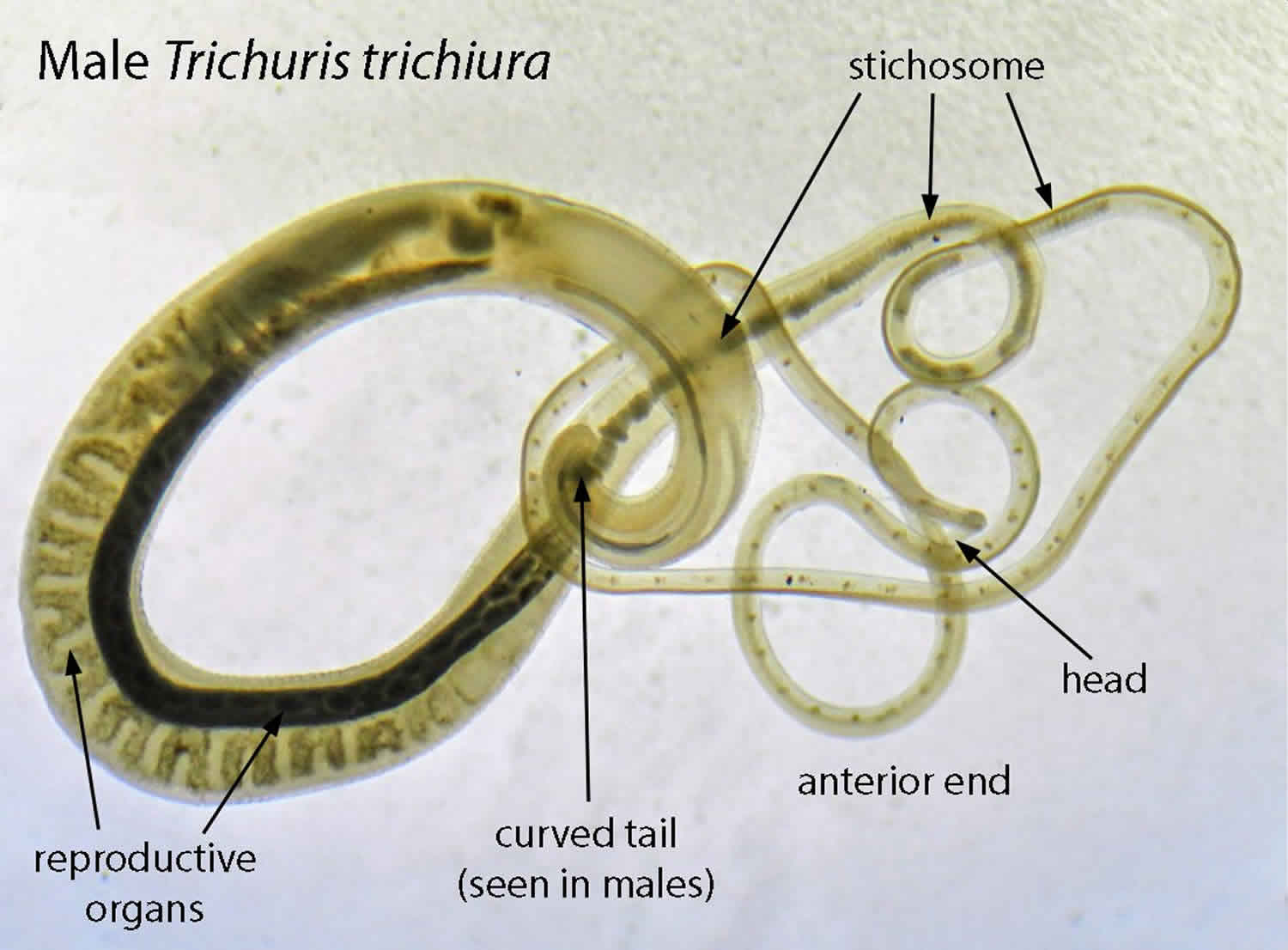
Trichuris trichiura
Trichuris trichiura also known as human whipworm, is an infection of the large intestine with Trichuris trichiura, a type of roundworm (nematode). Trichuris trichiura is referred to as the whipworm because it looks like a whip with wide handles at the posterior end. The whipworm has a narrow anterior esophagus and a thick posterior anus 1. Trichuris trichiura whipworms are usually pink and attach to the host via the slender anterior end. The size of Trichuris trichiura whipworms varies from 3 to 5 cm. The female usually larger than the male 2. The female Trichuris trichiura worm can lay anywhere from 2,000 to 10,000 eggs per day 1. The eggs are deposited in soil from human feces. After 14 to 21 days, the eggs mature and enter an infective stage. If humans ingest the embryonated eggs, the eggs start to hatch in the human small intestine and utilize the intestinal microflora and nutrients to multiply and grow. The majority of Trichuris trichiura larvae move to the cecum, penetrate the mucosa and mature to adulthood. Infections involving a high-worm burden will typically involve distal parts of the large intestine 2.
The egg of Trichuris trichiura whipworm is the infective stage, and favorable conditions for its maturation are warm and humid climate. This is why most of the disease burden is seen in tropical climates, specifically in Asia and less often, in Africa and South America. It is also found in rural parts of the southeast United States.
An estimated 450 million to 1 billion people in the world are infected with Trichuris trichiura with most active cases diagnosed in children 3. Trichuris trichiura is the third most common round worm of humans. Worldwide, with infections more frequent in areas with tropical weather and poor sanitation practices, and among children. It is estimated that 800 million people are infected worldwide 4. Trichuriasis occurs in the southern United States. Trichuris trichiura (human whipworm), hookworm, and Ascaris are known as soil-transmitted helminths (parasitic worms). Together, they account for a major burden of disease worldwide 3.
Trichuris trichiura whipworms live in the large intestine and Trichuris trichiura eggs are passed in the feces of infected persons. If the infected person defecates outside (near bushes, in a garden, or field) or if human feces as used as fertilizer, eggs are deposited on soil. They can then mature into a form that is infective. Trichuriasis or Trichuris trichiura infection is caused by ingesting eggs. This can happen when hands or fingers that have contaminated dirt on them are put in the mouth or by consuming vegetables or fruits that have not been carefully cooked, washed or peeled.
Patients will typically reside in or have visited areas that are endemic to the trichuris trichiura whipworm. People infected with trichuris trichiura whipworm can suffer light or heavy infections. People with light trichuris trichiura infections usually have no symptoms. People with heavy trichuris trichiura infections can experience frequent, painful passage of stool that contains a mixture of mucus, water, and blood. Rectal prolapse can also occur. Children with heavy infections can become severely anemic and growth-retarded. Whipworm infections are treatable with medication prescribed by your health care provider. Anthelminthic medications (drugs that rid the body of parasitic worms), such as albendazole and mebendazole, are the drugs of choice for treatment. Infections are generally treated for 3 days. The recommended medications are effective. Health care providers may decide to repeat a stool exam after treatment. Iron supplements may also be prescribed if the infected person suffers from anemia.
How is trichuris trichiura spread?
Trichuris trichiura whipworms live in the intestine and whipworm eggs are passed in the feces of infected persons. If the infected person defecates outside (near bushes, in a garden, or field), or if the feces of an infected person are used as fertilizer, then eggs are deposited on the soil. They can then mature into a form that is infective. Trichuris trichiura infection is caused by ingesting eggs. This can happen when hands or fingers that have contaminated dirt on them are put in the mouth, or by consuming vegetables or fruits that have not been carefully cooked, washed or peeled.
Trichuris trichiura infection cause
The most common cause of trichuris trichiura infection (trichuriasis) is ingestion of infected eggs that are found in soil, typically while eating food. This is often due to poor sanitary conditions, including open defecation and using human feces as fertilizer. Once the embryonated eggs are ingested, the larvae hatch in the small intestine. From there they migrate to the large intestine, where the anterior ends lodge within the mucosa. This leads to cell destruction and activation of the host immune system, recruiting eosinophils, lymphocytes and plasma cells. This causes the typical symptoms of rectal bleeding and abdominal pain.
Some recent studies show that people with certain chromosome traits may be predisposed or have increased susceptibility to acquiring trichuriasis 5.
Trichuris trichiura infection prevention
The best way to prevent trichuris trichiura is to improve personal hygiene, wash all fruit and vegetables, and teach everyone about the importance of hand washing. Global initiatives have been started which focus on improved sanitation, poverty reduction, and periodic chemotherapy.
The best way to prevent trichuris trichiura whipworm infection is to always:
- Avoid ingesting soil that may be contaminated with human feces, including where human fecal matter (“night soil”) or wastewater is used to fertilize crops.
- Wash your hands with soap and warm water before handling food.
- Teach children the importance of washing hands to prevent infection.
- Wash, peel, or cook all raw vegetables and fruits before eating, particularly those that have been grown in soil that has been fertilized with manure.
Transmission of trichuris trichiura infection to others can be prevented by:
- Not defecating outdoors.
- Effective sewage disposal systems.
What is preventive treatment?
In developing countries, groups at higher risk for soil-transmitted helminth infections (hookworm, Ascaris, and whipworm) are often treated without a prior stool examination. Treating in this way is called preventive treatment (or “preventive chemotherapy”). The high-risk groups identified by the World Health Organization are preschool and school-age children, women of childbearing age (including pregnant women in the 2nd and 3rd trimesters and lactating women) and adults in occupations where there is a high risk of heavy infections. School-age children are often treated through school-health programs and preschool children and pregnant women at visits to health clinics.
What is mass drug administration (MDA)?
The soil-transmitted helminths (hookworm, Ascaris, and whipworm) and four other “neglected tropical diseases” (river blindness, lymphatic filariasis, schistosomiasis and trachoma) are sometimes treated through mass drug administrations. Since the drugs used are safe and inexpensive or donated, entire risk groups are offered preventive treatment. Mass drug administrations are conducted periodically (often annually), commonly with drug distributors who go door-to-door. Multiple neglected tropical diseases are often treated simultaneously using MDAs.
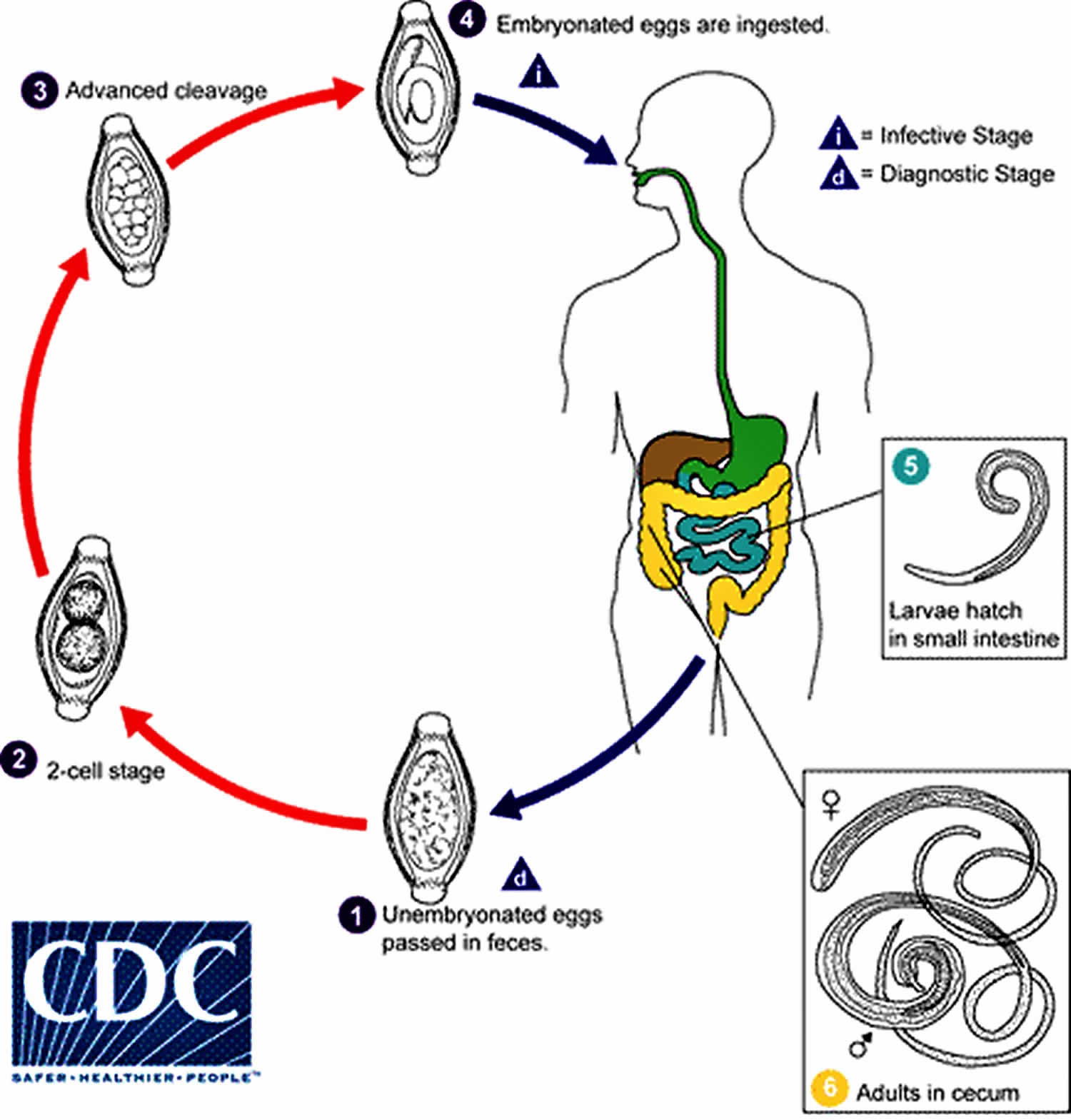
Trichuris trichiura life cycle
The unembryonated eggs are passed with the stool (number 1). In the soil, the eggs develop into a 2-cell stage (number 2), an advanced cleavage stage (number 3), and then they embryonate (number 4); eggs become infective in 15 to 30 days. After ingestion (soil-contaminated hands or food), the eggs hatch in the small intestine, and release larvae (number 5) that mature and establish themselves as adults in the colon (number 6). The adult worms (approximately 4 cm in length) live in the cecum and ascending colon. The adult worms are fixed in that location, with the anterior portions threaded into the mucosa. The females begin to oviposit 60 to 70 days after infection. Female worms in the cecum shed between 3,000 and 20,000 eggs per day. The life span of the adults is about 1 year.
Figure 1. Trichuris trichiura life cycle
Trichuris trichiura infection symptoms
Most trichuris trichiura infection are asymptomatic. Heavy trichuris trichiura infections, especially in small children, can cause gastrointestinal problems such as abdominal pain, diarrhea, painful passage of stools, abdominal discomfort, mucus discharge and rectal prolapse. Rectal prolapse is known to occur in a heavy infestation. Children may develop anemia, growth retardation, and even impaired cognitive development. The latter 2 are thought to be due to iron deficiency and poor nutrition secondary to worm burden and are not a direct cause of the infestation 6.
Trichuris trichiura infection complications
Trichuris trichiura dysentery syndrome can be found in children (with no adult cases noted) and is seen when there is a very high worm burden. This often leads to diarrhea, tenesmus, iron deficiency anemia and growth retardation. The growth retardation is typically secondary to poor nutrition and consequently causes the cognitive delay.
Trichuris trichiura infection diagnosis
The standard method for diagnosing the presence of trichuris trichiura whipworm is by microscopically identifying whipworm eggs in a stool sample. Because eggs may be difficult to find in light infections, a concentration procedure is recommended.
The diagnosis is made by using the Kato-Katz method for counting eggs per unit weight of feces. One caveat is that from the time the eggs are ingested to development of the mature worm, there is a time lag of about three months. During this period, there may be no signs of an infestation and the stools may not show evidence of any eggs or shedding.
There have been case reports of patients reporting symptoms in areas that are resource-rich where the diagnosis has been made with colonoscopy. The classic finding is the “coconut cake rectum.” There have recently been studies which show a whipworm dance on ultrasound, and this is a modality that can easily be used in resource-poor settings 7.
PCR assays are currently being developed and used. This has improved the specificity and sensitivity of detecting the whipworm 8.
Trichuris trichiura infection treatment
The treatment for trichuris trichiura infection is with albendazole, mebendazole or ivermectin 9. Each drug needs to be taken for 3 days. Dosage guidelines are the same for children as for adults. Albendazole should be taken with food. Ivermectin should be taken with water on an empty stomach and the safety of ivermectin for children weighing less than 15 kg has not been established. Neither albendazole nor ivermectin is FDA-approved for treating whipworm.
Table 1. Trichuris trichiura treatment
| Drug | Dosage for adults and children |
|---|---|
| Albendazole | 400 mg orally for 3 days |
| Mebendazole | 100 mg orally twice a day for 3 days |
| Ivermectin | 200 mcg/kg/day orally for 3 days |
The suggested dose of mebendazole 100 mg twice a day for 3 days or albendazole is 200 to 400 mg twice a day for 3 days. Mebendazole has been shown to be more effective and is considered the first-line treatment.
Ivermectin (200 mcg/kg daily) can be used; however, it is not as effective as the first 2.
It is important to keep in mind that there are often co-infections with other helminths so treatments with multiple medications may be required.
Adults and children should be treated appropriately for the anemia they experience. Many global organizations stress the importance of increased education for children who have been treated for whipworm infection. Not doing so keeps them behind in school when compared to peers of their same age group who were not infected.
Albendazole
Treatment in pregnancy
- Pregnancy Category C: Either studies in animals have revealed adverse effects on the fetus (teratogenic or embryocidal, or other) and there are no controlled studies in women or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus.
- Albendazole is pregnancy category C. Data on the use of albendazole in pregnant women are limited, though the available evidence suggests no difference in congenital abnormalities in the children of women who were accidentally treated with albendazole during mass prevention campaigns compared with those who were not. In mass prevention campaigns for which the World Health Organization (WHO) has determined that the benefit of treatment outweighs the risk, WHO allows use of albendazole in the 2nd and 3rd trimesters of pregnancy. However, the risk of treatment in pregnant women who are known to have an infection needs to be balanced with the risk of disease progression in the absence of treatment.
Treatment during breastfeeding
- It is not known whether albendazole is excreted in human milk. Albendazole should be used with caution in breastfeeding women.
Treatment in children
- The safety of albendazole in children less than 6 years old is not certain. Studies of the use of albendazole in children as young as one year old suggest that its use is safe. According to WHO guidelines for mass prevention campaigns, albendazole can be used in children as young as 1 year old. Many children less than 6 years old have been treated in these campaigns with albendazole, albeit at a reduced dose.
Mebendazole
Treatment in pregnancy
- Pregnancy Category C: Either studies in animals have revealed adverse effects on the fetus (teratogenic or embryocidal, or other) and there are no controlled studies in women or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus.
- Mebendazole is in pregnancy category C. Data on the use of mebendazole in pregnant women are limited. The available evidence suggests no difference in congenital anomalies in the children of women who were treated with mebendazole during mass treatment programs compared with those who were not. In mass treatment programs for which the World Health Organization (WHO) has determined that the benefit of treatment outweighs the risk, WHO allows use of mebendazole in the 2nd and 3rd trimesters of pregnancy. The risk of treatment in pregnant women who are known to have an infection needs to be balanced with the risk of disease progression in the absence of treatment.
Treatment during breastfeeding
- It is not known whether mebendazole is excreted in breast milk. The WHO classifies mebendazole as compatible with breastfeeding and allows the use of mebendazole in lactating women.
Treatment in children
- The safety of mebendazole in children has not been established. There is limited data in children age 2 years and younger. Mebendazole is listed as an intestinal antihelminthic medicine on the WHO Model List of Essential Medicines for Children, intended for the use of children up to 12 years of age.
Ivermectin
Treatment in pregnancy
- Pregnancy Category C: Either studies in animals have revealed adverse effects on the fetus (teratogenic or embryocidal, or other) and there are no controlled studies in women or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus.
- Ivermectin is pregnancy category C. Data on the use of ivermectin in pregnant women are limited, though the available evidence suggests no difference in congenital abnormalities in the children of women who were accidentally treated during mass prevention campaigns with ivermectin compared with those who were not. The World Health Organization (WHO) excludes pregnant women from mass prevention campaigns that use ivermectin. However, the risk of treatment in pregnant women who are known to have an infection needs to be balanced with the risk of disease progression in the absence of treatment.
Treatment during breastfeeding
- Ivermectin is excreted in low concentrations in human milk. Ivermectin should be used in breast-feeding women only when the risk to the infant is outweighed by the risk of disease progress in the mother in the absence of treatment.
Treatment in children
The safety of ivermectin in children who weigh less than 15kg has not been demonstrated. According to the WHO guidelines for mass prevention campaigns, children who are at least 90 cm tall can be treated safely with ivermectin. The WHO growth standard curves show that this height is reached by 50% of boys by the time they are 28 months old and by 50% of girls by the time they are 30 months old, many children less than 3 years old been safely treated with ivermectin in mass prevention campaigns, albeit at a reduced dose.
Trichuris trichiura infection prognosis
Trichuris trichiura whipworm tends to be more resistant to treatment than other helminths, with some studies listing cure rates as low as 28% to 36%. Trichuris trichiura whipworms can still be present after treatment however it is thought that a low worm count leads to no significant disease burden.
References- Viswanath A, Williams M. Trichuris Trichiura (Whipworm, Roundworm) [Updated 2019 Apr 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507843
- Bansal R, Huang T, Chun S. Trichuriasis. Am. J. Med. Sci. 2018 Feb;355(2):e3.
- Parasites – Trichuriasis (also known as Whipworm Infection). https://www.cdc.gov/parasites/whipworm
- Trichuriasis. https://www.cdc.gov/dpdx/trichuriasis/
- Williams-Blangero S, Vandeberg JL, Subedi J, Jha B, Dyer TD, Blangero J. Two quantitative trait loci influence whipworm (Trichuris trichiura) infection in a Nepalese population. J. Infect. Dis. 2008 Apr 15;197(8):1198-203.
- Brooker SJ, Mwandawiro CS, Halliday KE, Njenga SM, Mcharo C, Gichuki PM, Wasunna B, Kihara JH, Njomo D, Alusala D, Chiguzo A, Turner HC, Teti C, Gwayi-Chore C, Nikolay B, Truscott JE, Hollingsworth TD, Balabanova D, Griffiths UK, Freeman MC, Allen E, Pullan RL, Anderson RM. Interrupting transmission of soil-transmitted helminths: a study protocol for cluster randomised trials evaluating alternative treatment strategies and delivery systems in Kenya. BMJ Open. 2015 Oct 19;5(10):e008950
- Vijayaraghavan SB. Sonographic whipworm dance in trichuriasis. J Ultrasound Med. 2009 Apr;28(4):555-6.
- Pilotte N, Papaiakovou M, Grant JR, Bierwert LA, Llewellyn S, McCarthy JS, Williams SA. Improved PCR-Based Detection of Soil Transmitted Helminth Infections Using a Next-Generation Sequencing Approach to Assay Design. PLoS Negl Trop Dis. 2016 Mar;10(3):e0004578
- Parasites – Trichuriasis (also known as Whipworm Infection). https://www.cdc.gov/parasites/whipworm/health_professionals/index.html