Velamentous cord insertion
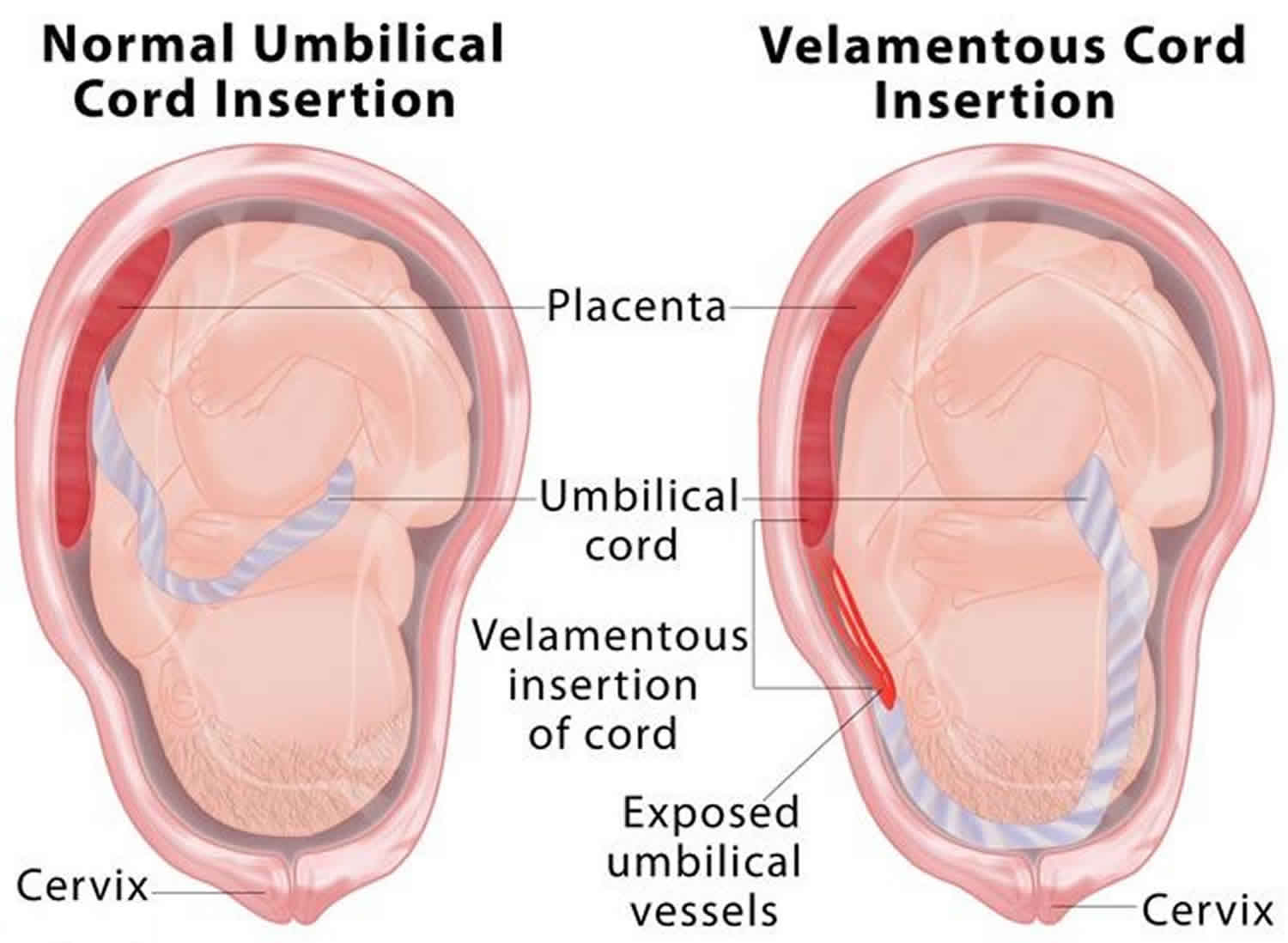
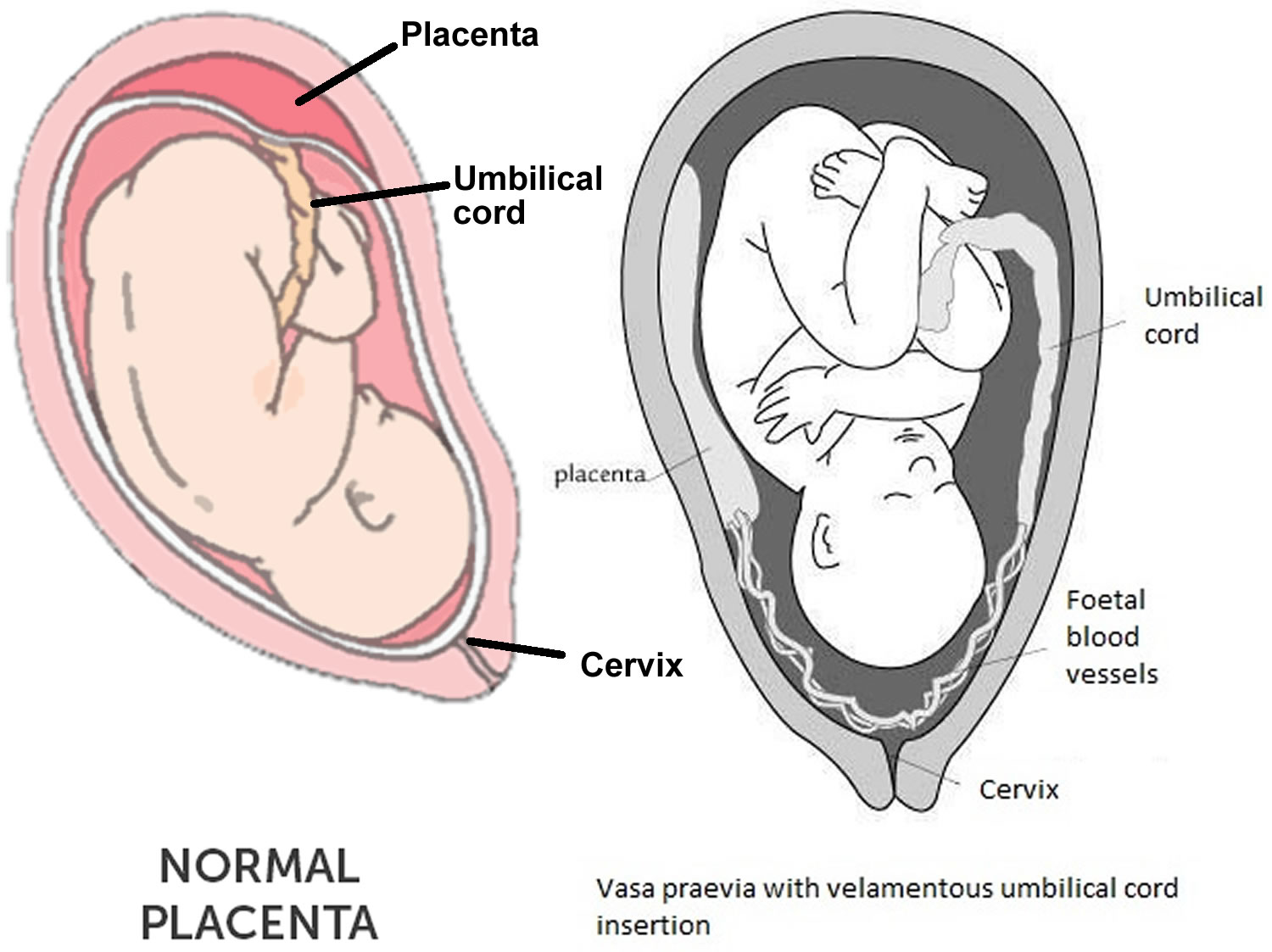
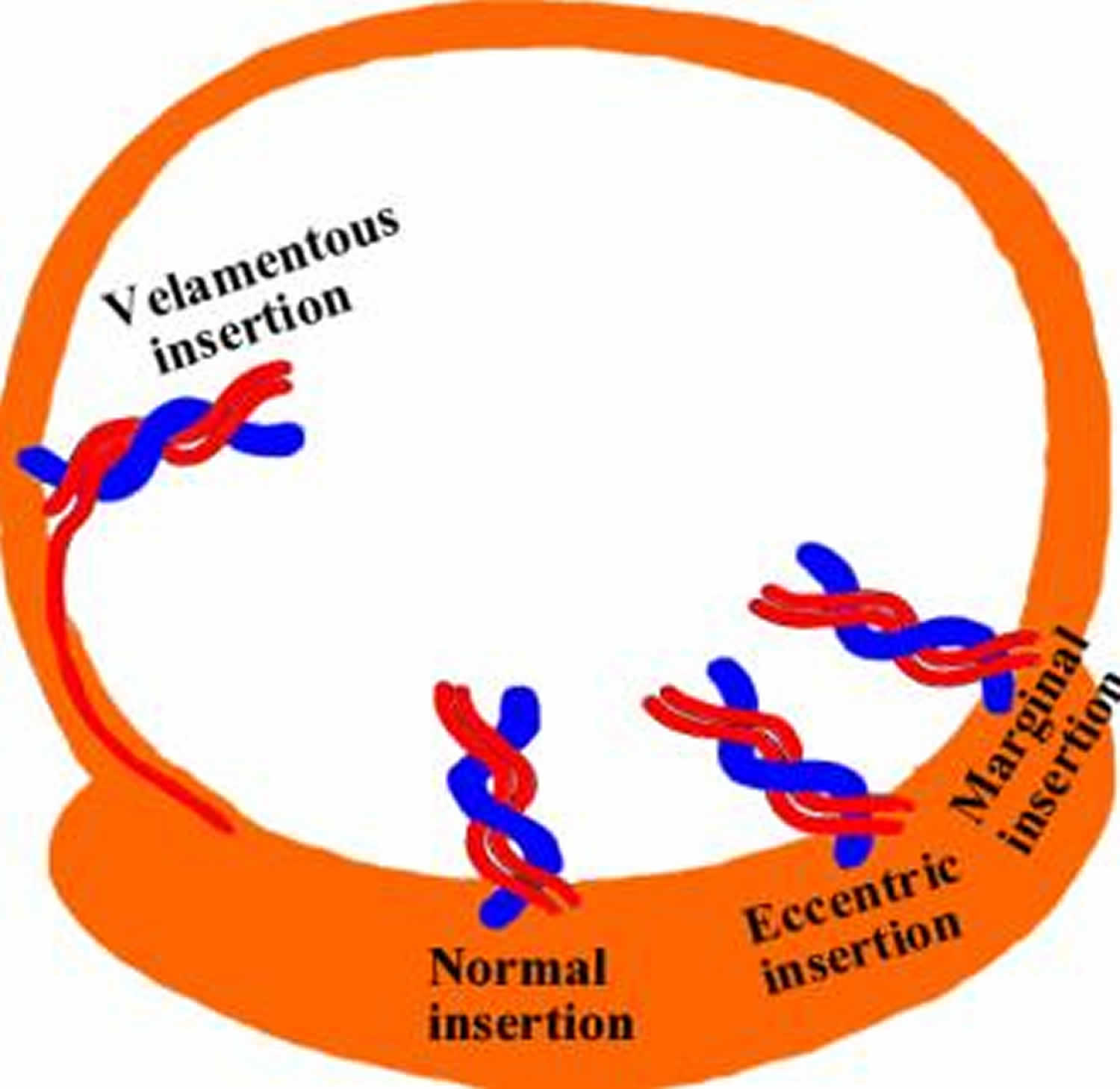
Velamentous cord insertion also called marginal cord insertion (Battledore placenta), refers to abnormal insertion of the umbilical cord at the edge of placental disk into the membranes rather than directly into the placenta 1. The umbilical vessels course between the membranes before reaching the placenta 2. Velamentous insertion means that the fetal blood vessels, unprotected by Wharton’s jelly or placental tissue, traverse the membranes freely before they come together into the umbilical cord to insert into the placenta (Figure 1). When velamentous vessels overlie the cervix, the condition is known as vasa previa (Figure 2). In vasa previa, spontaneous or artificial rupture of the membranes carries an extremely high risk of fetal exsanguination and death. Normally the blood vessels of the baby run from the middle of the placenta via the umbilical cord to the baby (Figure 3). The umbilical cord inserts on the placental mass in about 99% of cases. The insertion site may vary from the center of the fetal surface to the border of the placenta. The term velamentous insertion is used to describe the condition in which the umbilical cord inserts on the chorioamniotic membranes rather than on the placental mass. Therefore, a variable segment of the umbilical vessels runs between the amnion and the chorion, losing the protection of the Wharton’s jelly.
When a velamentous insertion occurs away from the cervix (i.e. when there is no vasa previa), most pregnancies proceed normally with no complications. Occasionally, however, intrauterine growth restriction may occur. Also, rarely, velamentous vessels that are not in the lower segment of the uterus may rupture spontaneously, resulting in fetal death. However, this is exceedingly uncommon.
Pregnancies complicated with velamentous cord insertion are at risk of adverse maternal outcome such as preterm labor, placental abruption, vasa previa, manual removal of placenta and post partum hemorrhage; and adverse perinatal outcome such as abnormal fetal heart tracing, low Apgar score, neonatal intensive unit admission as well as neonatal death 3.
When velamentous cord insertion is present in a monochorionic twin pregnancy, there may be unequal placental sharing with risks of growth restriction and death in the twin whose cord inserts into the membranes. Also, velamentous cord insertion in a monochorionic twin pregnancy increases the risk for Twin-Twin transfusion syndrome. Velamentous cord insertion also may exist in association with chromosomal abnormalities and syndromes, and in association with fetal anomalies.
Velamentous insertion occurs (1) when placental tissue grows laterally, leaving the centrally located umbilical cord in an area that becomes atrophic, or (2) when the cord implants in the trophoblast anterior to the decidua capsularis rather than the trophoblast tissue that is destined to become the placental mass.
Velamentous insertion estimated incidence is approximately 1% of all singleton pregnancies and 9-15% in twin pregnancies 4. Velamentous cord insertion is more common among multi-fetal pregnancies, and has been estimated to occur in up to 10% of twin pregnancies, with increasing incidence with increasing number of fetuses in a multifetal gestation. In twin pregnancies it is higher in monochorionic placentation or when the placentas are fused than when the placentas are non-fused. The incidence of velamentous insertion is even higher in early pregnancy; in spontaneous abortions it has been estimated to be 33% between the 9th and 12th weeks and 26% between the 13th and 16th weeks. Velamentous cord insertion is also more common in placenta previa than in normally located placentas. The prevalence may be slightly higher in stillbirths, particularly from multifetal pregnancies.
Velamentous insertion has been diagnosed by ultrasonography with a sensitivity of 67% and specificity of 100% in the second trimester 5; first trimester diagnosis is also possible 6. Velamentous cord insertion is associated with a lower maternal serum alpha-fetoprotein (AFP) and higher maternal serum human chorionic gonadotropin (hCG) 7.
Velamentous insertion can cause hemorrhage if the vessels are torn when the membranes are ruptured, most often with a vasa previa. Velamentous cord insertion is associated with low birth weight, prematurity, and abnormal fetal heart patterns in labor 8, as well as hypoxic ischemic encephalopathy (HIE) of the newborn 9. If detected, fetal growth may be monitored with ultrasonography in the third trimester. Consider an elective cesarean delivery to avoid a vasa previa rupture or fetal distress if the velamentous insertion is in the lower segment 5.
Figure 1. Velamentous cord insertion
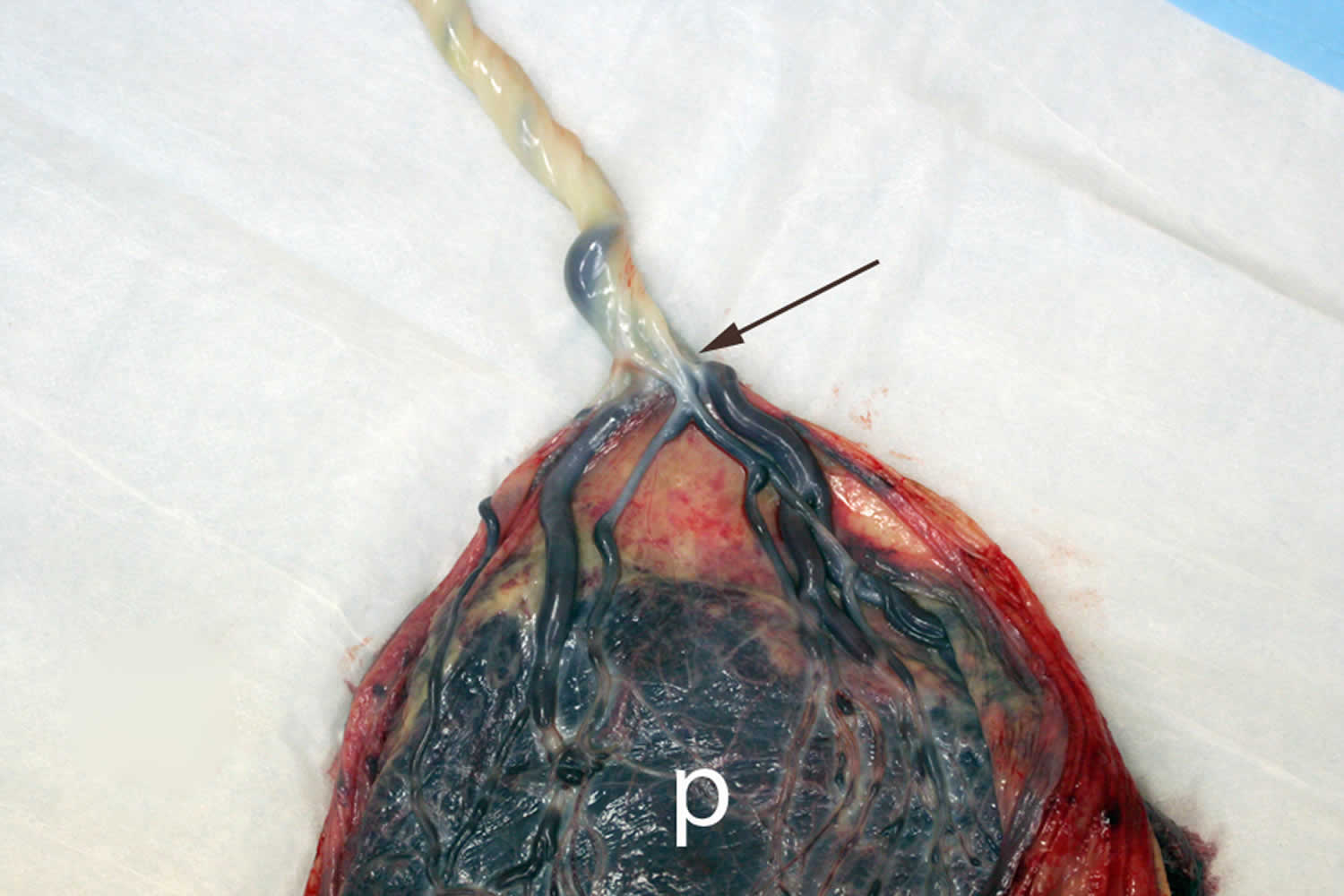
Footnote: A placenta with velamentous cord insertion: Velamentous cord insertion involves abnormal cord insertion in which the umbilical vessels diverge as they traverse between the amnion and chorion before reaching the placenta. Velamentous insertion forms during abnormal placental development starting in early gestation. The velamentous vessels of the umbilical cord easily compressed due to a lack of Wharton’s jelly (arrows) during uterine contractions or fetal movement.
[Source 1 ]Figure 2. Vasa previa with velamentous cord insertion
Figure 3. Velamentous cord insertion
Velamentous cord insertion causes
Velamentous cord insertion cause is not well established. However, velamentous cord insertion was more commonly found in placenta praevia, assisted reproductive conception and multiple pregnancies 10.
The placenta is a maternal-fetal organ which begins development at implantation of the blastocyst, and it is delivered at birth with the fetus. The fetus relies on the placenta for nutrition and many developmentally essential functions.
In a velamentous cord insertion, the umbilical cord inserts into the fetal (chorio-amniotic) membranes outside the placental margin and then travels within the membranes to the placenta (between the amnion and the chorion). It is thought to result from remodelling of the placenta as a response to factors that affect the distribution of uterine blood flow, a process known as trophotropism 11. A marginal cord insertion may evolve into a velamentous cord insertion as the pregnancy progresses 12. Some also support an abnormal primary implantation due to the obliquity of the embryo during implantation as a cause.
Velamentous cord insertion associations:
- bilobed placenta
- increased incidence in twin pregnancy
- uterine anomalies 13
- presence of an intrauterine contraceptive device (IUCD) 13
- single umbilical artery 12
- placenta previa.
Risk factors for developing velamentous cord insertion
Risk factors for developing velamentous cord insertion include:
- In vitro fertilization (IVF)
- Multiple pregnancy
- An advanced maternal age
- Having a history of an abnormal umbilical cord insertion in a previous pregnancy
- Placenta previa 14
- C-section in a previous pregnancy 15
- Preeclampsia
- Gestational diabetes 16
It may also be associated with placental trophotropism, a process in which the placenta tends to move to areas with better blood flow, so it can provide the fetus properly with oxygen and nutrients. An abnormal cord insertion is believed to have an association with higher levels of maternal serum hCG and lower maternal serum AFP 17.
Velamentous cord insertion risks
Pregnancies complicated with velamentous cord insertion are at risk of adverse maternal outcome such as preterm labor, placental abruption, vasa previa, manual removal of placenta and post partum hemorrhage; and adverse perinatal outcome such as abnormal fetal heart tracing, low Apgar score, neonatal intensive unit admission as well as neonatal death 18.
The most significant clinical problem arising from a velamentous insertion of the umbilical cord is vasa previa, a dangerous condition in which the velamentous umbilical vessels traverse the fetal membranes in the lower uterine segment below the presenting part (Figure 2). In 6% of singleton pregnancies with a velamentous insertion, vasa previa is a coexisting condition. These unprotected vessels may rupture at any time during pregnancy, causing fetal exsanguination and death. Although spontaneous rupture has been reported before labor and with or without intact membranes, this accident occurs most often during amniotomy.
When velamentous cord insertion is present in a monochorionic twin pregnancy, there may be unequal placental sharing with risks of growth restriction and death in the twin whose cord inserts into the membranes. Also, velamentous cord insertion in a monochorionic twin pregnancy increases the risk for Twin-Twin transfusion syndrome. Velamentous cord insertion also may exist in association with chromosomal abnormalities and syndromes, and in association with fetal anomalies.
Velamentous cord insertion complications
Potential complications associated with velamentous insertion include 19:
- type 1 vasa previa,
- increased risk of intrauterine growth restriction (IUGR),
- increased risk of growth discordance if in a twin pregnancy,
- increased risk of twin to twin transfusion syndrome,
- fetal distress,
- miscarriage,
- prematurity,
- low birth weight,
- fetal malformation,
- perinatal death,
- low Apgar scores,
- retained placenta.
Velamentous cord insertion diagnosis
The gold standard for diagnosis of velamentous cord insertion is based on inspection of the placenta and cord following delivery. When the cord insertion is specifically looked for using prenatal ultrasound, velamentous insertion may be diagnosed prenatally. In the absence of prenatal ultrasound, it is not possible to make the diagnosis prior to placental delivery. Velamentous insertion also should be suspected when the cord easily avulses from the placenta in the third stage of labor with little traction.
Antenatal ultrasound
Velamentous cord insertion can be identified on prenatal ultrasound. Ultrasound allows direct visualization of the cord implantation to a site outside the placenta. A velamentous cord insertion may be detected as early as the first-trimester scan 20. Antenatal ultrasound is considered to have a variable sensitivity (69-100%) but high specificity (99-100%) for revealing abnormal placental cord insertion sites, including velamentous insertions 21.
The ultrasound appearance of a velamentous insertion is of a cord that does not insert centrally into the placenta. Generally, location of the cord insertion into the placental body is possible using prenatal ultrasound, especially if color or power Doppler are used. Failure to recognize this highly suggests a velamentous insertion. Using a combination of gray scale ultrasound and color, power and pulsed wave Doppler, one may determine that the cord inserts directly into the membranes.
The appearance on gray scale ultrasound is of echolucent linear structures running along the uterine wall and ending in the edge of the placenta. Color and power Doppler will reveal flow through these structures. Pulsed wave Doppler will demonstrate a fetal umbilical arterial or venous signal through these structures, confirming fetal vessels.
Velamentous cord insertion delivery
Treatment somewhat depends on the location of velamentous vessels and if in the lower segment, a cesarean section to avoid the risks of a vasa previa is often considered 5. Since there is no way to correct the velamentous insertion, close monitoring is the only way to make sure the baby stays healthy and grows properly. Regular ultrasonography usually helps with the purpose. An immediate cesarean section is necessary to save the baby if there is a rupture of the umbilical vessels or any sign of fetal distress 22. In case of vasa previa, an urgent C-section is done before the start of cervical dilation and labor.
References- Ng, Beng. (2016). Velamentous Cord Insertion: A Rare Cause of Emergency Caesarean Section. Journal of Surgical Academia. 6. 37-39. 10.17576/JSA.2016.0602.09
- Hasegawa J, Matsuoka R, Ichizuka K, Sekizawa A, Okai T. Ultrasound diagnosis and management of umbilical cord abnormalities. Taiwan J Obstet Gynecol 2009; 48(1): 23-7.
- Baumfeld Y, Gutvirtz G, Shoham I, Sheiner E. Fetal heart rate patterns of pregnancies with vasa previa and velamentous cord insertion. Arch Gynecol Obstet 2016; 293(2): 361-7.
- Fadl S, Moshiri M, Fligner CL, Katz DS, Dighe M. Placental Imaging: Normal Appearance with Review of Pathologic Findings. Radiographics : a review publication of the Radiological Society of North America, Inc. 37 (3): 979-998. doi:10.1148/rg.2017160155
- Hasegawa J, Matsuoka R, Ichizuka K, Sekizawa A, Okai T. Velamentous cord insertion: significance of prenatal detection to predict perinatal complications. Taiwan J Obstet Gynecol. 2006 Mar. 45(1):21-5.
- Sepulveda W. Velamentous insertion of the umbilical cord: a first-trimester sonographic screening study. J Ultrasound Med. 2006 Aug. 25(8):963-8; quiz 970.
- Heinonen S, Ryynanen M, Kirkinen P, Saarikoski S. Elevated midtrimester maternal serum hCG in chromosomally normal pregnancies is associated with preeclampsia and velamentous umbilical cord insertion. Am J Perinatol. 1996 Oct. 13(7):437-41.
- Feldman DM, Borgida AF, Trymbulak WP, Barsoom MJ, Sanders MM, Rodis JF. Clinical implications of velamentous cord insertion in triplet gestations. Am J Obstet Gynecol. 2002 Apr. 186(4):809-11.
- Nasiell J, Papadogiannakis N, Lof E, Elofsson F, Hallberg B. Hypoxic ischemic encephalopathy in newborns linked to placental and umbilical cord abnormalities. J Matern Fetal Neonatal Med. 2015 Jul 26. 1-6.
- Ruiter L, Kok N, Limpens J, et al. Incidence of and risk factors for vasa praevia: a systematic review. BJOG 2016; 123(8): 1278-87.
- Sonographic evaluation of the placental cord insertion site. American Journal of Roentgenology. 1998;170: 1295-1298. 10.2214/ajr.170.5.9574605 https://www.ajronline.org/doi/abs/10.2214/ajr.170.5.9574605
- Placental cord insertion visualization with prenatal ultrasonography. J Ultrasound Med. 1996 Aug;15(8):585-93. https://doi.org/10.7863/jum.1996.15.8.585
- Dudiak CM, Salomon CG, Posniak HV et-al. Sonography of the umbilical cord. Radiographics. 1995;15 (5): 1035-50. https://doi.org/10.1148/radiographics.15.5.7501849
- Velamentous umbilical cord insertion and vasa previa. https://www.uptodate.com/contents/velamentous-umbilical-cord-insertion-and-vasa-previa
- Prevalence, Risk Factors and Outcomes of Velamentous and Marginal Cord Insertions: A Population-Based Study of 634,741 Pregnancies. https://doi.org/10.1371/journal.pone.0070380
- Franks,, Adam MD; Curtis,, Carolyn MD; and Barker,, Shawndra MD (2015) “Velamentous Cord: A Dangerous Case Complicated by a Rural Population,” Marshall Journal of Medicine: Vol. 1: Iss. 1, Article 8. DOI: http://dx.doi.org/10.18590/mjm.2015.vol1.iss1.8
- Umbilical Cord Complications. https://emedicine.medscape.com/article/262470-overview#a4
- Yerlikaya G, Pils S, Springer S, Chalubinski K, Ott J. Velamentous cord insertion as a risk factor for obstetric outcome: a retrospective casecontrol study. Arch Gynecol Obstet 2016; 293(5): 975-81.
- Sepulveda W, Sebire NJ, Harris R, Nyberg DA. The placenta, umbilical cord, and membranes. In: Nyberg DA, McGahan JP, Pretorius DH, Pilu G (eds). Diagnostic Imaging of Fetal Anomalies. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:85–132.
- Sepulveda W. Velamentous insertion of the umbilical cord: a first-trimester sonographic screening study. J Ultrasound Med. 2006;25 (8): 963-8. https://doi.org/10.7863/jum.2006.25.8.963
- Di salvo DN, Benson CB, Laing FC et-al. Sonographic evaluation of the placental cord insertion site. American Journal of Roentgenology. 1998;170: 1295-1298. 10.2214/ajr.170.5.9574605 https://www.ajronline.org/doi/abs/10.2214/ajr.170.5.9574605
- Rocha, Juliana & Carvalho, Joana & Costa, Fernanda & Meireles, Isabel & Carmo, Olímpia. (2012). Velamentous Cord Insertion in a Singleton Pregnancy: An Obscure Cause of Emergency Cesarean—A Case Report. Case reports in obstetrics and gynecology. 2012. 308206. 10.1155/2012/308206.