What is tracheomalacia
Tracheomalacia is a localized or generalized weakness and floppiness of the walls of the windpipe (trachea, or airway), which creates airway obstruction resulting in different degrees of symptoms 1. Tracheomalacia can be acquired tracheomalacia also known as secondary tracheomalacia, that develops after birth. Or congenital tracheomalacia, which is weakness and floppiness of the walls of the windpipe (trachea) that it is present at birth. All types of tracheomalacia are extremely rare; no definite incidence rates are available 2.
Tracheomalacia can be isolated or associated with other anomalies such as anterior vascular compression, esophageal atresia with tracheo-esophageal fistula or gastro-esophageal reflux 1.
Tracheomalacia is often undetected or misdiagnosed, often as asthma, recurrent croup or simply noisy breathing. You may have been told that this is the “new normal” for your child or that your child will eventually grow out of it. Many families — and even some doctors — do not know that there are surgical treatments for tracheomalacia.
Tracheomalacia can be categorized into three groups on the basis of histologic, endoscopic, and clinical presentation, as follows:
- Type 1 presents as congenital or intrinsic tracheal abnormalities that can be associated with a tracheoesophageal fistula or esophageal atresia
- Type 2 presents as extrinsic defects or anomalies, such as a vascular ring causing undue pressure on the trachea
- Type 3 presents as acquired tracheomalacia that occurs with prolonged intubation, chronic tracheal infections, or inflammatory conditions like relapsing polychondritis
Immaturity of the tracheobronchial cartilage is thought to be the cause in type 1, whereas degeneration of previously healthy cartilage is thought to produce other types. Inflammatory processes, extrinsic compression from vascular anomalies, or neoplasms may produce degeneration.
Although, in some cases, spontaneous improvement can occur, some children don’t outgrow more severe forms of tracheomalacia. Instead, they adapt to it and learn to live with the discomfort and complications. Tracheomalacia can also result in severe cough, respiratory distress episodes or “near-death” spells (acute life-threatening events).
Amongst several possible treatments, including tracheostomy and non invasive ventilation, airway stenting, and surgical approaches, aortopexy is a favored option in many centers 1. Aortopexy means lifting anteriorly the aorta and suturing it to the posterior surface of the sternum. As the anterior tracheal wall is attached through pre-tracheal fascia to the posterior aortic wall, the tracheal lumen is opened by aortopexy.
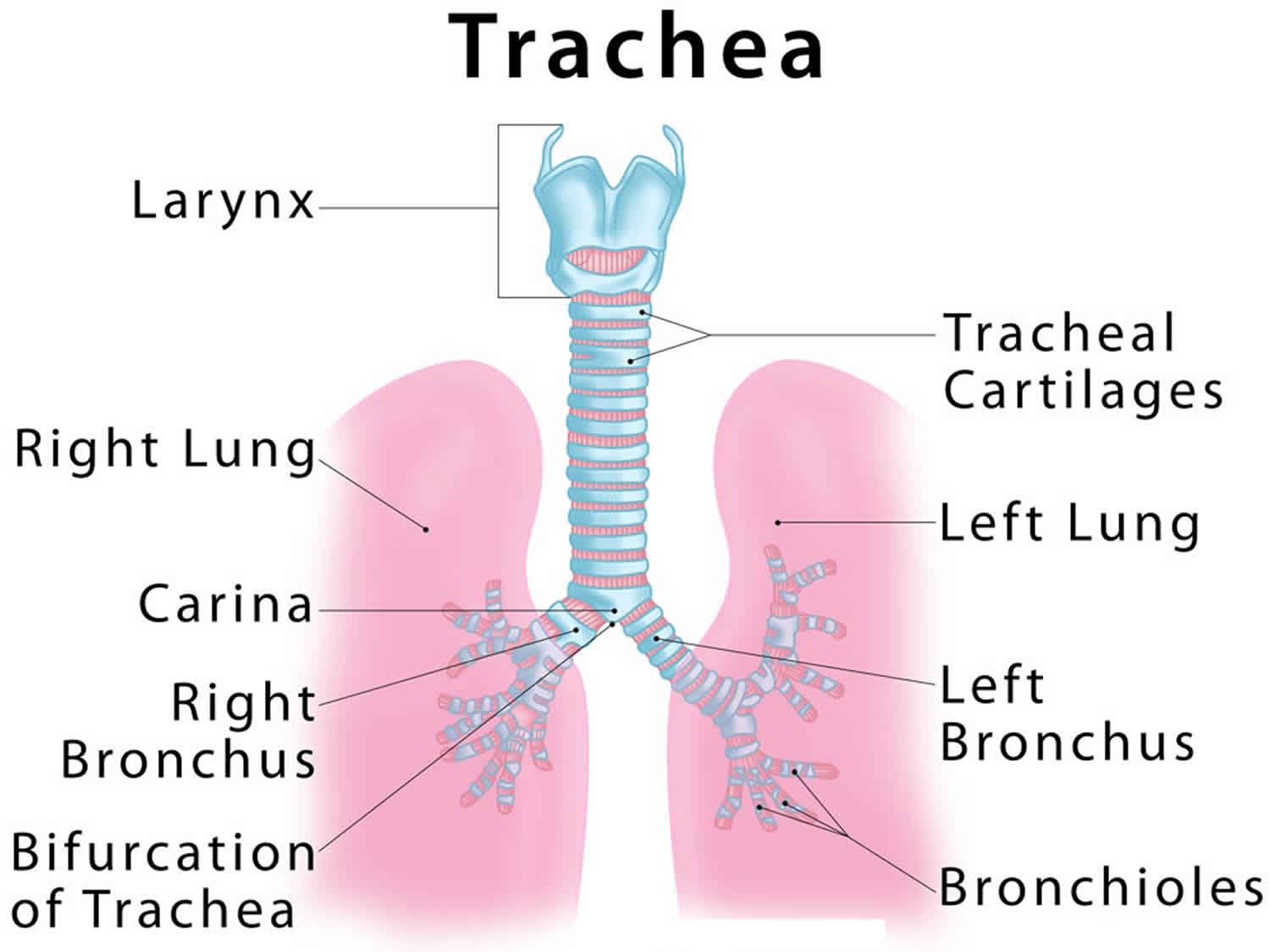
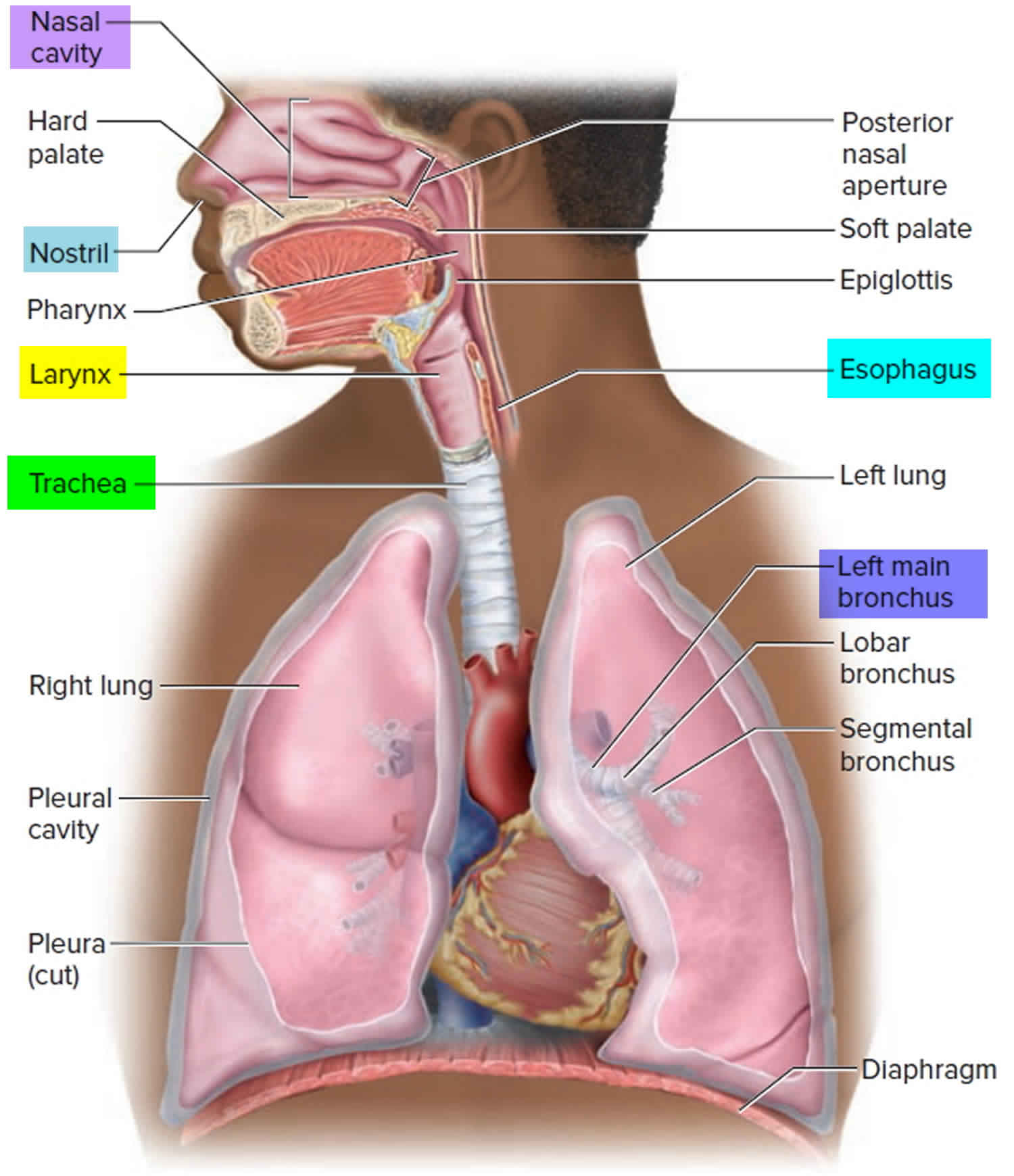
Trachea anatomy
The trachea commences at the cricoid cartilage and terminates at the fifth thoracic vertebra. It lengthens and dilates during inspiration and narrows and shortens during expiration. Fifteen to 20 incomplete rings of cartilage prevent it from collapsing.
The trachea is separated from the vertebral column by the esophagus posteriorly.
In the thorax, the jugular venous arch lies anteriorly at the sternum; the brachiocephalic trunk and left common carotid artery lie at the level of the third thoracic vertebra.
The arch of the aorta is to the left and front of the distal trachea just before it bifurcates. On the right of the trachea are pleura, on the left is the aortic arch, and posterolaterally is the left subclavian artery.
The relation of the trachea to the aortic arch makes it liable to compress from aneurysm or from vascular rings, which occur with abnormal arterial development. Therefore, for distal tracheomalacia, whether associated with tracheoesophageal fistula or with vascular anomalies, aortopexy is the procedure of choice.
Figure 1. Trachea anatomy
How does tracheomalacia affect feeding, sleeping, and breathing?
Signs and symptoms of tracheomalacia vary depending on where the narrowing occurs and if it is mild or severe. If a large area is involved the symptoms tend to begin earlier. The child may have noisy, rattling breathing that changes with body position and improves during sleep. Breathing problems can get worse with coughing, crying, and feeding. Also, children with tracheomalacia tend to have more frequent upper respiratory infections that can also make breathing problems worse 3.
Are babies with tracheomalacia more likely to have other congenital defects?
Congential tracheomalacia can occur alone, but often occurs along with other birth defects of the airway, such as laryngomalacia, bronchomalacia, largelaryngeal clefts, tracheo-esophageal fistulae or esophageal atresia. These airway problems can occur alone or in association with a variety of conditions/syndromes such as craniofacial disorders, chromosome anomalies, mucopolysaccharidase deficiency, and heritable connective tissue disorders 4.
What is the long-term outlook for congenital tracheomalacia?
The long-term outlook of congenital tracheomalacia is good in children with no associated problems. These children tend to improve by age 2 4. Children who have tracheomalacia in combination with other malformations tend to have symptoms that last into later childhood 5. Studies suggest that some people with tracheomalacia have exercise intolerance as adults 5.
Possible serious complications of tracheomalacia include complete airway blockage, repeat infection, respiratory failure, and failure to thrive. Treatments to prevent these complications include positive pressure ventilatory support (cPAP) or surgery (e.g., aortopexy, tracheopexy, tracheal stent). Surgery is reserved for treatment of very serious cases 5.
Tracheomalacia causes
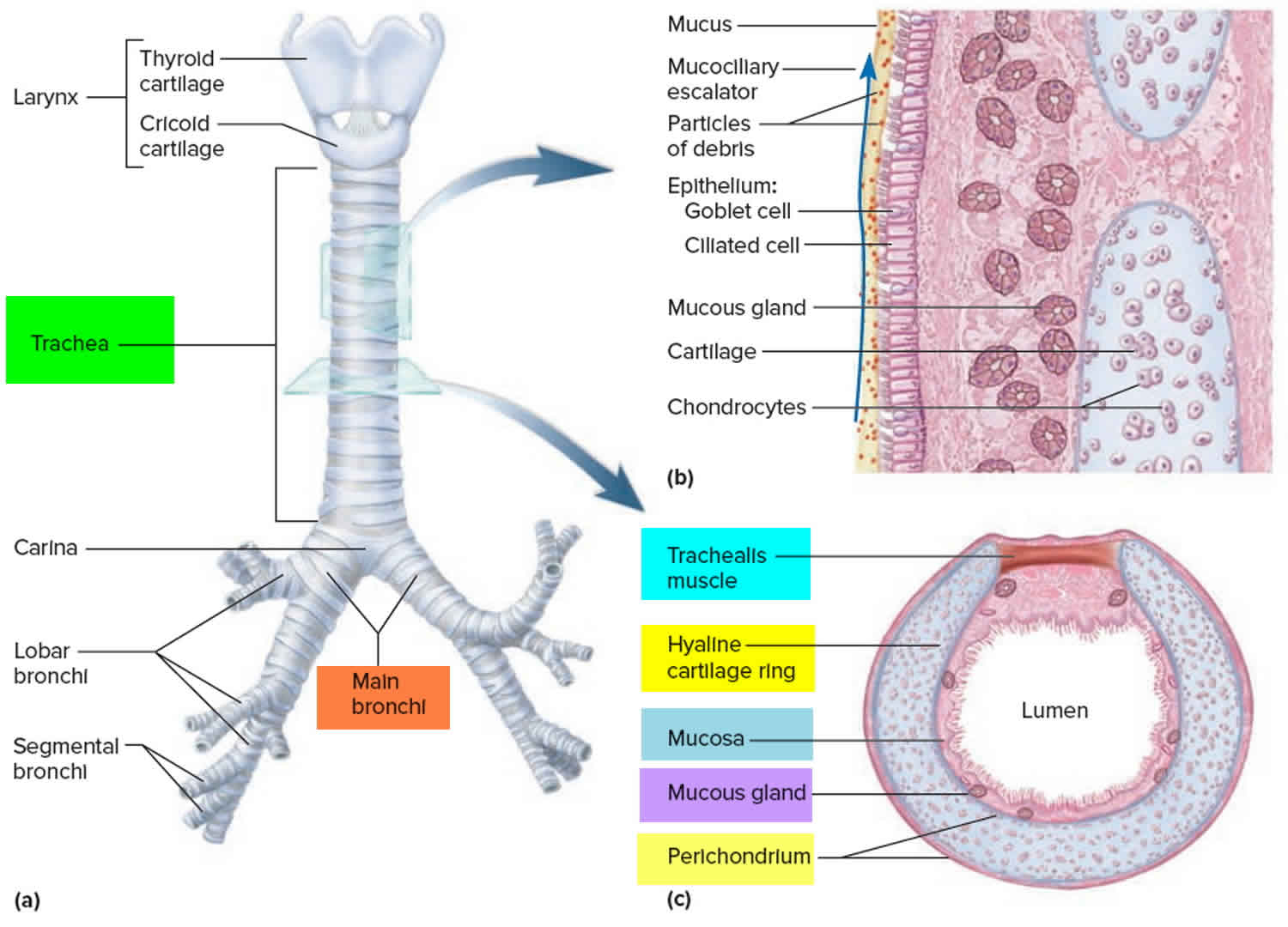
Tracheomalacia is a structural abnormality of the tracheal cartilage allowing collapse of its walls and airway obstruction. A deficiency and/or malformation of the supporting cartilage exists, with a decrease in the cartilage-to-muscle ratio.
A healthy trachea is supported by a series of C-shaped rings made of cartilage that help your child’s airway to stay open during exhalation. The most common form of tracheomalacia occurs when the rings are wide and shaped more like a letter “U,” causing the membrane at the back of the airway to interfere with breathing and restrict airflow.
Tracheomalacia most commonly affects the distal third of the trachea. By virtue of its intrinsic flexibility, or compliance, the trachea changes caliber during the respiratory cycle. Tracheal dilatation and lengthening occurs during inspiration; narrowing and shortening occurs during expiration. Accentuation of this cyclic process may cause excessive narrowing of tracheal lumen, thus deforming the entire length or a localized segment. However, it is rarely found in combination with laryngomalacia because of the separate developmental pathways for the trachea and the larynx.
In general, abnormal collapsibility denotes a loss of structural rigidity, such as softening, better expressed as abnormally increased compliance. Any disease process affecting the integrity of the tracheal wall is apt to cause a change in tracheal compliance. The anatomic defect may be trivial or even may escape detection. The functional interference with ventilation may cause expiratory flow obstruction and interfere with clearance of secretions.
Functional impairment is proportional to the length of the involved segment and the degree of stenosis. Furthermore, kinking may occur at the transition between healthy tracheal wall and the indurated segment, as well as in the malacic segment. In diffuse tracheal disease or extensive peritracheal adhesions, the trachea usually distends unevenly during inspiration and collapses during expiration, thus interfering with the tracheal function.
It is often incorrectly assumed that the condition results when the cartilage that goes around the trachea isn’t strong enough to fully support it. While this theory has long been taught, newer research and our extensive experience has proven this to be an uncommon cause. Other types of tracheomalacia occur in the lower trachea or bronchi (the branching airways) — known as bronchomalacia — or are caused by a cyst (mass) in the chest or in the airway itself. Almost all babies with esophageal atresia have some degree of tracheomalacia. Sometimes, however, tracheomalacia can occur on its own, without another condition.
Tracheomalacia may be congenital (present at birth), or acquired later. Acquired tracheomalacia, also known as secondary tracheomalacia, can be caused by:
- a previous treatment for esophageal atresia or another medical condition
- heart anomalies, such as vascular rings
- other internal structures or masses that push on the trachea, causing it to narrow
- recurrent infection
- tracheostomy tubes
Congenital tracheomalacia
Congenital tracheomalacia is very uncommon. Tracheomalacia in infants occurs when the cartilage in the windpipe (trachea) has not developed properly that makes it difficult to keep the airway open 6. Instead of being rigid, the walls of the trachea are floppy. The trachea can collapse when breathing out. Because the windpipe (trachea) is the main airway, breathing problems begin soon after birth.
Congenital tracheomalacia can be associated with a variety of congenital anomalies, including cardiovascular defects, developmental delay, esophageal anomalies, and gastro-esophageal reflux. Congenital tracheomalacia can be caused by a diffuse process of congenital origin or by a localized abnormality such as a vascular ring, anomalous innominate artery, esophageal atresia 7 and tracheoesophageal fistula. Internal compression by an endobronchial or tracheostomy tube also may be the culprit. Tracheal cartilage deficiency may be present in 75% of the patients with tracheoesophageal fistula. Tracheomalacia rarely is found in combination with laryngomalacia.
Congenital tracheomalacia symptoms vary from mild to severe and may include noisy breathing (stridor), shortness of breath, difficulty breathing, and bluish skin (cyanotic spells). Symptoms typically worsen during periods of activity. Tracheomalacia can occur on its own or along with other airway problems. It can also occur with congenital abnormalities that affect other parts of the body 8. Tracheomalacia often resolves on its own by the second year of life 6. Treatment of symptoms may include humidified air, chest physical therapy, or continuous positive airway pressure (CPAP) for respiratory distress 8. Severe tracheomalacia may need to be treated with surgery 6.
Congenital tracheomalacia symptoms
Symptoms can range from mild to severe.
There are many types of tracheomalacia, and each child is different, but some common signs include:
- Breathing noises that may change with position and improve during sleep
- Breathing problems that get worse with coughing, crying, feeding, or upper respiratory infections (such as cold)
- High-pitched breathing
- Rattling or noisy breathing (stridor)
- Frequent infections in the airway, such as bronchitis or pneumonia (because your child can’t cough or otherwise clear his lungs)
- Frequent noisy cough
- Exercise intolerance
More severe signs may include:
- Choking during feeding
- A halt in breathing, particularly when crying or during strenuous activity
- Blue spells (child appears blue because his heart isn’t beating quickly enough)
Congenital tracheomalacia possible complications
Babies born with tracheomalacia may have other congenital abnormalities, such as heart defects, developmental delay, or gastroesophageal reflux.
Aspiration pneumonia can occur from inhaling food into the lungs or windpipe.
Congenital tracheomalacia diagnosis
A physical exam confirms the symptoms. A chest x-ray will be done to rule out other problems. The x-ray may show narrowing of the trachea when breathing in.
A procedure called laryngoscopy provides the most reliable diagnosis. In this procedure, an otolaryngologist (ear, nose, and throat doctor, or ENT) will look at the structure of the airway and determine how severe the problem is.
The best is a procedure called a three-phase dynamic bronchoscopy. During this test, your child’s doctor will use a thin instrument called a bronchoscope to look in your child’s airway in three different situations: during shallow breathing, during vigorous coughing and when the airways have been distended with water.
The physician may also use a dynamic airway CT scan, a noninvasive procedure that uses x-ray equipment and computers to create detailed, cross-sectional images of your child’s body.
Other tests may include:
- Airway fluoroscopy — a kind of x-ray that shows the images on a screen
- Barium swallow
- Bronchoscopy — camera down the throat to see the airways and lungs
- CT scan
- Lung function tests
- Magnetic resonance imaging (MRI)
Congenital tracheomalacia treatment
Most infants respond well to humidified air, careful feedings, and antibiotics for infections. Babies with tracheomalacia must be closely monitored when they have respiratory infections.
Your infant should be monitored closely by their care team and may benefit chest physical therapy and perhaps a continuous positive airway pressure (CPAP) device and a pulmonary clearance regimen supervised by a pulmonologist.
Often, the symptoms of tracheomalacia improve as the infant grows.
However, if your child is having blue spells, choking when eating, episodes of choking, problems with noisy breathing, coughing or exercise intolerance or recurrent pneumonia despite maximal medical treatment, surgery may be necessary. The treatments depend on the type and locations of the tracheomalacia, and each treatment is customized for the individual child.
Rarely, surgery is needed.
Surgical options include:
Aortopexy
- This safe and reliable procedure provides immediate and permanent relief of some types of severe tracheomalacia. This surgery opens up the trachea by moving up the aorta (the body’s main blood vessel) and attaching it to the back of the breastbone (sternum).
Tracheopexy
- Similar to an aortopexy, this procedure opens up and supports the airway by suspending the front of the tracheal wall from the back of the sternum. Sometimes the thymus gland is removed to create more space between the aorta and the sternum.
Bronchopexy
- This procedure may improve airflow in airway collapse that involves the bronchial airways.
Posterior aortopexy
In some situations, the aorta on the spine compresses the back of the airway. In these children, moving the aorta back can take the pressure off the airway and improve airflow.
Posterior tracheopexy
- This operation was developed at Boston Children’s Hospital where surgeons can support the flexible back wall of the trachea against the spine. It is much more effective than the other options alone and can be used in combination with the other procedures.
Tracheal diverticulum resection
- After esophageal atresia with tracheoesophageal fistula repair, a pouch or diverticulum usually remains that can trap secretions and collapse the airway. Our surgeons have developed techniques to completely resect (remove) these lesions to make the airway nearly normal.
Combined procedures
Surgeons very often discover more than one problem with the airway, esophagus or both. Using a variety of techniques, they will correct all the problems in one combined procedure. This procedure may include:
- Anterior/posterior tracheopexies
- Slide tracheoplasty: A technique to make the airway larger
- Rotational esophagolplasty: A technique to rotate the esophagus away from the trachea so that there is no chance for recurrence of a tracheoesophageal fistula
- Descending aortopexy
Placing a stent
- Your child’s doctor may also choose to temporarily place a stent in your child’s trachea. This narrow tube holds the trachea open and encourages the tissues to grow around it. Stents are generally avoided in favor of other surgical options; however, our doctors have the most experience with tracheal stents in the country.
Congenital tracheomalacia prognosis
The long-term outlook of congenital tracheomalacia is good in children with no associated problems. Congenital tracheomalacia most often goes away on its own by the age of 18 to 24 months 9. As the cartilage gets stronger and the trachea grows, the noisy and difficult breathing slowly improves. People with tracheomalacia must be monitored closely when they have respiratory infections.
Children who have tracheomalacia in combination with other malformations tend to have symptoms that last into later childhood 10. Studies suggest that some people with tracheomalacia have exercise intolerance as adults 10.
Possible serious complications of tracheomalacia include complete airway blockage, repeat infection, respiratory failure, and failure to thrive. Treatments to prevent these complications include positive pressure ventilatory support (cPAP) or surgery (e.g., aortopexy, tracheopexy, tracheal stent). Surgery is reserved for treatment of very serious cases 11.
Tracheomalacia in adults (acquired tracheomalacia)
Acquired tracheomalacia is a weakness and floppiness of the walls of the windpipe (trachea, or airway) that develops after birth.
The cases of acquired tracheomalacia occur with increasing frequency both in children and in adults, and the tracheomalacia often is not recognized clearly. Acquired tracheomalacia usually cause focal tracheomalacia and may result from indwelling tracheostomy 12 and endobronchial tube, chest trauma, chronic tracheobronchitis, and inflammation (relapsing polychondritis). Acquired tracheomalacia may be secondary to pulmonary resection and tracheal malignancy (cylindroma), and they may be idiopathic.
Secondary (acquired) adult tracheomalacia may be classified as follows 13:
- Posttraumatic (postintubation, posttracheotomy, external chest trauma, post-lung transplantation)
- Emphysema
- Chronic bronchitis
- Chronic inflammation (relapsing polychondritis) 14
- Chronic external compression of trachea (malignancy, benign tumors, cysts, abscesses, aortic aneurysm)
- Vascular rings (undiagnosed in childhood)
Acquired tracheomalacia causes
Acquired tracheomalacia is very uncommon at any age. It occurs when normal cartilage in the wall of the windpipe begins to break down.
Acquired tracheomalacia may result:
- When large blood vessels put pressure on the airway
- As a complication after surgery to repair birth defects in the windpipe and esophagus (the tube that carries food from the mouth to the stomach)
- After having a breathing tube or trachea tube (tracheostomy) for a long time
Acquired tracheomalacia symptoms
Symptoms of tracheomalacia include:
- Breathing problems that get worse with coughing, crying, or upper respiratory infections, such as a cold
- Breathing noises that may change when body position changes, and improve during sleep
- High-pitched breathing
- Rattling, noisy breaths
Acquired tracheomalacia possible complications
Aspiration pneumonia (a lung infection) can occur from breathing in food.
Adults who develop tracheomalacia after being on a breathing machine often have serious lung problems.
Acquired tracheomalacia diagnosis
A physical exam confirms the symptoms. A chest x-ray may show narrowing of the trachea when breathing out. Even if the x-ray is normal, it is needed to rule out other problems.
A procedure called a laryngoscopy is used to diagnose the condition. This procedure allows the otolaryngologist (ear, nose, and throat doctor, or ENT) to see the structure of the airway and determine how severe the problem is.
Other tests may include:
- Airway fluoroscopy
- Barium swallow
- Bronchoscopy
- CT scan
- Lung function tests
- Magnetic resonance imaging (MRI)
Acquired tracheomalacia treatment
Acquired tracheomalacia may improve without treatment. However, people with tracheomalacia must be monitored closely when they have respiratory infections.
Adults with breathing problems may need continuous positive airway pressure (CPAP). Rarely, surgery is needed. A hollow tube called a stent may be placed to hold the airway open.
Tracheomalacia symptoms
Infants present after a few weeks after birth with expiratory stridor (also called laryngeal crow). Expiratory stridor may worsen with supine position, crying, and respiratory infections. Feeding difficulties are reported sometimes. Hoarseness, aphonia, and breathing also may be reported.
There are many types of tracheomalacia, and each person is different, but some common signs include:
- Breathing noises that may change with position and improve during sleep
- Breathing problems that get worse with coughing, crying, feeding, or upper respiratory infections (such as cold)
- High-pitched breathing
- Rattling or noisy breathing (stridor)
- Frequent infections in the airway, such as bronchitis or pneumonia (because your child can’t cough or otherwise clear his lungs)
- Frequent noisy cough
- Exercise intolerance
More severe signs may include:
- Choking during feeding
- A halt in breathing, particularly when crying or during strenuous activity
- Blue spells (child appears blue because his heart isn’t beating quickly enough)
Tracheomalacia diagnosis
A physical exam confirms the symptoms. A chest x-ray will be done to rule out other problems. The x-ray may show narrowing of the trachea when breathing in.
A procedure called laryngoscopy provides the most reliable diagnosis. In this procedure, an otolaryngologist (ear, nose, and throat doctor, or ENT) will look at the structure of the airway and determine how severe the problem is.
The best is a procedure called a three-phase dynamic bronchoscopy. During this test, your child’s doctor will use a thin instrument called a bronchoscope to look in your child’s airway in three different situations: during shallow breathing, during vigorous coughing and when the airways have been distended with water.
The physician may also use a dynamic airway CT scan, a noninvasive procedure that uses x-ray equipment and computers to create detailed, cross-sectional images of your child’s body.
Other tests may include:
- Airway fluoroscopy — a kind of x-ray that shows the images on a screen
- Barium swallow
- Bronchoscopy — camera down the throat to see the airways and lungs
- CT scan
- Lung function tests
- Magnetic resonance imaging (MRI)
Tracheomalacia treatment
Current recommendations for treatment of tracheomalacia include the following:
- Forms of milder primary tracheomalacia are best treated by nonsurgical means 15
- For distal tracheomalacia that is idiopathic, pulsatile, or associated with tracheoesophageal fistula or vascular anomalies, aortopexy with concomitant intraoperative bronchoscopy appears to be the procedure of choice
- For proximal or diffuse tracheomalacia, tracheostomy (despite its related high morbidity) or the use of stents is beneficial 13
- As experience accumulates, a direct surgical approach to treating tracheomalacia may replace tracheostomy in the management of proximal and diffuse tracheomalacia; these procedures include prosthetic stenting, tracheoplasty, and tracheal resection with end-to-end anastomosis
In one study 16, silicone stents were inserted into the trachea or left main-stem bronchus in 14 children (aged 2-69 months) for tracheomalacia or airway kinking (7 cases), vascular compression (5 cases), and surgically-corrected congenital tracheal stenoses (2 cases). The best results were obtained in tracheomalacia. Six cases out of 14 (43%) were considered successful, and five cases were considered failures, primarily because of stent migration.
An earlier study 17 reviewed conservative therapy, tracheostomy, aortopexy, or tracheal reconstruction in 41 infants with tracheomalacia. Fifteen patients with mild primary tracheomalacia had resolution of symptoms by the age of 2 years; five treated with tracheostomy developed secondary tracheomalacia at the site. In nine patients with primary tracheomalacia treated with aortopexy, five were symptom-free, one was improved, and three procedures were unsuccessful.
Of the 10 patients in the acquired group treated with aortopexy, six were cured, two were improved, and treatment failed in two 17. Of the 6 patients with tracheostomy, three eventually were extubated, one had major reconstruction, and two had tracheostomies for an extended period.
Medical Therapy
Tracheomalacia of the milder primary variety is best treated by nonsurgical means.
Most infants who have mild-to-moderate symptoms should be offered conservative therapy because these patients improve by age 18-24 months 12. The majority respond to such therapy, consisting of humidified air, chest physical therapy, slow and careful feedings, and control of infection and secretions with antibiotics.
The use of continuous positive airway pressure (CPAP) has been recommended in patients having respiratory distress and may be successful in patients requiring a short-term intervention as the disorder spontaneously resolves 13.
Surgical Therapy
Tracheomalacia generally is benign; most infants outgrow the symptoms by age 18-24 months 13. Surgical therapy is required when conservative measures are not adequate or when reflex apnea is present. Surgery includes correction of the underlying cause, such as vascular ring when present, tracheostomy, and aortopexy 12.
The indications for tracheostomy are severe symptoms, failure of conservative therapy, and proximal or diffuse tracheomalacia. The indications for aortopexy are dying spells or reflex apnea, recurrent pneumonia, intermittent respiratory obstruction, and inability to extubate airway in an infant who is intubated. Surgery only is recommended for severe symptoms and failure of conservative therapy 12.
During surgery, a careful search should be made for tracheoesophageal fistula, which should be treated surgically if present. Other causes of tracheal compression, such as mediastinal tumors or vascular rings, also need to be corrected surgically. Patients identified as having vascular anomalies compressing distal trachea should have constricting vessels surgically divided and affixed to other structures to eliminate impingement on the trachea 18.
Tracheomalacia following long-standing tracheotomies may be helped by anterior cricoid/tracheal suspension, where muscular tissue of the overlying trachea is sutured to the fascia of strap muscles.
Acquired tracheomalacia, if severely symptomatic, can be treated by internal stenting, external stenting, or tracheostomy.
The use of various types of tubes and stents for the management of tracheomalacia is helpful 19. Reports exist of success with Montgomery and Dumon tubes in the literature. Short-term satisfactory results also have been reported with the use of expandable metallic stent (Palmaz Stent) placement in patients with intractable respiratory symptoms caused by tracheomalacia.
A report of aortopexy in 28 children with severe and localized tracheomalacia utilized a left lateral muscle-sparing approach. The indications included acute life-threatening events in 22 patients, failure to extubate in five, and recurrent pneumonia in one. Associated esophageal atresia was present in 15 patients, and 13 had primary tracheomalacia. Most symptoms of tracheomalacia resolved in 26 of the 28 patients after aortopexy 20.
Video-assisted posterior tracheopexy, with or without robotic guidance, has been suggested as a possible option for select children with severe tracheomalacia 21.
Tracheostomy
Tracheostomy helps maintain an airway while the child grows and the trachea regains structural integrity, but the problem with this procedure is that the tracheostomy tube may not support the distal trachea. The tracheostomy can be performed as an open procedure or via a percutaneous approach.
With the patient in supine position, the neck is placed in moderate hypertension. Identify cricoid cartilage and the thyroid isthmus, and aim to place the opening over the third tracheal ring. A transverse incision is made, the pretracheal fascia is divided, and the tracheal rings are counted. The third tracheal ring is identified and divided in the midline; the tracheal incision must be vertical. The second and fourth rings may need to be divided as well. No amount of tracheal tissue is removed during the procedure.
The stoma is enlarged by gently spreading the blades of the hemostat against the margins of the tracheal opening. A lubricated tracheostomy tube is inserted through this opening. Transtracheal injection of lidocaine reduces coughing and eases tube placement. The tube is secured to the neck and adjusted so that the distal end is at least 2 cm above the carina.
Percutaneous tracheostomy
The percutaneous tracheostomy can be performed in the intensive care unit (ICU) and requires specially designed introducer sets. After prepping the patient’s neck, a 3-cm longitudinal incision is made over the second and third cartilaginous tracheal rings. The endotracheal tube is withdrawn somewhat, and the introducer catheter is advanced into the tracheal lumen. Confirm the intratracheal location either under bronchoscopic guidance or though the withdrawal of air bubbles. The introducer catheter is advanced into the trachea, and the syringe and steel needle of the introducer catheter are withdrawn.
The flexible J-tipped guide wire is inserted into the trachea through the introducer catheter, and the catheter is removed. Thereafter, an introducing dilator is advanced into the trachea until the black positioning mark. The tapered sequential dilators are used successively to dilate the anterior tracheal wall to a diameter larger than the tracheostomy tube. A tracheostomy tube over the tapered dilator is advanced into the trachea, and dilator, guiding catheter, and wire guide are removed. The inner cannula is inserted, and the patient is attached to the ventilator. A chest radiograph should confirm the correct positioning.
Aortopexy
Aortopexy can provide relief of tracheal compression and relieves the external pressure on the flaccid trachea. This is not a perfect operation, because of the low but significant failure rate and the potential for complications.
The patient is positioned with the left shoulder elevated at a 30º-45º angle. A bronchoscopy is performed to confirm the diagnosis of tracheal compression. Through a left anterior thoracotomy, partial thymectomy improves the exposure and increases the effective cross-sectional area of the upper mediastinum. The apex of the left upper lobe is retracted inferiorly and posteriorly. The search for the vascular ring is conducted, and the esophagus is examined.
A single row of interrupted monofilament sutures is placed from the arch of the aorta to the undersurface of the sternum and tied down to displace the arch anteriorly. The bites into the aorta must be deep enough to include media and adventitia; sometimes, the sutures are passed through the sternum to a subcutaneous pocket. The dissection around the aorta must be avoided because these attachments help to draw open the lumen of the trachea when aortopexy has been achieved.
Aortopexy attaches the aorta to the sternum, pulling the anterior wall of the trachea forward and, therefore, preventing its collapse.
Treatment of tracheomalacia in adult patients
The finding may be incidental in many adults with tracheomalacia; these patients are asymptomatic and do not require therapy.
In symptomatic patients, care is initially supportive. Tracheomalacia frequently occurs in patients who also have chronic obstructive pulmonary disease (COPD), and the obstructive disorder optimally should be treated first. If conservative measures fail, noninvasive, positive-pressure ventilation can be used in the short term to keep the airway open and to facilitate secretion drainage. In selected patients, surgery may be used. Tracheostomy alone may be effective because the tracheostomy tube might bypass the malacic segment, or the tube itself might splint the airway open. If the patient has generalized and extensive disease, a longer tube may be necessary.
Surgical placation of the posterior wall of the trachea with crystalline polypropylene and high-density polyethylene mesh has been used recently 22. Via a right posterolateral thoracotomy, the mesh is fashioned into a 2.5-cm-wide strip, which is sutured to the posterior membranous wall. Thereafter, 2-cm sheets of mesh can be sutured to the right and left mainstem bronchi.
A range of stents can be utilized to keep the airway open mechanically. Metal stents have been used to manage airway obstruction. Such stents can be easily placed by flexible bronchoscopy, are visible on plain radiographs, expand dynamically, and preserve mucociliary function. Formation of granulation tissue, which can cause severe problems including airway obstruction, airway perforation, and death, is a potential complication. Silicone stents are easy to insert, reposition, and remove. However, placing these stents requires rigid bronchoscopy and general anesthesia.
Stents have resulted in both subjective and objective improvement 23. Most patients report immediate improvement in their respiratory symptoms, and airflow improves, but success is not universal. Gotway et al reported long-term pulmonary function improvement with stents placed for both tracheal stenosis and tracheomalacia 19.
Postoperative Care
Postoperative care of these patients is very similar to that of patients undergoing thoracic surgery. In the immediate postoperative period, patients may need to be monitored closely in an ICU setting because several days may pass before improvement in airway function occurs. These patients require long-term follow-up for evaluating the success or failure of the surgical procedure and the development of complications.
Complications of surgery
Long-standing tracheostomies lead to several complications, which include bilateral vocal cord paralysis; compression and erosion of the innominate artery; formation of secondary granulation tissue, which results in protraction of tracheomalacia; and speech delay in several instances.
During aortopexy, as the sutures are placed through aortic wall, there is an immediate risk of hemorrhage and a later potential for postoperative aneurysm formation. Deaths have occurred as a result of operative failures, other structural anomalies, and chronic ventilatory insufficiency.
Complications of percutaneous tracheostomy are bleeding, infection, accidental endotracheal extubation, extratracheal dilator position, esophageal perforation, and mucosal endobronchial flap. Some advantages exist over usual tracheostomy; the procedure is inexpensive and is easy to learn.
Tracheomalacia prognosis
With conservative measures, the symptoms often resolve spontaneously by age 18-24 months 13. Diffuse malacia of the airway of the congenital origin improves by age 6-12 months as the structural integrity of the trachea is restored gradually with resolution of the process.
Tracheostomy has been used to stent the airway until natural maturation of cartilage occurs. This often imposes a heavy penalty on the child; therefore, treatment alternatives should be explored.
Aortopexy has proven to be a safe, expedient way to relieve the problem of tracheomalacia in most patients. The success of aortopexy has been reported at about 75% in several small studies. Aortopexy has less long-term morbidity than tracheostomy. While not altering the structural characteristics of the tracheal wall, it widens the anterior-posterior tracheal dimension to maintain a patent lumen. The only treatment failures with aortopexy were patients with diffuse or proximal tracheal involvement.
References- Torre M, Carlucci M, Speggiorin S, Elliott MJ. Aortopexy for the treatment of tracheomalacia in children: review of the literature. Ital J Pediatr. 2012;38:62. Published 2012 Oct 30. doi:10.1186/1824-7288-38-62 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3502176/
- Jaquiss RD. Management of pediatric tracheal stenosis and tracheomalacia. Semin Thorac Cardiovasc Surg. 2004 Fall. 16(3):220-4.
- Tracheomalacia Symptoms & Causes. Boston Children’s Hospital. http://www.childrenshospital.org/conditions-and-treatments/conditions/t/tracheomalacia/symptoms-and-causes
- Hysinger EB, Panitch HB. Paediatric Tracheomalacia. Paediatr. Respir. Rev. January 2016; 17:9-15. https://www.ncbi.nlm.nih.gov/pubmed/25962857
- Oermann CM. Congenital anomalies of the intrathoracic airways and tracheoesophageal fistula. UpToDate. Dec 7, 2016.
- Snijders D, Barbato A. An Update on Diagnosis of Tracheomalacia in Children. Eur J Pediatr Surg. August, 2015; 25(4):333-335. https://www.ncbi.nlm.nih.gov/pubmed/26276910
- Beasley SW, Qi BQ. Understanding tracheomalacia. J Paediatr Child Health. 1998 Jun. 34(3):209-10.
- Tracheomalacia. https://emedicine.medscape.com/article/426003-overview
- Tracheomalacia – congenital. https://medlineplus.gov/ency/article/001084.htm
- Oermann CM. Congenital anomalies of the intrathoracic airways and tracheoesophageal fistula. UpToDate. Dec 7, 2016
- Tracheomalacia. http://www.childrenshospital.org/conditions-and-treatments/conditions/t/tracheomalacia
- Anton-Pacheco JL, Garcia-Hernandez G, Villafruela MA. The management of tracheobronchial obstruction in children. Minerva Pediatr. 2009 Feb. 61(1):39-52.
- Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005 Mar. 127(3):984-1005.
- Adliff M, Ngato D, Keshavjee S, Brenaman S, Granton JT. Treatment of diffuse tracheomalacia secondary to relapsing polychondritis with continuous positive airway pressure. Chest. 1997 Dec. 112 (6):1701-4.
- McNamara VM, Crabbe DC. Tracheomalacia. Paediatr Respir Rev. 2004 Jun. 5(2):147-54.
- Fayon M, Donato L, de Blic J, Labbé A, Becmeur F, Mely L, et al. French experience of silicone tracheobronchial stenting in children. Pediatr Pulmonol. 2005 Jan. 39 (1):21-7.
- Greenholz SK, Karrer FM, Lilly JR. Contemporary surgery of tracheomalacia. J Pediatr Surg. 1986 Jun. 21 (6):511-4.
- Sandu K, Monnier Y, Hurni M, Bernath MA, Monnier P, Wang Y, et al. Repair of tracheomalacia with inflammatory defect and mediastinitis. Ann Thorac Surg. 2011 Jan. 91(1):e14-6.
- Gotway MB, Golden JA, LaBerge JM, Webb WR, Reddy GP, Wilson MW, et al. Benign tracheobronchial stenoses: changes in short-term and long-term pulmonary function testing after expandable metallic stent placement. J Comput Assist Tomogr. 2002 Jul-Aug. 26 (4):564-72.
- Dave S, Currie BG. The role of aortopexy in severe tracheomalacia. J Pediatr Surg. 2006 Mar. 41(3):533-7.
- Kamran A, Hamilton TE, Zendejas B, Nath B, Jennings RW, Smithers CJ. Minimally Invasive Surgical Approach for Posterior Tracheopexy to Treat Severe Tracheomalacia: Lessons Learned from Initial Case Series. J Laparoendosc Adv Surg Tech A. 2018 Jul 5.
- Wright CD. Tracheomalacia. Chest Surg Clin N Am. 2003 May. 13(2):349-57, viii.
- Zinman R. Tracheal stenting improves airway mechanics in infants with tracheobronchomalacia. Pediatr Pulmonol. 1995 May. 19(5):275-81.