What are toll like receptors
Toll-like receptors are members of the pattern-recognition receptors family and play a key role in the innate immune system in the activation of innate immune cells including monocytes, macrophages and dendritic cells (DCs) 1. Macrophage activation by toll like receptors is pivotal in the initiation of the rapid expression of pro-inflammatory cytokines tumor necrosis factor (TNF), type I interferons (IFN-1), interleukin-1β (IL-1β) and interleukin-6 (IL-6) whilst promoting T-helper cell 17 (Th17) responses, all of which play critical roles in autoimmunity.
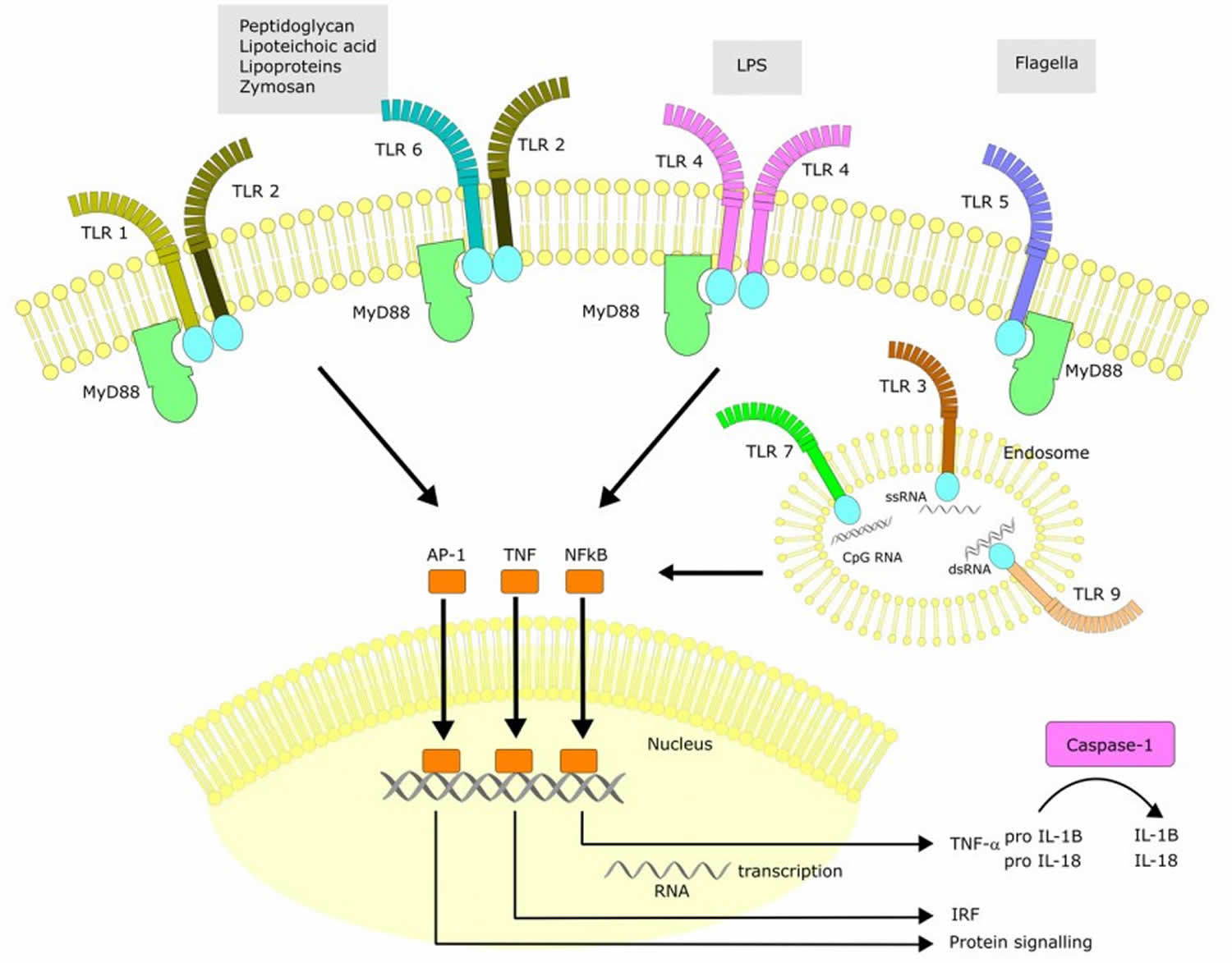
Toll-like receptors are type 1 transmembrane glycoproteins that play a key role in the immune response against microbes 1. Ten human toll like receptors have been identified to date and a subset of toll like receptors recognizes forms of nucleic acids, including double-stranded RNA, single-stranded RNA, and DNA. Toll like receptor1, 2, 4, 5, 6, and 10 exist in the cell surface, whereas toll like receptor 3, 7, 8, and 9 occur in endosomal membranes 2. All ten toll like receptors are expressed in human macrophages and mice express toll like receptors 11, 12, and 13 3,
Toll like receptors are cell surface and intracellular (toll like receptor-3, -7, -8 and -9) single, membrane-spanning, non-catalytic receptors expressed mainly on sentinel cells such as macrophages and dendritic cells 4. Their name derives from homology to the Drosophila Toll molecule—an important component of dorsal-ventral patterning and antifungal defense 4.
Figure 1. Toll like receptors location
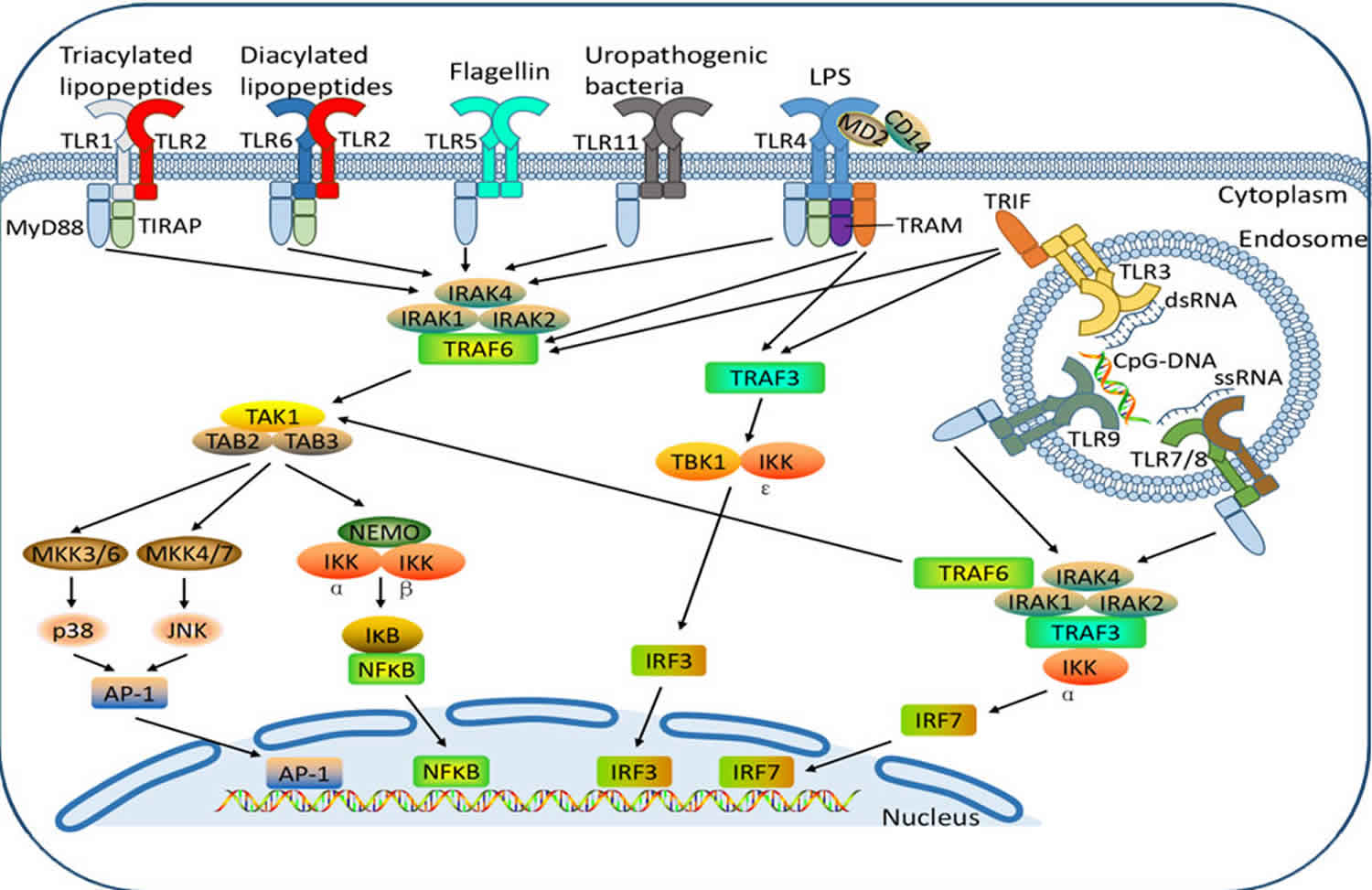
 Footnote: Toll like receptor ligands and toll like receptor signaling pathways. Cell surface toll like receptors, including toll like receptor-1, -2, -4, -5, -6, -10, and -11, and intracellular toll like receptors, including toll like receptor-3, -7, -8, and -9, recognize their specific PAMPs (pathogen associated molecular patterns) to activate toll like receptor signaling cascades.
Footnote: Toll like receptor ligands and toll like receptor signaling pathways. Cell surface toll like receptors, including toll like receptor-1, -2, -4, -5, -6, -10, and -11, and intracellular toll like receptors, including toll like receptor-3, -7, -8, and -9, recognize their specific PAMPs (pathogen associated molecular patterns) to activate toll like receptor signaling cascades.
Abbreivations: TLR = Toll-like receptor; PAMPs = pathogen associated molecular patterns
[Source 5 ]Toll like receptors are composed of an extracellular or ectodomain, a single-path transmembrane domain and an intracellular domain and are classified as Pattern Recognition Receptors (PRRs) as they recognize conserved molecular structures in microbes termed Pathogen Associated Molecular Patterns (PAMPs) 6. The ectodomain is involved in the recognition of ligands, which induce the dimerization of the intracellular domain, termed TIR (Toll/IL-1 resistance) domain and the activation of the signalling pathways. Recent crystal structures of ligands-toll like receptor ectodomains have shed lights to the way that these recognitions take place. The ectodomains of toll like receptors are composed of an N-terminal cap, a leucine-rich repeat domain (LRR domain), and a cysteine rich domain 6. The most important ligands for human toll like receptors are summarized in Table 1.
Table 1. Toll like receptors and their corresponding endogenous and microbial ligands
| Type of toll like receptors (TLR) | Microbial ligands (PAMPs) | Potential endogenous toll like receptors ligands (DAMPs) |
|---|---|---|
| TLR2 (in association with TLR1 or 6) | Lipomannan (Mycobacterium), Lipoteichoic Acids (Gram-positive bacteria), di-acylated and try-acylated bacterial lipopeptides | HSP 60, HSP70, HSP 96, HMGB-1, gp96, Biglycan , SP-D, |
| TLR4 | LPS (Gram-negative bacteria) | Biglycan, HSP 60, HSP 70,HSP 96, fibrinogen, fibronectin, hyaluronic acid, HMGB- 1,OxLDL( in association with TLR6), beta amyloid (in association with TLR6) |
| TLR5 | Flagellin (Gram-negative bacteria) | undetermined |
| TLR3 | dsRNA (virus) | mRNA (necrotic cells) |
| TLR7 | ssRNA(virus) | ssRNA, imiquimod |
| TLR8 | ssRNA(virus) | ssRNA, microRNAs |
| TLR9 | CpG motif (bacteria, virus) | Self-DNA |
The activation of toll like receptors— except for toll like receptor-3 leads to the recruitment of the adaptor protein MyD88 (myeloid differentiation factor 88) to the TIR (toll-interleukin receptor) domain on toll like receptors 7. IRAK-4 (interleukin-1 receptor-associated kinase 4) binds to MyD88, leading to the phosphorylation of IRAK-1, which in turn activates the transcription factor NF-κB4 (nuclear factor κ-light-chain-enhancer of activated B4 cells). NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells) translocates to the nucleus, which then regulates the expression of some genes, including pro-inflammatory cytokine- and survival-related genes 8.
Different toll like receptor signals induced by different cell types could activate MAPKs, JNK, p38, and ERK, leading to the activation of various transcription factors, including AP-1 and CREB 9. Toll like receptor-3 transmits signals via the TRIF (TIR-domain-containing adaptor inducing interferon-β) adapter molecule, and toll like receptor-4 recruits
TRIF using a bridging adapter TRAM (TRIF-related adaptor molecule) 10. Therefore, toll like receptor4 is a unique toll like receptor that is able to transmit signals via MyD88 and TRIF. In addition, TRIF can also lead to the activation of NF-κB, ERK, p38, and JNK7.
What do toll like receptors do?
Toll like receptors recognize conserved microbial structures called PAMPs (pathogen-associated molecular patterns) as well as host biomolecules associated with cell damage or necrosis called DAMPs (danger-associated molecular patterns) and induce an immune response 4. Toll like receptors utilize leucine-rich-repeat motifs (similar to interleukin (IL)-1) to bind the ligands and a shared cytoplasmic domain to recruit the following adaptors for downstream signaling: MyD88 (myeloid differentiation factor 88), TRIF (TIR-domain-containing adaptor inducing interferon-β), TIRAP (TIR domain-containing adaptor protein) and/or TRAM (TRIF-related adaptor molecule) 11. The crucial end-point of the cascade reactions is unmasking the nuclear localization domain of NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells), which after its translocation into the nucleus, activates multiple pro-inflammatory genes 12. Toll like receptor stimulation via PAMPs (e.g., microbial nucleic acids, bacterial lipoglycans, carbohydrates and peptides, protozoan glycosylphosphatidylinositol anchors, fungal glucans and chitin) or DAMPs (e.g., heat-shock proteins, HMGB1 (high-mobility group box 1), uric acid, ATP and DNA) leads to synthesis of type 1 interferons via the TRIF-dependent pathway and pro-inflammatory cytokines via the MyD88-dependent pathway, as well as the maturation of dendritic cells and even in some cases an induction of the adaptive immune system 13. Additionally, toll like receptors play a role in the regulation of immune responses (suppression and contrasupression) by direct and indirect influence on the function of CD4+ CD25+ (cluster of differentiation) T regulatory cells (Tregs) 4.
Finally, mounting evidence demonstrates that toll like receptors and the innate immune system are involved in the pathogenesis of disorders associated with chronic inflammation such as diabetes mellitus, asthma, Crohn’s disease, systemic lupus erythematosus, cancer and cardiovascular diseases, which suggests new possible therapeutic targets 14.
Toll like receptors play a critical role in tumor development
Toll like receptor signaling inhibits tumor growth
The toll like receptors play an immune surveillance role mainly by inducing the production of multiple cytokines and the activation of immune cells. Cytokines such as type I interferon (IFN-I) and interleukin 12 (IL-12) promote the activation of NK cells and enhance the scavenging capacity of the host with tumor cells 15. Other cytokines such as IL-2 and IFN-γ can enhance the ability of tumor-specific cytotoxic T lymphocyte (CTL) in the host to recognize and scavenge tumor cells. Intriguingly, some toll like receptors agonists were found capable of inhibiting tumor growth 16. It has been reported that the combination of toll like receptor agonists, chemotherapy drugs and tumor vaccine could improve the efficacy of eliminating tumor cells, an effect mainly based on the activation of antigen-presenting cells and the enhancement of T-cell immune response by toll like receptors 17. The increased expression of MHCII, CD88 and CCR7 in the activated antigen presenting cells of the toll like receptors signaling pathway significantly enhances recognition and presenting to tumor antigen. Also, toll like receptor1/2 acting on CD8+ CTLs increases the secretion of IFN-γ, TNF-α and IL-2 to promote the secretion of granzyme B and perforin by CD8+ T cells, which play a key role in elimination of tumor cells 18.
In addition, toll like receptors also act directly on tumor cells; toll like receptor3 is thought to be effective in promoting tumor cell apoptosis in a variety of tumors. When activated by dsRNA, an agonist of toll like receptor3, breast cancer cells generate autocrine type I IFN, which mediates toll like receptor-3 dependent cell apoptosis 19. In type I and II lung cancer cells, the engagement of toll like receptor-3 by dsRNA induces an atypical caspase-8-containing complex, which activates apoptotic pathways leading to tumor cell death 19. In the development of tumors, vigorous metabolism leads to metabolic disorders and local hypoxia, through which large amounts of tissue cell debris and proteins are released. The debris and proteins are recognized by toll like receptors as DAMPs, which are considered signals of danger, and this recognition consequently influences the various biological behaviors of tumor cells. It has been reported that HMGB1, an endogenous ligand of toll like receptor2 which binds to toll like receptor2 and activates toll like receptor-2 signaling pathways in glioblastoma, mediates antitumor immune response by inducing the activation of DCs and their migration into the brain tumor 20.
Toll like receptor signaling promotes tumor growth
The activation of toll like receptors can also promote tumor growth in many situations. Recent studies have found that the combination of highly expressed toll like receptors and DAMPs in tumors changes the homeostasis of the immune system, which leads to the suppression of immune function. HMGB1 has been identified as a cause of tumors of the skin, liver and pancreas. Furthermore, toll like receptor4 recognizes and combines with HMGB1 released by necrotic cells, and this recognition may eventually cause immune tolerance by activating the downstream pro-inflammatory signaling pathway. At the same time, HMGB1 aggregates in the cell membrane and promotes the invasion and growth of tumor cells 21.
Although the specific mechanisms of toll like receptor-mediated immune escape are still unknown, the high expression of toll like receptors in tumors often leads to immunosuppression while enhancing the invasiveness of tumors. Studies have found that the activation of the toll like receptors signaling pathway may lead to increased secretion of IL-10 and TGF-β, both of which are major immune suppressors in vivo 22. In addition, the activation of toll like receptors is also accompanied by the expression of PD-L1, HLA-G and other inhibitory costimulatory molecules 23. In a mouse model of colon cancer, toll like receptor4 has prolonged the survival time of tumor cells by up-regulating programmed death ligand 1 (PD-L1/B7-H1), inducible costimulator ligand (B7-H2) and down-regulating the expression level of Fos 24. Supernatants generated from murine colon cancer cells stimulated with LPS were found to play a significant role in the inhibition of T cell proliferation and NK cell cytotoxicity. The effect can be reversed after the toll like receptor4 signaling pathway is blocked, which may explain the pathway’s immunosuppressive effect 25. In addition to the inhibiting role, toll like receptors also promote the proliferation of tumor cells and enhances tumor invasion, promoting immune escape, while toll like receptor2 in human gastric cancer cell lines promotes tumor progression through the induction of COX-2, PGE-2 and IL-8 24.
The toll like receptor signaling can lead macrophage polarization change, from M1 (inhibiting tumor) to M2 (promoting tumor), which might explain, at least partially, why toll like receptor signaling promotes tumor growth. The M1/M2 polarization model has been reported in many cancer research studies in recent years. The M1 of tumor-associate macrophages (TAM) express high levels of IL-12 and IL-23, and function as inducers of Th1 responses. During tumor progression, TAM polarizes toward M2 TAM, an alternatively activated macrophage, with a tumor growth-promoting phenotype. However, this M1/M2 polarization has only been well established in vitro, not in vivo. Therefore, the role of toll like receptor signaling in M1/Me polarization calls for further investigation.
The role of toll like receptors in cancer progression: a double-edged sword
Overall, as discussed above, the activation of toll like receptors can both promote and inhibit tumor growth and cancer progression, and the underlying mechanism remains elusive 26. Current knowledge shows that different toll like receptors share similar signaling pathways, but this cannot explain why the activation of different toll like receptors in cancers has opposite effects on tumor growth. Also, toll like receptor agonists themselves might have direct pro- or anti-tumor effects, but current evidence shows that these effects, at least in majority of cases, are very minor. Another potential mechanism is that different toll like receptors might trigger different signaling pathways in cancer cells. We recently found that activation of toll like receptors in cancer cells may induce cancer cells to secrete various soluble factors, which might play distinct roles in cancer development. The role of toll like receptors in cancer progression needs to be further investigated, and understanding the underlying mechanism is essential for the further development of toll like receptor agonists as therapeutic agents.
Toll-like receptors and cardiovascular diseases
Apart from being expressed in immune cells, toll like receptors are expressed in other cells found in the epithelium, endothelium, as well as adipocytes and those of the cardiovascular system 27. Messenger RNA for toll like receptor 1–10 has been detected in the human heart 28. Nevertheless, the role of the innate immune system in the pathogenesis of cardiovascular diseases has been discovered only recently. The most investigated receptor in this area is toll like receptor-4. Aggregated data suggest that short-term activation of toll like receptors has a cytoprotective effect on the cardiovascular system, whereas prolonged or excessive activation of toll like receptors induces chronic low-grade inflammation, which leads to endothelial dysfunction, increased cell death, adverse cardiac remodeling and subsequently coronary and cerebrovascular atherosclerosis, heart failure, septic cardiomyopathy, viral myocarditis, valvular diseases, thrombosis and/or hypertension 29. Furthermore, cardiovascular risk factors such as diabetes, obesity and insulin resistance, are also associated with a low-grade inflammation that mimics the activation of innate immunity associated with metabolic, environmental, and genetic factors 30. Taking into account that cardiovascular diseases are the leading cause of mortality worldwide (17.6 million deaths in 2016 with 14.5% rise from 2006 to 2016), thorough knowledge of the pathomechanisms of toll like receptors is very important 31.
Toll like receptor activation affects vascular function and remodeling, and these molecular events prime antigen-specific adaptive immune responses. Despite the presence of toll like receptors in vascular cells, the exact mechanisms whereby toll like receptor signaling affects the function of vascular tissues are largely unknown. Cardiovascular diseases are considered chronic inflammatory conditions, and accumulating data show that toll like receptors and the innate immune system play a determinant role in the initiation and development of cardiovascular diseases. This evidence unfolds a possibility that targeting toll like receptors and the innate immune system may be a novel therapeutic goal for these conditions. Toll like receptor inhibitors and agonists are already in clinical trials for inflammatory conditions such as asthma, cancer, and autoimmune diseases, but their study in the context of cardiovascular diseases is in its infancy.
Toll-like receptors in cancer therapy
Toll-like receptors are associated with tumor growth and immunosuppression, as well as apoptosis and immune system activation 32. Toll-like receptors can activate apoptosis and innate and adaptive immunity pathways, which can be pharmacologically targeted for the development of anti-cancer oncotherapies 32. Several studies and clinical trials indicate that toll like receptor agonists are promising adjuvants (add-on therapies) or elements of novel therapies, particularly when used in conjunction with chemotherapy or radiotherapy. An increasing number of studies suggests that the activation of toll like receptors in various cancer types is related to oncotherapy; however, before this finding can be applied to clinical practice, additional studies are required. Research suggests that toll like receptor agonists may have potential applications in cancer therapy; nevertheless, because toll like receptor signaling can also promote tumorigenesis, a critical and comprehensive evaluation of toll like receptor action is warranted.
The pro-tumor or anti-tumor effects of the toll like receptor signaling pathway depends on the activation of specific toll like receptors, cell types, and downstream signaling pathways. Research advances involving toll like receptors show promising applications to cancer therapy. However, understanding the molecular mechanism of toll like receptors in all types of cancer cells remains the biggest obstacle in clinical therapeutic application. The activation of toll like receptors not only plays an important role in innate immunity, but it is also linked with the induction of autophagy, apoptosis, or pyroptosis in cancer cells.
The function of toll like receptor signaling in cancer oncotherapy applications remains controversial. Toll like receptor-4 has both protective and destructive functions in colitis-associated colorectal cancer 33. Toll like receptor activation leads to cell proliferation in the head and neck and
prostate cancers via the NF-κB pathway and is time- and lipopolysaccharide (LPS) dose-dependent 34. Research by Paone and coworkers 35 demonstrated that toll like receptor-3 stimulation induces apoptosis in prostate cancer, suggesting that toll like receptor-3 and toll like receptor-4 play important roles in cancer development and should be investigated in various tumorigenic settings, including colorectal, head and neck, and prostate cancers. toll like receptor-4 facilitates tumor immune escape and decreases apoptosis in colon cancer. The activation of toll like receptor-4 also induces production of immunosuppressive agents and chemokines, which increase tumor progression and metastasis, including nitric oxide, IL-8, and MMP-9. Additionally, silencing toll like receptor-4 in the context of liver cancer has the potential to decrease tumor cell metastasis 36.
The results of recent studies indicate that the best application of toll like receptor agonists in oncotherapy is the combination of toll like receptor agonists with other therapy method, including toll like receptor agonists, monoclonal antibodies, cancer associated antigens, siRNA, and conventional therapies, including chemotherapy, radiotherapy, or surgery 32. New delivery systems, including nanomaterials which can produce nanovaccines or deliver drugs/genes/proteins to designated location should also requires further
investigations. The advantages of combination therapy include lower dosage of the drug and lower side effects, as well as a potential decrease in therapeutic resistance or activation of immune response. Therefore, the combination treatment is likely to be more effective and provide better anti-tumor effects. However, many studies still report that toll like receptor ligands have pro-tumor effects in various cancers, and increasing expression or toll like receptors in different tumor tissues is related to poor prognosis, recurrence, and low survival rate in cancer patients. Additionally, some studies demonstrate that the existence of endogenous toll like receptor ligands may be derived from cell death induced by chemotherapy and can promote immune evasion or cancer growth. In summary, additional in vitro (test tube) and in vivo (animals and humans) studies are needed to assess the role of toll like receptor ligands in cancer progression and cancer prevention 32.
Toll like receptors in autoimmunity
Toll like receptors have also been implicated in the pathogenesis and severity of some autoimmune diseases. Toll like receptors play an important role in direct and indirect activation of T cells in autoimmunity as recently reviewed and also contribute to the activation of auto-reactive B cells 37. For example, the overexpression of toll like receptor-2 has been reported in the synovial fluid cells of patients with rheumatoid arthritis 38. DNA specific IgG can trigger low affinity autoreactive antibodies like rheumatoid factor when complexed with autologous CpG DNA in the circulation of autoimmune prone mice 39. Moreover, one of the defects that is associated with susceptibility to systemic autoimmune diseases is the defective clearance of dying cells and self DNA. This would exacerbate the potential for an autoimmune response.
Cross-linking of rheumatoid factor (RF) surface receptor with complex DNA-immunoglobulin has been shown to be necessary in the activation of auto-reactive B cells 40. However, a second signal due to the activation of toll like receptor9 by un-methylated CpG motifs leads to auto-reactive B cell activation and RF antibody secretion 41. Similarly, an increased production of autoantibodies in mice harboring a duplicated toll like receptor-7 gene is observed 42. The BCR/toll like receptor two-signal mechanism explains the high prevalence of autoantibodies against nuclear proteins in autoimmune diseases like systemic lupus erythematosus (SLE) 43. Furthermore, M2 macrophages produced pro-inflammatory cytokines in the presence of IgG-toll like receptor ligands, increasing the pro-inflammatory cytokine secretion and the polarization towards Th17, which are critical in the pathology of rheumatoid arthritis 44. Th17 is also involved in bone destruction via osteoclastogenesis 45. Therefore, the activation of toll like receptors have multiple roles in exacerbating the progression of various inflammatory arthritides and their relevance have been highlighted by the increasing number polymorphism in toll like receptors associated with rheumatoid arthritis and psoriatic arthritis 46.
The current traditional therapies for rheumatoid arthritis, based on targeting TNF (eg. infliximab, etarnecep), are, not completely effective, very expensive and come with very undesirable side effects. Alternative therapies are also needed as the anti TNF therapies also induce drug resistance One interesting novel approach would be the employment of synthetic ligands inhibitors which bind but not activate the toll like receptor2, toll like receptor4 to decrease the inflammation in rheumatoid arthritis 47. Synthetic oligodeoxynucleotides with immuno-regulatory sequences that blocks signaling via toll like receptor-7 and/or via toll like receptor-9 could also be employed to inhibit auto-antibody production 48. Furthermore, chemical inhibitors that bind the BB loop of the TIR domain of MyD88 have been developed 49. Peptides inhibitors of TRAF6, which inhibit osteoclastogenesis by blocking both the toll like receptor (MyD88 dependent) and RANKL-RANK signaling pathways have been used with some relative success 50.
References- Jiménez-Dalmaroni MJ, Gerswhin ME, Adamopoulos IE. The critical role of toll-like receptors–From microbial recognition to autoimmunity: A comprehensive review. Autoimmun Rev. 2016;15(1):1–8. doi:10.1016/j.autrev.2015.08.009 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4679489
- Boozari M, Butler AE, Sahebkar A. Impact of curcumin on toll-like receptors. J Cell Physiol. 2019.[E-pub ahead of print]
- Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors. Pandey S, Kawai T, Akira S. Cold Spring Harb Perspect Biol. 2014 Oct 9; 7(1):a016246.
- Adamczak DM. The Role of Toll-Like Receptors and Vitamin D in Cardiovascular Diseases-A Review. Int J Mol Sci. 2017;18(11):2252. Published 2017 Oct 27. doi:10.3390/ijms18112252 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5713222
- Toll-like Receptors (TLR). https://www.clinisciences.com/en/read/toll-like-receptors-tlr-1179.html
- Pandey S, Kawai T, Akira S. Microbial Sensing by Toll-Like Receptors and Intracellular Nucleic Acid Sensors. Cold Spring Harb Perspect Biol. 2015;7
- Thomas C, Bazan JF, Garcia KC. Structure of the activating IL-1 receptor signaling complex. Nat Struct Mol Biol. 2012;19(4):455-7
- Anwar MA, Shah M, Kim J, Choi S. Recent clinical trends in Toll-like receptor targeting therapeutics. Med Res Rev. 2018.[E-pub ahead of print].
- Scharf S, Hippenstiel S, Flieger A, Suttorp N, N’Guessan PD. Induction of human β-defensin-2 in pulmonary epithelial cells by Legionella pneumophila: involvement of TLR2 and TLR5, p38 MAPK, JNK, NF-κB, and AP-1. Am J Physiol Lung Cell Mol Physiol. 2010;298(5):L687-95
- Ropert C. How toll-like receptors reveal monocyte plasticity: the cutting edge of antiinflammatory therapy. Cell Mol Life Sci. 2019;76(4):745-55.
- Recognition and signaling by toll-like receptors. West AP, Koblansky AA, Ghosh S. Annu Rev Cell Dev Biol. 2006; 22():409-37.
- Toll-like receptor signaling pathways. Kawasaki T, Kawai T. Front Immunol. 2014; 5():461.
- Toll-like Receptors in the Vascular System: Sensing the Dangers Within. Goulopoulou S, McCarthy CG, Webb RC. Pharmacol Rev. 2016 Jan; 68(1):142-67.
- Toll Like Receptors Signaling Pathways as a Target for Therapeutic Interventions. Jezierska A, Kolosova IA, Verin AD. Curr Signal Transduct Ther. 2011; 6(3):428-440.
- Yuminamochi E, Koike T, Takeda K, Horiuchi I, Okumura K. Interleukin-12- and interferon-gamma-mediated natural killer cell activation by Agaricus blazei Murill. Immunology 2007;121:197-206.
- Sharma S, Zhu L, Davoodi M, Harris-White M, Lee JM, St John M, Salgia R, Dubinett S. TLR3 agonists and proinflammatory antitumor activities. Expert Opin Ther Targets 2013;17:481-3.
- Davis MB, Vasquez-Dunddel D, Fu J, Albesiano E, Pardoll D, Kim YJ. Intratumoral administration of TLR4 agonist absorbed into a cellular vector improves antitumor responses. Clin Cancer Res 2011;17:3984-92
- Geng D, Zheng L, Srivastava R, Asprodites N, Velasco-Gonzalez C, Davila E. When Toll-like receptor and T-cell receptor signals collide: a mechanism for enhanced CD8 T-cell effector function. Blood 2010;116:3494-504
- Estornes Y, Toscano F, Virard F, Jacquemin G, Pierrot A, Vanbervliet B, Bonnin M, Lalaoui N, Mercier-Gouy P, Pacheco Y, Salaun B, Renno T, Micheau O, Lebecque S. dsRNA induces apoptosis through an atypical death complex associating TLR3 to caspase-8. Cell Death Differ 2012;19:1482-94.
- Fucikova J, Moserova I, Urbanova L, Bezu L, Kepp O, Cremer I, Salek C, Strnad P, Kroemer G, Galluzzi L, Spisek R. Prognostic and predictive value of DAMPs and DAMP-associated processes in cancer. Front Immunol 2015;6:402.
- Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, Muhammad AK, Clark MC, Arditi M, Comin-Anduix B, Ribas A, Lowenstein PR, Castro MG. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med 2009;6:e10
- Jin Y, Wi HJ, Choi MH, Hong ST, Bae YM. Regulation of anti-inflammatory cytokines IL-10 and TGF-beta in mouse dendritic cells through treatment with Clonorchis sinensis crude antigen. Exp Mol Med 2014;46:e74
- Luddy KA, Robertson-Tessi M, Tafreshi NK, Soliman H, Morse DL. The role of toll-like receptors in colorectal cancer progression: evidence for epithelial to leucocytic transition. Front Immunol 2014;5:429
- Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol 2013;93:847-63.
- Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res 2005;65:5009-14
- Targeting Toll-like receptors against cancer. Cancer Metastasis Treat 2016;2:463-70. https://jcmtjournal.com/article/view/1774
- Salvador B., Arranz A., Francisco S., Córdoba L., Punzón C., Llamas M.Á., Fresno M. Modulation of endothelial function by toll like receptors. Pharmacol. Res. 2016;108:46–56. doi: 10.1016/j.phrs.2016.03.038
- Nishimura M., Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol. Pharm. Bull. 2005;28:886–892. doi: 10.1248/bpb.28.886
- Mann D.L. The emerging role of innate immunity in the heart and vascular system: For whom the cell tolls. Circ. Res. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936
- Spirig R., Tsui J., Shaw S. The emerging role of TLR and innate immunity in cardiovascular disease. Cardiol. Res. Pract. 2012;2012:1–12. doi: 10.1155/2012/181394
- Abajobir A.A., Abbafati C., Abbas K.M., Abd-Allah F., Abera S.F., Aboyans V., Adetokunboh O., Afshin A., Agrawal A., Ahmadi A., et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9
- The Role of Toll-like Receptors in Oncotherapy. Oncol Res. 2019 Mar 25. doi: 10.3727/096504019X15498329881440. [Epub ahead of print] https://www.ingentaconnect.com/content/cog/or/pre-prints/content-orm-a-2617_liu;jsessionid=fmph35d032bc.x-ic-live-03
- Ying J, Zhou HY, Liu P, You Q, Kuang F, Shen YN, Hu ZQ. Aspirin inhibited the metastasis of colon cancer cells by inhibiting the expression of toll-like receptor 4. Cell Biosci. 2018;8:1
- Rehman SU, Ali T, Alam SI, Ullah R, Zeb A, Lee KW, Rutten BPF, Kim MO. Ferulic Acid Rescues LPS-Induced Neurotoxicity via Modulation of the TLR4 Receptor inthe Mouse Hippocampus. Mol Neurobiol. 2018.
- Paone A, Starace D, Galli R, Padula F, De Cesaris P, Filippini A, Ziparo E, Riccioli A. Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-alpha-dependent mechanism. Carcinogenesis 2008;29(7):1334-42
- Zheng Q, Xu J, Lin Z, Lu Y, Xin X, Li X, Yang Y, Meng Q, Wang C, Xiong W, Lu D. Inflammatory factor receptor Toll-like receptor 4 controls telomeres through heterochromatin protein 1 isoforms in liver cancer stem cell. J Cell Mol Med. 2018;22(6):3246-58.
- Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11:807–22.
- Seibl R, Birchler T, Loeliger S. et al. Expression and regulation of toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–1227
- Leadbetter EA, Rifkin IR, Hohlbaum AM. et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607
- Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–41
- Sweet RA, Cullen JL, Shlomchik MJ. Rheumatoid factor B cell memory leads to rapid, switched antibody-forming cell responses. J Immunol. 2013;190:1974–81.
- Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–72
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7
- Vogelpoel LT, Hansen IS, Rispens T, Muller FJ, van Capel TM, Turina MC, et al. Fc gamma receptor-TLR cross-talk elicits pro-inflammatory cytokine production by human M2 macrophages. Nat Commun. 2014;5:5444
- Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. The Journal of experimental medicine. 2006;203:2673–82
- Davis ML, LeVan TD, Yu F, Sayles H, Sokolove J, Robinson W, et al. Associations of toll-like receptor (TLR)-4 single nucleotide polymorphisms and rheumatoid arthritis disease progression: An observational cohort study. Int Immunopharmacol. 2015;24:346–52
- Popa C, Abdollahi-Roodsaz S, Joosten LA, Takahashi N, Sprong T, Matera G, et al. Bartonella quintana lipopolysaccharide is a natural antagonist of Toll-like receptor 4. Infect Immun. 2007;75:4831–7
- Pawar RD, Ramanjaneyulu A, Kulkarni OP, Lech M, Segerer S, Anders HJ. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J Am Soc Nephrol. 2007;18:1721–31
- Bartfai T, Behrens MM, Gaidarova S, Pemberton J, Shivanyuk A, Rebek J., Jr A low molecular weight mimic of the Toll/IL-1 receptor/resistance domain inhibits IL-1 receptor-mediated responses. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7971–6
- Chatzigeorgiou A, Seijkens T, Zarzycka B, Engel D, Poggi M, van den Berg S, et al. Blocking CD40-TRAF6 signaling is a therapeutic target in obesity-associated insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2686–91