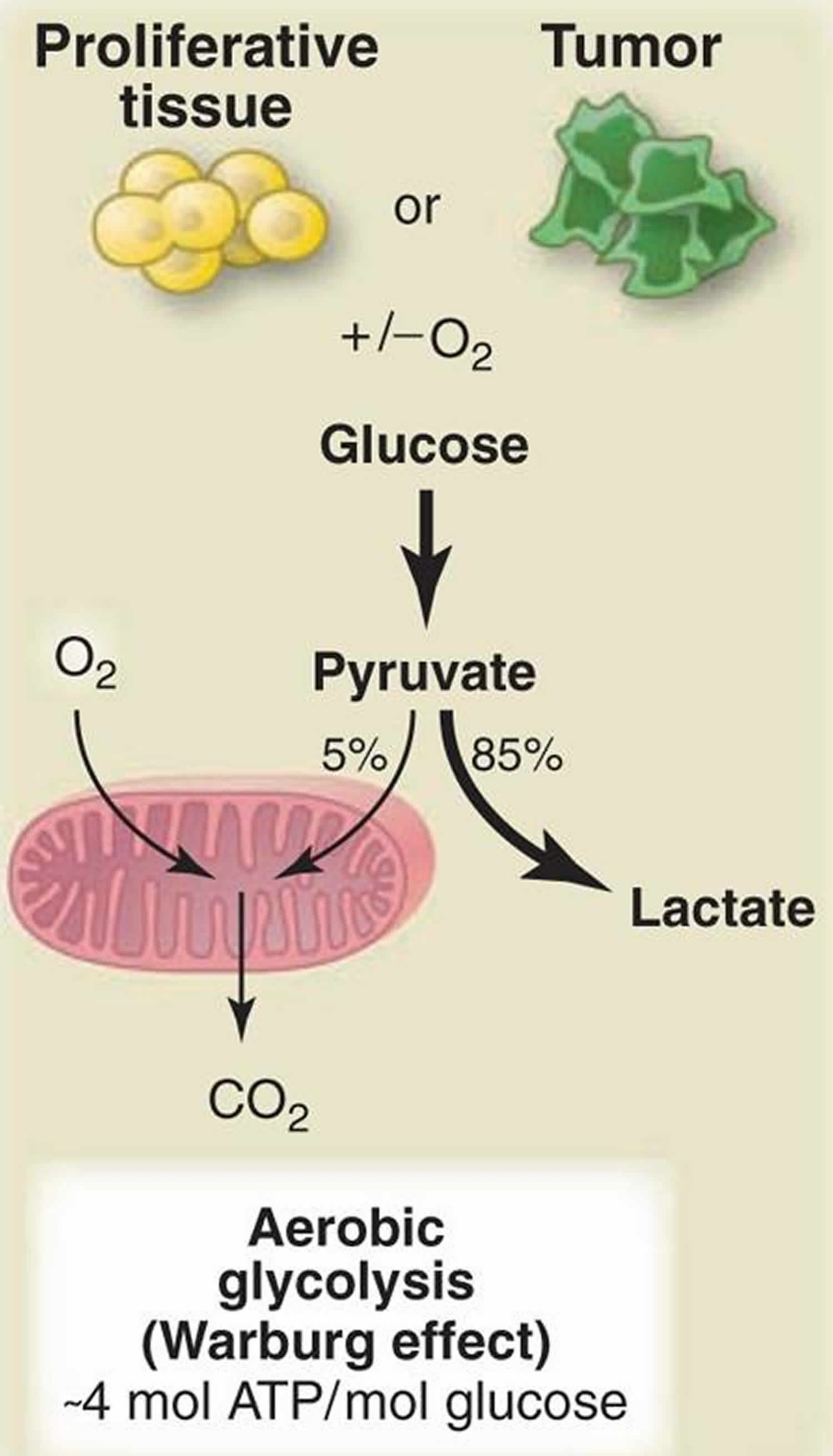
Warburg effect
Warburg effect also called aerobic glycolysis, where cancerous cells transform significant amounts of glucose into lactate and adenosine 5′-triphosphate (ATP) irrespective of oxygen availability to meet the energetic demands of cancer cells and provides them with growth and survival advantages (Figure 1) 1. Adaptive responses through the induction of the hypoxia-inducible factor 1 (HIF-1) and cell autonomous changes that activate AKT, HIF-1, MYC, or RAS oncogenes or inactivate the tumor suppressor P53 or vHL could all contribute to the Warburg effect 2. In the presence of oxygen, nonproliferating (differentiated) tissues first metabolize glucose to pyruvate via glycolysis and then completely oxidize most of that pyruvate in the mitochondria to CO2 during the process of oxidative phosphorylation 3. Because oxygen is required as the final electron acceptor to completely oxidize the glucose, oxygen is essential for this process. When oxygen is limiting, cells can redirect the pyruvate generated by glycolysis away from mitochondrial oxidative phosphorylation by generating lactate (anaerobic glycolysis). This generation of lactate during anaerobic glycolysis allows glycolysis to continue (by cycling NADH back to NAD+), but results in minimal ATP production when compared with oxidative phosphorylation. Warburg observed that cancer cells tend to convert most glucose to lactate regardless of whether oxygen is present (aerobic glycolysis). This property is shared by normal proliferative tissues. Mitochondria remain functional and some oxidative phosphorylation continues in both cancer cells and normal proliferating cells. Nevertheless, aerobic glycolysis is less efficient than oxidative phosphorylation for generating ATP. In proliferating cells, ~10% of the glucose is diverted into biosynthetic pathways upstream of pyruvate production.
This realization has brought renewed attention to Otto Warburg’s observation in 1924 that cancer cells metabolize glucose in a manner that is distinct from that of cells in normal tissues 4. By examining how Louis Pasteur’s observations regarding fermentation of glucose to ethanol might apply to mammalian tissues, Warburg found that unlike most normal tissues, cancer cells tend to “ferment” glucose into lactate even in the presence of sufficient oxygen to support mitochondrial oxidative phosphorylation. A definitive explanation for Warburg’s observation has remained elusive, at least in part because the energy requirements of cell proliferation appear at first glance to be better met by complete catabolism of glucose using mitochondrial oxidative phosphorylation to maximize adenosine 5′-triphosphate (ATP) production.
In Otto Warburg’s opinion, aerobic glycolysis, which releases lactate under normal oxygen conditions, was a preferential manner to meet the energy requirements for cancer cells’ rapid growth and due to the mitochondrial dysfunction common in cancer cells 5. For each molecule of glucose consumed, 2 ATP are produced by glycolysis, while 32 ATP molecules are produced by the oxidative phosphorylation pathway (Figure 2). Therefore, although the ATP generation via aerobic glycolysis is less efficient than oxidative phosphorylation, cancer cells are able to quickly produce sufficient energy by consuming huge quantities of glucose. Although inefficient, this allows cancer cells to maintain their abnormal biological functions, including rapid proliferation.
Since glycolysis yields only two molecules of ATP, cancer cells require a large amount of glucose for energy production 6. In fact, it has been shown that the glucose concentration in colonic or gastric cancer tissue is only one tenth of that in normal tissue, whereas the concentration of lactate, the final product of glycolysis, is significantly elevated 7. However, rapid proliferation of cancer cells results in abnormal angiogenesis, which means that cancer tissues often become depleted of oxygen and nutrients, including glucose 8. However, the physiology of cancer cells including cancer stem cells under glucose free condition remains unclear 6.
The factors that drive cancer cells to choose such an inefficient pathway, and the biological significance of the Warburg effect, have remained elusive. It was theorized that the increased rate of glycolysis could compensate for the energy requirements for cancer cells’ rapid growth 9. This speculation was supported by later evidence 10, and was considered to be a mechanism responsible for the fast energy production and biosynthesis. Nevertheless, this theory has been challenged by many investigators. Early in 1967, Weinhouse S et al. 11 observed that the glycolmetabolism of slow-growing rat hepatocytes was typically supported by oxidation. Not only in cells with normal mitochondrial function 12, but also some tumor cells, have been reported to use oxidative phosphorylation as the major ATP supplier, regardless of the metabolic rate of glycolysis 13. The mitochondrial membrane potential is mainly generated by the end-step of ATP production. NADH or FADH2 (dihydroflavine-adenine dinucleotide) transfers electrons to oxygen molecules via the electron transport chain to form water, NAD+ or FAD2+, thus releasing protons from the mitochondrial matrix outside of the mitochondrial inner membrane 9. As a result, the membrane potential can function as a powerful index for assessing mitochondrial activity 14. When compared with the adjacent normal tissues, tumors always exhibit elevations in the mitochondrial membrane potential 15. This suggests that oxidative phosphorylation may be preferred over glycolysis for efficient ATP production. Therefore, Warburg’s theory about mitochondrial malfunction is not a reasonable explanation for the dominance of aerobic glycolysis in cancer cells 9. Thus, additional research has been performed to try to elucidate the main driving factor(s) underlying the high lactate release in tumor cells.
The Pyruvate Kinase Muscle (PKM) catalyzes one of the rate-limiting steps of glycolysis and the cancer-specific spliced isoform of pyruvate kinase, PKM-2 is known to promote the Warburg effect and therefore facilitates the tumor growth 16. The Pyruvate Kinase Muscle (PKM) has two spliced isoforms: the alternative inclusion of mutually exclusive exon 9 and exon 10 leads to the generation of PKM-1 and PKM-2 isoform respectively 17. The PKM-1 isoform is expressed in the normal cells 16 and is associated with normal glucose metabolism wherein PKM-2 isoform is overexpressed in cancer cells 18 and is associated with increased aerobic glycolysis, termed as Warburg effect, which is associated with the increased cell proliferation and reduced apoptosis 19, thereby PKM-2 may be a potential therapeutic target for cancer treatment 20. Therefore, it becomes important to understand the mechanism of splicing switch from PKM-1 to PKM-2 in cancer cells. Recent studies have shown that PKM-1 can promote tumor cell proliferation and activate glucose catabolism in small-cell lung cancer (SCLC) 21. But more results confirm that among the four mammalian pyruvate kinase isoforms (i.e.,PKL, PKR, PKM-1, and PKM-2), PKM-2 is most closely related to tumor cell proliferation. PKM-2 is an important regulator of the Warburg effect and is highly expressed in many tumors. Tetrameric PKM-2 has pyruvate kinase activity in normal cells. PKM-2 tetramers are tyrosine-phosphorylated at Y105 to transform dimeric PKM-2 in tumor cells 22. Then, PKM-2 dimers promote the synthesis of deoxynucleotides, providing tumor cells with a growth and proliferation advantage. Dimeric PKM-2 can enhance aerobic glycolysis, increase lactate production, and regulate the expression of genes that mediate the Warburg effect in many tumors 23, including hepatocellular carcinoma 24. This study 1 shows that signal transducer and activator of transcription 3 (STAT3) can increase the Warburg effect by stimulating hexokinase 2 in breast cancer and upregulate lactate dehydrogenase A and pyruvate dehydrogenase kinase 1 in myeloma. STAT3 and pyruvate kinase M2 (PKM-2) can also be activated and enhance the Warburg effect in hepatocellular carcinoma.
Recently, regulating the nutrient intake of cancer cells has been considered a possible therapeutic strategy, which may involve the exploitation of glycolytic inhibitors or PKM-2 25, PKM-1 26 or metformin 27. Obtaining a better understanding of the key principles and mechanisms underlying the Warburg effect in cancer cells will aid in the development of preventive and therapeutic approaches using dietary and pharmacological interventions.
Figure 1. Warburg effect
[Source 3 ]Figure 2. Oxidative phosphorylation pathway
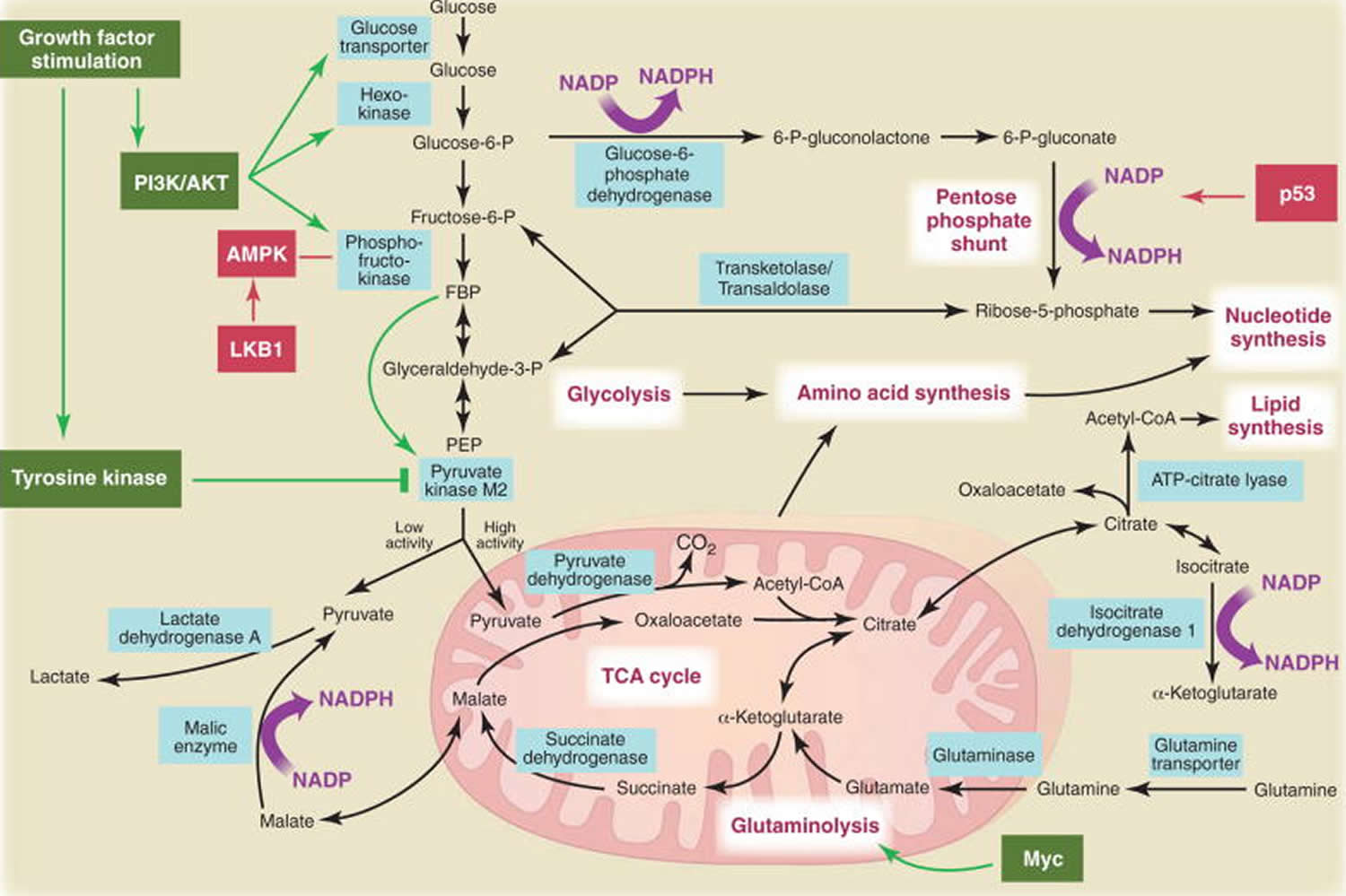
[Source 3 ]Figure 3. Metabolic pathways active in proliferating cells
Footnote: Metabolic pathways active in proliferating cells are directly controlled by signaling pathways involving known oncogenes and tumor suppressor genes. This schematic shows our current understanding of how glycolysis, oxidative phosphorylation, the pentose phosphate pathway, and glutamine metabolism are interconnected in proliferating cells. This metabolic wiring allows for both NADPH production and acetyl-CoA flux to the cytosol for lipid synthesis. Key steps in these metabolic pathways can be influenced by signaling pathways known to be important for cell proliferation. Activation of growth factor receptors leads to both tyrosine kinase signaling and PI3K activation. Via AKT, PI3K activation stimulates glucose uptake and flux through the early part of glycolysis. Tyrosine kinase signaling negatively regulates flux through the late steps of glycolysis, making glycolytic intermediates available for macromolecular synthesis as well as supporting NADPH production. Myc drives glutamine metabolism, which also supports NADPH production. LKB1/AMPK signaling and p53 decrease metabolic flux through glycolysis in response to cell stress. Decreased glycolytic flux in response to LKB/AMPK or p53 may be an adaptive response to shut off proliferative metabolism during periods of low energy availability or oxidative stress. Tumor suppressors are shown in red, and oncogenes are in green. Key metabolic pathways are labeled in purple with white boxes, and the enzymes controlling critical steps in these pathways are shown in blue. Some of these enzymes are candidates as novel therapeutic targets in cancer. Malic enzyme refers to NADP+-specific malate dehydrogenase [systematic name (S)-malate:NADP+ oxidoreductase (oxaloacetate-decarboxylating)].
[Source 3 ] References- Bi YH, Han WQ, Li RF, et al. Signal transducer and activator of transcription 3 promotes the Warburg effect possibly by inducing pyruvate kinase M2 phosphorylation in liver precancerous lesions. World J Gastroenterol. 2019;25(16):1936–1949. doi:10.3748/wjg.v25.i16.1936 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6487376
- Dang C.V., Gao P., Kim J. (2017) Warburg Effect. In: Schwab M. (eds) Encyclopedia of Cancer. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-46875-3
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi:10.1126/science.1160809 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2849637
- Warburg O, Posener K, Negelein E. Biochem Z. 1924;152:319.
- Warburg O. On respiratory impairment in cancer cells. Science 1956;124(3215):269-70.
- Yoshikawa, N.; Saito, Y.; Manabe, H.; Nakaoka, T.; Uchida, R.; Furukawa, R.; Muramatsu, T.; Sugiyama, Y.; Kimura, M.; Saito, H. Glucose Depletion Enhances the Stem Cell Phenotype and Gemcitabine Resistance of Cholangiocarcinoma Organoids through AKT Phosphorylation and Reactive Oxygen Species. Cancers 2019, 11(12), 1993; https://doi.org/10.3390/cancers11121993
- Hirayama, A.; Kami, K.; Sugimoto, M.; Sugawara, M.; Toki, N.; Onozuka, H.; Kinoshita, T.; Saito, N.; Ochiai, A.; Tomita, M.; et al. Quantitative Metabolome Profiling of Colon and Stomach Cancer Microenvironment by Capillary Electrophoresis Time-of-Flight Mass Spectrometry. Cancer Res. 2009, 69, 4918–4925.
- Heddleston, J.M.; Li, Z.; Lathia, J.D.; Bao, S.; Hjelmeland, A.B.; Rich, J.N. Hypoxia inducible factors in cancer stem cells. Br. J. Cancer 2010, 102, 789–795.
- Zhang, Pi & Xing, Ya & Niu, Yong. (2019). Fundamentals of the Warburg Effect in Cancer. journal of nutritional oncology. 4. 108-114. 10.34175/jno201903002.
- Baracca A, Chiaradonna F, Sgarbi G, Solaini G, Alberghina L, Lenaz G. Mitochondrial Complex I decrease is responsible for bioen-ergetic dysfunction in K-ras transformed cells. Biochim Biophys Acta 2010;1797(2):314-323.
- Weinhouse S. Hepatomas. Science 1967;158(3800):542-3
- Jose C, Bellance N, Rossignol R. Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim Biophys Acta 2011;1807(6):552-61.
- Mandujano-Tinoco EA, Gallardo-Perez JC, Marin-Hernandez A, Moreno-Sanchez R, Rodriguez-Enriquez S. Anti-mitochondrial therapy in human breast cancer multi-cellular spheroids. Biochim Biophys Acta 2013;1833(3):541-51.
- Teodoro JS, Palmeira CM, Rolo AP. Mitochondrial membrane po-tential (DeltaPsi) fluctuations associated with the metabolic states of mitochondria. Methods Mol Biol 2018;1782:109-19.
- Kang JH, Lee SH, Lee JS, Nam B, Seong TW, Son J, Jang H, Hong KM, Lee C, Kim SY. Aldehyde dehydrogenase inhibition combined with phenformin treatment reversed NSCLC through ATP depletion. Oncotarget 2016;7(31):49397-410
- Dayton TL, Jacks T, Vander Heiden MG. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016;17(12):1721–1730.
- Yadav S, Bhagat SD, Gupta A, Samaiya A, Srivastava A, Shukla S. Dietary-phytochemical mediated reversion of cancer-specific splicing inhibits Warburg effect in head and neck cancer. BMC Cancer. 2019;19(1):1031. Published 2019 Nov 1. doi:10.1186/s12885-019-6257-1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6823945
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233.
- Rajala RV, Rajala A, Kooker C, Wang Y, Anderson RE. The Warburg effect mediator pyruvate kinase M2 expression and regulation in the retina. Sci Rep. 2016;6:37727.
- Vander Heiden MG, Christofk HR, Schuman E, Subtelny AO, Sharfi H, Harlow EE, Xian J, Cantley LC. Identification of small molecule inhibitors of pyruvate kinase M2. Biochem Pharmacol. 2010;79(8):1118–1124.
- Morita M, Sato T, Nomura M, Sakamoto Y, Inoue Y, Tanaka R, Ito S, Kurosawa K, Yamaguchi K, Sugiura Y, Takizaki H, Yamashita Y, Katakura R, Sato I, Kawai M, Okada Y, Watanabe H, Kondoh G, Matsumoto S, Kishimoto A, Obata M, Matsumoto M, Fukuhara T, Motohashi H, Suematsu M, Komatsu M, Nakayama KI, Watanabe T, Soga T, Shima H, Maemondo M, Tanuma N. PKM1 Confers Metabolic Advantages and Promotes Cell-Autonomous Tumor Cell Growth. Cancer Cell. 2018;33:355–367.e7.
- Yang W, Lu Z. Pyruvate kinase M2 at a glance. J Cell Sci. 2015;128:1655–1660.
- Israelsen WJ, Vander Heiden MG. Pyruvate kinase: Function, regulation and role in cancer. Semin Cell Dev Biol. 2015;43:43–51.
- Iansante V, Choy PM, Fung SW, Liu Y, Chai JG, Dyson J, Del Rio A, D’Santos C, Williams R, Chokshi S, Anders RA, Bubici C, Papa S. PARP14 promotes the Warburg effect in hepatocellular carcinoma by inhibiting JNK1-dependent PKM2 phosphorylation and activation. Nat Commun. 2015;6:7882.
- Stone OA, El-Brolosy M, Wilhelm K, Liu X, Romao AM, Grillo E, Lai JKH, Günther S, Jeratsch S, Kuenne C, Lee IC, Braun T, Santoro MM, Locasale JW, Potente M, Stainier DYR. Loss of pyruvate kinase M2 limits growth and triggers innate immune signaling in endothelial cells. Nat Commun 2018;9(1):4077.
- Morita M, Sato T, Nomura M, Sakamoto Y, Inoue Y, Tanaka R, Ito S, Kurosawa K, Yamaguchi K, Sugiura Y, Takizaki H, Yamashita Y, Katakura R, Sato I, Kawai M, Okada Y, Watanabe H, Kondoh G, Matsumoto S, Kishimoto A, Obata M, Matsumoto M, Fukuhara T, Motohashi H, Suematsu M, Komatsu M, Nakayama KI, Watanabe T, Soga T, Shima H, Maemondo M, Tanuma N. PKM1 confers metabolic advantages and promotes cell-autonomous tumor cell growth. Cancer Cell 2018;33(3):355-67.
- Lee J, Yesilkanal AE, Wynne JP, Frankenberger C, Liu J, Yan J, Elbaz M, Rabe DC, Rustandy FD, Tiwari P, Grossman EA, Hart PC, Kang C, Sanderson SM, Andrade J, Nomura DK, Bonini MG, Locasale JW, Rosner MR. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 2019;568(7751):254-8.