Striae gravidarum
Striae gravidarum are pregnancy stretch marks, which are fine atrophic linear scars on your body that occur from tissue under your skin tearing from rapid growth or over-stretching. Striae gravidarum is the second most common skin condition after skin pigmentation during pregnancy 1. Striae gravidarum affects between 55% 2 and 90% of women 3. Striae gravidarum does not cause any significant medical problems but can be of cosmetic concern for some women. Striae gravidarum can cause emotional and psychological distress for many women 4.
The cause of striae gravidarum is uncertain, but it is assumed that changes in the structure of collagen, mediated by hormonal changes during pregnancy, are the causative factor. These pregnancy stretch marks normally appear during the third trimester of pregnancy and disappear several months after delivery. The most significant risk factors for striae gravidarum during pregnancy identified in this review 4 are younger age, maternal and family history of striae gravidarum, increased pre-pregnancy and pre-delivery weight, and increased birth weight. For prevention of striae gravidarum, creams with Centella asiatica extract such as Trofolastin cream and a daily massage seem the most supported treatment options by the literature, but further studies are necessary. This information can be helpful for future expectant mothers who would like to try preventative treatments for striae gravidarum. With regard to the treatment of striae gravidarum, skin rubbing of almond oil as well as extracts from the Centella tree have proved to be of limited therapeutic value. The current most effective therapies for striae gravidarum include tretinoin cream ≥ 0.05% and modalities such as nonablative fractional lasers. Laser treatment appears to yield on average greater mean improvement and in a much shorter time than topical treatments, but no head-to-head studies have been conducted to date. Tretinoin cream and laser treatments resulted in increased elastin content and collagen production in the treated lesions, which can partly explain the improvement observed. Many new studies that test novel laser treatments, microdermabrasion, and microneedling are underway.
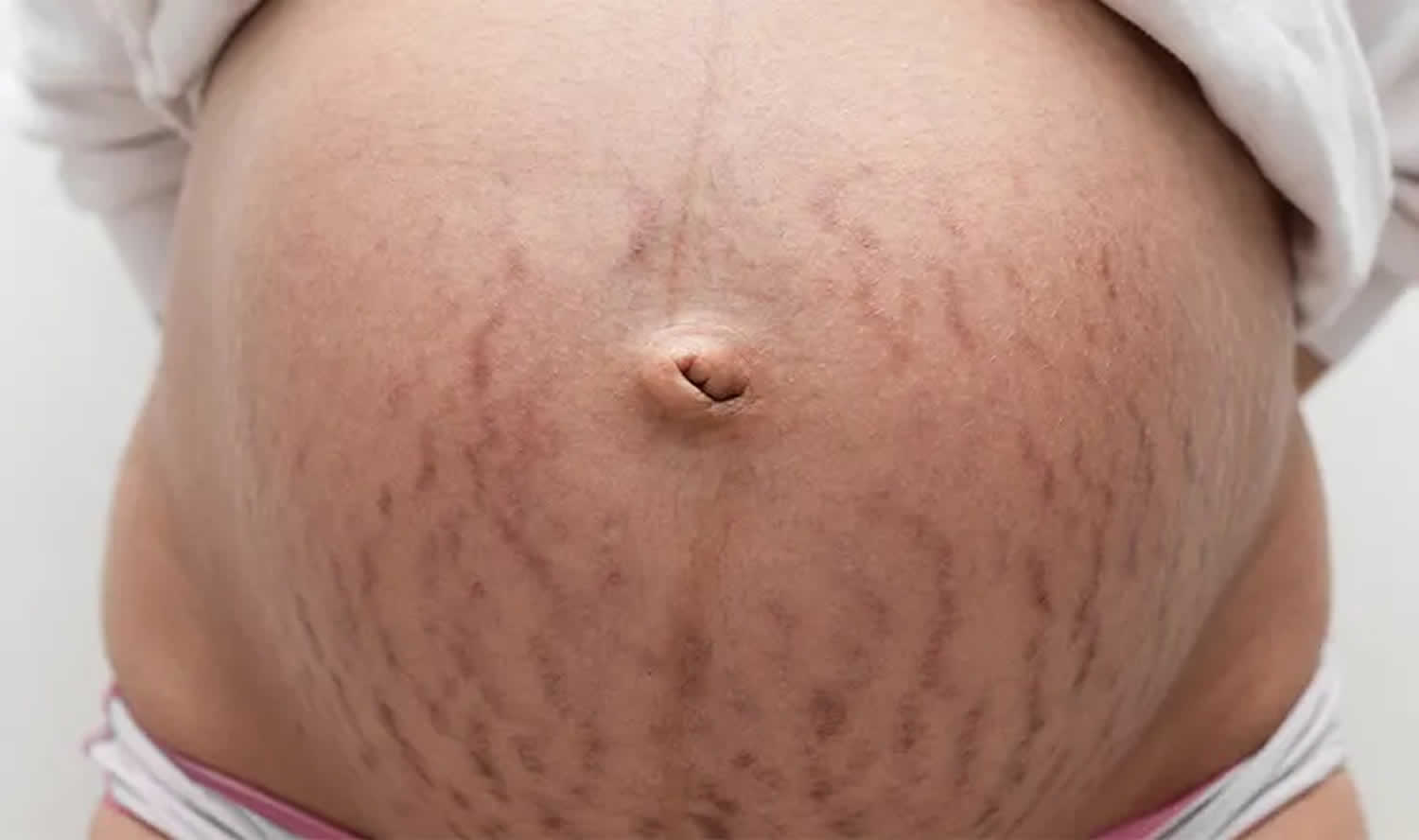
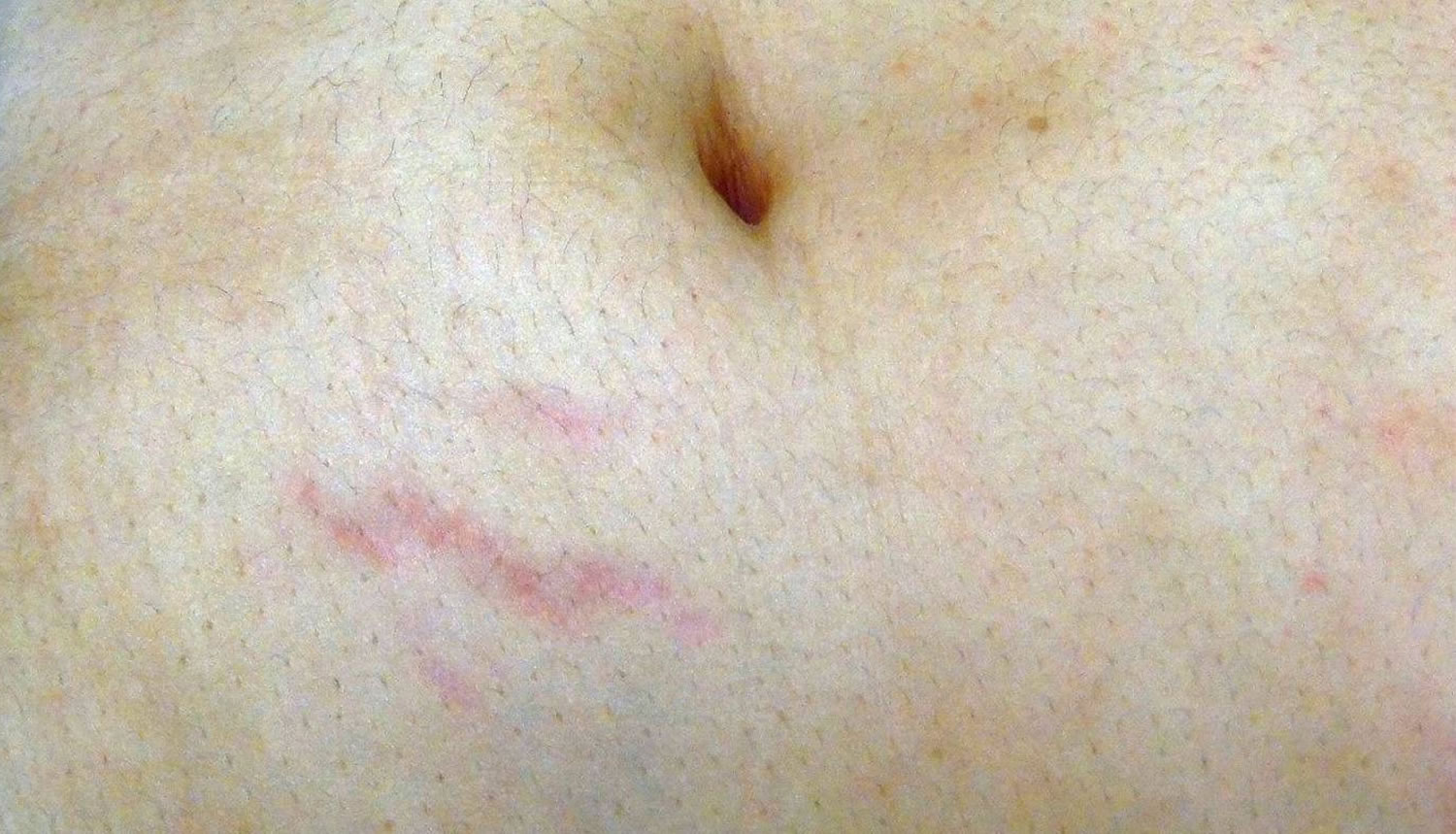
Figure 1. Immature striae gravidarum (striae rubra) on the abdomen
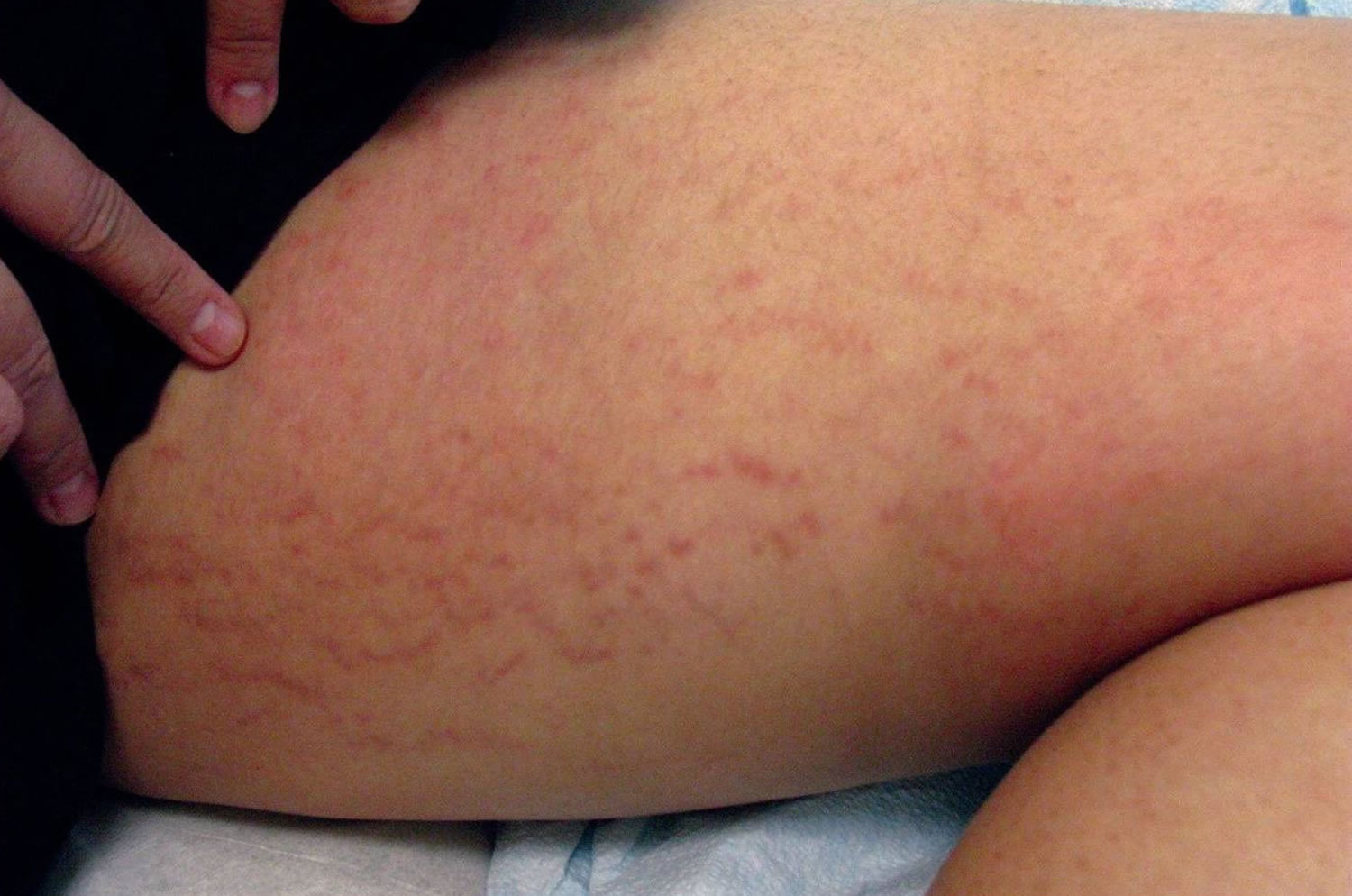
[Source 4 ]Figure 2. Immature striae gravidarum (striae rubra) on the thigh
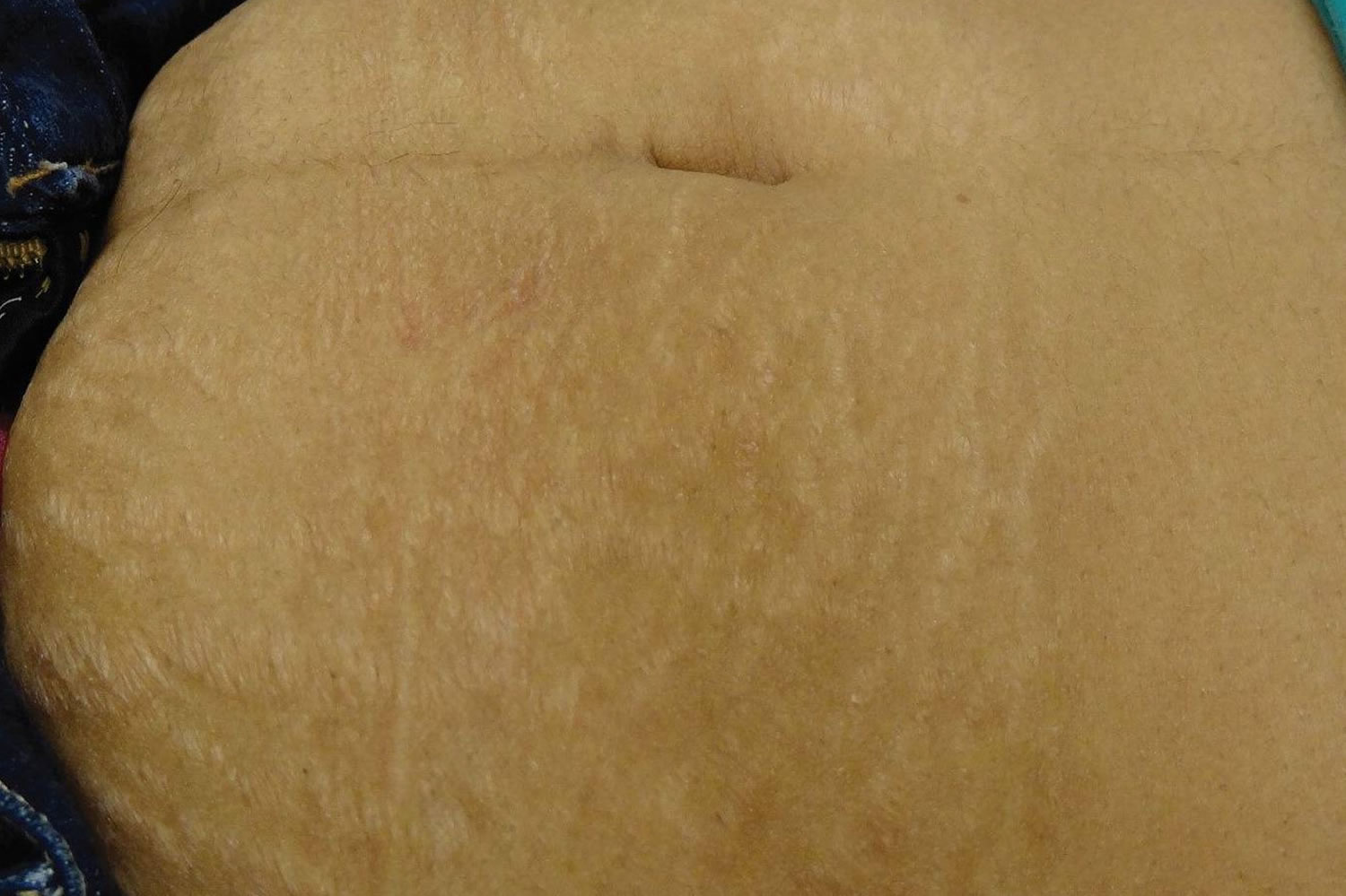
Figure 3. Mature striae gravidarum (striae alba) on the abdomen
[Source 4 ]Striae gravidarum causes
The cause of striae gravidarum during pregnancy involves a combination of genetic factors 5, hormonal factors 6 and increased mechanic stress on connective tissue 7. Interestingly, skin stretch has been a controversial trigger as studies have demonstrated an inconsistent association of striae gravidarum with maternal weight gain and abdominal and hip girth stretch 8. With regard to hormonal factors, twice as many estrogen receptors and elevated androgen and glucocorticoid receptors have been observed in striae compared with those in healthy skin 9. Pregnancy’s distinct hormonal environment is thought to influence connective tissue that is susceptible to striae gravidarum when stretched. Ultimately, abnormalities in elastic fibers 10, collagen fibrils 11 and other extracellular membrane components 12 are believed to underlie the pathogenesis of striae gravidarum 13.
Risk factors for developing striae gravidarum during pregnancy
The most common risk factors for striae gravidarum include younger age, maternal and family history of striae gravidarum, higher pre-pregnancy and pre-delivery weight, and higher birth weight (see Table 1). Most studies showed a statistically significant association between these risk factors and striae gravidarum, although Findik et al. 14 and Chang et al. 15 did not confirm pre-pregnancy weight or maternal age as a risk factor. Most studies also demonstrated that a history of striae (stretch marks) on the breasts, hips, and thighs was associated with formation of striae gravidarum; however, a study of 299 Caucasian women showed that although striae on the breasts increased risk of striae gravidarum, striae on the thighs decreased the risk of striae gravidarum. The study by Chang et al. 15 found a higher prevalence of striae gravidarum in non-white women. With regard to socioeconomic status, multiple studies showed that unemployment, receiving state medical assistance, and lower education level were also associated with striae gravidarum. However, confounding factors should be considered. Increased alcohol intake, decreased water consumption, decreased blood vitamin C levels, and expecting a male baby were also found to be more common among those women who developed striae gravidarum in select studies 4. Even though it has been speculated that diabetes and increased serum glucose levels could play a part in the pathogenesis of striae gravidarum, the studies included here did not reveal an association with diabetes or glycosylated hemoglobin levels. Studies were limited by study type, size, and patient population 4.
Table 1. Risk factors for striae gravidarum
| Investigators and Study Type | Number of Subjects and Subject profiles | Risk Factors Identified⁎ | Treatments |
| Picard et al. 16 Cross-sectional study | 800 primiparous women examined postpartum with mean age of 26.3 years | ▪ Younger age ▪ Higher pre-pregnancy weight ▪ Body mass index ▪ Higher weight at delivery ▪ Higher gestational weight gain ▪ Fitzpatrick skin types 1 and 4 ▪ Absence of employment ▪ Family history of striae gravidarum | Topical treatments to reduce the occurrence of striae gravidarum were not found to be effective |
| Kasielska-Trojan et al. 17 Cross-sectional study | 299 Caucasian women up to 6 months after delivery, without distinguishing primiparous or multiparas. | ▪ Previous history of striae gravidarum ▪ Family history of striae gravidarum ▪ Higher BMI before pregnancy ▪ Lack of chronic diseases ▪ Higher birthweight ▪ Striae distensae (stretched skin) on the breasts increased risk (71.4% with striae on breasts vs. 28.6% without) ▪ Striae distensae (stretched skin) on thighs decreased risk (23% with striae vs. 77% without striae) | Progesterone treatment was not found to be related to striae gravidarum |
| J-Orh et al. 18 Cross-sectional study | 280 Thai women who had just given birth to first child, in the immediate postpartum period. | ▪ Younger age (22.8 yr vs. 26.6 yr) ▪ Higher pre-pregnancy BMI (21.2 kg/m2 vs. 19.8 kg/m2) ▪ Higher maternal BMI at pregnancy (27.3 kg/m2 vs. 25.6 kg/m2) ▪ Higher gestational age at delivery (39.1 wk vs. 38.6 wk) ▪ Higher birth weight of baby (3,078.8 g vs. 2,895.8) ▪ Alcohol drinker (91.4% vs. 8.6%) ▪ Had little water intake (7.4 glasses vs. 8.3 glasses) ▪ Family history of striae gravidarum (82.8% vs. 17.2%) | Did not assess |
| Osman et al. 19 Cross-sectional study | 112 primiparous Lebanese women assessed during the immediate postpartum period | ▪ Younger age (26.5 yr vs. 30.5) ▪ Increased weight gain during pregnancy (15.6 kg vs. 38.4 kg) ▪ Birth weight, gestational age at delivery, and family history of striae gravidarum associated with moderate-to-severe striae gravidarum | Did not assess |
| Atwal et al. 8 Cross-sectional study with questionnaire | 309 primiparous women within 48 hours of delivery | ▪ Most significant was low maternal age ▪ 20% (14 of 71) of teenagers had severe striae, not seen in ♀ over 30 yr of age. ▪ Pre-pregnancy BMI greater than 26 ▪ Maternal weight gain of more than 15 kg ▪ High neonatal birth weight | Did not assess |
| Chang et al. 15 Cross-sectional study with anonymous survey | 161 women who had just given birth | ▪ Most significant was a history of breast or thigh striae (81% who developed striae gravidarum had striae history vs. 31% without striae gravidarum who had history of striae) ▪ Having a mother with striae gravidarum ▪ Additional family history (sisters, daughters, grandmothers, aunts, cousins) of striae gravidarum ▪ Non-white ♀ had higher association with striae gravidarum (odds ratio = 4.2). ▪ Pre-pregnancy BMI not significantly different | Did not assess |
| Ersoy et al. 20 Prospective observational study | 211 singleton primiparous pregnant women who were hospitalized for birth and who did not have systemic diseases or other risk factors, like drug use or polyhydramnios. | ▪ Younger age ▪ Higher pre-conceptional BMI ▪ Family history ▪ Having a male baby ▪ Lower educational level ▪ Smoking status, skin type, water intake, and level of financial income did not significantly predict striae gravidarum | Use of preventive oil or drugs, did not affect development of striae gravidarum |
| Findik et al. 14 Prospective study | 69 primigravidas using prophylactic iron and vitamin preparations at 36 weeks gestation or greater | ▪ Family history ▪ Reduced blood vitamin C levels ▪ No significant relation with age, weight gain during pregnancy, abdominal/thigh circumference, or smoking status | Did not assess |
| Thomas and Liston 21 Prospective observational study | 128 primigravid women who presented in labor or for induction of labor | ▪ Younger age ▪ Higher pre-delivery BMI ▪ Higher baby weight | Did not assess |
| Davey 22 Prospective study | 76 primiparous women | ▪ Younger age ▪ Higher weight ▪ Higher baby weight | Striae gravidarum were less common in skin messaged with olive oil |
| Madlon-Kay 23 Retrospective cohort study | 48 nulliparous women at 34 to 36 weeks’ estimated gestational age | ▪ Younger age ▪ More likely to receive state medical assistance ▪ More likely to have hip striae ▪ Greater weight gain during pregnancy ▪ Diabetes tests and glycosylated hemoglobin levels were similar in ♀ with and without striae. | ♀ who used oils or creams formed striae as frequently as those who did not |
Abbreviations: ♀=women; BMI = body-mass index; mos = months; SG = striae gravidarum; yrs = years.
Striae gravidarum prevention
Preventative treatments have met with limited success. Creams that contain Centella asiatica extract, especially Trofolastin cream, are best supported by data for the prevention or reduction of the severity of striae gravidarum (see Table 2) 24. Centella asiatica is a medicinal herb that is thought to increase the production of collagen and elastic fibers 24. Mallol et al. 25 demonstrated that Trofolastin cream with Centella asiatica extract, α-tocopherol, and collagen–elastin hydrolysates that is applied daily from gestational week 12 until delivery significantly reduced the incidence of striae gravidarum compared with placebo. Both Mallol et al. 25 and García Hernández et al. 24 found that creams that contained Centella asiatica significantly reduced the intensity and/or severity of striae gravidarum among women who did develop striae gravidarum. García Hernández et al. 24 also demonstrated that the severity of previous striae significantly increased in the patient group treated with placebo but did not change in the patient group treated with Centella cream.
The application of almond oil, olive oil, or cocoa butter consistently failed to significantly lower the incidence of striae gravidarum compared with placebo group. Two studies did find that when olive oil or almond oil were applied with a massage daily, they were associated with a lower incidence of striae gravidarum development. However, these results may reflect the benefits of massage alone 26.
Alphastria cream and verum cream, two proprietary creams that contain hyaluronic acid combined with various vitamins and fatty acids, were shown to significantly lower the incidence of striae gravidarum in two studies 27. Hyaluronic acid, the active ingredient in both creams, is thought to increase resistance to mechanical forces and oppose atrophy through stimulation of fibroblast activity and collagen production 28. In both studies, the creams were applied through massage during the second trimester, which poses the question of whether the creams were truly beneficial or whether the results reflected the benefits of massage alone.
Table 2. Striae gravidarum prevention
| Investigators and Study Type | Number of Subjects and Subject Profiles | Preventive Methods Used | Results of Preventive Methods⁎ |
| Mallol et al. 25 Randomized, double-blind, placebo-controlled study | 80 pregnant ♀ during their first 12 weeks of a healthy pregnancy | Trofolastin cream with Centella asiatica extract, α-tocopherol and collagen–elastin hydrolysates; applied daily from 12th week of pregnancy until delivery | ▪ Development of striae gravidarum: 56% in placebo group vs. 34% in treatment group ▪ Intensity of striae gravidarum was significantly lower in ♀ treated with cream vs. placebo ▪ In ♀ with history of striae during puberty, cream prevented striae gravidarum in 89% of cases, whereas all ♀ formed striae gravidarum in the placebo group |
| García Hernández et al. 24 Randomized, double-blind, placebo-controlled study | 183 pregnant patients over age 18 at week 12 +/- 2 | Cream containing hydroxyprolisilane-C, rosehip oil, Centella asiatica triterpenes and vitamin E; applied twice a day around 12 weeks of pregnancy | ▪ Effective in preventing striae gravidarum only in ♀ without a history of striae (6% developed striae gravidarum on treatment vs. 35% on placebo) ▪ Severity of previous stretch marks increased in the control group during the study, but not in the treated group ▪ Among ♀ who developed new striae gravidarum, there was increased severity in control vs. treated group |
| Taavoni et al. 29 Randomized clinical study | 70 nulliparous ♀ aged between 20-30 yrs old, in 18–20th week of gestation with BMI ranging between 18.5-25. 35 used treatment, 35 did not | Olive oil applied topically onto abdomen twice daily, without massaging, vs. no olive oil | ▪ Striae gravidarum occurred at the end of the second quarter of pregnancy in 45.7% in intervention group vs. 62.9% in control group (p = 0.115) ▪ Difference NOT statistically significant |
| Soltanipoor et al. 30 Randomized controlled clinical study | 100 nulliparous pregnant ♀; 50 used treatment, 50 did not | Olive oil applied topically onto abdomen twice daily, without massaging, vs. no olive oil | ▪ Frequency of severe striae gravidarum was lower in group that used olive oil. ▪ Difference NOT statistically significant |
| de Buman et al 31 Randomized controlled study | 90 pregnant ♀; 30 received treatment cream, 30 received vitamin cream, 30 received placebo | Alphastria cream (hyaluronic acid, allantoin, vitamin A, vitamin E and calcium pantothenate) vs. vitamin cream vs. placebo cream; messaged for a few minutes daily to the thighs, abdomen and chest, starting at the 3rd month of pregnancy and ending 3 mos after childbirth | ▪ Striae gravidarum developed in 10% of alphastria treated group vs. 40% of vitamin treated group vs. 37% in placebo treated group |
| Wierrani et al. 27 Randomized controlled study | 50 pregnant ♀; 24 received treatment, 26 did not receive any treatment | Verum cream (vitamin E, essential fatty acids, panthenol, hyaluronic acid, elastin and menthol); massaged onto the abdomen, thighs and breasts starting at the 20th week of pregnancy | ▪ Striae gravidarum developed in 29% of verum treated group vs. 62% in no treatment group |
| Soltanipour et al. 32 Parallel randomized controlled clinical study | 150 nulliparous ♀ at their second trimester of pregnancy in Iran. 50 subjects in each group | Olive oil vs. Saj® cream that contains lanolin, stearin, triethanolamine, almond oil, and bizovax glycerin amidine vs. placebo | ▪ Striae gravidarum occurred in 72% of olive oil group vs. 64% in Saj® cream group vs. 60% in control group. ▪ Differences NOT statistically significant |
| Osman et al. 33 Randomized double-blind placebo-controlled study | 175 nulliparous ♀ in Lebanon with singleton pregnancies between week 12 and 18 weeks of gestation. 91 with study treatment, 84 with placebo | Cocoa butter lotion vs. placebo lotion, daily from weeks 12-18 | ▪ Striae gravidarum developed in 45% of patients using cocoa butter cream vs. 48% using placebo ▪ Difference NOT statistically significant |
| Buchanan et al. 34 Randomized, double-blind, placebo-controlled study | 300 pregnant ♀; 150 received treatment, 150 placebo | Cocoa butter lotion vs. placebo lotion, daily from 16 weeks to delivery | ▪ Striae gravidarum developed in 44% of patients using cocoa butter cream vs. 55% using placebo ▪ Difference NOT statistically significant |
| Timur Taşhan and Kafkasli 26 Posttest-only quasi-experimental design with a control group | 141 primiparous ♀ who visited the pregnancy unit in Turkey between February 1st, 2010 and April 15th, 2011. 47 subjects in oil + massage, 48 in oil – massage, 46 in control | Bitter almond oil applied with or without massage vs. control; applied every other day in weeks 19–32 of pregnancy, followed by daily until delivery | ▪ Frequency of striae gravidarum: 20% among ♀ who applied oil with massage vs. 38.8% among those who applied without massage vs. 41.2% in control group |
| Poidevin 35 Prospective study | 116 primigravidas;♀ 55 used treatment, 66 did not | Olive oil vs. no olive oil applied daily | ▪ Striae gravidarum developed in 68% of olive oil group vs. 55% not using olive oil. ▪ Difference NOT statistically significant |
| Davey 22 Prospective study | 76 primiparous ♀; 35 used treatment, 41 did not | Olive oil massaged into abdomen daily vs. no olive oil | ▪ The prophylactic use of olive oil to massage the abdomen was associated with a lower incidence of striae gravidarum |
Abbreviations: ♀=women; BMI = body-mass index; mos = months; SG = striae gravidarum; yrs = years.
Striae gravidarum signs and symptoms
Striae gravidarum first present as flat, pink-to-red bands (striae rubra or immature striae) that become raised, longer, wider, and violet-red (Figures 1 and 2). Over a period of months to years, the stretch marks fade and become hypopigmented (striae alba or mature striae), appearing parallel to skin tension lines as scar-like, wrinkled, white, and atrophic marks (Figure 3) 36. Striae gravidarum can cause itching, burning, and discomfort and typically present on the breasts, abdomen, hips, and thighs. Up to 90% of striae gravidarum appear in a woman who is pregnant for the first time (primigravida) 15. Onset has typically been reported in the late second and early third trimester; however, one study has demonstrated that 43% of women develop striae gravidarum prior to 24 weeks of gestation 15.
Striae gravidarum treatment
Striae gravidarum treatment should be instituted during the early stages of striae gravidarum rather than when striae have matured and permanent changes have occurred 4. Many homeopathic and alternative therapies, including fruit and vegetable oils that hydrate the skin, are employed but limited by insufficient evidence.
Table 3. Striae gravidarum treatment options
| Investigators and Study Type | Number of Subjects and Subject Profiles | Type of Striae | Treatment | Efficacy⁎ | Adverse Effects |
| Malekzad et al. 37 Prospective pilot study | 10 ♀ aged 26-50, Fitzpatrick skin types 3 to 5 | Striae alba | 1540-nm non-ablative fractional laser | ▪ Clinical improvement in striae ranging from 1-24% ▪ Improvement between the 4-week treatment and the 16-week treatment was identified ▪ 3 mos after final treatment, patients had observable improvement in the striae, compared with baseline | Mild post-inflammatory hyperpigmentation in one patient after 8-week treatment and mild acne in another patient after 4 weeks of treatment |
| Rangel et al. 38 Open-label, multicenter, prospective study | 26 ♀ with abdominal pregnancy-related striae | Not reported | 0.1% tretinoin cream daily for 3 mos applied to SG | ▪ At treatment conclusion, global improvement was achieved from baseline in all stretch marks ▪ Pre-selected target lesion decreased in length by 20% and width by 23% | Erythema and scaling were the most common adverse events |
| Pribanich et al. 39 Double-blind placebo controlled study | 11 non-pregnant ♀ who had SG, 6 received treatment and 5 placebo | Not reported | 0.025% tretinoin cream applied daily for 7 mos | ▪ No statistically significant difference in treated group compared to control group | Did not assess |
| Kang et al. 40 Double-blind, randomized, vehicle-controlled study | 22 healthy white ♀ with erythematous stretch marks, 10 received treatment and 12 vehicle | Striae rubra | 0.1% tretinoin (n = 10) or vehicle (n = 12) daily for 6 mos to the affected areas | ▪ At 2 mos, tretinoin patients had significant improvements in severity scores of SG vs. vehicle patients ▪ At 6 mos, 80% of tretinoin patients had improvement vs. 8% of vehicle patients ▪ Targeted stretch marks treated with tretinoin had decrease in mean length and width of 14% and 8%, respectively, vs. an increase of 10% and 24%, respectively, in vehicle patients | Erythema and scaling, with itching and burning |
| Ash et al. 41 Randomized controlled study | 10 American nonpregnant ♀ of varying skin types, age 23 to 49 yr. Striae age ranged from 8 mos to 31 yr and all were white striae. All patients had abdominal striae, 50% also had striae on thighs | Striae alba | 20% glycolic acid + 0.05% tretinoin vs. 20% glycolic acid + 10% ascorbic acid, applied daily to abdomen or thighs for 12 weeks (each regimen was applied to half the treatment area) | ▪ At treatment conclusion, 47% improvement with 0.05% tretinoin vs. 43% improvement with ascorbic acid ▪ Results not statistically significant from each other, but significant for both compared to pretreatment ▪ 0.05% tretinoin increased elastin content at sites vs. untreated striae by 22% in the papillary and reticular dermis combined ▪ 10% L-ascorbic acid failed to improve elastin content in either the papillary or reticular dermis ▪ Both regimens increased epidermal thickness and decreased papillary dermal thickness | 70% of patients experienced mild irritation at treatment initiation on both treatment sites. A single patient developed a mild irritant dermatitis |
Abbreviations: ♀=women; BMI = body-mass index; mos = months; SG = striae gravidarum; yrs = years.
Topical medications
Tretinoin cream and a combination of 20% glycolic acid + 10% ascorbic acid were shown to improve striae gravidarum in clinical studies (see Table 3 above). Use of tretinoin 0.05% and 0.1% creams on a daily basis for 3 to 7 months consistently resulted in overall global improvement of striae gravidarum up to 47% 41 and decreased in mean length and width up to 20% and 23% respectively 38, of lesions. A study by Pribanich et al. 39 showed that the minimum effective concentration of tretinoin cream is 0.05%. Twenty percent glycolic acid combined with either 10% ascorbic acid or 0.05% tretinoin improved the appearance of striae gravidarum although there was no statistically significant difference between the two combinations 41. Tretinoin increased elastin content in the papillary and reticular dermis of the lesions but ascorbic acid and untreated areas did not show such improvement. Both treatments increased epidermal thickness and decreased papillary dermal thickness in striae gravidarum lesions.
Laser treatments
A 1540-nm non-ablative fractional laser demonstrated a statistically-significant clinical improvement in striae gravidarum that ranged from 1 to 24% and an observable difference at 3 months post-treatment37. For nongestational striae distensae (stretched skin), both fractional and non-fractional lasers have been employed with varying efficacies.
Among fractional lasers, both non-ablative Erbium (Er):Glass and ablative carbon dioxide (CO2) lasers have been studied. An average of 50 to 75% improvement in lesions after 2 to 6 nonablative Er:Glass treatments has been reported 42. Histologic studies showed an increase in elastic fibers and collagen production. This laser was generally safe and treatments were well-tolerated by patients. In a study by Lee et al. (2010), ablative CO2 lasers were demonstrated to have improvements of 50 to 75%, especially in striae alba. However, other studies have shown inconsistent results 43. CO2 lasers are more painful and may have longer recovery times than non-ablative lasers.
Among non-fractional lasers, excimer, pulsed dye, neodymium-doped yttrium aluminum garnet (Nd:YAG), copper bromide, and diode have been studied in the treatment of patients with nongestational striae distensae (stretched skin). The excimer 308-nm laser is used to treat mature striae alba by producing repigmentation and has achieved up to 75% increase in pigmentation; however, results are generally temporary and pigmentation of the normal surrounding skin is an unfavorable consequence 44. Pulsed dye laser results in textural improvements but has shown a limited benefit to treat striae alba 45. It may be beneficial in striae rubra by reducing erythema 46. Nd:YAG laser, also a vascular laser, demonstrated excellent improvement of up to 70% or more, even though it is specifically for immature striae rubra 47. Thirteen of 15 women experienced a complete resolution or modest improvement of striae for up to 2 years in a small study that used copper bromide laser 48. The diode laser was used to treat striae distensae (stretched skin) in dark-skinned individuals but this laser was ineffective and 64% of patients developed undesirable hyperpigmentation 49.
Light treatments
Light therapy modalities such as intense pulsed light (IPL), ultraviolet (UV) light, and infrared light have been employed for the treatment of nongestational striae distensae (stretched skin). Intense pulsed light (IPL) seems to result in at least moderate improvement of striae 50, but persistent erythema and post-inflammatory hyperpigmentation may complicate this treatment. UV light, especially a combination of UV-B and UV-A, has been shown to consistently repigment striae alba. However, the results are not permanent and maintenance treatment is required 51. Infrared light at 800 to 1800 nm can result in 25 to 50% improvement in striae alba after only four treatment sessions 52. Long-term studies with larger sample sizes are needed to confirm these results.
Other treatments
Bipolar radiofrequency demonstrated clinical and histologic improvements in striae distensae (stretched skin) 53, while tripolar third generation radiofrequency (Tripollar) resulted in 25 to 75% improvement at 1 week post-final treatment 54. Modalities such as microdermabrasion and microneedling have been found to be effective to improve nongestational striae in multiple studies. Microdermabrasion has been especially effective for striae rubra 55. Microdermabrasion involves the blowing and subsequent vacuuming of abrasive substances to a treated area. Another study found that although microdermabrasion with sonophoresis improved striae, needling therapy yielded an even greater, statistically-significant improvement in striae compared with microdermabrasion 56. Needling therapy causes controlled skin injury with the goal of producing new collagen and elastin in the papillary dermis.
References- Rabinerson D, Melzer H, Gabbay-Ben-Ziv R. [STRIAE GRAVIDARUM – ETIOLOGY, PREVALENCE AND TREATMENT]. Harefuah. 2018 Dec;157(12):787-790.
- Picard D., Sellier S., Houivet E., Marpeau L., Fournet P., Thobois B. Incidence and risk factors for striae gravidarum. J Am Acad Dermatol. 2015;73:699–700.
- Rathore SP, Gupta S, Gupta V. Pattern and prevalence of physiological cutaneous changes in pregnancy: a study of 2000 antenatal women. Indian J Dermatol Venereol Leprol. 2011 May-Jun;77(3):402. doi: 10.4103/0378-6323.79741
- Farahnik B, Park K, Kroumpouzos G, Murase J. Striae gravidarum: Risk factors, prevention, and management. Int J Womens Dermatol. 2016;3(2):77-85. Published 2016 Dec 6. doi:10.1016/j.ijwd.2016.11.001 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5440454
- Di Lernia V, Bonci A, Cattania M, Bisighini G. Striae distensae (rubrae) in monozygotic twins. Pediatr Dermatol. 2001 May-Jun;18(3):261-2. doi: 10.1046/j.1525-1470.2001.018003261.x
- Lurie S, Matas Z, Fux A, Golan A, Sadan O. Association of serum relaxin with striae gravidarum in pregnant women. Arch Gynecol Obstet. 2011 Feb;283(2):219-22. doi: 10.1007/s00404-009-1332-5
- Fitzpatrick T.B., Wolff K. McGraw-Hill Medical; New York: 2008. Fitzpatrick’s dermatology in general medicine.
- Atwal GS, Manku LK, Griffiths CE, Polson DW. Striae gravidarum in primiparae. Br J Dermatol. 2006 Nov;155(5):965-9. doi: 10.1111/j.1365-2133.2006.07427.x
- Cordeiro RC, Zecchin KG, de Moraes AM. Expression of estrogen, androgen, and glucocorticoid receptors in recent striae distensae. Int J Dermatol. 2010 Jan;49(1):30-2. doi: 10.1111/j.1365-4632.2008.04005.x
- Sheu HM, Yu HS, Chang CH. Mast cell degranulation and elastolysis in the early stage of striae distensae. J Cutan Pathol. 1991 Dec;18(6):410-6. doi: 10.1111/j.1600-0560.1991.tb01376.x
- Shuster S. The cause of striae distensae. Acta Derm Venereol Suppl (Stockh). 1979;59(85):161-9.
- Watson RE, Parry EJ, Humphries JD, Jones CJ, Polson DW, Kielty CM, Griffiths CE. Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol. 1998 Jun;138(6):931-7. doi: 10.1046/j.1365-2133.1998.02257.x
- Wang F, Calderone K, Smith NR, Do TT, Helfrich YR, Johnson TR, Kang S, Voorhees JJ, Fisher GJ. Marked disruption and aberrant regulation of elastic fibres in early striae gravidarum. Br J Dermatol. 2015 Dec;173(6):1420-30. doi: 10.1111/bjd.14027
- Findik RB, Hascelik NK, Akin KO, Unluer AN, Karakaya J. Striae gravidarum, vitamin C and other related factors. Int J Vitam Nutr Res. 2011 Jan;81(1):43-8. doi: 10.1024/0300-9831/a000049
- Chang AL, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol. 2004 Dec;51(6):881-5. doi: 10.1016/j.jaad.2004.05.030
- Picard D, Sellier S, Houivet E, Marpeau L, Fournet P, Thobois B, Bénichou J, Joly P. Incidence and risk factors for striae gravidarum. J Am Acad Dermatol. 2015 Oct;73(4):699-700. doi: 10.1016/j.jaad.2015.06.037
- Kasielska-Trojan A, Sobczak M, Antoszewski B. Risk factors of striae gravidarum. Int J Cosmet Sci. 2015 Apr;37(2):236-40. doi: 10.1111/ics.12188
- J-Orh R, Titapant V, Chuenwattana P, Tontisirin P. Prevalence and associate factors for striae gravidarum. J Med Assoc Thai. 2008 Apr;91(4):445-51.
- Osman H, Rubeiz N, Tamim H, Nassar AH. Risk factors for the development of striae gravidarum. Am J Obstet Gynecol. 2007 Jan;196(1):62.e1-5. doi: 10.1016/j.ajog.2006.08.044
- Ersoy E, Ersoy AO, Yasar Celik E, Tokmak A, Ozler S, Tasci Y. Is it possible to prevent striae gravidarum? J Chin Med Assoc. 2016 May;79(5):272-5. doi: 10.1016/j.jcma.2015.12.007
- Thomas RG, Liston WA. Clinical associations of striae gravidarum. J Obstet Gynaecol. 2004 Apr;24(3):270-1. doi: 10.1080/014436104101001660779
- Davey CM. Factors associated with the occurrence of striae gravidarum. J Obstet Gynaecol Br Commonw. 1972 Dec;79(12):1113-4. doi: 10.1111/j.1471-0528.1972.tb11896.x
- Madlon-Kay DJ. Striae gravidarum. Folklore and fact. Arch Fam Med. 1993 May;2(5):507-11. doi: 10.1001/archfami.2.5.507
- García Hernández JÁ, Madera González D, Padilla Castillo M, Figueras Falcón T. Use of a specific anti-stretch mark cream for preventing or reducing the severity of striae gravidarum. Randomized, double-blind, controlled trial. Int J Cosmet Sci. 2013 Jun;35(3):233-7. doi: 10.1111/ics.12029
- Mallol J, Belda MA, Costa D, Noval A, Sola M. Prophylaxis of Striae gravidarum with a topical formulation. A double blind trial. Int J Cosmet Sci. 1991 Feb;13(1):51-7. doi: 10.1111/j.1467-2494.1991.tb00547.x
- Timur Taşhan S, Kafkasli A. The effect of bitter almond oil and massaging on striae gravidarum in primiparaous women. J Clin Nurs. 2012 Jun;21(11-12):1570-6. doi: 10.1111/j.1365-2702.2012.04087.x
- Wierrani F, Kozak W, Schramm W, Grünberger W. Versuch einer vorbeugenden Behandlung der Striae gravidarum mittels prophylaktischer Massagesalbenapplikation [Attempt of preventive treatment of striae gravidarum using preventive massage ointment administration]. Wien Klin Wochenschr. 1992;104(2):42-4. German.
- Korgavkar K, Wang F. Stretch marks during pregnancy: a review of topical prevention. Br J Dermatol. 2015 Mar;172(3):606-15. doi: 10.1111/bjd.13426
- Taavoni S, Soltanipour F, Haghani H, Ansarian H, Kheirkhah M. Effects of olive oil on striae gravidarum in the second trimester of pregnancy. Complement Ther Clin Pract. 2011 Aug;17(3):167-9. doi: 10.1016/j.ctcp.2010.10.003
- Soltanipoor F, Delaram M, Taavoni S, Haghani H. The effect of olive oil on prevention of striae gravidarum: a randomized controlled clinical trial. Complement Ther Med. 2012 Oct;20(5):263-6. doi: 10.1016/j.ctim.2012.05.001
- de Buman M, Walther M, de Weck R. Wirksamkeit der Alphastria-Creme bei der Vorbeugung von Schwangerschaftsstreifen (Striae distensae). Ergebnisse einer Doppelblind-Studie [Effectiveness of Alphastria cream in the prevention of pregnancy stretch marks (striae distensae). Results of a double-blind study]. Gynakol Rundsch. 1987;27(2):79-84. German.
- Soltanipour F, Delaram M, Taavoni S, Haghani H. The effect of olive oil and the Saj® cream in prevention of striae gravidarum: A randomized controlled clinical trial. Complement Ther Med. 2014 Apr;22(2):220-5. doi: 10.1016/j.ctim.2013.11.011
- Osman H, Usta IM, Rubeiz N, Abu-Rustum R, Charara I, Nassar AH. Cocoa butter lotion for prevention of striae gravidarum: a double-blind, randomised and placebo-controlled trial. BJOG. 2008 Aug;115(9):1138-42. doi: 10.1111/j.1471-0528.2008.01796.x
- Buchanan K, Fletcher HM, Reid M. Prevention of striae gravidarum with cocoa butter cream. Int J Gynaecol Obstet. 2010 Jan;108(1):65-8. doi: 10.1016/j.ijgo.2009.08.008
- POIDEVIN LO. Striae gravidarum. Their relation to adrenal cortical hyperfunction. Lancet. 1959 Sep 26;2(7100):436-9. doi: 10.1016/s0140-6736(59)90421-0
- Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006 Mar-Apr;24(2):97-100. doi: 10.1016/j.clindermatol.2005.10.008
- Malekzad F, Shakoei S, Ayatollahi A, Hejazi S. The Safety and Efficacy of the 1540nm Non-Ablative Fractional XD Probe of Star Lux 500 Device in the Treatment of Striae Alba: Before-After Study. J Lasers Med Sci. 2014 Fall;5(4):194-8.
- Rangel O, Arias I, García E, Lopez-Padilla S. Topical tretinoin 0.1% for pregnancy-related abdominal striae: an open-label, multicenter, prospective study. Adv Ther. 2001 Jul-Aug;18(4):181-6. doi: 10.1007/BF02850112
- Pribanich S, Simpson FG, Held B, Yarbrough CL, White SN. Low-dose tretinoin does not improve striae distensae: a double-blind, placebo-controlled study. Cutis. 1994 Aug;54(2):121-4.
- Kang S, Kim KJ, Griffiths CE, Wong TY, Talwar HS, Fisher GJ, Gordon D, Hamilton TA, Ellis CN, Voorhees JJ. Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol. 1996 May;132(5):519-26.
- Ash K, Lord J, Zukowski M, McDaniel DH. Comparison of topical therapy for striae alba (20% glycolic acid/0.05% tretinoin versus 20% glycolic acid/10% L-ascorbic acid). Dermatol Surg. 1998 Aug;24(8):849-56. doi: 10.1111/j.1524-4725.1998.tb04262.x
- Tretti Clementoni M, Lavagno R. A novel 1565 nm non-ablative fractional device for stretch marks: A preliminary report. J Cosmet Laser Ther. 2015 Jun;17(3):148-55. doi: 10.3109/14764172.2015.1007061
- Cho SB, Lee SJ, Lee JE, Kang JM, Kim YK, Oh SH. Treatment of striae alba using the 10,600-nm carbon dioxide fractional laser. J Cosmet Laser Ther. 2010 Jun;12(3):118-9. doi: 10.3109/14764171003706117
- Goldberg DJ, Sarradet D, Hussain M. 308-nm Excimer laser treatment of mature hypopigmented striae. Dermatol Surg. 2003 Jun;29(6):596-8; discussion 598-9. doi: 10.1046/j.1524-4725.2003.29144.x
- McDaniel DH, Ash K, Zukowski M. Treatment of stretch marks with the 585-nm flashlamp-pumped pulsed dye laser. Dermatol Surg. 1996 Apr;22(4):332-7. doi: 10.1111/j.1524-4725.1996.tb00326.x
- Aldahan AS, Shah VV, Mlacker S, Samarkandy S, Alsaidan M, Nouri K. Laser and Light Treatments for Striae Distensae: A Comprehensive Review of the Literature. Am J Clin Dermatol. 2016 Jun;17(3):239-56. doi: 10.1007/s40257-016-0182-8
- Goldman A, Rossato F, Prati C. Stretch marks: treatment using the 1,064-nm Nd:YAG laser. Dermatol Surg. 2008 May;34(5):686-91; discussion 691-2. doi: 10.1111/j.1524-4725.2008.34129.x
- Longo L, Postiglione MG, Marangoni O, Melato M. Two-year follow-up results of copper bromide laser treatment of striae. J Clin Laser Med Surg. 2003 Jun;21(3):157-60. doi: 10.1089/104454703321895617
- Tay YK, Kwok C, Tan E. Non-ablative 1,450-nm diode laser treatment of striae distensae. Lasers Surg Med. 2006 Mar;38(3):196-9. doi: 10.1002/lsm.20281
- Al-Dhalimi MA, Abo Nasyria AA. A comparative study of the effectiveness of intense pulsed light wavelengths (650 nm vs 590 nm) in the treatment of striae distensae. J Cosmet Laser Ther. 2013 Jun;15(3):120-5. doi: 10.3109/14764172.2012.748200
- Sadick NS, Magro C, Hoenig A. Prospective clinical and histological study to evaluate the efficacy and safety of a targeted high-intensity narrow band UVB/UVA1 therapy for striae alba. J Cosmet Laser Ther. 2007 Jun;9(2):79-83. doi: 10.1080/14764170701313767
- Trelles MA, Levy JL, Ghersetich I. Effects achieved on stretch marks by a nonfractional broadband infrared light system treatment. Aesthetic Plast Surg. 2008 May;32(3):523-30. doi: 10.1007/s00266-008-9115-0
- Montesi G, Calvieri S, Balzani A, Gold MH. Bipolar radiofrequency in the treatment of dermatologic imperfections: clinicopathological and immunohistochemical aspects. J Drugs Dermatol. 2007 Sep;6(9):890-6.
- Manuskiatti W, Boonthaweeyuwat E, Varothai S. Treatment of striae distensae with a TriPollar radiofrequency device: a pilot study. J Dermatolog Treat. 2009;20(6):359-64. doi: 10.3109/09546630903085278
- Abdel-Latif A.M., Elbendary A. Treatment of striae distensae with microdermabrasion: A clinical and molecular study. J Egypt Wom Dermatol Soc. 2008;5:24–30
- Nassar A, Ghomey S, El Gohary Y, El-Desoky F. Treatment of striae distensae with needling therapy versus microdermabrasion with sonophoresis. J Cosmet Laser Ther. 2016 Oct;18(6):330-4. doi: 10.1080/14764172.2016.1175633