What is alopecia
Alopecia means hair loss regardless of the cause 1. Hair loss is not exclusive to the scalp, it can be anywhere in your body. Alopecia occurs in:
- Men and women
- Children and adults
- People with any color or type of hair.
Alopecia can be an isolated problem or associated with another disease or condition, including:
- A family history of balding on your mother’s or father’s side
- Age
- Significant weight loss
- Certain medical conditions, such as diabetes and lupus
- Stress
- Poor nutrition
Alopecia can be temporary or permanent, depending on the cause.
- Alopecia may be localized or diffuse.
- Alopecia can affect the scalp or other parts of the body.
- Alopecia may be due to hair shedding, poor quality hair, or hair thinning.
- There may be areas of skin that are completely bald.
- There may be associated skin disease or scarring.
Since hair loss may be an early sign of a disease, it is important to find the cause so that it can be treated.
If you suspect that you may have excessive hair loss, talk to your doctor. He or she will probably ask you some questions about your diet, any medicines you’re taking, and whether you’ve had a recent illness, and how you take care of your hair. If you’re a woman, your doctor may ask questions about your menstrual cycle, pregnancies, and menopause. Your doctor may want to do a physical exam to look for other causes of hair loss. Finally, your doctor may order blood tests or a biopsy (taking a small sample of cells to examine under a microscope).
Depending on your type of hair loss, treatments are available. If a medicine is causing your hair loss, your doctor may be able to prescribe a different medicine. Recognizing and treating an infection may help stop the hair loss. Correcting a hormone imbalance may prevent further hair loss.
Medicines may also help slow or prevent the development of common baldness. One medicine, minoxidil (brand name: Rogaine), is available without a prescription. It is applied to the scalp. Both men and women can use it. Another medicine, finasteride, is available with a prescription. It comes in pills and is only for men. It may take up to 6 months before you can tell if one of these medicines is working.
See your doctor if you are distressed by persistent hair loss in you or your child and want to pursue treatment. For women who are experiencing a receding hairline (frontal fibrosing alopecia), talk with your doctor about early treatment to avoid significant permanent baldness.
Also talk to your doctor if you notice sudden or patchy hair loss or more than usual hair loss when combing or washing your or your child’s hair. Sudden hair loss can signal an underlying medical condition that requires treatment.
Also if you are having significant, persistent hair loss or if there is redness, itching, or skin changes associated with the hair loss, seek medical advice, as there are sometimes other causes for hair loss that can be treated.
Lastly, if you have hair loss that is cosmetically concerning and other causes have been ruled out, you might consult a surgical specialist in hair replacement.
Physiology of hair growth
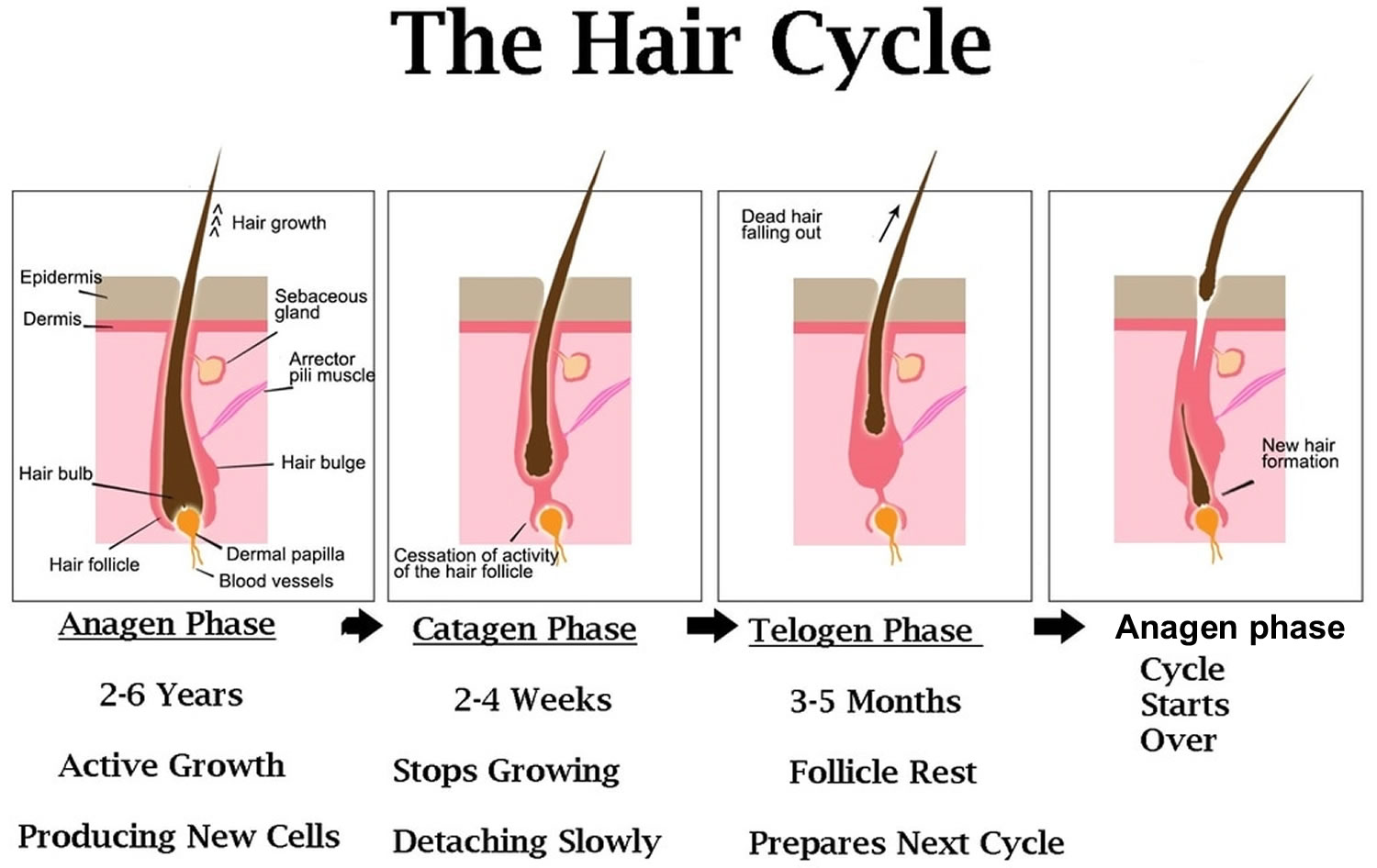
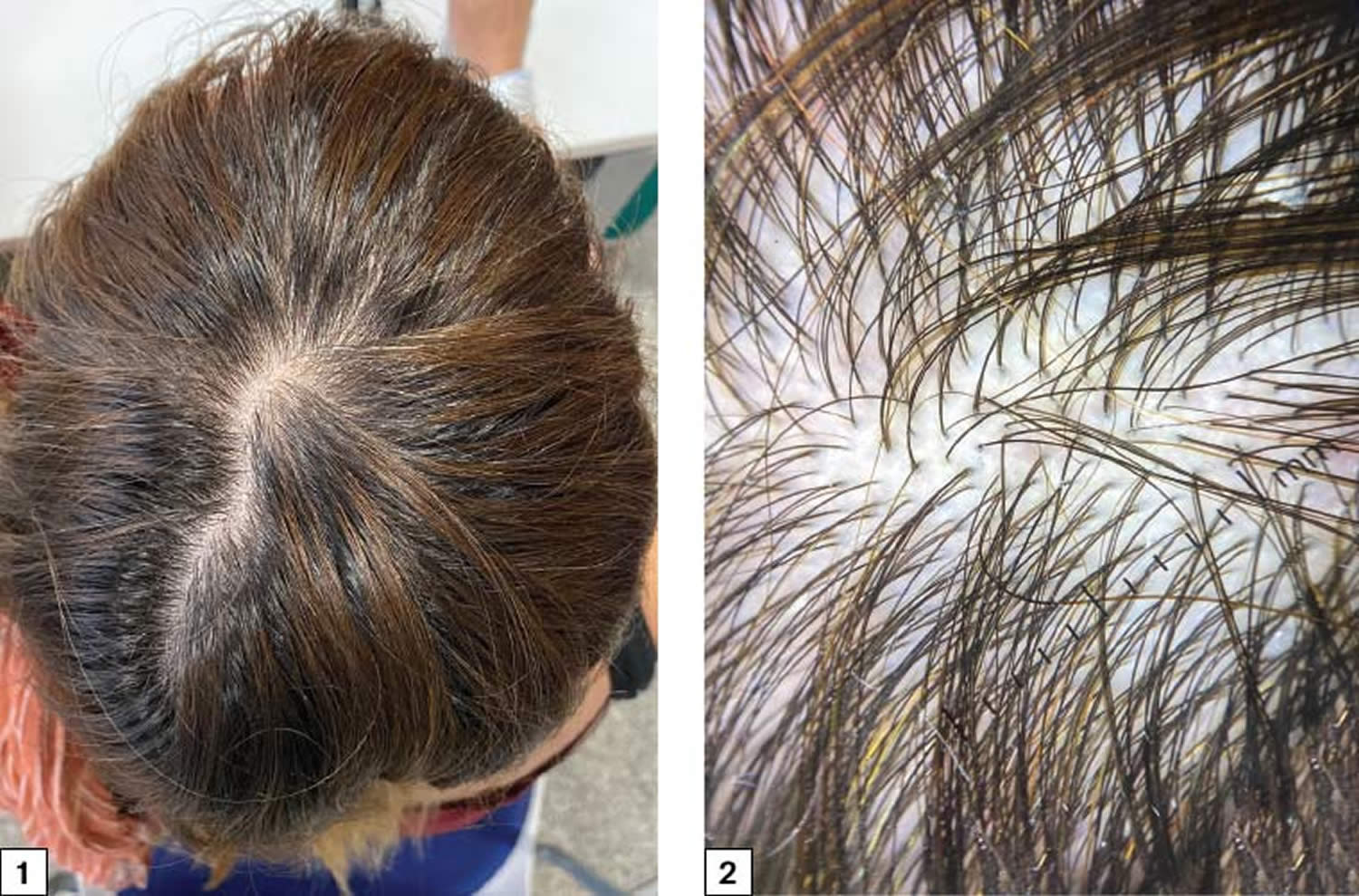
The scalp contains, on average, 100,000 hairs 2. You lose up to 100 hairs from your scalp every day. That’s normal, and in most people, those hairs grow back. A hair shaft grows within a hair follicle at a rate of about 1 – 1.5 cm per month. It is due to cell division within the hair bulb at the base of the hair follicle. The cells produce the three layers of the hair shaft (medulla, cortex, cuticle), which are mainly made of the protein keratin (which is also the main structure of skin and nails). Hair growth follows a cycle and the hair growth cycle is divided into three phases: anagen (active growing stage, about 90 % of hairs), catagen (degeneration stage, less than 10% of hairs) and telogen (resting stage, 5% to 10% of hairs). Hair is shed during the telogen phase. When telogen hairs are shed, new anagen hairs grow to replace them, beginning a new cycle 3, 4. These phases are not synchronized, and any hair may be at a particular phase at random. Hair length depends on the duration of anagen. Short hairs (eyelashes, eyebrows, hair on arms and legs) have a short anagen phase of around one month. Anagen lasts up to 6 years or longer in scalp hair. In addition to the ratio of anagen hair to telogen hair, the diameter of the hair follicles determines scalp coverage. Vellus hairs have a hair-shaft diameter of less than 0.03 mm, whereas terminal hairs have a diameter greater than 0.06 mm. The optimal hairs for scalp-hair growth and scalp coverage are anagen and terminal hairs.
Timespan of the hair growth cycle
- The anagen phase constitutes about 90% (1000 days or more) of the growth cycle. Anagen hairs are anchored deeply into the subcutaneous fat and cannot be pulled out easily.
- The catagen phase (10 days) and telogen phase (100 days) constitute only 10% of the hair growth cycle.
- During the catagen and telogen phase of the hair growth cycle, as hairs are at the shedding and rest-from-growth period, no bald spots are shown as hairs are randomly distributed over the scalp.
Anagen (active growing stage, about 90 % of hairs) stage
Your hair grows around 1 – 1.5 cm per month, faster in summer than in winter.
- The anagen stage is the growing period of a hair follicle.
- This stage typically lasts about 3 to 5 years. Asian hair can last 5-7 years
- Full length hair can be upto 100 cm long
Catagen (degeneration stage, less than 10% of hairs) stage
At the end of the anagen phase, your hair enters the catagen phase.
The catagen stage is the intermediate period of hair growth.
- Hair follicles prepare themselves for the resting phase.
- It lasts around 1-2 weeks.
- During this phase, the deeper portions of the hair follicles start to collapse.
Telogen (resting stage, 5% to 10% of hairs) stage
During the telogen phase each hair is released and falls out
- The telogen stage is the resting and shedding period of the hair cycle.
- The follicle remains inactive for 3 to 4 months.
- At the end of this period, older hairs that have finished their life will fall out and newer hairs will begin to grow.
- As compared with anagen hair, telogen hair is located higher in the skin and can be pulled out relatively easily. Normally, the scalp loses approximately 100 telogen hairs per day.
Hair loss, hair thinning and problems with hairgrowth occur when the growth cycle is interrupted/disrupted. This can be triggered by conditions such as nutritional and medical situations, illness or stress. For instance 6 weeks after intensive dieting or stress you can experience hair loss. This occurs because the growing stage (Anagen) is cut short and hairs enter the falling (Telogen) stage at the same time.
Figure 1. Hair growth cycle
Causes of alopecia
People typically lose 50 to 100 hairs a day. This usually isn’t noticeable because new hair is growing in at the same time. Hair loss occurs when new hair doesn’t replace the hair that has fallen out.
Hair loss is typically related to one or more of the following factors:
- Family history (heredity). The most common cause of hair loss is a hereditary condition that happens with aging. This condition is called androgenic alopecia (androgenetic alopecia), male-pattern baldness and female-pattern baldness. It usually occurs gradually and in predictable patterns — a receding hairline and bald spots in men and thinning hair along the crown of the scalp in women.
- Hormonal changes and medical conditions. A variety of conditions can cause permanent or temporary hair loss, including hormonal changes due to pregnancy, childbirth, menopause and thyroid problems. Medical conditions include alopecia areata, which is autoimmune hair loss and causes patchy hair loss, scalp infections such as ringworm, and a hair-pulling disorder called trichotillomania (traction alopecia or traumatic alopecia).
- If your thyroid gland is overactive or underactive, your hair may fall out. This hair loss usually can be helped by treating your thyroid disease. Hair loss may occur if male or female hormones, known as androgens and estrogens, are out of balance. Correcting the hormone imbalance may stop your hair loss.
- Many women notice hair loss about 3 months after they’ve had a baby. This loss is also related to hormones. During pregnancy, high levels of certain hormones cause the body to keep hair that would normally fall out. When the hormones return to pre-pregnancy levels, that hair falls out and the normal cycle of growth and loss starts again.
- Certain infections can cause hair loss. Fungal infections of the scalp (tinea capitis) can cause hair loss in adults and children. The infection is treated with antifungal medicines.
- Systemic diseases resulting in reversible patchy hair thinning, poor hair quality and bald patches include:
- Diabetes
- Iron deficiency
- Thyroid hormone deficiency (hypothyroidism)
- Systemic lupus erythematosus (SLE)
- Syphilis
- Severe acute or chronic illness.
- Dermatological disease resulting in reversible patchy hair thinning, poor hair quality and bald patches include:
- Localized alopecia areata
- A localized infection, such as tinea capitis
- Severe local skin disease, such as psoriasis, seborrhoeic dermatitis, atopic dermatitis, pityriasis rubra pilaris, cutaneous lupus erythematosus, cutaneous T-cell lymphoma
- Generalized skin disease (erythroderma).
- Medications and supplements. Hair loss can be a side effect of certain drugs, such as those used for cancer, arthritis, depression (antidepressants), birth control pills, vitamin A (if you take too much of it), heart problems, gout, blood thinners (anticoagulants) and high blood pressure. This type of hair loss improves when you stop taking the medicine.
- Radiation therapy to the head. The hair may not grow back the same as it was before.
- A very stressful event. Many people experience a general thinning of hair several months after a physical or emotional shock. This type of hair loss is temporary.
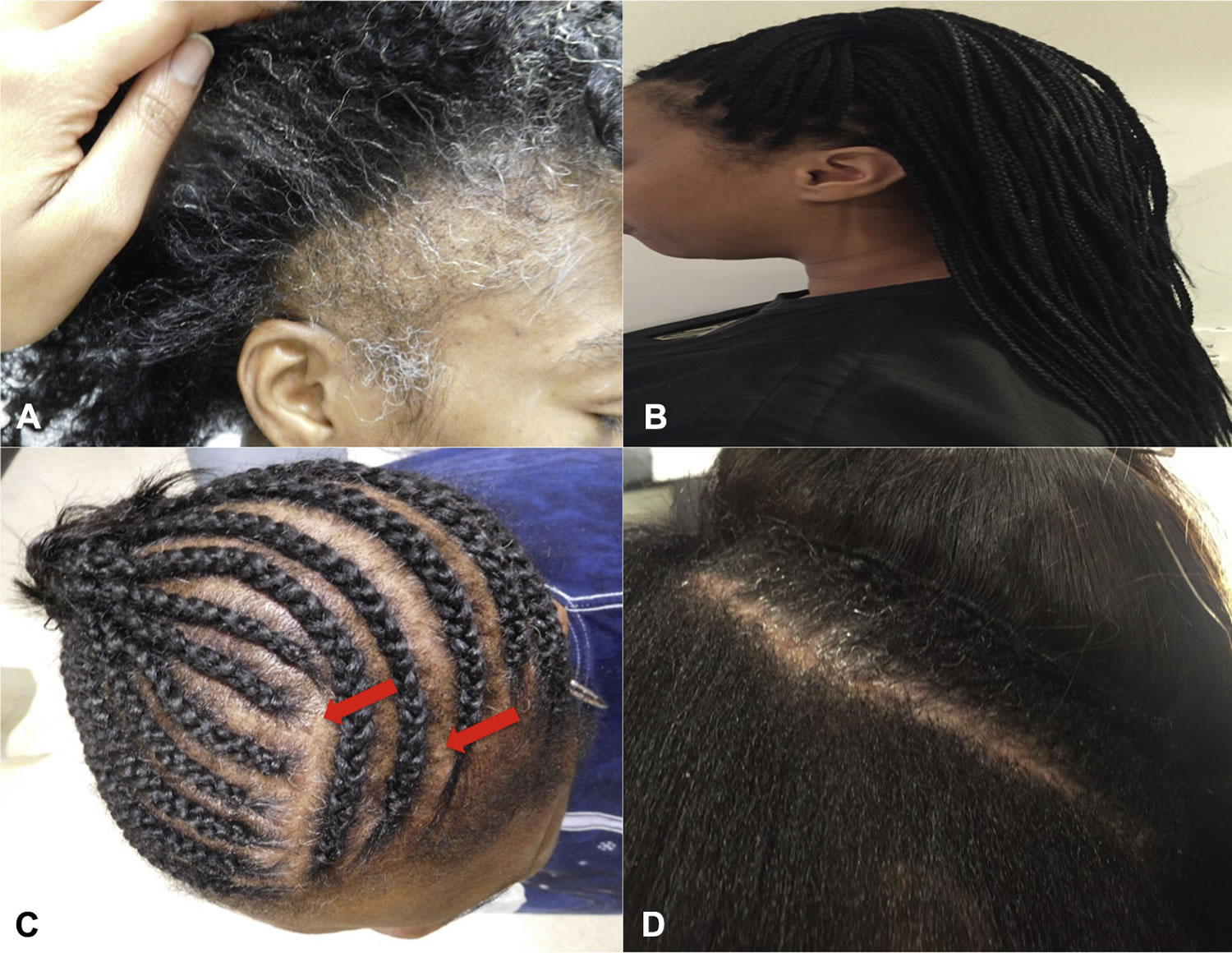
- Hairstyles and treatments. Excessive hairstyling or hairstyles that pull your hair tight, such as pigtails, cornrows or use tight hair rollers, the pull on your hair can cause a type of hair loss called traction alopecia. If the pulling is stopped before scarring of the scalp develops, your hair will grow back normally. However, scarring can cause permanent hair loss. Hot-oil hair treatments and chemicals used in permanents (also called “perms”) may cause inflammation (swelling) of the hair follicle cause the hair to fall out. If scarring occurs, hair loss could be permanent.
Alopecia can be subdivided into two main categories: scarring alopecia and non-scarring alopecia 1:
Non-scarring alopecia
Non-scarring alopecia falls into six major categories:
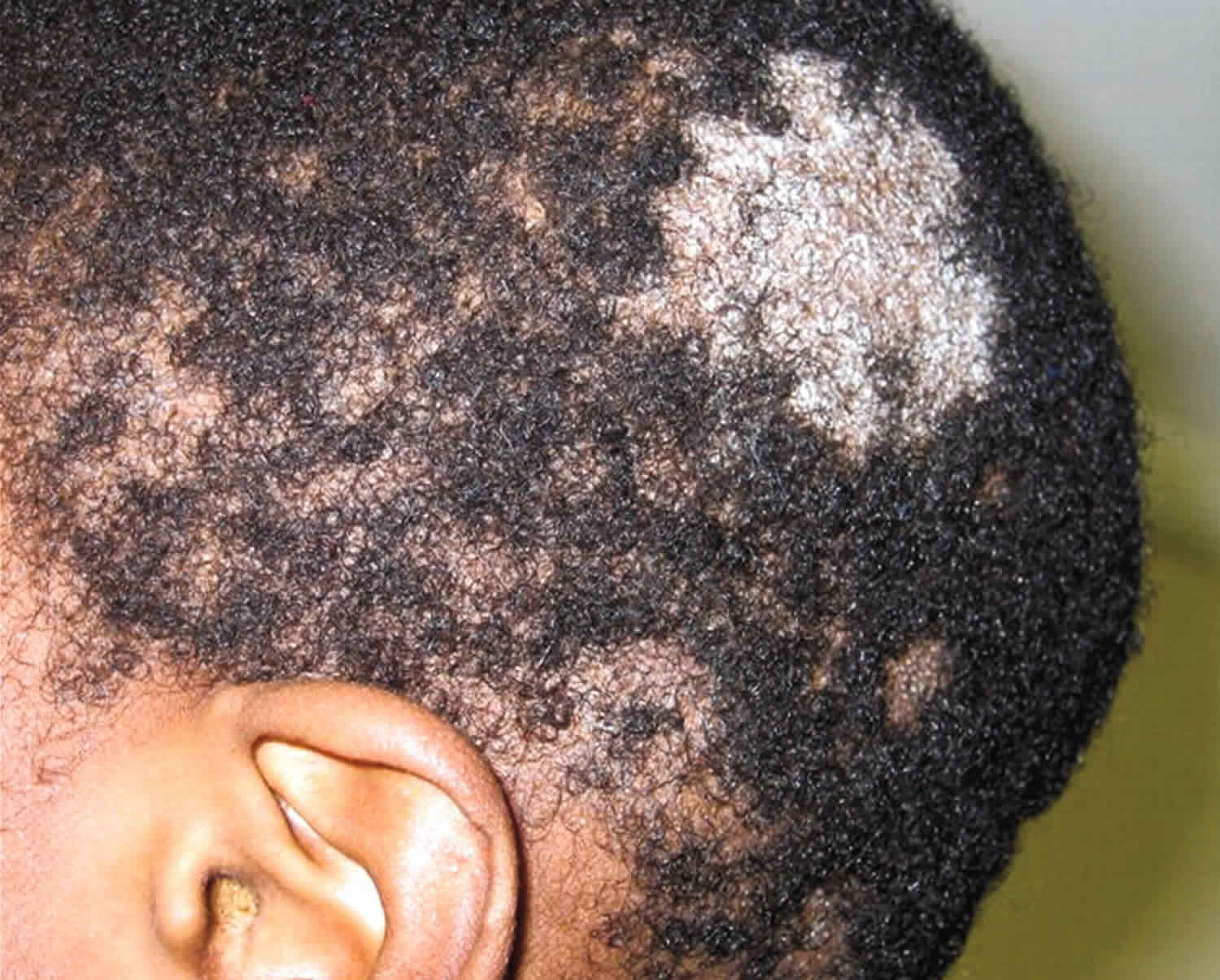
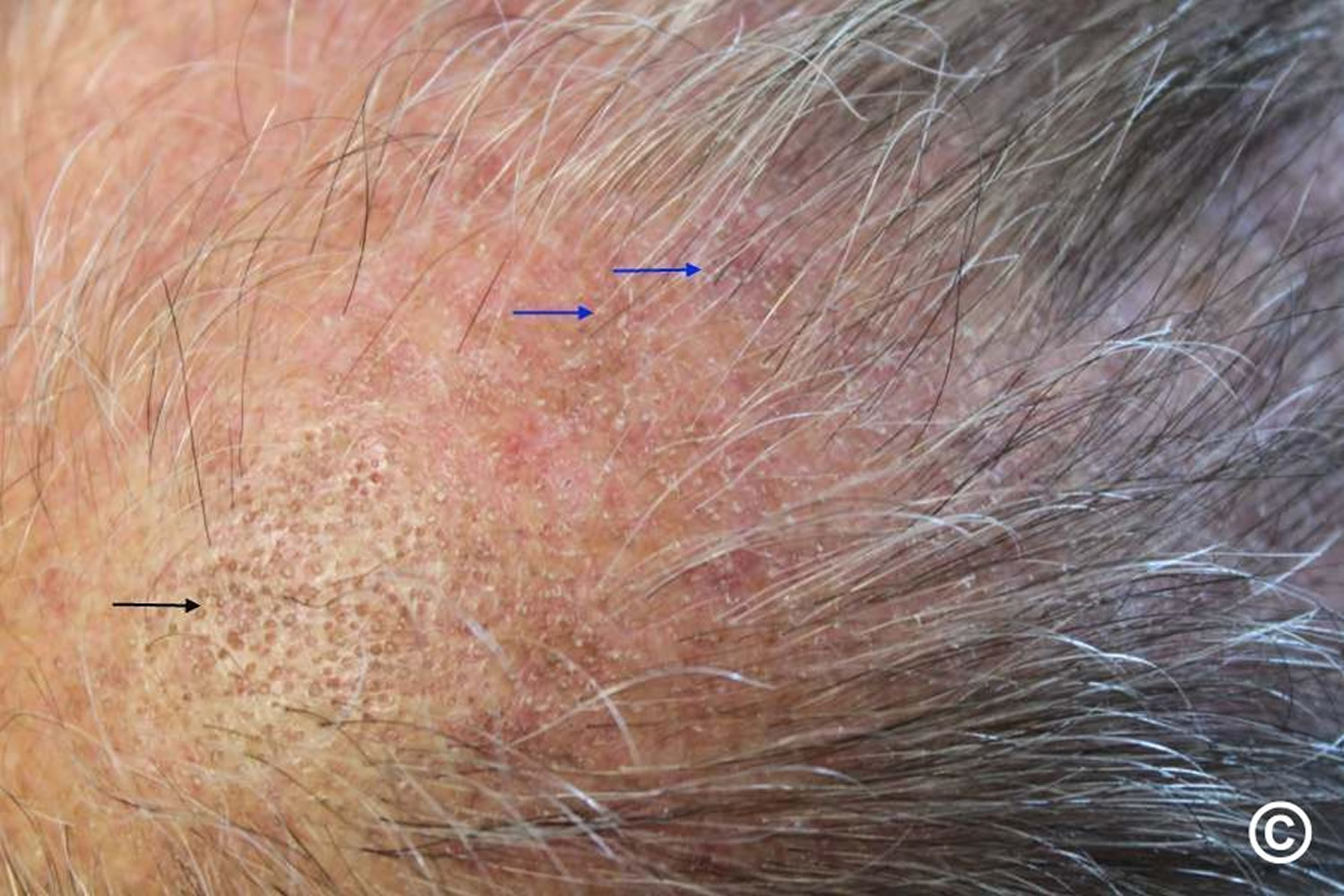
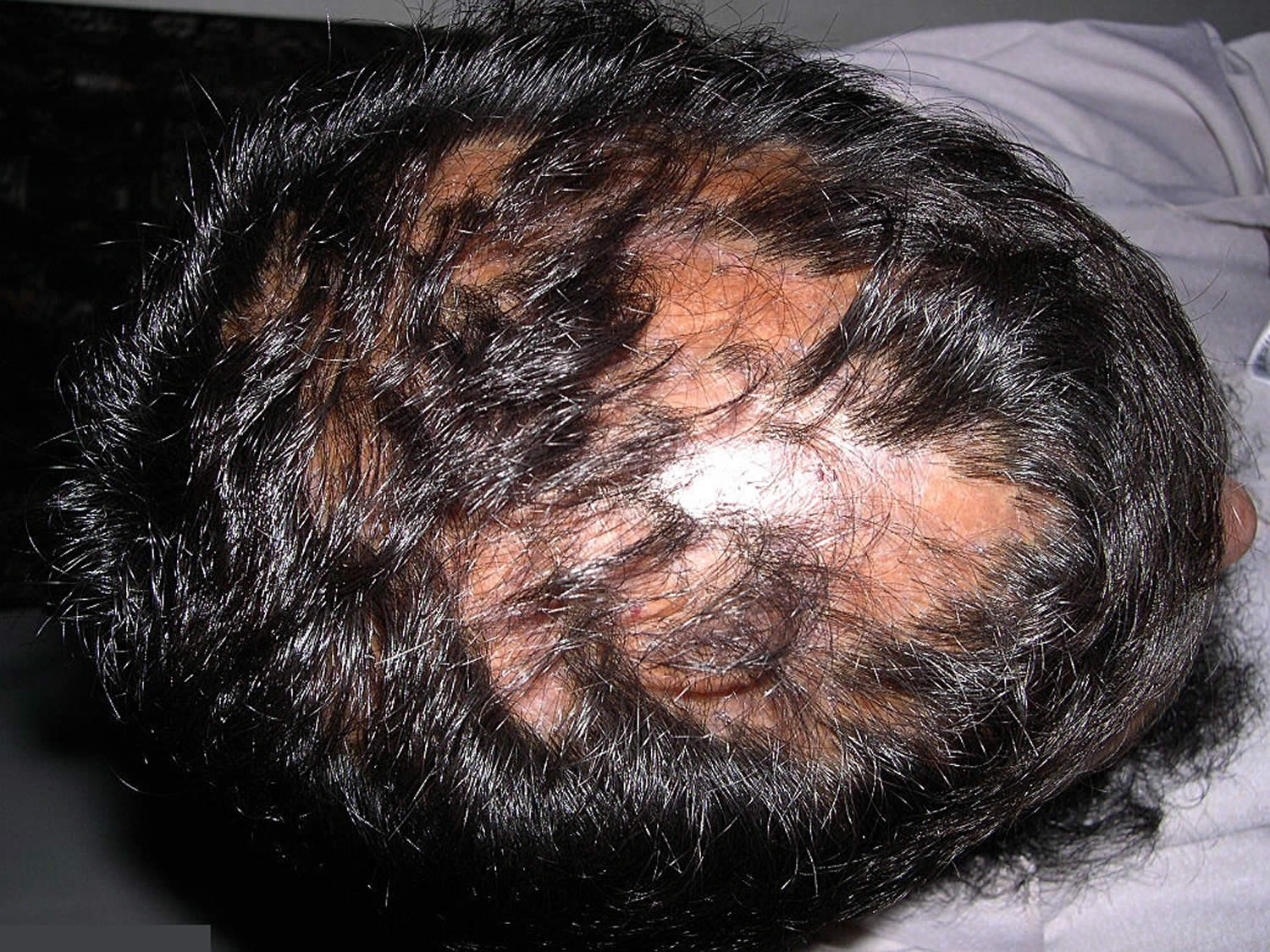
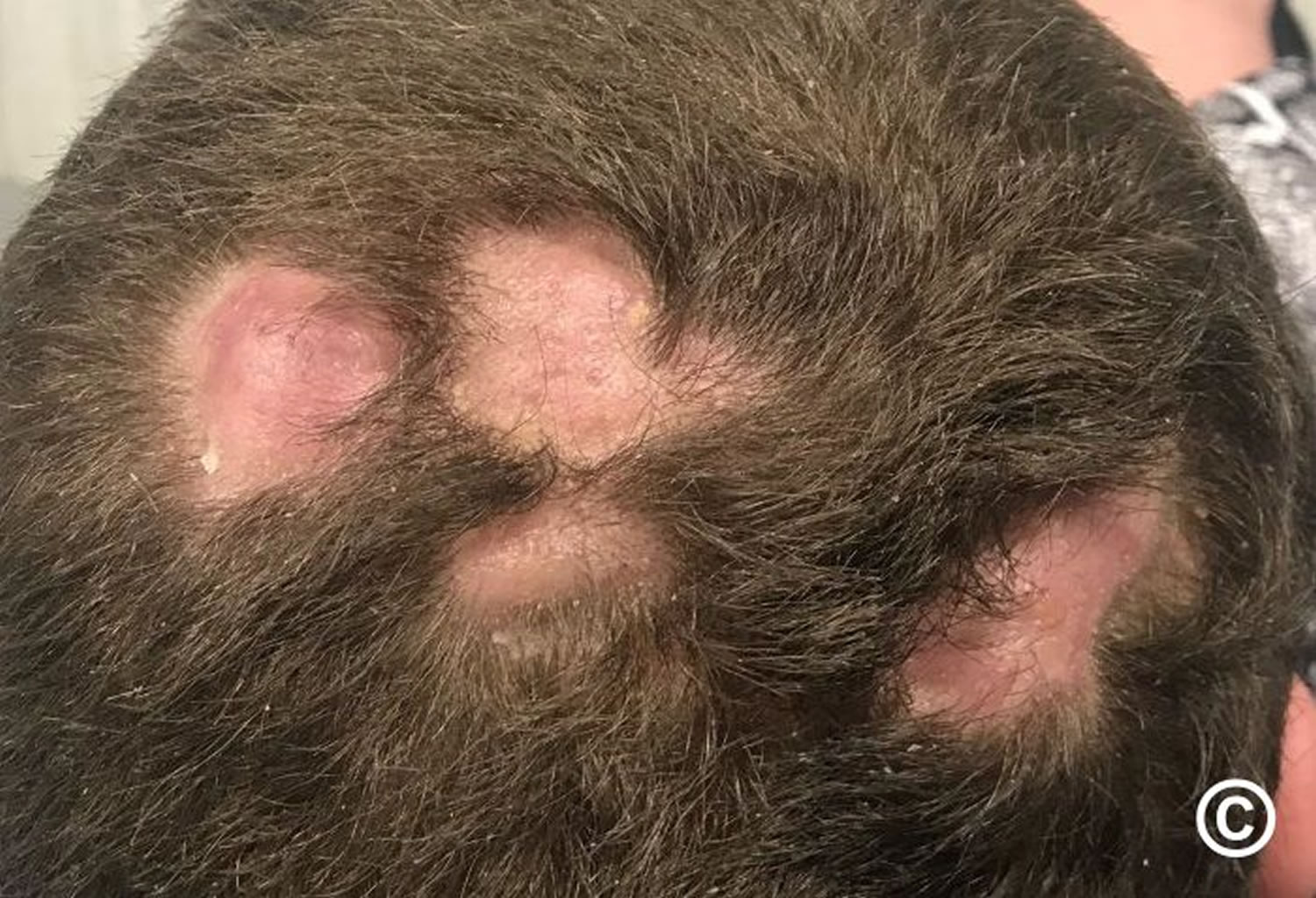
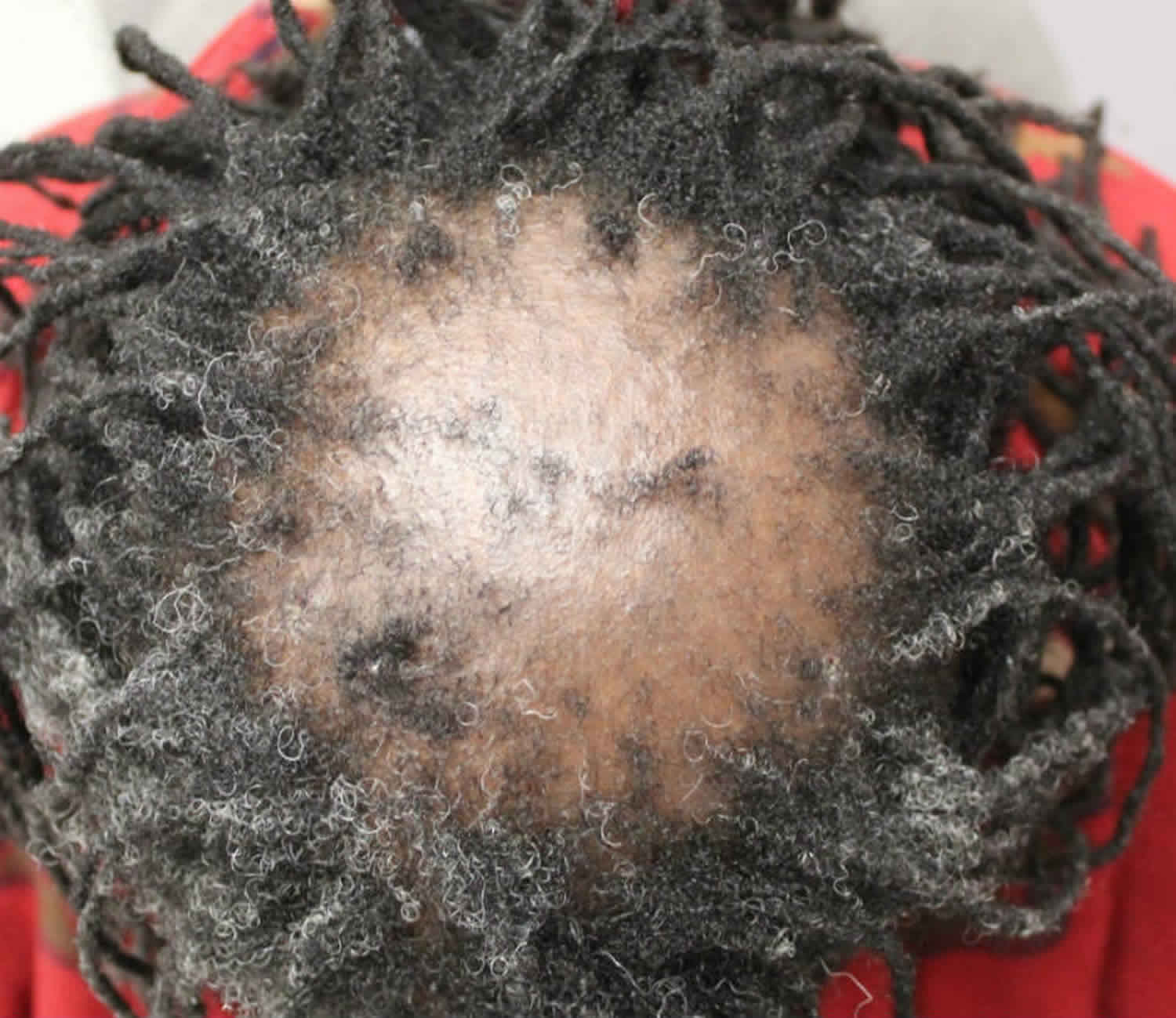
- Alopecia areata: Alopecia areata also called autoimmune alopecia or autoimmune hair loss, is a common autoimmune skin disease, where your body’s immune system attacks your hair cells, causing hair loss on the scalp, face and sometimes on other areas of your body 5. The term “alopecia” means hair loss and “areata” refers to the patchy nature of the hair loss that is typically seen with alopecia areata (see Figure 2). Alopecia areata represent an attack on the hair roots by the body’s own immune system. Alopecia areata hair loss that can affect every part of the body, including the scalp, face, trunk, and extremities. When it affects only a portion of the body, it is called alopecia areata. When it affects an entire site, it is called alopecia totalis. When it involves the whole body, it is called alopecia universalis. The cause is unknown, but it might be related to an autoimmune disease 6. In 80% of patients with a single bald patch, spontaneous regrowth occurs within a year. Even in the most severe cases of alopecia totalis and alopecia universalis, recovery may occur at some future date. This is an important difference between alopecia areata and the scarring forms of alopecia, which destroy the hair follicle and result in irreversible hair loss. Referral centers indicate that 34–50% of patients will recover spontaneously within 1 year, although most will experience multiple episodes of the alopecia, and 14–25% of patients will progress to alopecia totalis or alopecia universalis, from which full recovery is unusual (<10% of patients) 7.
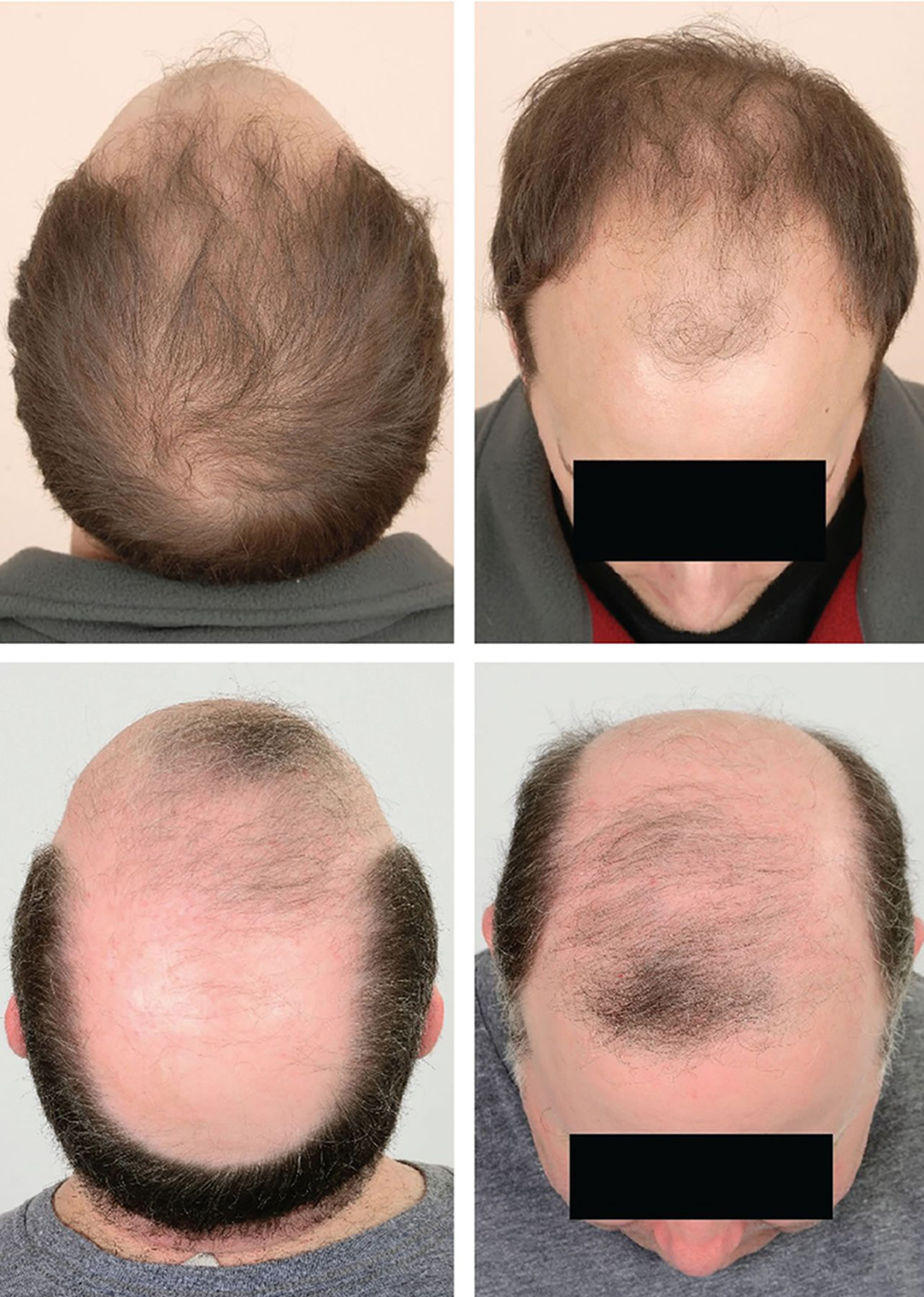
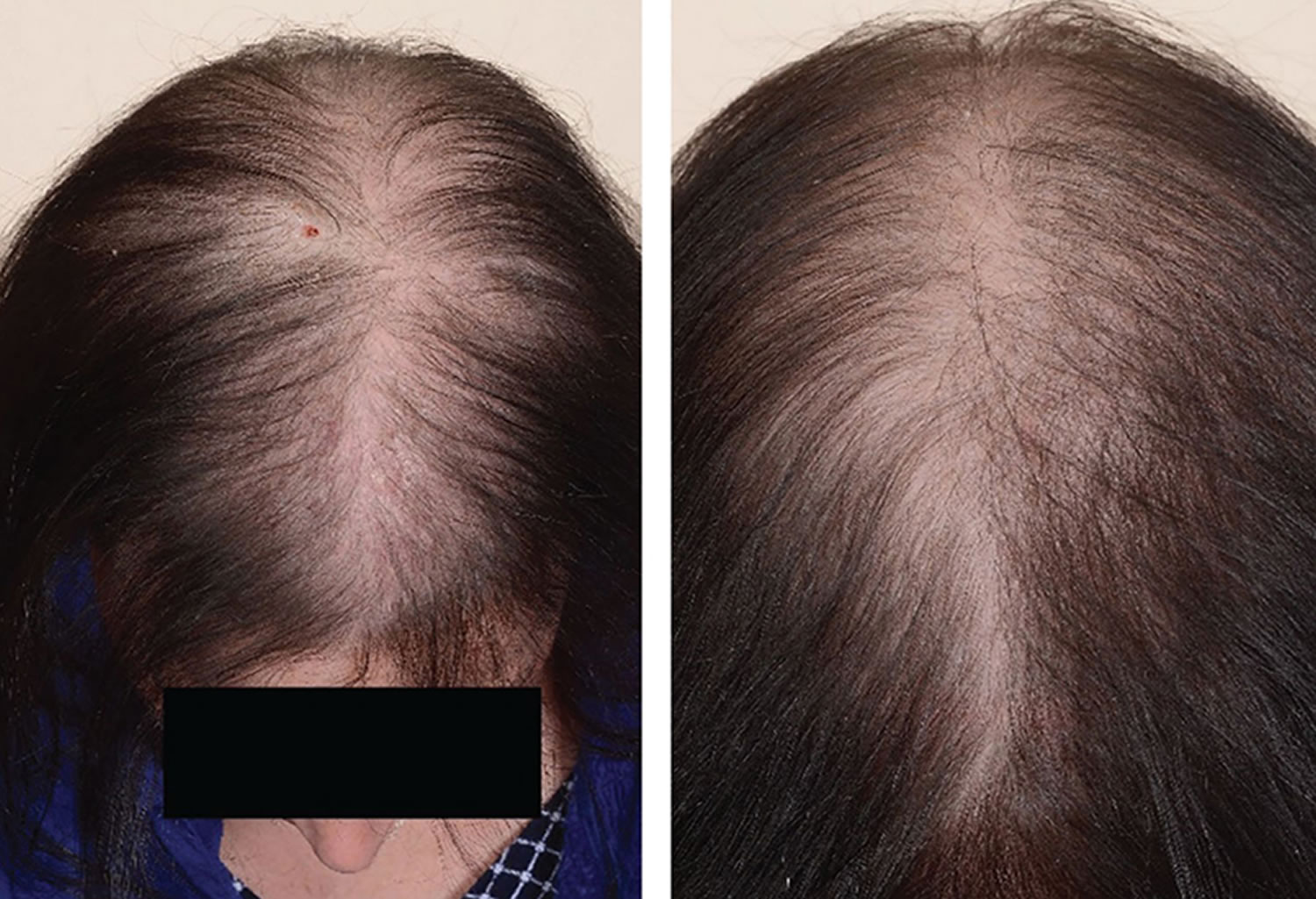
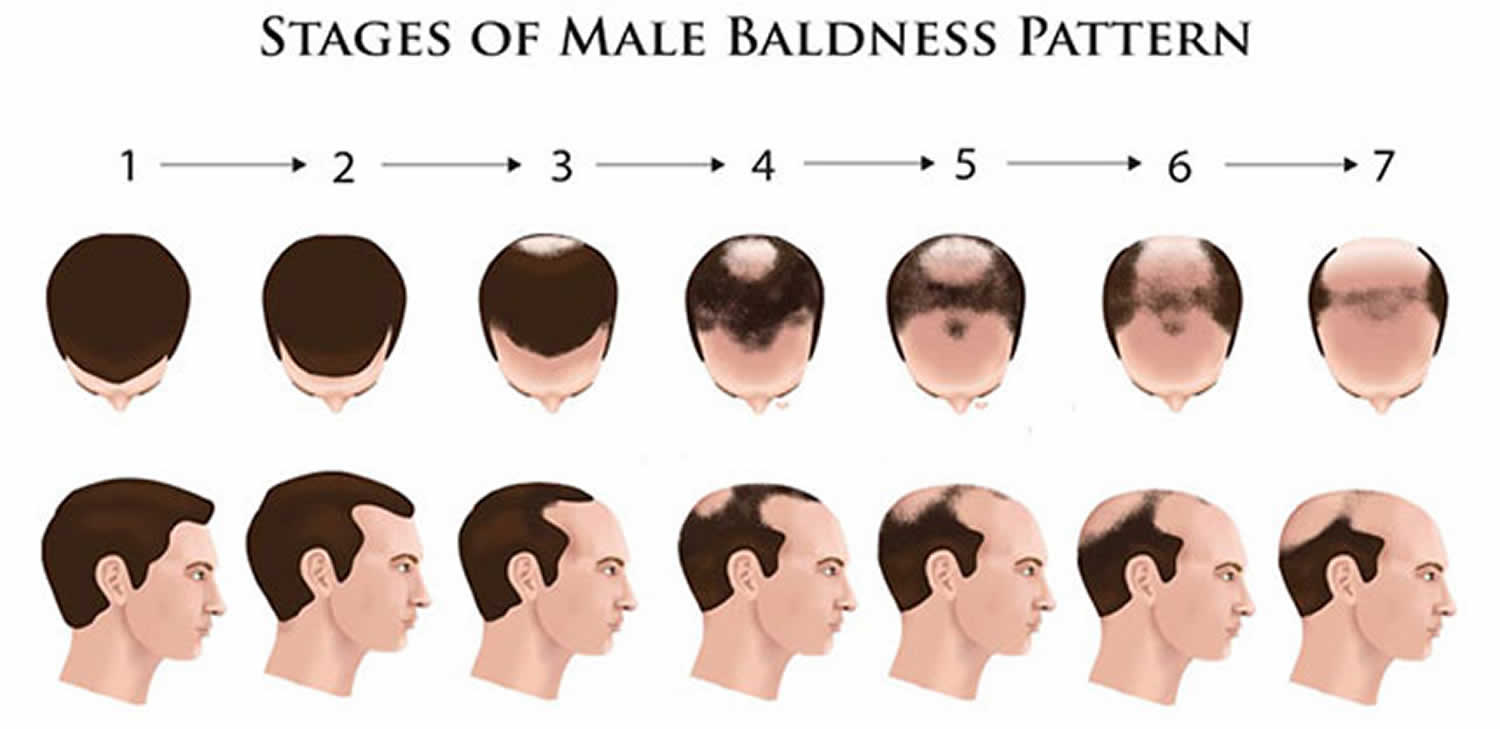
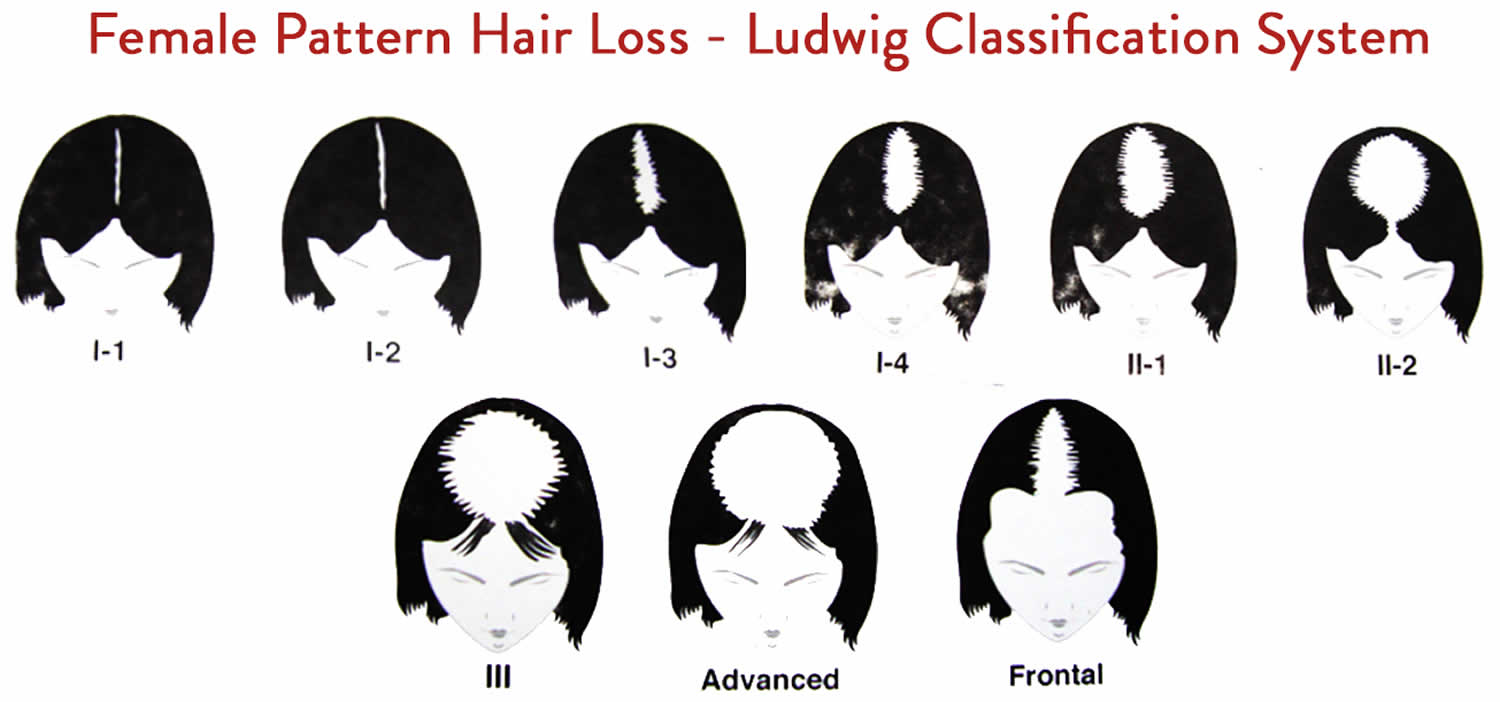
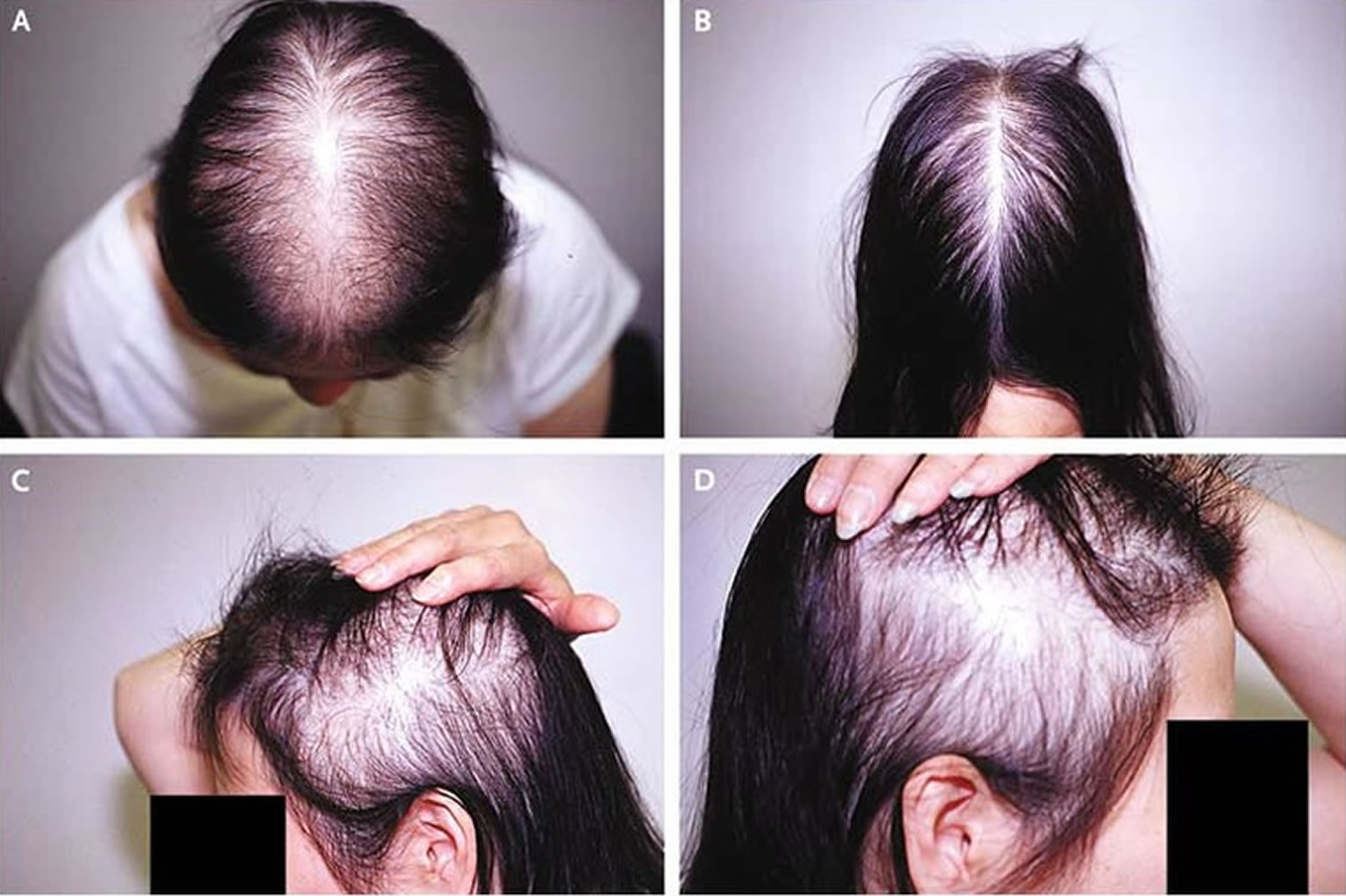
- Androgenetic alopecia: Androgenetic alopecia is a pattern of hair loss that is affected by the genes and hormones (androgenic alopecia). Androgenetic alopecia is the most common form of hair loss in men and women and is a normal physiologic variant. Androgenetic alopecia is most prevalent in white men, with 30%, 40%, and 50% experiencing androgenetic alopecia at 30, 40, and 50 years of age, respectively (see Figure 3). Although androgenetic alopecia is less common in women, 38% of women older than 70 years may be affected (see Figure 4) 8. Androgenetic alopecia hair loss follows a gradual progressive course. Many patients with androgenetic alopecia have a family history of this condition. Hair thinning occurs in a sex-specific pattern.
- Androgenic alopecia in men: bitemporal thinning of the frontal and vertex scalp, complete hair loss with some hair at the occiput and temporal fringes 9. Minoxidil and oral finasteride are the only treatments currently approved by the U.S. Food and Drug Administration for the treatment of androgenetic alopecia. Both of these drugs stimulate hair regrowth in some men, but are more effective in preventing progression of hair loss. Although there are a number of other treatments listed in various texts, there is not good evidence to support their use 10. Topical minoxidil (2% or 5% solution) is approved for the treatment of androgenetic alopecia in men. Hair regrowth is more robust at the vertex than in the frontal area, and will take six to 12 months to improve 9. Treatment should continue indefinitely because hair loss reoccurs when treatment is discontinued. Adverse effects include irritant and contact dermatitis. Finasteride (Propecia), 1 mg per day orally, is approved to treat androgenetic alopecia in men for whom topical minoxidil has been ineffective. Adverse effects of finasteride include decreased libido, erectile dysfunction, and gynecomastia (increase in the amount of breast gland tissue in men) 11.
- Androgenic alopecia in women: diffuse hair thinning of the vertex with sparing of the frontal hairline. Treatment involves topical minoxidil (2% solution). Adverse effects include irritant or contact dermatitis.
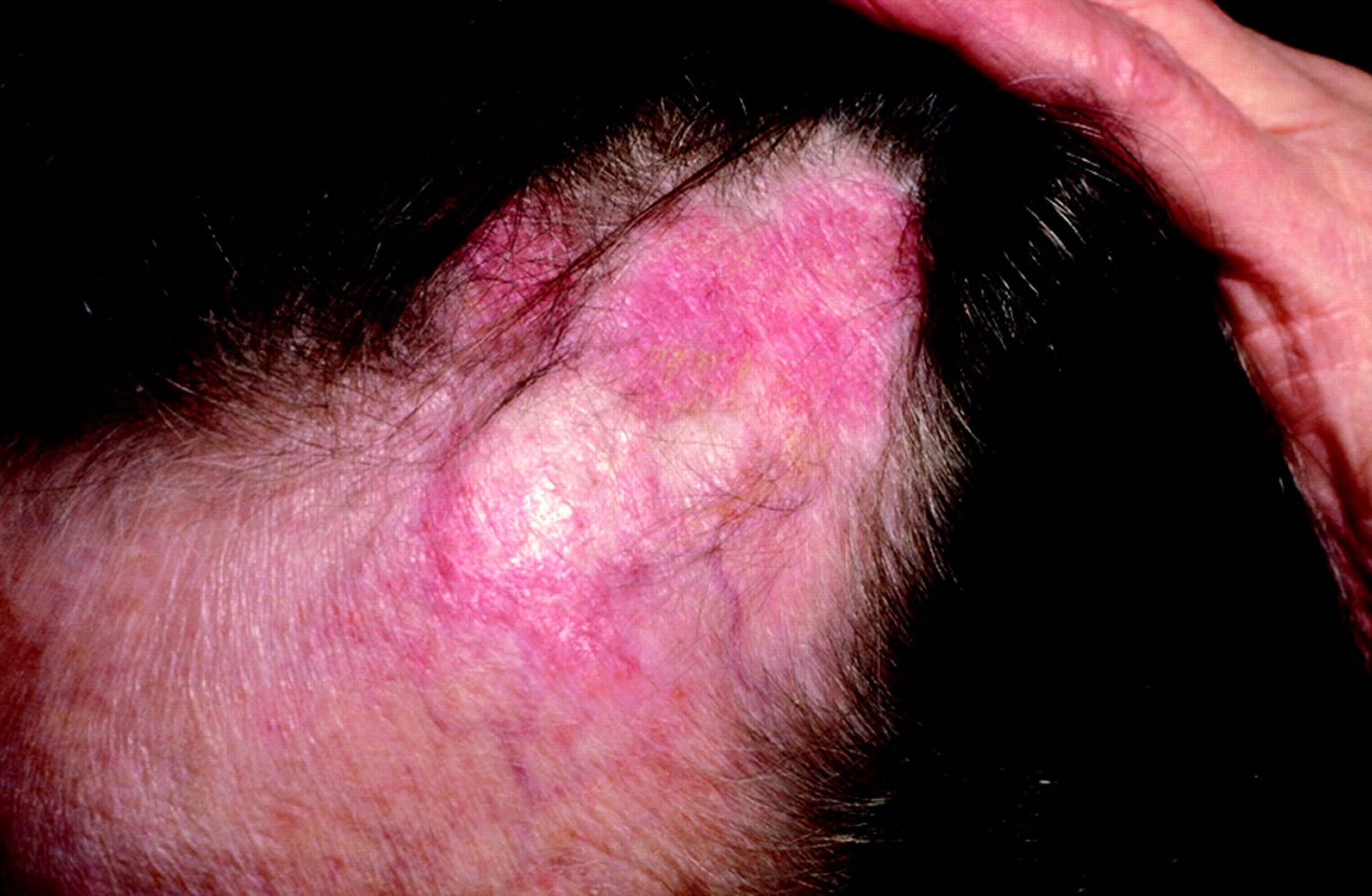
- Telogen effluvium: Telogen effluvium results from shifting of the hair cycle growth (anagen) phase towards the shedding (telogen) phase. Clumps of hair come out in the shower or in hairbrush; associated with physiologic or emotional stress (Figure 5). Patients typically report significant hair loss and a decrease in hair volume (they commonly complain about their ponytail reducing in diameter) without well-defined alopecic patches. A pull test is typically positive 12. Telogen effluvium may result from an illness like hypothyroidism (underactive thyroid) or hyperthyroidism (overactive thyroid). Also, it can arise from stress like major surgery. A crash diet, poor feeding, and drugs can cause telogen effluvium 13. Telogen effluvium is usually self-limited and resolves within two to six months. Treatment involves removing the underlying cause and providing reassurance about the reversible nature of alopecia.
- Traumatic alopecia: This is similar to traction alopecia, which results from forceful traction of the hair commonly seen in children (Figure 6). Also, trichotillomania is a type of traumatic alopecia in which the patient pulls on his/her hair repeatedly 14.
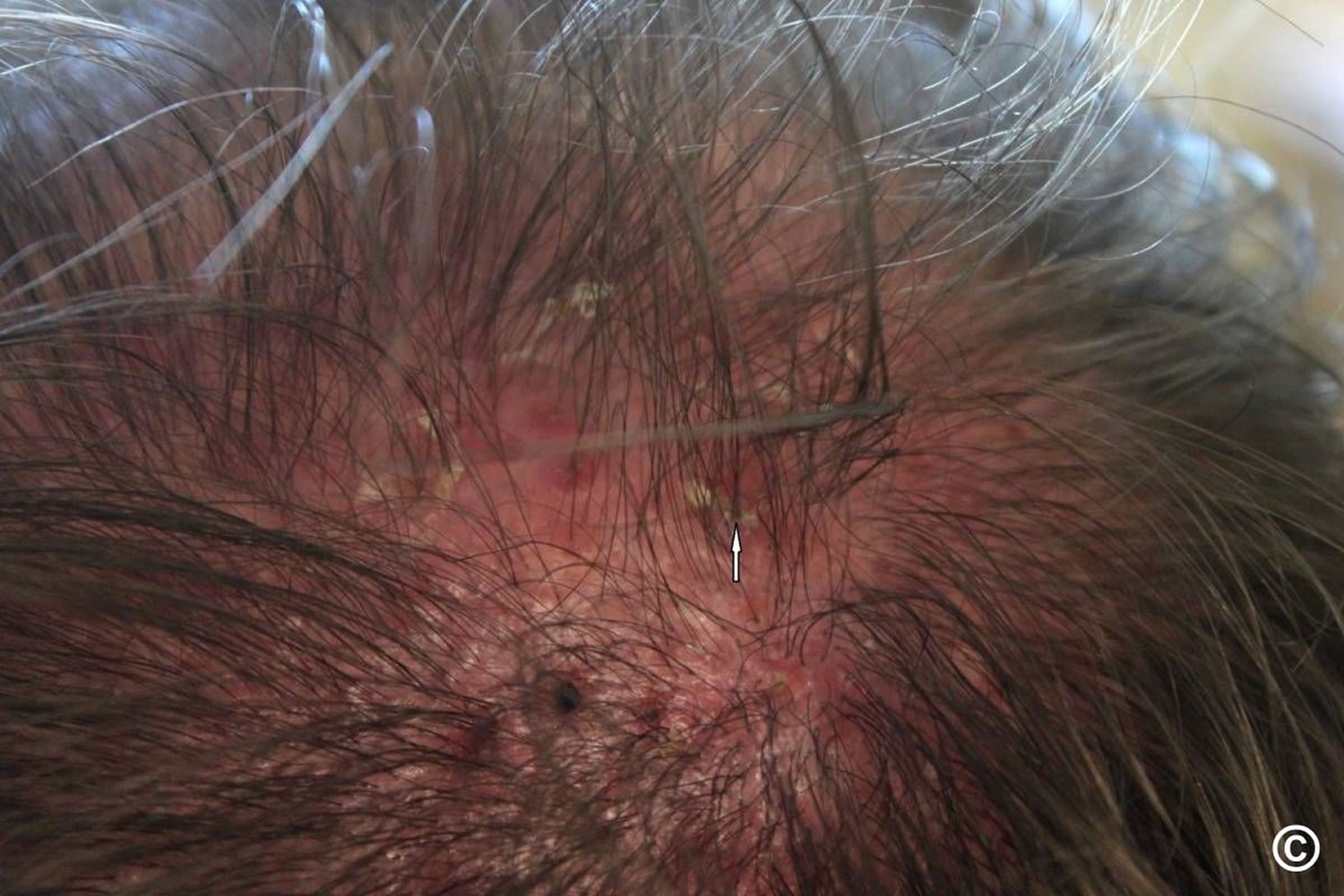
- Tinea capitis (ringworm of the scalp): Tinea capitis is a fungal infection of the scalp and hair shafts. It is caused primarily by the dermatophyte species Microsporum and Trichophyton 15. The fungi can penetrate the hair follicle outer root sheath and ultimately may invade the hair shaft. Clinically, tinea capitis divides into inflammatory and non-inflammatory types. The non-inflammatory type usually will not be complicated by scarring alopecia. The inflammatory type may result in a kerion (painful nodules with pus) as well as scarring alopecia 16. Tinea capitis, a highly contagious infection, occurs primarily in children between 3 and 14 years of age, but it might affect any age group. It may also involve the eyelashes and eyebrows. The signs and symptoms of ringworm of the scalp may vary, but it usually appears as itchy, scaly, bald patches on the head (see Figure 7). Tinea capitis can is treatable with systemic antifungal medications because topical antifungals do not penetrate hair follicles. The treatment is for 4 to 8 weeks. Topical treatment is not recommended, as it is ineffective 17.
- Trichophyton species: oral terbinafine (Lamisil), itraconazole (Sporanox), fluconazole (Diflucan), or griseofulvin
- Microsporum species: griseofulvin
- Anagen effluvium: This is hair shedding (usually abrupt) that occurs during the anagen phase of the cell cycle due to an event that impairs the mitotic or metabolic activity of the hair follicle (Figure 8). Seen in cancer patients who are receiving chemotherapeutic agents or it can be an inherited or congenital condition, such as loose anagen syndrome. Patients typically present with diffuse hair loss that begins days to weeks after exposure to a chemotherapeutic agent and is most apparent after one or two months 18. In cancer patients who are receiving chemotherapeutic agents, short broken hairs and empty hair follicles may be observed. The incidence of anagen effluvium after chemotherapy is approximately 65% 19; it is most commonly associated with cyclophosphamide, nitrosoureas, and doxorubicin (Adriamycin). Other causative medications include tamoxifen, allopurinol, levodopa, bromocriptine (Parlodel), and toxins such as bismuth, arsenic, and gold. Other medical and inflammatory conditions, such as mycosis fungoides or pemphigus vulgaris, can lead to anagen effluvium 20. Anagen effluvium is usually reversible, with regrowth one to three months after cessation of the offending agent. Permanent alopecia is rare. No pharmacologic intervention has been proven effective. A large meta-analysis of clinical trials concluded that scalp cooling was the only intervention that significantly reduced the risk of chemotherapy-induced anagen effluvium 21. However, scalp cooling should be discouraged because it may minimize delivery of chemotherapeutic drugs to the scalp, leading to cutaneous scalp metastases 21. Minoxidil may help during regrowth period.
Figure 2. Alopecia areata
Figure 3. Male pattern hair loss
Figure 4. Female pattern hair loss
Figure 5. Telogen effluvium
Footnote: 1) Diffuse loss of hair volume without any defined alopecic patches. 2) Numerous follicular units of only one hair and without anisotrichosis (hair shafts with different diameters); no trichoscopic signs of alopecia areata or other kinds of alopecia
Figure 6. Traction alopecia
Figure 7. Tinea capitis
Figure 8. Anagen effluvium
Scarring alopecia
Scarring alopecia is divided into four major types:
- Tinea capitis: the inflammatory variety of tinea capitis (favus) may culminate with scarring alopecia.
- Alopecia mucinosa also known as follicular mucinosis: Alopecia mucinosa is a benign condition that occurs when mucinous material accumulates in the hair follicles and the sebaceous glands. The mucinous material causes an inflammatory response that hinders the growth of hair.
- Alopecia neoplastica: This is the metastatic infiltration of the scalp hair with malignant cells.
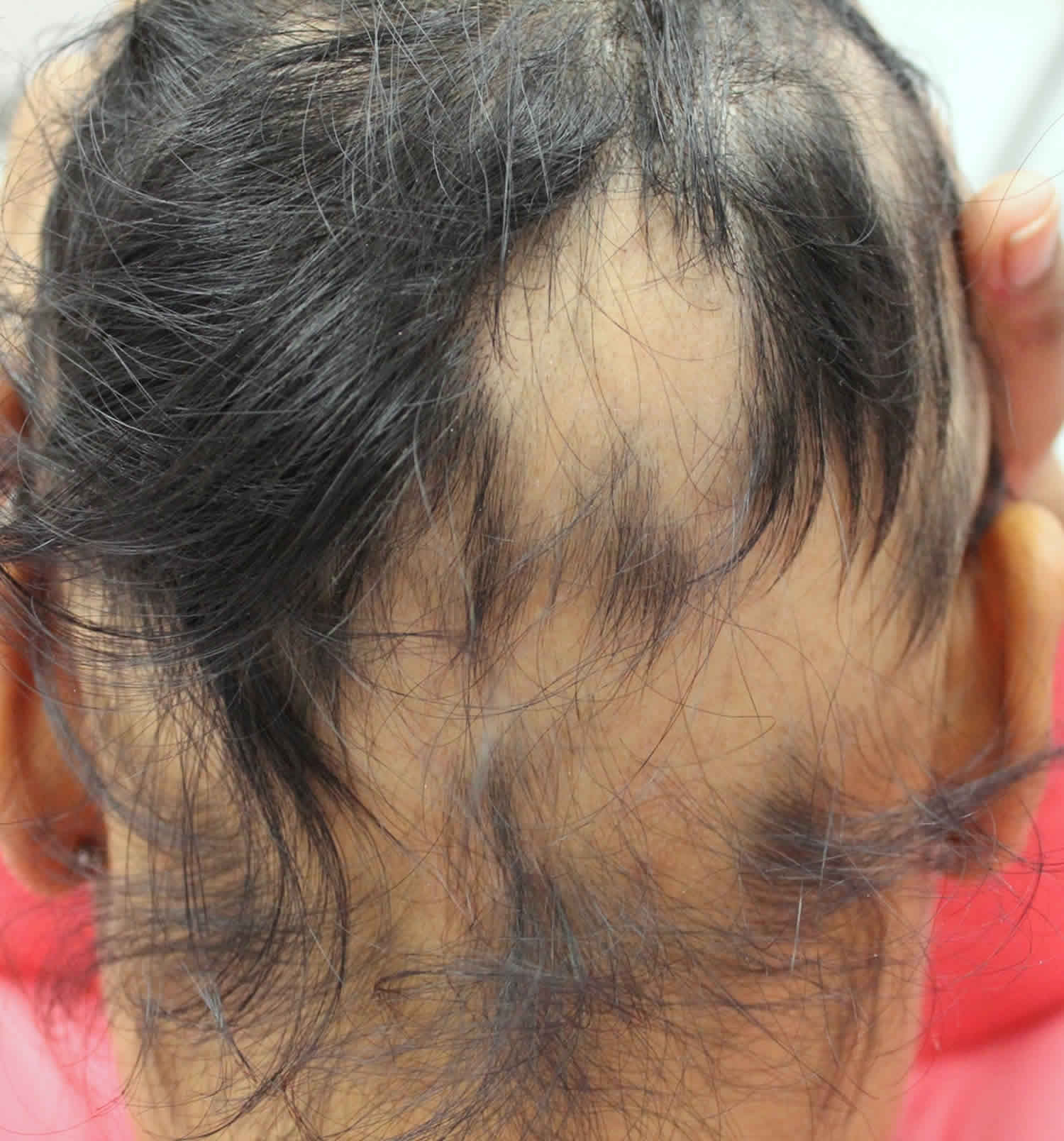
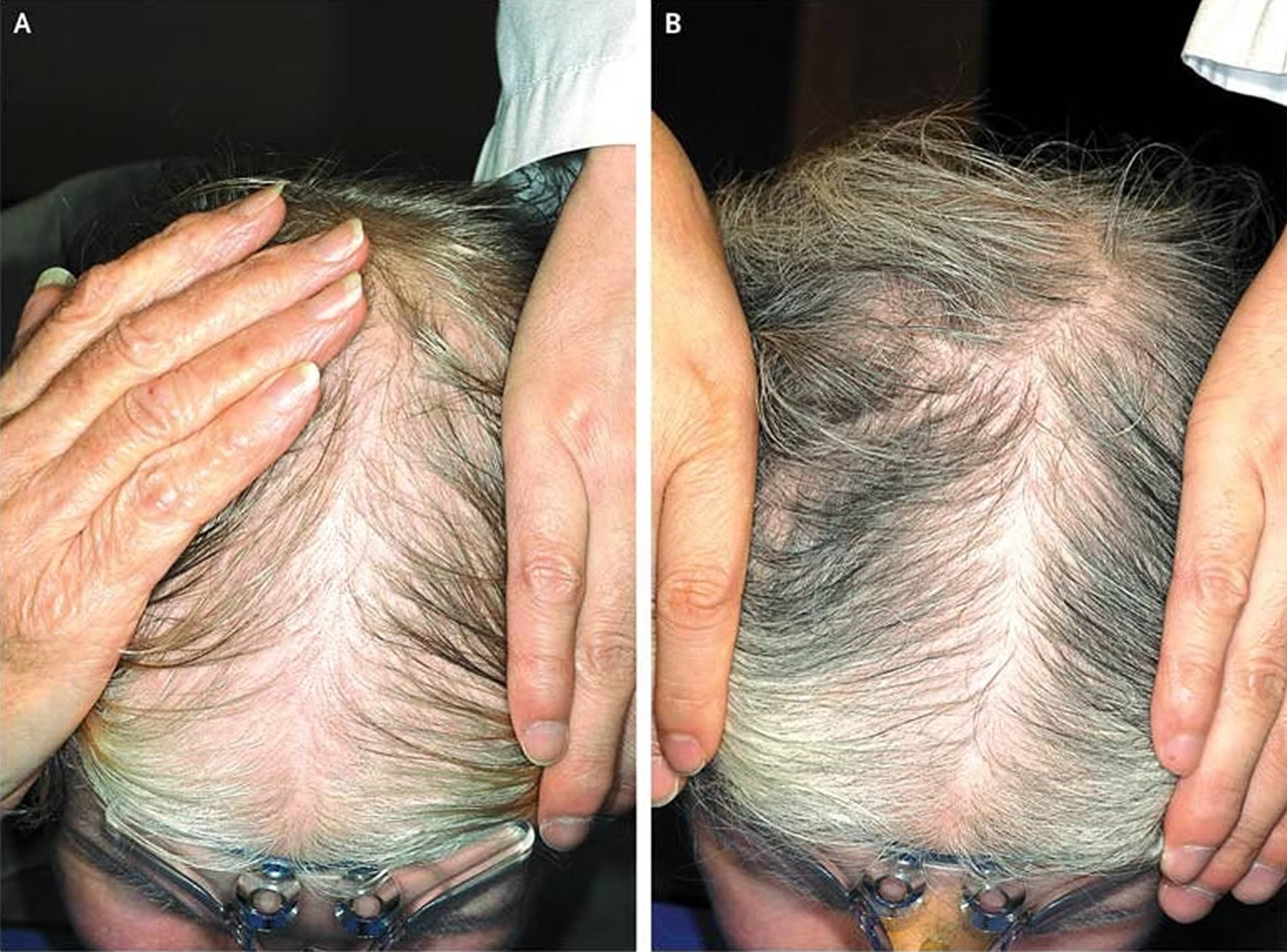
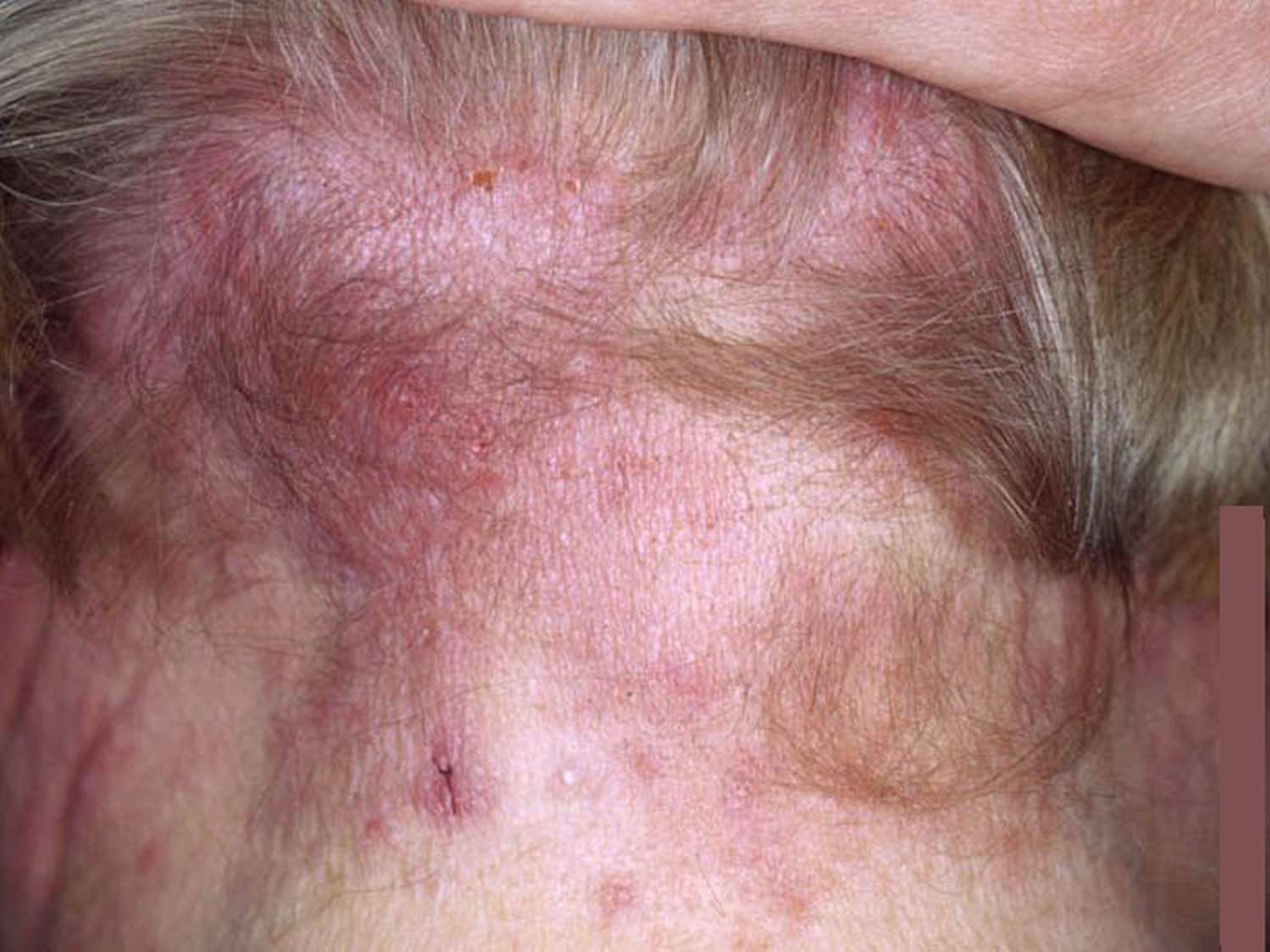
- Frontal fibrosing alopecia. Frontal fibrosing alopecia is a form of scarring hair loss affecting the hair margin on the front of the scalp (i.e. the forehead and sideburns) (Figure 8). This happens due to inflammation and destruction of the hair follicles. There may also be hair loss from the scalp near the ears and from the eyebrows. Sometimes hair loss can also occur from other parts of the body, but this is less common. Frontal fibrosing alopecia occurs mostly in white postmenopausal women but can occur in premenopausal women, men, and people of other ethnicities. Frontal fibrosing alopecia is thought to be a variant of another condition called lichen planopilaris. There are a number of treatments that are used for frontal fibrosing alopecia to help to slow down or halt further hair loss in some people. Unfortunately, their success is variable and some people cannot find a treatment that is effective for them. Treatments used to slow the progression of the condition include oral corticosteroids, intralesional steroid injections, anti-inflammatory antibiotics such as tetracyclines, or anti-malarial tablets (hydroxychloroquine). All these treatments aim to lower the activity of the immune system and slow down the attack on the hair follicles.
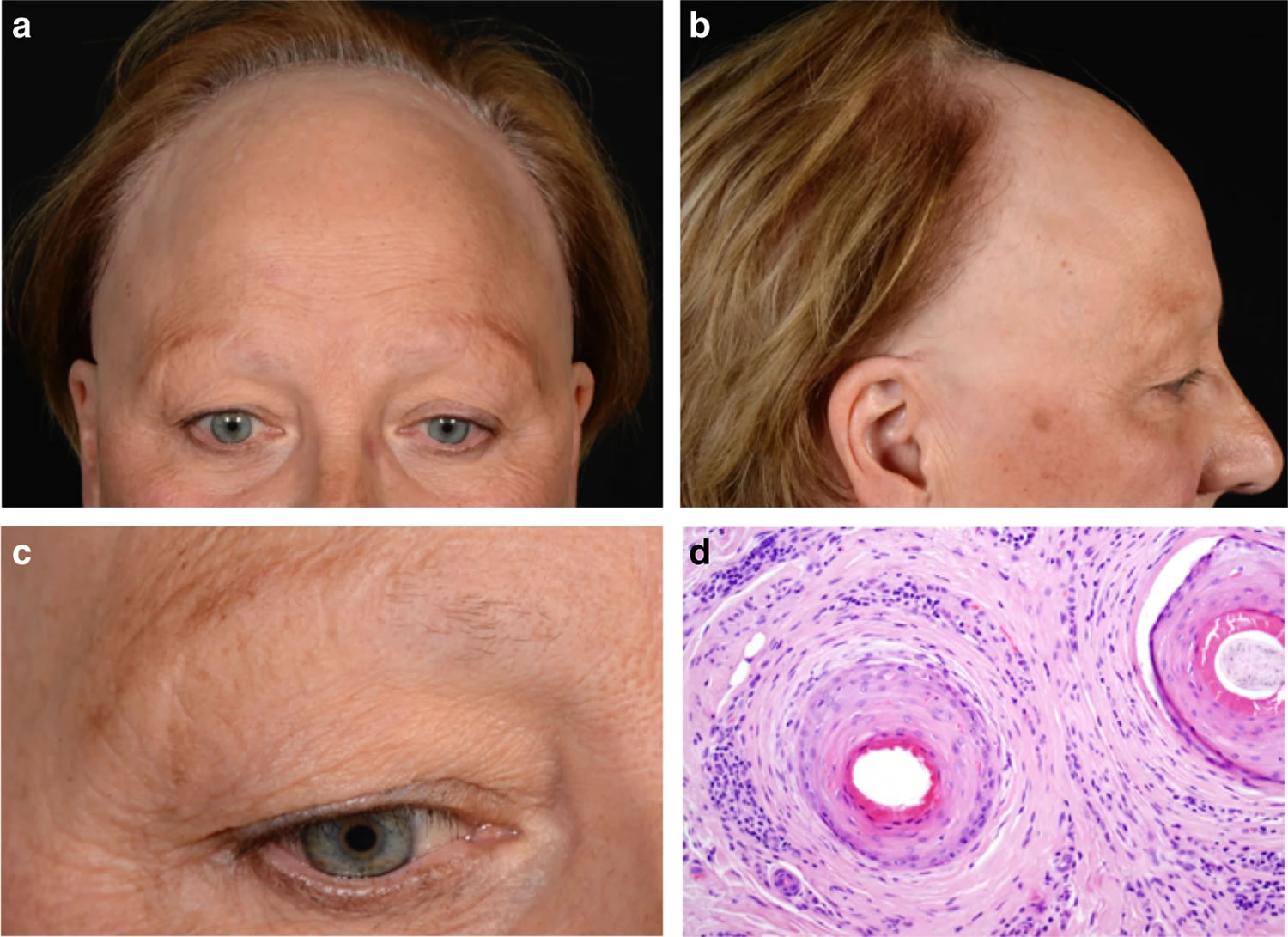
Figure 9. Frontal fibrosing alopecia
Footnote: Clinical features of frontal fibrosing alopecia. Scalp with frontal hairline recession (a) involving the temporal areas bilaterally (b), as well as eyebrows (c). Histopathology (d) shows two hair follicles with focal interface changes, and a moderately dense perifollicular lymphoid cell infiltrate with perifollicular fibrosis, characteristic of frontal fibrosing alopecia.
Alopecia prevention
There is no way to prevent male-pattern baldness or female-pattern baldness (androgenetic alopecia), because it is a genetic trait, meaning you inherited a gene for baldness from your parents. This type of hair loss is not preventable.
Some other causes of excessive hair loss can be prevented. These tips may help you avoid preventable types of hair loss:
- Be gentle with your hair. Use a detangler and avoid tugging when brushing and combing, especially when your hair is wet. A wide-toothed comb might help prevent pulling out hair. Avoid harsh treatments such as hot rollers, curling irons, hot-oil treatments and permanents. Limit the tension on hair from styles that use rubber bands, barrettes and braids.
- Ask your doctor about medications and supplements you take that might cause hair loss.
- Protect your hair from sunlight and other sources of ultraviolet light.
- Stop smoking. Some studies show an association between smoking and baldness in men.
- If you’re being treated with chemotherapy, ask your doctor about a cooling cap. This cap can reduce your risk of losing hair during chemotherapy.
Alopecia symptoms
Alopecia can appear in many different ways, depending on what’s causing it. Hair loss can come on suddenly or gradually and affect just your scalp or your whole body.
Signs and symptoms of hair loss may include:
- Gradual thinning on top of head. This is the most common type of hair loss, affecting people as they age. In men, hair often begins to recede at the hairline on the forehead. Women typically have a broadening of the part in their hair. An increasingly common hair loss pattern in older women is a receding hairline (frontal fibrosing alopecia).
- Circular or patchy bald spots. Some people lose hair in circular or patchy bald spots on the scalp, beard or eyebrows. Your skin may become itchy or painful before the hair falls out.
- Sudden loosening of hair. A physical or emotional shock can cause hair to loosen. Handfuls of hair may come out when combing or washing your hair or even after gentle tugging. This type of hair loss usually causes overall hair thinning but is temporary.
- Full-body hair loss. Some conditions and medical treatments, such as chemotherapy for cancer, can result in the loss of hair all over your body. The hair usually grows back.
- Patches of scaling that spread over the scalp. This is a sign of ringworm. It may be accompanied by broken hair, redness, swelling and, at times, oozing.
Alopecia diagnosis
Before making a diagnosis, your doctor will likely give you a physical exam and ask about your diet, your hair care routine, and your medical and family history. You might also have tests, such as the following:
- Blood test. This might help uncover medical conditions that can cause hair loss.
- Pull test. Your doctor gently pulls several dozen hairs to see how many come out. This helps determine the stage of the shedding process.
- Scalp biopsy. Your doctor scrapes samples from the skin or from a few hairs plucked from the scalp to examine the hair roots under a microscope. This can help determine whether an infection is causing hair loss.
- Light microscopy. Your doctor uses a special instrument to examine hairs trimmed at their bases. Microscopy helps uncover possible disorders of the hair shaft.
Alopecia treatment
Treatment for hair loss depends on the cause. In some cases, treating the underlying cause will correct the problem. With some conditions, such as patchy hair loss (alopecia areata), hair may regrow without treatment within a year. Treatments for hair loss include medications and hair restoration surgery.
Medication
If your hair loss is caused by an underlying disease, treatment for that disease will be necessary. If a certain medication is causing the hair loss, your doctor may advise you to stop using it for a few months.
Medications are available to treat androgenetic alopecia or pattern (hereditary) baldness. The most common options include:
- Minoxidil (Rogaine). Over-the-counter (nonprescription) minoxidil comes in liquid, foam and shampoo forms. To be most effective, apply the product to the scalp skin once daily for women and twice daily for men. Many people prefer the foam applied when the hair is wet. Products with minoxidil help many people regrow their hair or slow the rate of hair loss or both. It’ll take at least six months of treatment to prevent further hair loss and to start hair regrowth. It may take a few more months to tell whether the treatment is working for you. If it is helping, you’ll need to continue using the medicine indefinitely to retain the benefits. Possible side effects include scalp irritation and unwanted hair growth on the adjacent skin of the face and hands.
- Finasteride (Propecia). This is a prescription drug for men. You take it daily as a pill. Many men taking finasteride experience a slowing of hair loss, and some may show new hair growth. It may take a few months to tell whether it’s working for you. You’ll need to keep taking it to retain any benefits. Finasteride may not work as well for men over 60. Rare side effects of finasteride include diminished sex drive and sexual function and an increased risk of prostate cancer. Women who are or may be pregnant need to avoid touching crushed or broken tablets.
- Other oral medications include spironolactone (Carospir, Aldactone) and oral dutasteride (Avodart).
Hair transplant surgery
In the most common type of permanent hair loss, only the top of the head is affected. Hair transplant, or restoration surgery, can make the most of the hair you have left.
During a hair transplant procedure, a dermatologist or cosmetic surgeon removes hair from a part of the head that has hair and transplants it to a bald spot. Each patch of hair has one to several hairs (micrografts and minigrafts). Sometimes a larger strip of skin containing multiple hair groupings is taken. This procedure doesn’t require hospitalization, but it is painful so you’ll be given a sedation medicine to ease any discomfort. Possible risks include bleeding, bruising, swelling and infection. You may need more than one surgery to get the effect you want. Hereditary hair loss will eventually progress despite surgery.
Surgical procedures to treat baldness are not usually covered by insurance.
Laser therapy
The Food and Drug Administration (FDA) has approved a low-level laser device as a treatment for hereditary hair loss in men and women. A few small studies have shown that it improves hair density. More studies are needed to show long-term effects.
Camouflaging hair loss
Scalp
A hairpiece is often the best solution to disguise the presence of hair loss. These cover the whole scalp or only a portion of the scalp, using human or synthetic fibers tied or woven to a fabric base.
- A full wig is a cap that fits over the whole head.
- A partial wig must be clipped or glued to existing hair.
- A hair integration system is a custom-made hair net that provides artificial hair where required, normal hair being pulled through the net.
- Hair additions are fibers glued to existing hair and removed after 8 weeks
Styling products include gels, mousses and sprays to keep hair in place and add volume. They are reapplied after washing or styling the hair.
If your hair loss is due to a medical condition, the cost of a wig might be covered by insurance.
Eyelashes
Artificial eyelashes come as singlets, demilashes and complete sets. They can be trimmed if necessary. The lashes can irritate the eye and eyelids. They are stuck on with methacrylate glue, which can also irritate and sometimes causes contact allergic dermatitis.
Eyeliner tattooing is permanent and should be undertaken by a professional cosmetic tattooist. The color eventually fades and may move slightly from the original site. It is extremely difficult to remove the pigment, should the result turn out to be unsatisfactory.
Eyebrows
Artificial eyebrows are manufactured from synthetic or natural human hair on a net that is glued in place.
Eyebrow pencil can be obtained in a variety of colors made from inorganic pigments.
Tattooing can also be undertaken to disguise the loss of eyebrows, but tends to look rather unnatural because of the shine of hairless skin.
Living with hair loss
Losing your hair can be devastating. Many people consider a thick head of hair a symbol of youth and vitality. So losing it — no matter how young you are — can make you feel old. It can make you feel less attractive. It can lower your overall self-esteem.
Remember that it is okay to feel what you’re feeling. It is also okay to seek out a strategy for stopping or even reversing hair loss. Wanting hair doesn’t mean that you are vain. You should not feel guilty about doing something about your hair loss.
If adequate treatment is not available for your type of hair loss, you may consider trying different hairstyles or wigs, hairpieces, hair weaves or artificial hair replacement.
Alopecia areata
Alopecia areata is also called autoimmune alopecia, is a common autoimmune skin disease, where your body’s immune system attacks your hair cells, causing hair loss on the scalp, face and sometimes on other areas of your body 5. The term “alopecia” means hair loss and “areata” refers to the patchy nature of the hair loss that is typically seen with alopecia areata. Alopecia areata represent an attack on the hair roots by the body’s own immune system. The hair loss can be total (including facial hair such as the eyelashes and eyebrows) or partial, resulting in a bald spot. Any disorder in which the body attacks its own cells is called an autoimmune disorder, and alopecia areata is an example of this kind of disorder.
Alopecia areata affects people of all ages, both sexes and all ethnic groups can develop alopecia areata. Approximately 20% of affected patients are children 22. Alopecia areata affects as many as 6.8 million people in the U.S. with a lifetime risk of 2.1% 23. Alopecia areata affects 0.1–0.2% of the population worldwide 24. Alopecia areata often first appears suddenly during childhood and young adults with one or more round bald patches appear suddenly, most often on the scalp, but it can be different for everyone who has it. Alopecia areata’s manifestations vary, from a well-defined alopecic patch, multiple patches, total scalp alopecia (alopecia totalis), to complete body hair loss (alopecia universalis) 25. The hair loss associated with alopecia areata is not painful or disabling. However, alopecia areata impacts quality of life because it causes changes in a person’s appearance and has major psychological effects for men and women, especially in social acceptance and psychological well-being 26. In some people, alopecia areata can lead to depression, anxiety, and other emotional or psychological issues. The good news is that with alopecia areata, your hair follicles remain alive and hair can regrow at any time and those affected by alopecia areata sometimes experience regrowth of their hair. Hairs that do grow back often lack color or may be either temporarily or permanently white. This hypopigmentation is not seen in other forms of alopecia.
People with mild early alopecia areata may need no treatment, as their hair is likely to come back anyway without it. Some treatments can induce hair growth, though none is able to alter the overall course of the disease. Any treatments that carry serious risks should be avoided, as alopecia areata itself has no adverse effect on physical health. Alopecia areata treatment for adults with less than 50% of scalp involvement is intralesional triamcinolone acetonide injected intradermally using a 0.5-inch, 30-gauge needle 27. Maximal volume is 3 mL per session 28. Treatment may be repeated every four to six weeks until resolution or for a maximum of six months. Local adverse effects include transient atrophy and telangiectasia.
Other therapies for the treatment of alopecia areata include topical mid- to high-potency corticosteroids, minoxidil, anthralin, immunotherapy (squaric acid dibutylester [SADBE] and diphenylcyclopropenone [diphencyprone, DPCP]), and systemic corticosteroids 29. Currently available therapies often yield unsatisfactory results, and some clinicians rely on the high rate of spontaneous remission or recommend a hairpiece or wig if remission does not occur 30.
Key facts
- Alopecia areata is the third most common cause of hair loss. Hair loss occurs over a period of weeks. The hair usually grows back after several months, although it may fall out again. In some cases, unpredictable cycles of hair loss followed by regrowth can last for years. In addition to hair loss, some affected individuals have fingernail and toenail abnormalities, such as pits on the surface of the nails.
- The lifetime risk in the general population is 1.7%.
- Alopecia areata represents a T-cell-mediated immune attack on the hair causing bald spots.
- The target allergen is related to melanin. Thus, patients with both black and white hair can preferentially loose the dark hair.
- About half of patients have onset of the alopecia before 15 years of age.
- About 10% of patients have nail changes including pitting, trachyonychia, and longitudinal ridging.
- A study of treatment with intralesional steroid Kenalog (triamcinolone acetonide) concentration showed 2.5 mg/mL to be just as effective as 5 or 10 mg/mL.
- Many studies have found Vitamin D levels to be lower in alopecia areata patients compared to controls.
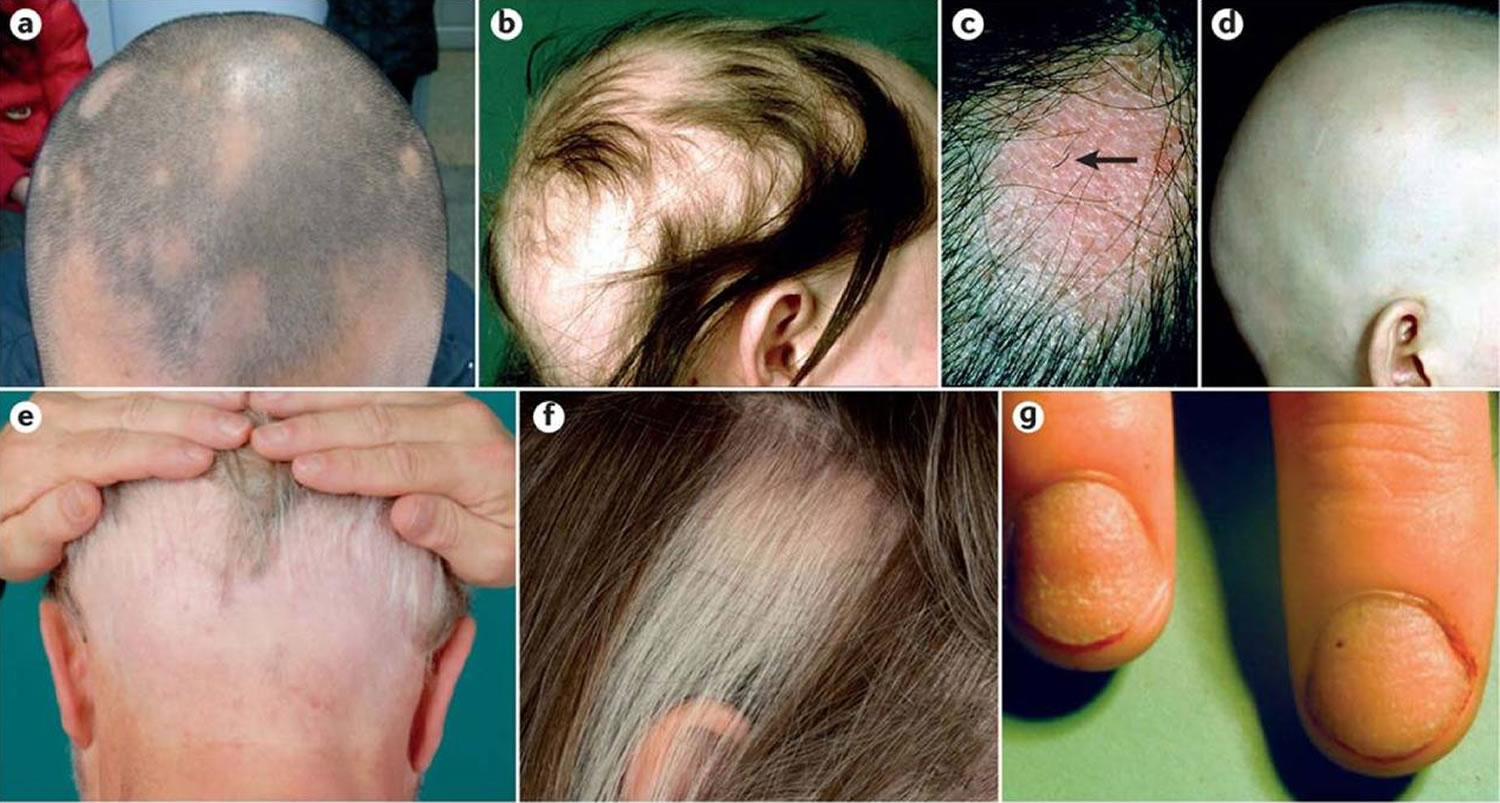
Figure 10. Alopecia areata
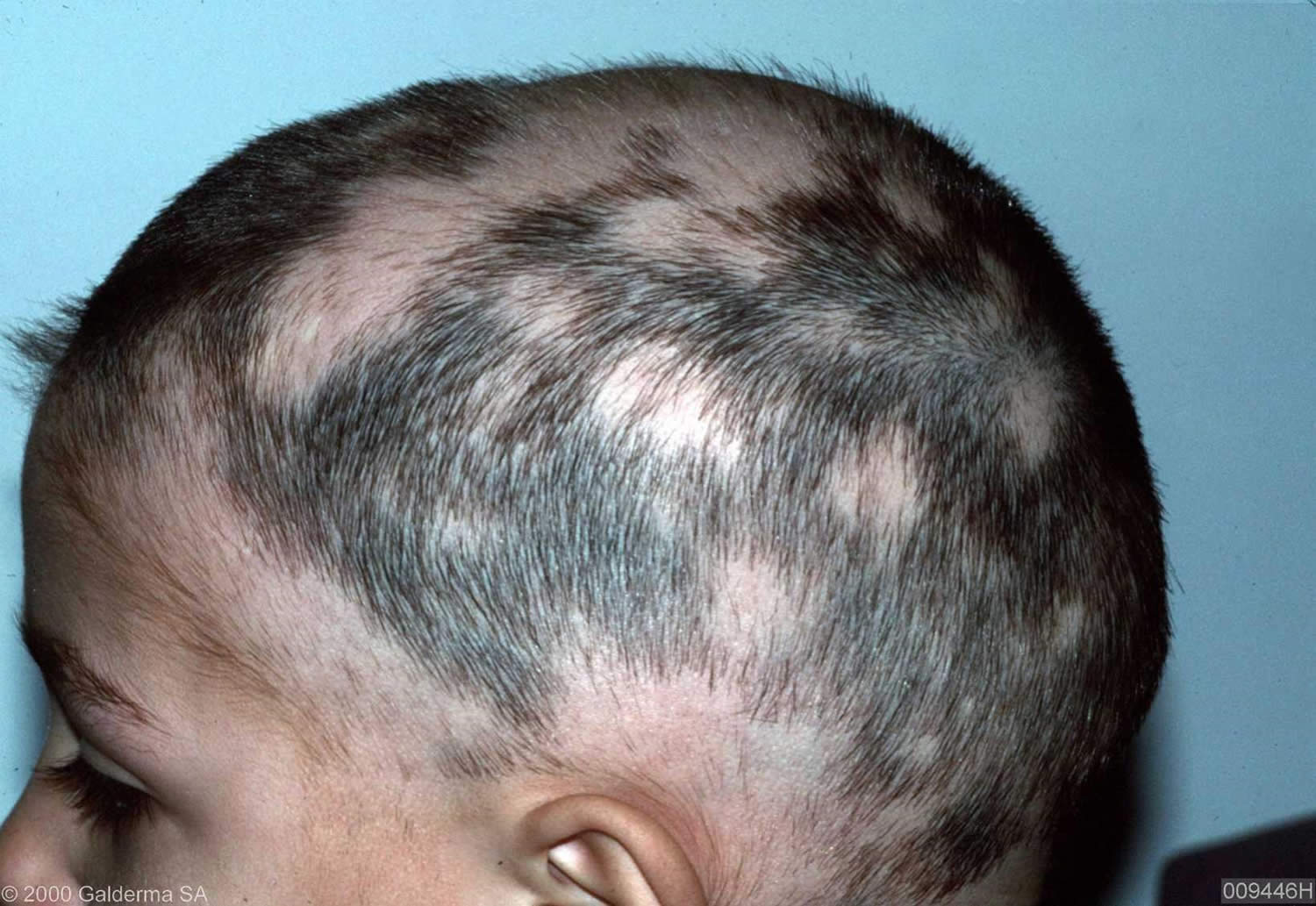
Figure 11. Alopecia areata in children (hair loss involving the eyebrows and eyelashes)
Who gets alopecia areata?
Alopecia areata can affect males and females at any age. Alopecia areata starts in childhood in about 50% of cases and before the age of 40 years in 80%. Lifetime risk is 1–2% and is independent of ethnicity.
- A family history of alopecia areata and/or of other autoimmune conditions is present in 10–25% of patients.
- At least 8 susceptibility genes have been detected.
- Patients with alopecia areata have higher than expected rates of thyroid disease, vitiligo and atopic eczema.
- There is increased prevalence in patients with chromosomal disorders such as Down syndrome.
- It’s possibly drug-induced when arising in patients on biologic medicines.
Is alopecia areata hereditary?
There is a genetic predisposition to alopecia areata. About 20% of people with alopecia areata have a family history. The inheritance pattern of alopecia areata is unclear because multiple genetic and environmental factors appear to be involved. Overall, the risk of developing alopecia areata is greater for first-degree relatives (such as siblings or children) of affected individuals than it is in the general population. People with alopecia areata are also more likely to have family members with other autoimmune disorders.
Can alopecia areata be cured?
No, alopecia areata cannot be cured. Depending on the extent of hair loss there is a good chance that, for 4 out of 5 affected people, complete regrowth will occur within 1 year without treatment. There may, however, be further episodes of hair loss in the future. If there is very extensive hair loss from the start, the chances of it regrowing are not as good. Those with more than half the hair lost at the beginning or with complete hair loss at any stage have only about a 1 in 10 chance of full recovery. The chances of regrowth are not so good in young children and those with the condition affecting the hairline at the front, side or back.
Types of alopecia areata
Alopecia areata most commonly begins as isolated patchy hair loss, usually in one or more coin-sized (usually round or oval) patches on the scalp or other places on the body that grow hair — such as the beard, eyebrows, eyelashes or extremities (arms, legs, hands and feet).
- Patchy alopecia areata: Patchy alopecia areata is the form with one or more coin-sized (usually round or oval) patches on the scalp or other places on the body that grow hair. This type may convert into either alopecia totalis (hair loss across the entire scalp) or alopecia universalis (hair loss across the entire body), but most commonly it remains patchy.
- Persistent patchy alopecia areata: Persistent patchy alopecia areata is characterized by patchy scalp hair loss that continues over a long period of time without ever developing into extensive alopecia areata such as totalis or universalis.
- Alopecia totalis: total or near-total loss of hair on the scalp.
- Alopecia universalis: total to near-total loss of hair on all haired surfaces of the body. Alopecia universalis results in hair loss across the entire scalp and face (including eyebrows and eyelashes), plus the rest of the body (including pubic hair).
- Alopecia incognita: diffuse total hair loss with positive pull test, yellow dots, short, miniaturized regrowing hairs, but without nail involvement.
- Diffuse alopecia areata: Diffuse alopecia areata results in sudden and unexpected thinning of the hair all over the scalp. It can be hard to diagnose because it looks a lot like other forms of hair loss such as telogen effluvium or male or female pattern hair loss.
- Ophiasis: hair loss in a band-like shape along the circumference of the head, more specifically along the border of the temporal and occipital bones. Ophiasis alopecia areata can be more difficult to treat, because it does not respond as quickly to medication.
- Sisaipho: extensive alopecia except around the periphery of the scalp.
- Marie Antoinette syndrome also called canities subita: acute episode of diffuse alopecia with very sudden “overnight” greying with preferential loss of pigmented hair 31.
What does alopecia areata look like?
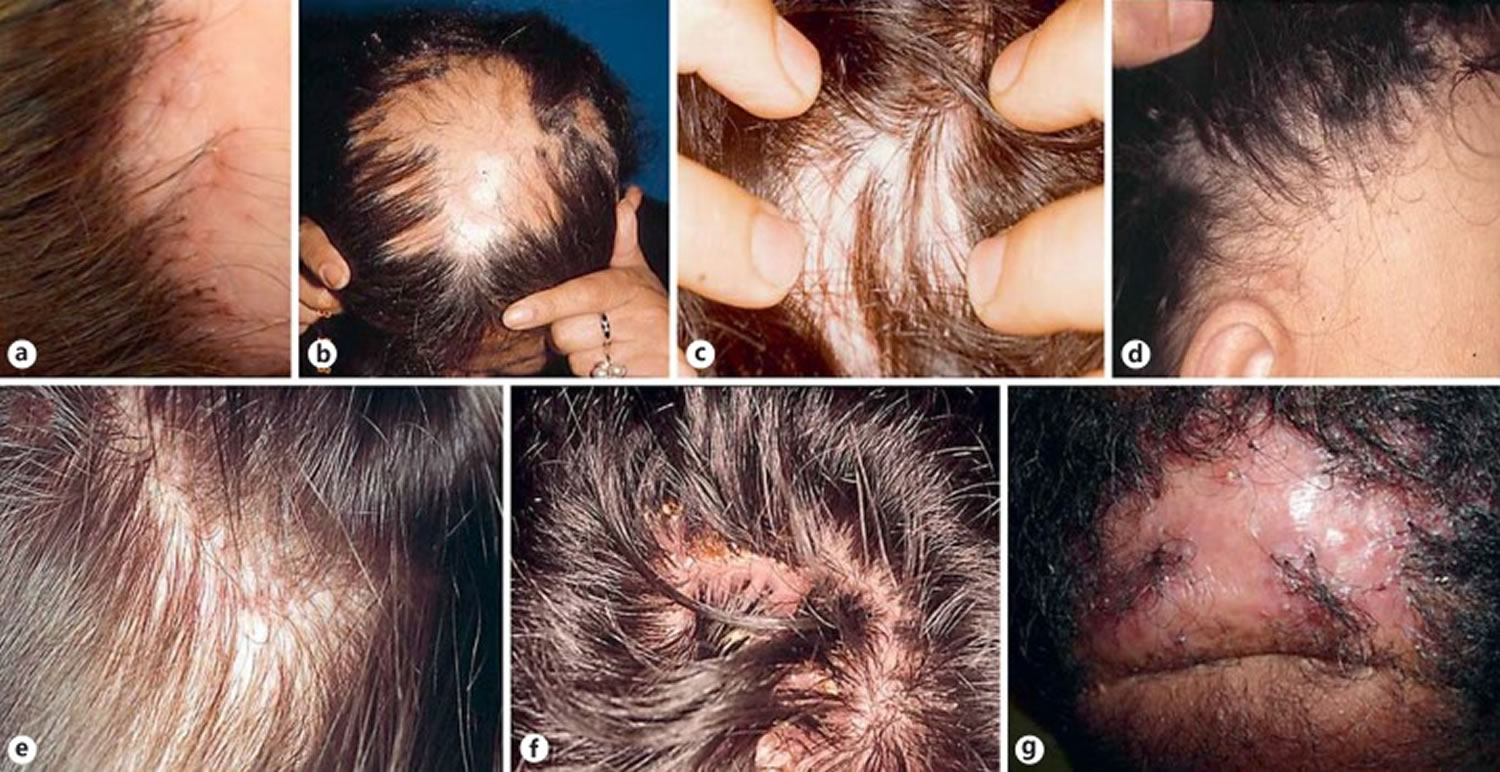
Typically, alopecia areata starts as one or more bald, smooth patches on the scalp, which are not inflamed or scaly. Alopecia areata tends to affect the pigmented hair so there may be some white hairs left within the bald area in older people. Sometimes the hair loss is diffused rather than well-circumscribed patches. Short, tapered hairs, known as exclamation mark hairs that are characteristic of alopecia areata, may be seen at the edge of the bald patch. Regrowth usually starts at the centre of the bald patch with fine white hair that thickens with time and usually regains its color. Some people with alopecia areata develop small pits on their nails, similar to the dimples seen on a thimble.
Figure 12. Clinical manifestations of alopecia areata
Footnotes: a) Limited patchy alopecia areata (<50% scalp involvement). b) Extensive patchy alopecia areata (>50% scalp involvement). c) Active patch of alopecia areata showing exclamation point hairs (arrow) and slight skin erythema. d) Alopecia universalis. e) Ophiasis pattern of alopecia areata. f) Sparing of white hairs in alopecia areata. g) Nail pitting and longitudinal striations (trachyonychia) associated with alopecia areata.
[Source 5 ]Alopecia areata causes
Alopecia areata is classified as an autoimmune disorder with a genetic predisposition, progressing from disruption of immune privilege of hair follicles 5. Emerging evidence suggests that a collapse in hair follicle immune privilege is the leading cause of alopecia areata 32. When this process develops, hair follicles present surface autoantigens, resulting in inflammatory cells attacking hair follicles and eventually resulting in an alopecic patch. Other factors such as genetics, stress, and environment are also responsible for development of alopecia areata 33.
A 55% concordance rate between identical twins has also been observed 34. Recent genome-wide association studies metanalysis have localized the human leukocyte antigen (HLA) signal of alopecia areata mostly to the HLA-DRB1 34. One locus harboring the genes that encode the natural killer cell receptor D (NKG2D) was implicated in alopecia areata and not in other autoimmune diseases, which suggests a key role in pathogenesis. Therefore, CD8+ NKG2D T cells have been a subject of study and found to be the major effectors in alopecia areata 5.
Alopecia areata is histologically characterized by T (lymphocyte) cells around the hair follicles. These CD8(+)NK group 2D-positive (NKG2D(+)) T cells release pro-inflammatory cytokines and chemokines that reject the hair. The exact mechanism is not yet understood.
Immune privilege protects hair follicle components from immune attacks by various mechanisms. Physical barriers, including the extracellular matrix, have reduced lymphatic permeability and guard hair bulbs against infiltrating immune cells 35. It also downregulates major histocompatibility complex (MHC) class I expression and MHC class I pathway molecules (β2-microglobulin and transporter associated with antigen processing [TAP-2]). Downregulation of MHC class I is caused by the local production of immunosuppressive factors, such as α-melanocyte-stimulating hormone (α-MSH), transforming growth factor-β (TGF-β), indoleamine‐2,3‐dioxygenase (IDO), protein red encoded by IK gene (red/IK), interleukin (IL)‐10, calcitonin gene‐related peptide, insulin‐like growth factor‐1, and somatostatin 36. Reduction of MHC class II expression on hair follicle Langerhans cells impairs antigen-presenting cell (APC) function 37. In addition, immune privilege expresses “no danger” signals using type-1 transmembrane glycoprotein CD200, which lowers antigen-presenting cell (APC) activity and pro-inflammatory cytokines secretion 38.
The immune privilege environment normally suppresses natural killer (NK) cell activation by downregulating MHC class I chain-related gene A (MICA) and UL16-binding protein (ULBP) in resident immune cells. These would otherwise bind to NKG2D‐activating receptors on CD8+ T cells and NK cells inducing inflammation and damaging local tissues. Supporting evidence shows that there are few perifollicular NK cells in healthy hair follicles 39. Next, killer cell Ig-like receptors, which are MHC class I inhibitory receptors, were significantly higher in controls than in alopecia areata patients 40. Killer cell Ig-like receptors help NK cells distinguish between normal cells and target cells, which prevents damage to healthy cells. Lastly, macrophage migration inhibitory factor, a pleiotropic cytokine presented in several immune privilege sites, prevents the release of cytolytic perforin granules from NK cells 41.
The onset or recurrence of hair loss is sometimes triggered by:
- Viral infection
- Trauma
- Hormonal change
- Emotional/physical stressors
Moreover, sporadic cases of alopecia areata developing during anti-TNF-α therapy have been reported 42. One third of the cases had a positive (personal or family) history of alopecia areata. Most of presented with rapid extensive alopecia areata, usually involving the ophiasis area. Prognosis was usually poor, with slight response to treatments. In the cases where anti-TNF-α therapy was maintained, the course did not seem to change.
Table 1. Alopecia areata associations
| Age of Onset | Associated Disease |
|---|---|
| < 10 years | atopic dermatitis, lupus |
| 11-20 years | psoriasis, rheumatoid arthritis |
| 21-60 years | atopic and autoimmune diseases |
| > 60 years | thyroid disease |
| All ages | anxiety, depression, and obsessive compulsive disorders |
Alopecia areata genetic factors
Several lines of evidence support the notion that alopecia areata has a genetic basis. In general, the prevalence of adult patients with a family history is estimated to be between 0% and 8.6%2 43, whereas in children data between 10% and 51.6% are reported 44. One study found that men were more likely to have a positive family history than women 45. The occurrence of alopecia areata in identical twins 46, siblings 47 and families with several generations of affected individuals 48 indicates that this alopecia areata has a heritable basis. Most of the early human genetic studies were candidate gene association studies, in which linkage to specific genes or groups of genes was the focus. These studies focused on the human leukocyte antigen class II (HLA-D) region on human chromosome 6 as the most likely region for genes that regulate susceptibility or resistance to alopecia areata 49. Family-based linkage studies and genome-wide association studies (GWAS) analyses, which were greatly enabled by the repository of the National Alopecia Areata Registry, identified linkage or association on many chromosomes, which suggests that alopecia areata is a very complex polygenic disease 50. These results confirmed earlier quantitative trait locus (QTL) analysis studies using an alopecia areata mouse model, often with similar, if not identical results 51.
Alopecia areata pathophysiology
The pathomechanism of alopecia areata involves the complex interaction between innate and adaptive immunities 52. The hair follicle immune privilege environment is highly regulated and usually prevents autoimmune hair loss. Emerging evidence suggests that the collapse of hair follicle immune privilege contributes to alopecia areata pathogenesis. MHC class I and class II expression on the hair matrix and follicular epithelium are found in alopecia areata-affected patients 37. The local production of immunosuppressive factors including α-MSH, TGF-β, IDO, and red/IK are downregulated in peri-lesional and lesional alopecia areata 53. Histological features from alopecia areata patient scalp biopsies showed infiltrating peri-follicular CD4+ T cells, intrafollicular CD8+T cells 5, mast cells 54, NK cells 40 and APCs.28 MICA immunoreactivity occurred throughout alopecia areata-affected hair follicles, which activated NKG2D+ NK cells and CD8+ T cells around alopecia areata lesions, but not in normal hair follicles 55.
Recent hypothesis of the disease mechanism focuses on the collapse of immune privilege of hair follicles and autoreactive lymphocytes. Under normal conditions, hair follicles form an area where autoantigens cannot be recognized due to the lack of major histocompatibility complex (MHC) in the proximal outer root sheath and matrix cells 56. In alopecia areata, the immune privilege is disrupted by specific triggers, such as microtrauma, viral infection, or endocrine dysfunction, resulting in immune dysregulation 53. Furthermore, ectopic expression of MHC class I, recognized by autoreactive CD8+ T cells, could directly and adversely affect anagen hair follicles, leading to follicular apoptosis 53.
Several studies have demonstrated the role of inflammatory cytokines, especially Th1-mediated cytokines, in the occurrence of alopecia areata via two possible mechanisms, either activation of the CD8+ T cell pathway or induction of cessation of hair growth cycle. Interferon (IFN)-γ, the hallmark cytokine of Th1-mediated pathway, is regarded as a key cytokine in alopecia areata 56. A large amount of IFN-γ is produced by autoactivated CD8+ cells and antigen presenting cells (APCs) after initial inflammatory insult on hair follicles 57, resulting in further upregulation of MHC class I and II molecules in the bulb of hair follicles and activation of CD8+ and CD4+ T cells 58. Serum from patients has been reported to contain a higher level of IFN-γ, interleukin (IL)-2, IL-12, and IL-18 compared to that from control subjects 59. Serum levels of IFN-γ tend to be elevated with disease severity 60. IL-1B, IL-2, and IL-6 are also present in human scalp lesions 61. Furthermore, the Th17 pathway may be involved in disease development by collaboration with the Th1 pathway via IL-17A and IL-17F 62.
Recent studies have reported significantly increased serum levels of Th2 cytokines, such as IL-4 and IL-10, which were suspected to be critical players in disease suppression. Serum level of IL-4 was found to be higher in patients with patch-type alopecia areata, a mild form of the disease, than in those with other subtypes 63. Apart from the Th2-mediated pathway, regulatory T cells are also responsible for the suppression of exaggerated Th1- and Th2-related inflammation via TGF-β and IL-10 60. Without a definite conclusion about its mechanism, the majority of the studies have shown no significant difference in regulatory cytokines between patients with alopecia areata and normal controls 64.
Alopecia areata triggers
In the majority of cases, no obvious explanation for the onset of an episode of alopecia areata can be found, but a variety of trigger factors have been proposed. The most commonly reported is emotional or physical stress, such as following bereavement or injury 65. Others include vaccinations, febrile illness, and drugs. A low frequency of alopecia areata was reported to arise shortly after vaccinations against a variety of human pathogens including Japanese encephalitis 66, hepatitis B virus 67, Clostridium tetani 68, herpes zoster virus 69 and papillomavirus 70. By contrast, one report showed that alopecia areata was triggered or exacerbated by swine flu virus infection 71. However, hepatitis B vaccine trials using large numbers of C3H/HeJ spontaneous, adult-onset mouse model of alopecia areata, in which diphtheria and tetanus toxoids were added as controls, suggested that alopecia areata associated with vaccination was in the normal, predicted incidence range 72.
Alopecia areata prevention
Scientists do not yet know how to prevent the onset of alopecia areata.
Alopecia areata prognosis
In 80% of patients with a single bald patch, spontaneous regrowth occurs within a year. Even in the most severe cases of alopecia totalis and alopecia universalis, recovery may occur at some future date. This is an important difference between alopecia areata and the scarring forms of alopecia, which destroy the hair follicle and result in irreversible hair loss. Referral centers indicate that 34–50% of patients will recover spontaneously within 1 year, although most will experience multiple episodes of the alopecia, and 14–25% of patients will progress to alopecia totalis or alopecia universalis, from which full recovery is unusual (<10% of patients) 7. One Japanese study reported spontaneous remission in 80% of patients within 1 year for those with a small number of circumscribed patches of hair loss 73. The best indication for a prognosis is the extent of hair loss when first diagnosed 7. A less favorable prognosis is observed with childhood onset alopecia areata and ophiasis 74. A later age of onset correlates with less extensive alopecia 25. Severe alopecia areata (alopecia totalis and alopecia universalis) usually occurs before 30 years of age 25 and is often associated with nail dystrophy (trachyonychia) 75.
Research has shown:
- 40% of patients with a single patch of hair loss have full hair regrowth within 6 months.
- 27% of patients with multiple patches of hair loss have full regrowth within 12 months.
- 33% of patients with alopecia areata have chronic hair loss.
Poor prognostic factors include:
- Extensive disease
- Bald patches persisting for more than 1 year
- Ophiasis pattern of hair loss
- Alopecia areata of the nails
- Onset of alopecia areata before puberty
- Family members with alopecia areata
- Personal or family history of other autoimmune diseases
- Down syndrome
New monoclonal antibody biologic agents targeting cytokine pathways offer promise for future treatment of alopecia areata.
Alopecia areata symptoms
Hair loss most commonly occurs on the scalp, but it can also target the eyebrows, eyelashes, beard, and other body sites. Symptoms may include the following:
- Round, patchy areas of non-scarring hair loss, ranging from mild to severe
- Mild: 1–5 scattered areas of hair loss on the scalp and beard
- Moderate: More than 5 scattered areas of hair loss on the scalp and beard
- Severe: loss of all of the hair on the scalp and body
- Scalp burning (without redness), accompanying lesions
- Pitting and ridging of the fingernails
Several clinical patterns are described. More severe disease is associated with young age, concurrent atopic eczema, and chromosomal abnormalities.
Most patients have no symptoms, and a bald patch or thinning hair is noted incidentally, often discovered by a hairdresser. Other patients describe a burning, prickly discomfort in the affected areas—this is known as trichodynia.
Alopecia areata typically presents with round, bald spots on the scalp. The beard, eyebrows, or eyelashes may be affected but it is unusual for isolated lesions elsewhere on the body. One or more may be present at any one time. The exclamation point hair is characteristic and appears as a short terminal hair, tapered at the proximal end. There is no scarring, scale, or other alteration of the scalp skin.
The follicular openings are not lost in contrast to a scarring alopecia.
Patients may rarely go on to lose extensive amounts of hair of the scalp and body, but again, this is unusual. Most patients regrow their hair. The term alopecia totalis refers to patients who have lost all of their scalp hair. The term alopecia universalis refers to patients who have lost all hair on the scalp and body. All nasal hair may be lost and this can lead to increased nasal inflammation and irritation.
A new subtype of alopecia areata called “acute diffuse and total alopecia of the female scalp” has been described where the woman suffers complete hair loss within one month of presentation. The histology is that of alopecia areata except for a significant eosinophilic tissue infiltrate. Fortunately, the vast majority of these women do well with cosmetically acceptable hair regrowth at six months with or without steroid administration.
Patchy alopecia areata
Patch alopecia areata can affect any hair-bearing area, most often the scalp, eyebrows, eyelashes and beard.
Patchy alopecia areata has three stages:
- Sudden loss of hair
- Enlargement of bald patch or patches
- Regrowth of hair
The bald areas may have a smooth surface, completely devoid of hair or with scattered “exclamation mark” hairs.
- Exclamation mark hairs are 2- to 3-mm in length, broken or tapered, with a club-shaped root. Microscopy shows a thin proximal shaft and normal caliber distal shaft.
- Regrowing hairs are often initially colored white or grey; they may be curly when previously straight.
- It may take months and sometimes years to regrow all the hair.
- One patch can be falling out while another is regrowing.
Alopecia totalis
- Affects up to 5% of patients with autoimmune hair loss
- All or nearly all scalp hair is lost
Alopecia universalis
- Affects less than 1% of cases
- All hair or nearly all hair on the entire body is lost
Ophiasis
- Pattern of alopecia areata affecting occipital and lateral scalp
- Bald area can encircle scalp
Diffuse alopecia areata
- Sometimes called alopecia areata incognita
- Presents with sudden diffuse thinning of scalp hair
- Persisting hair tends to grey, thus descriptions of ‘turning white overnight’
- Positive hair pull test
- May be confused with telogen effluvium or hair loss due to medications
Alopecia areata of the nails
- Nail disease affects 10–50% of those with alopecia areata
- Regular pitting and ridging are the most common findings
- May also cause koilonychia, trachyonychia, Beau lines, onychorrhexis, onychomadesis, onycholysis and red spots on the lunula
Figure 13. Alopecia areata of fingernails (nail pitting)
Alopecia areata complications
Alopecia areata patients are at risk for psychosocial consequences of their disease, such as depression and anxiety. Psychological support may be beneficial.
Alopecia areata patients should be assessed for atopy, vitiligo, thyroid disease, and other autoimmune conditions.
Alopecia areata is associated with several concurrent diseases (comorbidities) including depression, anxiety, and several autoimmune diseases including thyroid disease (hyperthyroidism, hypothyroidism, goiter ant thyroiditis), lupus erythematosus, vitiligo, psoriasis, rheumatoid arthritis and inflammatory bowel disease 65. The frequency of these concurrent diseases varies between geographically separate populations, which may suggest genetic variability within these different populations. A retrospective study in Taiwan found that patients with alopecia areata had higher hazard ratios for autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus and psoriasis within the 3-year follow-up period than healthy controls 76. In addition, increased prevalence of other forms of inflammatory skin disease such as atopic dermatitis, vitiligo, psoriasis and lichen planus were found than in controls, suggesting that patients with alopecia areata are at increased risk of developing variety of T-cell driven inflammatory skin diseases 77. Severe alopecia areata might be accompanied by nail changes 78. Atopic diseases, such as sinusitis, asthma, rhinitis, and especially atopic dermatitis, are also more common than expected in populations with alopecia areata 79 and are associated with early-onset and more severe forms of hair loss. In a Korean population, atopic dermatitis was significantly more common in patients with early-onset alopecia areata, whereas thyroid disease was the most common in late-onset disease 80; findings were similar in Sri Lanka 78. In a review of 17 studies, investigators found higher odds of atopic dermatitis in patients with alopecia totalis or alopecia universalis compared to those with patchy alopecia areata 80. In a large-scale epidemiological study in Taiwan investigators found a correlation between prior herpes zoster outbreaks with alopecia exposure within 3 years, suggesting that stress might trigger alopecia areata 81. Several studies, with and without controls, demonstrated a high prevalence of thyroid autoimmunity associated with alopecia areata 82, whereas others found lower frequencies than in earlier studies, indicating that there is no need for detailed investigations into these diseases without a clinical history to suggest they are present 83.
Alopecia areata diagnosis
Alopecia areata is diagnosed clinically. Although usually straightforward, additional tests are sometimes needed to confirm the diagnosis.
- Trichoscopy (use of a dermatoscope to examine hair and scalp)
- Skin biopsy (histopathology)
Alopecia areata treatment
There is not yet any reliable cure for alopecia areata and other forms of autoimmune hair loss. Because spontaneous regrowth is common in alopecia areata, and research has often been of poor quality, the effectiveness of reported treatments is mostly unknown.
- Observation
- Intralesional injection triamcinolone 2.5 mg/mL monthly
- Hydroxychloroquine
- Methotrexate or azathioprine
- Topical immunotherapy with diphenylcyclopropenone (DPCP) and anthralin
- Fexofenadine
- Measure Vitamin D and supplement if low.
Systemic therapy is reserved for patients with:
- More than 20% of scalp hair loss
- Rapid hair loss
- Chronic hair loss
- Severe distress.
Alopecia areata need not be treated as it is a benign condition and regrowth is typical. In fact, spontaneous remission occurs in up to 80% of patients with limited disease within a year. However, alopecia areata often causes great embarrassment and thus therapy is often desired to speed regrowth.
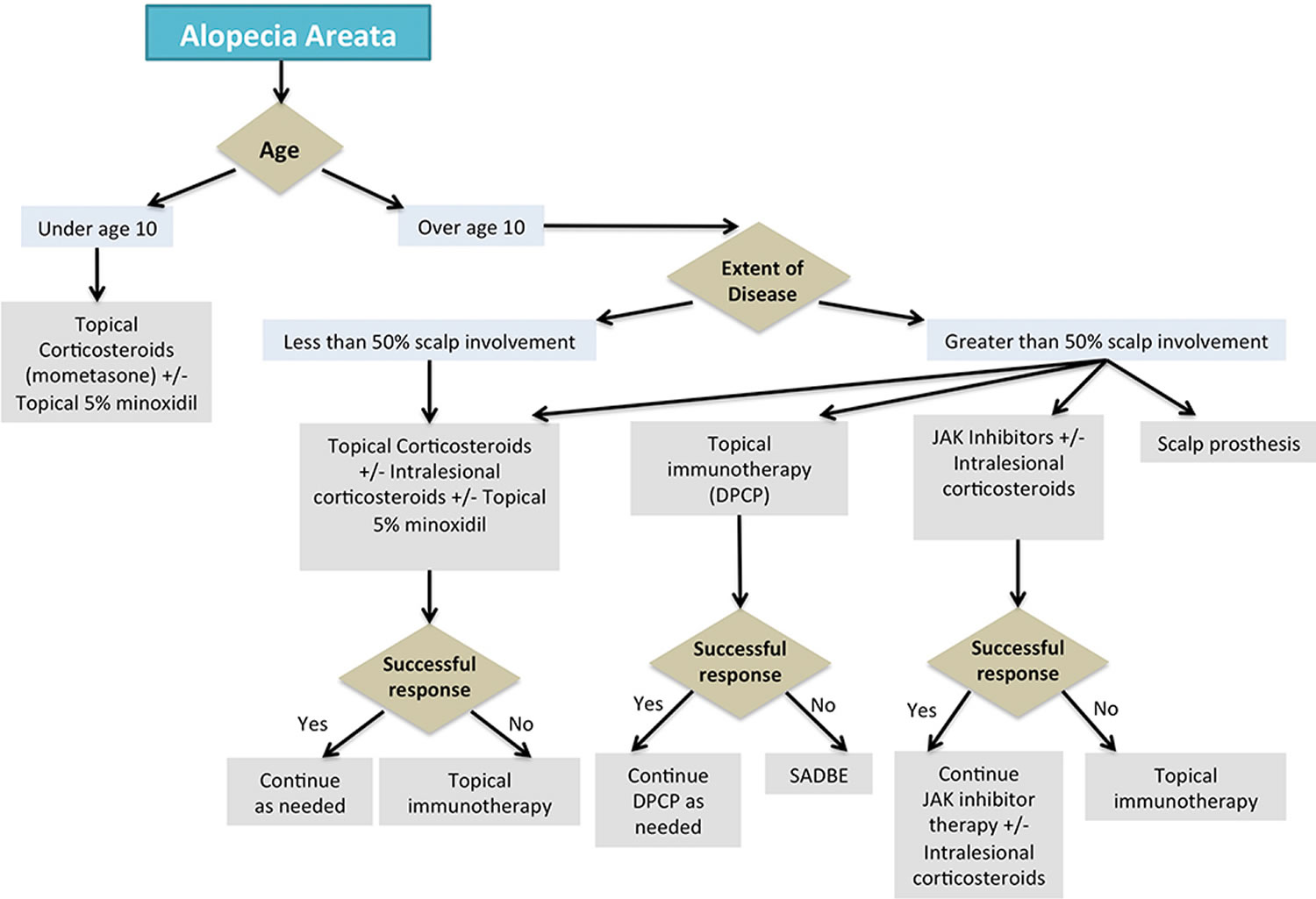
Figure 14. Alopecia areata treatment algorithm
[Source 84 ]Intralesional steroids, first-line
Individual lesions may be injected with Kenalog (triamcinolone acetonide) every month. This usually induces hair regrowth for isolated areas although atrophy of the skin may occur. The injection should be done into the deep dermal/upper subcutaneous plane using a 0.5-inch, 30-gauge needle 27. One may cover several square centimeters of skin with one injection point by fanning out in various directions with the needle and injecting while withdrawing. This helps distribute the medication more evenly than multiple small injection points. The maximum dose often cited is 20 mg per month, e.g., 8 mL of 2.5 mg/mL 28. In a double blind placebo controlled clinical trial comparing injection with 2.5, 5, and 10 mg/mL in alopecia areata of < 50% of the scalp, the 2.5 mg/mL concentration was just as effective as the 5 and 10 85. Treatment may be repeated every four to six weeks until resolution or for a maximum of six months. Local adverse effects include transient atrophy and telangiectasia.
Vitamin D
Deficient serum 25(OH)D (calcidiol or 25-hydroxyvitamin D) levels are present in patients with alopecia areata and inversely correlate with disease severity. Thus, screening patients and supplementing, if indicated, seems prudent 86. It would also be reasonable to screen patients for iron and zinc deficiency and supplement as appropriate 87.
Fexofenadine
Some use fexofenadine 120-180 mg/day for adults either as monotherapy or as an adjunct for other therapies. In a retrospective study of extensive alopecia treated with contact immunotherapy, the mean regrowth score of the fexofenadine group was 1.33 and that of the control 0.47 88.
Topical treatments
Several topical treatments used for alopecia areata are reported to result in temporary improvement in some people. Their role and efficacy are unknown. The hair may fall out when they are stopped. These include 29:
- Potent or ultrapotent topical steroids
- Minoxidil solution or foam
- Dithranol (anthralin) ointment
- Immunotherapy (diphenylcyclopropenone, squaric acid dibutylester)
Any alopecia areata patient may benefit from a class 1 topical steroid for several months, although the area should be monitored for the development of atrophy. For example, topical clobetasol (given as a foam) grew modestly more hair than placebo in a double blind placebo controlled clinical trial.
Steroid creams and scalp applications
Potent or ultrapotent topical steroids are applied to the bald patches, usually twice a day, for a limited time.
Topical Minoxidil
Minoxidil 5% is recommended by many, but its use is off-label in the US. In one study, topical minoxidil applied twice daily and nightly occluded with petrolatum for 1 year outperformed placebo modestly, but the hair is often fine and not of much use.
Topical Bimatoprost
A study in which 30 patients applied mometasone cream once daily or bimatoprost 0.03% solution (Lumigan, Allergan, 3 ml) twice daily to two separate patches of alopecia areata found that bimatoprost grew hair sooner and better than mometasone 89.
Dithranol cream
Dithranol cream which is usually used to treat another skin condition called psoriasis, causes irritation of the skin, and occasionally this appears to stimulate the hair to regrow when applied to the bald areas. There is only weak evidence for this but it is safe to use so doctors may offer it. Dithranol stains the skin and hair a purple-brown colour, which is particularly prominent in blond and fair-headed people.
Alopecia of Eyebrows
- Intralesional steroid injection.
- Topical Bimatoprost.
Intralesional injection of steroid may be done by an ophthalmologist. Forty-one subjects with alopecia areata universalis without ocular disease applied 0.03% bimatoprost to the eyelid margin once a day over the course of 1 year 90. 43% of patients had moderate or total regrowth.
One may use intralesional triamcinolone 2.5 mg/mL every month, but the patient must accept the rare risk of ocular complications. For example, intralesional Kenalog (triamcinolone acetonide) 40 mg for vitiligo of the forehead (a much higher dose than would be used for alopecia areata) caused immediate stroke and blindness in a 15-year-old boy 91. One patient who had not had eyebrows for 20 years developed reasonable regrowth after several injections.
Intralesional triamcinolone 2.5 mg/mL may be injected every cm–about 0.1 mL per injection. When the patient returns often there is regrowth in tufts. One can then inject in between the tufts.
Alopecia Areata in a Child
- First-line 5% minoxidil and topical steroid (e.g., clobetasol, mometasone cream)
- Second-line, if extensive, immunocontact therapy
- Hydroxychloroquine
- Once over 10 years of age, or can tolerate, add intralesional triamcinolone as above
- Oral Tofacitinib did well in 8 patient 12-19 years of age with alopecia universals 92.
Treatment Options for Extensive Disease
Hydroxychloroquine
Hydroxychloroquine improved the clinical appearance in 5/9 children after 6 months 93. Patients were 12-16 years of age. A typical dose was 200 mg twice daily.
Systemic Steroids
Systemic steroids are usually only considered for extensive disease because of potential side effects. Most physicians agree that long-term systemic steroid treatment is not justified because of potential and actual adverse effects. Taking steroids by mouth over a period of time can cause many side effects including raised blood pressure, diabetes, stomach ulcers, cataracts and osteoporosis as well as weight gain. In one study, pulse oral prednisone 300 mg every 4 weeks was used. Otherwise, one may give the steroid daily orally and taper to the lowest effective dose. Alternatively, Kenalog (triamcinolone acetonide) 60 mg intramuscular monthly x 3 may be tried but no more should be given and the patient risks the hair falling out once treatment is stopped.
Azathioprine
In a small study, 14 patients with alopecia universalis were treated with azathioprine 2.5 mg/kg body weight per day. Forty-three percent achieved complete regrowth at a mean of 4.7 months 94 reviewed treatment of 31 patients and found regrowth greater than 50% was observed in 67.7% of patients, with the best responses observed in those with <5 years of disease progression (79%), age over 40 years (73.3%), male patients (72.8%), cumulative dose of methotrexate 1000-1500 mg, and multifocal alopecia areata (93%).
Short Contact Anthralin
Inducing an irritant contact dermatitis can sometimes cause regrowth. Typically, one has the patient apply 1% anthralin for 1 hour and then wash off. The main side effect is irritation, but that is the goal. Rarely, there can be facial edema, vesicles, blisters, etc., if the reaction is too severe.
Tofacitinib and other JAK Inhibitors
Recently, the efficacy of leveraging Janus Kinase inhibitors (JAKis) in various autoimmune and hematologic diseases has seen increased interest. Janus Kinase inhibitors (JAKis) are selective, competitive inhibitors of adenosine triphosphate-binding sites on JAK/STAT 95. It predominantly blocks the downstream IFN-γ and γc cytokine receptors and reduces the recruitment of CD8+NKG2D+ T cells 96. It also interferes with Th1 cell and Th17 cell differentiation. Notably, activation and proliferation of hair follicle stem cells are promoted by JAKis, which accelerates hair follicle reentry into the anagen phase 97. Treatment of alopecia areata with JAK1/2 (IFN-γ pathway) and JAK3 (γc cytokines) blockers showed promising results. The therapeutic efficacy of oral tofacitinib (Xeljanz) and oral ruxolitinib (Jakafi) in treating severe and recalcitrant alopecia areata has an overall response rate of 30%–75%, with transient and minimal side effects 98.
Ninety patients with severe alopecia areata were treated with tofacitinib 5 mg twice daily for 2-3 months initially. Then, non-responders were eligible for pulse prednisone or higher doses of tofacitinib 99. Seventy-seven percent achieved a clinical response, with 58% achieving a greater than 50% improvement in SALT score over 4-18 months. There were no serious adverse events.
68% (9/13) pediatric patients with severe alopecia areata experienced clinically significant regrowth of hair in a study using tofacitinib (5 mg twice daily x 5 months) 100. Most of the patients studied had either alopecia universalis or totalis. No serious side effects were reported.
Topical 2% tofacitinib ointment applied twice daily (topical) had a modest effect, perhaps similar to clobetasol ointment under occlusion 101.
Oral ruxolitinib (a JAK1 and JAK2 blocker) led to almost complete hair regrowth in 3 alopecia areata patients in 5 months 102. In another study, 9/12 patients achieved at least 50% regrowth most by 6 months 103. Also Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia 104.
Other Therapy
A wig or hair piece may be needed for more extensive disease. Patients with more diffuse hair loss, e.g., alopecia totalis or universalis, may desire more aggressive therapy. Intramuscular triamcinolone 40-60 mg or a tapering course of systemic steroids may be tried although controlled studies are lacking. Cyclosporin has been tried. Topical immunotherapy, with e.g., phenol, has been reported.
Adalimumab did not help alopecia areata in one report but did in another 105. Apremilast did not help 9 patients with severe alopecia areata 106.
Treatment with platelet-rich plasma was studied in 45 patients with alopecia areata 107. Intralesional platelet-rich plasma grew more hair than intralesional triamcinolone 2.5 mg/mL or placebo without significant side effects. The platelet-rich plasma was prepared by drawing the patient’s own blood, centrifuging it for 8 minutes at 70 “G”s, and separating the platelet-rich plasma fraction.
A combination of the lipid lowering agents simvastatin and ezetimibe (which have immunomodulating effects) has been reported to be effective 108. Besides their efficacy in reducing atherosclerotic cardiovascular risk, statins are also anti-inflammatory and immunomodulatory agents. In vitro (test tube) and in vivo (animal) studies showed that statins downregulate Th1 cytokines and upregulate Th2 cytokines via modulation of the JAK/STAT pathway. Furthermore, it can directly modulate APCs to increase Treg cell activation 109. Evidence also suggests that statins downregulate leukocyte activation, proliferation, differentiation, adhesion, and extravasation into target tissues 110. The combination of statins and ezetimibe (non-statin lipid-lowering medication) showed promising results with 30%–80% hair regrowth in 28% of recalcitrant alopecia areata patients 108. However, another study reported unsatisfactory results as none of the patients achieved hair regrowth 111. The relapse rate was significantly lower in statin-treated patients than in the control group 112. Thus, lipid-lowering agents, when combined with other therapies, show promise for preventing disease relapses; however, further studies to elucidate this are required.
There is no convincing data to support the use of methotrexate, sulfasalazine, azathioprine, ciclosporin or phototherapy.
Topical immunotherapy
Topical immunotherapy is currently considered as the first-line treatment, representing the most effective modality for treating extensive or recalcitrant alopecia areata 113. Despite being commonly used, the exact mechanism underlying topical immunotherapy for the treatment of alopecia areata has not yet been elucidated. Accumulating evidence has shown advantage of topical immunotherapy over no treatment; however, comparison of the efficacies across different clinical studies and different substances is difficult owing to variations of treatment protocols, evaluation methods, and study durations.
Diphenylcyclopropenone
Diphenylcyclopropenone (DPCP) is a topical sensitizer, efficacy of which was primarily reported by Happle et al in 1983 114. Currently, diphenylcyclopropenone (DPCP) is the most commonly used substance owing to the following reasons. First, it is nonmutagenic in Ames assay, with no report of systemic absorption 115. Second, no long-term adverse effect has been documented yet. Finally, it is less expensive and more stable in acetone solution compared to squaric acid dibutylester (SADBE) 116. In 2012, the British Association of Dermatologists’ guideline also recommended the use of diphenylcyclopropenone (DPCP) as the first-line topical sensitizer for the treatment of alopecia areata 117.
Several studies have evaluated the efficacy of diphenylcyclopropenone (DPCP) in patients with alopecia areata; hair regrowth rate was found to be 6–77%. A systematic review had previously reported an overall hair regrowth rate of 53.75% in diphenylcyclopropenone (DPCP)-treated patients 118. Severity of alopecia areata was found to be a significant factor associated with hair regrowth. The highest efficacy of diphenylcyclopropenone (DPCP) was reported by Tosti et al 119 with 77% complete hair regrowth in patients with mild alopecia areata. The largest retrospective study involving 757 patients with all subtypes of alopecia areata was published in 2020 120. The overall hair regrowth rate has been reported to be 60.1% and the satisfactory hair regrowth (>75% hair regrowth) rate was 16.3% 120. Comparison across subtypes of alopecia areata showed patch-type alopecia areata to have 2.56 times higher satisfactory hair regrowth while alopecia universalis had 2.6 times lower response in comparison to other subtypes 120. The satisfactory hair regrowth rate of diphenylcyclopropenone (DPCP) for patch-type alopecia areata was reported ranging between 55.4% and 63.4% 121, 122. When the efficacy of diphenylcyclopropenone (DPCP) on alopecia totalis or alopecia universalis subtype was considered, two meta-analyses showed different results. Lee et al 121 reported 28.3% of patients with satisfactory hair regrowth while Gupta et al 122 reported a higher rate of 87.9%.
Very few studies to date have demonstrated the efficacy of diphenylcyclopropenone (DPCP) in children with alopecia areata. The efficacy of satisfactory hair regrowth has been reported to range from 11–33%. A prospective study using diphenylcyclopropenone (DPCP) in 12 pediatric patients with extensive alopecia areata reported initial hair regrowth in 67% of patients and complete hair regrowth in 33% after a mean treatment duration of 7.3 months 123. Another retrospective study investigated the efficacy of diphenylcyclopropenone (DPCP) in 108 children with alopecia areata, and found only 13% and 11% of patients to have achieved complete hair regrowth after six and 12 months of treatment, respectively 124.
Although efficacy of diphenylcyclopropenone (DPCP) has been widely investigated for hair regrowth, few studies have focused on the relapse rate after cessation of treatment. A comparative study in this regard showed that patients who continued using diphenylcyclopropenone (DPCP) as a maintenance therapy had a lower relapse rate (24.4%) compared to those who did not (68.2%) 125. Hull and Cunliffe 126 reported 63% relapse rate after six months of successful therapy without maintenance treatment. Male gender, high severity of disease, and body hair involvement were established as negative factors determining recurrence. In contrast, a study on 25 patients with complete hair regrowth showed no relapse after discontinuing diphenylcyclopropenone (DPCP), over a mean period of 15 months 127. The importance of maintenance therapy in topical immunotherapy still remains inconclusive.
Although diphenylcyclopropenone (DPCP) is the best-documented treatment for extensive or recalcitrant alopecia areata, not all patients achieve a good response, and some might withdraw due to adverse effects. Patients with high serum IgE levels may have more severe adverse events following diphenylcyclopropenone (DPCP) application 128. Regarding safety, most patients were found to be tolerant to diphenylcyclopropenone (DPCP) and no systemic absorption has yet been reported 129. Most adverse effects have been recorded without long-term complications; common side effects include dermatitis and urticaria 130. Angioedema, anaphylaxis, fever, erythema multiforme-like reactions, postinflammatory hypopigmentation, and depigmentation have been reported as infrequent complications 131.
Topical combination therapy using diphenylcyclopropenone (DPCP)
Topical combination therapy using diphenylcyclopropenone (DPCP) and anthralin on 25 patients with severe alopecia areata resulted in complete regrowth of hair in 72% of patients 132. On the contrary, two studies and one case series showed nonsuperior efficacy of diphenylcyclopropenone (DPCP) and anthralin combination therapy compared to diphenylcyclopropenone (DPCP) monotherapy 133, 134. Regarding diphenylcyclopropenone (DPCP) combination with 5% minoxidil, Shapiro et al 135 found no significant difference in satisfactory hair regrowth between combined regimen and diphenylcyclopropenone (DPCP) monotherapy. When combined with imiquimod, however, superior efficacy of combination therapy to diphenylcyclopropenone (DPCP) monotherapy was evident 136.
Note that topical immunotherapy diphenylcyclopropenone (diphencyprone) provokes contact allergic dermatitis in treated areas. The resultant dermatitis is irritating and may be unsightly. It is often accompanied by a swollen lymph gland.
Since the standard treatment protocol of contact sensitizers may take a long time until acceptable treatment response is achieved, modified protocols have been introduced to address this issue. Sriphojanart et al 137 and Thuangtong et al 138 had reported the use of a multiconcentration diphenylcyclopropenone (DPCP) treatment on patients’ scalps at the first visit. A concentration that created an optimal eczematous reaction would then be selected as the starting solution for application. Multi-concentration protocol showed similar efficacy as the standard protocol, with a shorter duration till significant hair regrowth is achieved 137. Another study had also investigated the efficacy across various application intervals. Results showed the three-week treated group to have higher efficacy than the one-week interval group, with 54% and 46% response rates, respectively 139. A subsequent retrospective study reported no significant difference of efficacy between home-based diphenylcyclopropenone (DPCP) therapy and clinic-based therapy; however, the home-based group had better compliance compared to the clinic-based group 140.
Topical Squaric Acid Dibutylester
Squaric acid dibutylester (SADBE) or 3,4-dibutoxycyclobut-3-ene-1,2-dione, its topical application induces an allergic contact dermatitis and can grow hair both in adults and children 141. Various protocols exist, but one common approach is to first do an application on the arm to induce sensitivity and then one month later, start topical application of 5% squaric acid dibutylester (SADBE) on the affected areas of the scalp. Patients return monthly to monitor progress and for reapplication of the 5% if the hair is regrowing. If there is too much reaction (e.g., burning, blistering), the concentration is reduced to 2.5%. If no hair is growing, then the concentration can be increased to 10%.
The response rate of squaric acid dibutylester (SADBE) in the treatment of alopecia areata ranges between 19% and 79.6% 113. A meta-analysis by Lee et al 121, including 45 studies with 2227 patients, showed 51.8% of patients with patch-type alopecia areata and 25% of patients with alopecia totalis or alopecia universalis to have achieved satisfactory hair regrowth. A network meta-analysis by Gupta et al 122 suggested that 35% and 49.7% of patients with patch-type alopecia areata and alopecia totalis or alopecia universalis, respectively, demonstrated satisfactory hair regrowth.
One randomized placebo-controlled trial of squaric acid dibutylester (SADBE) was published in 1986, which demonstrated complete hair regrowth in 64% of patients with patch-type alopecia areata 119. The largest prospective study of squaric acid dibutylester (SADBE), conducted in 144 patients with variable severities of alopecia areata, reported 80% and 49% response rates in mild and severe forms of alopecia areata, respectively 142. Satisfactory results were reported by Happle et al 141, Dall’oglio et al 143 and Chua et al 144, with complete hair regrowth rates of 87%, 79.6%, and 68%, respectively. In contrast, Caserio 145 and Gianetti and Orecchia 146 reported unsatisfactory results with complete response rate of 28.5% and 19%, respectively.
Adverse effects of squaric acid dibutylester (SADBE) include redness, swelling, and itching at the application site. However, some patients might experience more severe reactions, such as blistering, burning of the skin, and spreading of rash to other areas. Uncommon adverse effects reported include spread of generalized eczema, persistent contact dermatitis, and severe angioedema 147. Postinflammatory hypopigmentation and depigmentation have also been reported after the application of squaric acid dibutylester (SADBE), and lesions were obviously visible in patients with skin phototype V or VI 148.
Dinitrochlorobenzene
Dinitrochlorobenzene (DNCB) or 1-chloro-2,4-dinitrobenzene was the earliest contact allergen introduced in 1912 113. Dinitrochlorobenzene (DNCB) used to be popular for the treatment of extensive or recalcitrant alopecia areata, with hair regrowth ranging around 25–89% and complete regrowth rates being 6.7–25% 113. However, in 1985, dinitrochlorobenzene (DNCB) was found to be mutagenic and carcinogenic in Ames test and had to be discontinued thereafter 149.
Androgenic alopecia
Androgenetic alopecia is a pattern of hair loss that is affected by the genes and hormones (androgenic). Androgenetic alopecia is the most common form of hair loss in men and women and is a normal physiologic variant. Androgenetic alopecia is the most common type of hair loss, affecting approximately 50% of men over the age of 50 and around 50% of women over the age of 65. Androgenetic alopecia is most prevalent in white men, with 30%, 40%, and 50% experiencing androgenetic alopecia at 30, 40, and 50 years of age, respectively (see Figure 3). Although androgenetic alopecia is less common in women, 38% of women older than 70 years may be affected (see Figure 4) 8. Androgenetic alopecia hair loss follows a gradual progressive course. Many patients with androgenetic alopecia have a family history of this condition.
Androgenetic alopecia tends to look different between males and females. Androgenetic alopecia is characterized by progressive loss of terminal hair of the scalp any time after puberty, in a characteristic distribution in both males and females. Hair is lost in a well-defined pattern, beginning above both temples. Over time, the hairline recedes to form a characteristic “M” shape. Hair also thins at the crown (near the top of the head), often progressing to partial or complete baldness. Unlike other areas of the body, hairs on the scalp to grow in tufts of 3–4. In androgenetic alopecia, the tufts progressively lose hairs. Eventually, when all the hairs in the tuft are gone, bald scalp appears between the hairs.
Hair thinning occurs in a sex-specific pattern:
- Men typically present with bitemporal thinning, thinning of the frontal and vertex scalp, or complete hair loss with residual hair at the occiput and temporal fringes, often in a classic M-shaped pattern 9
- Women typically present with diffuse hair thinning at the crown of the head with sparing of the frontal hairline. Some women experience thinning over the lateral scalp. It is less likely that a woman will experience total baldness as a result of androgenetic alopecia.
Common conditions that mimic androgenetic alopecia include thyroid disease, iron deficiency anemia, and malnutrition.
Androgenetic alopecia is a frequent cause of hair loss in both men and women. This form of hair loss affects an estimated 50 million men and 30 million women in the United States. Androgenetic alopecia can start as early as a person’s teens and risk increases with age; more than 50 percent of men over age 50 have some degree of hair loss. In women, hair loss is most likely after menopause.
White patients are most affected followed by Asians and African Americans, then Native Americans and Eskimos. The incidence approximates the age in Caucasian males, with 50% affected by 50 years old and up to 80% affected by 70 years old. In females, the disorder is quite common, with an increase in incidence after menopause 150.
Androgenetic alopecia in men has been associated with several other medical conditions including coronary heart disease and enlargement of the prostate. Additionally, prostate cancer, disorders of insulin resistance (such as diabetes and obesity), and high blood pressure (hypertension) have been related to androgenetic alopecia. In women, androgenetic alopecia is associated with an increased risk of polycystic ovary syndrome (PCOS). PCOS is characterized by a hormonal imbalance that can lead to irregular menstruation, acne, excess hair elsewhere on the body (hirsutism), and weight gain.
Multiple studies demonstrate a strong relationship between pattern baldness, especially early onset or vertex loss of hair, with cardiovascular disease including hypertension, myocardial infarction, insulin resistance, death from diabetes or heart disease, abnormal lipids, obesity, and infertility.
The data is mixed regarding the androgenetic alopecia relationship with benign prostate hypertrophy. However, there is some data suggesting a two-fold risk of prostate cancer and a higher incidence of death from the prostate cancer. There is also a modest increase in the risk of colon cancer and urolithiasis in patients with androgenetic alopecia.
As with other types of alopecia, there is no cure for androgenetic alopecia. However, the effects of androgenetic alopecia may be slowed down with treatments. For men, potential treatments include oral finasteride and/or topical minoxidil solution or foam, and for women topical minoxidil solution or foam. Hair transplantation can be used to improve the appearance of androgenetic alopecia.
Androgenic alopecia causes
Androgenetic alopecia is caused by both genetic and hormonal factors, many of which are not yet understood. This form of hair loss is related to hormones called androgens, particularly an androgen called dihydrotestosterone (DHT). Increased levels of androgens such as DHT (dihydrotestosterone) in hair follicles can lead to a shorter cycle of hair growth and the growth of shorter and thinner strands of hair. Follicles can also stay in the resting phase for longer periods of time. The production of DHT is regulated by an enzyme called 5-alpha reductase.
Although researchers suspect that several genes play a role in androgenetic alopecia, however, to date, only changes in one gene, the androgen receptor gene, have been linked to development of androgenetic alopecia. Androgen receptors allow the body to respond appropriately to dihydrotestosterone and other androgens. Studies suggest that changes in the androgen receptor gene lead to more active androgen receptors in hair follicles. It remains unclear, however, how these genetic changes increase the risk of hair loss in men and women with androgenetic alopecia.
Androgenic alopecia signs and symptoms
Androgenetic alopecia tends to look different between males and females. In men, the typical pattern of hair loss is a receding hair line with loss of hair from the top and front of the head, often in a classic M-shaped pattern. In women, the usual pattern of hair loss is thinning at the crown of the head, with the frontal hairline over the forehead remaining. It is less likely that a woman will experience total baldness as a result of androgenetic alopecia.
Androgenic alopecia diagnosis
Your doctor will usually diagnose androgenetic alopecia by examining the pattern of hair loss on the scalp. They may also perform blood tests to measure hormone levels, serum ferritin and thyroid function.
Androgenic alopecia treatment
As with other types of alopecia, there is no cure for androgenetic alopecia. However, the effects of androgenetic alopecia may be slowed down with treatments. For men, potential treatments include oral finasteride and/or topical minoxidil solution or foam, and for women topical minoxidil solution or foam. Hair transplantation can be used to improve the appearance of androgenetic alopecia.
Male pattern baldness
Male pattern baldness or androgenetic alopecia, is the patterned balding of a man. Although the condition may affect both the appearance and self-esteem of some men, one should note that the condition is not a medical disorder. The hair loss is non-scarring and has a genetic basis. Sex steroids (androgens) – specifically, dihydrotestosterone – play a role in this form of balding.
Male pattern hair loss occurs in men who are genetically predisposed to be more sensitive to the effects of dihydrotestosterone (DHT). Researchers now believe that the condition can be inherited from either side of the family. DHT (dihydrotestosterone) is found in several tissues in the body including the prostate gland and skin. 5-alpha reductase is an enzyme that regulates the production of dihydrotestosterone (DHT). An enzyme is a protein that acts as a catalyst to speed up a chemical reaction. 5-alpha reductase can be inhibited by specially synthesized drugs.
- Male pattern hair loss is due to a combination of hormones (androgens) and a genetic predisposition.
- Male pattern hair loss is also called androgenetic alopecia.
- It is characterised by a receding hairline and hair loss on the top and front of the head.
- A similar type of hair loss in women, female pattern hair loss, results in thinning hair on the mid-frontal area of the scalp and is generally less severe than occurs in males.
Male pattern hair loss affects nearly all men at some point in their lives. It affects different populations at different rates, probably because of genetics. Up to half of male Caucasians will experience some degree of hair loss by age 50, and possibly as many as 80% by the age of 70 years, while other population groups such as Japanese and Chinese men are far less affected.
Male pattern hair loss can have a negative psychological impact. Studies have shown that hair loss can be associated with low self-esteem, depression, introversion, and feelings of unattractiveness. This is reinforced by attitudes in Western society, which place great value on youthful appearance and attractiveness. Some studies have shown that based on appearance alone, men with hair loss are seen as less attractive, less assertive, less likeable, and less successful than men without hair loss.
The severity of hair loss can be classified in several ways. Sinclair systems are shown below.
Figure 15. Androgenetic alopecia male
What causes male pattern baldness?
Male pattern hair loss is an inherited condition, caused by a genetically determined sensitivity to the effects of dihydrotestosterone (DHT) in some areas of the scalp. DHT (dihydrotestosterone) is believed to shorten the growth, or anagen, phase of the hair cycle, from a usual duration of 3–6 years to just weeks or months. This occurs together with miniaturisation of the follicles and progressively produces fewer and finer hairs. The production of DHT is regulated by an enzyme called 5-alpha reductase.
Male pattern hair loss occurs in men who are genetically predisposed to be more sensitive to the effects of DHT. Researchers now believe that the condition can be inherited from either side of the family.
Several genes are involved, accounting for differing age of onset, progression, pattern and severity of hair loss in family members. The susceptibility genes are inherited from both mother and father. At this time, genetic testing for prediction of balding is unreliable.
A few women present with male pattern hair loss because they have excessive levels of androgens as well as genetic predisposition. These women also tend to suffer from acne, irregular menses and excessive facial and body hair. These symptoms are characteristic of polycystic ovarian syndrome (PCOS) although the majority of women with PCOS do not experience hair loss. Less often, congenital adrenal hyperplasia may be responsible. Females that are losing their hair with age are more likely to present with female pattern hair loss, in which hormone tests are normal.
Is male pattern hair loss hereditary?
Yes. It is believed male pattern hair loss can be inherited from either or both parents.
Can male pattern hair loss be cured?
No, there is no cure. However, it tends to progress very slowly, from several years to decades. An earlier age of onset may lead to quicker progression.
Male pattern baldness signs and symptoms
Male pattern hair loss affects nearly all men at some point in their lives. It affects different populations at different rates, probably because of genetics. Up to half of male Caucasians will experience some degree of hair loss by age 50, and possibly as many as 80% by the age of 70 years, while other population groups such as Japanese and Chinese men are far less affected.
The severity of hair loss can be classified in several ways (see Figure 14 above). The usual pattern of hair loss is a receding frontal hairline and loss of hair from the top of the head. Hairs in the affected areas are initially smaller in diameter, and shorter compared to hairs in unaffected areas, before they become absent.
Male pattern baldness diagnosis
Your doctor will usually diagnose androgenetic alopecia by examining the pattern of hair loss on the scalp. They may also perform blood tests to measure hormone levels, serum ferritin and thyroid function.
Male pattern baldness treatment
Treatment is based on patient preference. Current treatment options include:
- Hair replacement / transplantation
- Cosmetics
- Micropigmentation (tattoo) to resemble shaven scalp
- Hairpieces
- Minoxidil solution
- Finasteride tablets (type II 5-alpha-reductase inhibitor)
- Dutasteride.
Minoxidil and oral finasteride are the only treatments currently approved by the U.S. Food and Drug Administration for the treatment of androgenetic alopecia. Both of these drugs stimulate hair regrowth in some men, but are more effective in preventing progression of hair loss. Although there are a number of other treatments listed in various texts, there is not good evidence to support their use 10.
Topical minoxidil (2% or 5% solution) is approved for the treatment of androgenetic alopecia in men 151. Minoxidil comes as a solution or foam – the easiest way to measure the correct amount is to use a large tangerine-sized blob of the foam. Hair regrowth is more robust at the vertex than in the frontal area, and will take six to 12 months to improve 9. The response to treatment should be assessed at six months – if beneficial treatment needs to be continued to maintain efficacy. Up to 40% of patients may benefit. Treatment should continue indefinitely because hair loss reoccurs when treatment is discontinued. Adverse effects include irritant and contact dermatitis.
Finasteride (Propecia), 1 mg per day orally, although a single dose of 5 mg per week is probably as effective and much cheaper for the patient and it is approved to treat androgenetic alopecia in men for whom topical minoxidil has been ineffective. Adverse effects of finasteride include decreased libido, erectile dysfunction, and gynecomastia (increase in the amount of breast gland tissue in men) 11. There is some evidence that dutasteride 2.5 mg oral once daily may be more effective than finasteride.
There is some evidence that ketoconazole shampoo may also be of benefit, perhaps because it is effective in seborrheic dermatitis and dandruff.
Low-level laser therapy is of unproven benefit in pattern balding; one device has been approved by the FDA for marketing. Platelet-rich plasma injections are also under investigation. Further studies are required to determine the magnitude of the benefit if any.
Wigs and hair pieces
Some affected individuals find wigs, toupees and even hair extensions very helpful in disguising hair loss. There are two types of postiche (false hairpiece) available to individuals; these can be either synthetic or made from real hair. Synthetic wigs and hairpieces, such as a toupee, usually last about 6 to 9 months, are easy to wash and maintain, but can be susceptible to heat damage and may be hot to wear. Real hair wigs or hairpieces can look more natural, can be styled with low heat and are cooler to wear.
Skin camouflage
Spray preparations containing small pigmented fibres are available from the internet and may help to disguise the condition in some individuals. These preparations however, may wash away if the hair gets wet i.e. rain, swimming, perspiration, and they only tend to last between brushing/shampooing.
Surgical treatments
Surgical treatment includes (i) hair transplantation, a procedure where hair follicles are taken from the back and sides of the scalp and transplanted onto the bald areas; and (ii) scalp reduction, where a section of the bald area is removed and the hair-bearing scalp stretched to cover the gap. Tissue expanders may be used to stretch the skin.
Hair loss in women
Female pattern baldness is frequently referred to as androgenetic alopecia, is a form of hair loss affecting women due to an inherited susceptibility. Female pattern baldness is most commonly noticed after menopause, although it may begin any time after puberty 152. In the majority of cases women have normal levels of androgens in their blood, nevertheless there is a subset of women with alopecia who have associated hyperandrogenism eg secondary to the polycystic ovarian syndrome (PCOS). Female pattern baldness is due to a combination of a family history of balding (in men or women from either parent’s side of the family), aging, and hormones 153. The incidence of androgenetic alopecia in women is thought to be less than that in males, although some argue that differences in expression only make it seem that this is the case. Both frequency and severity increase with age. Onset of hair loss seems most common at either 20–30 or 40–50 years of age. Because there is a genetic basis to female pattern baldness, different racial populations are affected at different rates. The incidence is highest in Caucasians followed by Asians and African Americans, and the lowest incidence of hair loss is in Native Americans. Female pattern baldness is not due to a vitamin deficiency, poor circulation, dandruff, or wearing hats. There is progressive shrinking of the hair follicles until they produce only a fine, wispy hair or cease functioning.
Almost half of men, and perhaps as many women who are postmenopausal, are affected by hair loss to some degree. Female-pattern hair loss can develop any time after the onset of puberty; by 70 years of age, 38% of women have female-pattern hair loss 154. Female pattern baldness affects the central portion of the scalp, sparing the frontal hairline, and is characterized by a wider midline part on the crown than on the occipital scalp (Figure 15). In some women, hair thinning over the side area of the scalp also occurs. The severity of hair loss is staged according to the Ludwig classification, in which increasing stages (I to III) correspond to increasing widths of the midline part 155. If hair thinning is more evident in the frontal portion of the scalp, the part may resemble a fir tree in what is known as a “Christmas tree pattern” behind the frontal hair line (Figure 17). This pattern is referred to as “frontal accentuation” 152.
The hair loss associated with female pattern baldness, although permanent, requires no treatment if you are comfortable with your appearance.
There is no known prevention for hair loss; shampooing and other hair products have no adverse effects other than harsh products or practices that may damage the hair shaft, causing breakage.
Hair loss can have a significant psychologic impact, particularly in western society, which puts such a large emphasis on appearance.
For mild to moderate hair thinning, creative hair styling, hair weaving, or hairpieces may be adequate to improve appearance. Protect the scalp from sunburn with a hat.
Treatment for hair loss in women in the US is topical minoxidil; the 2% preparation recommended for women is available over the counter 156. Adverse effects include irritant and contact dermatitis. This may help hair to grow in a quarter of the women using it, and it will stop or slow hair loss in the majority of users. The medication is expensive, however, and the hair will fall out when its use is discontinued.
Other therapies are aimed at stimulating regrowth of terminal hairs might include the oral medications spironolactone or cyproterone acetate (not available in the US). Finasteride, also an oral medication, is approved for male balding only, and studies show no effect for female pattern hair loss.
Finasteride, spironolactone, and cyproterone should not be used in women of childbearing potential.
Surgical therapy to improve the appearance includes scalp reduction, flaps, and hair transplants (micrografting). Not everyone is a good candidate for these procedures.
Figure 16. Androgenetic alopecia women
Figure 17. Female pattern baldness
Figure 18. Female pattern hair loss with frontal accentuation (Christmas tree pattern)
Footnote: Panel A shows hair loss before topical 5% Minoxidil treatment, and Panel B shows regrowth after 6 months of treatment.
Female pattern baldness causes
Female pattern baldness is caused by a combination of genetic and hormonal factors. The hairs produced by the affected follicles become progressively smaller in diameter, shorter in length and lighter in colour until eventually the follicles shrink completely and stop producing hair. The mode of inheritance is polygenic, indicating that there are many genes that contribute to female pattern baldness and these genes could be inherited from either parent or both. Genetic testing to assess the risk of balding is currently not recommended, as it is unreliable.
Female pattern hair loss can be associated with conditions in which androgen (a group of hormones) levels are elevated such as polycystic ovarian syndrome (PCOS). Acne, increased facial hair, irregular periods and infertility are all signs of PCOS.
Currently, it is not clear if androgens (male sex hormones) play a role in female pattern baldness, although androgens have a clear role in male pattern baldness. The majority of women with female pattern hair loss have normal levels of androgens in their bloodstream. Due to this uncertain relationship, the term androgenetic alopecia female is preferred to ‘female androgenetic alopecia’.
The role of estrogen is uncertain. Androgenetic alopecia female is more common after the menopause suggesting estrogens may be stimulatory for hair growth. But laboratory experiments have also suggested estrogens may suppress hair growth.
Is female pattern hair loss inherited?
Yes. It is believed that it can be inherited from either or both parents.
Can female pattern hair loss be cured?
No, there is no cure for female pattern hair loss. However, it tends to progress very slowly, from several years to decades. An earlier age of onset may lead to quicker progression.
Female pattern baldness signs and symptoms
Female pattern hair loss is different in women than men; the hairline is preserved while there is diffuse thinning of the hair of the crown and frontal scalp. Total hair loss is very rare.
Female pattern hair loss is not usually associated with any scalp symptoms. Hair loss can, however, cause psychological consequences and have an impact on quality of life.
In women, the age of onset is later compared to male pattern hair loss, usually occurring in the 50s or 60s. Occasionally, female pattern hair loss in women may start earlier than this, in the 30s or 40s.
How long does it take for female pattern hair loss to progress?
Female pattern hair loss can affect women in any age group, but it occurs more commonly after menopause. The hair loss process is not constant and usually occurs in fits and bursts. It is not uncommon to have accelerated phases of hair loss for 3–6 months, followed by periods of stability lasting 6–18 months. Without medication, it tends to progress in severity over the next few decades of life.
Female pattern baldness diagnosis
The diagnosis of female pattern baldness is usually easy for the physician because of the typical pattern. However, certain blood tests will help to rule out other causes such as anemia (low blood count) or a thyroid disorder. A skin biopsy may be recommended.
The majority of women affected by female pattern hair loss do not have underlying hormonal abnormalities. However, a few women with female pattern hair loss are found to have excessive levels of androgens. These women also tend to suffer from acne, irregular menses and excessive facial and body hair. These symptoms are characteristic of the polycystic ovarian syndrome (PCOS) although the majority of women with PCOS do not experience hair loss. Less often, congenital adrenal hyperplasia may be responsible.
Female hair loss treatment
Treatments are available for female pattern hair loss although there is no cure. It is important to manage expectations when seeking treatment, as the aim is to slow or stop the progression of hair loss rather than to promote hair regrowth. However, some women do experience hair regrowth with treatment. Results are variable, and it is not possible to predict who may or may not benefit from treatment.
A Cochrane systematic review published in 2012 concluded that minoxidil solution was effective for female pattern hair loss. Minoxidil is available as 2% and 5% solutions; the stronger preparation is more likely to irritate and may cause undesirable hair growth unintentionally on areas other than the scalp.
Hormonal treatment, i.e. oral medications that block the effects of androgens (e.g. spironolactone, cyproterone, finasteride and flutamide) is also often tried.
A combination of low dose oral minoxidil (0.25 mg daily) and spironolactone (25 mg daily) has been shown to significantly improve hair growth, reduce shedding and improve hair density.
Once started, treatment needs to continue for at least six months before the benefits can be assessed, and it is important not to stop treatment without discussing it with your doctor first. Long term treatment is usually necessary to sustain the benefits.
Cosmetic camouflages include coloured hair sprays to cover thinning areas on the scalp, hair bulking fibre powder, and hair wigs. Hair transplantation for female pattern hair loss is becoming more popular although not everyone is suitable for this procedure.
Low-level laser therapy is of unproven benefit in pattern balding, but one device has been approved by the FDA for marketing. Platelet-rich plasma injections are also under investigation. Further studies are required to determine the magnitude of the benefit if any.
Topical and oral medications
- Applying 2% or 5% minoxidil solution to the scalp every day may help to slow down the progression and partially restore hair in some women. Only the 2% strength is licensed for women; the 5% minoxidil solution can be used under the advice of a medical doctor and is expensive. Minoxidil solution should be applied to the affected scalp (not the hair) using a dropper or pump spray device and should be spread over the affected area lightly, it does not need to be massaged in. Minoxidil can cause skin reactions such as dryness, redness, scaling and/or itchiness at the site of application and should not be applied if there are cuts or open wounds. Minoxidil solution should only be applied to the scalp. Any spillage to the forehead or cheeks should be cleansed to avoid increased hair growth in these areas. Minoxidil should be used for at least 6 months before any benefit may be noted. Any benefit will only be maintained for as long as the treatment is used. Minoxidil solution may cause an initial hair fall in the first 2-8 weeks of treatment, and this usually subsides when the new hairs start to grow.
- Oral treatments such as spironolactone, cyproterone acetate, flutamide and cimetidine can block the action of dihydrotestosterone (DHT) on the scalp, which may lead to some improvement in hair loss. These treatments are not licensed for use in androgenetic alopecia female. Spironolactone and cyproterone acetate should be avoided in pregnancy since they can cause feminization of a male fetus; both should be avoided during breast feeding. Flutamide carries a risk of damaging the liver.
It is important to note that all of these topical and oral treatments only work for as long as the treatment is continued.
Wigs and hair pieces
Some affected individuals find wigs, toupees and even hair extensions can be very helpful in disguising androgenetic alopecia female. There are two types of postiche (false hairpiece) available to individuals; these can be either synthetic or made from real hair. Synthetic wigs and hairpieces, usually last about 6 to 9 months, are easy to wash and maintain, but can be susceptible to heat damage and may be hot to wear. Real hair wigs or hairpieces can look more natural, can be styled with low heat and are cooler to wear.
Skin camouflage
Spray preparations containing small pigmented fibers are available from the internet and may help to disguise the condition in some individuals. These preparations however, may wash away if the hair gets wet (i.e. rain, swimming, perspiration), and they only tend to last between brushing/shampooing.
Surgical treatments
Surgical hair transplantation is a procedure where hair follicles are taken from the back and sides of the scalp and transplanted onto the bald areas.
Traction alopecia
Traction alopecia is a form of acquired hair loss that results from prolonged or repetitive tension on the scalp hair, often from tight hairstyles. The continuous strain on the hair follicles pulls out strands of hair and can damage or destroy the follicles. Traction alopecia can occur if you wear pigtails, braids or cornrows, or use tight hair rollers. Traction alopecia can happen to anyone who wears their hair pulled back tightly, whether in braids, dreadlocks, or a ponytail. It can also occur when tight headwear (like a cycle helmet) is used in the same way every day, from using chemical relaxers or even hair extensions. Traction alopecia was first described in 1907 in subjects from Greenland who had developed hair loss along the hairline due to prolonged wearing of tight ponytails 157.
Traction alopecia can happen in any area where the hair is under strain, including the top of the head and the beard area. Traction alopecia can also be due to the weight of excessively long hair. Traction alopecia is commonly associated with African American females who wear tight braids resulting in hair loss on sides of the head (temporal scalp). Traction alopecia has also been described in Sikh males who twist their uncut scalp hair tightly on the scalp (resulting in scalp alopecia) or their uncut beard below the chin (causing submandibular traction alopecia).
Traction alopecia affects people of any ethnic background or age. The likelihood of developing traction alopecia increases with age, likely due to a prolonged history of these hair practices.
The first step in treating traction alopecia is taking away the strain on the hair. This can mean not wearing certain hairstyles or helmets or stopping use of chemical relaxers. Sometimes dermatologists will suggest minoxidil to stimulate hair growth, or topical corticosteroids to help with itching or redness.
Is traction alopecia permanent?
Traction alopecia is not usually permanent, especially if it is diagnosed early. Often, recognizing the problem and avoiding putting strain on the hair will allow full regrowth. If traction alopecia is diagnosed too late, the hair follicles may have been destroyed and hair will not regrow.
Traction alopecia causes
Traction alopecia is caused by repeated strain (or pulling) on the hair, over a long period of time. It can affect people of all ages but is more common in older people as the hair follicles weaken over time. Some of the things that can cause traction alopecia include:
- Tight hairstyles, like dreadlocks, braids, cornrows, and tight ponytails.
- Hair extensions and weaves.
- Tight headwear, like such as helmets, tight hair grips or tight elastic headbands.
- Very long hair, including beard hair can pull on the hair follicles.
- Hair relaxers and other chemical treatments.
- Using extensions and relaxers together.
The hair loss seen in traction alopecia is thought to be caused by the exertion of excessive pulling forces, leading to mechanical damage of the hair follicles 158. The damage induces an inflammatory response, which presents as perifollicular erythema with pustules and/or papules in areas of traction 159. Chronic and repeated traction causes repeated follicular damage and eventually hair loss 158.
Traction alopecia can also be due to the weight of excessively long hair. Traction alopecia is commonly associated with African American females who wear tight braids resulting in hair loss on sides of the head (temporal scalp). Traction alopecia has also been described in Sikh males who twist their uncut scalp hair tightly on the scalp (resulting in scalp alopecia) or their uncut beard below the chin (causing submandibular traction alopecia).
Traction alopecia signs and symptoms
There is a large variation in the pattern of clinical presentation of traction alopecia. It there is no suspicion of traction, it can be difficult to diagnose.
The signs of traction alopecia may include:
- Pimples
- Redness
- Itching
- Scaling
- Ulcers on the scalp
- Patches of thin or short broken hair in places where the hair has been under strain
- Patches of shiny, scarred skin
- Folliculitis or pustules
- Thinning and hair loss
Traction alopecia mostly affects the front (frontal) and sides of the scalp but the location of traction alopecia wholly depends on an individual’s hair care practice, which may or may not is related to their ethnic background. “Fringe sign” is commonly found in patients with traction alopecia of the marginal hairline – this means that some hair is retained along the frontal and/or temporal rim of the hairline. Initially, traction alopecia is noncicatricial (without scarring), but prolonged and excessive tension leads to destruction of the hair follicles and permanent alopecia.
Traction alopecia diagnosis
Your doctor will diagnose traction alopecia using your medical history, and examination of hair styling habits, trichoscopy (an examination of the scalp using a microscope) and a scalp biopsy if necessary.
Traction alopecia histological findings
The histological findings of a skin biopsy taken from an area of traction alopecia differ depending on its stage of progression.
- Early stages present with:
- Trichomalacia (thinned out hair)
- An increased number of catagen (intermediate stage of hair cycle) and telogen hairs (bulb hair)
- The normal number of telogen follicle
- Preserved sebaceous (oil) glands.
- At a later stage:
- Vellus hairs (fine short hairs) develop
- Sebaceous glands and terminal hair follicles reduce and are replaced by fibrotic fibrous tracts (scars).
- Inflammation is mild to absent.
Traction alopecia treatment
People with traction alopecia should consider changing hair care and styling practice to prevent further deterioration.
- Loosen the hairstyle.
- Cut long hair.
- Avoid exposing affected hair and scalp to chemicals and heat.
Medical treatment options reported to have been used in traction alopecia include:
- Antibiotics to prevent infection
- Topical or intralesional steroids
- Topical antifungal shampoos
- Biotin supplements
- Minoxidil
- Hair replacement surgery.
Khumaloand Ngwanya reported two cases of women suffering late stage traction alopecia who experienced hair regrowth at 3 months and significant hair regrowth after 6 and 9 months, respectively, with the topical application of 2% minoxidil 160. Same patients had previously experienced no response with 1– 2 years of abstention from traumatic hairstyling practices. Additionally, Callender et al 161 reported anecdotal success with topical minoxidil in a subset of traction alopecia patients. Further study is required in order to determine the optimal duration and concentration of minoxidil therapy in the treatment of traction alopecia. Although there are no reports on the use of 5% minoxidil in traction alopecia, it may be considered as an alternative therapeutic option. Latanoprost has not been studied in traction alopecia.
The advanced stages of traction alopecia, characterized by scarring and follicular atrophy, are less amenable to medical therapy, but surgical treatment through hair transplantation may be an option for some patients. Successful hair transplantation for patients with advanced traction alopecia has been documented with multiple techniques, including punch grafting with rotation flaps 162, micro (1–2 follicular unit grafts), and mini (3–4 follicular unit grafts) grafting 163. Ozcelik 163 reported a case of a 23-year-old woman with a 5-year history of wearing an extremely tight ponytail every day in order to elevate her eyebrows who presented with bilateral temporal scarring alopecia and underwent one session of micro- and minigraft transplantation. At 1-year follow-up, the patient had 90%–95% survival of hair at the recipient site with a natural direction of hair growth and reported satisfaction with cosmesis. With hair transplantation, it is important to counsel the patient on realistic outcomes and likelihood of multiple sessions in order to achieve cosmetically favorable outcomes.
Scarring alopecia
Scarring alopecia also known as cicatricial alopecia or permanent alopecia, are an uncommon and clinically diverse set of disorders that result in permanent and irreversible (scalp) hair loss generally as a result of inflammatory mechanisms (eg, in the context of autoimmune disease), often leading to major disfigurement, discomfort, and psychological distress 164. In all forms of cicatricial alopecia, fibrous tissue replaces the hair follicles. In most conditions, the inflammatory cells destroy all the appendages (hair, oil and sweat glands) in an area of the scalp and an area with hair is replaced by hairless skin that appears “slick”, without the usual visible pores, and may be slightly depressed on palpation. It is “like a scar” but does not necessarily have the appearance of a scar that occurs after trauma, that is it may not look very different from normal skin to most people. Scarring alopecia is not the result of a break in the skin being closed by scar tissue. In cicatricial aloplecia, the scar is mostly underneath the surface where there is gradual thickening of the fibrous tissue.
Clinically, this form of irreversible hair loss is characterized by the disappearance of visible follicular ostia within an area of alopecia. Histologically, this corresponds to hair follicle destruction and subsequent replacement with fibrous tissue. Scarring alopecia may be primary scarring alopecia with the follicle itself being the target of the disease process, or secondary scarring alopecia where the hair follicles are destroyed coincidentally as part of a more generalized tissue-damaging event (eg, deep skin infection, thermal burn, trauma, or ionizing radiation) 165.
In primary scarring alopecia the hair follicle is the main target of the inflammatory process, which can be microscopically observed as a preferential destruction of the follicular epithelium and/or adventitial dermis, with a relative preservation of the interfollicular reticular dermis 166. Primary scarring alopecia group includes the following clinical entities: chronic cutaneous lupus erythematosus, lichen planopilaris, classic pseudopelade of Brocq, folliculitis decalvans and dissecting folliculitis among others. The term secondary scarring alopecia refers to when the destruction of the hair follicle is not the primary pathological event and the condition is rather caused by exogenous factors such as trauma (e.g., burns, radiation, traction) or endogenous infiltrative and inflammatory processes (e.g., sarcoidosis, pemphigus vulgaris, and scleroderma) 167.
There are currently various primary scarring alopecia classifications, but the most accepted is the one proposed by the North American Hair Research Society 168, whose last revision was in 2003. This classification divides primary scarring alopecia s into 2 large groups based on the dominant type of inflammatory cell infiltrate.
Classification of primary scarring alopecias (cicatricial alopecias) according to the North American Hair Research Society 168:
- Lymphocytic
- Chronic cutaneous lupus erythematosus (CCLE)
- Lichen planopilaris and its variants
- Classic pseudopelade of Brocq
- Central centrifugal cicatricial alopecia (CCCA)
- Alopecia mucinosa
- Keratosis follicularis spinulosa decalvans
- Neutrophilic
- Folliculitis decalvans
- Dissecting cellulitis/folliculitis
- Mixed
- Folliculitis (acne) keloidalis (folliculitis keloidalis nuchae)
- Folliculitis (acne) necrotica
- Erosive pustular dermatosis
- Nonspecific idiopathic scarring alopecia
It important to note that it is impossible to perform a precise diagnosis in almost a third of the patients, which represents a true diagnostic and therapeutic challenge for both dermatologists and patients 169.
The epidemiology of primary scarring alopecia in the general population is unknown. There are 2 retrospective studies that have been carried out in clinical centers specializing in hair research, in which the estimated prevalence was 3.2% in one center and 7.3% in the other 170.
There are few data in the literature about the origin of primary scarring alopecia. In the majority of primary scarring alopecia cases, histopathology reveals inflammation affecting the upper section of the hair follicle 171. This would explain why the process is irreversible, as this is the spot where stem cells are located, at the level of the bulge (where the hair erector muscle is inserted), below the infundibulum 171. The location of this inflammatory response is sometimes the result of antigenic stimulation of the Langerhans cells that are mostly found at the infundibulum and in lower numbers in the isthmus. Examples of these possible antigenic stimuli would be ultraviolet light in the case of lupus erythematosus, certain drugs in the case of lichen planopilaris and Staphylococcus aureus in the case of folliculitis decalvans. New knowledge about primary scarring alopecia origin proposes that a loss in the immune protection of the hair bulb stem cells might be involved172, as well as a dysfunction in stem cell self-perpetuation, increased autoimmunity by proinflammatory cytokines, and genetic/environmental predisposition 165. Recent data also suggest an association between lipid metabolism alteration and primary scarring alopecia development, in which dysfunction of the sebaceous glands could play an important role in its pathogenesis. Independent of the initial event, obliteration or permanent functional alteration of the crucial elements for follicular reconstitution causes permanent baldness 173.
Treatment for cicatricial alopecia remains poor. Once the hair is destroyed, the hair loss becomes permanent. It is primarily the hair at the periphery of the hair loss and/or the islands of remaining hair that are at risk of being destroyed that is the main focus of treatment. The main goals of treatment for cicatricial alopecia are to prevent further hair loss and to eradicate or at least lessen the redness, scale and itching associated with the process. There are no current FDA approved treatments for cicatricial alopecia. All treatment now for cicatricial alopecia is strictly based on the experience of the prescribing physician or anecdotal reports as there has never been any multicenter clinical trial in this area.
Figure 19. Scarring alopecia
Footnote: a) Follicular. b) Single large patch. c) Multiple patches. d) Marginal. e) “Footprints in the snow.” f) Folliculitis decalvans. g) Acne keloidalis.
[Source 171 ]Scarring alopecia signs and symptoms
In clinical practice, experts observe 2 distinct clinical patterns of presentation for scarring alopecia. The first, called “footprints in the snow,” is characterized by multiple irregular patches; the second, called “great patch,” corresponds to a large central patch of scarring alopecia surrounded by various smaller similar patches in the form of cicatricial satellitosis scarring 171. Other forms of clinical presentations of scarring alopecia, there are characteristic patterns: marginal (associated with frontal fibrosing alopecia and traction alopecia), follicular (associated with lichen planopilaris, folliculitis decalvans, alopecia parvimaculata, acne necrotica), tufted pattern (folliculitis in tufts, acne keloidalis nuchae type), abscessing inflammatory pattern (erosive pustular scalp dermatosis, perifolliculitis capitis abscedens et suffodiens), and diffuse pattern (acute lichen planopilaris, “red scalp” syndrome) 169.
In a clinical series, the most frequent clinical patterns in lymphocytic scarring alopecias were follicular and multiple patches, which coincides with the pattern described as characteristic of lichen planopilaris 171 . This was followed by the great central patch pattern, which fundamentally corresponded to lupus erythematosus, normally initiating as a single patch that gradually expanded. In the case of nonspecific alopecias, the 2 main patterns were the follicular and large single patch; this could be explained by the likelihood of final or inactive stages of some types of lymphocytic scarring alopecias. It should be emphasized that clinical cases with the “footprints in the snow” pattern that are described as characteristic of the classic pseudopelade of Brocq were finally classified as lichen planopilaris or lupus erythematosus using other complementary study techniques (e.g., direct immunofluorescence); this would support the belief that this entity is probably not an entity in itself, but rather a final process of another. In the neutrophilic scarring alopecias, the most frequent clinical pattern was folliculitis decalvans, characterized by pustules in the follicular areas and sometimes associated with follicles in tufts; however, there were also 3 cases that presented as a large central patch. From these results, the authors concluded that the clinical pattern can allow clinicians to infer the type of inflammatory infiltrate of primary scarring alopecia, but that it does not contribute to making an etiological diagnosis.
Scarring alopecia diagnosis
Scarring alopecia diagnosis is based on the history and clinical features, including the pattern of hair loss and appearance of the scalp skin and hair. A skin biopsy is generally required to establish the diagnosis, and to guide treatment. Taking a correct scalp biopsy is crucial for diagnosing primary scarring alopecia 171. Consequently, it should be performed at the initial presentation of the disorder, or shortly afterwards, taking a sample from the active (inflamed) borders of the affected area; this increases the possibility of finding more inflammatory infiltrate than fibrosis, therefore establishing a more accurate diagnosis and initiating proper and early treatment to achieve a better response. However, histopathology might not be diagnostic if the biopsy is taken from the incorrect site. This is especially relevant in primary scarring alopecia s since the disorder can be focal and the activity might be hard to see with the naked eye 174. For these reasons, experts emphasize the importance of dermoscopy in studying scalp pathology, in addition to allowing the clinician to examine the morphology of primary scarring alopecia macro- and microscopically. It also makes possible to identify subtle clinical changes, confirm the naked-eye diagnoses, monitor the treatment and guide the scalp biopsy for optimum results. This last usefulness was confirmed by Miteva and Tosti 175 in a study with 80 patients with primary scarring alopecia in which the biopsies were selected based on the dermoscopy findings, reaching a definitive diagnosis in 95% of the cases; it is a quick, accurate method, even for identifying individually affected follicles in focal primary scarring alopecia or in early stages 171.
Some types of hair loss are best diagnosed under the microscope based on slices of the specimen cut vertically from the skin surface down to the deep fat (vertical sections). Other types of hair loss are best diagnosed by horizontal sections cut sideways through the specimen (horizontal sections). Each of these types of examination requires a separate biopsy specimen. Biopsies are best done of active, inflamed sites on the scalp which still have remaining hair. A biopsy of an older scarred area may be helpful to predict the likelihood of regrowth of hair, and to help establish the diagnosis by evaluating the pattern of scar formation. If certain types of cicatricial alopecia are suspected, your doctor may send a biopsy specimen for additional special tests including direct immunofluorescence, and special stains for bacteria, fungi and elastic tissue. In some infectious disorders that can cause cicatricial alopecia, a biopsy must be sent for tissue culture.
Lichen planopilaris
Lichen planopilaris (LPP) is a type of scarring alopecia that occurs when a relatively common skin disease, known as lichen planus, affects areas of skin where there is hair. Lichen planopilaris destroys the hair follicle with a lymphocytic inflammatory process and then replaces it with scarring (cicatricial alopecia), resulting in permanent hair loss. Lichen planopilaris can be divided into three different subtypes:
- Classic lichen planopilaris, also known as follicular lichen planus
- Frontal fibrosing alopecia (FFA)
- Graham Little syndrome.
Although lichen planopilaris is rare, it is one of the common causes of scarring hair loss of the scalp.
The cause of lichen planopilaris (LPP) is unknown. Lichen planopilaris is between 2 and 5 times more common in women than it is in men with the commonest age of onset being in the mid-40s (between 40 and 60 years). Lichen planopilaris commonly develops in association with lichen planus affecting the skin, mucosa and nails.
Figure 20. Lichen planopilaris
Is lichen planopilaris hereditary?
No, lichen planopilaris is not inherited. However, there may be genes responsible for increasing the risk of developing the condition. These genes affect the immune system and its responsiveness.
What causes lichen planopilaris?
The cause of lichen planopilaris is unknown, but it may be linked with the body’s immune system. T-lymphocytes, a type of white blood cell, are known to be involved, however, the trigger is not yet known. The hair loses its protection from the immune system. The immune system then starts to attack the hair follicle. Both lichen planopilaris and lichen planus are not contagious.
Only about 25% of patients will have signs of lichen planus elsewhere.
Lichen planopilaris signs and symptoms
Lichen planopilaris typically causes an itchy scalp. Lichen planopilaris typically presents as smooth white patches of scalp hair loss. The crown and vertex (top of the scalp) are most commonly affected, and symptoms of pain, burning and scalp tenderness may occasionally be experienced. Gradually, areas of hair loss may be noticed. No hair follicle openings can be seen in the areas of hair loss. Scale and redness surround each hair follicle at the edges of these patches, which may be spiny on palpation. Hairs can be easily pulled out. It is multifocal and small areas may merge to form larger irregular areas. Common sites of involvement are the sides, front and lower back of the scalp.
Lichen planus can also affect the skin, mouth, genitals and nails. However, only about 25% of patients will have signs of lichen planus elsewhere.
Lichen planopilaris symptoms are often absent, but they may include:
- Itch
- Pain
- Tenderness
- Discomfort
- Burning.
Lichen planopilaris is usually slowly progressive.
Diffuse hair loss is uncommon.
Dermoscopic findings:
- In the centre of the bald areas there is a lack follicular orifices
- On the margin, the pink / red translucent inflammation is clearly perifollicular, with keratin scale surrounding and extending along the proximal part of the hair shafts
Differentiation from discoid lupus erythematosus (DLE) of the scalp can sometimes be difficult. In discoid lupus erythematosus (DLE), inflammation is not restricted to surrounding hairs, and the affected skin can become telangiectatic. Although follicular lichen planopilaris and discoid lupus erythematosus (DLE) can be seen in the same patient, this is very rare
Lichen planopilaris diagnosis
Lichen planopilaris is suspected on the clinical presentation and careful examination of the mouth, nails and skin for evidence of lichen planus elsewhere.
Trichoscopy reveals absent follicles, white dots, tubular perifollicular scale and perifollicular erythema.
The diagnosis may be confirmed on a scalp biopsy that includes hairs with surrounding redness and scale at the edge of an area of hair loss. Lichen planopilaris is an example of a primary lymphocytic folliculitis.
However, it is not always possible to make a diagnosis on biopsy. Biopsy from an already scarred area of hair loss is unhelpful. Where there is only patchy scarring hair loss and no evidence of inflammation the diagnosis may not be able to be confirmed.
Lichen planopilaris treatment
Although lichen planopilaris often burns itself out within 2-3 years, treatment should be initiated early because, as with other cause of scarring alopecia, hair loss is irreversible. The aim of treatment is to slow the progression of the disease and relieve symptoms. Hair loss may continue, although at a slower rate.
Anti-inflammatory treatment options include:
- Corticosteroids – potent topical, intralesional, oral
- Dermovate scalp application applied daily
- Intralesional injections of triamcinolone acetonide can be used as an additional treatment in areas of local and severe inflammation
- Topical tacrolimus
- Hydroxychloroquine
- Tetracycline antibiotic, which have anti-inflammatory effects e.g. Lymecycline 408 mg twice daily or doxycycline 100 mg twice daily
- Acitretin
- Methotrexate
- Ciclosporin
- Mycophenolate mofetil
- Pioglitazone (an oral PPAR-γ agonist).
Response to treatment is variable and some published studies contradict others as to the efficacy. The best patients can expect is to stop the progression of hair loss and to minimise scalp discomfort.
Monitoring
In addition to serial photography, the amount of scale can be a good guide to treatment success. A reduction/absence of scale suggests treatment is helping. Erythema is not a good guide of activity, not least because steroids and UV exposure can give an erythematous appearance
A management protocol was suggested by Mirmirani et al in 2003 176:
- Make a diagnosis of lichen planopilaris clinically and with a scalp biopsy.
- The severity of symptoms, the extent of hair loss, and presence of disease activity are documented at each visit, approximately every 3 months.
- Oral hydroxychloroquine (usually 200 mg twice daily) is started after appropriate laboratory tests and eye check if the patient is symptomatic, has progressive hair loss or signs of active disease.
- Intralesional and potent topical corticosteroids may also be used.
- After 2-4 months, hydroxychloroquine is changed to ciclosporin (3-5mg/kg/d) if symptoms continue, the extent of hair loss progresses, or there are clinical signs of disease activity.
- Ciclosporin is used according to the ciclosporin consensus guidelines.
Since 2009, there have been several reports of the use of the antidiabetic agent pioglitazone (off-label) for the treatment of lichen planopilaris. Its efficacy has varied; up to 50–70% of patients have reduced symptoms, inflammation, and disease progression. Side effects include ankle swelling and weight gain.
Adalimumab, a tumor necrosis factor-alpha (TNFα) inhibitor, has also been reported to be effective in a few patients.
Camouflage with careful hair styling and hair colouring. Hairpieces may be required for areas of permanent hair loss.
Surgery such as scalp reduction and hair transplantation has been used for end-stage disease with large areas of scarring but is not always successful.
Folliculitis decalvans
Folliculitis decalvans belongs to a group of disorders called cicatricial or scarring alopecias that destroy the hair follicle, replace it with scar tissue, and eventually causes permanent hair loss. Decalvans is a term derived from the Latin meaning “making bald.” Folliculitis decalvans is further classified as a neutrophilic scarring alopecia, based upon the type of inflammation (neutrophils) seen on routine biopsies in the early stages of the disease. Folliculitis decalvans typically starts with crops of red bumps around the base of the hair follicles, which then develop marked inflammation, pustules formation, crusting and eventually patchy hair loss.
Tufted hair folliculitis is probably a subset of folliculitis decalvans although tufting can be seen in other forms of cicatricial alopecia as well.
Although extensive epidemiologic studies are not available, the incidence of folliculitis decalvans comprises about 11% of all scarring alopecia cases. Folliculitis decalvans typically occurs in young and middle-aged adults with a slight male predominance. There are probably no racial differences in prevalence, although it has been claimed to be more common in African-American compared to Caucasians. Folliculitis decalvans can be seen families, thereby supporting a genetic predisposition. Familial cases have been rarely reported.
Folliculitis decalvans usually presents in the 4th and 5th decades, with a male predominance. Children are not affected.
Figure 21. Folliculitis decalvans
Folliculitis decalvans causes
The cause of folliculitis decalvans is unknown, however it has been speculated that the disease reflects an abnormal immune response to bacteria, most commonly Staphylococcus aureus, although this is not yet proven. Such organisms may act as “superantigens” and may escape detection by the host immune system. Interestingly, patients with folliculitis decalvans typically do not have evidence of bacterial infection elsewhere on the skin, nor do they have any evidence of immune deficiency.
Folliculitis decalvans signs and symptoms
Folliculitis decalvans typically affects the scalp, often around the crown, but may affect the beard area, axillae, limbs, and pubic hair. The characteristic clinical features include:
- Irregular, atrophic white patches of scarring and hair loss — solitary or multiple
- Induration of the scalp
- Follicular pustules and perifollicular crusts at the patch periphery
- Follicular hyperkeratosis, scale, and erosions
- Tufting — multiple hair shafts emerge from a single hair follicle, resulting in a ‘doll’s hair’ appearance
- Mild itch, discomfort, or pain.
The initial lesions of folliculitis decalvans involves a red bump around the base hair follicle usually along the crown or posterior scalp. As the inflammatory process continues bright inflammation, pus formation, scaling, and crusting takes place. Patients occasionally report spontaneous bleeding and frequently complain about pain, itching, and/or burning sensations. As the disease progresses small discrete to extensive, irregularly shaped smooth scarred patches of hair loss develop.
Dermoscopy of folliculitis decalvans:
- Tufted hairs
- White dots
- Perifollicular erythema and scale
- Scattered follicular pustules
Folliculitis decalvans diagnosis
Experienced dermatologists will suspect the diagnosis of folliculitis decalvans from a detailed clinical history, thorough physical examination of the scalp, bacterial cultures, and a scalp biopsy.
It is important to know if the patient has a history of recurrent Staphylococcus aureus or other bacterial infections. A scalp biopsy the gold standard of diagnosis of scarring hair loss and are often performed when considering a diagnosis of folliculitis decalvans.
Skin biopsy of an early lesion shows a neutrophilic infiltrate dilating the infundibulum of the hair follicle. The follicle in later lesions has ruptured resulting in perifollicular scarring and mixed inflammatory infiltrate including foreign body giant cells.
The most helpful information from the biopsy is the type and pattern of inflammation (especially predominance of neutrophils) and the presence of bacterial pathogens. A bacterial culture from a pustule or a tissue culture from the scalp tissue can be helpful in determining the best treatment plan.
Folliculitis decalvans treatment
Early diagnosis and therapeutic intervention can often prevent permanent damage to hair follicles. Once a definitive diagnosis is made, we can determine the best treatment plan for everyone. Some regrowth is even possible with treatment.
Since bacteria (most commonly Staphylococcus aureus) seems to play an important role in the pathogenesis of folliculitis decalvans, appropriate and long-term antimicrobial therapy directed at the predominant pathogen is needed to control the disease process. Repeated culture of pustules is needed to guide the choice of antimicrobial agents, and this may change over time. Several different oral antibiotics, such as the tetracyclines, trimpethoprim-sulfamethoxale, and erythromycin have been shown to be beneficial. Rifampin 300 mg twice daily over 10–12 weeks is believed to be the best treatment for S. aureus eradication and may lead to successful long-term remission. It is strongly recommended to use rifampin in combination with clindamycin 300 mg twice daily to avoid rapid emergence of resistance.
Topical antibiotics such as mupirocin, clindamycin or topical anti-septics such as chlorhexidine can be helpful in conjunction with oral antibiotics.
Topical corticosteroids can help decrease inflammation and calm associated symptoms (itching, redness, or pain). Topical tacrolimus may also be helpful as a steroid sparing topical agent. Additionally, injections of corticosteroid, such as triamcinolone acetonide, may be used in inflamed and symptomatic areas, every 4-6 weeks.
Other oral anti-inflammatory agents that can be used include oral corticosteroids (for short periods of time), oral isotretinoin, and dapsone.
Other systemic agents (off-label):
- Oral isotretinoin, dapsone, tacrolimus, apremilast
- Intravenous immunoglobulin, tumour necrosis factor inhibitors
Physical therapies
- Photodynamic therapy
- Surgery for permanent hair loss if in remission.
Folliculitis decalvans prognosis
Folliculitis decalvans usually follows a chronic fluctuating course of exacerbations and remissions over many years. It is not clear that treatment influences the long-term prognosis despite successfully reducing inflammation in the short-term. Early diagnosis and treatment are important but permanent hair loss is to be expected.
Cutaneous lupus erythematosus
Cutaneous lupus erythematosus is a diverse group of autoimmune connective tissue disorders localized to the skin that can be associated with systemic lupus erythematosus (SLE) to varying degrees.
Cutaneous lupus erythematosus is classified as:
- Acute cutaneous lupus erythematosus (ACLE)
- Subacute cutaneous lupus erythematosus (SCLE)
- Intermittent cutaneous lupus erythematosus (lupus tumidus)
- Chronic cutaneous lupus erythematosus (CCLE) eg, discoid lupus (DLE), lupus profundus, chilblain lupus erythematosus.
Chronic lupus erythematosus occurs more frequently in females than males and more commonly in adults than children. Most patients with chronic cutaneous lupus erythematosus (CCLE) only have evidence of lupus in their skin, and do not have systemic lupus erythematosus (SLE) and will never develop internal problems related to lupus. However, up to 75% of patients with SLE develop cutaneous signs, and these may be the first indication of SLE in 25%. A physical examination together with blood and urine tests can be used to determine who is likely to have internal problems with their lupus. Blood tests may be necessary to rule out SLE. It is important to determine which patients with cicatricial alopecia secondary to lupus do have SLE, because they may need special treatment for internal organ involvement, especially kidney disease.
There is a marked female predominance with cutaneous lupus erythematosus particularly affecting women 20 to 50 years of age. However, all age groups and both sexes can be affected. Skin of color is an important predisposing factor.
Depending on the severity of the skin lupus, you may require courses of oral treatment in addition to topical treatments. Cicatricial alopecia caused by lupus may actually show some hair regrowth with treatment which is different from other types of cicatricial alopecia where prevention of further loss and not regrowth is the hoped for result of effective therapy.
Treatments utilized for cicatricial alopecia caused by chronic cutaneous lupus erythematosus (CCLE) include corticosteroids (topical, intralesional or internal), antimalarial pills such as plaquenil, vitamin A derivatives, Dapsone and even Thalidomide. Each drug has its own potential side effects for which you would need to be monitored. Surgery can also be helpful to remove areas of scar but surgical removal of bald areas should be approached cautiously, as it can sometimes result in a flare of the skin disease in surrounding skin.
Figure 22. Cutaneous lupus erythematosus
Cutaneous lupus erythematosus causes
The pathogenesis of cutaneous lupus erythematosus is multifactorial:
- Genetic susceptibility: High incidence among family members
- Environmental factors
- Cigarette smoking
- Sun exposure
- Medications
- Innate and adaptive immune responses
- Autoantibodies.
The cause of cutaneous lupus erythematosus is not fully understood but is thought to be an autoimmune disease in which the body’s immune system, which protects against infection, mistakenly attacks various parts of the body, including the skin. The mistake made by the immune system in cutaneous lupus erythematosus is to view the cells in your skin as ‘foreign’ and to make antibodies that damage them.
Cutaneous lupus erythematosus is more common in women than men and sunlight (ultraviolet light) can often trigger the rash. Cutaneous lupus erythematosus can sometimes be caused by medication, including some diuretics (i.e. water tablets). However, any treatment should not be stopped without discussing it with the doctor. Cutaneous lupus erythematosus is not infectious.
Cutaneous lupus erythematosus signs and symptoms
Chronic cutaneous lupus erythematosus (CCLE) is the most common form of cutaneous lupus erythematosus and about 25% of SLE patients have some form of chronic cutaneous lupus erythematosus (CCLE).
Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI)
The Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) scores activity and damage in each of 12 anatomical locations.
- The total activity score:
- Degree of redness (0-3) and scale (0-2)
- Mucous membrane involvement (0-1)
- Recent hair loss (0-1), nonscarring alopecia (0-3)
- Total damage score:
- Degree of dyspigmentation (0-2) and scarring (0-2)
- Persistence of dyspigmentation more than 12 months doubles the dyspigmentation score
- Scalp scarring (0, 3, 4, 5, 6).
Cutaneous lupus erythematosus diagnosis
A doctor may be able to make a cutaneous lupus erythematosus diagnosis after an examination, but in most cases it is necessary to take a small sample of skin (a biopsy) to be examined under a microscope in order to confirm the diagnosis. The doctor will probably also take a sample of blood to test for specific antibodies (known as “ENA” [extractable nuclear antigen] or “Ro and La”) which appear to be important in the condition. A routine blood screen may also be carried out.
- Skin biopsy — diagnostic histopathology and direct immunofluorescence is seen only in specific-lupus erythematosus lesions
- Blood tests — full blood count, renal function test, inflammatory markers
- Serology — including antinuclear antibody (ANA), extractable nuclear antigen (ENA) – are often negative in chronic cutaneous lupus erythematosus (CCLE)
- Immunofluorescence – may occasionally aid in diagnosis. Deposition of immunoglobulin, predominantly IgG, and/or complement at the dermoepidermal junction is a characteristic feature of lupus erythematosus. Skin biopsies for immunofluorescence testing are best taken from skin lesions
Cutaneous lupus erythematosus treatment
Because cutaneous lesions of lupus are known to be induced or exacerbated by exposure to ultraviolet light, a logical approach in the management of cutaneous lupus erythematosus must include sun avoidance and the liberal application of SPF 50+ broad spectrum sunscreen and UPF 50+ sun-protective clothing 177. Topical corticosteroid ointments are the mainstay of treatment of cutaneous lupus erythematosus. Patients usually start with a potent topical steroid applied twice a day, then switch to a lower-potency steroid as soon as possible. The minimal use of steroids reduces the recognized side effects like atrophy, telengiaectasiae, striae, and purpura.
Intralesional steroids are particularly useful to treat chronic lesions, hyperkeratotic lesions, and those that do not respond adequately to topical steroids. Lesions at particular sites, eg, the scalp, may also benefit. Recognized side effects of intralesional steroids include cutaneous atrophy and dyspigmentation, which are not significant risks in experienced hands 178. Oral steroids may be required for the control of systemic lupus but are not generally beneficial in discoid lupus erythematosus (DLE). For patients with progressive or disseminated disease or in those with localized disease that does not respond to topical measures, the addition of systemic agents should be considered.
Other treatments used by dermatologists and reported to be helpful, include tacrolimus and pimecrolimus ointments, which can be used together on the skin with corticosteroids.
Other forms of treatment have recently been found to be useful in the treatment of discoid lupus erythematosus. Gul et al 179 described a case of generalized discoid lupus erythematosus successfully treated with 5% Imiquimod cream applied to lesions once a day 3 times a week. After 20 applications all of the lesions regressed significantly. Usmani and Goodfield 180 reported good to excellent responses in 12 out of 13 patients with discoid lupus erythematosus who were treated with efalizumab, a monoclonal antibody directed against CD 11a (discoid lupus is known to be predominantly t-cell mediated). Finally, Koch et al 181 suggest cryotherapy as a treatment option in cases of discoid lupus erythematosus lesions that are resistant to local or systemic recommended therapy. The standard therapies for the management of cutaneous lupus, including sunscreens, protective clothing, and behavioral alteration, and topical steroids with or without an antimalarial agent are often not used appropriately and can result in a situation in which the patient has a refractory disease 182.
Sometimes tablets are also needed if ointments and sunscreens do not work, or if general health is affected. Treatment with antimalarial drugs constitutes first-line systemic therapy for discoid lupus erythematosus (DLE). Therapy with antimalarials, either used singly or in combination, is usually effective 183. The 3 commonly used preparations include chloroquine, hydroxychloroquine and mepacrine. Mepacrine is not freely commercially available in the United States but is freely available in other countries. It is customary to start hydroxychloroquine at a dose of 200 mg per day for an adult and, if there are no untoward gastrointestinal or other side effects, to increase the dose to twice a day. No more than 6.5 mg/kg/day should be administered. It is important to emphasize to the patient that it may take between 4 to 8 weeks for any clinical improvement. In some patients who do not respond to hydroxychloroquine, chloroquine may be more effective. Some patients do not respond well to monotherapy with either hydroxychloroquine or chloroquine, and in such cases the addition of mepacrine may be of benefit 184.
In general, hydroxychloroquine and mepacrine are safe, well-tolerated drugs and adverse effects are relatively few, the most widely recognized being retinal toxicity 185. Chloroquine causes macular pigmentation that progresses to a typical bull’s eye lesion and then to widespread retinal pigment epithelial atrophy resembling retinitis pigmentosa 185. This is dose related and can largely be avoided. The side effect spectrum between chloroquine and hydroxychloroquine is different, with ocular toxicity being mainly, although perhaps not exclusively, seen after chloroquine use. To prevent overdosing, doses should be calculated not on the actual weight of the patient but on ideal (lean) body weight 186; this substantially reduces the risk of retinal toxicity.
Other adverse effects of antimalarials include gastrointestinal symptoms, eg, nausea and vomiting, and cutaneous side effects including pruritus, lichenoid drug reactions, annular erythema, hyperpigmentation, and hematological disturbances like leukopenia and thrombocytopenia 187. Hemolysis is reported in individuals who are deficient in the enzyme glucose-6-phosphate-dehydrogenase. Hydroxychloroquine has, on rare occasions, caused toxic psychosis when used for the treatment of discoid lupus 188. Prolonged mepacrine therapy may produce a yellow discoloration of the skin and urine. Hepatitis and aplastic anemia have also been reported.
A few patients may require other drugs such as methotrexate, acitretin, mycophenolate, dapsone, ciclosporin, thalidomide or oral corticosteroids.
Thalidomide
Thalidomide may provide one of the most useful therapeutic alternatives for chronic refractory discoid lupus erythematosus, although its distribution is limited to a few countries because of the risk of teratogenicity and polyneuropathy 189. However, in a retrospective study of 18 patients with chronic discoid lupus erythematosus, Brocard et al 190 found low-dose thalidomide treatment was efficacious with good tolerance, with the most frequent side effect being usually mild asthenia.
Methotrexate
In 1995, Bottomley and Goodfield 191 found that methotrexate may be of help to patients with discoid lupus erythematosus resistant to conventional treatment; short-term treatment is unlikely to be complicated by any significant side effects. Full blood count and liver function along with renal function need to be checked before commencing treatment with methotrexate and regularly thereafter because it can cause myelosuppresion and hepatic and renal impairment.
Cyclosporin A
Cyclosporin A is a potent immunosuppressant because of its immunomodulating effect on helper T-cell function, inhibiting lymphocyte activation and proliferation. Because discoid lupus erythematosus is an inflammatory dermatosis with T-cell infilterate it should not be surprising if cyclosporine is effective in the management of the condition. In 1994 Yell and Burge 192 tried cyclosporine in 2 patients with severe discoid lupus erythematosus and concluded that it was effective at a dose of 4 to 5 mg/kg/day, but others have not confirmed this finding. Blood pressure and kidney function need to be monitored, and hypertension is a common side effect. It can also cause gingival hyperplasia and hirsutism. Lipid disturbances can also occur and therefore serum cholesterol and triglycerides have to be monitored.
Tacrolimus
Tacrolimus is a macrolide derived from the fungus Streptomyces tsukubaensis and has been used in recent years to treat a number of inflammatory and autoimmune conditions. When used as an ointment it acts as a local immunosuppressive agent. Walker et al 193 reported 2 patients with severe recalcitrant chronic discoid lupus that had not responded to potent topical steroids or antimalarials but dramatically responded to topical tacrolimus ointment in one case and a combination of clobetasol ointment and tacrolimus in the other. Recently, Tzung et al 194 conducted a randomized double-blind study in which 20 patients were enrolled but only 11 women and 7 men (13 with malar rash of SLE, 4 with discoid lupus erythematosus, and 1 with subacute cutaneous lupus erythematosus [SCLE]) completed the study. All patients had facial cutaneous lupus erythematosus and were instructed to apply 0.1% tacrolimus ointment twice daily to the affected areas on one side of the face and 0.05% clobetasol propionate ointment on the other side; this was randomly assigned for each patient. The severity of lesions was assessed at each visit (weeks 0–4 and posttreatment week 4) using a 7-point rating scale. They found tacrolimus was as efficient as clobetasol in treating cutaneous lupus erythematosus 194.
Mycophenolate mofetil
Mycophenolate mofetil is an immunosuppressive agent that has been added relatively recently to the other drugs in this group and has been used increasingly in recent years for the treatment of various dermatoses that are inflammatory or autoimmune in origin. Mycophenolate is an ester prodrug of mycophenolic acid, initially isolated from Penicillium species 195. Goyal and Nousari 196 described 2 cases of refractory discoid lupus involving the palms and soles that responded satisfactorily to mycophenolate mofetil.
Azathioprine
Azathioprine, a potentially toxic drug, has been used in refractory cases of discoid lupus, with particular success among those with the involvement of the palms of the hands and the soles of the feet 197. Azathioprine is a synthetic derivative of 6-mercaptopurine and is an immunosuppressive drug. There are wide differences in the activity of the enzyme thiopurine methyltransferase in different individuals, which can be measured by a blood test. The chances of myelosuppression in a patient with very low levels of thiopurine methyltransferase are significantly greater than in others.
Cutaneous lupus erythematosus prognosis
Cutaneous lupus erythematosus can be the presenting sign of SLE, as in acute cutaneous lupus erythematosus, or may evolve into SLE.
Female patients with cutaneous lupus erythematosus and Ro/La autoantibodies should be advised of the risk their baby may have neonatal lupus erythematosus including congenital heart block.
Chronic cutaneous lupus erythematosus tends to follow a chronic relapsing course for years, with flares in spring and summer, and resolution with scarring if untreated.
Classic pseudopelade of Brocq
Pseudopelade of Brocq is a type of scarring hair loss condition. The hair loss in the bald areas is permanent. Pseudopelade, a French word for “imitator of alopecia” describes a type of scarring hair loss that at first glance looks like alopecia areata (a non-scarring autoimmune type of hair loss). But on closer inspection one sees smooth shiny patches of hair loss with loss of follicular openings. The term has been confusing because it can represent either new onset scarring alopecia with no evident inflammation or symptoms, or late-stage lichen planopilaris which is considered ‘burnt-out.” Many have suggested that the term Pseudopelade of Brocq be abandoned in favor of a late stage lichen planopilaris (LPP).
Both men and women can develop pseudopelade, although it is 3 times more common in middle-aged and older women. However, pseudopelade of Brocq can present in both sexes and all age groups including children. Pseudopelade of Brocq has rarely been reported to affect more than one family member.
Several features lead to this diagnosis, including areas of scarring hair loss, some redness around the hair follicles, depression of the skin, and findings on the biopsy showed very little inflammation, loss of the fat glands and scarring
Pseudopelade is challenging to treat. Even with treatment, some cases to spread, albeit very slowly.
Pills may be used such as Doxycycline, Plaquenil, Isotretinoin (Accutane), Mycophenolate mofetil. Injection of steroids may be advised. Use of topical medicines you apply yourself at home may also be advised (Clobetasol, Protopic, Clobex).
Figure 23. Pseudopelade of Brocq
Pseudopelade of Brocq causes
The cause of pseudopelade of Brocq is unknown, as is the mechanism by which the hair loss occurs. Recent studies have shown the cells in the inflammatory infiltrate and the switched-on genes are different in pseudopelade of Brocq compared to lichen planopilaris and discoid lupus erythematosus, thus providing further evidence this is a distinct entity.
Pseudopelade of Brocq is not an inflammatory form of alopecia and the hair loss seems to be due to atrophy of the hair follicles rather than scarring.
Pseudopelade of Brocq signs and symptoms
Pseudopelade of Brocq is often found accidentally as a small patch of hair loss on the scalp. Single hairs may persist in the otherwise hairless patch. The hair loss usually begins at the vertex, the highest point on the scalp. The sides of the scalp (parietal scalp) is another area commonly involved. Rarely it has also been reported in the beard area.
The clinical features of the patch(es) of hair loss are:
- Single or multiple
- Smooth
- Soft
- Slightly depressed
- Round or oval
- Small
- May merge to become larger and irregular in shape
- Asymptomatic
- Not scaly
- Skin-coloured
- May see pale red/pink around individual hairs initially
- Hairs may be easily pulled from the edges if active
The bald areas have been described as looking like ‘footprints in the snow’.
The progress of pseudopelade of Brocq is unpredictable however it is generally a slow process. Some patients are left with only one small area of hair loss whereas for others it continues for many years resulting in a significant cosmetic defect.
Pseudopelade of Brocq diagnosis
Pseudopelade of Brocq must be distinguished from ‘pseudopelade’, which is an umbrella term for any form of patchy scarring alopecia such as lichen planopilaris or discoid lupus erythematosus.
A scalp biopsy from a smooth patch shows a thin epidermis and sclerotic dermis with streamers of fibrosis down to the fat layer. There is no inflammation seen. Biopsy early in the process may show a small number of lymphocytes around the upper 2/3 of the hair follicle.
Braun-Falco proposed clinical and histological criteria for making the diagnosis 198:
- Clinical criteria
- Irregular and confluent patches of hair loss
- Moderate atrophy (late stage)
- Mild redness around hair follicles (early stage)
- Female predominance (3:1)
- A long course of more than 2 years
- Slow progression
- Spontaneous termination possible
- Histological criteria
- No marked inflammation
- No widespread scarring
- No significant plugging of hair follicles
- No sebaceous (oil) glands
- Normal epidermis
- Fibrotic streamers in dermis
- Immunohistochemistry
- Negative
Pseudopelade of Brocq treatment
Pseudopelade is challenging to treat. No treatment has yet been found to stop the process or to regain lost hair. Pills may be used such as Doxycycline, Plaquenil, Isotretinoin (Accutane), Mycophenolate mofetil. Injection of steroids may be advised. Use of topical medicines you apply yourself at home may also be advised (Clobetasol, Protopic, Clobex). Even with treatment, some cases to spread, albeit very slowly.
Surgery may help to improve the cosmetic appearance once the condition is no longer active.
Dissecting cellulitis
Dissecting cellulitis of the scalp also known as perifolliculitis capitis abscedens et suffodiens, dissecting folliculitis or Hoffman disease, is an uncommon cause of scarring alopecia. Dissecting cellulitis of the scalp is characterized by multiple painful sometime fluid filled nodules, interconnecting sinus tracts, and draining pus over large areas of the scalp. The condition tends to be chronic and progressive, and ultimately leads to scarring and extensive hair loss. Dissecting cellulitis of the scalp sometimes occurs in people who have other forms of follicular occlusion syndrome.
Dissecting cellulitis of the scalp is most commonly seen in men of African descent in the third, fourth and fifth decades of life. African women, Caucasians and other ethnicities, and occasionally children, can also present with this condition.
In some patients, only the scalp hair follicles are affected. As a follicular occlusion disorder, there is a defect in normal keratin production in the hair follicles. As a result, the hair follicles become obstructed by oil (sebaceous contents) and keratin debris. Ultimately the follicles burst resulting in an intense inflammatory reaction. It has been suggested that the cause of dissecting cellulitis may involve an abnormal host response to bacteria. However, patients with dissecting cellulitis have no evidence of immune deficiency or presence of infection at other skin sites.
Figure 24. Dissecting cellulitis of the scalp
Dissecting cellulitis causes
Dissecting cellulitis is considered part of the follicular occlusion triad that also includes acne conglobata and hidradenitis suppurativa. A defect in follicular keratinization causes occlusion and subsequent inflammatory destruction of the hair follicle. An aberrant immune to response to commensal bacteria may be involved with the pathogenesis, particularly coagulase-negative staphylococci.
Dissecting cellulitis signs and symptoms
Dissecting cellulitis can affect single or multiple areas of the scalp, it frequently begins in the posterior scalp or crown although any region of the scalp may be involved. Dissecting cellulitis is characterized by multiple painful sometime fluid filled nodules, interconnecting sinus tracts, and purulent drainage may be present over large areas of the scalp. The condition tends to be chronic and progressive, and ultimately leads to scarring and extensive hair loss.
Hair loss is temporary initially, but the deep inflammation eventually leads to patchy cicatricial alopecia, which can be very extensive.
Dissecting cellulitis signs can include:
- Perifollicular and follicular pustules
- Nodules and pseudocysts, often with purulent exudate
- Interconnecting sinuses
- Abscess
- Hair loss
- Keloid scars
- Cutaneous squamous cell carcinoma has been reported.
Patients with dissecting cellulitis may also have hidradenitis suppurativa, nodulocystic acne and/or pilonidal disease.
Dissecting cellulitis can be complicated by secondary bacterial infection due to Staphylococcus aureus, Pseudomonas, and anaerobic bacteria.
Dissecting cellulitis is also associated with arthritis and spondyloarthropathy. Sacroiliitis is reported in three-quarters of sufferers.
Dermoscopy features of dissecting cellulitis
Three stages of disease progression have been described on dermoscopy.
- Early phase resembles alopecia areata with black dots, broken hairs, and yellow dots.
- Abscedens phase shows three-dimensional large yellow dots with a soap-bubble appearance, and yellow structureless areas.
- Fibrotic phase resembles the end stage of any cicatricial alopecia with white areas lacking follicular openings, but with a characteristic feature of cutaneous clefts with hair tufts.
Dissecting cellulitis diagnosis
Dermatologists will suspect the diagnosis of dissecting cellulitis from a detailed clinical history, thorough physical examination of the scalp, bacterial cultures, and a scalp biopsy. It is important to know if the patient has a history of recurrent Staphylococcus aureus or other bacterial infections. A scalp biopsy the gold standard of diagnosis of scarring hair loss and are often performed when considering a diagnosis of dissecting cellulitis. The most helpful information from the biopsy is the type and pattern of inflammation (mixed inflammatory infiltrate), presence of scarring and granuloma formation and sometimes the presence of bacterial pathogens.
A swab of the exudate for culture is recommended to identify secondary bacterial infection. A fungal culture may also be performed to rule out tinea capitis (fungal infection of the scalp). However the swab is often sterile.
Dissecting cellulitis treatment
Treatment of dissecting cellulitis often requires a multi-pronged approach, especially initially and during acute flares.
Medical treatment
- Oral antibiotics, such as tetracyclines or erythromycin for their anti-inflammatory and antibacterial action
- Oral isotretinoin
- Oral corticosteroids
- Intralesional corticosteroid injection
- Analgesia
- Topical antiseptic cleansers
- Oral zinc
- Biological agents such as antitumour necrosis factor-alpha (TNF) inhibitors, such as adalimumab.
Surgical treatment
- Incision and drainage of abscesses
- Surgical excision
- Surgical resection and skin grafting
- Laser epilation
- Carbon dioxide laser ablation
- Radiotherapy
- Photodynamic therapy.
Treatment of dissecting cellulitis is challenging in terms of permanent arrest of the condition and full hair regrowth. In patients with a positive bacterial culture, antibiotics should be directed at eradication of that organism. In patients with a negative bacterial culture, oral isotretinoin may be helpful, though it must be started in small doses to prevent worsening inflammation. However, relapses are frequent upon discontinuation of the above medications, and treatment is invariably long-term. Partial hair regrowth may occur if the dissecting cellulitis is treated early.
Recently several reports have shown favorable response to anti-tumor necrosis factor (TNF) therapy for dissecting cellulitis and hidradenitis suppurativa. Infliximab and adalimumab have been used in a small number of patients with dissecting cellulitis with good results while on therapy. However, relapses are common when the drugs are discontinued, and the underlying sinus tracts are not altered. Long-term medical treatment combined with surgical drainage and resection of affected areas are often needed in dissecting cellulitis.
Dissecting cellulitis prognosis
Dissecting cellulitis usually follows a chronic course with variable relapses. The subsequent scarring alopecia results in permanent, patchy hair loss.
Alopecia mucinosa
Alopecia mucinosa also called follicular mucinosis, is a clinic-pathological entity which presents with several erythematous papules or acneiform eruptions and plaques mainly over the head and face regions with associated loss of hair 199. Early signs of the disease are the presence of grouped follicular papules arising in patches or plaques, usually 2-5 cm in diameter. Hair loss is common from the affected hair follicles. In some patients the condition resolves spontaneously and the hair regrows. In more severe disease complete follicular destruction prevents normal hair growth. Other features can include acneiform lesions.
Alopecia mucinosa has been divided into the primary (idiopathic) alopecia mucinosa and the secondary alopecia mucinosa associated with cutaneous T cell lymphoma, hematopoietic stem cell transplantation (HSCT), hematological malignancies (Hodgkin’s lymphoma, acute myeloblastic leukemia and chronic lymphocytic leukemia) 200, 201, 202 and other malignant and benign skin disorders 203, 204, 205. However, alopecia mucinosa is typically reported in the context of cutaneous T-cell lymphomas, specifically folliculotropic mycosis fungoides 206. It is a matter of controversy whether alopecia mucinosa (follicular mucinosis), especially when seen in a generalized eruption, may represent an early variant or a precursor stage of mycosis fungoides 207.
Primary (idiopathic) alopecia mucinosa is an uncommon benign and transient disorder that is typically reported in children and young adults as well as older adults with locally distributed follicular-based papules, patches or plaques 208. Primary (idiopathic) alopecia mucinosa in young people tends to involve the head and neck, with resolution after 2 to 24 months 208. Most of the cases have a benign course and demonstrate spontaneous resolution in the pediatric population. However, rare case reports of the development of Hodgkin’s disease, other lymphomas, and leukemia have been seen 204. Primary (idiopathic) alopecia mucinosa in older adults usually has widespread lesions and can last indefinitely 199.
Mycosis fungoides-associated alopecia mucinosa is more commonly described in older patients with a diffusely distributed eruption 209. Mycosis fungoides-associated alopecia mucinosa and idiopathic alopecia mucinosa frequently look similar clinically, and their histopathologic features, immunohistochemical phenotype and T-cell receptor gene-rearrangement status overlap, thus diagnosis is based on a combination of clinical, histopathologic, immunohistochemical and molecular data 210.
The exact mechanism underlying the deposition of mucin selectively within the hair follicle is unknown 208. Follicular keratinocytes have been postulated to be the source of mucin and an etiologic role has been proposed for cell-mediated immune mechanisms and possible stimulation by cytokines released from perifollicular T lymphocytes 203.
Unfortunately, no clinical or histopathological pattern is predictive of its course or can reliably differentiate primary (idiopathic) alopecia mucinosa from secondary alopecia mucinosa 204. Many have proposed criteria to differentiate primary (idiopathic) alopecia mucinosa from secondary alopecia mucinosa with considerable difficulty. It has been suggested that patients with clonal TCR gene rearrangements may be at a higher risk for the development of lymphoma, although long-term follow-up of seven patients younger than forty years of age failed to show progression to alopecia mucinosa despite the presence of a clonal TCR gene rearrangement 211.
There are no specific treatments for idiopathic alopecia mucinosa. A wait and see approach is usually recommended and most cases resolve within 2 to 24 months 204. Current treatment options include: topical, intralesional and systemic corticosteroids, dapsone, antimalarials, isotretinoin, indomethacin, dapsone, and interferon, minocycline. And photodynamic therapy. Sufficient long-term follow-up may be the ultimate criteria to assess the biologic nature of this entity 212.
Figure 25. Alopecia mucinosa
Central centrifugal cicatricial alopecia
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring alopecia which starts in the center of the scalp that results in permanent hair loss. Central centrifugal cicatricial alopecia is the most common form of scarring hair loss seen in black women (African ethnicity). However, it may be seen in men and among persons of all races and hair color (though rarely). Middle-aged women are most commonly affected. Central centrifugal cicatricial alopecia usually presents in the fourth decade with a female to male ratio of approximately 3:1.
Central centrifugal cicatricial alopecia (CCCA) is a diagnostic category adopted by the North American Hair Research Society to encompass terms such as hot comb alopecia, follicular degeneration syndrome, pseudopelade in African Americans, and central elliptical pseudopelade in whites 213. Despite the many attempts to clarify and unify the terminology of central centrifugal cicatricial alopecia patterns of scarring alopecia, CCCA is not clearly a diagnostic entity in and of itself.
Clinically there is a diffuse scarring alopecia that begins at the crown and spreads forwards. The alopecia is incomplete with a number of hairs remaining within the area of scarring. There tends to be little or no redness.
The hair loss caused by CCCA is permanent, but treatment can help to slow down or stop any more hair loss from happening.
However, no ideal treatment currently exists. Minimal hair grooming is recommended, but many patients find this difficult. If there are signs of inflammation (clinically or histologically) the use of a potent topical or intralesional corticosteroid may arrest or slow progression. Occasionally pustules are seen in which case a systemic tetracycline such as doxycycline or lymecycline may be of value. Other treatments occasionally used in the presence of inflammation include those used for lichen planopilaris.
Figure 26. Central centrifugal cicatricial alopecia
Central centrifugal cicatricial alopecia causes
The exact cause of CCCA is unknown, but it has often been linked to hair styling practices, which include: heat (hot combs/hair straighteners, hair dryers and curling irons); traction (tight braids/cornrows, weaves, tight ponytails or hair extensions that pull on the hair); and use of chemical relaxers (especially lye relaxers).
More recently, changes in a gene called PADI3, which encodes peptidyl arginine deiminase type III (PADI3), an enzyme that modifies proteins that are essential to formation of the hair-shaft have been linked with development of the condition in about 25% of cases. Other proposed causative factors include fungal infections, bacterial infections, autoimmune disease, and genetics. Several studies have also reported an association of type 2 diabetes with hair loss, and potentially with CCCA.
Traction (from heated styling instruments or chemical straighteners), pattern hair loss and iron-deficiency may co-exist. Central centrifugal cicatricial alopecia is also associated with hirsutism.
Central centrifugal cicatricial alopecia signs and symptoms
In CCCA hair tends to start falling out in the middle of the scalp. Hair loss then gradually spreads out from that point in a centrifugal manner. Often, people with CCCA notice hair being more brittle and breaking more easily. Other symptoms include itching, pain or tenderness of the scalp, a spongy texture to the scalp, and flaking or redness of the affected areas.
There is loss of the follicular openings on examination of the scalp. Thus, the scalp may appear shiny. While some persons do not have symptoms, tenderness, itch and burning are common. Hair breakage may also be an early sign of central centrifugal cicatricial alopecia. Hair loss is slowly progressive. A photographic scale has been developed to rate the severity of the central hair loss.
Central centrifugal cicatricial alopecia diagnosis
Early diagnosis of CCCA is important because medical intervention can prevent further progression that often results in extensive, permanent hair loss. Your doctor will use your medical history, examine your scalp and often take a scalp biopsy, from an area at the edge of the patch of hair loss, to diagnose CCCA. Histopathology reveals a lymphocytic inflammatory infiltrate (inflammatory cells) around the infundibulum (base of the hair follicle), and fibrosis (scarring). Premature desquamation (peeling) of the inner root sheath is a common feature.
Doctors may also take a skin swab to rule out an infection of the scalp.
Central centrifugal cicatricial alopecia treatment
The hair loss caused by CCCA cannot regrow, but there are ways of slowing down and potentially stopping more hair loss from happening. In areas where the hair follicle has been replaced with fibrosis, regrowth is not possible. As the exact cause is not known, targeted therapy for CCCA is not available.
Usually, your doctor will prescribe topical or injected corticosteroids, which help to dampen down the inflammation. Other treatments include tetracyline (an antibiotic), ciclosporin (a drug that slows down the immune attack on the hair follicles) or hydroxychloroquine (an anti-malaria drug that also helps to suppress the immune system). People with CCCA are also recommended to use natural hair styles.
Treatment options for CCCA include anti-inflammatory agents such as:
- Potent topical steroids (eg clobetasol) or intralesional steroids
- Calcineurin inhibitors: tacrolimus ointment, pimecrolimus cream
- Tetracyclines (eg doxycycline 100 mg twice daily, taken for several weeks to months)
- Hydroxychloroquine
- Ciclosporin.
Hair transplantation can be considered in individuals with well-controlled CCCA for at least one year. However, graft survival is low.
Minoxidil solution may help stimulate growth in viable follicles. Seborrhoeic dermatitis should be treated with appropriate medicated shampoos and topical anti-inflammatory agents as needed.
Discontinuation of traumatic hair care practices is an essential aspect of treatment of CCCA.
Women with CCCA are encouraged to consider natural hairstyles.
- Relaxers should be performed by a professional, no more frequently than every 6-8 weeks. The scalp should not burn as a result of relaxer application.
- Minimize heat application (hooded dryers, blow dryers, hot combs and flat irons)
- Avoid tight braids and weaves/extensions
- Avoid hair style practices associated with discomfort, scalp irritation or scale
- It is important for providers to know that frequency of shampooing the hair varies among Black women. Many shampoo every 1–2 weeks. This is the norm and prevents excessive dryness.
Frontal fibrosing alopecia
Frontal fibrosing alopecia is a type of scarring hair loss along the frontal scalp hair margin (i.e. the forehead and sideburns). This happens due to inflammation and destruction of the hair follicles. There may also be hair loss from the scalp near the ears and from the eyebrows. Sometimes hair loss can also occur from other parts of the body, but this is less common 214. Frontal fibrosing alopecia was first described in a group of Australian post-menopausal women in 1994 215. Frontal fibrosing alopecia is believed to be localized form of lichen planopilaris. It most often affects white postmenopausal women over the age of 50, but it can also affect men and younger women and people of other ethnicities. Frontal fibrosing alopecia incidence is reported to be increasing in white-skinned women (possibly because of greater awareness of the condition), and it is uncommon in women with dark skin.
Frontal fibrosing alopecia may be due to hormonal changes or an autoimmune response, the exact cause of this condition is not yet known.
Frontal fibrosing alopecia presents as a progressive symmetric band-like alopecia (hair loss), affecting the frontal hair line, the preauricular scalp and, less commonly and distinctively, the retroauricular areas. ”Orphaned” hairs ie isolated hairs, may remain in areas of hair loss. The eyebrows are often affected and this may occur before the frontal scalp. Clinical inflammation is not observed in the eyebrows. The other vellus or terminal hair of the face can be involved, including the eyelashes. Additional clinical features – atrophy of affected sites with prominent forehead veins and facial papules. Progression, for the majority, is relatively slow
Dermoscopic features – clearly perifollicular, with keratin scale surrounding and extending along the proximal part of the hair shafts. Erythema often mild or absent.
There are a number of treatments that are used for frontal fibrosing alopecia to help to slow down or halt further hair loss in some people. Unfortunately, their success is variable and some people cannot find a treatment that is effective for them. Treatments used to slow the progression of the condition include oral corticosteroids, intralesional steroid injections, anti-inflammatory antibiotics such as tetracyclines, or anti-malarial tablets (hydroxychloroquine). All these treatments aim to lower the activity of the immune system and slow down the attack on the hair follicles.
What is lichen planopilaris?
Lichen planopilaris is a rare inflammatory condition that results in patchy progressive permanent hair loss mainly on the scalp. Three forms are recognized 216:
- Classic lichen planopilaris, also known as follicular lichen planus
- Frontal fibrosing alopecia
- Graham Little syndrome
Lichen planopilaris usually affects young adult women, although the age range is wide and it also affects men. It commonly develops in association with lichen planus affecting the skin, mucosa and nails.
The cause of lichen planopilaris is unknown.
Although lichen planopilaris is rare, it is one of the common causes of scarring hair loss of the scalp.
The hair loss caused by lichen planopilaris cannot be reversed, however, progression of the condition can be limited in some people. Treatments used to slow the progression of the condition include anti-inflammatory agents, including corticosteroids, ciclosporin and hydroxychloroquine. Some recent reports have also shown success using JAK-inhibitors to treat lichen planopilaris in a small number of patients.
Is frontal fibrosing alopecia permanent?
Yes, in frontal fibrosing alopecia, the hair follicles are destroyed and turned into scar tissue. Frontal fibrosing alopecia is a slowly progressive condition, which means that the areas of the scalp that are affected by the condition will gradually increase over time. In some people, the condition stops progressing and there have been some rare reports of regrowth.
Can frontal fibrosing alopecia be cured?
There are treatments that help to slow down or halt further hair loss in some people. These may be of help in follicles that are inflamed but not yet lost. Unfortunately once hair follicles have been completely lost, the skin develops a smooth shiny appearance, and the follicles and their hair cannot then regrow. There is no treatment that can cure frontal fibrosing alopecia, in areas where the follicles have gone.
Who gets frontal fibrosing alopecia?
Although the typical patient with frontal fibrosing alopecia was described as a Caucasian post-menopausal woman over the age of 50, younger women, men, and children, and all ethnic groups including Asians, Hispanics, and those of African descent can be affected.
The incidence is reported to be increasing worldwide.
Frontal fibrosing alopecia is frequently reported in patients with hypothyroidism, contact allergy to fragrances, regular sunscreen use, and autoimmune diseases including lupus erythematosus and rheumatoid arthritis.
How do clinical features differ in various types of skin?
Women of African descent with frontal fibrosing alopecia present differently from Caucasian women. Typically they present at a younger age, often in their early 40s before menopause. Itch, redness, and scale are less obvious. Lichen planus pigmentosus is commonly associated, and usually precedes the hair loss. Speckled pigmentation of hair follicles along the frontal hair margin is seen on dermoscopy. Frontal fibrosing alopecia may be overlooked due to associated traction alopecia.
Frontal fibrosing alopecia causes
The exact underlying cause of frontal fibrosing alopecia is unknown. Genetic studies of frontal fibrosing alopecia suggest that this is an autoimmune condition, in which an affected person’s immune system mistakenly attacks the hair follicles (structures in the skin that make hair) and destroys them. There is a disturbed immune response to some component of the intermediate-sized and vellus scalp hair follicles. Although triggers are not yet clear, hormones and other environmental factors are thought be involved. A 2016 study suggests that there are both genetic and environmental components involved in frontal fibrosing alopecia. An androgen-dependent etiology has been suggested by the predominance of post-menopausal patients. Contact allergy or photocontact allergy to cosmetics, moisturizing creams, hair dye, and sunscreens have been suggested as possible but unconfirmed causative factors 217.
There are several descriptions of multiple family members all being affected by frontal fibrosing alopecia, so there is likely to be a hereditary element to this condition, although not everyone with frontal fibrosing alopecia has a relative with the condition. Genetic studies, however, have been lacking.
Frontal fibrosing alopecia has been considered a variant of lichen planopilaris due to the resemblance on histology and an association with various forms of lichen planus, but there are also many differences that raise doubts.
Frontal fibrosing alopecia signs and symptoms
Frontal fibrosing alopecia is characterized primarily by a usually symmetrical band of hair loss (alopecia) and scarring on the front and sides of the scalp, resulting in a receding frontal hair line. The edge may appear moth-eaten, and single ‘lonely’ hairs may persist in the bald areas. The band of hair loss on the front and sides of the scalp is usually symmetrical and slowly progressive (worsening over time). Atypical patterns of loss include a diffuse zig-zag pattern, a pseudo-fringe-sign, or continuous involvement all the way around the hair margin both front and back. Approximately half of all affected people experience eyebrow thinning or loss (madarosis) as well, which often precedes the scalp changes. Less commonly, the eyelashes may also be involved. Some people with frontal fibrosing alopecia develop hair loss in areas other than the scalp and face. Hair loss can affect all parts of the body, and almost total loss from limbs is common. In men, loss of beard and sideburns is described and may be the only site of involvement. Some women with frontal fibrosing alopecia also have female pattern hair loss, which is associated with thinning of hair on the scalp due to increased hair shedding and/or a reduction in hair volume.
Itch and pain are common early symptoms, and may occur before any obvious loss of hair density. Facial rashes are another potentially early sign. These may present as skin colored or yellowish follicular papules located on the forehead and temples, diffuse erythema or red dots around hairs 218.
The skin in the affected area lacks the sundamage seen on the forehead, allowing assessment of the extent of the recession. It looks pale, shiny, or mildly scarred, without visible hair follicle openings. During the active phase, close inspection or dermatoscopy, shows redness and scaling is visible around involved hairs. Single ‘lonely’ hairs often persist in the bald areas. The hair pull test is negative.
Trichoscopy (dermoscope for the scalp and hair) reveals absent follicles, white dots, tubular perifollicular scale and perifollicular erythema. In skin that tans easily, perifollicular pigmentation may be observed.
Androgenetic alopecia (male pattern hair loss, female pattern hair loss) is commonly associated and may lead to missed diagnosis.
Frontal fibrosing alopecia diagnosis
The clinical features of frontal fibrosing alopecia are characteristic. Frontal fibrosing alopecia is usually diagnosed using a combination of trichoscopy (examining the hair and scalp using a microscope) often accompanied by a scalp biopsy from the edge of the affected area to confirm the diagnosis. The histopathological features of frontal fibrosing alopecia are identical to those of lichen planopilaris.
Biopsy of skin papules may also show a lichenoid pattern of inflammation, fibrosing alopecia, and sebaceous gland hyperplasia.
Frontal fibrosing alopecia diagnostic criteria have been proposed:
- Major criteria:
- scarring hair loss of the frontal, frontotemporal, or temporal scalp in the absence of follicular keratotic papules on the body
- scarring loss of eyebrows.
- Minor criteria:
- redness and scale around hair follicles, or solitary ‘lonely’ hairs, best seen on dermoscopy
- characteristic histology on skin biopsy
- similar clinical signs involving other body sites
- noninflammatory facial papules
- itch or pain preceding or concurrently at sites of involvement.
Diagnosis requires two major criteria or one major and two minor criteria.
Recommended blood tests include hematology, biochemistry, thyroid function tests and ANA. Hormone status may assessed if there are other clinical features to suggest hyperandrogenism. Patch testing should be considered.
Frontal fibrosing alopecia treatment
The hair loss caused by frontal fibrosing alopecia cannot be reversed and there are no truly effective treatments to date. Treatments used to slow the progression of hair loss include oral corticosteroids, intralesional steroid injections, anti-inflammatory antibiotics such as tetracyclines, or anti-malarial tablets (hydroxychloroquine). All these treatments aim to lower the activity of the immune system and slow down the attack on the hair follicles.
Frontal fibrosing alopecia treatments include:
- Topical corticosteroids. Potent steroid gels, lotions or creams applied to the skin on the front of the scalp can be helpful. They may also be used alongside other treatments as they may not slow hair line recession on their own.
- Topical Tacrolimus. An ointment or cream that acts by suppressing the immune system and calming the inflammation where it is applied.
- Intralesional steroids. Injections of steroid into the skin on the front of the scalp can be used. This helps to settle the inflammation and slow or halt the progression of hair line recession. This treatment may need to be repeated.
- Antibiotics e.g. tetracycline, doxycycline. Thesemedicines are chiefly used to help reduce inflammation and not for their antibiotic action. They can help to relieve the symptoms and redness of the scalp.
- Hydroxychloroquine. This may help frontal fibrosing alopecia become inactive in some people. It will usually require a trial of 4-6 months. It is uncertain how this drug works in this condition. It carries a small risk of damage to your eyesight and annual eye tests are required.
- Immunomodulatory drugs e.g. mycophenolate mofetil. These can help to dampen down the immune system and prevent the inflammation around the hair follicles. In some people it can slow or halt hair loss.
- Antiandrogen treatments e.g. oral finasteride and dutasteride. These treatments work by maintaining levels of testosterone (a hormone naturally found in men and women) in the hair follicles. Reduced levels of testosterone have been associated with a different type of hair loss called female/male pattern alopecia. This may occur with frontal fibrosing alopecia also contributing to hair loss. Treatment with antiandrogens may be of benefit where the two conditions occur together.
The 5-alpha-reductase inhibitors finasteride and dutasteride have been reported to stop further hair loss but this may be due to associated androgenetic alopecia. Immunosuppressants tried include ciclosporin and mycophenolate mofetil.
The use of the antidiabetic agent pioglitazone (off-label) for the treatment of frontal fibrosing alopecia was reported to reduce symptoms, inflammation, and progression of frontal fibrosing alopecia but its use has not been supported by further investigations. Side effects include ankle swelling and weight gain.
Rituxomab and adalimumab are novel new treatments.
Hair grafting may be considered once disease activity has settled.
Frontal fibrosing alopecia prognosis
The long-term outlook (prognosis) for people with frontal fibrosing alopecia varies. Usually, frontal fibrosing alopecia is slowly progressive (worsening over time); however, the condition does stabilize after a few years in some patients. The hair line recedes on average of 1.8-2.6 cm. As it is a scarring alopecia, hair does not regrow unless treatment is instituted early in the process. Hair regrowth has been reported in some patients.
References- Al Aboud AM, Zito PM. Alopecia. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538178
- Hair Loss in Women. N Engl J Med 2007; 357:1620-1630. https://www.nejm.org/doi/full/10.1056/nejmcp072110
- Meephansan J, Thummakriengkrai J, Ponnikorn S, Yingmema W, Deenonpoe R, Suchonwanit P. Efficacy of topical tofacitinib in promoting hair growth in non-scarring alopecia: possible mechanism via VEGF induction. Arch Dermatol Res. 2017;309(9):729–738. doi:10.1007/s00403-017-1777-5
- Rojhirunsakool, S., & Suchonwanit, P. (2017). Parietal scalp is another affected area in female pattern hair loss: an analysis of hair density and hair diameter. Clinical, cosmetic and investigational dermatology, 11, 7–12. https://doi.org/10.2147/CCID.S153768
- Pratt, C. H., King, L. E., Jr, Messenger, A. G., Christiano, A. M., & Sundberg, J. P. (2017). Alopecia areata. Nature reviews. Disease primers, 3, 17011. https://doi.org/10.1038/nrdp.2017.11
- Lee YB, Jun M, Lee WS. Alopecia areata and poliosis: A retrospective analysis of 258 cases. J Am Acad Dermatol. 2019 Jun;80(6):1776-1778. doi: 10.1016/j.jaad.2018.11.033
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006 Sep;55(3):438-41. doi: 10.1016/j.jaad.2006.05.008
- Shapiro J. Clinical practice. Hair loss in women. N Engl J Med. 2007;357(16):1620–1630. DOI: 10.1056/NEJMcp072110
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341(13):964–973.
- Banka N, Bunagan MJ, Shapiro J. Pattern hair loss in men: diagnosis and medical treatment. Dermatol Clin. 2013;31(1):129–140.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330(2):120–125.
- Asghar, F., Shamim, N., Farooque, U., Sheikh, H., & Aqeel, R. (2020). Telogen Effluvium: A Review of the Literature. Cureus, 12(5), e8320. https://doi.org/10.7759/cureus.8320
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019 Jun;80(6):1778-1779. doi: 10.1016/j.jaad.2018.11.037
- Chen P, Chen F, Zhou B. The risk of dermatological toxicities of combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma patients: a systematic review and meta-analysis. Cutan Ocul Toxicol. 2019 Jun;38(2):105-111. doi: 10.1080/15569527.2018.1553180
- Dei-Cas I, Carrizo D, Giri M, Boyne G, Domínguez N, Novello V, Acuña K, Dei-Cas P. Infectious skin disorders encountered in a pediatric emergency department of a tertiary care hospital in Argentina: a descriptive study. Int J Dermatol. 2019 Mar;58(3):288-295. doi: 10.1111/ijd.14234
- Souissi A, Ben Lagha I, Toukabri N, Mama M, Mokni M. Morse code-like hairs in tinea capitis disappear after successful treatment. Int J Dermatol. 2018 Dec;57(12):e150-e151. doi: 10.1111/ijd.14224
- Al Aboud AM, Crane JS. Tinea Capitis. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536909
- McGarvey EL, Baum LD, Pinkerton RC, Rogers LM. Psychological sequelae and alopecia among women with cancer. Cancer Pract. 2001;9(6):283–289.
- Trüeb RM. Chemotherapy-induced alopecia. Semin Cutan Med Surg. 2009;28(1):11–14.
- Kanwar AJ, Narang T. Anagen effluvium. Indian J Dermatol Venereol Leprol. 2013;79(5):604–612.
- Thiedke CC. Alopecia in women. Am Fam Physician. 2003;67(5):1007–1014.
- Alkhalifah A. Alopecia areata update. Dermatol Clin. 2013;31(1):93–108.
- Gordon KA, Tosti A. Alopecia: evaluation and treatment. Clin Cosmet Investig Dermatol. 2011;4101–106.
- Perera E, Yip L, Sinclair R. Alopecia areata. Curr Probl Dermatol. 2015;47:67-75. doi: 10.1159/000369406
- Tan E, Tay YK, Goh CL, Chin Giam Y. The pattern and profile of alopecia areata in Singapore–a study of 219 Asians. Int J Dermatol. 2002 Nov;41(11):748-53. doi: 10.1046/j.1365-4362.2002.01357.x
- Cash TF. The psychology of hair loss and its implications for patient care. Clin Dermatol. 2001 Mar-Apr;19(2):161-6. doi: 10.1016/s0738-081x(00)00127-9
- Hair Loss: Common Causes and Treatment. Am Fam Physician. 2017 Sep 15;96(6):371-378. https://www.aafp.org/afp/2017/0915/p371.html
- Kumaresan M. Intralesional steroids for alopecia areata. Int J Trichology. 2010;2(1):63–65.
- Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366(16):1515–1525.
- Delamere FM, Sladden MM, Dobbins HM, Leonardi-Bee J. Interventions for alopecia areata. Cochrane Database Syst Rev. 2008(2):CD004413.
- Navarini AA, Nobbe S, Trüeb RM. Marie Antoinette syndrome. Arch Dermatol. 2009 Jun;145(6):656. doi: 10.1001/archdermatol.2009.51
- Suchonwanit, P., Kositkuljorn, C., & Pomsoong, C. (2021). Alopecia Areata: An Autoimmune Disease of Multiple Players. ImmunoTargets and therapy, 10, 299–312. https://doi.org/10.2147/ITT.S266409
- Ikeda T. Produced alopecia areata based on the focal infection theory and mental motive theory. Dermatologica. 1967;134(1):1–11. doi:10.1159/000254233
- Lepe K, Zito PM. Alopecia Areata. [Updated 2021 Aug 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537000
- Harnchoowong, S., & Suchonwanit, P. (2017). PPAR-γ Agonists and Their Role in Primary Cicatricial Alopecia. PPAR research, 2017, 2501248. https://doi.org/10.1155/2017/2501248
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55(6):e338–343. doi:10.1111/ijd.13061
- Brocker EB, Echternacht-Happle K, Hamm H, Happle R. Abnormal expression of class I and class II major histocompatibility antigens in alopecia areata: modulation by topical immunotherapy. J Invest Dermatol. 1987;88(5):564–568. doi:10.1111/1523-1747.ep12470166
- Rosenblum MD, Yancey KB, Olasz EB, Truitt RL. CD200, a “no danger” signal for hair follicles. J Dermatol Sci. 2006;41(3):165–174. doi:10.1016/j.jdermsci.2005.11.003
- Bertolini M, Meyer KC, Slominski R, Kobayashi K, Ludwig RJ, Paus R. The immune system of mouse vibrissae follicles: cellular composition and indications of immune privilege. Exp Dermatol. 2013;22(9):593–598. doi:10.1111/exd.12205
- Ito T, Ito N, Saatoff M, et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128(5):1196–1206. doi:10.1038/sj.jid.5701183
- Gilhar A, Kalish RS. Alopecia areata: a tissue specific autoimmune disease of the hair follicle. Autoimmun Rev. 2006;5(1):64–69. doi:10.1016/j.autrev.2005.07.001
- J Am Acad Dermatol. 2014;70:1146
- Guzmán-Sánchez DA, Villanueva-Quintero GD, Alfaro N, McMichael A. A clinical study of alopecia areata in Mexico. Int J Dermatol. 2007;46:1308–1310. doi: 10.1111/j.1365-4632.2007.03320.x
- Xiao FL, et al. The epidemiology of childhood alopecia areata in China: a study of 226 patients. Pediatr Dermatol. 2006;23:13–18.
- Lundin M, Chawa S, Sachdev A, Bhanusali D, Seiffert-Sinha K, Sinha AA. Gender differences in alopecia areata. J Drugs Dermatol. 2014 Apr;13(4):409-13.
- Werth VP, White WL, Sanchez MR, Franks AG. Incidence of alopecia areata and lupus erythematosus. Arch Dermatol. 1992;128:368–371.
- Insler MS, Helm CJ. Alopecia areata including the cilia and eyebrows of two sisters. Ann Ophthalmol. 1989;21:451–453.
- Dawn G, Kumar B. Profile of alopecia areata in northern India. Intnatl J Dermatol. 1996;35:22–27.
- Barahmani N, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240–243.
- Betz RC, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nature Commun. 2015;6:5966. doi: 10.1038/ncomms6966
- Sundberg JP, Silva KA, Li R, Cox GA, King LE. Adult-onset Alopecia areata is a complex polygenic trait in the C3H/HeJ mouse model. J Invest Dermatol. 2004 Aug;123(2):294-7. doi: 10.1111/j.0022-202X.2004.23222.x
- Petukhova, L., Duvic, M., Hordinsky, M., Norris, D., Price, V., Shimomura, Y., Kim, H., Singh, P., Lee, A., Chen, W. V., Meyer, K. C., Paus, R., Jahoda, C. A., Amos, C. I., Gregersen, P. K., & Christiano, A. M. (2010). Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature, 466(7302), 113–117. https://doi.org/10.1038/nature09114
- Kang H, Wu WY, Lo BK, et al. Hair follicles from alopecia areata patients exhibit alterations in immune privilege-associated gene expression in advance of hair loss. J Invest Dermatol. 2010;130(11):2677–2680. doi:10.1038/jid.2010.180
- Bertolini M, Zilio F, Rossi A, et al. Abnormal interactions between perifollicular mast cells and CD8+ T-cells may contribute to the pathogenesis of alopecia areata. PLoS One. 2014;9(5):e94260. doi:10.1371/journal.pone.0094260
- Ito T, Suzuki T, Sakabe JI, Funakoshi A, Fujiyama T, Tokura Y. Plasmacytoid dendritic cells as a possible key player to initiate alopecia areata in the C3H/HeJ mouse. Allergol Int. 2020;69(1):121–131. doi:10.1016/j.alit.2019.07.009
- Gregoriou, S., Papafragkaki, D., Kontochristopoulos, G., Rallis, E., Kalogeromitros, D., & Rigopoulos, D. (2010). Cytokines and other mediators in alopecia areata. Mediators of inflammation, 2010, 928030. https://doi.org/10.1155/2010/928030
- Natpracha W, Sukanjanapong S, Chanprapaph K, Suchonwanit P. Characterization and classification of different female hairline patterns in the Thai population. J Cosmet Dermatol. 2021;20(3):890–896. doi:10.1111/jocd.13642
- Paus R, Bertolini M. The role of hair follicle immune privilege collapse in alopecia areata: status and perspectives. J Investig Dermatol Symp Proc. 2013;16(1):S25–27. doi:10.1038/jidsymp.2013.7
- Lee D, Hong SK, Park SW, et al. Serum levels of IL-18 and sIL-2R in patients with alopecia areata receiving combined therapy with oral cyclosporine and steroids. Exp Dermatol. 2010;19(2):145–147. doi:10.1111/j.1600-0625.2009.00937.x
- Katagiri K, Arakawa S, Hatano Y. In vivo levels of IL-4, IL-10, TGF-beta1 and IFN-gamma mRNA of the peripheral blood mononuclear cells in patients with alopecia areata in comparison to those in patients with atopic dermatitis. Arch Dermatol Res. 2007;298(8):397–401. doi:10.1007/s00403-006-0700-2
- Bodemer C, Peuchmaur M, Fraitaig S, Chatenoud L, Brousse N, De Prost Y. Role of cytotoxic T cells in chronic alopecia areata. J Invest Dermatol. 2000;114(1):112–116. doi:10.1046/j.1523-1747.2000.00828.x
- Kryczek I, Wei S, Gong W, et al. Cutting edge: IFN-gamma enables APC to promote memory Th17 and abate Th1 cell development. J Immunol. 2008;181(9):5842–5846. doi:10.4049/jimmunol.181.9.5842
- Teraki Y, Imanishi K, Shiohara T. Cytokines in alopecia areata: contrasting cytokine profiles in localized form and extensive form (alopecia universalis). Acta Derm Venereol. 1996;76(6):421–423. doi:10.2340/0001555576421423
- Suchonwanit P, Rojhirunsakool S, Khunkhet S. A randomized, investigator-blinded, controlled, split-scalp study of the efficacy and safety of a 1550-nm fractional erbium-glass laser, used in combination with topical 5% minoxidil versus 5% minoxidil alone, for the treatment of androgenetic alopecia. Lasers Med Sci. 2019;34(9):1857–1864. doi:10.1007/s10103-019-02783-8
- Villasante Fricke, A. C., & Miteva, M. (2015). Epidemiology and burden of alopecia areata: a systematic review. Clinical, cosmetic and investigational dermatology, 8, 397–403. https://doi.org/10.2147/CCID.S53985
- Chu CH, Cheng YP, Chan JY. Alopecia Areata After Vaccination: Recurrence with Rechallenge. Pediatr Dermatol. 2016 May;33(3):e218-9. doi: 10.1111/pde.12849
- Wise RP, Kiminyo KP, Salive ME. Hair loss after routine immunizations. JAMA. 1997 Oct 8;278(14):1176-8.
- Sánchez-Ramón S, Gil J, Cianchetta-Sívori M, Fernández-Cruz E. Alopecia universal en un adulto tras la vacunación de rutina con toxoide tetánico [Alopecia universalis in an adult after routine tetanus toxoid vaccine]. Med Clin (Barc). 2011 Mar 19;136(7):318. Spanish. doi: 10.1016/j.medcli.2010.03.010
- Lai YC, Yew YW. Severe Autoimmune Adverse Events Post Herpes Zoster Vaccine: A Case-Control Study of Adverse Events in a National Database. J Drugs Dermatol. 2015 Jul;14(7):681-4.
- Geier, D. A., & Geier, M. R. (2015). A case-control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clinical rheumatology, 34(7), 1225–1231. https://doi.org/10.1007/s10067-014-2846-1
- Ito T, Tokura Y. Alopecia areata triggered or exacerbated by swine flu virus infection. J Dermatol. 2012;39:863–864. doi: 10.1111/j.1346-8138.2011.01437
- Sundberg, J. P., Silva, K. A., Zhang, W., Sundberg, B. A., Edwards, K., King, L. E., Davis, R. L., & Black, S. (2009). Recombinant human hepatitis B vaccine initiating alopecia areata: testing the hypothesis using the C3H/HeJ mouse model. Veterinary dermatology, 20(2), 99–104. https://doi.org/10.1111/j.1365-3164.2008.00692.x
- Ikeda T. A new classification of alopecia areata. Dermatologica. 1965;131(6):421-45. doi: 10.1159/000254503
- De Waard-van der Spek FB, Oranje AP, De Raeymaecker DM, Peereboom-Wynia JD. Juvenile versus maturity-onset alopecia areata–a comparative retrospective clinical study. Clin Exp Dermatol. 1989 Nov;14(6):429-33. doi: 10.1111/j.1365-2230.1989.tb02604.x
- Goh C, Finkel M, Christos PJ, Sinha AA. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006 Oct;20(9):1055-60. doi: 10.1111/j.1468-3083.2006.01676.x
- Chen CH, Wang KH, Lin HC, Chung SD. Follow-up study on the relationship between alopecia areata and risk of autoimmune diseases. J Dermatol. 2016 Feb;43(2):228-9. doi: 10.1111/1346-8138.13165
- Garzorz N, Alsisi M, Todorova A, Atenhan A, Thomas J, Lauffer F, Ring J, Schmidt-Weber C, Biedermann T, Eyerich S, Eyerich K. Dissecting susceptibility from exogenous triggers: the model of alopecia areata and associated inflammatory skin diseases. J Eur Acad Dermatol Venereol. 2015 Dec;29(12):2429-35. doi: 10.1111/jdv.13325
- Ranawaka RR. An observational study of alopecia areata in Sri Lankan adult patients. Ceylon Med J. 2014 Dec;59(4):128-31. doi: 10.4038/cmj.v59i4.7865
- Chu SY, Chen YJ, Tseng WC, Lin MW, Chen TJ, Hwang CY, Chen CC, Lee DD, Chang YT, Wang WJ, Liu HN. Comorbidity profiles among patients with alopecia areata: the importance of onset age, a nationwide population-based study. J Am Acad Dermatol. 2011 Nov;65(5):949-56. doi: 10.1016/j.jaad.2010.08.032
- Lee, N. R., Kim, B. K., Yoon, N. Y., Lee, S. Y., Ahn, S. Y., & Lee, W. S. (2014). Differences in Comorbidity Profiles between Early-Onset and Late-Onset Alopecia Areata Patients: A Retrospective Study of 871 Korean Patients. Annals of dermatology, 26(6), 722–726. https://doi.org/10.5021/ad.2014.26.6.722
- Chen CH, Wang KH, Hung SH, Lin HC, Tsai MC, Chung SD. Association between herpes zoster and alopecia areata: A population-based study. J Dermatol. 2015 Aug;42(8):824-5. doi: 10.1111/1346-8138.12912
- Díaz-Angulo S, López-Hoyos M, Muñoz-Cacho P, López-Escobar M, González-López MA. High prevalence of thyroid autoimmunity in patients with alopecia areata and vitiligo: a controlled study. Australas J Dermatol. 2015 May;56(2):142-3. doi: 10.1111/ajd.12321
- Saylam Kurtipek, G., Cihan, F. G., Erayman Demirbaş, Ş., & Ataseven, A. (2015). The Frequency of Autoimmune Thyroid Disease in Alopecia Areata and Vitiligo Patients. BioMed research international, 2015, 435947. https://doi.org/10.1155/2015/435947
- Alopecia areata: An appraisal of new treatment approaches and overview of current therapies. Journal of the American Academy of Dermatology Volume 78, Issue 1, January 2018, Pages 15-24. https://doi.org/10.1016/j.jaad.2017.04.1142
- JAAD 2015;73;338
- BJD 2014;170;1299–304
- J Drugs Dermatol 2016;15;1235
- J Dermatol. 2009 Jun;36(6):323-7
- Dermatology 2015;230:308-313
- Int J Trichology. 2010 Jul-Dec; 2(2): 86–88
- Indian J Dermatol Venereol Leprol 2014;80:177
- Jalopecia areataD 2017;76;754
- PD 2018;35;361
- Jalopecia areataD 2016;74;1007).
Methotrexate
Methotrexate has been used with benefit for alopecia areata in several small series of patients. It is usually combined with intralesional or oral steroids–often the steroids are given early and then the patient treated with methotrexate alone or in combination with topical minoxidil. Hammerschmidt and Brenner ((An. Bras. Dermatol 89;5;Sept./Oct. 201
- Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi:10.1146/annurev.immunol.16.1.293
- Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;17(1):78. doi:10.1038/nrd.2017.267
- Harel, S., Higgins, C. A., Cerise, J. E., Dai, Z., Chen, J. C., Clynes, R., & Christiano, A. M. (2015). Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Science advances, 1(9), e1500973. https://doi.org/10.1126/sciadv.1500973
- Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. 2018 Nov;179(5):1033-1048. doi: 10.1111/bjd.16808
- JAAD 2017;76;22
- JAAD 2017;76;29
- JAAD 2018;78;403
- Nature Med 2014;20;1043-9
- JCI Insight 2016;1(15);e89790
- Am J Hematol. 2015 Jan;90(1):82-3
- JAMA Derm 2014;150;1341
- JAAD 2017;77;773
- BJD 2013;169;690
- Choi, J. W., Suh, D. W., Lew, B. L., & Sim, W. Y. (2017). Simvastatin/Ezetimibe Therapy for Recalcitrant Alopecia Areata: An Open Prospective Study of 14 Patients. Annals of dermatology, 29(6), 755–760. https://doi.org/10.5021/ad.2017.29.6.755
- Forero-Peña, D. A., & Gutierrez, F. R. (2013). Statins as modulators of regulatory T-cell biology. Mediators of inflammation, 2013, 167086. https://doi.org/10.1155/2013/167086
- Cervantes J, Jimenez JJ, DelCanto GM, Tosti A. Treatment of alopecia areata with simvastatin/ezetimibe. J Investig Dermatol Symp Proc. 2018;19(1):S25–S31. doi:10.1016/j.jisp.2017.10.013
- Loi C, Starace M, Piraccini BM. Alopecia areata (AA) and treatment with simvastatin/ezetimibe: experience of 20 patients. J Am Acad Dermatol. 2016;74(5):e99–e100. doi:10.1016/j.jaad.2015.09.071
- Lattouf C, Jimenez JJ, Tosti A, et al. Treatment of alopecia areata with simvastatin/ezetimibe. J Am Acad Dermatol. 2015;72(2):359–361. doi:10.1016/j.jaad.2014.11.006
- Mahasaksiri, T., Kositkuljorn, C., Anuntrangsee, T., & Suchonwanit, P. (2021). Application of Topical Immunotherapy in the Treatment of Alopecia Areata: A Review and Update. Drug design, development and therapy, 15, 1285–1298. https://doi.org/10.2147/DDDT.S297858
- Happle R, Hausen BM, Wiesner-Menzel L. Diphencyprone in the treatment of alopecia areata. Acta Derm Venereol. 1983;63(1):49-52.
- Buckley DA, Du Vivier AW. The therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol. 2001;145(3):385–405. doi:10.1046/j.1365-2133.2001.04399.x
- Borowska K, Wasylyszyn T. Stability of solutions of 2,3-diphenylcyclopropenone in various solvents. A novel formula – diphenylcyclopropenone in isopropanol may be useful in topical therapy of patients with Alopecia Areata. Acta Pol Pharm. 2017;74(2):459–464.
- Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166(5):916–926. doi:10.1111/j.1365-2133.2012.10955.x
- Jang YH, Jung HJ, Moon SY, et al. Systematic review and quality analysis of studies on the efficacy of topical diphenylcyclopropenone treatment for alopecia areata. J Am Acad Dermatol. 2017;77(1):170–172. doi:10.1016/j.jaad.2017.03.015
- Tosti A, Caponeri GM, Primativo R, Melino M, Veronesi S. Squaric acid dibutyl ester and diphencyprone in the therapy of alopecia areata. G Ital Dermatol Venereol. 1985;120(5):371–373.
- Nasimi M, Abedini R, Ghandi N, Seirafi H, Sadat Mehdizade M, Tootoonchi N. Topical immunotherapy with diphenylcyclopropenone in patients with alopecia areata: a large retrospective study of 757 patients. Dermatol Ther. 2020;33(6):e13808. doi:10.1111/dth.13808
- Lee, S., Kim, B. J., Lee, Y. B., & Lee, W. S. (2018). Hair Regrowth Outcomes of Contact Immunotherapy for Patients With Alopecia Areata: A Systematic Review and Meta-analysis. JAMA dermatology, 154(10), 1145–1151. https://doi.org/10.1001/jamadermatol.2018.2312
- Gupta, A. K., Carviel, J. L., Foley, K. A., Shear, N. H., Piraccini, B. M., Piguet, V., & Tosti, A. (2019). Monotherapy for Alopecia Areata: A Systematic Review and Network Meta-Analysis. Skin appendage disorders, 5(6), 331–337. https://doi.org/10.1159/000501940
- Hull SM, Pepall L, Cunliffe WJ. Alopecia areata in children: response to treatment with diphencyprone. Br J Dermatol. 1991;125(2):164–168. doi:10.1111/j.1365-2133.1991.tb06064.x
- Salsberg JM, Donovan J. The safety and efficacy of diphencyprone for the treatment of alopecia areata in children. Arch Dermatol. 2012;148(9):1084–1085. doi:10.1001/archdermatol.2012.1622
- Choe SJ, Lee S, Lee H, Choi J, Lee WS. Efficacy of topical diphenylcyclopropenone maintenance treatment for patients with alopecia areata: a retrospective study. J Am Acad Dermatol. 2018;78(1):205–207 e201. doi:10.1016/j.jaad.2017.07.028
- Hull SM, Cunliffe WJ. Post-therapy relapse rate in alopecia areata after successful treatment with diphencyprone. J Dermatolog Treat. 1989;1(2):71–74.
- van der Steen PH, Boezeman JB, Happle R. Topical immunotherapy for alopecia areata: re-evaluation of 139 cases after an additional follow-up period of 19 months. Dermatology. 1992;184(3):198–201. doi:10.1159/000247540
- Gong Y, Zhao Y, Zhang X, et al. Serum level of IL-4 predicts response to topical immunotherapy with diphenylcyclopropenone in alopecia areata. Exp Dermatol. 2020;29(3):231–238. doi:10.1111/exd.13758
- Gordon PM, Aldrige RD, McVittie E, Hunter JA. Topical diphencyprone for alopecia areata: evaluation of 48 cases after 30 months’ follow-up. Br J Dermatol. 1996;134(5):869–871. doi:10.1111/j.1365-2133.1996.tb06317.x
- Francomano M, Seidenari S. Urticaria after topical immunotherapy with diphenylcyclopropenone. Contact Dermatitis. 2002;47(5):310–311. doi:10.1034/j.1600-0536.2002.4705102.x
- Sanger J, Zahir A, Driscoll M, Gaspari AA. Erythema multiforme major after immunotherapy with diphenylcyclopropenone for Alopecia Areata. Dermatitis. 2018;29(6):348–349. doi:10.1097/DER.0000000000000415
- Durdu M, Ozcan D, Baba M, Seckin D. Efficacy and safety of diphenylcyclopropenone alone or in combination with anthralin in the treatment of chronic extensive alopecia areata: a retrospective case series. J Am Acad Dermatol. 2015;72(4):640–650. doi:10.1016/j.jaad.2015.01.008
- Kagami S, Kishi Y, Hino H. Topical immunotherapy in combination with anthralin in the treatment of refractory alopecia areata. J Cosmet Dermatol. 2020;19(9):2411–2414. doi:10.1111/jocd.13588
- Nasimi M, Ghandi N, Abedini R, Mirshamsi A, Shakoei S, Seirafi H. Efficacy and safety of anthralin in combination with diphenylcyclopropenone in the treatment of alopecia areata: a retrospective case series. Arch Dermatol Res. 2019;311(8):607–613. doi:10.1007/s00403-019-01940-x
- Shapiro J, Tan J, Ho V, Abbott F, Tron V. Treatment of chronic severe alopecia areata with topical diphenylcyclopropenone and 5% minoxidil: a clinical and immunopathologic evaluation. J Am Acad Dermatol. 1993;29(5):729–735. doi:10.1016/0190-9622(93)70238-O
- Wasylyszyn T, Borowska K. Possible advantage of imiquimod and diphenylcyclopropenone combined treatment versus diphenylcyclopropenone alone: an observational study of nonresponder patients with alopecia areata. Australas J Dermatol. 2017;58(3):219–223. doi:10.1111/ajd.12478
- Sriphojanart, T., Khunkhet, S., & Suchonwanit, P. (2017). A retrospective comparative study of the efficacy and safety of two regimens of diphenylcyclopropenone in the treatment of recalcitrant alopecia areata. Dermatology reports, 9(2), 7399. https://doi.org/10.4081/dr.2017.7399
- Thuangtong R, Varothai S, Triwongwaranat D, Rujitharanawong C. Multi-concentration level patch test guided Diphenyl Cyclopropenone (DPCP) treatment in alopecia totalis or alopecia universalis. J Med Assoc Thai. 2017;100(1):86–92.
- Nowicka, D., Maj, J., Jankowska-Konsur, A., & Hryncewicz-Gwóźdź, A. (2018). Efficacy of diphenylcyclopropenone in alopecia areata: a comparison of two treatment regimens. Postepy dermatologii i alergologii, 35(6), 577–581. https://doi.org/10.5114/ada.2018.77608
- Lee S, Lee WS. Home-based contact immunotherapy with diphenylcyclopropenone for alopecia areata is as effective and safe as clinic-based treatment in patients with stable disease: a retrospective study of 40 patients. J Am Acad Dermatol. 2018;78(3):599–601.e591. doi:10.1016/j.jaad.2017.09.037
- Happle R, Kalveram KJ, Büchner U, Echternacht-Happle K, Göggelmann W, Summer KH. Contact allergy as a therapeutic tool for alopecia areata: application of squaric acid dibutylester. Dermatologica. 1980;161(5):289–297. doi:10.1159/000250380
- Micali G, Cicero RL, Nasca MR, Sapuppo A. Treatment of alopecia areata with squaric acid dibutylester. Int J Dermatol. 1996;35(1):52–56. doi:10.1111/j.1365-4362.1996.tb01618.x
- Dall’oglio F, Nasca MR, Musumeci ML, et al. Topical immunomodulator therapy with squaric acid dibutylester (SADBE) is effective treatment for severe alopecia areata (AA): results of an open-label, paired-comparison, clinical trial. J Dermatolog Treat. 2005;16(1):10–14. doi:10.1080/09546630410023601
- Chua SH, Goh CL, Ang CB. Topical squaric acid dibutylester therapy for alopecia areata: a double-sided patient-controlled study. Ann Acad Med Singapore. 1996;25(6):842–847.
- Caserio RJ. Treatment of alopecia areata with squaric acid dibutylester. Arch Dermatol. 1987;123(8):1036–1041. doi:10.1001/archderm.1987.01660320078016
- Giannetti A, Orecchia G. Clinical experience on the treatment of alopecia areata with squaric acid dibutyl ester. Dermatologica. 1983;167(5):280–282. doi:10.1159/000249797
- Chen CA, Carlberg V, Kroshinsky D. angioedema after squaric acid treatment in a 6-year-old girl. Pediatr Dermatol. 2017;34(1):e44–e46. doi:10.1111/pde.12993
- Valsecchi R, Pansera B, Rossi A, Cainelli T. Pigmentation abnormalities in the course of topical immunotherapy of alopecia areata. G Ital Dermatol Venereol. 1989;124(1–2):31–32.
- Black HS, Castrow FF 2nd, Gerguis J. The mutagenicity of dinitrochlorobenzene. Arch Dermatol. 1985;121(3):348–349. doi:10.1001/archderm.1985.01660030070021
- He H, Xie B, Xie L. Male pattern baldness and incidence of prostate cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2018 Jul;97(28):e11379
- Jimenez-Cauhe J, Saceda-Corralo D, Rodrigues-Barata R, Hermosa-Gelbard A, Moreno-Arrones OM, Fernandez-Nieto D, Vaño-Galvan S. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J Am Acad Dermatol. 2019 May 2. pii: S0190-9622(19)30685-1. doi: 10.1016/j.jaad.2019.04.054. [Epub ahead of print]
- Olsen EA. Female pattern hair loss. J Am Acad Dermatol. 2001 Sep;45(3 Suppl):S70-80. doi: 10.1067/mjd.2001.117426
- Birch MP, Lalla SC, Messenger AG. Female pattern hair loss. Clin Exp Dermatol. 2002 Jul;27(5):383-88. doi: 10.1046/j.1365-2230.2002.01085.x
- Birch MP, Messenger JF, Messenger AG. Hair density, hair diameter and the prevalence of female pattern hair loss. Br J Dermatol. 2001 Feb;144(2):297-304. doi: 10.1046/j.1365-2133.2001.04018.x
- Ludwig E. Androgenetic alopecia. Arch Dermatol. 1977 Jan;113(1):109. doi: 10.1001/archderm.1977.01640010111023
- van Zuuren EJ, Fedorowicz Z, Schoones J. Interventions for female pattern hair loss. Cochrane Database Syst Rev. 2016(5):CD007628.
- Traction alopecia. https://dermnetnz.org/topics/traction-alopecia
- Khumalo NP, Jessop S, Gumedze F, Ehrlich R. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59(3):432–438.
- Fox GN, Stausmire JM, Mehregan DR. Traction folliculitis: an underreported entity. Cutis. 2007;79(1):26–30.
- Khumalo N, Ngwanya R. Traction alopecia: 2% topical minoxidil shows promise. Report of two cases. J Eur Acad Dermatol Venereol. 2007;21(3):433–434.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17(2):164–176.
- Earles RM. Surgical correction of traumatic alopecia marginalis or traction alopecia in black women. J Dermatol Surg Oncol. 1986;12(1):78–82.
- Özçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29(4):325–327.
- Harries, M. J., & Paus, R. (2010). The pathogenesis of primary cicatricial alopecias. The American journal of pathology, 177(5), 2152–2162. https://doi.org/10.2353/ajpath.2010.100454
- Harries MJ, Trueb RM, Tosti A, Messenger AG, Chaudhry I, Whiting DA, Sinclair R, Griffiths CE, Paus R. How not to get scar(r)ed: pointers to the correct diagnosis in patients with suspected primary cicatricial alopecia. Br J Dermatol. 2009 Mar;160(3):482-501. doi: 10.1111/j.1365-2133.2008.09008.x
- Ohyama M. Primary cicatricial alopecia: recent advances in understanding and management. J Dermatol. 2012 Jan;39(1):18-26. doi: 10.1111/j.1346-8138.2011.01416.x
- Whiting DA. Cicatricial alopecia: clinico-pathological findings and treatment. Clin Dermatol. 2001 Mar-Apr;19(2):211-25. doi: 10.1016/s0738-081x(00)00132-2
- Olsen EA, Bergfeld WF, Cotsarelis G, Price VH, Shapiro J, Sinclair R, Solomon A, Sperling L, Stenn K, Whiting DA, Bernardo O, Bettencourt M, Bolduc C, Callendar V, Elston D, Hickman J, Ioffreda M, King L, Linzon C, McMichael A, Miller J, Mulinari F, Trancik R; Workshop on Cicatricial Alopecia. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003 Jan;48(1):103-10. doi: 10.1067/mjd.2003.68
- Villablanca S, Ferrando J. Management of acquired primary cicatricial alopecia. In: Bouhanna P, Bouhanna E, editors. The Alopecias: Diagnosis and Treatments. Boca Raton: CRC Press; 2016.
- Tan E, Martinka M, Ball N, Shapiro J. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004 Jan;50(1):25-32. doi: 10.1016/j.jaad.2003.04.001
- Villablanca, S., Fischer, C., García-García, S. C., Mascaró-Galy, J. M., & Ferrando, J. (2017). Primary Scarring Alopecia: Clinical-Pathological Review of 72 Cases and Review of the Literature. Skin appendage disorders, 3(3), 132–143. https://doi.org/10.1159/000467395
- Harries MJ, Meyer KC, Chaudhry IH, Griffiths CE, Paus R. Does collapse of immune privilege in the hair-follicle bulge play a role in the pathogenesis of primary cicatricial alopecia? Clin Exp Dermatol. 2010 Aug;35(6):637-44. doi: 10.1111/j.1365-2230.2009.03692.x
- Karnik, P., Tekeste, Z., McCormick, T. S., Gilliam, A. C., Price, V. H., Cooper, K. D., & Mirmirani, P. (2009). Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. The Journal of investigative dermatology, 129(5), 1243–1257. https://doi.org/10.1038/jid.2008.369
- Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006 Mar;25(1):41-50. doi: 10.1016/j.sder.2006.01.006
- Miteva M, Tosti A. Dermoscopy guided scalp biopsy in cicatricial alopecia. J Eur Acad Dermatol Venereol. 2013 Oct;27(10):1299-303. doi: 10.1111/j.1468-3083.2012.04530.x
- Mirmirani, P., & Karnik, P. (2009). Lichen planopilaris treated with a peroxisome proliferator-activated receptor gamma agonist. Archives of dermatology, 145(12), 1363–1366. https://doi.org/10.1001/archdermatol.2009.283
- Early Diagnosis and Treatment of Discoid Lupus Erythematosus. Suresh Panjwani. The Journal of the American Board of Family Medicine Mar 2009, 22 (2) 206-213; DOI: 10.3122/jabfm.2009.02.080075
- Callen JP. Cutaneous lupus erythematosus: a personal approach to management. Australas J Dermatol. 2006 Feb;47(1):13-27. doi: 10.1111/j.1440-0960.2006.00217.x
- Gül U, Gönül M, Cakmak SK, Kiliç A, Demiriz M. A case of generalized discoid lupus erythematosus: successful treatment with imiquimod cream 5%. Adv Ther. 2006 Sep-Oct;23(5):787-92. doi: 10.1007/BF02850319
- Usmani N, Goodfield M. Efalizumab in the treatment of discoid lupus erythematosus. Arch Dermatol. 2007 Jul;143(7):873-7. doi: 10.1001/archderm.143.7.873
- Koch M, Horwath-Winter J, Aberer E, Salmhofer W, Klein A. Kryotherapie bei diskoidem Lupus erythematodes [Cryotherapy in discoid lupus erythematosus (DLE)]. Ophthalmologe. 2008 Apr;105(4):381-3. German. doi: 10.1007/s00347-007-1548-3
- Callen JP. Management of “refractory” skin disease in patients with lupus erythematosus. Best Pract Res Clin Rheumatol. 2005 Oct;19(5):767-84. doi: 10.1016/j.berh.2005.05.003
- Callen JP. Treatment of cutaneous lesions in patients with lupus erythematosus. Dermatol Clin. 1994 Jan;12(1):201-6.
- von Schmiedeberg S, Rönnau AC, Schuppe HC, Specker C, Ruzicka T, Lehmann P. Kombination der Antimalariamittel Mepacrin und Chloroquin bei therapieresistentem kutanem Lupus erythematodes [Combination of antimalarial drugs mepacrine and chloroquine in therapy refractory cutaneous lupus erythematosus]. Hautarzt. 2000 Feb;51(2):82-5. German. doi: 10.1007/s001050050017
- Cruz DD. Antimalarial therapy: a panacea for mild lupus. Lupus 2001; 10: 148–51.
- Mackenzie AH. Dose refinements in long-term therapy of rheumatoid arthritis with antimalarials. Am J Med. 1983 Jul 18;75(1A):40-5. doi: 10.1016/0002-9343(83)91269-x
- Lo JS, Berg RE, Tomecki KJ. Treatment of discoid lupus erythematosus. Int J Dermatol. 1989 Oct;28(8):497-507. doi: 10.1111/j.1365-4362.1989.tb04599.x
- Ward WQ, Walter-Ryan WG, Shehi GM. Toxic psychosis: a complication of antimalarial therapy. J Am Acad Dermatol. 1985 May;12(5 Pt 1):863-5. doi: 10.1016/s0190-9622(85)70109-0
- Fabbri P, Cardinali C, Giomi B, Caproni M. Cutaneous lupus erythematosus: diagnosis and management. Am J Clin Dermatol. 2003;4(7):449-65. doi: 10.2165/00128071-200304070-00002
- Brocard A, Barbarot S, Milpied B, Stalder JF. Lupus érythémateux chronique: traitement par thalidomide [Thalidomide in the treatment of chronic discoid lupus erythematosus]. Ann Dermatol Venereol. 2005 Nov;132(11 Pt 1):853-6. French. doi: 10.1016/s0151-9638(05)79503-x
- Bottomley WW, Goodfield MJ. Methotrexate for the treatment of discoid lupus erythematosus. Br J Dermatol. 1995 Oct;133(4):655-6. doi: 10.1111/j.1365-2133.1995.tb02726.x
- Yell JA, Burge SM. Cyclosporin and discoid lupus erythematosus. Br J Dermatol. 1994 Jul;131(1):132-3. doi: 10.1111/j.1365-2133.1994.tb08471.x
- Walker SL, Kirby B, Chalmers RJ. The effect of topical tacrolimus on severe recalcitrant chronic discoid lupus erythematosus. Br J Dermatol. 2002 Aug;147(2):405-6. doi: 10.1046/j.1365-2133.2002.488616.x
- Tzung TY, Liu YS, Chang HW. Tacrolimus vs. clobetasol propionate in the treatment of facial cutaneous lupus erythematosus: a randomized, double-blind, bilateral comparison study. Br J Dermatol. 2007 Jan;156(1):191-2. doi: 10.1111/j.1365-2133.2006.07595.x
- Nousari HC, Sragovich A, Kimyai-Asadi A, Orlinsky D, Anhalt GJ. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol. 1999 Feb;40(2 Pt 1):265-8. doi: 10.1016/s0190-9622(99)70203-3
- Goyal S, Nousari HC. Treatment of resistant discoid lupus erythematosus of the palms and soles with mycophenolate mofetil. J Am Acad Dermatol. 2001 Jul;45(1):142-4. doi: 10.1067/mjd.2001.114297
- Abu Shakra M, Shoenfield Y. Azathioprine therapy for patients with systemic lupus erythematosus. Lupus 2001; 10: 152–3.
- Braun-Falco O, Imai S, Schmoeckel C, Steger O, Bergner T. Pseudopelade of Brocq. Dermatologica 1986; 172: 18-23.
- Lockshin BN, Khachemoune A, Cohen C. Follicular mucinosis in a 4-year-old boy. Int J Dermatol. 2004 Dec;43(12):950-2. doi: 10.1111/j.1365-4632.2004.01954.x
- Zvulunov A, Shkalim V, Ben-Amitai D, Feinmesser M. Clinical and histopathologic spectrum of alopecia mucinosa/follicular mucinosis and its natural history in children. J Am Acad Dermatol. 2012 Dec;67(6):1174-81. doi: 10.1016/j.jaad.2012.04.015
- Sumner WT, Grichnik JM, Shea CR, Moore JO, Miller WS, Burton CS. Follicular mucinosis as a presenting sign of acute myeloblastic leukemia. J Am Acad Dermatol. 1998 May;38(5 Pt 2):803-5. doi: 10.1016/s0190-9622(98)70462-1
- Rongioletti F, Rebora A. Follicular mucinosis in exaggerated arthropod-bite reactions of patients with chronic lymphocytic leukemia. J Am Acad Dermatol. 1999 Sep;41(3 Pt 1):500. doi: 10.1016/s0190-9622(99)70133-7
- Rongioletti F, De Lucchi S, Meyes D, Mora M, Rebora A, Zupo S, Cerruti G, Patterson JW. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010 Jan;37(1):15-9. doi: 10.1111/j.1600-0560.2009.01338.x
- Lewars, M., Levin, J., & Purcell, S. (2013). Follicular mucinosis. Indian dermatology online journal, 4(4), 333–335. https://doi.org/10.4103/2229-5178.120667
- Mir-Bonafé JM, Cañueto J, Fernández-López E, Santos-Briz A. Follicular mucinosis associated with nonlymphoid skin conditions. Am J Dermatopathol. 2014 Sep;36(9):705-9. doi: 10.1097/01.dad.0000451564.29918.31
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005 May 15;105(10):3768-85. doi: 10.1182/blood-2004-09-3502
- Böer A, Guo Y, Ackerman AB. Alopecia mucinosa is mycosis fungoides. Am J Dermatopathol. 2004 Feb;26(1):33-52. doi: 10.1097/00000372-200402000-00006
- Geller, S., Gomez, C. J., Myskowski, P. L., & Pulitzer, M. (2019). Follicular mucinosis in patients with hematologic malignancies other than mycosis fungoides: A clinicopathologic study. Journal of the American Academy of Dermatology, 80(6), 1704–1711. https://doi.org/10.1016/j.jaad.2019.01.062
- Geller, S., Pulitzer, M., & Myskowski, P. L. (2018). Acneiform follicular mucinosis: an indolent follicular mucinosis variant unrelated to mycosis fungoides?. Clinical and experimental dermatology, 43(8), 921–924. https://doi.org/10.1111/ced.13671
- Emge DA, Lewis DJ, Aung PP, Duvic M. How to Discern Folliculotropic Mycosis Fungoides From Follicular Mucinosis Using a Pediatric Case. J Cutan Med Surg. 2018 May/Jun;22(3):336-340. doi: 10.1177/1203475417752366
- Brown HA, Gibson LE, Pujol RM, Lust JA, Pittelkow MR. Primary follicular mucinosis: long-term follow-up of patients younger than 40 years with and without clonal T-cell receptor gene rearrangement. J Am Acad Dermatol. 2002 Dec;47(6):856-62. doi: 10.1067/mjd.2002.124604
- Parker SRS, Murad E. Follicular mucinosis: clinical, histologic, and molecular remission with minocycline. J Am Acad Dermatol. 2010 Jan;62(1):139-141. doi: 10.1016/j.jaad.2009.01.031
- Zinkernagel MS, Trüeb RM. Fibrosing alopecia in a pattern distribution: patterned lichen planopilaris or androgenetic alopecia with a lichenoid tissue reaction pattern?. Arch Dermatol. 2000 Feb. 136(2):205-11.
- Arakelyan, Hayk. (2019). Frontal Fibrosing Alopecia. https://www.researchgate.net/publication/337006698_Frontal_Fibrosing_Alopecia
- Kossard S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution [published correction appears in Arch Dermatol 1994 Nov;130(11):1407]. Arch Dermatol. 1994;130(6):770–774. doi:10.1001/archderm.1994.01690060100013
- Lichen planopilaris. https://dermnetnz.org/topics/lichen-planopilaris
- Kumaran MS, Razmi T M, Vinay K, Parsad D. Clinical, dermoscopic, and trichoscopic analysis of frontal fibrosing alopecia associated with acquired dermal macular hyperpigmentation: A cross sectional observational case-control study. J Am Acad Dermatol. 2018;79(3):588–591. doi:10.1016/j.jaad.2018.03.001
- Pirmez, Rodrigo et al. Histopathology of facial papules in frontal fibrosing alopecia and therapeutic response to oral isotretinoin. Journal of the American Academy of Dermatology , Volume 78 , Issue 2 , e45. https://doi.org/10.1016/j.jaad.2017.10.038