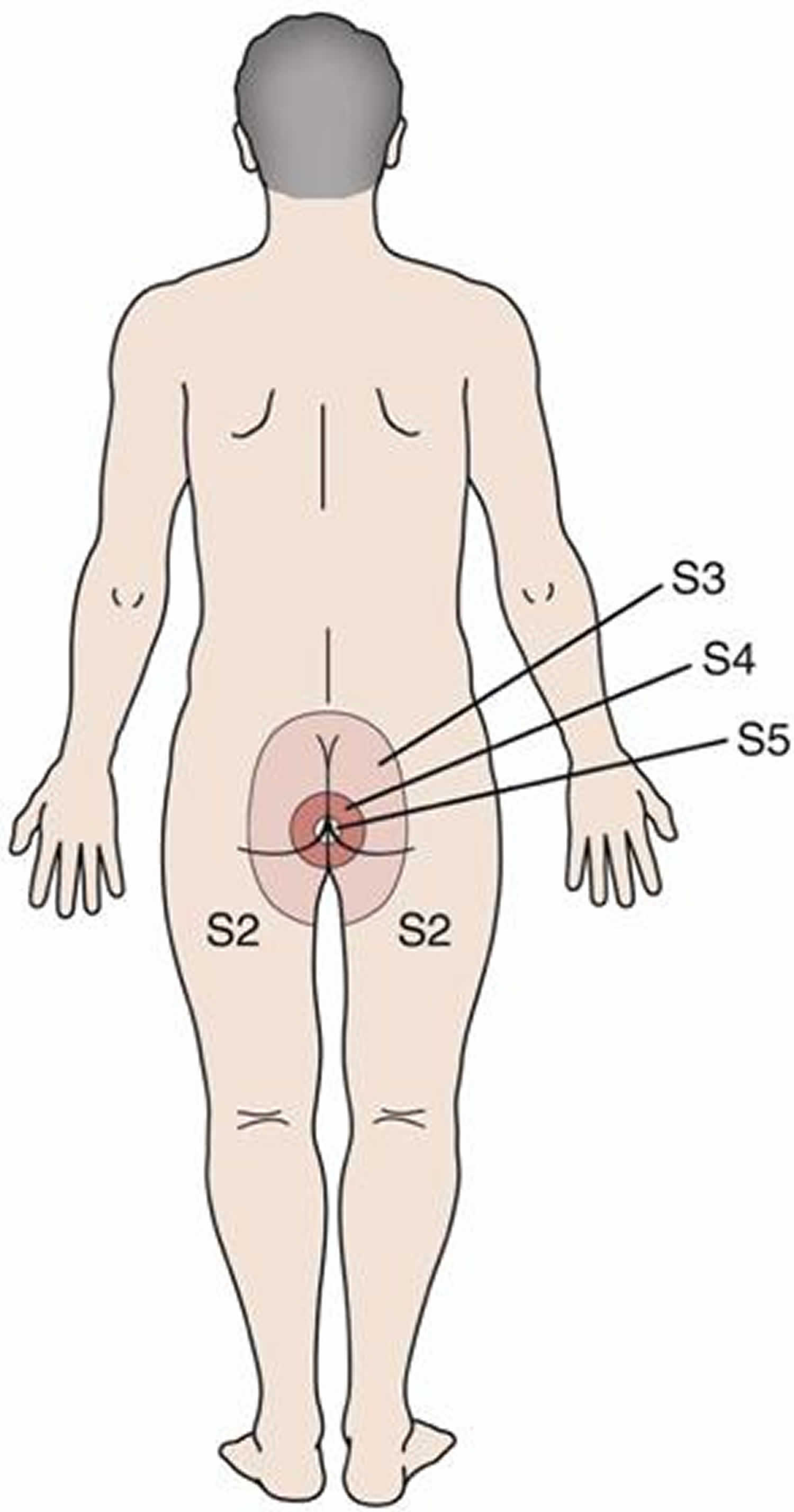
Saddle anesthesia
Saddle anesthesia is a loss of sensation (anesthesia) restricted to the area of the buttocks, the perineum (the area between the anus and the scrotum or vulva) and the inner surfaces of the thighs. Saddle anesthesia is frequently associated with the spine-related injury cauda equina syndrome. Saddle anesthesia is also seen in conus medullaris syndrome. Saddle anesthesia may also occur following intrathecal fentanyl 15 μg for anorectal surgery 1.
The nerves at the end of the spinal cord (approximately just above the waist) are called cauda equina. These nerves are responsible for the supply of sensation to the skin around the bottom and back passage. When the Cauda Equina nerves become compressed or squashed they can stop functioning. A loss of function can mean messages of physical sensation in the lower body do not reach the brain. This can potentially cause an individual to suffer from saddle anesthesia.
Cauda equina syndrome is a medical emergency that results from compression and disruption of the function of these nerves and can be inclusive of the conus medullaris or distal to it, and most often occurs when damage occurs to the L3-L5 nerve roots 2. Cauda equina syndrome can present with back pain radiating to the legs, motor and sensory dysfunction of the lower extremities, bladder and/or bowel dysfunction, sexual dysfunction and saddle anesthesia 3. Cauda equina syndrome also carry a high risk of litigation as delays in diagnosis and management can lead to devastating life-long impairment 4.
Individuals with cauda equina syndrome may experience varying degrees of the following problems:
- Loss of feeling between the legs;
- Numbness in or around the back passage and / or genitals;
- Inability to feel the toilet paper when wiping;
- Tingling sensation in the saddle area;
- Weakness in the saddle area.
If you experience saddle anesthesia, seek immediate medical care from your doctor or nearest emergency department. They should be able to diagnose whether you are suffering from symptoms of cauda equina syndrome or conus medullaris syndrome.
Cauda equina syndrome and conus medullaris syndrome are rare, with an estimated prevalence of 1 in 30,000 to 100,000 people per year 5. Estimates of annual incidence are between 1.5 to 3.4 per million people 5. It occurs in 3% of all disc herniations 5. Cauda equina syndrome and conus medullaris syndrome are most common in young men, possibly due to this population group being more likely to experience compressive thoracolumbar trauma 5. One study estimated that in the U.S. we would expect to see 1016 new causes of cauda equina syndrome and 449 new cases of conus medullaris per year 5.
Cauda equina anatomy
As spinal nerves branch from the spinal cord, they pass laterally to exit the vertebral canal through the intervertebral foramina between adjacent vertebrae. However, because the spinal cord is shorter than the vertebral column, nerves that arise from the lumbar, sacral, and coccygeal regions of the spinal cord do not leave the vertebral column at the same level they exit the spinal cord. The roots of these lower spinal nerves angle inferiorly alongside the filum terminale in the vertebral canal like wisps of hair. Accordingly, a rope-like tail of nerve fibers at the caudal end of the spinal cord are collectively named the cauda equina, from the Latin translation meaning “horse’s tail”. The cauda equina is a group of nerves and nerve roots stemming from the distal end of the spinal cord, typically levels L1-L5 and contains axons of nerves that give both motor and sensory innervation to the legs, bladder, anus, and perineum 6. Cauda equina syndrome is a medical emergency that results from compression and disruption of the function of these nerves and can be inclusive of the conus medullaris or distal to it, and most often occurs when damage occurs to the L3-L5 nerve roots 2. Cauda equina syndrome can present with back pain radiating to the legs, motor and sensory dysfunction of the lower extremities, bladder and/or bowel dysfunction, sexual dysfunction and saddle anesthesia 3. Cauda equina syndrome also carry a high risk of litigation as delays in diagnosis and management can lead to devastating life-long impairment 4.
The human spinal cord terminates at the L1-L2 vertebral level in a conical structure called the conus medullaris, which lies just caudad to the anatomical landmark of the 12th rib. The cauda equina contains a bundle of nerves which project distally within the enclosed cavity of the lumbar cistern from the spinal cord and conus medullaris toward the coccyx. Each nerve exits at its respective vertebral level toward targets which are supplied by the L2-S5 spinal cord level. Nerves contained within the cauda equina provide somatic efferent innervation to muscles of the lower extremity and somatic afferent sensations such as vibration, proprioception, pain, and temperature. Parasympathetic nerves provide visceral efferent signals to the urinary bladder from the S2 to S4 spinal cord levels and are responsible for micturition, accomplished by stimulating the detrusor muscle to contract while simultaneously relaxing the internal urethral sphincter. Sympathetic fibers from T11 through L2 exert urinary bladder filling by relaxing the detrusor muscle and contracting the internal urethral sphincter 7.
The cauda equina is supplied by arteries of the same name, which are small and may not be visualized radiographically. Each spinal nerve root has a corresponding medullary artery. A vasocorona surrounding the conus medullaris and the high degree of arterial anastomoses among the nerve roots predispose the vasculature patterns to significant diversity. Lymphatic capillaries occur nearly everywhere in the body except for a small number of sites, including the central nervous system (CNS); consequently, immune surveillance occurs within the layers of the meninges in a medium of cerebrospinal fluid (CSF). Cerebrospinal fluid bathes the brain, spinal cord, and cauda equina and is thought to serve as a protective layer of lubrication which provides nutrients and removes waste.
Figure 1. Cauda equina
Saddle anesthesia causes
Cauda equina and conus medullaris syndromes have overlap in anatomy and clinical presentation 8. The conus medullaris is the terminal end of the spinal cord, which typically occurs at the L1 vertebral level in the average adult 9. Conus medullaris syndrome results when there is compressive damage to the spinal cord from T12-L2 9. The cauda equina is a group of nerves and nerve roots stemming from the distal end of the spinal cord, typically levels L1-L5 and contains axons of nerves that give both motor and sensory innervation to the legs, bladder, anus, and perineum 10. Cauda equina syndrome results from compression and disruption of the function of these nerves and can be inclusive of the conus medullaris or distal to it, and most often occurs when damage occurs to the L3-L5 nerve roots 9. Both syndromes are neurosurgical emergencies as they can present with back pain radiating to the legs, motor and sensory dysfunction of the lower extremities, bladder and/or bowel dysfunction, sexual dysfunction and saddle anesthesia 11. Conus medullaris syndrome and cauda equina syndrome also carry a high risk of litigation as delays in diagnosis and management can lead to devastating life-long impairment 8.
Cauda equina syndrome and conus medullaris syndrome result from compression of the spinal cord and nerves/nerve roots arising from L1-L5 levels. The most common cause of compression in 45% of cauda equina syndrome is a herniated lumbar intervertebral disc 11. Other causes include epidural abscess, spinal epidural hematoma, diskitis, tumor (either metastatic or a primary brain and spinal cord cancer), trauma (particularly when there is retropulsion of bone fracture fragments), spinal stenosis and aortic obstruction 12. Rare reported cases exist in which cauda equina syndrome was associated with chiropractic manipulation, placement of interspinous devices, and thrombosis of the inferior vena cava 12.
Saddle anesthesia symptoms
Patients can present with symptoms of isolated cauda equina syndrome, isolated conus medullaris syndrome, or a combination. The symptoms and signs of cauda equina syndrome tend to be mostly lower motor neuron in nature, while those of conus medullaris syndrome are a combination of lower motor neuron and upper motor neuron effects (see Table 1, below). The history of onset, the duration of symptoms, and the presence of other features or symptoms could point to the possible causes.
Table 1. Symptoms and signs of Conus Medullaris and Cauda Equina Syndromes
| Conus Medullaris Syndrome | Cauda Equina Syndrome | |
| Presentation | Sudden and bilateral | Gradual and unilateral |
| Reflexes | Knee jerks preserved but ankle jerks affected | Both ankle and knee jerks affected |
| Radicular pain | Less severe | More severe |
| Low back pain | More | Less |
| Sensory symptoms and signs | Numbness tends to be more localized to perianal area; symmetrical and bilateral; sensory dissociation occurs | Numbness tends to be more localized to saddle area; asymmetrical, may be unilateral; no sensory dissociation; loss of sensation in specific dermatomes in lower extremities with numbness and paresthesia; possible numbness in pubic area, including glans penis or clitoris |
| Motor strength | Typically symmetric, hyperreflexic distal paresis of lower limbs that is less marked; fasciculations may be present | Asymmetric areflexic paraplegia that is more marked; fasciculations rare; atrophy more common |
| Impotence | Frequent | Less frequent; erectile dysfunction that includes inability to have erection, inability to maintain erection, lack of sensation in pubic area (including glans penis or clitoris), and inability to ejaculate |
| Sphincter dysfunction | Urinary retention and atonic anal sphincter cause overflow urinary incontinence and fecal incontinence; tend to present early in course of disease | Urinary retention; tends to present late in course of disease |
Symptoms of cauda equina syndrome include the following:
- Low back pain
- Unilateral or bilateral sciatica
- Saddle and perineal hypoesthesia or anesthesia
- Bowel and bladder disturbances
- Lower extremity motor weakness and sensory deficits
- Reduced or absent lower extremity reflexes
Low back pain can be divided into local and radicular pain. Local pain is generally a deep, aching pain resulting from soft-tissue and vertebral body irritation. Radicular pain is generally a sharp, stabbing pain resulting from compression of the dorsal nerve roots. Radicular pain projects in dermatomal distributions. Low back pain in cauda equina syndrome may have some characteristic that suggests something different from the far more common lumbar strain. Patients may report severity or a trigger, such as head turning, that seems unusual.
Severe pain is an early finding in 96% of patients with cauda equina syndrome secondary to spinal neoplasm. Later findings include lower extremity weakness due to involvement of the ventral roots. Patients generally develop hypotonia and hyporeflexia. Sensory loss and sphincter dysfunction are also common.
Urinary manifestations of cauda equina syndrome include the following:
- Retention
- Difficulty initiating urination (micturition)
- Decreased urethral sensation
- Typically, urinary manifestations begin with urinary retention and are later followed by an overflow urinary incontinence.
Bell et al 13 demonstrated that the accuracy of urinary retention, urinary frequency, urinary incontinence, altered urinary sensation, and altered perineal sensation as indications of possible disk prolapse justified urgent MRI assessment.
Bowel disturbances may include the following:
- Incontinence
- Constipation
- Loss of anal tone and sensation
The initial presentation of bladder/bowel dysfunction may be of difficulty starting or stopping a stream of urine. It may be followed by frank incontinence, first of urine then of stool. The urinary incontinence is on the basis of overflow. It is usually with associated saddle (perineal) anesthesia (the examiner can inquire if toilet paper feels different when the patient wipes).
Saddle anesthesia complications
Complications in cauda equina syndrome and conus medullaris syndrome occur in a large percentage of those diagnosed. One study looked at 63-day outcomes on micturition, defecation, saddle anesthesia, sexual function, and sciatica in cauda equina syndrome. The data indicate that a large percentage of patients still experience residual symptoms irrespective of their time to surgical decompression 11. Micturition deficits such as retention requiring self-catheterization or presence of suprapubic or indwelling catheters and incontinence still presented in 47.7% of patients 11. Dysfunction with defecation decreased post-operatively significantly, but 41.8% of patients still had problems at 63 days post-operatively.[3] Sexual dysfunction persisted in 53.3% of patients, and saddle anesthesia in 56.6% 11. Sciatica was present in 47.5% of patients 11. The best predictors of outcome are neurological status at presentation and degree of injury. Incomplete injuries tend to have better outcomes 11.
Saddle anesthesia diagnosis
A thorough history is necessary, with detailed questions regarding recent falls, trauma or injuries, use of anticoagulation, presented spinal instrumentation, intravenous drug use, history of malignancy, chiropractic manipulation, and constitutional symptoms like fevers/chills 11.
Patients can present with:
- Back pain and sciatica (seen in as many as 97% of patients)
- Weakness and changes in sensation in the lower extremities
- Bladder dysfunction (disruption of autonomic fibers results in either retention or incontinence in up to 92% of patients)
- Bowel dysfunction (retention or incontinence in up to 72% of patients)
- Saddle anesthesia or decreased sensation in the perineum (in up to 93% of patients)
- Sexual dysfunction (impotence in men) 11
The symptoms above when presenting in isolation, are neither specific nor sensitive for cauda equina syndrome or conus medullaris syndrome. However, several of the signs and symptoms mentioned above taken as a constellation should raise clinical suspicion 10. These symptoms also lack significant positive predictive value for the syndromes, especially early on 14. However, the onset of perineal anesthesia associated with bladder dysfunction is typical of the start of CES and the time at which the clock starts on diagnosis and management 10. It is important also to note that painless urinary retention often has the greatest predictive value as a stand-alone symptom, but it is unfortunately indicative of late, often irreversible cauda equina syndrome or conus medullaris syndrome 15.
Once clinical suspicion is established based on history, a thorough neurological examination is paramount. Findings to watch for include:
- Motor or sensory deficits in the legs – usually bilateral but can also be unilateral and asymmetrical (particularly in cases with an incomplete injury)
- Lower motor neuron signs in the legs – areflexia, hypotonia, atrophy (in cases of chronic cord compression resulting in cauda equina syndrome)
- Saddle anesthesia
- Absent or decreased rectal tone
- Absent or decreased bulbocavernosus reflex
- Palpable bladder indicating urinary retention 11
It is important to note that in the case of isolated conus medullaris syndrome, deficits of the lower extremities are more often bilateral and symmetric. Also, upper motor neuron signs can be present, such as spasticity and hyperreflexia 9.
Saddle anesthesia test
The gold standard method of evaluation for cauda equina syndrome or conus medullaris syndrome is obtaining urgent MRI imaging with sagittal and axial T1 and T2 sequences 10. There has not been a specific timeframe established for “door to MRI time” in the emergency department, but early MRI and neurosurgical or orthopedic consultation is imperative. An ideal goal for MRI is one hour from the patient presentation 10. For patients with contraindications to MRI, such as those with metal implants, a CT myelogram is a viable option 10. This imaging modality has limited utility as it requires injecting contrast through a spinal tap to visualize the spinal cord and its associated structures. A bladder scan checking for a post-void residual volume should also be obtained to evaluate for urinary retention 10.
Saddle anesthesia treatment
Prompt neurosurgical or orthopedic consultation is necessary in cases of cauda equina and conus medullaris syndromes, as the treatment is surgical decompression via laminectomy with or without subsequent discectomy, or via sequestrectomy 10. The timing of decompression is controversial, with immediate, early, and late surgical decompression showing varying results 16.
Other medical treatment options are useful in certain patients, depending on the underlying cause of the cauda equina syndrome 17. Anti-inflammatory agents and steroids can be effective in patients with inflammatory processes, including ankylosing spondylitis.
Patients with cauda equina syndrome secondary to infectious causes should receive appropriate antibiotic therapy. Patients with spinal neoplasms should be evaluated for the suitability of chemotherapy and radiation therapy.
Methylprednisolone should be administered. It treatment must be started within 8 hours of injury. No evidence exists of any benefit if it is started more than 8 hours after injury; on the contrary, late treatment may have detrimental effects.
Administration of ganglioside GM1 sodium salt beginning within 72 hours of injury may be beneficial; the dose is 100 mg IV once daily for 18-32 days.
Tirilazad mesylate (a nonglucocorticoid 21-aminosteroid) has been proven to be of benefit in animals and is currently under investigation. It inhibits lipid peroxidation and hydrolysis in the same manner as glucocorticoids.
Caution should be used in all forms of medical management for cauda equina syndrome. Any patient with true cauda equina syndrome with symptoms of saddle anesthesia and/or bilateral lower extremity weakness or loss of bowel or bladder control should undergo no more than 24 hours of initial medical management. If no relief of symptoms is achieved during this period, immediate surgical decompression is necessary to minimize the chances of permanent neurologic injury.
Surgical decompression
In acute compression of the conus medullaris or cauda equina, surgical decompression as soon as possible becomes mandatory. The goal is to relieve the pressure on the nerves of the cauda equina by removing the compressing agent and increasing the space in the spinal canal. Traditionally, cauda equina syndrome has been considered a surgical emergency, with surgical decompression considered necessary within 48 hours after the onset of symptoms, and preferably performed within 6 h of injury 18.
For patients in whom a herniated disk is the cause of cauda equina syndrome, a laminotomy or laminectomy to allow for decompression of the canal is recommended, followed by gentle retraction and discectomy.
In a more chronic presentation with less severe symptoms, decompression could be performed when medically feasible and should be delayed to optimize the patient’s medical condition; with this precaution, decompression is less likely to lead to irreversible neurological damage.
Surgical treatment may be necessary for decompression or tumor removal, especially if the patient presents with acute onset of symptoms. Surgical treatment may include laminectomy and instrumentation/fusion for stabilization or discectomy. Other surgical care may entail wound care (eg, debridement, skin graft, and skin flap/myocutaneous flap).
Many clinical and experimental reports have presented data on the functional outcome based on the timing of surgical decompression 19. Several investigators have reported no significant differences in the degree of functional recovery as a function of the timing of surgical decompression 20. Even with these findings, however, most investigators recommend surgical decompression as soon as possible after the onset of symptoms to offer the greatest chance of complete neurologic recovery.
On discharge from the surgical ward, patients often are transferred to an acute rehabilitation unit, from which they may be discharged, transferred to a subacute unit, or transferred to long-term care, depending on the level of long-term disability.
Rehabilitation
The rehabilitation team, especially the spinal cord injury rehabilitation physician and occupational and physical therapists, should be involved as soon as possible. The team will set goals in the rehabilitation unit toward maintaining and improving endurance, with the ability to be independent in activities of daily living on discharge from the hospital or long-term care facility.
The rehabilitation goals are to maximize the medical, physical, psychological, educational, vocational, and social function of the patient. To maximize medical function, ensure adequate prevention and treatment of possible medical complications already discussed, especially deep venous thrombosis, bladder and bowel problems, and decubitus ulcers
Physical therapy
Perform range of motion and strengthening exercises, sitting balance, transfer training, and tilt table as tolerated (because of tendency to orthostatic hypotension). Tilt table should start at 15 degrees, progressing by 10 degrees every 15 minutes up to about 80 degrees with the necessary precautions.
Other activities include the following:
- Wheelchair propulsion training
- Standing table exercises
- Functional electrical stimulation for increased muscle tone
- Lower extremity orthoses to aid balance and walking
- Ambulation exercises
- Family training and community skills
- A home exercise program
Occupational therapy
Conduct the following:
- Wheelchair training, especially for advanced wheelchair activities
- Transfer training
- Activities of daily living program with assistive devices for dressing, feeding, grooming, bathing, and toileting
- Motor coordination skills training
- Shower program
- Upper extremities training to increase strength for the increased demands of wheelchair propulsion and walking with assistive devices
- Home evaluation
- Family training
- A home exercise program
Orthotic/assistive devices may be needed for functional household ambulation and, if possible, community ambulation. This entails prescribing and training in proper use of knee-ankle-foot orthoses (KAFO) with forearm crutches for support; for lower lesions, knee-ankle-foot orthoses (KAFOs) or AFOs (ankle-foot orthoses) with canes or crutches may be needed. In addition to the above, bathtub bench, transfer boards, pressure-relieving seats, and wheelchairs are devices that may be needed. The patient should be assessed for these needs prior to discharge from the acute rehabilitation setting.
Long-term monitoring
Follow up with the rehabilitation team, including the spinal cord injury rehabilitation physician, physical therapist, and occupational therapist. These professionals are responsible for monitoring community and home integration and following improvements in the patient’s strength, coordination, transfer, activities of daily living, and ambulation.
Follow up with a primary care physician to monitor posthospital medications and other laboratory tests.
Patients with any renal or bladder complications and impotence should undergo regular follow-up, because they have an increased tendency for recurrent urinary tract infection and calculi 21. Yearly cystoscopy is recommended for patients with suprapubic catheters to help detect early bladder malignancies.
Regular follow-up urodynamic studies, renal ultrasound, and general cancer screening should be performed.
Prevention and treatment of complications
For deep venous thrombosis/pulmonary embolism, patients should use antiembolic compression stockings and subcutaneous heparin for 3 months as prophylaxis. Low-molecular-weight heparin also has been approved for prophylaxis. Ultrasound of the lower extremities may need to be done as an initial screening test with follow-up later. For neurogenic bladder, patients may require bladder catheterization.
In August 2011, onabotulinumtoxin A was approved by the US Food and Drug Administration for urinary incontinence in patients with neurologic conditions (eg, spinal cord injury, multiple sclerosis) who have overactive bladder. Therapy consists of 30 intradetrusor injections via cystoscopy. Trials have shown patients who received onabotulinumtoxinA had significant reduction in urinary incontinence episodes and improved urodynamics compared with placebo at 12 weeks 22.
Pressure ulcers may be prevented by eliminating pressure, optimizing wound-healing environment, and debriding if necessary.
For impotence, use of a phosphodiesterase type 5 inhibitor (eg, sildenafil [Viagra]) is becoming popular. Other drugs for erectile dysfunction include yohimbine, papaverine, and alprostadil. Methods to promote coitus and/or ejaculation could also be used; these include implantable penile prostheses or vibrator stimulation.
Patients may require use of stool softener or manual evacuation for fecal incontinence.
Heterotopic ossification can be confirmed by a triple-phase bone scan with associated elevation in serum levels of alkaline phosphatase and phosphate, especially in the early stage. Treatment includes stretching exercises, disodium etidronate (20 mg/kg once daily x 2 wk, then 10 mg/kg for as long as 12 weeks), radiation, and surgical excision. Surgery is done only when the heterotopic ossification has matured or stabilized, which is evident by stable plain x-ray, normal alkaline phosphatase level, and decline in triple-phase bone scan activity.
Pain should be treated appropriately based on its origin; treatment may include narcotics in the acute setting and tricyclic antidepressants later. Patient education, biofeedback, and relaxation techniques may also be used.
Nerve root ischemia is partially responsible for the pain and decreased motor strength associated with cauda equina syndrome. As a result, vasodilatory treatment can be useful in some patients. Mean arterial blood pressure should be maintained above 90 mm Hg to maximize blood flow to the spinal cord and nerve roots.
Treatment with lipoprostaglandin E1 and its derivatives has been reported to be effective in increasing blood flow to the cauda equina region and reducing symptoms of pain and motor weakness. This treatment option should be reserved for patients with modest spinal stenosis with neurogenic claudication. No benefit has been reported in patients with more severe symptoms or patients with radicular symptoms.
Use of orthoses is advised to prevent contractures. Use of antispasticity medications also is encouraged. Other medications include dantrolene, diazepam, clonidine, and tizanidine. Nerve blocks also could be done to relieve spasticity; appropriate agents include phenol, botulinum toxin, or local anesthetics.
Saddle anesthesia prognosis
Several studies have looked at prognosis and outcomes based on the timing of surgical decompression. Early intervention by surgical decompression in patients with conus medullaris and cauda equina syndromes is associated with a better prognosis, particularly when surgery occurs within 48 hours of initial presentation 14. The longer the compression continues, the worse the permanent structural and functional impairment, and the poorer the prognosis 10. It is important to note that the presence of bladder dysfunction prior to surgery has been linked to poorer outcomes regardless of the timing of decompression, although early decompression is still the recommendation for a better prognosis irrespective of clinical status at initial presentation 10.
References- Shim SM, Park JH, Hyun DM, Jeong EK, Kim SS, Lee HM. The effects of adjuvant intrathecal fentanyl on postoperative pain and rebound pain for anorectal surgery under saddle anesthesia. Korean J Anesthesiol. 2018;71(3):213–219. doi:10.4097/kja.d.18.27097 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5995019
- Brouwers E, van de Meent H, Curt A, Starremans B, Hosman A, Bartels R. Definitions of traumatic conus medullaris and cauda equina syndrome: a systematic literature review. Spinal Cord. 2017 Oct;55(10):886-890
- Korse NS, Pijpers JA, van Zwet E, Elzevier HW, Vleggeert-Lankamp CLA. Cauda Equina Syndrome: presentation, outcome, and predictors with focus on micturition, defecation, and sexual dysfunction. Eur Spine J. 2017 Mar;26(3):894-904
- Rider IS, Marra EM. Cauda Equina And Conus Medullaris Syndromes. [Updated 2019 Jan 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537200
- Podnar S. Epidemiology of cauda equina and conus medullaris lesions. Muscle Nerve. 2007 Apr;35(4):529-31.
- Quaile A. Cauda equina syndrome-the questions. Int Orthop. 2018 Oct 29
- Ridley LJ, Han J, Ridley WE, Xiang H. Cauda equina: Normal anatomy. J Med Imaging Radiat Oncol. 2018 Oct;62 Suppl 1:123
- Rider IS, Marra EM. Cauda Equina And Conus Medullaris Syndromes. [Updated 2019 Jan 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537200
- Brouwers E, van de Meent H, Curt A, Starremans B, Hosman A, Bartels R. Definitions of traumatic conus medullaris and cauda equina syndrome: a systematic literature review. Spinal Cord. 2017 Oct;55(10):886-890.
- Quaile A. Cauda equina syndrome-the questions. Int Orthop. 2019 Apr;43(4):957-961.
- Korse NS, Pijpers JA, van Zwet E, Elzevier HW, Vleggeert-Lankamp CLA. Cauda Equina Syndrome: presentation, outcome, and predictors with focus on micturition, defecation, and sexual dysfunction. Eur Spine J. 2017 Mar;26(3):894-904.
- Lavy C, James A, Wilson-MacDonald J, Fairbank J. Cauda equina syndrome. BMJ. 2009 Mar 31;338:b936.
- Bell DA, Collie D, Statham PF. Cauda equina syndrome: what is the correlation between clinical assessment and MRI scanning?. Br J Neurosurg. 2007 Apr. 21(2):201-3.
- Thakur JD, Storey C, Kalakoti P, Ahmed O, Dossani RH, Menger RP, Sharma K, Sun H, Nanda A. Early intervention in cauda equina syndrome associated with better outcomes: a myth or reality? Insights from the Nationwide Inpatient Sample database (2005-2011). Spine J. 2017 Oct;17(10):1435-1448.
- Todd NV. Guidelines for cauda equina syndrome. Red flags and white flags. Systematic review and implications for triage. Br J Neurosurg. 2017 Jun;31(3):336-339.
- Olivero WC, Wang H, Hanigan WC, et al. Cauda equina syndrome (CES) from lumbar disc herniations. J Spinal Disord Tech. 2009 May. 22(3):202-6.
- Cauda Equina and Conus Medullaris Syndromes Treatment & Management. https://emedicine.medscape.com/article/1148690-treatment
- Weninger P, Schultz A, Hertz H. Conservative management of thoracolumbar and lumbar spine compression and burst fractures: functional and radiographic outcomes in 136 cases treated by closed reduction and casting. Arch Orthop Trauma Surg. 2009 Feb. 129(2):207-19.
- Delamarter RB, Sherman JE, Carr JB. 1991 Volvo Award in experimental studies. Cauda equina syndrome: neurologic recovery following immediate, early, or late decompression. Spine (Phila Pa 1976). 1991 Sep. 16(9):1022-9.
- McCarthy MJ, Aylott CE, Grevitt MP, Hegarty J. Cauda equina syndrome: factors affecting long-term functional and sphincteric outcome. Spine (Phila Pa 1976). 2007 Jan 15. 32(2):207-16.
- Gleave JR, MacFarlane R. Prognosis for recovery of bladder function following lumbar central disc prolapse. Br J Neurosurg. 1990. 4(3):205-9.
- Cruz F, Herschorn S, Aliotta P, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: a randomised, double-blind, placebo-controlled trial. Eur Urol. 2011 Oct. 60(4):742-50.