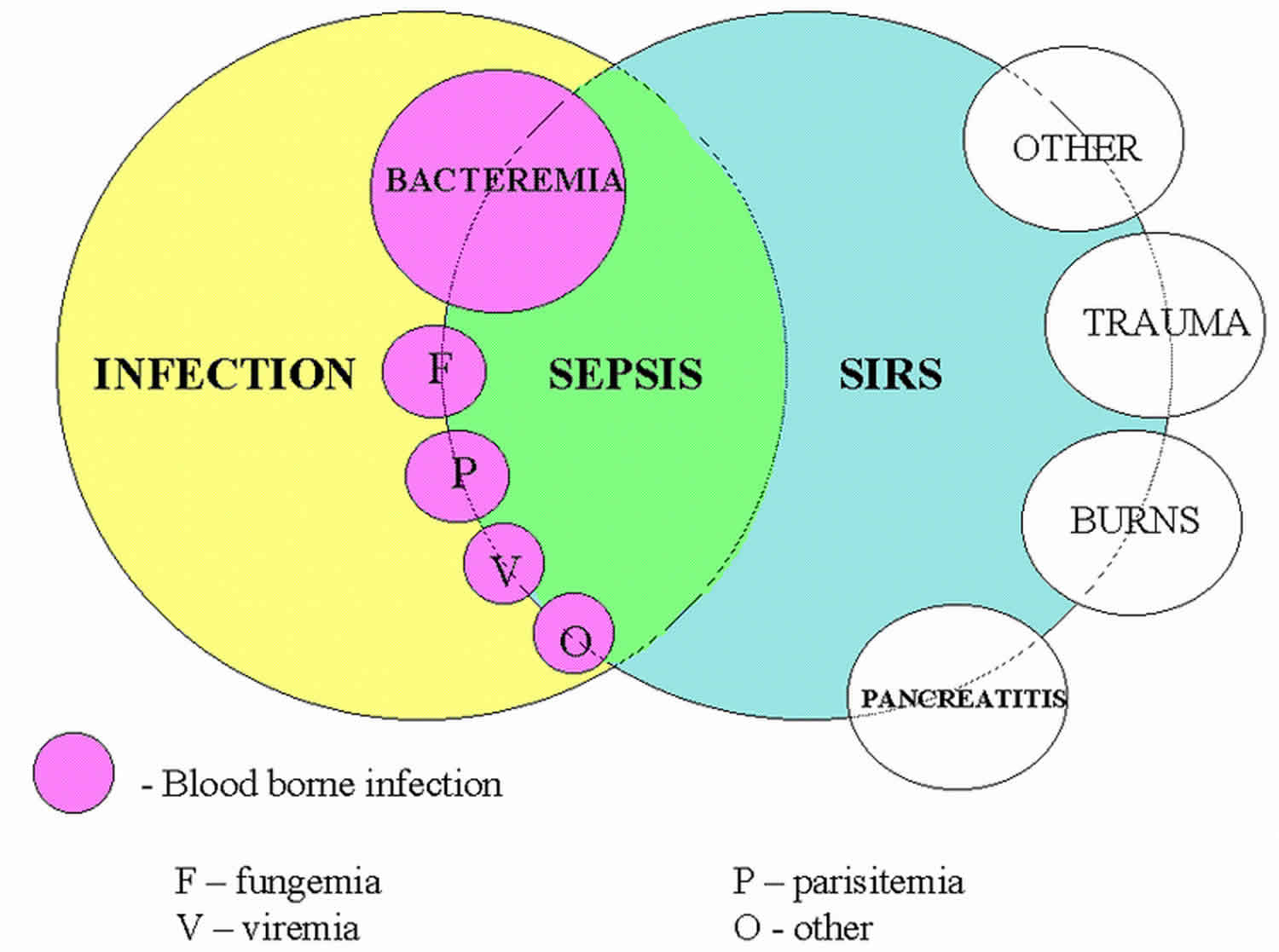
Systemic inflammatory response syndrome
Systemic inflammatory response syndrome (SIRS) is an exaggerated defense response of the body to a noxious stressor (infection, trauma, surgery, acute inflammation, ischemia or reperfusion, or malignancy to name a few) to localize and then eliminate the endogenous or exogenous source of the insult 1. Systemic inflammatory response syndrome involves the release of acute-phase reactants which are direct mediators of widespread autonomic, endocrine, hematological and immunological alteration in the subject. Even though the purpose is defensive, the dysregulated cytokine storm has the potential to cause massive inflammatory cascade leading to reversible or irreversible end-organ dysfunction and even death.
SIRS with a suspected source of infection is termed sepsis. Confirmation of infection with positive cultures is therefore not mandatory, at least in the early stages. Sepsis with one or more end-organ failure is called severe sepsis and with hemodynamic instability in spite of intravascular volume repletion is called septic shock. Together they represent a physiologic continuum with progressively worsening balance between pro and anti-inflammatory responses of the body.
The American College of Chest Physicians and the Society of Critical Care Medicine-sponsored sepsis definitions consensus conference also identified the entity of multiple organ dysfunction syndrome (MODS) as the presence of altered organ function in acutely ill septic patients such that homeostasis is not maintainable without intervention 2.
Objectively, SIRS is defined by the satisfaction of any two of the criteria below 1:
- Body temperature over 38 or under 36 degrees Celsius (over 100.4 °F or under 96.8 °F).
- Heart rate greater than 90 beats/minute
- Respiratory rate greater than 20 breaths/minute or partial pressure of CO2 less than 32 mmHg
- Leucocyte count greater than 12,000 or less than 4000 /microliters or over 10% immature forms or bands.
In the pediatric population, the definition is modified to a mandatory requirement of abnormal leukocyte count or temperature to establish the diagnosis, as abnormal heart rate and respiratory rates are more common in children.
To summarize, almost all septic patients have SIRS, but not all SIRS patients are septic. Kaukonen et al. explained exceptions to this theory by suggesting that there are subgroups of hospitalized patients particularly at extremes of age who do not meet criteria for SIRS on presentation but progress to severe infection and multiple organ dysfunction and death. Establishing laboratory indices to identify such subgroup of patients along with the clinical criteria that we currently rely upon has been therefore gaining prominence over the recent years 3.
Several scores exist to assess the severity of organ system damage. The Acute Physiology and Chronic Health Evaluation (APACHE) score version II and III, Multiple organ dysfunction (MOD) score, sequential organ failure assessment (SOFA) and logistic organ dysfunction (LOD) score are to name a few.
Systemic inflammatory response syndrome causes
At a molecular level, the etiopathogenesis of systemic inflammatory response syndrome broadly divides into
- Damage Associated Molecular Pattern (DAMP)
- Pathogen Associated Molecular Pattern (PAMP)
Although the list is not all-inclusive some common etiologies from clinical perspective include:
Damage Associated Molecular Pattern (DAMP)
- Burns
- Trauma
- Surgical procedure-related trauma
- Acute aspiration
- Acute pancreatitis
- Substance abuse and related intoxications
- Acute end-organ ischemia
- Acute exacerbation of autoimmune vasculitis
- Medication adverse reaction
- Intestinal ischemia and perforation
- Hematologic malignancy
- Erythema multiforme
Pathogen Associated Molecular Pattern (PAMP)
- Bacterial infection
- Viral syndrome-like influenza
- Disseminated fungal infection in immunosuppressed
- Toxic shock syndrome derived from both exotoxins and endotoxins
Pathogen Associated Molecular Pattern (PAMP) can also be classified based on the location and extent of dissemination of infection, which ranges from localized organ-specific infection to disseminated bacteremia and sepsis.
Systemic inflammatory response syndrome pathophysiology
Inflammation triggered by an infectious or noninfectious stimuli sets forth a complex interplay of the humoral and cellular immune response, cytokines, and complement pathway — eventually, systemic inflammatory response syndrome results when the balance between proinflammatory and anti-inflammatory cascades tip over towards the former.
Roger et al. 4 laid out a five-stage overlapping sepsis cascade that starts with SIRS and progresses to multiple organ dysfunction syndrome (MODS), if not appropriately countered by compensatory anti-inflammatory response or alleviation of the primary inciting etiology.
Stage 1
Stage 1 is a local reaction at the site of injury that aims at containing the injury and limit spread.
Immune effector cells at the site release cytokines that in turn stimulate the reticuloendothelial system promoting wound repair through local inflammation. There is local vasodilatation induced by nitric oxide and prostacyclin (rubor) and disruption of the endothelial tight junction to allow margination and transfer of leucocytes into tissue space. The leakage of cells and protein-rich fluid in extravascular space causes swelling (tumor) and increased heat (calor). Inflammatory mediators impact the local somatosensory nerves causing pain (dolor) and loss of function (functio laesa). That loss of function also allows the part of the body to repair instead of persistent use.
Stage 2
Stage 2 is an early compensatory anti-inflammatory response syndrome (CARS) in an attempt to maintain immunological balance. There is stimulation of growth factors and recruitment of macrophages and platelets as the level of pro-inflammatory mediators decrease to maintain homeostasis.
Stage 3
Stage 3 is when the scale tips over towards proinflammatory SIRS resulting in progressive endothelial dysfunction, coagulopathy, and activation of the coagulation pathway. It results in end-organ micro thrombosis, and a progressive increase in capillary permeability, eventually resulting in loss of circulatory integrity.
Stage 4
Stage 4 is characterized by compensatory anti-inflammatory response syndrome (CARS) taking over SIRS, resulting in a state of relative immunosuppression. The individual, therefore, becomes susceptible to secondary or nosocomial infections, thus perpetuating the sepsis cascade.
Stage 5
Stage 5 manifests in multiple organ dysfunction syndrome (MODS) with persistent dysregulation of both SIRS and compensatory anti-inflammatory response syndrome (CARS) response.
At a cellular level, non-infectious noxious stimuli, an infectious agent or an endotoxin or exotoxin produced by an infection activates a multitude of cells including neutrophils, macrophages, mast cells, platelets, and endothelial cells.
The early response mediated by these inflammatory cells involves three major pathways:
- Activation of interleukin 1 (IL1) and tumor necrosis factor alfa (TNF-alpha).
- Activation of prostaglandin and Leukotriene pathway
- Activation of C3a – C5a complement pathway
Interleukin 1 (IL1) and tumor necrosis factor alfa (TNF-alpha) are the early mediators within the first hour. Their role is of paramount importance in tilting the scale towards a proinflammatory overdrive.
Their actions can broadly divide into three categories:
- Propagation of cytokine pathway
- Alteration of coagulation causing microcirculatory abnormalities
- Release of stress hormones
Propagation of cytokine pathway
Release of IL1 and TNF-alpha results in dissociation of nuclear factor-kB (NF-kB) from its inhibitor. NF-kB is thus able to induce the mass release of other proinflammatory cytokines including IL-6, IL-8, and Interferon-gamma.IL-6 induces the release of acute-phase reactants including procalcitonin and C reactive protein. Infectious triggers tend to produce a greater surge of TNF-alpha and thus IL-6 and IL-8. Another potent proinflammatory cytokine is High mobility group box 1 (HMGB1) protein which is involved in the delayed cytotoxic response of SIRS and sepsis. It has been established as an independent predictor of 1-year mortality in an observational study of traumatic brain injury patients 5.
Alteration of coagulation causing microcirculatory abnormalities
Like most other early responses in SIRS, alteration of coagulation pathway also gets triggered by IL-1 and TNF-alpha. Fibrinolysis becomess impaired by activation of plasminogen activator inhibitor-1. There is direct endothelial injury, thus resulting in the release of tissue factor, which triggers the coagulation cascade. Also, the anti-inflammatory mediators Activated protein C and antithrombin get inhibited. As a result, there is widespread microvascular thrombosis, increase in capillary permeability, as well as fragility and impairment of tissue perfusion contributing to progressive organ dysfunction.
Release of stress hormones
Primarily the catecholamine, vasopressin, and activation of the renin-angiotensin-aldosterone axis result in an increased surge of endogenous steroids. Catecholamines are responsible for the tachycardia and tachypnea component of sepsis while glucocorticoids contribute to leucocyte count increase as well as their margination in the peripheral circulation.
Compensatory anti-inflammatory response syndrome (CARS)
The compensatory anti-inflammatory response is mediated by Interleukins IL-4 and IL-10 which tend to inhibit the production of TNF-alpha, IL-1, IL-6, and IL-8. The balance of SIRS and CARS decides where the termination point in the continuum of SIRS to multiple organ dysfunction syndrome (MODS) is CARS has its own perils. If allowed to perpetuate, it subjects the surviving individual to a prolonged state of immunosuppression. The individual thus becomes susceptible to nosocomial infection, which can thus reinitiate the septic cascade.
Systemic inflammatory response syndrome signs and symptoms
SIRS is defined as 2 or more of the following variables:
- Fever of more than 38°C (100.4°F) or body temperature less than 36°C (96.8°F)
- Heart rate of more than 90 beats per minute
- Respiratory rate of more than 20 breaths per minute or arterial carbon dioxide tension (PaCO2) of less than 32 mm Hg
- Abnormal white blood cell count (>12,000/µL or < 4,000/µL or >10% immature [band] forms)
Early clinical presentation irrespective of etiology, mirrors the pathological phenomena of rubor, calor, dolor, tumor, and loss of function or a disturbance of function (functio laesa). A thorough history of location, character, radiation, and exacerbating – relieving factors of pain, duration, and time correlation of symptom are important. Where the etiology and primary source is not as obvious, history should focus on any alteration from usual activities, including new medications, food intake, exposure, travel, or recreational agents of abuse.
Identification of specific risk factors through history may help prioritize intensive treatment strategy, e.g., preexisting immunosuppression, diabetes mellitus, solid tumors and leukemia, dysproteinemias, cirrhosis of the liver, and extremes of age.
A complete physical examination is not only helpful in localizing the source but also to assess the true extent of involvement and complications related to end-organ involvement. It also helps in guiding the appropriate investigations and imaging studies.
Systemic inflammatory response syndrome complications
Complications of a systemic inflammatory response syndrome can include the progression of the disease state along the continuum of sepsis (for infectious etiology) to severe sepsis to shock and multiorgan dysfunction syndrome. Complications can also be related to individual end-organ dysfunction. Some important ones are as below:
- Central nervous system – Acute encephalopathy
- Respiratory – Acute respiratory distress syndrome (ARDS), acute aspiration pneumonitis related to encephalopathy
- Cardiac – Demand perfusion mismatch causing troponin elevation, tachyarrhythmia
- Gastrointestinal – Stress ulcer, acute transaminitis
- Renal – Acute tubular necrosis and acute kidney injury, metabolic acidosis, electrolyte abnormalities.
- Hematological – Thrombocytosis or thrombocytopenia, disseminated intravascular coagulation, hemolysis, deep venous thrombosis.
- Endocrine – Hyperglycemia, acute adrenal insufficiency
Systemic inflammatory response syndrome diagnosis
Over the years, a gradual paradigm shift has occurred from placing sepsis on the shoulders of clinicians to the incorporation of more objective parameters. While it is unquestionably a clinical diagnosis and cannot be defined by merely diagnostic assays without clinician’s recognition of signs, prompt identification of uniform clinical criteria became increasingly important.
As newer inroads were made at the end of the 20th century in the complex pathophysiology, etiology and pharmacotherapy targets, the need for early diagnosis and intervention became obvious to make an impact on mortality and morbidity. The recognition of the continuum from early inflammation to multiorgan dysfunction added more incentive. Thus was born the necessity to diagnose systemic inflammatory response syndrome both in the backdrop of infection and in noninfectious stress where the body later becomes susceptible to a secondary infection.
Establishment of clinical criteria was where the initial endeavor lay. Thus were born APACHE score, SIRS score, sequential organ failure assessment score (SOFA score) and quick SOFA (q SOFA) score, logistic organ dysfunction (LOD) score. Each one of them evolved with an intent to find a simpler, easily applicable prompt scoring system that can be used in any clinical setting to predict:
- Identification of sepsis
- Risk of organ dysfunction
- In-hospital mortality
If the etiology of SIRS is identified early, investigations are individualized to the organ in focus. In the absence of an apparent source, a time-sensitive search for infectious source becomes a priority. Health care facilities across US and society guidelines endorse a routine collection of specimens from blood, sputum, urine and any other obvious wound for culture within the first hour of assessment and before initiation of antimicrobial therapy.
Depending on the severity of presentation, routine investigations involve periodic evaluation of basic metabolic panel, and lactic acid level to assess the extent of end-organ injury and perfusion impairment.
With time, there has also been an emerging discussion in the community about the importance of distinguishing sepsis earlier in SIRS with the help of biomarkers, even before microbial cultures come positive.
Biomarkers also become important in identifying SIRS due to secondary infection in patients who were initially admitted with a noninfectious etiology, e.g., trauma or burns or for a planned surgical intervention. Mere clinical criteria are not enough to capture the change in etiopathogenesis midway through hospitalization 6.
Procalcitonin
A glycoprotein precursor of calcitonin, procalcitonin is produced by C cells of thymus and also from leucocytes, liver, kidney, adipose, and muscle tissue 7. In healthy individuals, serum levels are usually below 0.1 mg/dl but can be significantly abnormal in bacterial, fungal, or parasitic infections. Levels can mildly elevate in viral infection or noninfectious acute inflammation, and can also rise in individuals with neuroendocrine tumors or post-surgical stress 8. Serum concentrations rise within 2 to 4 hours of the inflammatory surge and fall rapidly after halting the primary insult. Half-life is about 25 to 30 hours. The peak serum concentration, therefore, seems to parallel the timeline of disease severity and outcome 9.
Research has mostly focused around the utility of procalcitonin in differentiating infectious from an infectious cause of SIRS, as well as its value in serial assessment to determine the duration of antimicrobial therapy. Kumar et al. 10 showed a favorability for procalcitonin over CRP in the diagnosis and prognosis of sepsis but only in conjunction with clinical parameters. Karzai et al. 7 also confirmed its value in predicting a systemic infectious process, although the cutoff value seemed to differ based on the disease process. Ciriello et al. 11, in their comparison of a wide assembly of biomarkers in trauma patients, found only procalcitonin to be of benefit in predicting sepsis. Persistently high levels correlated well with increased mortality and severity scores. Agarwal and Schwartz 12 demonstrated that serial procalcitonin measurements in ICU contributed to a significant reduction of ICU days and duration of antimicrobial therapy.
Selberg et al. 13 in their study demonstrated that plasma concentrations of procalcitonin, C3a, and IL-6 obtained up to 8 hours after the clinical onset of sepsis or SIRS were significantly higher in patients with infectious etiologies. Procalcitonin, IL-6, and C3a were more reliable in distinguishing SIRS from sepsis 13.
Lactate
Lactic acid elevation can be a type A lactic acidosis with excessive production from tissue hypoperfusion related anaerobic metabolism or type B lactic acidosis from inadequate clearance due to liver dysfunction. Use of epinephrine as a vasopressor agent can also lead to excessive lactate production due to alteration of the pyruvate cycle.
Interleukin 6
An interleukin 6 (IL-6) level of greater than 300 pg/ml correlates with an increased incidence of MODS and death. Similarly, a reduction in level by the second day of antimicrobial therapy has been shown to be a positive prognostic sign 14.
Leptin
Serum leptin levels above a cutoff of 38 mcg/L correlate serum levels of IL-6 and TNF-alpha and helps in differentiating between infectious and noninfectious causes of SIRS with a sensitivity of 91.2% and a specificity of 85% 15. It is a centrally acting hormone generated by adipocytes acting on hypothalamus.
Endothelial markers
Angiopoietin 1 and 2 are ligands for the Tie-2 receptor in endothelial cells. During acute inflammation, there is increased binding of Angiopoietin 2 (Ang-2) with Tie-2 receptor, triggering microvascular thrombosis, and capillary permeability. The circulating levels of Ang- 2 appears to correlate with 28-day mortality in SIRS as well as with severity scores like APACHE and SOFA 16. Similar significance has been attached to soluble E- selectin and P- selectin levels, which can help distinguish between septic and non-septic etiologies of SIRS. Andrejaitiene et al. 17 in a study of 92 SIRS patients found soluble E selectin to be most useful in identifying early SIRS and prognosticating severity. Soluble Intracellular adhesion molecule (s-ICAM 1) helped in distinguishing septic and non-septic patients. However, none of their analytic methods are standardized and cut off levels still need to be established to bring them into the market anywhere soon.
Emerging biomarkers
Other emerging biomarkers in research to distinguish septic and non-septic etiology of SIRS include triggering receptor expression on myeloid cells 1 (TREM-1), Decoy receptor 3 (DcR3) (belongs to the tumor necrosis factor family) and suPAR (soluble urokinase-type plasminogen activator receptor) 18. Among them, suPAR correlated particularly well with disease severity scores and identification of nonsurvivors in the sepsis group.
Transcriptome analysis
In recent years there has been an emerging idea behind SIRS pathophysiology suggesting immune dysregulation as a key phenomenon than a mere inflammatory surge in SIRS and sepsis. Utilizing high-throughput sequencing of cDNAs from mononuclear cells, a genetic profile of endotoxin tolerance (called endotoxin tolerance signature or ETS) has been identified which is expressed more often in septic patients, and was more commonly associated with organ failure and disease severity. It may thus provide an opportunity of identifying a subpopulation of septic patients early for ICU admission and intensive therapy impacting mortality and morbidity 19.
Systemic inflammatory response syndrome treatment
Systemic inflammatory response syndrome is a conglomeration of clinical manifestations of a triggering cause; management focuses on the treatment of the primary triggering condition 1.
Management is thus designed around a parallel search for the underlying etiology and its resolution along with time-sensitive interventions that may not be cause-specific, but get targeted towards preventing end-organ injury. The goal is to disrupt progression along the continuum of shock and multi-organ dysfunction syndrome.
Ensuring hemodynamic stability is of utmost importance 1. In severe sepsis and septic shock, the surviving sepsis guidelines recommend an initial administration of isotonic crystalloids at a rate of 30 ml/kg bolus 1. Such an arbitrary establishment of volume standards across the patient spectrum with variable cardiac, renal, and intravascular protein reserve can be a topic of clinical debate. Therefore some practice standards are consistent with subsequent volume administration guided by dynamic measures of volume responsiveness. For a spontaneously breathing patient not in cardiac arrhythmia, the indices relied upon include measurement of pulse pressure variability or stroke volume variability with passive leg raising. For a patient on mechanical ventilator support, pulse pressure variability, stroke volume variability or IVC diameter variability with respiration is an option. In an era where Swan Ganz catheter is not commonly used, other newer devices can be used to measure some of these indices while newer less invasive ones are in the pipeline.
Vasopressors and inotropes are useful in shock nonresponsive to volume repletion. A detailed description of their use will fall in the purview of discussion of management of shock in specific.
Primary source control may involve surgical intervention, e.g., incision and drainage of wound infection, tube drainage of a contained abscess and collection, or more exploratory surgery.
When the clinician suspects sepsis as the cause of SIRS, and in specific predisposed individuals, e.g., generalized debilitation, immunosuppression, neutropenia or asplenia, broad-spectrum empiric antibiotic therapy is indicated immediately after collection of culture specimen.
Broad-spectrum antibiotics should still be guided by:
- Suspicion of community vs. hospital-acquired infection
- Prior microbiology patterns in the individual
- Antibiogram for the facility
Prompt de-escalation is the recommendation once culture results are available.
Antiviral therapy is considered only with respiratory exacerbation and systemic inflammatory response syndrome in the influenza season. Neutropenic patients and those on total parenteral nutrition with central venous access may need empiric antifungal if they continue to show SIRS response after empiric antibiotics.
Glucocorticoids in low dose (200 to 300 mg hydrocortisone or equivalent) have been shown to improve survival and help in the reversal of shock in patients with persistent shock in spite of fluid resuscitation and vasopressor use. There is no evidence in serum cortisol level or ACTH stimulation testing to determine the indication for steroids in septic shock. The rationale is decreased responsiveness at receptor level rather than an absolute reduction in serum cortisol level as a cause of relative adrenal insufficiency in SIRS syndromes.
Blood glucose control- Van den Berghe et al. in their landmark study in surgical ICU patients reported a reduction of in-hospital mortality rates with intensive insulin therapy (maintenance of blood glucose at 80 to 110 mg/dL) by 34%. However, subsequently, the large NICE-SUGAR trial failed to replicate the outcome benefit of tight glucose control with an increased incidence of complications of hypoglycemia and hypokalemia. The surviving sepsis guidelines recommend blood glucose control less than 180 mg/dl 20.
Systemic inflammatory response syndrome prognosis
Time being of supreme essence in the outcome of SIRS and sepsis, early identification holds the key to a favorable outcome. Prognosis depends on the etiologic source of SIRS, as well as on associated comorbidities. A systemic inflammatory response syndrome score of 2 or more on day 1 of hospitalization are more likely to develop multiorgan dysfunction syndrome (MODS), have more prolonged ICU stay and have a higher need for mechanical ventilation, vasopressor support, blood and blood products. The median time interval from SIRS to sepsis in the continuum is inversely related to the number of SIRS criteria met on admission 21. The median time interval from SIRS to sepsis was inversely related to the number of SIRS criteria met. Morbidity is related to the causes of SIRS, complications of organ failure, and the potential for prolonged hospitalization.
Comstedt et al. 22, in a study of systemic inflammatory response syndrome (SIRS) in acutely hospitalized medical patients, demonstrated a 6.9 times higher 28-day mortality in SIRS patients than in non-SIRS patients. Most deaths occurred in SIRS patients with an associated malignancy.
Interestingly the mortality rates in Rangel-Fausto et al. 23 study were 7% (SIRS), 16% (sepsis), 20% (severe sepsis), and 46% (septic shock).
Whereas in a similar study on in-hospital mortality, Shapiro et al evaluated mortality in patients with suspected infection in the emergency department and found the following in-hospital mortality rates of 1.3% (sepsis), 9.2 % (severe sepsis), and 28% (septic shock) 24. Another interesting observation of the study by Shapiro et al. 25 was that the presence of SIRS criteria alone did not have any correlation with in-hospital or 1-year mortality. The authors concluded that organ dysfunction, rather than SIRS criteria, to be a better predictor of mortality, thus validating the significance of sequential organ failure assessment score (SOFA score) and quick SOFA (q SOFA) score 26.
The difference reflects upon a change in practice patterns over a decade with more adherence to early goal-directed therapy, and use of proven risk reduction approaches like DVT prophylaxis, blood glucose control, lung-protective tidal volume in mechanical ventilation, daily awakening and early ambulation.
Sinning et al 27 evaluated the SIRS criteria in patients who underwent transcatheter aortic valve implantation (TAVI) and found that SIRS appeared to be a strong predictor of mortality. The occurrence of SIRS was characterized by a significantly elevated release of IL-6 and IL-8, with subsequent increase in the leukocyte count, C-reactive protein (CRP), and procalcitonin. The occurrence of SIRS was related to 30-day and 1-year mortality (18% vs 1.1% and 52.5% vs 9.9%, respectively) and independently predicted 1-year mortality risk 27.
Heffner et al study 28, patients without an identified infection had a lower hospital mortality rate than did patients with an infectious etiology for their SIRS (9% vs 15%, respectively).
A large retrospective study of all of Australia and New Zealand ICU care from 2000-2012 demonstrated a clear progressive decline in severe sepsis and septic shock mortality from 35% to 18% over this period, with equal trends across all age groups and treatment settings 29. These data suggest that attention to detail, using best practices and overall quality care, has nearly halved mortality from severe sepsis independent of any specific treatment. Thus, attention to overall patient status and use of proven risk reduction approaches (eg, stress ulcer prophylaxis, DVT prophylaxis, daily awakening, and weaning trials in ventilator-dependent patients) are central to improving outcome from severe sepsis.
Pittet et al 30 showed that control patients had the shortest hospital stay, while patients with SIRS, sepsis, and severe sepsis, respectively, required progressively longer hospital stays.
References- Chakraborty RK, Burns B. Systemic Inflammatory Response Syndrome. [Updated 2019 Sep 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547669
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992 Jun;101(6):1644-55.
- Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015 Apr 23;372(17):1629-38.
- Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997 Jul;112(1):235-43.
- Wang KY, Yu GF, Zhang ZY, Huang Q, Dong XQ. Plasma high-mobility group box 1 levels and prediction of outcome in patients with traumatic brain injury. Clin. Chim. Acta. 2012 Nov 12;413(21-22):1737-41.
- Sayampanathan AA. Systematic review of complications and outcomes of diabetic patients with burn trauma. Burns. 2016 Dec;42(8):1644-1651.
- Karzai W, Oberhoffer M, Meier-Hellmann A, Reinhart K. Procalcitonin–a new indicator of the systemic response to severe infections. Infection. 1997 Nov-Dec;25(6):329-34.
- Martiny P, Goggs R. Biomarker Guided Diagnosis of Septic Peritonitis in Dogs. Front Vet Sci. 2019;6:208.
- Wong HR. Sepsis Biomarkers. J Pediatr Intensive Care. 2019 Mar;8(1):11-16.
- Kumar S, Tripathy S, Jyoti A, Singh SG. Recent advances in biosensors for diagnosis and detection of sepsis: A comprehensive review. Biosens Bioelectron. 2019 Jan 15;124-125:205-215.
- Ciriello V, Gudipati S, Stavrou PZ, Kanakaris NK, Bellamy MC, Giannoudis PV. Biomarkers predicting sepsis in polytrauma patients: Current evidence. Injury. 2013 Dec;44(12):1680-92.
- Agarwal R, Schwartz DN. Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin. Infect. Dis. 2011 Aug;53(4):379-87.
- Selberg O, Hecker H, Martin M, Klos A, Bautsch W, Köhl J. Discrimination of sepsis and systemic inflammatory response syndrome by determination of circulating plasma concentrations of procalcitonin, protein complement 3a, and interleukin-6. Crit. Care Med. 2000 Aug;28(8):2793-8.
- Wolf TA, Wimalawansa SJ, Razzaque MS. Procalcitonin as a biomarker for critically ill patients with sepsis: Effects of vitamin D supplementation. J. Steroid Biochem. Mol. Biol. 2019 Oct;193:105428.
- Yousef AA, Amr YM, Suliman GA. The diagnostic value of serum leptin monitoring and its correlation with tumor necrosis factor-alpha in critically ill patients: a prospective observational study. Crit Care. 2010;14(2):R33.
- Vassiliou AG, Mastora Z, Orfanos SE, Jahaj E, Maniatis NA, Koutsoukou A, Armaganidis A, Kotanidou A. Elevated biomarkers of endothelial dysfunction/activation at ICU admission are associated with sepsis development. Cytokine. 2014 Oct;69(2):240-7.
- Andrejaitiene J, Sirvinskas E, Zebrauskiene I. [Procalcitonin: a new infection marker. Its use in intensive care]. Medicina (Kaunas). 2002;38(5):491-8.
- Oku R, Oda S, Nakada TA, Sadahiro T, Nakamura M, Hirayama Y, Abe R, Tateishi Y, Ito M, Iseki T, Hirasawa H. Differential pattern of cell-surface and soluble TREM-1 between sepsis and SIRS. Cytokine. 2013 Jan;61(1):112-7.
- Pena OM, Hancock DG, Lyle NH, Linder A, Russell JA, Xia J, Fjell CD, Boyd JH, Hancock RE. An Endotoxin Tolerance Signature Predicts Sepsis and Organ Dysfunction at Initial Clinical Presentation. EBioMedicine. 2014 Nov 01;1(1):64-71.
- Bellomo R. Acute glycemic control in diabetics. How sweet is oprimal? Pro: Sweeter is better in diabetes. J Intensive Care. 2018;6:71.
- Kurmyshkina OV, Bogdanova AA, Volkova TO, Poltoraka AN. [Septic Shock: Innate Molecular Genetic Mechanisms of the Development of Generalized Inflammation]. Ontogenez. 2015 Jul-Aug;46(4):225-39.
- Comstedt P, Storgaard M, Lassen AT. The Systemic Inflammatory Response Syndrome (SIRS) in acutely hospitalised medical patients: a cohort study. Scand J Trauma Resusc Emerg Med. 2009 Dec 27. 17(1):67.
- Rangel-Fausto MS, Pittet D, Costigan M. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995. 273:117-123.
- Shapiro N, Howell MD, Bates DW, Angus DC, Ngo L, Talmor D. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med. 2006 Nov;48(5):583-90, 590.e1
- Shapiro N, Howell MD, Bates DW, Angus DC, Ngo L, Talmor D. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med. 2006 Nov;48(5):583-90, 590.e1.
- Shapiro N, Howell MD, Bates DW, Angus DC, Ngo L, Talmor D. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med. 2006 Nov. 48 (5):583-90, 590.e1.
- Sinning JM, Scheer AC, Adenauer V, Ghanem A, Hammerstingl C, Schueler R, et al. Systemic inflammatory response syndrome predicts increased mortality in patients after transcatheter aortic valve implantation. Eur Heart J. 2012 Jun. 33 (12):1459-68.
- Heffner AC, Horton JM, Marchick MR, Jones AE. Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clin Infect Dis. 2010 Mar 15. 50(6):814-20.
- Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014 Apr 2. 311(13):1308-16.
- Pittet D, Rangel-Fausto MS, Li N. Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: incidence, morbidities and outcomes in surgical ICU patients. Int Care Med. 1995. 21:302-309.