Waldenstrom macroglobulinemia
Waldenstrom macroglobulinemia also called Waldenstrom’s macroglobulinemia, Waldenström macroglobulinemia or lymphoplasmacytic lymphoma, is an uncommon type of blood cancer of the B lymphocytes (a type of white blood cell) 1. Waldenstrom macroglobulinemia is a type of non-Hodgkin’s lymphoma and more closely resembles indolent non-Hodgkin’s lymphoma in its progression as a disease 2. In addition, Waldenstrom macroglobulinemia can have symptoms that are similar to other non-Hodgkin’s lymphoma.
The cancer cells in people with Waldenstrom macroglobulinemia are similar to those of multiple myeloma and non-Hodgkin lymphoma (NHL) 3. Multiple myeloma is considered a cancer of plasma cells, and non-Hodgkin lymphoma is a cancer of lymphocytes. Waldenstrom macroglobulinemia cells have features of both plasma cells and lymphocytes and are called lymphoplasmacytoid 3. Even though Waldenstrom macroglobulinemia has a monoclonal gammopathy and is sometimes grouped into other plasma cell disorders, it is considered a type of non-Hodgkin lymphoma (NHL).
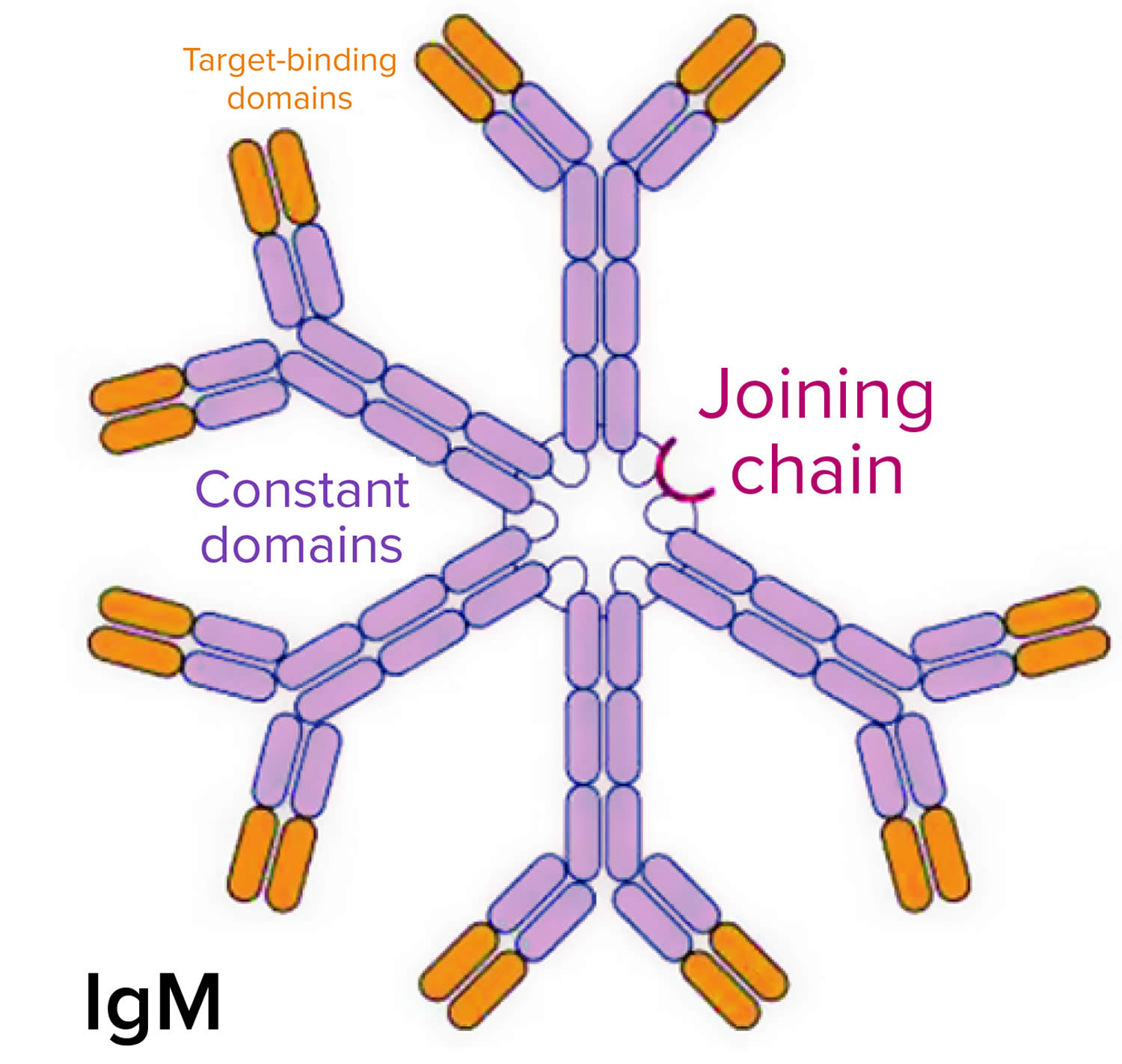
Waldenstrom macroglobulinemia cells (B lymphocytes that are turning into plasma cells) make large amounts of a protein called monoclonal immunoglobulin M (IgM) antibody, which is known as a macroglobulin 4. The “M” in immunoglobulin M (IgM) stands for macroglobulin and is where the naming macroglobinemia is derived from. Each antibody (protein) made by the Waldenstrom macroglobulinemia cells is the same, so it is called a monoclonal protein, or just an M protein. The buildup of this M protein (high levels of IgM antibody) in the bone marrow, spleen and blood, causing the blood plasma to become thicker and blood flow to various body organs may be impaired. This may cause signs or symptoms such as trouble seeing or hearing loss, heart problems, shortness of breath, headaches, dizziness, abnormal bleeding, confusion, and numbness or tingling of the hands and feet. Patients can also experience lethargy and fatigue, and are at increased risk of infection. This complication occurs in approximately 10-30% of people with Waldenstrom’s macroglobulinemia. Sometimes there are no signs or symptoms of Waldenstrom macroglobulinemia. It may be found when a blood test is done for another reason.
The Waldenstrom macroglobulinemia cells grow mainly in the bone marrow, where they can crowd out the normal cells that make the different types of blood cells. This can lead to low levels of red blood cells (called anemia), which can make people feel tired and weak. It can also cause low numbers of white blood cells (called leukopenia), which makes it hard for your body to fight infection. The numbers of platelets in the blood can also drop (called thrombocytopenia), leading to increased bleeding and bruising.
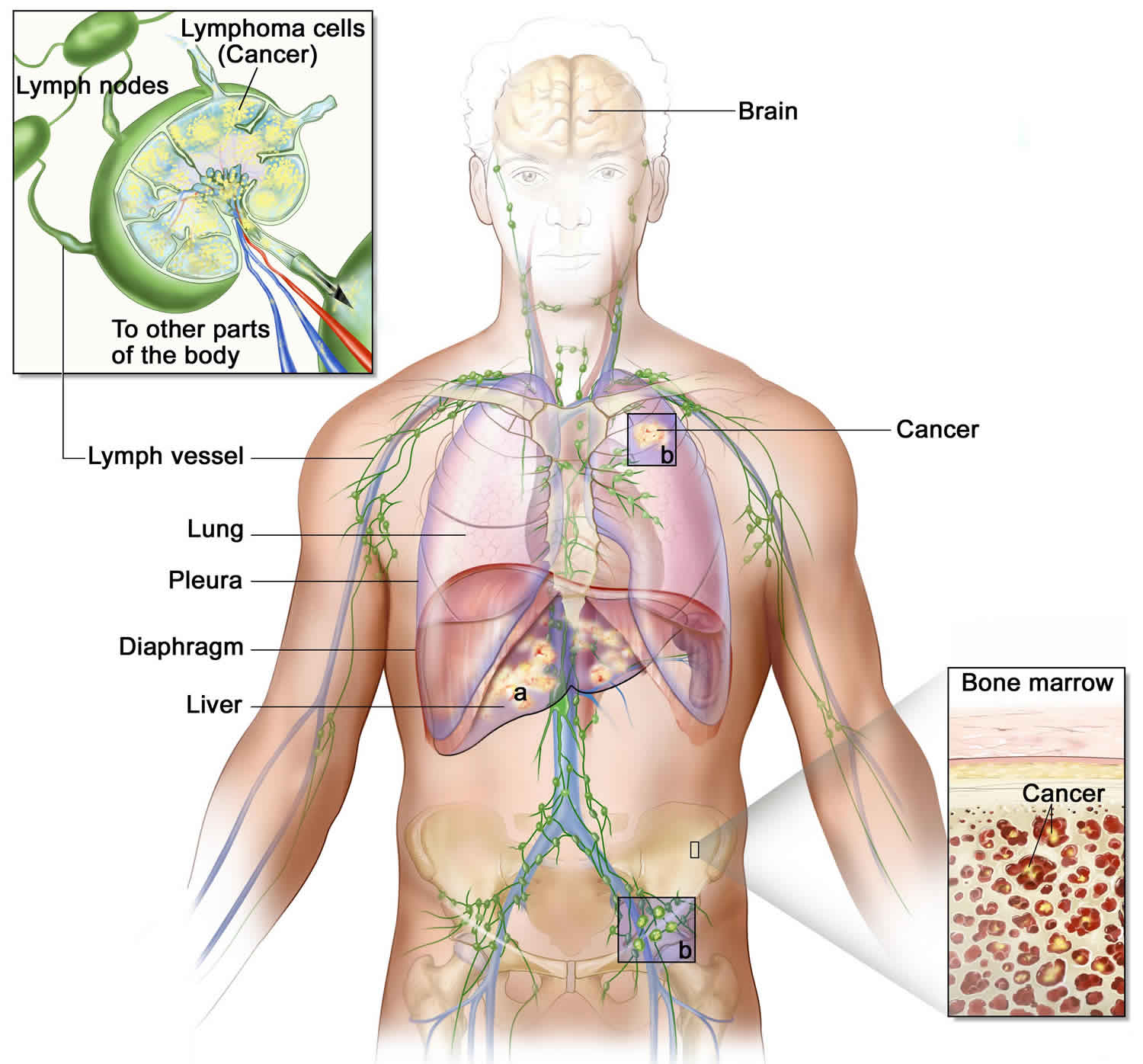
Lymphoma cells can also grow in organs like the liver and spleen, causing these organs to swell and leading to abdominal pain 5.
Waldenstrom macroglobulinemia is rare, with an incidence rate of about 3 cases per million people per year in the United States 6. About 1,000 to 1,500 people are diagnosed with Waldenstrom macroglobulinemia each year in the United States 6.
Waldenstrom macroglobulinemia is more common in men than it is in women and it is much more common among Whites than African Americans 6.
There are few cases of Waldenstrom macroglobulinemia in younger people, but the chance of developing this disease goes up as people get older. The average age of people when they are diagnosed with Waldenstrom macroglobulinemia is 70 6.
Waldenstrom macroglobulinemia typically progresses slowly and may be managed with a careful watch-and-wait approach for about 20% of patients 7. More often, Waldenstrom macroglobulinemia requires treatment. Treatment for Waldenstrom macroglobulinemia includes drugs used to treat multiple myeloma and non-Hodgkin lymphoma (NHL). The current therapies can control the disease for many years. The five-year-survival-rate is approximately 75% for patients with newly diagnosed Waldenstrom macroglobulinemia 7. New therapies have significantly increased our ability to control the disease and may extend the median overall survival of Waldenstrom macroglobulinemia patients for up to 15 years after diagnosis, although we are still collecting data to prove this projection. Nevertheless, recurrence is common in Waldenstrom macroglobulinemia patients, and the disease is still considered incurable 7.
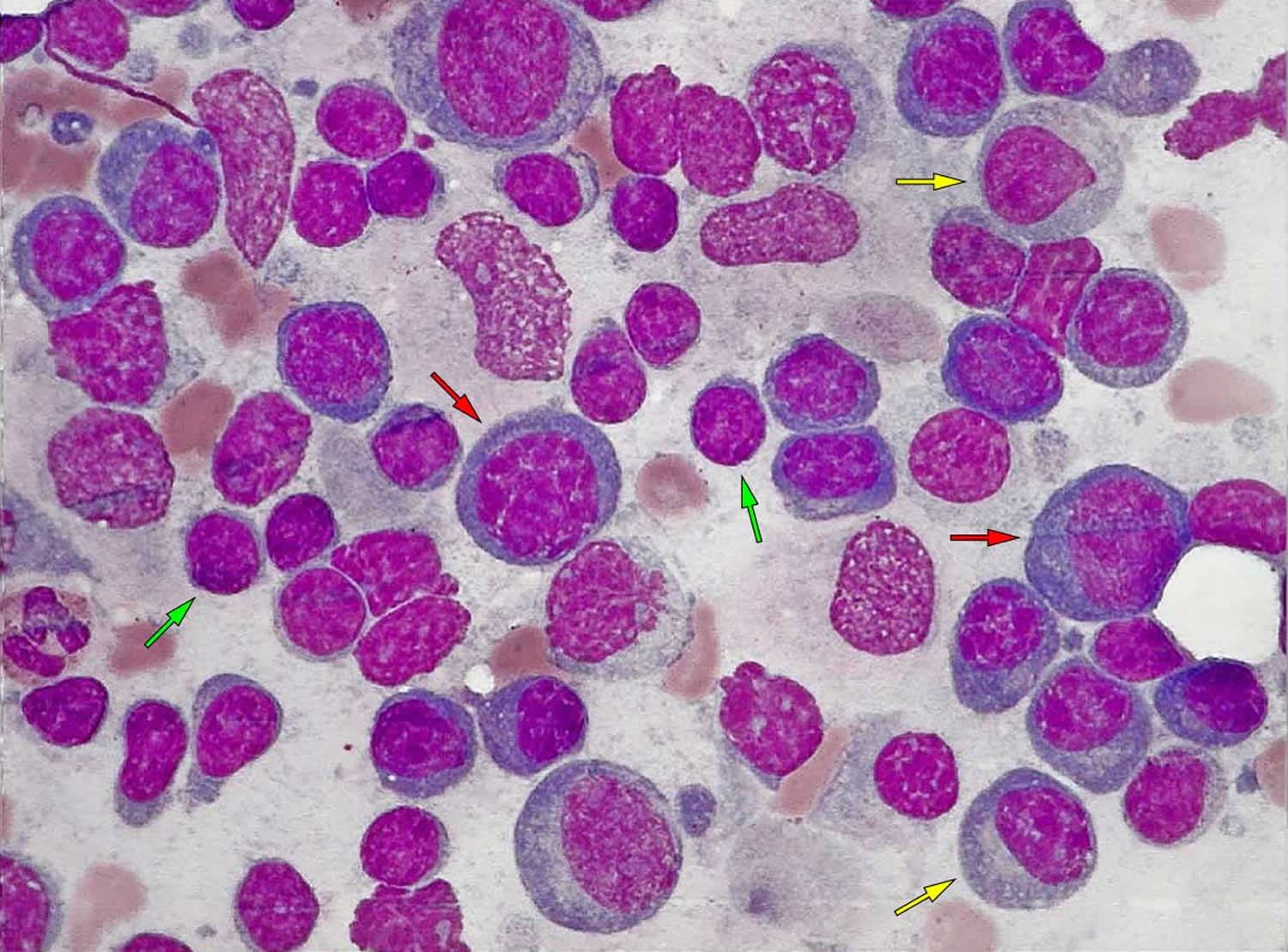
Figure 1. Lymphoplasmacytic lymphoma
Footnote: Image of lymph node shows an infiltration by lymphoplasmacytic lymphoma. The tumor cells comprise a variable mixture of small lymphocytes with a high nuclear-cytoplasmic ratio, condensed nuclear chromatin and inconspicuous nucleoli (green arrow), medium-sized to large plasmacytoid lymphocytes characterized by an eccentric location of the nucleus, abundant darkly basophilic cytoplasm (yellow arrow) and plasma cells (red arrow).
[Source 8 ]Figure 2. Immunoglobulin M
The Lymphatic System
To understand Waldenstrom macroglobulinemia, it helps to know about the lymph system (also known as the lymphatic system) and the functions of lymphoid tissue in the body.
The lymph system is part of the immune system, which helps fight infections and some other diseases. The lymphatic system plays a role in:
- fighting bacteria and other infections
- destroying old or abnormal cells, such as cancer cells
The lymphatic system also helps the flow of fluids in the body.
The lymph system is made up mainly of cells called lymphocytes, a type of white blood cell. There are 2 main types of lymphocytes:
- B lymphocytes (B cells): B lymphocytes (B cells) respond to an infection by changing into a different type of cell called a plasma cell. Plasma cells make proteins called antibodies (also called immunoglobulins) that help the body attack and kill disease-causing germs like bacteria and viruses.
- T lymphocytes (T cells): There are several types of T cells. Some T cells destroy germs or abnormal cells in the body. Other T cells help boost or slow the activity of other immune system cells.
- Natural killer (NK) cells, which attack virus-infected cells or tumor cells.
The lymphatic system is a vast collection of cells and biochemicals that travel in lymphatic vessels, and the organs and glands that produce them. The lymphatic system includes a network of vessels (like the arteries and veins that carry blood) that assist in circulating body fluids (a colorless liquid called lymph), so it is closely associated with your cardiovascular system. Lymphatic vessels transport excess fluid away from interstitial spaces in most tissues and return it to the bloodstream (Figure 5). This fluid carries food to the cells and bathes the body tissues to form tissue fluid. The fluid then collects waste products, bacteria, and damaged cells. It also collects any cancer cells if these are present. This fluid then drains into the lymph vessels. Without the lymphatic system, this fluid would accumulate in tissue spaces. Special lymphatic capillaries, called lacteals, are located in the lining of the small intestine. They absorb digested fats and transport them to the venous circulation.
The lymphatic system is a system of thin tubes and lymph nodes that run throughout the body. Lymph nodes are bean shaped glands. The thin tubes are called lymph vessels or lymphatic vessels. Tissue fluid called lymph circulates around the body in these vessels and flows through the lymph nodes.
The lymph system is an important part of your immune system. It plays a role in fighting bacteria and other infections and destroying old or abnormal cells, such as cancer cells.
The major sites of lymphoid tissue are:
- Lymph nodes: Lymph nodes are bean-sized collections of lymphocytes and other immune system cells throughout the body, including inside the chest, abdomen, and pelvis. They are connected to each other by a system of lymphatic vessels.
- Spleen: The spleen is an organ under the lower ribs on your left side. The spleen makes lymphocytes and other immune system cells. It also stores healthy blood cells and filters out damaged blood cells, bacteria, and cell waste.
- Bone marrow: The bone marrow is the spongy tissue inside certain bones. New blood cells (including some lymphocytes) are made there.
- Thymus: The thymus is a small organ behind the upper part of the breastbone and in front of the heart. Thymus is an organ in which T lymphocytes mature and multiply.
- Adenoids and tonsils: These are collections of lymphoid tissue in the back of your throat. They help make antibodies against germs that are breathed in or swallowed.
- Digestive tract: The stomach, intestines, and many other organs also have lymph tissue.
The lymphatic system has a second major function— it enables you to live in a world with different types of organisms. Some of them live in or on the human body and in some circumstances may cause infectious diseases. Cells and biochemicals of the lymphatic system launch both generalized and targeted attacks against “foreign” particles, enabling the body to destroy infectious agents. This immunity against disease also protects against toxins and cancer cells. When the immune response is abnormal, persistent infection, cancer, allergies, and autoimmune disorders may result.
The larger lymphatic vessels lead to specialized organs called lymph nodes. After leaving the lymph nodes, the vessels merge to form still larger lymphatic trunks.
Figure 3. Locations of major lymph nodes
Figure 4. Functions of lymph nodes in the lymphatic system
Figure 5. Schematic representation of lymphatic vessels transporting fluid from interstitial spaces to the bloodstream. Depending on its origin, lymph enters the right or left subclavian vein.
Waldenstrom macroglobulinemia signs and symptoms
Waldenstrom macroglobulinemia is slow growing and may not cause signs and symptoms for many years.
When Waldenstrom macroglobulinemia does cause symptoms, some of them can be like those seen with other types of non-Hodgkin lymphoma (NHL). For example, weight loss, fever, night sweats, and swollen lymph nodes can be seen in many types of non-Hodgkin lymphoma.
Waldenstrom macroglobulinemia signs and symptoms may include:
- Easy bruising
- Bleeding from the nose or the gums
- Fatigue
- Weight loss
- Numbness in your hands or feet
- Fever
- Headache
- Shortness of breath
- Changes in vision
- Confusion
Other Waldenstrom macroglobulinemia symptoms are caused by the large amounts of abnormal IgM antibody (M protein) made by the cancer cells:
- In hyperviscosity syndrome, too much of the M protein in the blood can cause it to become too “thick.” (This is not the kind of thickness that can be treated with drugs known as blood thinners.) When the blood gets too thick, it has trouble moving through blood vessels. This can cause problems such as poor circulation to the brain, which can lead to symptoms like those from a stroke.
- If the M protein only thickens the blood in cooler parts of the body (like in the tip of the nose, ears, fingers, and toes), it is called a cryoglobulin. Cryoglobulins can cause pain or other problems in these areas if a person is exposed to cooler temperatures.
- A condition called amyloidosis can occur when a part of the IgM antibody (called the light chain) builds up in organs like the heart and kidneys. This buildup can lead to heart and kidney problems.
Not all people with Waldenstrom macroglobulinemia develop hyperviscosity, cryoglobulins, or amyloidosis.
Common symptoms of Waldenstrom macroglobulinemia
The most common signs and symptoms to first appear in people with Waldenstrom macroglobulinemia are weakness and extreme tiredness (fatigue) caused by a shortage of red blood cells (anemia). Affected individuals can also experience general symptoms such as fever, night sweats, and weight loss. Some people with Waldenström macroglobulinemia develop a loss of sensation and weakness in the limbs (peripheral neuropathy). Doctors are unsure why this feature occurs, although they speculate that the IgM protein attaches to the protective covering of nerve cells (myelin) and breaks it down. The damaged nerves cannot carry signals normally, leading to neuropathy.
Other features of Waldenström macroglobulinemia are due to the accumulation of lymphoplasmacytic cells in different tissues. For example, accumulation of these cells can lead to an enlarged liver (hepatomegaly), spleen (splenomegaly), or lymph nodes (lymphadenopathy). In the bone marrow, the lymphoplasmacytic cells interfere with normal blood cell development, causing a shortage of healthy blood cells (pancytopenia).
- Weakness: This is one of the most common symptoms of Waldenstrom macroglobulinemia. It can be caused by anemia (too few red blood cells), which can happen when the Waldenstrom macroglobulinemia cells crowd out normal cells in the bone marrow. Some people also feel weak when the blood thickens from the buildup of the abnormal protein.
- Loss of appetite: Some people with Waldenstrom macroglobulinemia lose their appetite.
- Fever, sweats, weight loss: Waldenstrom macroglobulinemia, like other lymphomas, can cause fevers (without an infection), drenching night sweats, and weight loss (without trying). These are called B symptoms.
- Neuropathy: In some people with Waldenstrom macroglobulinemia, the abnormal antibody can attack and damage nerves outside the brain. This can lead to numbness or a painful “pins and needles” sensation in the feet and legs, which is called neuropathy.
Less common signs and symptoms of Waldenstrom macroglobulinemia
Several other signs and symptoms of Waldenström macroglobulinemia are related to the excess amounts of IgM. Increased IgM can thicken blood and impair circulation, causing a condition known as hyperviscosity syndrome. Features related to hyperviscosity syndrome include bleeding in the nose or mouth, blurring or loss of vision, headache, dizziness, and confusion. In some affected individuals, IgM and other immunoglobulins react to cold temperatures to form gel-like clumps that block blood flow in areas exposed to the cold, such as the hands and feet. These clumped proteins are referred to as cryoglobulins, and their clumping causes a condition known as cryoglobulinemia. Cryoglobulinemia can lead to pain in the hands and feet or episodes of Raynaud phenomenon, in which the fingers and toes turn white or blue in response to cold temperatures. The IgM protein, along with another protein called amyloid, can build up in organs and interfere with their normal function. This buildup causes a condition called amyloidosis. Organs that are typically affected by amyloidosis include the heart, kidneys, liver or spleen. Affected individuals can experience weakness, fatigue, shortness of breath, irregular heartbeat, or joint pain.
- Enlarged lymph nodes: These usually appear as lumps under the skin around the neck, in the groin, or in the armpits. Enlarged lymph nodes are usually about 1 or 2 inches (2.5-5 cm) across. They are seen less often in Waldenstrom macroglobulinemia than in most other lymphomas.
- Swollen abdomen (belly): Waldenstrom macroglobulinemia can sometimes make the spleen or liver bigger, making the belly look swollen. In the upper part of the abdomen, the liver is on the right and the spleen on the left. When the spleen gets larger, it can press on the stomach, which makes people feel full when they eat even a small amount.
- Circulation system symptoms: In hyperviscosity syndrome, the thickened blood causes poor brain circulation, leading to problems like headache, confusion, and dizziness. It can also cause symptoms like those seen with a stroke, including slurred speech and weakness on one side of the body. Patients with these symptoms should contact their doctor right away.
- Abnormal bleeding: High levels of abnormal antibody can damage blood vessels, which can lead to problems like nosebleeds and bleeding gums.
- Vision problems: Bleeding around the small blood vessels inside the eyes or poor circulation in these vessels caused by thickened blood might lead to blurred vision or blind spots.
- Kidney problems: High levels of the M protein can damage the kidneys directly or through the development of amyloidosis. When the kidneys don’t work well, excess salt, fluid, and body waste products stay in the blood. This can cause symptoms like weakness, trouble breathing, and fluid buildup in body tissues.
- Heart problems: High levels of the M protein can damage heart tissue directly or through the development of amyloidosis, in which the protein builds up in the heart muscle. This weakens the heart, affecting its ability to pump blood. In addition, because the blood of people with Waldenstrom macroglobulinemia is thicker than normal, their hearts have to work harder to pump blood throughout the body. This strain can wear down the heart muscle, leading to a condition called congestive heart failure. Symptoms can include heart palpitations, feeling tired and weak, cough, shortness of breath, rapid weight gain, and swelling in the feet and legs.
- Infections: The high levels of abnormal antibody in Waldenstrom macroglobulinemia can slow the body’s normal antibody production. This makes it harder for the body to fight infections.
- Digestive symptoms: In some people with Waldenstrom macroglobulinemia, the buildup of the M protein in the intestines can lead to problems such as diarrhea, poor absorption of vitamins, or gastrointestinal bleeding (seen as blood in the stools or dark stools).
- Sensitivity to cold: In people with cryoglobulins, exposure to cold temperatures can lead to pain, itching, a bluish color, or even sores on the tip of the nose, ears, fingers, or toes due to reduced blood flow to these areas.
Waldenstrom macroglobulinemia causes
It’s not clear what causes Waldenstrom macroglobulinemia. Doctors know that Waldenstrom macroglobulinemia begins with one abnormal white blood cell that develops errors (mutations) in its genetic code. The errors tell the cell to continue multiplying rapidly. Because cancer cells don’t mature and then die as normal cells do, they accumulate, eventually overwhelming production of healthy cells. In the bone marrow — the soft, blood-producing tissue that fills in the center of most of your bones — Waldenstrom macroglobulinemia cells crowd out healthy blood cells.
Waldenstrom macroglobulinemia cells continue trying to produce antibodies, as healthy white blood cells do, but instead they produce abnormal proteins that the body can’t use. The protein immunoglobulin M (IgM) accumulates in the blood, impairs circulation and causes complications.
Some people inherit DNA changes from a parent that increase their risk for certain types of cancer. Researchers are studying families that have many cases of Waldenstrom macroglobulinemia to try to find the genes that might cause this disorder in some people.
The DNA changes found in Waldenstrom macroglobulinemia cells are usually acquired after birth (not passed on from a parent). Some of these acquired changes may have outside causes, but often they occur for no apparent reason. They seem to happen more often as you age, which might help explain why Waldenstrom macroglobulinemia usually occurs in older people.
Recent research has found that about 9 times out of 10, Waldenstrom macroglobulinemia cells have a mutation (change) in a gene known as MYD88, which normally helps immune system cells signal each other and helps keep them alive 9. The DNA change in MYD88 gene might make it stay turned on all the time, which might help the Waldenstrom macroglobulinemia cells survive longer than they should. In approximately 30 percent of affected individuals (most of whom also have the MYD88 gene variant), the Waldenstrom macroglobulinemia cells have changes in the CXCR4 gene 10, 11. Changes in these genes have been linked with a greater chance of Waldenstrom macroglobulinemia causing symptoms and requiring treatment, and they seem to affect survival as well 12.
The proteins produced from the MYD88 and CXCR4 genes are both involved in signaling within cells. The MyD88 protein relays signals that help prevent the self-destruction (apoptosis) of cells, thus aiding in cell survival. The CXCR4 protein stimulates signaling pathways inside the cell that help regulate cell growth and division (proliferation) and cell survival. Variants in these genes lead to production of proteins that are constantly turned on (overactive). Excessive signaling through these overactive proteins allows survival and proliferation of abnormal cells that should undergo apoptosis, which likely contributes to the accumulation of lymphoplasmacytic cells in Waldenström macroglobulinemia.
Sometimes, Waldenstrom macroglobulinemia cells have other kinds of DNA changes. In each human cell, the DNA is packaged in 23 pairs of chromosomes. In some Waldenstrom macroglobulinemia cells, a piece of a chromosome is missing. This is called a deletion. The most common chromosome defect seen in Waldenstrom macroglobulinemia is a deletion of part of chromosome 6. It’s not clear exactly which genes this might affect.
Another type of chromosome defect in Waldenstrom macroglobulinemia is called a translocation. In a translocation, a piece of one chromosome becomes attached to a different chromosome. Chromosome changes like these can cause oncogenes to be turned on or tumor suppressor genes turned off.
Researchers have found that some patients with Waldenstrom macroglobulinemia have important changes or defects in other bone marrow cells. These changes might also help cancer cells grow. Certain cells in the bone marrow called dendritic cells release a hormone called interleukin-6 (IL-6) that helps normal plasma cells and plasmacytoid lymphocytes grow. Excess IL-6 production by these cells appears to be an important factor in the development of Waldenstrom macroglobulinemia.
Scientists are learning about the exact gene changes that cause Waldenstrom macroglobulinemia. But even though they have found some of these gene changes, they still do not know why these changes occur.
Risk factors for developing Waldenstrom macroglobulinemia
A risk factor is anything that affects your chance of getting a disease such as cancer. Researchers have found a few risk factors that make a person more likely to develop Waldenstrom macroglobulinemia. But most people with these risk factors never develop Waldenstrom macroglobulinemia.
Factors that may increase your risk of Waldenstrom macroglobulinemia include:
- Being older. Waldenstrom macroglobulinemia can occur at any age, but it’s most often diagnosed in adults 65 and older.
- Being male. Males are more likely to be diagnosed with Waldenstrom macroglobulinemia.
- Being white. White people are more likely to develop the disease, compared with people of other races.
- Having a family history of lymphoma. If you have a relative who has been diagnosed with Waldenstrom macroglobulinemia or another type of B-cell lymphoma, you may have an increased risk.
- Hepatitis C. Hepatitis C is caused by infection with a virus (known as the hepatitis C virus, or HCV). Some studies have found that people with chronic hepatitis C infection might be more likely to develop Waldenstrom macroglobulinemia than people without the virus. But not all studies have found such a link.
- Certain autoimmune diseases. Some research has suggested that people with certain types of autoimmune disease, such as Sjögren (Sjogren) syndrome, might be at higher risk for Waldenstrom macroglobulinemia.
- Monoclonal gammopathy of undetermined significance (MGUS). Monoclonal gammopathy of undetermined significance (MGUS) is an abnormality of antibody-making cells that is related to multiple myeloma and Waldenstrom macroglobulinemia. In MGUS, like Waldenstrom macroglobulinemia and multiple myeloma, abnormal cells in the bone marrow make large amounts of one particular antibody. This antibody is called a monoclonal (or M) protein, and the condition is called a monoclonal gammopathy. MGUS itself does not cause health problems, but each year about 1% to 2% of people with MGUS go on to develop a related cancer (like multiple myeloma, Waldenstrom macroglobulinemia, or lymphoma) or another serious health problem (like amyloidosis).
Waldenstrom macroglobulinemia prevention
Most of the risk factors for Waldenstrom macroglobulinemia, such as older age or monoclonal gammopathy of undetermined significance (MGUS), can’t be changed or controlled, so there is no way to prevent cancers that might be related to these risk factors.
Some research suggests that people with hepatitis C might be more likely to develop Waldenstrom macroglobulinemia. There is currently no vaccine to prevent hepatitis C, but there are ways to lower your risk of getting it, such as avoiding known risk factors like injection drug use or unprotected sex with many partners. Hepatitis C can also be treated effectively in many cases, although it’s not known how this might affect a person’s risk of Waldenstrom macroglobulinemia.
Waldenstrom macroglobulinemia diagnosis
Waldenstrom macroglobulinemia is often found when a person goes to see their doctor because of symptoms they are having, or because they just don’t feel well and go in for a checkup. Sometimes it’s found in people without symptoms when they have blood tests done for some other reason.
If signs or symptoms suggest that a person might have Waldenstrom macroglobulinemia, exams and tests will be done to be sure. The most important tests will look for abnormal proteins in the blood and abnormal cells in the bone marrow. Because Waldenstrom macroglobulinemia is a type of lymphoma, like other lymphomas it can invade the bone marrow, lymph nodes, and other organs.
Tests and procedures used to diagnose Waldenstrom macroglobulinemia include:
Lab tests
Waldenstrom macroglobulinemia might be suspected if your doctor finds you have low blood cell counts or unusual protein levels on blood tests. If so, your doctor may order a blood test called serum protein electrophoresis to find out what the abnormal proteins are. It is usually only after these tests are done that a biopsy of either the bone marrow or a lymph node is considered.
Blood cell counts
The complete blood count (CBC) is a test that measures the levels of red blood cells, white blood cells, and platelets. If lymphoma cells occupy too much of the bone marrow, these blood levels may be low.
Immunoglobulin levels
This test measures the levels of the different antibodies (immunoglobulins) in the blood – IgA, IgE, IgG, and IgM – to see if any are abnormally high or low. In Waldenstrom macroglobulinemia the level of IgM is high but the IgG level is often low.
Electrophoresis
The abnormal immunoglobulin made in Waldenstrom macroglobulinemia is an IgM antibody. This antibody is monoclonal, meaning that it is many copies of the exact same antibody. Serum protein electrophoresis (or SPEP) is a test that measures the total amount of immunoglobulins in the blood and finds any monoclonal immunoglobulin. Another test, such as immunofixation electrophoresis, is then used to determine the type of antibody that is abnormal (IgM or some other type).
Finding a monoclonal IgM antibody in the blood is needed to diagnose Waldenstrom macroglobulinemia. This abnormal protein in Waldenstrom macroglobulinemia is known by many different names, including monoclonal immunoglobulin M, IgM protein, IgM spike, IgM paraprotein, M protein, and M-spike. High levels of other types of monoclonal immunoglobulins, like IgA or IgG, are seen in different disorders (like multiple myeloma and some other lymphomas).
Sometimes pieces of the IgM protein are excreted by the kidneys into the urine. These proteins can be detected with a test called urine protein electrophoresis.
Viscosity
Viscosity is a measure of how thick the blood is. If the IgM level is too high, the blood will become thick (viscous) and can’t flow freely (think about pouring honey compared to pouring water).
Cryocrit
This test measures the blood levels of cryoglobulins (proteins that clump together in cool temperatures and can block blood vessels).
Cold agglutinins
Cold agglutinins are antibodies that attack and kill red blood cells, especially at cooler temperatures. These dead cells can then build up and block blood vessels. A blood test can be used to detect these antibodies.
Beta-2 microglobulin (β2M)
This test measures another protein made by the cancer cells in Waldenstrom macroglobulinemia. This protein itself doesn’t cause any problems, but it’s a useful indicator of a patient’s prognosis (outlook). High levels of beta-2 microglobulin (β2M) are linked with a worse outlook.
Biopsies
The symptoms of Waldenstrom macroglobulinemia and non-Hodgkin lymphoma (NHL) are not distinctive enough for a doctor to know for certain if a person has one of them, based on symptoms alone. Most symptoms can also be caused by non-cancerous problems like infections or by other kinds of cancers. Blood tests can help point to the correct diagnosis, but a biopsy (removing samples of affected tissue to look at under a microscope) is the only way to be sure. Several types of biopsies might be used.
Bone marrow aspiration and biopsy
This is the most important type of biopsy for Waldenstrom macroglobulinemia, and is needed to confirm the diagnosis. It can be done at the doctor’s office or at the hospital.
The bone marrow aspiration and biopsy are usually done at the same time. The samples are taken from the back of the pelvic (hip) bone, although in some cases they may be taken from the sternum (breast bone) or other bones.
- In bone marrow aspiration, you lie on a table (either on your side or on your belly). The doctor cleans the skin over the hip and then numbs the area and the surface of the bone by injecting a local anesthetic. This may briefly sting or burn. A thin, hollow needle is then inserted into the bone, and a syringe is used to suck out a small amount of liquid bone marrow. Even with the anesthetic, most patients still have some brief pain when the marrow is removed.
- A bone marrow biopsy is usually done just after the aspiration. A small piece of bone and marrow is removed with a slightly larger needle that is pushed down into the bone. This may also cause some brief pain.
Once the biopsy is done, pressure is applied to the site to help stop any bleeding. There will be some soreness in the biopsy area when the numbing medicine wears off. Most patients can go home right after the procedure.
The bone marrow samples are then sent to a lab, where they are tested to see if they have lymphoma cells (see below). For a diagnosis of Waldenstrom macroglobulinemia, at least 10% of the cells in the bone marrow must be lymphoplasmacytoid lymphoma cells.
Fine needle aspiration (FNA) biopsy
In an FNA biopsy, the doctor uses a very thin, hollow needle with a syringe to withdraw a small amount of tissue from a tumor or lymph node. This type of biopsy is useful for sampling lymph nodes to see if they are enlarged because of cancer or an infection. FNA can help diagnose some lymphomas, but Waldenstrom macroglobulinemia is usually diagnosed with a bone marrow biopsy.
For an FNA on an enlarged node near the surface of the body, the doctor can aim the needle while feeling the node. If the enlarged node (or tumor) is deep inside the body, the needle can be guided while it is seen on a computed tomography (CT) scan or ultrasound (see the descriptions of imaging tests later in this section).
The main advantage of FNA is that it does not require surgery and can often be done in a doctor’s office. The main drawback is that in some cases it might not get enough tissue to make a definite diagnosis of lymphoma. However, advances in lab tests (discussed later in this section) and the growing experience of many doctors with FNA have improved the accuracy of this procedure.
Excisional or incisional biopsy
For these types of biopsies, a surgeon cuts through the skin to remove an entire lymph node or tumor (excisional biopsy) or just a small part of a large tumor or lymph node (incisional biopsy). These biopsies are rarely needed in people with Waldenstrom macroglobulinemia because the diagnosis is usually made with a bone marrow biopsy. They are used more often for other types of lymphoma.
If the area to be biopsied is near the skin surface, this can be done using local anesthesia (numbing medicine). If the area is inside the chest or abdomen, general anesthesia or deep sedation is used (where the patient is asleep). These types of biopsies almost always provide enough tissue to diagnose the exact type of lymphoma.
Fat pad fine needle aspiration
This type of biopsy may be used in some people with Waldenstrom macroglobulinemia to check for amyloid. In this procedure, a thin, hollow needle with a syringe attached is inserted into an area of fat (usually under the skin of the abdomen/belly). A small amount of fat is removed and sent to the lab for testing.
Lab tests on biopsy specimens
All biopsy specimens are looked at in the lab by a pathologist – a doctor with special training in using lab tests to diagnose diseases. In some cases, a hematopathologist, a doctor with further training in diagnosing blood and lymph node diseases, might also look at the biopsy. The doctors look at the size and shape of the cells and how they are arranged. Sometimes just looking at the cells doesn’t provide a clear answer, so other lab tests are needed.
Immunohistochemistry
In this test, a part of the biopsy sample is treated with special man-made antibodies that attach to cells only if they contain specific proteins. These antibodies cause color changes in the cells, which can be seen with a microscope. This test may help tell different types of lymphoma from one another and from other diseases.
Flow cytometry
In this test, cells are treated with special man-made antibodies. Each antibody sticks only to certain types of cells. The cells are then passed in front of a laser beam. If the cells now have antibodies attached to them, the laser will make them give off light, which is measured and analyzed by a computer.
This is the most common test for immunophenotyping – classifying lymphoma cells according to the proteins (antigens) on their surfaces. Different types of lymphocytes have different antigens on their surface. These antigens also change as each cell matures.
This test can help show if a lymph node is swollen because of lymphoma, some other cancer, or a non-cancerous disease. It has become very important in helping doctors determine the exact type of lymphoma so they can select the best treatment.
Cytogenetics
Doctors use this technique to look at the chromosomes (long strands of DNA) inside lymphoma cells. Cells (usually from the bone marrow) are first grown in the lab. Then the chromosomes are stained and looked at closely. Because it takes time for the cells to start dividing, this test can take a few weeks.
In some lymphomas, the cells may have too many chromosomes, too few chromosomes, missing parts of chromosomes (called deletions), or other abnormalities. These changes can help identify the type of lymphoma.
Molecular genetic tests
Molecular tests such as fluorescent in situ hybridization (FISH) and polymerase chain reaction (PCR) are not usually needed to diagnose Waldenstrom macroglobulinemia, but they are sometimes used to diagnose other types of non-Hodgkin lymphoma. These tests look at the cells’ DNA without having to grow the cells in the lab first. The tests can give results in less time than cytogenetics and can be done on cells from different sources (like lymph nodes, blood, and bone marrow). They are generally used to look for specific chromosome or gene changes, not just any change.
Imaging tests
Imaging tests use x-rays, magnetic fields, sound waves, or radioactive particles to produce pictures of the inside of the body. These tests are not needed to diagnose Waldenstrom macroglobulinemia, but one or more of them might be done to help show how much disease and where it is in the body.
Chest x-ray
An x-ray might be done to look at the chest for enlarged lymph nodes.
Computed tomography (CT) scan
The CT scan is an x-ray that makes detailed cross-sectional images of your body. Unlike a regular x-ray, CT scans can show the detail in soft tissues (such as internal organs). This scan can help show if any lymph nodes or organs in your body are enlarged. CT scans are useful for looking for signs of lymphoma in the chest, abdomen, and pelvis.
CT-guided needle biopsy: CT scans can also be used to guide a biopsy needle into a suspicious area. For this procedure, the patient lies on the CT scanning table while the doctor moves a biopsy needle through the skin and toward the area. CT scans are repeated until the needle is in the right place. A biopsy sample is then removed and sent to the lab.
Magnetic resonance imaging (MRI) scan
This test is rarely used in Waldenstrom macroglobulinemia, but if your doctor is concerned about the brain or spinal cord, MRI is very useful for looking at these areas.
Ultrasound
Ultrasound can be used to look at lymph nodes near the surface of the body or to look inside your abdomen for enlarged lymph nodes or organs such as the liver, spleen, and kidneys. (It can’t be used to look at organs or lymph nodes in the chest because the ribs block the sound waves.) It is sometimes used to help guide a biopsy needle into an enlarged lymph node.
Positron emission tomography (PET) scan
A PET scan can be helpful in spotting small collections of cancer cells. It is even more valuable when combined with a CT scan (PET/CT scan).
PET scans also can help tell if an enlarged lymph node contains lymphoma or not. It can help spot small areas that might be lymphoma, even if the area looks normal on a CT scan. These tests can be used to tell if a lymphoma is responding to treatment. They can also be used after treatment to help decide whether an enlarged lymph node still contains lymphoma or is merely scar tissue.
Waldenstrom macroglobulinemia stages
The stage of a cancer means how big it is and whether it has spread. It is important because the stage often decides the treatment. The tests and scans you have to diagnose Waldenstrom macroglobulinemia give some information about the stage.
However, there is no standard staging system for Waldenstrom macroglobulinemia based on the extent of the disease in the body because this hasn’t been shown to be important when looking at outcomes or deciding on treatment 13. Instead, doctors look at other factors, such as age, blood cell counts, the amount of immunoglobulin (IgM) in the blood, and the level of another protein in the blood called beta-2 microglobulin (β2M). People with lower levels of IgM and β2M tend to do better than those with higher levels. People with Waldenstrom macroglobulinemia who are older, are anemic (based on a low blood hemoglobin level), or have a low blood platelet count tend to have a poorer outlook.
Experts have used these factors to develop a system that helps predict prognosis (outlook) for patients with Waldenstrom macroglobulinemia. It is called the International Prognostic Scoring System for Waldenstrom Macroglobulinemia (ISSWM) (see Waldenstrom macroglobulinemia survival rate below).
Waldenstrom macroglobulinemia treatment
Waldenstrom macroglobulinemia is generally not considered to be curable, but it is treatable. Many different medicines can help keep Waldenstrom macroglobulinemia under control, often for long periods of time.
Not everyone with Waldenstrom macroglobulinemia needs treatment right away. In fact, some people are diagnosed with Waldenstrom macroglobulinemia before they even have symptoms from it. Most experts recommend that people with Waldenstrom macroglobulinemia should not usually be treated until the disease is causing problems. This lets people avoid the side effects of chemotherapy (chemo), targeted therapy, or immunotherapy drugs until they really need these medicines. In fact, studies suggest that patients who delay treatment until their Waldenstrom macroglobulinemia is causing problems do not live any less time than those who start treatment as soon as they are diagnosed.
The Last Consensus on treatment initiation criteria has been recently published by the Eighth International Workshop on Waldenstrom Macroglobulinemia 14.
Table 1. Indications for Waldenstrom macroglobulinemia treatment initiation
| Clinical Criteria | Laboratory Criteria |
|---|---|
| Systemic symptoms (recurrent fever, night sweats, weight loss, fatigue) | Symptomatic cryglobulinemia |
| Hyperviscosity | Cold agglutinin anemia |
| Symptomatic or bulky (>/=5 cm in maximum diameter) lympadenopathy | Immune hemolytic anemia and/or thrombocytopenia |
| Symptomatic hepatomegaly and/or splenomegaly | Nephropathy related to Waldenström macroglobulinemia |
| Symptomatic organomegaly and/or organ or tissue infiltration | Amyloidosis related to Waldenström macroglobulinemia |
| Peripheral neuropathy due to Waldenstrom’s macroglobulinemia | Hemoglobin </=10 g/dL |
| Platelet count <100 × 109/L |
Treatment options for Waldenstrom macroglobulinemia may include:
- Observation. If IgM proteins are found in your blood, but you don’t have any signs or symptoms, you may choose to wait before beginning treatment. Your doctor may recommend blood tests every few months to monitor your condition. You may go years without needing further treatment.
- Plasma exchange. If you experience signs and symptoms related to having too many IgM proteins in your blood, your doctor may recommend plasma exchange (plasmapheresis) to remove the proteins and replace them with healthy blood plasma.
- Chemotherapy. Chemotherapy is a drug treatment that kills quickly growing cells, such as the abnormal blood cells produced by Waldenstrom macroglobulinemia. Chemotherapy may be used alone or combined with other drug treatments as an initial treatment for people who experience signs and symptoms of Waldenstrom macroglobulinemia. High-dose chemotherapy may also be used to suppress your bone marrow production in preparation for a bone marrow transplant.
- Targeted therapy. Targeted therapy drugs kill cancer cells by focusing on the specific abnormalities present in the cancer cells that allow them to survive. Targeted therapy drugs may be used alone or combined with other medications, such as chemotherapy or biological therapy, as an initial treatment for Waldenstrom macroglobulinemia or in cases where the cancer returns despite treatment.
- Biological therapy. Biological therapy drugs use your immune system to kill cancer cells. Biological therapy drugs can be used alone or in combination with other medications as an initial treatment or as a treatment for recurrent Waldenstrom macroglobulinemia.
- Bone marrow transplant. In certain highly selected situations, a bone marrow transplant, also known as a stem cell transplant, may be used to treat Waldenstrom macroglobulinemia. During this procedure, high doses of chemotherapy are used to wipe out your diseased bone marrow. Healthy blood stem cells are infused into your body where they can rebuild healthy bone marrow.
- Clinical trials. Clinical trials give you a chance to try the latest in Waldenstrom macroglobulinemia treatment.
Doctors agree that hyperviscosity syndrome is a reason to treat with plasmapheresis right away, because it can be life threatening. Other reasons to start treatment include problems from amyloidosis or cryoglobulins, as well as anemia (too few red blood cells), kidney or heart problems, nerve damage, or any severe symptom from the Waldenstrom macroglobulinemia.
Once a decision has been made to start treatment, there are several options, depending on the patient’s age, general health, and symptoms. Treatment is also based on whether or not the patient might have a stem cell transplant in the future.
The drugs used to treat Waldenstrom macroglobulinemia can be given in a variety of combinations and schedules depending on the situation. Some doctors like to combine drugs (often some type of chemotherapy plus rituximab), while others prefer to start with a single drug. The patient’s age, overall health, and symptoms can also affect which treatments are recommended.
In general, rituximab is not usually given when the IgM level is very high because it can make the IgM level temporarily go up even higher. Plasmapheresis may be used first to lower the IgM level before starting rituximab. Another option is to give rituximab along with ibrutinib because the combination can rapidly reduce the level of IgM.
If a stem cell transplant might be used later on, many experts recommend not giving certain chemo drugs (chlorambucil, bendamustine, cladribine, or fludarabine) because they might affect the stem cells in the body.
Some of the drugs and combinations that might be used as the first treatment for Waldenstrom macroglobulinemia include:
- Ibrutinib, with or without rituximab
- Bendamustine, with or without rituximab
- Bortezomib, with or without dexamethasone and/or rituximab
- Chlorambucil
- Cladribine, with or without rituximab
- Cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab (CHOP-R)
- Cyclophosphamide, prednisone, and rituximab (CPR)
- Fludarabine, with or without rituximab
- Fludarabine, cyclophosphamide, and rituximab (FCR)
- Rituximab
- Rituximab, cyclophosphamide, and dexamethasone (RCD)
- Thalidomide, with or without rituximab
- Zanubrutinib
Other drugs and drug combinations can also be used. Talk to your doctor about which regimen might be best for you based on your situation.
During treatment, you’ll have regular visits with your doctor, who will ask you about your symptoms, do physical exams, and test your blood to see how well the treatment is working. In most people with Waldenstrom macroglobulinemia, the disease will respond to treatment (IgM levels will go down and symptoms will get better) within a few months, although this may take longer in people getting only rituximab . If the Waldenstrom macroglobulinemia responds, options include close monitoring for signs of disease progression or giving rituximab on a regular schedule to help keep the disease in check.
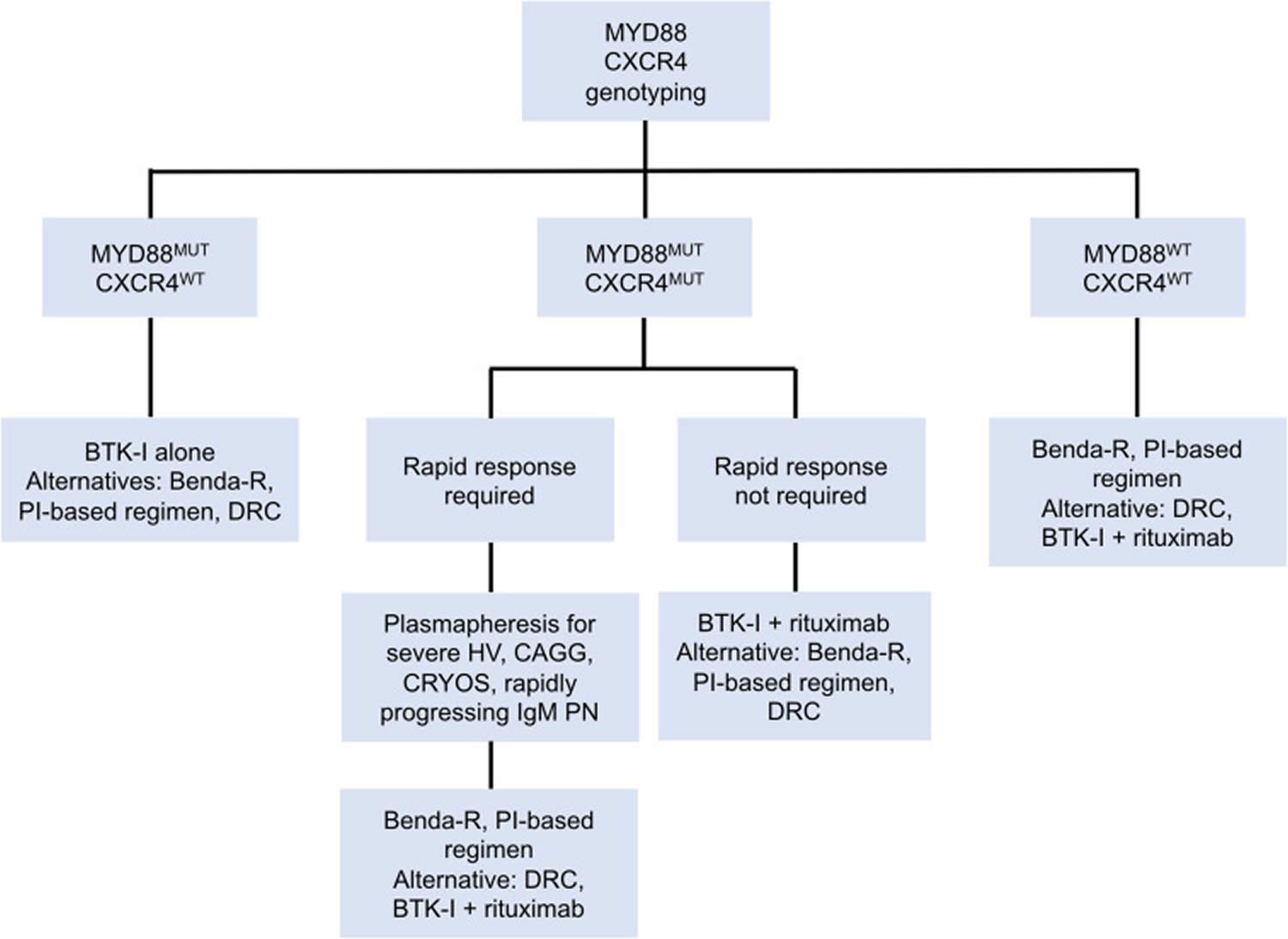
Figure 6. Genomic-based treatment algorithm for symptomatic treatment-naive patients with Waldenstrom macroglobulinemia
Abbreviations: Benda-R = bendamustine and rituximab; BTK-I = Bruton tyrosine kinase inhibitor; CAGG = cold agglutinin disease; CRYOS = cryoglobulins; DRC = dexamethasone, rituximab, cyclophosphamide; HV = hyperviscosity; PI = proteasome inhibitor; PN = progressive neuropathy.
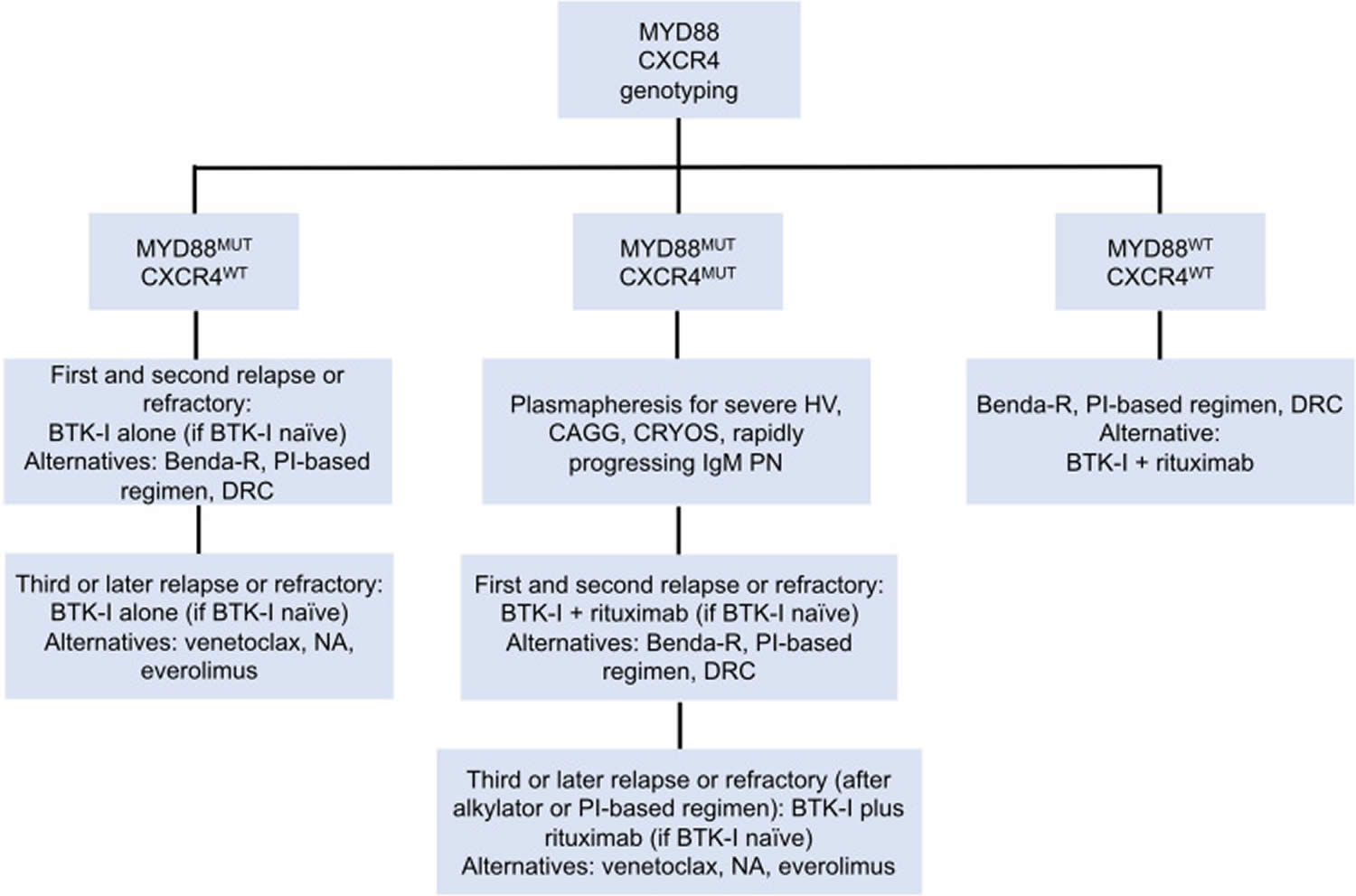
[Source 15 ]Figure 7. Genomic-based treatment algorithm for symptomatic, previously treated, or refractory patients with Waldenstrom macroglobulinemia
Abbreviations: Benda-R = bendamustine and rituximab; BTK-I = Bruton tyrosine kinase inhibitor; CAGG = cold agglutinin disease; CRYOS = cryoglobulins; DRC = dexamethasone, rituximab, cyclophosphamide; HV = hyperviscosity; NA, nucleoside analogs; PI = proteasome inhibitor; PN = progressive neuropathy.
[Source 15 ]Chemotherapy for Waldenstrom Macroglobulinemia
Chemotherapy (chemo) uses anti-cancer drugs that are taken by mouth, or injected into a vein, a muscle, or under the skin. These drugs enter the bloodstream and reach almost all areas of the body, making this treatment very useful for Waldenstrom macroglobulinemia.
Chemo is given in cycles. A period of treatment is followed by a rest period to allow the body time to recover. Each chemo cycle generally lasts for several weeks. Most chemo treatments are given on an outpatient basis (in the doctor’s office, clinic, or hospital outpatient department).
Many types of chemo drugs can be used to treat patients with Waldenstrom macroglobulinemia:
- Alkylating agents
- Cyclophosphamide (Cytoxan®)
- Bendamustine (Treanda®)
- Purine analogs
- Fludarabine (Fludara®)
- Cladribine (2-CdA, Leustatin®)
- Corticosteroids
- Prednisone
- Dexamethasone (Decadron®)
- Other chemo drugs
- Vincristine (Oncovin®)
- Doxorubicin (Adriamycin®)
Chemo drugs may be used alone or combined with other drugs, such as targeted drugs or immunotherapy drugs.
Chemotherapy side effects
Chemotherapy drugs attack cells that are dividing quickly, which is why they work against Waldenstrom macroglobulinemia cells. But other cells in the body, such as those in the bone marrow (where new blood cells are made), the lining of the mouth and intestines, and the hair follicles, also divide quickly. These cells are also likely to be affected by chemotherapy, which can lead to certain side effects.
The side effects of chemo depend on which drugs are used, the doses, and the length of time they are taken. Common side effects include:
- Nausea and vomiting
- Loss of appetite
- Hair loss
- Mouth sores
- Diarrhea or constipation
- Increased risk of infections (from having too few white blood cells)
- Problems with bleeding or bruising (from having too few blood platelets)
- Fatigue (tiredness) and shortness of breath (from having too few red blood cells)
Other side effects can be seen with certain drugs. For example, doxorubicin can damage the heart. Corticosteroid drugs can cause problems sleeping and an increased appetite.
If you have side effects, your cancer care team can suggest steps to ease them. For example, medicines can be taken to help prevent and control nausea and vomiting. Most side effects are temporary and go away after treatment is finished. If you have serious side effects, the chemo may have to be reduced or stopped, at least temporarily.
Long-term side effects of chemotherapy
Some chemo drugs cause long-term side effects that can affect almost any part of the body. One of the most serious complications with certain chemo drugs is the possibility of developing leukemia later on. It affects a very small percentage of patients, but it is more common in patients who take fludarabine or alkylating agents.
Targeted drug therapy for Waldenstrom macroglobulinemia
Cancer cells have changes in their genes (DNA) that make them different from normal cells. These changes mean that they behave differently. Cancer cells can grow faster than normal cells and they die less easily too. Cancer cells also sometimes spread.
Targeted cancer drugs work by ‘targeting’ those differences that help a cancer cell to survive and grow.
There are many different types of targeted drugs. These are grouped together depending on how they work.
Bruton tyrosine kinase (BTK) inhibitors
Ibrutinib (Imbruvica) and zanubrutinib (Brukinsa) block a protein called Bruton tyrosine kinase (BTK) inside lymphoma cells, which normally helps the cells grow and survive. Ibrutinib has become a key drug in Waldenstrom macroglobulinemia therapy because it is effective, generally well tolerated, and this oral medication is approved by the FDA for use in Waldenstrom macroglobulinemia. In patients who failed prior therapy for Waldenstrom macroglobulinemia the overall response rate with ibrutinib (which includes partial and complete responses) was 90%, although complete responses were rare. The estimated 2-year progression-free survival (i.e., no relapses due to advancing disease) and overall survival rates were 69% and 95% of patients, respectively. Ibrutinib also proved to be highly active in treatment-naïve patients, where the overall response exceeded 83% and the 18-month progression-free survival was estimated at 92% of patients. Patients with both MYD88 and CXCR4 mutations tended to have slower, less robust responses to ibrutinib than patients with MYD88 mutations only. Unfortunately, ibrutinib is not effective in patients without MYD88 mutations 7.
Ibrutinib can be used alone or in combination with rituximab to treat Waldenstrom macroglobulinemia, while zanubrutinib is typically used by itself. These drugs are taken by mouth as pills, typically once or twice a day.
While the efficacy of the two agents was similar, zanubrutinib had fewer side effects than ibrutinib (including, importantly, a reduced risk of atrial fibrillation of 2 vs. 15%).
Common side effects of BTK inhibitors include diarrhea, rash, muscle and bone pain, fatigue, cough, bruising, and low blood cell counts. More serious side effects can include bleeding, serious infections, and heart rhythm problems. Some people taking these drugs develop skin or other cancers, so it’s important to use sun protection when outside while taking one of these drugs.
Other BTK inhibitors, which bind and inhibit BTK in a manner distinct from ibrutinib, are now in clinical trials and may prove useful in patients who have failed ibrutinib or zanubrutinib therapy, especially if resistance is mediated by a mutation at a critical contact site between the drug and the BTK protein. Beyond these novel approaches to BTK inhibition, the development of CXCR4 inhibitors, which when used in combination with ibrutinib, may induce better responses in double-mutant Waldenstrom macroglobulinemia patients.
Proteasome inhibitors
These drugs stop enzyme complexes (proteasomes) inside cells from breaking down proteins that normally help keep cell division under control.
Bortezomib (Velcade) and carfilzomib (Kyprolis) are sometimes helpful in treating Waldenstrom macroglobulinemia. These drugs are given as an infusion into a vein (IV); bortezomib can also be given as an injection under the skin (sub-q).
Although these drugs work slightly differently from most chemo drugs, they can still cause many of the same types of side effects, including low blood counts, nausea, and loss of appetite. They can also damage nerves, causing pain in the feet and legs (peripheral neuropathy). The nerve damage usually gets better after the drug is stopped, but it might not go away completely.
mTOR inhibitors
These drugs block a cell protein known as mTOR, which normally helps cells grow and divide into new cells.
Everolimus (Afinitor) is used more often to treat some other types of cancer, but it has also been shown to be useful in treating Waldenstrom macroglobulinemia after other treatments have been tried. This drug is taken daily as a pill. Common side effects include fatigue (tiredness), mouth pain, rash, diarrhea, and infections.
Other mTOR inhibitors, such as temsirolimus (Torisel), are now being studied to see if they can help treat Waldenstrom macroglobulinemia as well.
Other drugs that target different parts of lymphoma cells are also being studied for use against Waldenstrom macroglobulinemia.
Biological therapy or immunotherapy for Waldenstrom macroglobulinemia
Biological therapies help the body’s immune system fight the cancer or use man-made versions of substances normally made by the immune system. These substances can kill Waldenstrom macroglobulinemia cells or slow their growth.
Monoclonal antibodies
Antibodies are proteins made by the immune system to help fight infections. Man-made versions, called monoclonal antibodies, can be designed to attack a specific target, such as a substance on the surface of lymphocytes (the cells in which Waldenstrom macroglobulinemia starts).
Rituximab (Rituxan) is the most widely used monoclonal antibody for Waldenstrom macroglobulinemia. It attaches to a protein called CD20 on the surface of lymphoma cells . This attachment tells the lymphoma cell to die. Patients get rituximab by infusion into a vein (IV) at the doctor’s office or clinic. Rituximab can be given alone or with chemotherapy or targeted therapy (or other drugs) as a part of treatment.
This drug has to be given carefully to Waldenstrom macroglobulinemia patients because sometimes it can actually raise the level of IgM in the blood at first, which can lead to problems with hyperviscosity (thickened blood). Side effects during the infusion are common, and can include chills, fever, nausea, rashes, fatigue, and headaches. Unlike regular chemotherapy, rituximab does not cause low blood counts or hair loss.
Ofatumumab (Arzerra) is another antibody that targets the CD20 antigen. It can be used for people who have trouble taking rituximab. Side effects are similar to those seen with rituximab, including an increased risk of IgM levels going up when the drug is first given.
Alemtuzumab (Campath) is directed at a different protein on lymphoma cells called CD52. This drug is more commonly used to treat patients with chronic lymphocytic leukemia, but it also helps some patients with Waldenstrom macroglobulinemia. It is given by infusion into a vein (IV) or under the skin, usually 3 times a week. A serious side effect of alemtuzumab is a large drop in blood cell counts that can last weeks or even months. People on this drug can develop life-threatening infections that are hard to treat while their white blood cells are low. Rare but serious side effects can include strokes, as well as tears in the blood vessels in the head and neck.
Immunomodulating drugs
Immunomodulating drugs (IMiDs) are thought to work against certain cancers by boosting parts of your immune system, although exactly how they work is not clear. These drugs are most often used to treat multiple myeloma, but they might also be helpful in treating Waldenstrom macroglobulinemia.
Thalidomide (Thalomid) is the immunomodulating drug with the most evidence showing it can help some patients with Waldenstrom macroglobulinemia. But many patients have trouble tolerating some of the side effects of this drug. These include drowsiness, fatigue (tiredness), severe constipation, and neuropathy (painful nerve damage). The neuropathy might not go away after the drug is stopped. There is also an increased risk of serious blood clots that start in the leg and can travel to the lungs. The best results with thalidomide in Waldenstrom macroglobulinemia have been seen when it is given along with other drugs, such as rituximab or dexamethasone.
Pomalidomide (Pomalyst) is a newer immunomodulating drug that generally cause less severe side effects than thalidomide. It is used mainly to treat multiple myeloma, but studies are now looking at whether it can help treat Waldenstrom macroglobulinemia as well.
Because of concerns these drugs can cause severe birth defects if taken during pregnancy, they can only be obtained through special programs run by the drug company that makes them.
Cytokines
Cytokines are hormone-like proteins normally made by white blood cells to help your immune system fight infections.
Interferon is a cytokine that can be made in the lab and given to patients. Some studies have suggested that interferon can make some lymphoma tumors shrink. Side effects of this treatment include moderate to severe fatigue, fever, chills, headaches, muscle and joint aches, and mood changes.
It is still not certain whether interferon is a good option for patients with Waldenstrom macroglobulinemia. It is most often used only in patients who continue to get sicker after treatment with other drugs.
Plasmapheresis (plasma exchange) for Waldenstrom macroglobulinemia
If the level of abnormal IgM protein in the blood gets very high in a patient with Waldenstrom macroglobulinemia, the blood becomes very thick (viscous). This is called hyperviscosity syndrome and can lead to brain damage (like a stroke) and bleeding problems. When this happens, the level of IgM needs to be lowered right away.
Plasmapheresis also known as plasma exchange uses a machine to separate the plasma (the liquid part of blood) that contains the abnormal IgM protein from the blood cells. The plasma containing the abnormal protein is discarded, while the blood cells are mixed with salt solution and plasma from a donor and given back to the patient.
Plasmapheresis is done over a few hours while the person lies in a bed or sits in a reclining chair. The blood is removed through an IV line (usually in a vein in the arm), goes through the machine where the plasma is replaced, and then is returned to the body through another IV line. Sometimes, minor surgery is done before the procedure to put a single large catheter in a large vein just below the neck or under the collar bone instead of using IV lines in the arms. This type of catheter, called a central line or central venous catheter (CVC), has both IVs built in.
Plasmapheresis is not painful (aside from the IV lines being put in), but it can be hard to stay sitting or lying down in the same place for 2 or 3 hours. Calcium levels can drop in some people during treatment, causing numbness and tingling (especially in the hands and feet and around the mouth) and muscle spasms, which can sometimes be painful. This can be treated by giving the patient calcium.
Plasmapheresis works quickly to bring down the IgM level. However, it does not treat the cause of the high IgM level (the cancer cells themselves), so it will go back up again without further treatment (like chemotherapy). Plasmapheresis is usually given to help the patient until chemotherapy or other drugs have a chance to work. It can also be used in people whose Waldenstrom macroglobulinemia is not controlled by other treatments.
Radiation therapy for Waldenstrom macroglobulinemia
Radiation therapy uses high-energy rays to kill cancer cells. This type of treatment is not used often to treat Waldenstrom macroglobulinemia. Rarely, it is used to shrink an enlarged spleen or lymph nodes if they are causing symptoms.
The type of radiation therapy used to treat Waldenstrom macroglobulinemia is called external beam radiation (EBRT). The treatment is much like getting an x-ray, but the radiation is much stronger. The procedure itself is painless. Before the treatments start, the radiation team takes careful measurements to determine the correct angles for aiming the radiation beams and the proper dose of radiation. Each treatment lasts only a few minutes, although the setup time — getting you into place for treatment — usually takes longer. Most often, radiation treatments are given 5 days a week for a few weeks.
Side effects of radiation therapy
Immediate side effects of radiation therapy can include sunburn-like skin problems, fatigue, and low blood cell counts. Other side effects depend on the area being treated. Radiation of the abdomen may cause nausea, vomiting, or diarrhea. Radiation to the head and neck area can lead to mouth sores and trouble swallowing. Often these effects go away a short while after treatment is finished.
A rare long-term side effect of radiation is a new cancer developing in the treated area.
Stem cell transplant for Waldenstrom macroglobulinemia
The doses of chemotherapy (chemo) drugs (and radiation) doctors can give are limited by the side effects they can cause. Higher doses can’t be used, even if they might kill more cancer cells, because they would severely damage the bone marrow, where new blood cells are made. This could lead to life-threatening infections, bleeding, and other problems due to low blood cell counts. Doctors can try to get around this problem by giving an infusion of blood-forming stem cells after treatment. These stem cells settle in the bone marrow, where they can create new blood cells.
A stem cell transplant is not a common treatment for Waldenstrom macroglobulinemia, but it might be an option in younger patients for whom other treatments are no longer working.
Blood-forming stem cells used for a transplant come either from the blood or from the bone marrow. Bone marrow transplants were more common in the past, but they have largely been replaced by stem cells taken from the blood.
The blood-forming stem cells can come either from the patient called an autologous stem cell transplant or from a donor called an allogeneic stem cell transplant.
Things to consider before having a stem cell transplant
A stem cell transplant is a complex treatment that can cause life-threatening side effects because of the high doses of chemotherapy used. Be sure you understand the possible benefits and risks. If the doctors think you might benefit from a transplant, it should be done at a hospital where the staff has experience with the procedure and with managing the recovery. Some stem cell transplant programs might not have experience in certain types of transplants, especially transplants from unrelated donors.
Stem cell transplants often require a long hospital stay and can be very expensive (costing well over $100,000). Because some insurance companies might view it as an experimental treatment, they might not pay for it. Even if the transplant is covered by your insurance, your co-pays or other costs could easily amount to tens of thousands of dollars. Find out what your insurer will cover before deciding on a transplant so you will have an idea of what you might have to pay.
Autologous stem cell transplant
Most transplants in people with Waldenstrom macroglobulinemia are autologous. The patient’s own blood-forming stem cells are removed from their bloodstream and stored to use later. Then the patient is given high doses of chemo (and sometimes radiation) to kill the Waldenstrom macroglobulinemia cells. The high doses of chemo kill the normal bone marrow cells as well as the cancer cells. After chemo, the frozen stem cells are thawed and returned to the body (like a blood transfusion).
Autologous transplants can help some people with Waldenstrom macroglobulinemia, but doctors are still trying to figure out which patients will benefit the most.
Allogeneic stem cell transplant
This is a treatment that is still being studied for Waldenstrom macroglobulinemia, and experts recommend it be done only as part of a clinical trial.
In an allogeneic stem cell transplant, the stem cells for the transplant come from someone else (a donor). The donor’s tissue type (also known as the HLA type) needs to match the patient’s tissue type as closely as possible to help prevent the risk of major problems with the transplant. Usually this donor is a brother or sister if they have the same tissue type as the patient. If there are no siblings with a good match, the cells may come from an HLA-matched, unrelated donor – a stranger who has volunteered to donate cells.
The stem cells for an allogeneic stem cell transplant are usually collected from a donor’s bone marrow or blood on several occasions. Regardless of the source, the stem cells are then frozen and stored until they are needed for the transplant.
Allogeneic transplants have more risks and side effects than autologous transplants, so patients typically need to be younger and relatively healthy to be good candidates. Another challenge is that it can sometimes be difficult to find a matched donor.
One of the most serious complications of allogeneic stem cell transplants is known as graft-versus-host disease (GVHD). It happens when the patient’s immune system is taken over by that of the donor. When this happens, the donor immune system may consider the patient’s own body tissues to be foreign and attacks them.
Symptoms of GVHD (graft-versus-host disease) can include severe skin rashes, itching, mouth sores (which can affect eating), nausea, and severe diarrhea. Liver damage can cause yellowing of the skin and eyes (jaundice). The lungs can also be damaged. The patient may also become easily tired and develop muscle aches. Sometimes GVHD can become disabling, and if it is severe enough, it can be life-threatening.
Non-myeloablative transplant: In this newer approach to allogeneic stem cell transplant (also called a mini-transplant), lower doses of chemo or radiation therapy are used than in a traditional allogeneic stem cell transplant. Patients are given drugs to suppress their immune system. This allows the donor cells to grow and partly take over the patient’s immune system. The donor cells then begin attacking the Waldenstrom macroglobulinemia cells (known as a graft-versus-lymphoma effect).
This type of transplant may be an option for some patients who couldn’t tolerate a regular allogeneic transplant because it would be too toxic. Most of the side effects with this type of transplant are less severe than with a standard allogeneic transplant. But this type of transplant can still cause GVHD, which can make patients very sick.
Doctors are trying to refine this treatment to work against the Waldenstrom macroglobulinemia cells without affecting the normal cells.
Stem cell transplant side effects
Side effects from a stem cell transplant are generally divided into early and long-term effects.
Early or short-term effects: The early complications and side effects are basically the same as those caused by any other type chemotherapy, but they tend to be more severe. One of the most common and serious short-term effects is the increased risk of infection. Antibiotics are often given to try to keep this from happening. Other side effects, like low red blood cell and platelet counts, may require blood product transfusions or other treatments.
A possible side effect of allogeneic transplants is graft-versus-host disease (GVHD), which is described above.
Long-term side effects: Some complications and side effects can remain for a long time or might not occur until months or years after the transplant. These include:
- Loss of fertility
- Damage to the thyroid gland
- Cataracts (damage to the lens of the eye)
- Damage to the lungs, causing shortness of breath
- Bone damage called aseptic necrosis (If damage is severe, the patient might need to have part of the affected bone and the joint replaced.)
- Development of another cancer (such as leukemia) years later
If treatment doesn’t work or if the disease comes back after treatment
No single treatment for Waldenstrom macroglobulinemia works for all patients. If the first drug or set of drugs doesn’t work, other drugs may be helpful.
Most people with Waldenstrom macroglobulinemia will require treatment with different drugs at some point. Often, a certain drug or combination of drugs will work at first, but over time it might stop working. Or a person might stop treatment if the Waldenstrom macroglobulinemia is under control, only to have it come back some time later. If the Waldenstrom macroglobulinemia remained under control for at least a year after the first treatment, then giving the same drug(s) again can often help bring the cancer back under control.
If the cancer comes back sooner, or if the initial treatment was not effective, then switching to another drug or drug combination is likely to be a better option. Many of the same drugs and combinations listed above as first-line treatments might be helpful here. Other drugs that might also be tried include alemtuzumab (Campath), ofatumumab (Arzerra), or everolimus (Afinitor). High-dose chemotherapy with stem cell transplant might also be an option for some patients.
If chemotherapy or other drugs are no longer slowing the growth of the Waldenstrom macroglobulinemia, some patients can still get relief from symptoms by getting plasmapheresis at regular intervals to lower the levels of the abnormal IgM protein in their blood.
Sometimes Waldenstrom macroglobulinemia can turn into an aggressive lymphoma. When this happens, the cancer grows much more quickly and causes symptoms that can soon become life threatening. These lymphomas are usually treated with a combination of several chemo drugs like those used for patients who are first diagnosed with an aggressive non-Hodgkin lymphoma. If combination chemo is not successful, high-dose chemo with a stem cell transplant may be an option.
New treatment for Waldenstrom macroglobulinemia
Many new drugs to treat Waldenstrom macroglobulinemia are being studied in clinical trials, as well as ways to use drugs already known to be effective by combining them in new ways, using different doses, or different sequences of drugs, one after another.
Some of the newer types of drugs that have shown promise or are being tested in Waldenstrom macroglobulinemia include 16:
- mTOR inhibitors, such as temsirolimus (Torisel)
- Proteasome inhibitors, such as ixazomib , carfilzomib (Kyprolis), and oprozomib
- Histone deacetylase (HDAC) inhibitors, such as panobinostat, romidepsin (Istodax), and belinostat (Beleodaq)
- Bruton tyrosine kinase (BTK) inhibitors, such as ACP-196, and AVL-292
- PI3K inhibitors, such as idelalisib (Zydelig) and buparlisib (BKM120)
- Aurora kinase inhibitors, such as alisertib
- BCL-2 inhibitor such as ABT-199
- A CXCR4 antibody such as ulocuplumab
Biological therapy
Another newer approach to Waldenstrom macroglobulinemia treatment is the use of biological response modifiers that stimulate the patient’s immune system to attack and destroy the lymphoma cells.
For example, it has recently been found that the bone marrow support tissues (stromal cells) make a substance called interleukin 6 (IL-6). IL-6 is a strong growth factor for multiple myeloma cells. IL-6 also helps cause the bone destruction seen in myeloma. Some current research efforts are trying to develop ways to block these functions of IL-6.
Bone marrow and peripheral blood stem cell transplant
Researchers are continually improving bone marrow and peripheral blood stem cell transplant methods, as well as trying to determine how helpful this type of treatment can be for people with Waldenstrom macroglobulinemia.
Vaccines
Doctors know it is possible for people with cancer to develop immune responses to their cancer. In rare instances, people’s immune systems have rejected their cancers, and they have been cured. Scientists are now studying ways to boost this immune reaction by using vaccines.
Unlike vaccines used to prevent infections, these vaccines create an immune reaction against the lymphoma cells in patients who have very early disease or whose disease is in remission but could come back or relapse. This is a major area of research in treating lymphomas (including Waldenstrom macroglobulinemia), but it is still being tested in clinical trials. You might want to consider enrolling in one of these studies.
Waldenstrom macroglobulinemia survival rate
Survival rates are often used by doctors as a way of discussing a person’s outlook. Survival rates tell you what percentage of people with the same type and stage of cancer are still alive a certain length of time (usually 5 years) after they were diagnosed. These numbers can’t tell you how long you will live, but they may help give you a better understanding about how likely it is that your treatment will be successful.
Cancer survival rates don’t tell the whole story. Survival rates are often based on previous outcomes of large numbers of people who had the disease, but they can’t predict what will happen in any particular person’s case. There are a number of limitations to remember:
- The numbers below are among the most current available. But to get 5-year survival rates, doctors look at people who were treated at least 5 years ago. As treatments are improving over time, people who are now being diagnosed with ovarian cancer may have a better outlook than these statistics show.
- The statistics below are based on the stage of the cancer when it was first diagnosed. They do not apply to cancers that come back later or spread, for example.
- Besides the cancer stage, many other factors can affect a person’s outlook, such as age and overall health, and how well the cancer responds to treatment.
Your doctor can tell you how these numbers may apply to you, as he or she is familiar with the aspects of your particular situation.
Experts have used these factors to develop a system that helps predict prognosis (outlook) for patients with Waldenstrom macroglobulinemia. It is called the International Prognostic Scoring System for Waldenstrom Macroglobulinemia (ISSWM). This system takes into account the factors that seem to predict a poorer outcome, such as 17:
- Older than 65
- Blood hemoglobin level 11.5 g/dL or less
- Platelet count 100,000/mcL or less
- Beta-2 microglobulin more than 3 mg/L
- Monoclonal IgM level more than 7 g/dL
Except for age, each of these factors is worth a single point. The points are added to make a score, which is used to divide patients into 3 risk groups:
- The low-risk group includes patients 65 or younger who have no more than 1 point.
- The intermediate-risk group includes those who are older than 65 with 2 or fewer points, and those younger than 65 who have 2 points.
- The high-risk group includes those of any age who have at least 3 points.
These groups can be used to help predict survival.
According to the National Cancer Institute’s SEER database (based on people diagnosed between 2001 and 2010), the overall relative 5-year survival of people with Waldenstrom macroglobulinemia is about 78% 18.
The group that created the International Prognostic Scoring System for Waldenstrom Macroglobulinemia (ISSWM) used data from about 600 patients with Waldenstrom macroglobulinemia who were diagnosed and treated before January 2002 to develop their risk groups:
| ISSWM risk group | 5-year survival rate |
|---|---|
| Low | 87% |
| Intermediate | 68% |
| High | 36% |
Median survival
Median survival is another way to look at survival. It is the length of time at which half of the patients in a group are still alive, and half have died. By definition, half of the patients live longer than the median survival. The group that developed the International Prognostic Scoring System for Waldenstrom Macroglobulinemia used data from Waldenstrom macroglobulinemia patients diagnosed and treated before January 2002 and found the following:
| ISSWM risk group | Median survival* |
|---|---|
| Low | 12 years |
| Intermediate | 8 years |
| High | 3.5 years |
Footnote: *Median survival is measured from the point that treatment is started. In the last decade (2001-2010), the median overall survival for all Waldenstrom macroglobulinemia groups has improved to just over 8 years compared to 6 years in the previous decade (1991-2000).
[Source 18 ]Waldenstrom macroglobulinemia prognosis
The median survival of Waldenstrom macroglobulinemia (lymphoplasmacytic lymphoma) patients is approximately five years 19. About 40% of patients survive for ten years or more 19. Typically, the cause of death is more due to advance age-associated comorbidities than Waldenstrom macroglobulinemia.
Several publications have been reported with the aim to identify variables that could be associated with reduced survival in Waldenstrom macroglobulinemia patients 5. Three population-based studies analysed survival data on large cohorts of patients. Significant shorter survival resulted to be related to older age. Nevertheless, a proportion of elderly patients died from causes unrelated to Waldenstrom macroglobulinemia, while disease-specific survival exceeded six years even for patients > 75 years 20. Moreover, a significant improvement in survival over time has been reported in patients with Waldenstrom macroglobulinemia during the last decade 21, 22.
At present, the only validated prognostic scoring system for patients with Waldenstrom macroglobulinemia is the International Scoring System for Waldenstrom macroglobulinemia (ISSWM) that was proposed in 2009 for patients requiring treatment. The International Prognostic Scoring System for Waldenstrom Macroglobulinemia (ISSWM) includes five parameters, giving 1 point to each one, except for age, which receives 2 points. Parameters are: Advance age (Age >65), Anemia (hemoglobin ≤ 11.5 g/dL), Thrombocytopenia (platelet count ≤ 100,000/mcL, Serum beta 2-microglobulin (β2M) >3 mg/L and serum monoclonal protein concentration (IgM > 7 g/dL) 17. Score at the beginning of treatment classifies them into low risk (score ≤ 1), intermediate-risk (score 2), or high risk (score ≥ 3) with 5-year survival rates of 87%, 68% and 36% respectivley 17.
Increased immunoblasts/transformed cells, deletion 6q, are considered independent adverse factors.
Waldenstrom macroglobulinemia (lymphoplasmacytic lymphoma) may transform to diffuse large B-cell lymphoma (DLBCL) and is associated with poor survival 23, 24.
Concerning molecular markers, the presence or absence of MYD88 L265P mutation demonstrated an impact on survival as patients carrying MYD88 L265P showed a significant improvement on survival when compared to absence of an MYD88 L265P mutation, thus independently from CXCR4 mutational status 25.
On the other hand, CXCR4 mutational status seems to modulate clinical presentation. In fact, patients with CXCR4 mutations present with a significantly lower rate of adenopathy, and those with CXCR4 nonsense mutations have an increased bone marrow disease burden, serum IgM levels, and/or risk of symptomatic hyperviscosity, while patients with MYD88 mutation seem to have high bone marrow disease involvement and serum IgM levels 26, 27.
Several reports of familial clustering of patients affected by Waldenstrom macroglobulinemia alone or with other malignancies showed a common predisposition for Waldenstrom macroglobulinemia with other lymphoproliferative diseases. A familial Waldenstrom macroglobulinemia was demonstrated in almost 20% of cases by Kristinsson et al 28. Furthermore, in a large single-center study 29, 26% of 924 consecutive patients with Waldenstrom macroglobulinemia had a first- or second-degree relative with either Waldenstrom macroglobulinemia or another B-cell disorder. The diagnosis of familial Waldenstrom macroglobulinemia represents an independent marker for disease progression being associated with a 1.3-fold increased risk of death compared to sporadic Waldenstrom macroglobulinemia, with an increasing hazard ratio for each additional relative with a lymphoproliferative disorder (defined as Waldenstrom macroglobulinemia, non-Hodgkin lymphoma, multiple myeloma, chronic lymphocytic leukemia, or monoclonal gammopathy of undetermined significance [MGUS]) 30. From a clinical point of view, greater bone marrow involvement and baseline IgM level were observed in familial compared to sporadic Waldenstrom macroglobulinemia, while no difference was noted in cytogenetic abnormalities or lymph node or spleen involvement 31.
While a report described a younger age at diagnosis of Waldenstrom macroglobulinemia in the familial cases 31, this observation was not confirmed in subsequent studies 32. In a single institution study, familial Waldenstrom macroglobulinemia was associated with inferior response rates to rituximab-combination regimens and shorter time-to-next therapy (TTNT) than the sporadic cases. Furthermore, time-to-progression (TTP) in familial Waldenstrom macroglobulinemia was significantly shorter (21 vs 45 months for sporadic). However, superior outcomes with bortezomib-containing regimens were observed in patients with familial Waldenstrom macroglobulinemia, regarding overall and major response rates and time-to-next therapy (TTNT) 33.
References- Leblond V, Kastritis E, Advani R, Ansell SM, Buske C, Castillo JJ, García-Sanz R, Gertz M, Kimby E, Kyriakou C, Merlini G, Minnema MC, Morel P, Morra E, Rummel M, Wechalekar A, Patterson CJ, Treon SP, Dimopoulos MA. Treatment recommendations from the Eighth International Workshop on Waldenström’s Macroglobulinemia. Blood. 2016 Sep 8;128(10):1321-8. https://doi.org/10.1182/blood-2016-04-711234
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 May 19;127(20):2375-90. doi: 10.1182/blood-2016-01-643569
- What Is Waldenstrom Macroglobulinemia? https://www.cancer.org/cancer/waldenstrom-macroglobulinemia/about/what-is-wm.html
- Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, Morra E, Pangalis GA, San Miguel JF, Branagan AR, Dimopoulos MA. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003 Apr;30(2):110-5. doi: 10.1053/sonc.2003.50082
- Mazzucchelli M, Frustaci AM, Deodato M, Cairoli R, Tedeschi A. Waldenstrom’s Macroglobulinemia: An Update. Mediterr J Hematol Infect Dis. 2018 Jan 1;10(1):e2018004. doi: 10.4084/MJHID.2018.004
- Key Statistics About Waldenstrom Macroglobulinemia. https://www.cancer.org/cancer/waldenstrom-macroglobulinemia/about/key-statistics.html
- Waldenström macroglobulinemia (WM). https://www.lls.org/research/waldenstrom-macroglobulinemia-wm
- Lymphoplasmacytic lymphoma. https://www.leukemia-cell.org/atlas/index.php?pg=images–mature-b-cell-neoplasms–lymphoplasmacytic-lymphoma–nos
- Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Sheehy P, Manning RJ, Patterson CJ, Tripsas C, Arcaini L, Pinkus GS, Rodig SJ, Sohani AR, Harris NL, Laramie JM, Skifter DA, Lincoln SE, Hunter ZR. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012 Aug 30;367(9):826-33. DOI: 10.1056/NEJMoa1200710
- Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014 May 1;123(18):2791-6. doi: 10.1182/blood-2014-01-550905
- Zachary R. Hunter, Lian Xu, Guang Yang, Yangsheng Zhou, Xia Liu, Yang Cao, Robert J. Manning, Christina Tripsas, Christopher J. Patterson, Patricia Sheehy, Steven P. Treon; The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014; 123 (11): 1637–1646. doi: https://doi.org/10.1182/blood-2013-09-525808
- What’s New in Waldenstrom Macroglobulinemia Research? https://www.cancer.org/cancer/waldenstrom-macroglobulinemia/about/new-research.html
- Waldenstrom Macroglobulinemia Stages. https://www.cancer.org/cancer/waldenstrom-macroglobulinemia/detection-diagnosis-staging/staging.html
- Véronique Leblond, Efstathios Kastritis, Ranjana Advani, Stephen M. Ansell, Christian Buske, Jorge J. Castillo, Ramón García-Sanz, Morie Gertz, Eva Kimby, Charalampia Kyriakou, Giampaolo Merlini, Monique C. Minnema, Pierre Morel, Enrica Morra, Mathias Rummel, Ashutosh Wechalekar, Christopher J. Patterson, Steven P. Treon, Meletios A. Dimopoulos; Treatment recommendations from the Eighth International Workshop on Waldenström’s Macroglobulinemia. Blood 2016; 128 (10): 1321–1328. doi: https://doi.org/10.1182/blood-2016-04-711234
- Castillo JJ, Treon SP. Management of Waldenström macroglobulinemia in 2020. Hematology Am Soc Hematol Educ Program. 2020 Dec 4;2020(1):372-379. doi: 10.1182/hematology.2020000121
- What’s New in Waldenstrom Macroglobulinemia Research and Treatment? https://www.cancer.org/cancer/waldenstrom-macroglobulinemia/about/new-research.html
- Pierre Morel, Alain Duhamel, Paolo Gobbi, Meletios A. Dimopoulos, Madhav V. Dhodapkar, Jason McCoy, John Crowley, Enrique M. Ocio, Ramon Garcia-Sanz, Steven P. Treon, Veronique Leblond, Robert A. Kyle, Bart Barlogie, Giampaolo Merlini; International prognostic scoring system for Waldenström macroglobulinemia. Blood 2009; 113 (18): 4163–4170. doi: https://doi.org/10.1182/blood-2008-08-174961
- Survival Rates for Waldenstrom Macroglobulinemia. https://www.cancer.org/cancer/waldenstrom-macroglobulinemia/detection-diagnosis-staging/survival-rates.html
- Kaseb H, Gonzalez-Mosquera LF, Parsi M, et al. Lymphoplasmacytic Lymphoma. [Updated 2022 May 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513356
- Kastritis E, Zervas K, Repoussis P, Michali E, Katodrytou E, Zomas A, Simeonidis A, Terpos E, Delimbassi S, Vassou A, Gika D, Dimopoulos MA; Greek Myeloma Study Group. Prognostication in young and old patients with Waldenström’s macroglobulinemia: importance of the International Prognostic Scoring System and of serum lactate dehydrogenase. Clin Lymphoma Myeloma. 2009 Mar;9(1):50-2. doi: 10.3816/CLM.2009.n.012
- Kristinsson SY, Eloranta S, Dickman PW, Andersson TM, Turesson I, Landgren O, Björkholm M. Patterns of survival in lymphoplasmacytic lymphoma/Waldenström macroglobulinemia: a population-based study of 1,555 patients diagnosed in Sweden from 1980 to 2005. Am J Hematol. 2013 Jan;88(1):60-5. doi: 10.1002/ajh.23351
- Castillo JJ, Olszewski AJ, Kanan S, Meid K, Hunter ZR, Treon SP. Overall survival and competing risks of death in patients with Waldenström macroglobulinaemia: an analysis of the Surveillance, Epidemiology and End Results database. Br J Haematol. 2015 Apr;169(1):81-9. doi: 10.1111/bjh.13264
- Kapoor P, Ansell SM, Braggio E. Waldenstrom Macroglobulinemia: Genomic Aberrations and Treatment. Cancer Treat Res. 2016;169:321-361. doi: 10.1007/978-3-319-40320-5_16
- Cao X, Medeiros LJ, Xia Y, Wang X, Thomas SK, Loghavi S, Li X, Shah JJ, Gustafson SA, Weber DM, Miranda RN, Xu-Monette ZY, Orlowski RZ, Young KH. Clinicopathologic features and outcomes of lymphoplasmacytic lymphoma patients with monoclonal IgG or IgA paraprotein expression. Leuk Lymphoma. 2016 May;57(5):1104-13. doi: 10.3109/10428194.2015.1096357
- Steven P. Treon, Yang Cao, Lian Xu, Guang Yang, Xia Liu, Zachary R. Hunter; Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenström macroglobulinemia. Blood 2014; 123 (18): 2791–2796. doi: https://doi.org/10.1182/blood-2014-01-550905
- Poulain S, Roumier C, Venet-Caillault A, Figeac M, Herbaux C, Marot G, Doye E, Bertrand E, Geffroy S, Lepretre F, Nibourel O, Decambron A, Boyle EM, Renneville A, Tricot S, Daudignon A, Quesnel B, Duthilleul P, Preudhomme C, Leleu X. Genomic Landscape of CXCR4 Mutations in Waldenström Macroglobulinemia. Clin Cancer Res. 2016 Mar 15;22(6):1480-8. doi: 10.1158/1078-0432.CCR-15-0646
- Schmidt J, Federmann B, Schindler N, Steinhilber J, Bonzheim I, Fend F, Quintanilla-Martinez L. MYD88 L265P and CXCR4 mutations in lymphoplasmacytic lymphoma identify cases with high disease activity. Br J Haematol. 2015 Jun;169(6):795-803. doi: 10.1111/bjh.13361
- Kristinsson SY, Björkholm M, Goldin LR, McMaster ML, Turesson I, Landgren O. Risk of lymphoproliferative disorders among first-degree relatives of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia patients: a population-based study in Sweden. Blood. 2008 Oct 15;112(8):3052-6. doi: 10.1182/blood-2008-06-162768
- Hanzis C, Ojha RP, Hunter Z, Manning R, Lewicki M, Brodsky P, Ioakimidis L, Tripsas C, Patterson CJ, Sheehy P, Treon SP. Associated malignancies in patients with Waldenström’s macroglobulinemia and their kin. Clin Lymphoma Myeloma Leuk. 2011 Feb;11(1):88-92. doi: 10.3816/CLML.2011.n.016
- Steingrímsson V, Lund SH, Turesson I, Goldin LR, Björkholm M, Landgren O, Kristinsson SY. Population-based study on the impact of the familial form of Waldenström macroglobulinemia on overall survival. Blood. 2015 Mar 26;125(13):2174-5. doi: 10.1182/blood-2015-01-622068
- Treon SP, Hunter ZR, Aggarwal A, Ewen EP, Masota S, Lee C, Santos DD, Hatjiharissi E, Xu L, Leleu X, Tournilhac O, Patterson CJ, Manning R, Branagan AR, Morton CC. Characterization of familial Waldenstrom’s macroglobulinemia. Ann Oncol. 2006 Mar;17(3):488-94. doi: 10.1093/annonc/mdj111
- Kristinsson SY, Goldin LR, Turesson I, Björkholm M, Landgren O. Familial aggregation of lymphoplasmacytic lymphoma/Waldenström macroglobulinemia with solid tumors and myeloid malignancies. Acta Haematol. 2012;127(3):173-7. doi: 10.1159/000335618
- Treon SP, Tripsas C, Hanzis C, Ioakimidis L, Patterson CJ, Manning RJ, Sheehy P, Turnbull B, Hunter ZR. Familial disease predisposition impacts treatment outcome in patients with Waldenström macroglobulinemia. Clin Lymphoma Myeloma Leuk. 2012 Dec;12(6):433-7. doi: 10.1016/j.clml.2012.08.006