Ventriculitis
Ventriculitis is the inflammation of the ependymal lining of the cerebral ventricles, usually secondary to infection. Ventriculitis has other names, such as ependymitis, ventricular empyema, pyocephalus, and pyogenic ventriculitis 1. Ventriculitis is an indolent but lethal infection and a source of persistent infection following meningitis treatment 2. Early diagnosis is essential for appropriate treatment. Ventriculitis is of particular concern in patients with external ventricular drains or intraventricular shunts 2.
There is no clear definition for ventricular infection and no accepted diagnostic criteria 2. It is unclear whether ventriculitis, catheter-related infections, and positive CSF cultures describe the same condition. True infection, contamination, colonization, or suspected ventriculostomy-related infection are hard to distinguish 3. This ambiguity means it is difficult to quantify the incidence.
Ventriculitis secondary to meningitis commonly known as pyogenic ventriculitis, is more common in infants. Risk factors are related to low host immunity (cancer, HIV, diabetes, alcoholism, etc.) and higher virulence of the causative organism. Often, when meningitis fails to respond to antibiotics, or when it recurs, ventriculitis should be considered 4. Suggested mechanisms include direct hematogenous spread to the choroid plexus. Chronically, septations within the ventricles can develop, resulting in multiloculated hydrocephalus, which worsens prognosis and is more common with bacterial infections.
Typical organisms include gram-negative species followed by Staphylococcus species 1. The incidence of gram-negative bacillary meningitis has increased, likely reflecting an increase in nosocomial meningitis, which presents a challenge due to its indolent course and its tendency to recur 5.
The incidence of ventricular catheter-related ventriculitis or healthcare-associated ventriculitis, ranges from 0 to 45% depending on the insertion technique and management (commonly less than 10%) 6. CSF shunt infection has ranged from 4 to 41% (usually in the range 4 to 17%), external ventricular drain ventriculitis has ranged from 0 to 22%, and lumbar drain meningitis rates are up to 5% 3. This has been difficult to assess due to the lack of clear definitions, the severity of the underlying illness, skin flora contamination, and the possibility that the indwelling catheter could induce a CSF pleocytosis. Most of the reported studies are single-center, retrospective studies with a small number of patients. A recent multi-center, UK-based study on external ventricular drains found rates vary between 3 to 18% and from 4.8 to 12.7 per 1000 external ventricular drain days 7.
Catheter-related ventriculitis is associated with significant morbidity and mortality, especially with gram-negative organisms (approaching 58% in some studies) 8. Gram-positive cocci consistent with skin flora present as isolates in 50 to 60% of infections, including coagulase-negative Staphylococcus (most common), Corynebacterium, Bacillus, Micrococcus, or Propionibacterium species 9. The rise in gram-negative organisms (Escherichia coli, Klebsiella, Enterobacter, Pseudomonas aeruginosa and Acinetobacter baumannii) and drug-resistant organisms has been attributed to the use of antibiotic prophylaxis targeting gram-positive bacteria and prolonged hospitalization 9.
In ventriculitis following head trauma, Streptococcus pneumoniae and gram-negative rods are the most common pathogens 9. Oral flora bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Streptococcus pyogenes) cause infections in patients with skull base fractures and persistent CSF leaks 9.
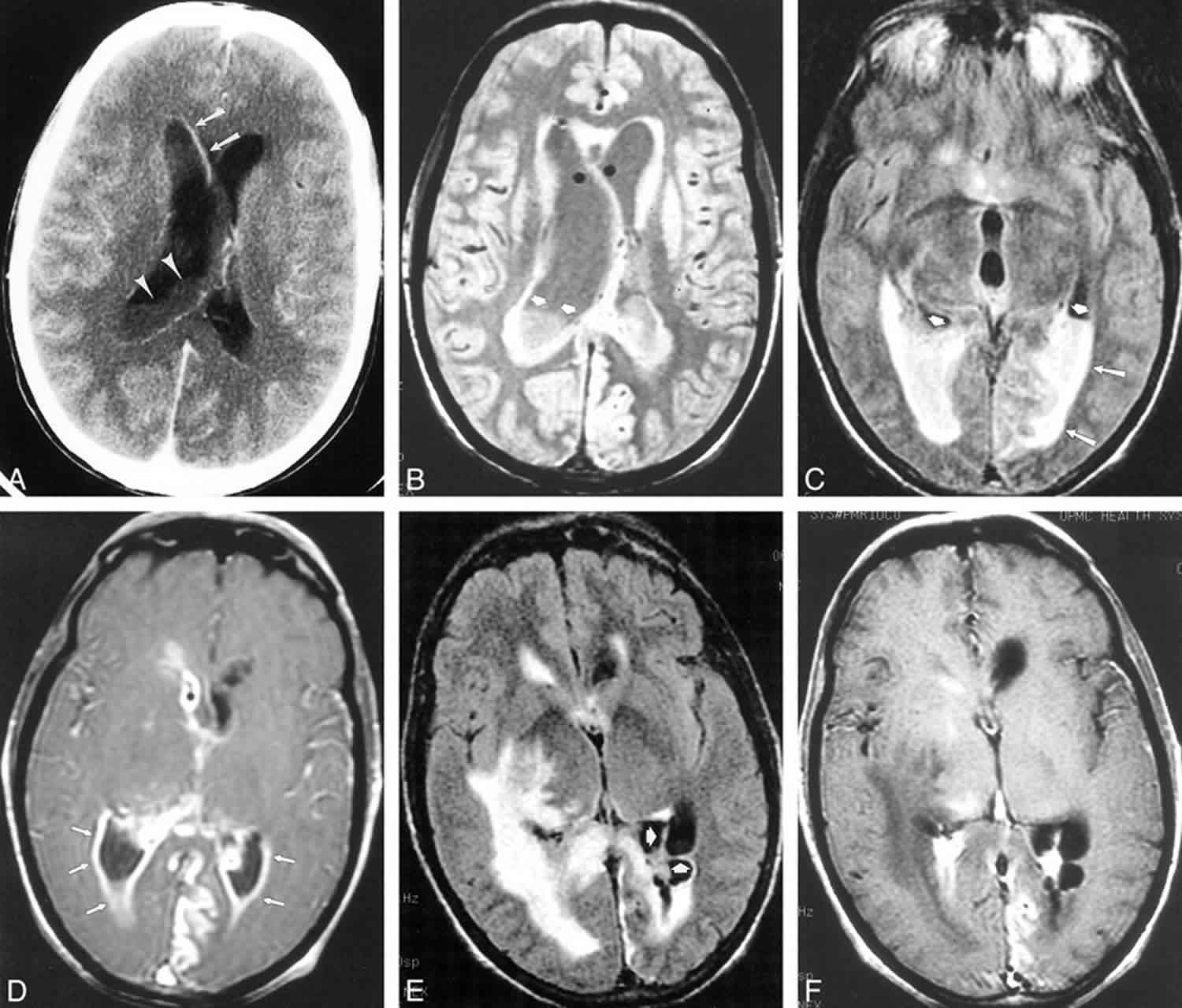
Figure 1. Pyogenic ventriculitis
Footnote: A 58-year-old patient with diabetes presented with altered mental status and multiple abscesses resulting from microaerophilic streptococcus. Gram-positive cocci were found on lumbar CSF analysis as well. Signal abnormality in the periventricular white matter and numerous abscesses (arrows) distributed throughout the deep and subcortical white matter are seen on proton density–weighted (2000/19/1 [TR/TEeff/excitation]) (A) and T2-weighted images (2800/256/1) (B). The irregular ventricular debris (arrowheads) is more clearly demonstrated on the proton density–weighted image (A). T1-weighted image (600/31/1) (C) obtained after intravenous administration of gadopentetate dimeglumine show minimal ependymal enhancement (arrows)
[Source 10]Ventriculitis causes
Ventriculitis can be secondary to 3:
- Meningitis – bacterial and viral
- Cerebral abscess with intraventricular rupture
- Catheter-related – shunt or external ventricular drain related
- Trauma
- Cerebrospinal fluid (CSF) leak
- Complication of neurosurgery
- Complications of intrathecal chemotherapy.
Ventriculitis, either alone or in the setting of meningitis, had choroid plexitis, with an inflammatory response to the ependymal lining of the ventricles.
Risk factors for catheter-related ventriculitis can categorize into three groups 11:
- Patient characteristics and the underlying condition e.g., head injury, diabetes, poor dentition, preexisting infections consisted of intracranial abscess, diskitis;
- Events that break the integrity of the closed system e.g., craniotomy, neurosurgical device;
- Environmental influences.
Risk factors include subarachnoid hemorrhage, neurosurgical operations, concurrent infection, external catheters (i.e., an external ventricular drain over a shunt), frequent manipulation of the external ventricular drain system, non-adherence to insertion and maintenance protocols and extended duration of external ventricular drain 12. There appears to be no association with age, sex, or race 11. CSF leaks are a significant risk factor for infection, allowing a long-standing conduit for retrograde microorganism migration 11.
Four mechanisms exist by which CSF shunts can become infected. Most commonly, colonization occurs at the time of surgery. Other mechanisms include; retrograde infection from the distal end of the shunt (e.g., bowel perforation), through the skin (e.g., after inserting a needle into the reservoir), and hematogenous seeding 3. For external ventricular drains, the introduction of infection is most likely at the time of placement, but retrograde infection also plays a role 3. Biofilm formation on devices protects the microorganisms from the host immune response and antimicrobial therapy 13.
Ventriculitis prevention
The literature is inconclusive about antibiotic prophylaxis for patients with ventriculostomies 14. Periprocedural prophylactic antibiotics are currently recommended for patients undergoing CSF shunt or drain insertion 3. Studies have compared perioperative antibiotics only, or prolonged antibiotics for the duration of the ventricular catheter. Findings show a reduction in the incidence of serious CSF infections, but selecting resistant or opportunistic organisms, including candida and methicillin-resistant Staphylococcus aureus (MRSA) 15. Improved catheter maintenance techniques have been shown to reduce infections 16.
Experience and the use of aseptic techniques reduce the risk of catheter-related ventriculitis, necessitating the need for education 6. The surgical technique is important, with lower rates when the device tunnels under the skin, with a distal skin puncture 17. Healthcare bundles are being increasingly used to minimize external ventricular drain- associated infections, including measures such as education, meticulous handling, sampling only when clinically necessary, pre-operative prophylactic antibiotics, with positive results 7.
Antibiotics prophylaxis is ineffective in trauma patients with CSF fistula 18. Prophylactic antibiotics for craniotomies help prevent surgical site infections, but has no effect on meningitis prevention and predisposes the patient to more resistant organisms 19.
Antibiotics-impregnated (0.054% rifampicin and 0.15% clindamycin) and silver-impregnated ventriculostomy catheters have been developed to reduce infection rates with some conflicting results 20. Silver-coated catheters provide an antimicrobial surface, which can inhibit the growth of bacteria, and biofilm formation, over an extended period without systemic side effects 21. One study 21 found complete growth inhibition of all microorganisms but Pseudomonas aeruginosa after 72 hours, with almost complete inhibition of bioform formation for E.Coli, Staphylococcus aureus, and Candida albicans, and reached more than 50% for Enterococcus, coagulase-negative Staphylococci, and Pseudomonas aeruginosa after 72 hours. There was a significantly lower infection rate with the silver-coated catheter in a randomized controlled trial (RCT), but not in the pooled non-randomized controlled trials on meta-analysis 22. An randomized controlled trial comparing antibiotic-impregnated catheters with an untreated catheter found that CSF cultures were seven times less frequent in patients with the antibiotic catheters (1.3% versus 9.4%) 23.
The British antibiotic and silver-impregnated catheters for ventriculoperitoneal shunts multi-center randomized controlled trial (BASICS) Trial is a 1200 patient, 17 center trial comparing silicone, antibiotic-coated or silver catheters 24. It is designed to determine whether impregnated catheters reduce early shunt infections, and results are forthcoming.
Controversy exists about whether the regular exchange of external ventricular drains can reduce infection. There is a body of literature reporting that infection rates increase after 4 to 5 days of catheter insertion 25. This data is the basis for the recommendation that external ventricular drain catheters should be removed and inserted at a different site if required for longer than five days. Other studies showed no benefit of routine catheter exchanges 26. Holloway’s study shows a rising risk of infection over the first ten days, but then infection becomes very unlikely, despite a population still at risk 27. This research has led to the recommendation of removing the catheters at the earliest opportunity clinically and routinely exchanging the catheter if the device becomes obstructed or if an infection develops 11.
Ventriculitis symptoms
Ventriculitis symptoms can include fever and signs of meningism (nuchal rigidity, headache, photophobia, decreased mental status, seizures, or moribund) 2.
With ventriculitis secondary to catheters, trauma, or neurosurgery, the onset is more subtle, and often there is a relative lack of fever and severe presenting symptoms, reflecting the predilection for immunocompromised patients 28. The patients may have fever unrelated to the infection (e.g., central fever, drug fever, chemical meningitis) 3. Erythema or tenderness over the subcutaneous shunt tubing is suggestive of infection 3.
Patients can present with features of obstructive hydrocephalus, particularly in infants with inflammatory aqueduct obstruction.
Typical symptoms and signs in patients with healthcare-associated ventriculitis and meningitis 3:
Cerebrospinal Fluid Shunts and Drains
- New headache, nausea, lethargy, and/or change in mental status are suggestive of cerebrospinal fluid (CSF) shunt infection (strong, moderate).
- Erythema and tenderness over the subcutaneous shunt tubing are suggestive of CSF shunt infection (strong, moderate).
- Fever, in the absence of another clear source of infection, could be suggestive of CSF shunt infection (weak, low).
- Symptoms and signs of peritonitis or abdominal tenderness in patients with ventriculoperitoneal shunts, in the absence of another clear etiology, are indicative of CSF shunt infection (strong, moderate).
- Symptoms and signs of pleuritis in patients with ventriculopleural shunts, in the absence of another clear etiology, are indicative of CSF shunt infection (strong, moderate).
- Demonstration of bacteremia in a patient with a ventriculoatrial shunt, in the absence of another clear source of bacteremia, is evidence of CSF shunt infection (strong, moderate).
- Demonstration of glomerulonephritis in a patient with a ventriculoatrial shunt is suggestive of CSF shunt infection (weak, low).
- New or worsening altered mental status in patients with external ventricular drains is suggestive of infection (weak, low).
- New fever and increased CSF white blood cell count in patients with external ventricular drains could be suggestive of infection (weak, low).
Neurosurgery or Head Trauma
- New headache, fever, evidence of meningeal irritation, seizures, and/or worsening mental status are suggestive of ventriculitis or meningitis in the setting of recent trauma or neurosurgery (strong, moderate).
- Fever, in the absence of another clear source of infection, is suggestive of central nervous system (CNS) infection in the setting of recent head trauma or neurosurgery (weak, low).
Intrathecal Infusion Pumps
- New fever and drainage from the surgical site in patients with intrathecal infusion pumps are suggestive of wound infection (weak, low).
Ventriculitis complications
Due to the risk of recurrence and hydrocephalus, long-term follow up is recommended. The ventricles and choroid plexus can serve as a reservoir of infection, even when the lumbar puncture yields sterile cultures 29.
Ventriculitis diagnosis
Investigations for ventriculitis include CSF sampling and imaging.
The CSF sample can demonstrate an elevated protein count (greater than 50mg/dL). This can be related to a decrease in CSF production, as seen in rabbit models of E.Coli ventriculitis 30. CSF can have low glucose (less than 25mg/dL), pleocytosis (over 10cells/microL with 50% or more polymorphonuclear neutrophils) and a positive culture or Gram stain 3. Cultures may be negative following antibiotic therapy, despite active ventriculitis. They may require several days or weeks of incubation, for example for low virulence organisms like Propionibacterium acnes which require at least ten days, however, treatment should not be delayed 3.
The presence of oligoclonal immunoglobulin G or M bands, CSF lactate, procalcitonin, and lysozymes help make an early diagnosis 31. An increase in CSF lactate, procalcitonin and lysozymes suggest bacterial over a viral infection 32. A meta-analysis reported a pooled sensitivity and specificity of 93% and 96% for CSF lactate, although this is reduced when antibiotics are administered prior to CSF collection 33.
CSF cultures are the most important test for establishing the diagnosis of healthcare-associated ventriculitis 3. However, repeated sampling from external ventricular drains, in the absence of clinical signs of ventriculitis, has been associated with higher infection rates and a low predictive value for diagnosis 34. Catheter-related ventriculitis is hard to diagnose on CSF as results are often subtle, and it is difficult to determine if the abnormalities are related to infection, device placement, or following neurosurgery 3.
Ultrasound, for neonates, can be performed using a high-frequency transducer through the anterior fontanelle in coronal and sagittal planes. Ventriculitis has increased thickness, irregularity and echogenicity of ependyma, with echogenic debris in the ventricle 4. Ependymal irregularity results from denudation of segments of ependyma, resulting in glial proliferation 4. At a later stage, when the inflammatory exudate organizes, there can be septa formation (composed of denuded and detached segments of ependyma), compartmentalization, intraventricular cysts, and obstructive hydrocephalus 4. Increased echogenicity may be seen in the periventricular region as a result of subependymal infiltration with lymphocytes and plasma cells and swollen subependymal astrocytes 4. Additionally, inflammation of the choroid plexus can show increased echogenicity and irregularity 4. Ultrasound can also be used to detect CSF loculations at the shunt terminus in infected ventriculoperitoneal shunts.
Non-contrast CT demonstrated non-specific findings, including dependent hyperdense ventricular debris, hydrocephalus, periventricular low density as well as features of the underlying abnormality (e.g. signs of meningitis – pial or dura/arachnoid signal abnormality or enhancement) 1. With contrast, the ependymal lining of the ventricles enhances homogeneously 1. Denuding of the ependyma could be a component of the breakdown of the blood-brain barrier, resulting in enhancement.
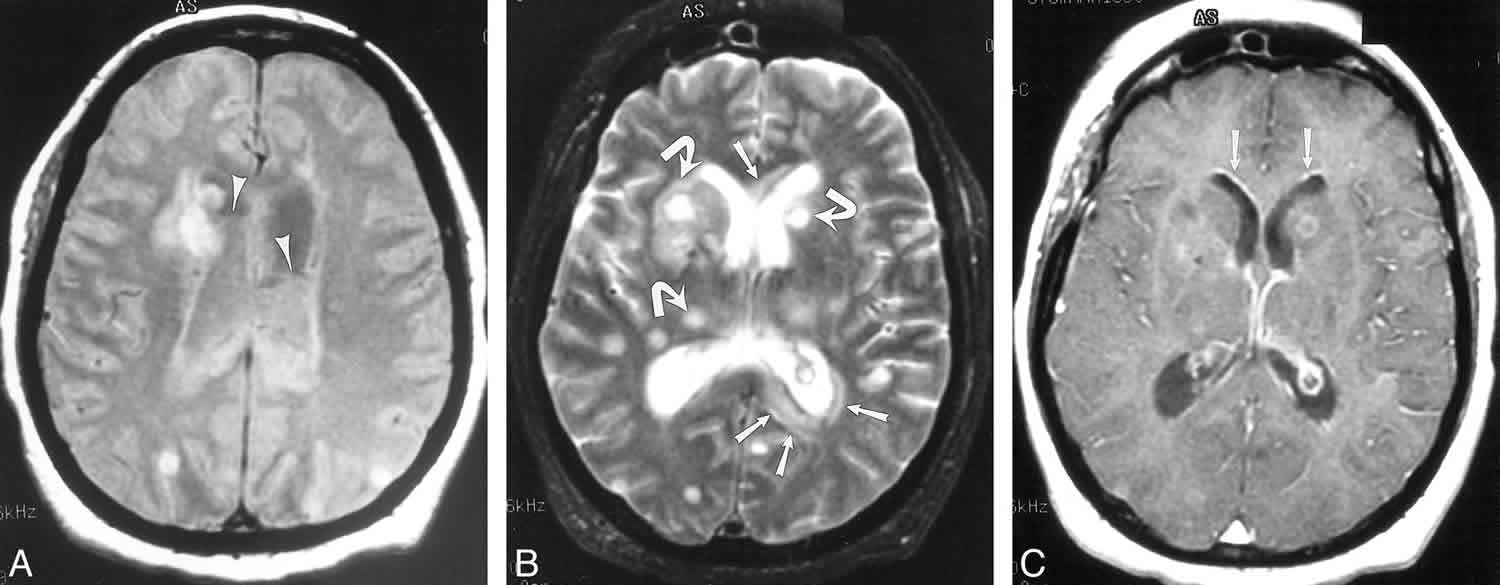
MRI shows the same features as CT, with ventricular debris hyperintense to CSF on T1-weighted images and hypointense to CSF on T2- weighted images 1. This is the most commonly observed imaging finding for ventriculitis and can be seen in up to 94% of cases 1. There can be intensely restricted diffusion of the intraventricular debris on DWI/ ADC as seen with cerebral abscesses, but not always 35. FLAIR images are sensitive to subtle periventricular hyperintense signal in 78% of cases 1.
Ventricular uptake in radionuclide brain scintigraphy using technetium-99 can be used.
Ventriculitis treatment
Antimicrobial therapy that can reach effective concentrations in the CSF is required. Immunocompromised patients need aggressive treatment. Initially, empirical therapy is used based on the patient’s age and etiology. For catheter-related ventriculitis, this is generally vancomycin and an anti-pseudomonal beta-lactam (cefepime, ceftazidime or meropenem) 3.
Specific antibiotics are chosen based on in vitro susceptibility and penetration into CSF when meningeal inflammation is present 32. A summary appears in Table 1 below. The duration of antibiotics depends on the microbe isolated, the CSF findings, and the clinical symptoms but varies between 10 and 21 days 3.
Intraventricular antibiotics are an option if the ventriculitis is refractory to systemic therapy. Commonly used antibiotics include vancomycin (5 to 20mg/day) or gentamicin (1 to 8mg/day) but depend on local microbial policies 3. This regimen results in higher levels achieved in the ventricular CSF than by intravenous administration 36. Antibiotic dosages have been used empirically, with dose adjustments and intervals based on the ability to achieve adequate CSF concentrations 32. After the initial dose, doses can be determined by the calculation of the inhibitory quotient (the trough CSF concentration divided by the minimal inhibitory concentration (MIC) of the agent for the isolated bacterial pathogen), which should exceed 10 to 20 for consistent CSF sterilization 32.
Removing all components of the infected shunt or external ventricular drain, in combination with antimicrobial therapy, is recommended for catheter-related infection 3. This action allows the infection to clear more rapidly, as the microorganisms can adhere to prostheses and survive despite antimicrobe therapy. The decision to re-implant the device depends on the patient, microorganism, severity of infection, and CSF findings 32.
Table 1. Ventriculitis antiobiotics
IDSA ventriculitis guidelines
IDSA (Infectious Diseases Society of America) ventriculitis guidelines 3:
Empiric antimicrobial approach for patients with suspected healthcare-associated ventriculitis and meningitis
- Vancomycin plus an anti-pseudomonal beta-lactam (such as cefepime, ceftazidime, or meropenem) is recommended as empiric therapy for healthcare-associated ventriculitis and meningitis; the choice of empiric beta-lactam agent should be based on local in vitro susceptibility patterns (strong, low).
- In seriously ill adult patients with healthcare-associated ventriculitis and meningitis, the vancomycin trough concentration should be maintained at 15–20 μg/mL in those who receive intermittent bolus administration (strong, low).
- For patients with healthcare-associated ventriculitis and meningitis who have experienced anaphylaxis to beta-lactam antimicrobial agents and in whom meropenem is contraindicated, aztreonam or ciprofloxacin is recommended for gram-negative coverage (strong, low).
- For patients with healthcare-associated ventriculitis and meningitis who are colonized or infected elsewhere with a highly antimicrobial-resistant pathogen, adjusting the empiric regimen to treat for this pathogen is recommended (strong, low).
Once a pathogen is identified, the specific antimicrobial agent(s) that should be administered include:
- For treatment of infection caused by methicillin-susceptible Staphylococcus aureus, nafcillin or oxacillin is recommended (strong, moderate). If the patient cannot receive beta-lactam agents, the patient can be desensitized or may receive vancomycin as an alternative agent (weak, moderate).
- For treatment of infection caused by methicillin-resistant Staphylococcus aureus, vancomycin is recommended as first-line therapy (strong, moderate), with consideration for an alternative antimicrobial agent if the vancomycin minimal inhibitory concentration (MIC) is ≥1 μg/mL (strong, moderate).
- For treatment of infection caused by coagulase-negative staphylococci, the recommended therapy should be similar to that for S. aureus and based on in vitro susceptibility testing (strong, moderate).
- If the staphylococcal isolate is susceptible to rifampin, this agent may be considered in combination with other antimicrobial agents for staphylococcal ventriculitis and meningitis (weak, low); rifampin is recommended as part of combination therapy for any patient with intracranial or spinal hardware such as a CSF shunt or drain (strong, low).
- For treatment of patients with healthcare-associated ventriculitis and meningitis caused by staphylococci in whom beta-lactam agents or vancomycin cannot be used, linezolid (strong, low), daptomycin (strong, low), or trimethoprim-sulfamethoxazole (strong, low) is recommended, with selection of a specific agent based on in vitro susceptibility testing.
- For treatment of infection caused by Propionibacterium acnes, penicillin G is recommended (strong, moderate).
- For treatment of infection caused by gram-negative bacilli, therapy should be based on in vitro susceptibility testing with agents that achieve good CNS penetration (strong, moderate).
- For treatment of infection caused by gram-negative bacilli susceptible to third-generation cephalosporins, ceftriaxone or cefotaxime is recommended (strong, moderate).
- For treatment of infection caused by Pseudomonas species, the recommended therapy is cefepime, ceftazidime, or meropenem (strong, moderate); recommended alternative antimicrobial agents are aztreonam or a fluoroquinolone with in vitro activity (strong, moderate).
- For treatment of infection caused by extended-spectrum beta-lactamase–producing gram-negative bacilli, meropenem should be used if this isolate demonstrates in vitro susceptibility (strong, moderate).
- For treatment of infection caused by Acinetobacter species, meropenem is recommended (strong, moderate); for strains that demonstrate carbapenem resistance, colistimethate sodium or polymyxin B (either agent administered by the intravenous and intraventricular routes) is recommended (strong, moderate).
- Prolonged infusion of meropenem (each dose administered over 3 hours) may be successful in treating resistant gram-negative organisms (weak, low).
- For treatment of infection caused by Candida species, based on in vitro susceptibility testing, liposomal amphotericin B, often combined with 5-flucytosine, is recommended (strong, moderate); once the patient shows clinical improvement, therapy can be changed to fluconazole if the isolated species is susceptible (weak, low).
- For treatment of infection caused by Aspergillus or Exserohilum species, voriconazole is recommended (strong, low).
Intraventricular antimicrobial therapy in patients with healthcare-associated ventriculitis and meningitis
- Intraventricular antimicrobial therapy should be considered for patients with healthcare-associated ventriculitis and meningitis in which the infection responds poorly to systemic antimicrobial therapy alone (strong, low).
- When antimicrobial therapy is administered via a ventricular drain, the drain should be clamped for 15–60 minutes to allow the agent to equilibrate throughout the CSF (strong, low).
- Dosages and intervals of intraventricular antimicrobial therapy should be adjusted based on CSF antimicrobial concentrations to 10–20 times the MIC of the causative microorganism (strong, low), ventricular size (strong, low), and daily output from the ventricular drain (strong, low).
Optimal duration of antimicrobial therapy in patients with healthcare-associated ventriculitis and meningitis
- Infections caused by a coagulase-negative staphylococcus or Propionibacterium acnes with no or minimal CSF pleocytosis, normal CSF glucose, and few clinical symptoms or systemic features should be treated for 10 days (strong, low).
- Infections caused by a coagulase-negative staphylococcus or Propionibacterium acnes with significant CSF pleocytosis, CSF hypoglycorrhachia, or clinical symptoms or systemic features should be treated for 10–14 days (strong, low).
- Infections caused by Staphylococcus aureus or gram-negative bacilli with or without significant CSF pleocytosis, CSF hypoglycorrhachia, or clinical symptoms or systemic features should be treated for 10–14 days (strong, low); some experts suggest treatment of infection caused by gram-negative bacilli for 21 days (weak, low).
- In patients with repeatedly positive CSF cultures on appropriate antimicrobial therapy, treatment should be continued for 10–14 after the last positive culture (strong, low).
Role of catheter removal in patients with cerebrospinal fluid shunts or drains
- Complete removal of an infected CSF shunt and replacement with an external ventricular drain combined with intravenous antimicrobial therapy is recommended in patients with infected CSF shunts (strong, moderate).
- Removal of an infected CSF drain is recommended (strong, moderate).
- Removal of an infected intrathecal infusion pump is recommended (strong, moderate).
- Removal of infected hardware in patients with deep brain stimulation infections is recommended (strong, moderate).
How are patients monitored for response to treatment?
- Patients with healthcare-associated ventriculitis and meningitis should be monitored for response to treatment based on clinical parameters (strong, low).
- In patients with healthcare-associated ventriculitis and meningitis and an external drainage device, monitoring of CSF cultures is recommended to ensure that they become negative (strong, low).
- In patients with no definitive clinical improvement, additional CSF analysis is recommended to ensure that the CSF parameters have improved and the cultures become negative (strong, low).
- For external CSF drains not being used in the treatment of CSF shunt infection, daily CSF cultures and analysis are not recommended unless clinically indicated (strong, low).
In patients with cerebrospinal fluid shunts who develop ventriculitis and meningitis, when can a new shunt be reimplanted?
- In patients with infection caused by coagulase-negative staphylococci or Propionibacterium acnes, with no associated CSF abnormalities and with negative CSF cultures for 48 hours after externalization, a new shunt should be reimplanted as soon as the third day after removal (strong, low).
- In patients with infection caused by a coagulase-negative staphylococcus or Propionibacterium acnes, with associated CSF abnormalities but negative repeat CSF cultures, a new shunt should be reimplanted after 7 days of antimicrobial therapy (strong, low); if repeat cultures are positive, antimicrobial treatment is recommended until CSF cultures remain negative for 7–10 consecutive days before a new shunt is placed (strong, low).
- In patients with infection caused by S. aureus or gram-negative bacilli, a new shunt should be reimplanted 10 days after CSF cultures are negative (strong, low).
- A period off antimicrobial therapy is not recommended to verify clearing of the infection before shunt reimplantation (strong, low).
What is the best approach to prevent infection in patients who are receiving cerebrospinal fluid shunts?
- Periprocedural prophylactic antimicrobial administration is recommended for patients undergoing CSF shunt or drain insertion (strong, moderate).
- Periprocedural prophylactic antimicrobial administration is recommended for patients undergoing placement of external ventricular drains (strong, moderate).
- Prolonged antimicrobial prophylaxis for the duration of the external ventricular drain is of uncertain benefit and not recommended (strong, moderate).
- Use of antimicrobial-impregnated CSF shunts and CSF drains is recommended (strong, moderate).
- In patients with external ventricular drains, fixed interval exchange is not recommended (strong, moderate).
- Use of a standardized protocol for insertion of CSF shunts and drains is recommended (strong, moderate).
Is there a role for prophylactic antimicrobial therapy in patients undergoing neurosurgery or in those with cerebrospinal fluid leak?
- For neurosurgical patients, perioperative antimicrobial agents are recommended to prevent infections of the incision (strong, high).
- In patients with basilar skull fractures and a CSF leak, prophylactic antimicrobial agents are not recommended (strong, moderate).
- In patients with basilar skull fractures and a prolonged CSF leakage (>7 days), an attempt to repair the leak is recommended (strong, low).
- In patients with basilar skull fractures and a CSF leak, pneumococcal vaccination is recommended (strong, moderate).
Ventriculitis prognosis
If untreated, ventriculitis could lead to poor neurology, hydrocephalus, and death 37. Early recognition and treatment are essential. High-quality studies evaluating prognosis are lacking.
References- Fukui MB, Williams RL, Mudigonda S. CT and MR imaging features of pyogenic ventriculitis. AJNR Am J Neuroradiol. 2001 Sep;22(8):1510-6.
- Harris L, Munakomi S. Ventriculitis. [Updated 2019 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544332
- Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis. 2017;64(6):e34–e65. doi:10.1093/cid/ciw861 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5848239
- Gupta N, Grover H, Bansal I, Hooda K, Sapire JM, Anand R, Kumar Y. Neonatal cranial sonography: ultrasound findings in neonatal meningitis-a pictorial review. Quant Imaging Med Surg. 2017 Feb;7(1):123-131.
- Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS, Swartz MN. Acute bacterial meningitis in adults. A review of 493 episodes. N. Engl. J. Med. 1993 Jan 07;328(1):21-8.
- Kitchen WJ, Singh N, Hulme S, Galea J, Patel HC, King AT. External ventricular drain infection: improved technique can reduce infection rates. Br J Neurosurg. 2011 Oct;25(5):632-5.
- Humphreys H, Jenks P, Wilson J, Weston V, Bayston R, Waterhouse C, Moore A., Healthcare Infection Society Working Party on Neurosurgical Infections. Surveillance of infection associated with external ventricular drains: proposed methodology and results from a pilot study. J. Hosp. Infect. 2017 Feb;95(2):154-160.
- Kitchen WJ, Singh N, Hulme S, Galea J, Patel HC, King AT. External ventricular drain infection: improved technique can reduce infection rates. Br J Neurosurg. 2011 Oct;25(5):632-5
- Martin RM, Zimmermann LL, Huynh M, Polage CR. Diagnostic Approach to Health Care- and Device-Associated Central Nervous System Infections. J Clin Microbiol. 2018;56(11):e00861-18. Published 2018 Oct 25. doi:10.1128/JCM.00861-18 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6204668
- CT and MR Imaging Features of Pyogenic Ventriculitis. Melanie B. Fukui, Robert L. Williams, Sanjay Mudigonda. American Journal of Neuroradiology Sep 2001, 22 (8) 1510-1516 http://www.ajnr.org/content/22/8/1510
- Lyke KE, Obasanjo OO, Williams MA, O’Brien M, Chotani R, Perl TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin. Infect. Dis. 2001 Dec 15;33(12):2028-33.
- Nilsson A, Uvelius E, Cederberg D, Kronvall E. Silver-Coated Ventriculostomy Catheters Do Not Reduce Rates of Clinically Diagnosed Ventriculitis. World Neurosurg. 2018 Sep;117:e411-e416.
- Martin RM, Zimmermann LL, Huynh M, Polage CR. Diagnostic Approach to Health Care- and Device-Associated Central Nervous System Infections. J. Clin. Microbiol. 2018 Nov;56(11).
- Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2008 Feb;62 Suppl 2:688-700.
- Poon WS, Ng S, Wai S. CSF antibiotic prophylaxis for neurosurgical patients with ventriculostomy: a randomised study. Acta Neurochir. Suppl. 1998;71:146-8.
- Bader MK, Littlejohns L, Palmer S. Ventriculostomy and intracranial pressure monitoring: in search of a 0% infection rate. Heart Lung. 1995 Mar-Apr;24(2):166-72.
- Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJ. Risk factors for infections related to external ventricular drainage. Acta Neurochir (Wien). 2008 Mar;150(3):209-14; discussion 214.
- Brown EM. Antimicrobial prophylaxis in neurosurgery. J. Antimicrob. Chemother. 1993 Feb;31 Suppl B:49-63.
- Korinek AM, Baugnon T, Golmard JL, van Effenterre R, Coriat P, Puybasset L. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2008 Feb;62 Suppl 2:532-9.
- Nilsson A, Uvelius E, Cederberg D, Kronvall E. Silver-Coated Ventriculostomy Catheters Do Not Reduce Rates of Clinically Diagnosed Ventriculitis. World Neurosurg. 2018 Sep;117:e411-e416
- Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet JB. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008 Apr;61(4):869-76.
- Atkinson RA, Fikrey L, Vail A, Patel HC. Silver-impregnated external-ventricular-drain-related cerebrospinal fluid infections: a meta-analysis. J. Hosp. Infect. 2016 Mar;92(3):263-72.
- Zabramski JM, Whiting D, Darouiche RO, Horner TG, Olson J, Robertson C, Hamilton AJ. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J. Neurosurg. 2003 Apr;98(4):725-30.
- Jenkinson MD, Gamble C, Hartley JC, Hickey H, Hughes D, Blundell M, Griffiths MJ, Solomon T, Mallucci CL. The British antibiotic and silver-impregnated catheters for ventriculoperitoneal shunts multi-centre randomised controlled trial (the BASICS trial): study protocol. Trials. 2014 Jan 03;15:4.
- Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, Narayan RK. Ventriculostomy-related infections. A prospective epidemiologic study. N. Engl. J. Med. 1984 Mar 01;310(9):553-9.
- Wong GK, Poon WS, Wai S, Yu LM, Lyon D, Lam JM. Failure of regular external ventricular drain exchange to reduce cerebrospinal fluid infection: result of a randomised controlled trial. J. Neurol. Neurosurg. Psychiatry. 2002 Dec;73(6):759-61.
- Holloway KL, Barnes T, Choi S, Bullock R, Marshall LF, Eisenberg HM, Jane JA, Ward JD, Young HF, Marmarou A. Ventriculostomy infections: the effect of monitoring duration and catheter exchange in 584 patients. J. Neurosurg. 1996 Sep;85(3):419-24.
- Beer R, Lackner P, Pfausler B, Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. J. Neurol. 2008 Nov;255(11):1617-24.
- Mactier H, Galea P, McWilliam R. Acute obstructive hydrocephalus complicating bacterial meningitis in childhood. BMJ. 1998 Jun 20;316(7148):1887-9.
- Breeze RE, McComb JG, Hyman S, Gilles FH. CSF production in acute ventriculitis. J. Neurosurg. 1989 Apr;70(4):619-22.
- Firth G, Rees J, McKeran RO. The value of the measurement of cerebrospinal fluid levels of lysozyme in the diagnosis of neurological disease. J. Neurol. Neurosurg. Psychiatry. 1985 Jul;48(7):709-12.
- Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 2004 Nov 01;39(9):1267-84.
- Sakushima K, Hayashino Y, Kawaguchi T, Jackson JL, Fukuhara S. Diagnostic accuracy of cerebrospinal fluid lactate for differentiating bacterial meningitis from aseptic meningitis: a meta-analysis. J. Infect. 2011 Apr;62(4):255-62.
- Williamson RA, Phillips-Bute BG, McDonagh DL, Gray MC, Zomorodi AR, Olson DM, Britz GW, Laskowitz DT, James ML. Predictors of extraventricular drain-associated bacterial ventriculitis. J Crit Care. 2014 Feb;29(1):77-82.
- Fujikawa A, Tsuchiya K, Honya K, Nitatori T. Comparison of MRI sequences to detect ventriculitis. AJR Am J Roentgenol. 2006 Oct;187(4):1048-53.
- Pfausler B, Spiss H, Beer R, Kampl A, Engelhardt K, Schober M, Schmutzhard E. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J. Neurosurg. 2003 May;98(5):1040-4.
- Shang F, Xu Y, Wang N, Cheng W, Chen W, Duan W. Diagnosis and treatment of severe neurosurgical patients with pyogenic ventriculitis caused by gram-negative bacteria. Neurol. Sci. 2018 Jan;39(1):79-84.