Systemic candidiasis
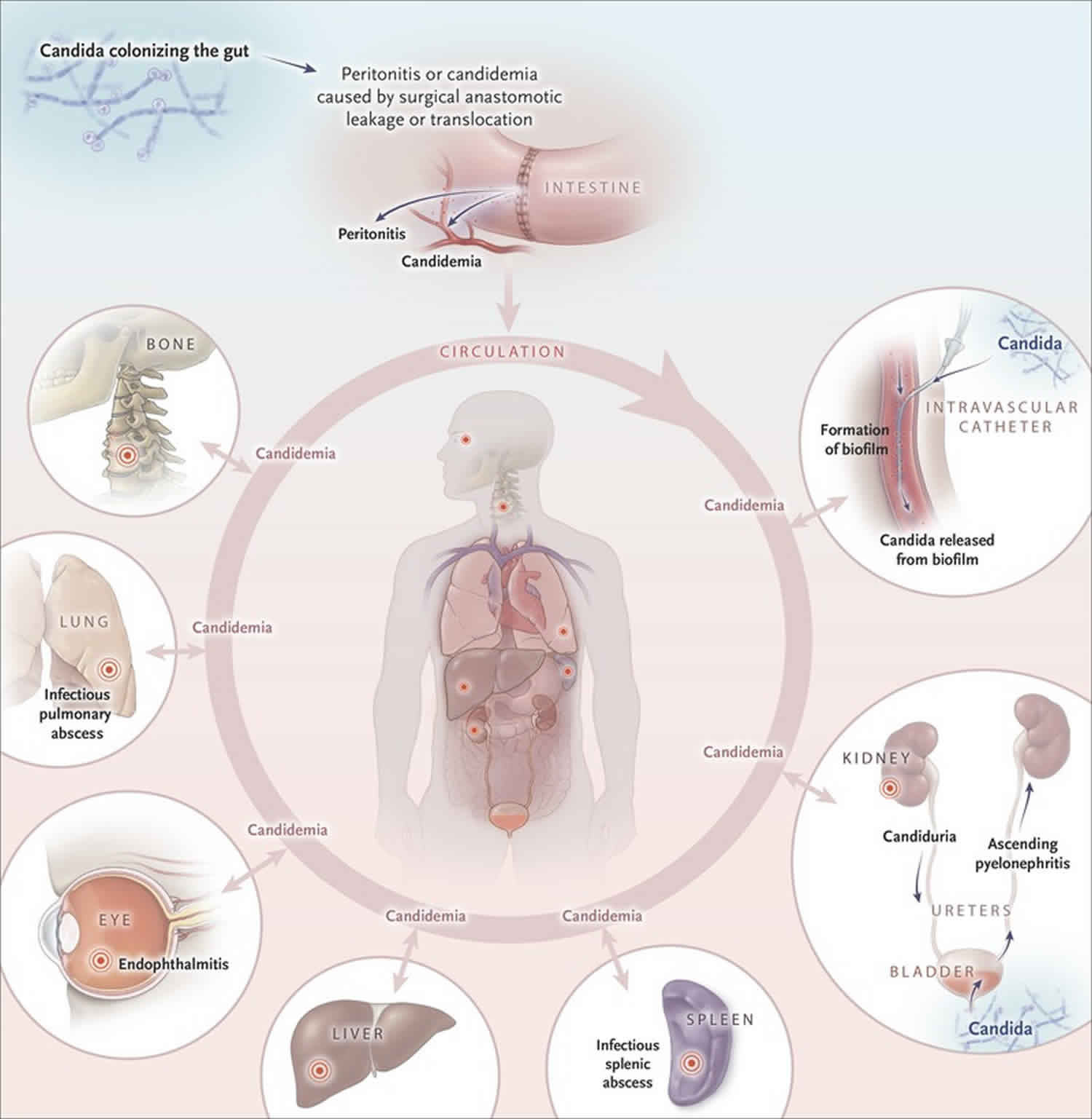
Systemic candidiasis includes a spectrum of yeast infections caused by different species (types) of Candida 1. Systemic candidiasis is a serious infection that can affect the blood, heart, brain, eyes, bones, or other parts of the body 2. Although there are over 200 species of Candida, five different species of Candida cause 90% of systemic candidiasis 2. Although mucocutaneous fungal infections, such as oral thrush and candidaesophagitis, are extremely common in patients with AIDS, candidemia (Candida infection in the blood) and disseminated candidiasis are uncommon. The most common form of this invasive yeast infection is when Candida enters the bloodstream (candidemia). Signs of candidemia include fever and chills that do not improve with antibiotics. Symptoms of other types of systemic candidiasis depend on the organ or system which is infected. Systemic candidiasis is the most common fungal infection among hosptilized people in high-income countries, including the United States. Diagnosis can be difficult, especially when the Candida is not found in the bloodstream 1. Treatment usually includes consists of oral or intravenous (IV) antifungal medications 3.
Systemic candidiasis causes
Systemic candidiasis is caused by Candida yeast. Although there are over 200 species (types), the five most common species of Candida to cause systemic infections include Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis, and Candida krusei. Candida normally live in the digestive tract (gastroinestinal system) and on skin and usually do not cause any problems. However if Candida enters the bloodstream, the yeast may overgrow. The overgrowth of Candida may also spread or occur in other parts of the body, including the liver, spleen, heart, eyes, lining of the abdomen and abdominal organs (peritoneum), kidney, bone, lungs and covering of the spinal cord and brain (meninges) 2.
People who may have an increased risk for systemic candidiasis include 2.:
- People who have a weakened immune system (for example, people who have had an organ transplant, have HIV/AIDS, or are on cancer chemotherapy)
- Intensive care unit (ICU) patients
- People who have had surgery, especially gastrointestinal surgery
- People who have a central venous catheter
- Very low-birth-weight infants
- People who have taken broad-spectrum antibiotics
- People who have a very low neutrophil (a type of white blood cell) count (neutropenia)
- People who have kidney failure or are on hemodialysis
- People who have diabetes
Systemic candidiasis symptoms
Common symptoms of candidemia (Candida infection of the bloodstream) include fever and chills that do not improve with antibiotics. Candidemia can cause septic shock and therefore may include symptoms such as low blood pressure, fast heart rate, and rapid breathing 3.
Systemic candidiasis may also affect other parts of the body such as the central nervous system (brain and spinal cord), abdomen, heart, kidneys, liver, bones, muscles, joints, spleen, and/or eyes 3. Candidemia may be present, but not in all cases 1. Signs and symptoms depend on the organ or system infected 1. For example, when Candida infects the eye, symptoms may include blurred vision with photophobia (the eye is overly senstive to light), whereas symptoms of candida endocarditis (Candida infection of the inner lining of the heart) may include fever, shortness of breath, fluid buildup in the arms or legs, tiny red spots on the skin, and weight loss 4. Since many people who develop systemic candidiasis are already sick, it can be difficult to know which symptoms are from Candida infection and which symptoms are from complications of other medical conditions 2.
Systemic candidiasis diagnosis
Systemic candidiasis is usually suspected in people who have an increased risk of developing an invasive Candida infection and have symptoms of an infection. Blood cultures (or cultures of other sterile fluids from the body such as cerebrospinal fluid) are then ordered to confirm the diagnosis 1.
However, in 40-50% of cases when systemic candidiasis affects another part of the body, the blood culture may be negative 2. In these cases, further testing will depend on which organ(s) or body system is infected 1. As of 2016, medical researchers are hoping T2 magnetic resonance testing will be able to more accurately and easily detect all forms of systemic candidiasis 5.
Systemic candida treatment
Systemic candidiasis is usually treated with oral or intravenous (IV) antifungal medications, including echinocandin (caspofungin, micafungin, or anidulafungin) fluconazole, and amphotericin B. The specific type of medication and length of treatment will depend on many factors, including the age and health of the infected person, the location and severity of the infection, and the specific species of Candida causing the infection 6
Antifungal medications may be given to certain high risk people in order to prevent the development of systemic candidiasis, however more studies are needed before it is clear if this practice is helpful 6.
Candidemia in nonneutropenic patients
Recommendations 6:
- An echinocandin (caspofungin: loading dose 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose 200 mg, then 100 mg daily) is recommended as initial therapy.
- Fluconazole, intravenous or oral, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily is an acceptable alternative to an echinocandin as initial therapy in selected patients, including those who are not critically ill and who are considered unlikely to have a fluconazole-resistant Candida species.
- Testing for azole susceptibility is recommended for all bloodstream and other clinically relevant Candida isolates. Testing for echinocandin susceptibility should be considered in patients who have had prior treatment with an echinocandin and among those who have infection with Candida glabrata or Candida parapsilosis.
- Transition from an echinocandin to fluconazole (usually within 5–7 days) is recommended for patients who are clinically stable, have isolates that are susceptible to fluconazole (eg, Candida albicans), and have negative repeat blood cultures following initiation of antifungal therapy.
- For infection due to Candida glabrata, transition to higher-dose fluconazole 800 mg (12 mg/kg) daily or voriconazole 200–300 (3–4 mg/kg) twice daily should only be considered among patients with fluconazole-susceptible or voriconazole-susceptible isolates.
- Lipid formulation amphotericin B (AmB) (3–5 mg/kg daily) is a reasonable alternative if there is intolerance, limited availability, or resistance to other antifungal agents.
- Transition from AmB to fluconazole is recommended after 5–7 days among patients who have isolates that are susceptible to fluconazole, who are clinically stable, and in whom repeat cultures on antifungal therapy are negative.
- Among patients with suspected azole- and echinocandin-resistant Candida infections, lipid formulation AmB (3–5 mg/kg daily) is recommended.
- Voriconazole 400 mg (6 mg/kg) twice daily for 2 doses, then 200 mg (3 mg/kg) twice daily is effective for candidemia, but offers little advantage over fluconazole as initial therapy (strong recommendation; moderate-quality evidence). Voriconazole is recommended as step-down oral therapy for selected cases of candidemia due to Candida krusei.
- All nonneutropenic patients with candidemia should have a dilated ophthalmological examination, preferably performed by an ophthalmologist, within the first week after diagnosis.
- Follow-up blood cultures should be performed every day or every other day to establish the time point at which candidemia has been cleared.
- Recommended duration of therapy for candidemia without obvious metastatic complications is for 2 weeks after documented clearance of Candida species from the bloodstream and resolution of symptoms attributable to candidemia.
- Central venous catheters should be removed as early as possible in the course of candidemia when the source is presumed to be the central venous catheter and the catheter can be removed safely; this decision should be individualized for each patient.
Candidemia in neutropenic patients
Recommendations 6:
- An echinocandin (caspofungin: loading dose 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose 200 mg, then 100 mg daily) is recommended as initial therapy.
- Lipid formulation AmB, 3–5 mg/kg daily, is an effective but less attractive alternative because of the potential for toxicity.
- Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, is an alternative for patients who are not critically ill and have had no prior azole exposure.
- Fluconazole, 400 mg (6 mg/kg) daily, can be used for step-down therapy during persistent neutropenia in clinically stable patients who have susceptible isolates and documented bloodstream clearance.
- Voriconazole, 400 mg (6 mg/kg) twice daily for 2 doses, then 200–300 mg (3–4 mg/kg) twice daily, can be used in situations in which additional mold coverage is desired. Voriconazole can also be used as step-down therapy during neutropenia in clinically stable patients who have had documented bloodstream clearance and isolates that are susceptible to voriconazole.
- For infections due to Candida krusei, an echinocandin, lipid formulation AmB, or voriconazole is recommended.
- Recommended minimum duration of therapy for candidemia without metastatic complications is 2 weeks after documented clearance of Candida from the bloodstream, provided neutropenia and symptoms attributable to candidemia have resolved.
- Ophthalmological findings of choroidal and vitreal infection are minimal until recovery from neutropenia; therefore, dilated funduscopic examinations should be performed within the first week after recovery from neutropenia.
- In the neutropenic patient, sources of candidiasis other than a central venous catheter (eg, gastrointestinal tract) predominate. Catheter removal should be considered on an individual basis.
- Granulocyte colony-stimulating factor (G-CSF)–mobilized granulocyte transfusions can be considered in cases of persistent candidemia with anticipated protracted neutropenia.
Chronic disseminated (hepatosplenic) candidiasis
Recommendations 6:
- Initial therapy with lipid formulation AmB, 3–5 mg/kg daily OR an echinocandin (micafungin: 100 mg daily; caspofungin: 70-mg loading dose, then 50 mg daily; or anidulafungin: 200-mg loading dose, then 100 mg daily), for several weeks is recommended, followed by oral fluconazole, 400 mg (6 mg/kg) daily, for patients who are unlikely to have a fluconazole-resistant isolate.
- Therapy should continue until lesions resolve on repeat imaging, which is usually several months. Premature discontinuation of antifungal therapy can lead to relapse.
- If chemotherapy or hematopoietic cell transplantation is required, it should not be delayed because of the presence of chronic disseminated candidiasis, and antifungal therapy should be continued throughout the period of high risk to prevent relapse.
- For patients who have debilitating persistent fevers, short-term (1–2 weeks) treatment with nonsteroidal anti-inflammatory drugs or corticosteroids can be considered.
Suspected systemic Candidiasis in nonneutropenic patients in the intensive care unit
Recommendations 6:
- Empiric antifungal therapy should be considered in critically ill patients with risk factors for invasive candidiasis and no other known cause of fever and should be based on clinical assessment of risk factors, surrogate markers for invasive candidiasis, and/or culture data from nonsterile sites. Empiric antifungal therapy should be started as soon as possible in patients who have the above risk factors and who have clinical signs of septic shock.
- Preferred empiric therapy for suspected candidiasis in nonneutropenic patients in the intensive care unit (ICU) is an echinocandin (caspofungin: loading dose of 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose of 200 mg, then 100 mg daily).
- Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, is an acceptable alternative for patients who have had no recent azole exposure and are not colonized with azole-resistant Candida species.
- Lipid formulation AmB, 3–5 mg/kg daily, is an alternative if there is intolerance to other antifungal agents.
- Recommended duration of empiric therapy for suspected invasive candidiasis in those patients who improve is 2 weeks, the same as for treatment of documented candidemia.
- For patients who have no clinical response to empiric antifungal therapy at 4–5 days and who do not have subsequent evidence of invasive candidiasis after the start of empiric therapy or have a negative non-culture-based diagnostic assay with a high negative predictive value, consideration should be given to stopping antifungal therapy.
Should prophylaxis be used to prevent systemic Candidiasis in the intensive care unit setting?
Recommendations 6:
- Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, could be used in high-risk patients in adult ICUs with a high rate (>5%) of invasive candidiasi.
- An alternative is to give an echinocandin (caspofungin: 70-mg loading dose, then 50 mg daily; anidulafungin: 200-mg loading dose and then 100 mg daily; or micafungin: 100 mg daily).
- Daily bathing of ICU patients with chlorhexidine, which has been shown to decrease the incidence of bloodstream infections including candidemia, could be considered.
Systemic Candidiasis and candidemia in neonates
Recommendations 6:
- AmB deoxycholate, 1 mg/kg daily, is recommended for neonates with disseminated candidiasis.
- Fluconazole, 12 mg/kg intravenous or oral daily, is a reasonable alternative in patients who have not been on fluconazole prophylaxis.
- Lipid formulation AmB, 3–5 mg/kg daily, is an alternative, but should be used with caution, particularly in the presence of urinary tract involvement.
- Echinocandins should be used with caution and generally limited to salvage therapy or to situations in which resistance or toxicity preclude the use of AmB deoxycholate or fluconazole.
- A lumbar puncture and a dilated retinal examination are recommended in neonates with cultures positive for Candida species from blood and/or urine.
- Computed tomographic or ultrasound imaging of the genitourinary tract, liver, and spleen should be performed if blood cultures are persistently positive for Candida species.
- Central venous catheter removal is strongly recommended.
- The recommended duration of therapy for candidemia without obvious metastatic complications is for 2 weeks after documented clearance of Candida species from the bloodstream and resolution of signs attributable to candidemia.
Central Nervous System Infections in Neonates
Recommendations 6:
- For initial treatment, AmB deoxycholate, 1 mg/kg intravenous daily, is recommended.
- An alternative regimen is liposomal AmB, 5 mg/kg daily.
- The addition of flucytosine, 25 mg/kg 4 times daily, may be considered as salvage therapy in patients who have not had a clinical response to initial AmB therapy, but adverse effects are frequent.
- For step-down treatment after the patient has responded to initial treatment, fluconazole, 12 mg/kg daily, is recommended for isolates that are susceptible to fluconazole.
- Therapy should continue until all signs, symptoms, and cerebrospinal fluid (CSF) and radiological abnormalities, if present, have resolved.
- Infected central nervous system (CNS) devices, including ventriculostomy drains and shunts, should be removed if at all possible.
Prophylaxis in the Neonatal Intensive Care Unit Setting
Recommendations 6:
- In nurseries with high rates (>10%) of invasive candidiasis, intravenous or oral fluconazole prophylaxis, 3–6 mg/kg twice weekly for 6 weeks, in neonates with birth weights <1000 g is recommended.
- Oral nystatin, 100 000 units 3 times daily for 6 weeks, is an alternative to fluconazole in neonates with birth weights <1500 g in situations in which availability or resistance preclude the use of fluconazole.
- Oral bovine lactoferrin (100 mg/day) may be effective in neonates <1500 g but is not currently available in US hospitals.
Intra-abdominal Candidiasis
Recommendations 6:
- Empiric antifungal therapy should be considered for patients with clinical evidence of intra-abdominal infection and significant risk factors for candidiasis, including recent abdominal surgery, anastomotic leaks, or necrotizing pancreatitis.
- Treatment of intra-abdominal candidiasis should include source control, with appropriate drainage and/or debridement.
- The choice of antifungal therapy is the same as for the treatment of candidemia or empiric therapy for nonneutropenic patients in the ICU.
- The duration of therapy should be determined by adequacy of source control and clinical response.
Does the isolation of Candida species from the respiratory tract require antifungal therapy?
Recommendation 6:
- Growth of Candida from respiratory secretions usually indicates colonization and rarely requires treatment with antifungal therapy.
Candida Endocarditis
Recommendations 6:
- For native valve endocarditis, lipid formulation AmB, 3–5 mg/kg daily, with or without flucytosine, 25 mg/kg 4 times daily, OR high-dose echinocandin (caspofungin 150 mg daily, micafungin 150 mg daily, or anidulafungin 200 mg daily) is recommended for initial therapy.
- Step-down therapy to fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended for patients who have susceptible Candida isolates, have demonstrated clinical stability, and have cleared Candida from the bloodstream.
- Oral voriconazole, 200–300 mg (3–4 mg/kg) twice daily, or posaconazole tablets, 300 mg daily, can be used as step-down therapy for isolates that are susceptible to those agents but not susceptible to fluconazole.
- Valve replacement is recommended; treatment should continue for at least 6 weeks after surgery and for a longer duration in patients with perivalvular abscesses and other complications.
- For patients who cannot undergo valve replacement, long-term suppression with fluconazole, 400–800 mg (6–12 mg/kg) daily, if the isolate is susceptible, is recommended.
- For prosthetic valve endocarditis, the same antifungal regimens suggested for native valve endocarditis are recommended. Chronic suppressive antifungal therapy with fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended to prevent recurrence.
Candida Infection of Implantable Cardiac Devices
Recommendations 6:
- For pacemaker and implantable cardiac defibrillator infections, the entire device should be removed.
- Antifungal therapy is the same as that recommended for native valve endocarditis.
- For infections limited to generator pockets, 4 weeks of antifungal therapy after removal of the device is recommended.
- For infections involving the wires, at least 6 weeks of antifungal therapy after wire removal is recommended.
- For ventricular assist devices that cannot be removed, the antifungal regimen is the same as that recommended for native valve endocarditis. Chronic suppressive therapy with fluconazole if the isolate is susceptible, for as long as the device remains in place is recommended.
Candida Suppurative Thrombophlebitis
Recommendations 6:
- Catheter removal and incision and drainage or resection of the vein, if feasible, is recommended (strong recommendation; low-quality evidence).
- Lipid formulation AmB, 3–5 mg/kg daily, OR fluconazole, 400–800 mg (6–12 mg/kg) daily, OR an echinocandin (caspofungin 150 mg daily, micafungin 150 mg daily, or anidulafungin 200 mg daily) for at least 2 weeks after candidemia (if present) has cleared is recommended.
- Step-down therapy to fluconazole, 400–800 mg (6–12 mg/kg) daily, should be considered for patients who have initially responded to AmB or an echinocandin, are clinically stable, and have a fluconazole-susceptible isolate.
- Resolution of the thrombus can be used as evidence to discontinue antifungal therapy if clinical and culture data are supportive.
Candida Osteomyelitis
Recommendations 6:
- Fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months OR an echinocandin (caspofungin 50–70 mg daily, micafungin 100 mg daily, or anidulafungin 100 mg daily) for at least 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months is recommended.
- Lipid formulation AmB, 3–5 mg/kg daily, for at least 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months is a less attractive alternative.
- Surgical debridement is recommended in selected cases.
Candida Septic Arthritis
Recommendations 6:
- Fluconazole, 400 mg (6 mg/kg) daily, for 6 weeks OR an echinocandin (caspofungin 50–70 mg daily, micafungin 100 mg daily, or anidulafungin 100 mg daily) for 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for at least 4 weeks is recommended.
- Lipid formulation AmB, 3–5 mg/kg daily, for 2 weeks, followed by fluconazole, 400 mg (6 mg/kg) daily, for at least 4 weeks is a less attractive alternative.
- Surgical drainage is indicated in all cases of septic arthritis.
- For septic arthritis involving a prosthetic device, device removal is recommended.
- If the prosthetic device cannot be removed, chronic suppression with fluconazole, 400 mg (6 mg/kg) daily, if the isolate is susceptible, is recommended.
Candida Endophthalmitis
Recommendations 6:
- All patients with candidemia should have a dilated retinal examination, preferably performed by an ophthalmologist, within the first week of therapy in nonneutropenic patients to establish if endophthalmitis is present. For neutropenic patients, it is recommended to delay the examination until neutrophil recovery.
- The extent of ocular infection (chorioretinitis with or without macular involvement and with or without vitritis) should be determined by an ophthalmologist.
- Decisions regarding antifungal treatment and surgical intervention should be made jointly by an ophthalmologist and an infectious diseases physician.
Candida chorioretinitis without vitritis
Recommendations 6:
- For fluconazole-/voriconazole-susceptible isolates, fluconazole, loading dose, 800 mg (12 mg/kg), then 400–800 mg (6–12 mg/kg) daily OR voriconazole, loading dose 400 mg (6 mg/kg) intravenous twice daily for 2 doses, then 300 mg (4 mg/kg) intravenous or oral twice daily is recommended.
- For fluconazole-/voriconazole-resistant isolates, liposomal AmB, 3–5 mg/kg intravenous daily, with or without oral flucytosine, 25 mg/kg 4 times daily is recommended.
- With macular involvement, antifungal agents as noted above PLUS intravitreal injection of either AmB deoxycholate, 5–10 µg/0.1 mL sterile water, or voriconazole, 100 µg/0.1 mL sterile water or normal saline, to ensure a prompt high level of antifungal activity is recommended.
- The duration of treatment should be at least 4–6 weeks, with the final duration depending on resolution of the lesions as determined by repeated ophthalmological examinations.
Candida chorioretinitis with vitritis
Recommendations 6:
- Antifungal therapy as detailed above for chorioretinitis without vitritis, PLUS intravitreal injection of either amphotericin B deoxycholate, 5–10 µg/0.1 mL sterile water, or voriconazole, 100 µg/0.1 mL sterile water or normal saline is recommended.
- Vitrectomy should be considered to decrease the burden of organisms and to allow the removal of fungal abscesses that are inaccessible to systemic antifungal agents.
- The duration of treatment should be at least 4–6 weeks, with the final duration dependent on resolution of the lesions as determined by repeated ophthalmological examinations.
Central Nervous System Candidiasis
Recommendations 6:
- For initial treatment, liposomal AmB, 5 mg/kg daily, with or without oral flucytosine, 25 mg/kg 4 times daily is recommended.
- For step-down therapy after the patient has responded to initial treatment, fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended.
- Therapy should continue until all signs and symptoms and CSF and radiological abnormalities have resolved.
- Infected CNS devices, including ventriculostomy drains, shunts, stimulators, prosthetic reconstructive devices, and biopolymer wafers that deliver chemotherapy should be removed if possible.
- For patients in whom a ventricular device cannot be removed, AmB deoxycholate could be administered through the device into the ventricle at a dosage ranging from 0.01 mg to 0.5 mg in 2 mL 5% dextrose in water.
Systemic candida prognosis
The long-term outlook (prognosis) for people with systemic candidiasis depends on many factors including the severity and location of the Candida infection, the general health of the infected person, and the timing of diagnosis and treatment. Because people who develop systemic candidiasis are usually already sick (often critically ill), it is hard to estimate the percentage of people who die from systemic candidiasis 1. Mortality rate (percentage of people who die) in critically ill people who develop systemic candidiasis is estimated to be between 45-50% 1. Mortality rates associated with candidemia (Candida infection in the blood) and disseminated candidiasis have not improved markedly over the past few years and remain in the range of 30%-40% 3.
Systemic candidiasis causes more case fatalities than any other systemic mycosis. Candidemia is associated with considerable prolongation in hospital stays (70 days vs 40 days in comparable patients without fungemia).
References- Antinori S, Milazzo L, Sollima S, Galli M, Corbellino M. Candidemia and invasive candidiasis in adults: A narrative review. Eur J Intern Med. 2016;34:21–28. doi:10.1016/j.ejim.2016.06.029 https://doi.org/10.1016/j.ejim.2016.06.029
- Invasive Candidiasis. https://www.cdc.gov/fungal/diseases/candidiasis/invasive
- Candidiasis. https://emedicine.medscape.com/article/213853-overview
- Endocarditis. https://medlineplus.gov/ency/article/001098.htm
- Pfaller MA, Wolk DM,and Lowery TJ. T2MR and T2Candida: novel technology for the rapid diagnosis of candidemia and invasive candidiasis. Future Microbiol. January 2016; 11(1):103-17. https://www.ncbi.nlm.nih.gov/pubmed/26371384
- Peter G. Pappas, Carol A. Kauffman, David R. Andes, Cornelius J. Clancy, Kieren A. Marr, Luis Ostrosky-Zeichner, Annette C. Reboli, Mindy G. Schuster, Jose A. Vazquez, Thomas J. Walsh, Theoklis E. Zaoutis, Jack D. Sobel, Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America, Clinical Infectious Diseases, Volume 62, Issue 4, 15 February 2016, Pages e1–e50, https://doi.org/10.1093/cid/civ933