Anti-inflammatory foods
Scientific evidence shows that eating mostly plant-based foods — variety of vegetables, fruits, whole grains, beans and other plant foods — plays a big role in preventing cancer and contributing to a healthier life. That’s because plant-based foods are high in the types of fiber, nutrients, minerals, vitamins and phytochemicals (natural substances) that may help to prevent cancer. Plus, plant-foods can help you manage your weight, and give you the energy you need to enjoy physical activity. A healthy diet emphasizes foods such as a variety of vegetables, fruits, whole grains, and beans that can reduce your risk for cancer and other chronic diseases. These foods are rich in fiber, vitamins, and other natural substances called phytochemicals that help keep you in good health, and protect against cancer. They are also naturally low in calories. The other most important consideration for any anti-inflammatory diet is calorie restriction 1. Any reduction of excess calorie intake will lead to a decrease in systemic oxidative stress. Calorie restriction has been the most successful therapeutic intervention to improve healthspan (defined as longevity minus years of disability) in virtually every species studied 2. Significant metabolic benefits have been achieved by calorie restriction in healthy overweight and normal-weight individuals who participated in the various CALERIE (Comprehensive Assessment of the Long-Term Effects of Reducing Intake of Energy) studies 3, 4.
In addition, several foods have been suggested to offer numerous health benefits 5, including long chain omega-3 polyunsaturated fatty acids [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] (chia seeds, flaxseeds, fatty fish) 6, 7, 8, 9, 10, monounsaturated fatty acids (MUFA) (avocado, sesame) 11, 12, antioxidants (N-acetylcysteine [NAC]) 13, phytochemicals from fruits, vegetables, and legumes 14, flavonoids 15, 16, vitamin D 17, fruits with enzymatic proteins such as papain and bromelain (papaya, mango, pineapple) 18, 19, 20, ginger 21, turmeric 22, 23, black pepper 24, 25, green tea 26, 27, 28, and legumes 24, 29. However, only a few randomized double-blind placebo-controlled clinical trials have attempted to determine whether supplementation with these ingredients are beneficial in patients with rheumatoid arthritis 9, 13, 15, 16, 21, 30. Rheumatoid arthritis (RA) is a chronic (long-lasting) autoimmune disease that mostly affects joints. Rheumatoid arthritis occurs when the immune system, which normally helps protect the body from infection and disease, attacks its own tissues. Rheumatoid arthritis causes pain, swelling, stiffness, and loss of function in joints. So far, most of the studies are pilot studies with small number of patients, studies on animal models, or test tube studies 31.
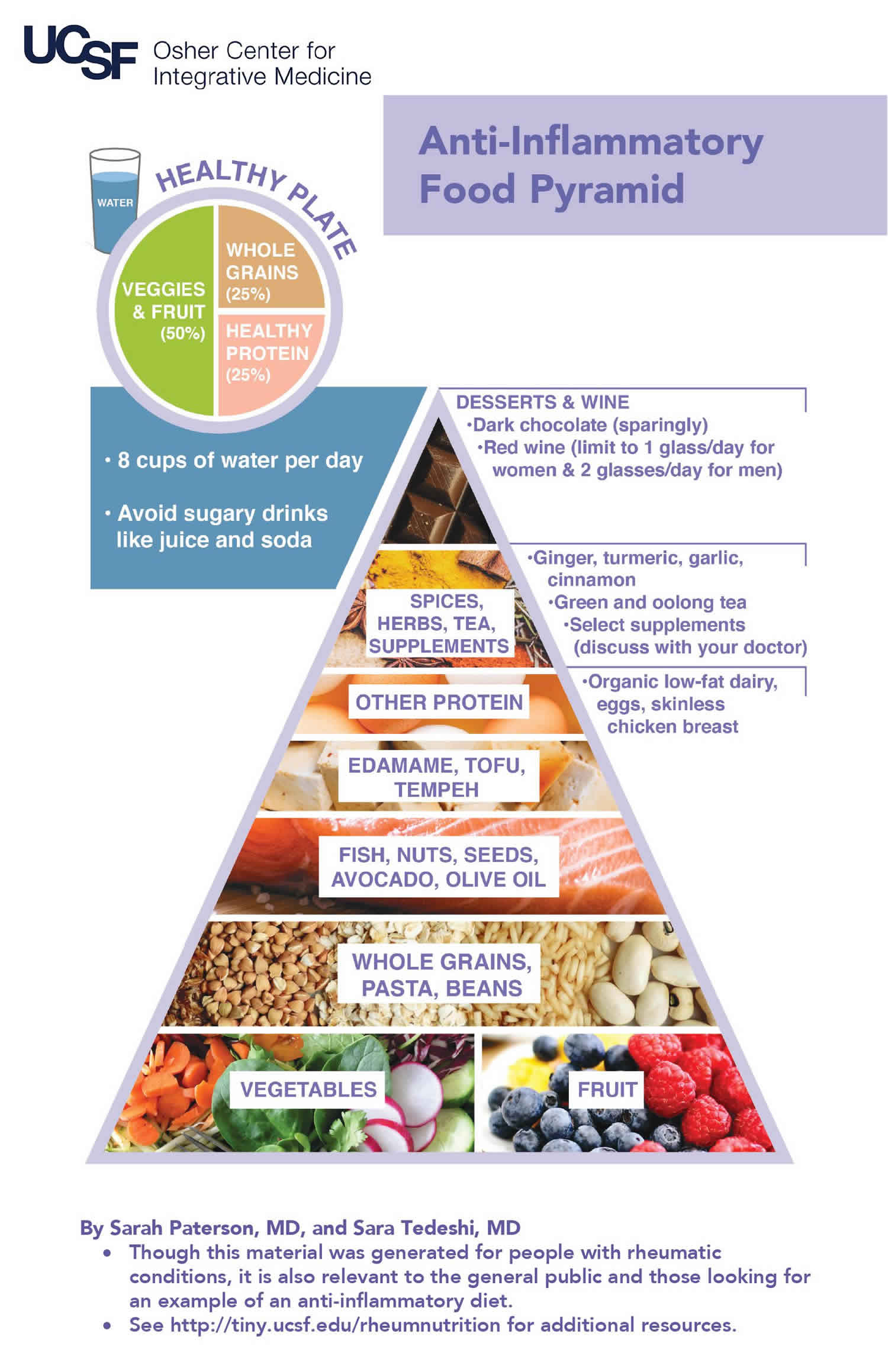
When it comes to anti-inflammatory foods, the goal should be to incorporate as many as you can into your overall diet. So make whole grains, vegetables, fruits and pulses (legumes) such as beans and lentils a major part of your normal diet. Get started by covering at least two-thirds (2/3) of your plate with plant foods such as whole grains, vegetables, fruit and beans. The remaining third (1/3) of your plate may be filled with animal-based protein rich foods such as seafood, poultry and dairy foods and occasionally with lean red meat.
Whole grains are healthier than refined grains because the process of refining carbohydrates results in the elimination of much of the fiber, vitamins, minerals, phytonutrients, and essential fatty acids 32. Furthermore, refined starches and sugars can rapidly alter blood glucose and insulin levels 32 and postprandial hyperglycemia can increase production of free radicals as well as proinflammatory cytokines 33.
There is consistent evidence that consumption of whole grains is protective against incident type 2 diabetes and cardiovascular disease 34, 35. However, it is unclear how these protective effects are mediated. Dietary fiber intake may reduce the risk of these diseases by mediating the pro-inflammatory process 36, 37. Two mechanistic hypotheses have emerged. First, dietary fiber may decrease oxidation of glucose and lipids, while maintaining a healthy intestinal environment. Second, dietary fiber may prevent inflammation by altering adipocytokines in adipose tissue and increasing enterohepatic circulation of lipids and lipophilic compounds 38. The link between dietary fiber intake and reduced high sensitivity serum C-reactive protein (hs-CRP) has been observed in several recent studies, including two analyses using cross-sectional data from NHANES 1999–2000 39, 40, an analysis using a longitudinal cohort of 524 healthy adults 41 and a small clinical trial 42.
Various dietary components including long chain omega-3 fatty acids, antioxidant vitamins, plant flavonoids, prebiotics and probiotics have the potential to modulate predisposition to chronic inflammatory conditions. These components act through a variety of mechanisms including decreasing inflammatory mediator production through effects on cell signaling and gene expression (omega-3 fatty acids, vitamin E, plant flavonoids), reducing the production of damaging oxidants (vitamin E and other antioxidants), and promoting gut barrier function and anti-inflammatory responses (prebiotics and probiotics) 43. In a large Danish study 44 involving over 7000 men and women who were followed over 15 years (1982-1998). What that study found was that in both men and women who frequently consume wholemeal bread, vegetables, fruits, and fish, was associated with a better overall survival rate as well as better cardiovascular survival 44.
A low-fat diet (≤30% of total calories) is still considered by many physicians to be a healthy choice for both primary and secondary prevention of cardiovascular disease (coronary heart disease) 45. An unintended consequence of emphasizing low-fat diets may have been to promote unrestricted carbohydrate intake, which reduces high-density lipoprotein cholesterol (HDL “good” cholesterol) and raises triglyceride levels, exacerbating the metabolic manifestations of the insulin resistance syndrome, also known as the metabolic syndrome 46.
Three dietary strategies may help prevent coronary heart disease (coronary artery disease) 47:
- Increase consumption of omega-3 fatty acids from fish or plant sources;
- Substitute nonhydrogenated unsaturated fats for saturated and trans-fats; and
- Consume a diet high in fruits, vegetables, nuts, and whole grains and low in refined grains.
The effects of diet on coronary heart disease (coronary artery disease) can be mediated through multiple biologic pathways other than serum lipids, including oxidative stress, subclinical inflammation, endothelial dysfunction, insulin sensitivity, blood pressure, and thrombotic tendency 48.
Endothelial dysfunction is one of the mechanisms linking diet and the risk of cardiovascular disease 49, 50. This study 51 suggests a mechanism for the role of dietary patterns in the pathogenesis of cardiovascular disease. For example, women in the Nurses’ Health Study who ate a “American” diet (high in red and processed meats, sweets, desserts, French fries, and refined grains) had higher proinflammatory cytokines (C-reactive protein, Interleukin-6, E-selectin, soluble vascular cell adhesion molecule 1 and soluble intercellular adhesion molecule 1) than those with the “healthier” pattern, characterized by higher intakes of fruit, vegetables, legumes, fish, poultry, and whole grains 51. The levels of these mediators amplify the inflammatory response, are destructive and contribute to the clinical symptoms 43.
Arachidonic acid (AA) derived (omega-6) eicosanoids (primarily from refined vegetable oils such as corn, sunflower, and safflower) increase the production of proinflammatory cytokines IL-1, TNF-α, and IL-6, operating as precursors of the proinflammatory eicosanoids of the prostaglandin (PG)2-series 52. In contrast, the omega-3 polyunsaturated fatty acids (PUFAs), found in fish, fish oil, walnuts, wheat germ, and some dietary supplements such as flax seed products can curb the production of arachidonic acid (AA)-derived eicosanoids 53. The Omega-6 and Omega-3 polyunsaturated fatty acids (PUFAs) compete for the same metabolic pathways, and thus their balance is important 54. Accordingly, it is not surprising that both higher levels of Omega-3 polyunsaturated fatty acids (PUFAs) as well as lower Omega-6:Omega-3 ratios are associated with lower proinflammatory cytokine production 55.
Furthermore, in another study a healthy dietary pattern rich in fruit, vegetables and olive oil, such as the Mediterranean diet, is associated with lower levels of inflammatory markers, perhaps because of the anti-inflammatory properties of antioxidants 32.
The antioxidant properties of vegetables and fruits are thought to be one of the fundamental mechanisms underlying their anti-inflammatory dietary contributions 32. Oxidants such as superoxide radicals or hydrogen peroxide that are produced during the metabolism of food can activate the NF-κB pathway, promoting inflammation 56. Higher fruit and vegetable intakes are associated with lower oxidative stress and inflammation 56. In fact, some evidence suggests that the addition of antioxidants or vegetables may limit or even reverse proinflammatory responses to meals high in saturated fat 57.
Chronic inflammatory processes contribute to the pathogenesis of many age-related diseases. In search of anti-inflammatory foods, researchers in the University of Wollongong, Australia, have systematically screened 115 variety of common dietary plants and mushrooms for their anti-inflammatory activity. Gunawardena and colleagues tested 115 commercially available plants and mushroom foods (including 115 identical samples heated with glucose) for their anti-inflammatory ability 58. The plant and mushroom samples were prepared by methods usually employed with food preparation, involving heating (i.e. ‘cooking’) and mechanical dispersion. Samples were prepared by blending in a food processor with water before heating in a microwave for 10 minutes under control conditions or including 1% glucose, to enhance Maillard reaction products. Using using the free radical nitric oxide (NO) and tumour necrosis factor–α (TNF-α) as pro-inflammatory markers, 10 foods demonstrated significant anti-inflammatory activity (see Table 3) 58. The activation of macrophages leads to secretion of inflammatory molecules such as the pro-inflammatory cytokine TNF-α and the free radical nitric oxide (NO), which play an important role in inflammation and nitroxidative stress in many age-related diseases, including Alzheimer’s disease 59.
To overcome silent inflammation requires an anti-inflammatory diet (with omega-3s and polyphenols). The most important aspect of such an anti-inflammatory diet is the stabilization of insulin and reduced intake of omega-6 fatty acids. The ultimate treatment lies in reestablishing hormonal and genetic balance to generate satiety instead of constant hunger. Anti-inflammatory nutrition with caloric restriction, should be considered as a form of gene silencing technology, in particular the silencing of the genes involved in the generation of silent inflammation. To this anti-inflammatory diet foundation supplemental omega-3 fatty acids at the level of 2–3 g of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) per day should be added. Finally, a diet rich in colorful, non-starchy vegetables would contribute adequate amounts of polyphenols to help not only to inhibit nuclear factor (NF)-κB (primary molecular target of inflammation) but also activate AMP kinase 60.
Figure 1. Foods that fight cancer
Table 1. Omega-3 Fatty Acid Foods EPA (Eicosapentaenoic acid) and DHA (Docosahexaenoic acid) – Fish and Seafood Sources
[Source 61]Table 2. Other sources of Omega-3 Alpha-Linolenic Acid (ALA) – Non-Seafood Sources
| Source of ALA | ALA content, g |
|---|---|
| Pumpkin seeds (1 tbsp) | 0.051 |
| Olive oil (1 tbsp) | 0.103 |
| Walnuts, black (1 tbsp) | 0.156 |
| Soybean oil (1 tbsp) | 1.231 |
| Rapeseed oil (1 tbsp) | 1.302 |
| Walnut oil (1 tbsp) | 1.414 |
| Flaxseeds (1 tbsp) | 2.350 |
| Walnuts, English (1 tbsp) | 2.574 |
| Flaxseed oil (1 tbsp) | 7.249 |
| Almonds (100 g) | 0.4 |
| Peanuts (100 g) | 0.003 |
| Beans, navy, sprouted (100 g) | 0.3 |
| Broccoli, raw (100 g) | 0.1 |
| Lettuce, red leaf (100 g) | 0.1 |
| Mustard (100 g) | 0.1 |
| Purslane (100 g) | 0.4 |
| Spinach (100 g) | 0.1 |
| Seaweed, spirulina, dried (100 g) | 0.8 |
| Beans, common, dry (100 g) | 0.6 |
| Chickpeas, dry (100 g) | 0.1 |
| Soybeans, dry (100 g) | 1.6 |
| Oats, germ (100 g) | 1.4 |
| Rice, bran (100 g) | 0.2 |
| Wheat, germ (100 g) | 0.7 |
| Avocados, California, raw (100 g) | 0.1 |
| Raspberries, raw (100 g) | 0.1 |
| Strawberries, raw (100 g) | 0.1 |
| Novel sources of ALA | ALA content, g |
| Breads and pasta (100 g) | 0.1–1.6 |
| Cereals (and granola bars) (55 g) | 1.0–4.9 |
| Eggs (50 g or 1 egg) | 0.1–0.6 |
| Processed meats (100 g) | 0.5 |
| Salad dressing (14 g – 31 g) | 2.0–4.0 |
| Margarine spreads (10 g – 100 g) | 0.3–1.0 |
| Nutrition bars (50 g) | 0.1–2.2 |
Footnote: 1 tablespoon (tbsp) oil = 13.6 g; 1 tbsp seeds or nuts = 12.35 g.
[Source 62]Table 3. Anti-inflammatory foods

Seven of these food preparations, including lime zest, English breakfast tea, honey brown mushroom, button mushroom, oyster mushroom, cinnamon and cloves demonstrated potent anti-inflammatory activity with IC 50 values between 0.1 and 0.5 mg/ml. However, the most active food samples were onion, followed by oregano and red sweet potato exhibiting IC 50 values below 0.1 mg/ml. In addition, English breakfast tea leaves, oyster mushroom, onion, cinnamon and button mushroom preparations suppressed TNF-α production, exhibiting with IC 50 values below 0.5 mg/ml in RAW 264.7 macrophages 63.
Secondary screen using RAW 264.7 macrophages, NO and TNF-α as pro-inflammatory markers, of the 10 products, oyster mushroom and cinnamon, demonstrated the most significant anti-inflammatory activities (with IC 50 values below 0.1 mg/ml), followed by cloves, oregano, onion, English breakfast tea leaves and lime zest (see Table 3) 63. The commonly used drug for the management of inflammatory conditions are non-steroidal anti-inflammatory drugs (NSAIDs), which have several adverse effects especially gastric irritation leading to the formation of gastric ulcers 64. Two anti-inflammatory drugs, including prednisone and the non-steroidal anti-inflammatory drug (NSAID) ibuprofen were also tested in the same study assay systems. Ibuprofen was toxic to cells at concentrations of 1.63 ± 0.44 mM, and NO and TNF-α production were down-regulated at the same concentrations (Table 4). Prednisone inhibited LPS + IFN-γ induced NO and TNF-α production with IC 50 values of 0.25 ± 0.09 mg/ml (Table 4).
Table 4. Anti-inflammatory foods re-tested in macrophages

Onions have been shown to exhibit anti-inflammatory properties, e.g. downregulation of adipokine expression in the visceral adipose tissue of rats or attenuation of vascular inflammation and oxidative stress in fructose-fed rats 65, 66. Among the polyphenols in onions, quercetin was suggested to be the responsible anti-inflammatory ingredient, as evidenced by the downregulation of COX2 transcription in human lymphocytes 67. Furthermore, the anti-inflammatory activity of the onion has been
studied also in relation to the presence of thiosulfinates and cepaenes 68, 69.
Anti-inflammatory properties of cinnamon has been demonstrated for Cinnamomum osmophloem kaneh 70, 71, but less is known about the ‘true’ cinnamon of India, Cinnamomum zeylanicum. Some authors reported significant inhibitory effects of inflammatory signalling by the extracts of C. cassia 72. Sodium benzoate appears to be one of the active ingredients in cinnamon, since it inhibits LPS-induced expression of inducible NO synthase (iNOS), pro-inflammatory cytokines (TNF-α and IL-1 β) and surface markers for inflammatory activation such as CD11b, CD11c, and CD68 in mouse microglia 73. However, E-cinnamaldehyde and o-methoxycinnamaldehyde are responsible for most of the anti-inflammatory activity of cinnamon 74.
Clove (Syzygium aromaticum) extracts have been identified as having potent free radical (including superoxide anion) scavenging properties, and metal chelating activities, which may be due to the presence of flavonoids. Cloves contain considerable concentrations of eugenol, beta-caryophyllene, quercetin and kaempferol as well as rhamnetin and kaempferol and their glycosides 75.
Red sweet potato (Ipomoea batatas), a species rich in β-carotene and anthocyanins 76, has been demonstrated to have anti-inflammatory properties for the first time by this study 63.
Lime (Citrus aurantifolia) rich in flavonol glycosides, especially of kaempferol-type are known for their anti-oxidant properties 77, this study 63 is also the first to report on its anti-inflammatory activities.
A handful studies have shown the anti-inflammatory properties of the various mushroom species. For example, oyster mushroom concentrate was shown to suppress LPS-induced secretion of TNF-α, IL-6 and IL-12p40 in RAW264.7 macrophages and also suppressed PGE2 and NO by down-regulation of COX-2 and iNOS expression, respectively. Oyster mushroom concentrate also inhibited LPS-dependent DNA binding activity of AP-1 and NF-κ B in RAW264.7 cells 78. In mushrooms, water-soluble polysaccharides, especially the β-glucans, are most likely to be the substances responsible for the anti-inflammatory properties. For example, β-glucans isolated Pleurotus ostreatus were able to potentiate the anti-inflammatory effects of methotrexate in rat models of experimental arthritis or colitis 79, 80. A further potential anti-inflammatory compound in mushrooms could be ergothioneine, a sulfur containing amino acid that functions as an antioxidant and is present in mushrooms at a concentration of up to 2.0mg/g 81. In Acute Respiratory Distress Syndrome (ARDS), ergothioneine given intravenously 1h before or 18 h after cytokine (IL-1 and IFN-γ) insufflation, decreased lung injury and lung inflammation in cytokine insufflated rats 82.
The anti-inflammatory activity in these food samples survived ‘cooking’ and suggesting these foods may be useful in limiting inflammation in a variety of age-related inflammatory diseases 63. Furthermore, these foods could be a source for the discovery of novel anti-inflammatory drugs 63. However, validation of these foods containing anti-inflammatory properties will require further clinical trials in human subjects before any recommendation can be given for their use to treat inflammatory conditions.

Fermented plant foods
Fermented plant foods are gaining wide interest worldwide as healthy foods due to their unique sensory features and their health-promoting potentials, such as antiobesity, antidiabetic, antihypertensive, and anticarcinogenic activities 83. Fermented fruits, vegetables, and grains are a rich source of nutrients and could enrich your diet with numerous live microorganisms (probiotic microbes), phytochemicals (chemical compounds produced by plants) and bioactive compounds. These compounds play a key role in the functional and health-promoting properties of fermented products. The excellent biological activities of these functional foods, such as anti-inflammatory and immunomodulatory functions, are widely attributable to their high antioxidant content and lactic acid-producing bacteria (LAB). Due to the high content of phenolic compounds with strong antioxidant activity, fermented blueberries and blackberries may protect against chronic inflammatory disorders by decreasing oxidative stress, modulating inflammatory signaling and responses, and improving immunity. Regarding fermented cabbage products, sauerkraut and kimchi, live lactic acid-producing bacteria are the key player in improving health and preventing chronic diseases through improving a healthy gut microbial balance and modulating inflammatory and immune responses. Fermented soybeans are an excellent source of isoflavones with known anti-inflammatory properties. Furthermore, probiotics found in fermented soy products contribute to the health benefits of these nutritious foods. Overall, growing evidence is strongly supporting the health benefits of fermented plant foods 83.
These human digestive-tract associated microbes are referred to as the gut microbiome (the collective genomes of the micro-organisms in a particular environment). Lactic acid-producing bacteria contribute to the maintenance of a healthy gut microbiota (the community of micro-organisms themselves) composition and improvement of local and systemic immunity 83. Approximately 100 trillion micro-organisms (most of them bacteria, but also viruses, fungi, and protozoa) exist in the human gastrointestinal tract 84—the microbiome is now best thought of as a virtual organ of the body. The term “microbiota,” “microflora,” or “normal flora” is used to designate this vast host of microbes which coexist with the host 85. The bacterial cells harbored within the human gastrointestinal tract (GIT) outnumber the host’s cells by a factor of 10 and the genes encoded by the bacteria resident within the gastrointestinal tract outnumber their host’s genes by more than 100 times 84. The human genome consists of about 23,000 genes, whereas the microbiome encodes over three million genes producing thousands of metabolites, which replace many of the functions of the host 86, consequently influencing the host’s fitness, phenotype, and health 87. The human gut microbiota consists of diverse microorganisms, including archaea, bacteria, viruses, and yeasts, which maintain a symbiotic relationship with the host 88. There is a mutual influence between gut microbiota and the immune system. Gut microbiota play a key role in the function and homeostasis of the immune system by the maturation of gut-associated lymphoid tissue and innate lymphoid cells, enhancing antimicrobial peptides, antibodies, and cytokines production, inducing immunoglobulin A (IgA)-producing B cells and T cells differentiation, and regulating T helper 17 (Th17)/regulatory T cells (Tregs) balance 89. Gut microbiota disturbance negatively affects the immune system and leads to inflammation 90.
The human gut microbiome and its role in both health and disease has been the subject of extensive research, establishing its involvement in human metabolism, nutrition, physiology, and immune function. Imbalance of the normal gut microbiota have been linked with gastrointestinal conditions such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS), and wider systemic manifestations of disease such as obesity, type 2 diabetes, and atopy 84. Atopy is a problem with your immune system that makes you more likely to develop allergic diseases.
Besides, antioxidant compounds are involved in several functional properties of fermented plant products by neutralizing free radicals, regulating antioxidant enzyme activities, reducing oxidative stress, ameliorating inflammatory responses, and enhancing immune system performance. Therefore, these products may protect against chronic inflammatory diseases, which are known as the leading cause of mortality worldwide. The gut remains the most important organ in which fermented foods exert their beneficial effects, either by systemically modulating the immune response or positively influencing the gut microbiota 91.
The anti-inflammatory and immunomodulatory properties of plant-based fermented foods are well documented 92. The functional properties of fermented products are, in part, related to the probiotics content of the products 91. Numerous health-promoting benefits have been attributed to probiotics due to their anti-inflammatory and immunomodulatory activities at the gut level and beyond 93. Probiotic consumption in the form of fermented foods can improve gut barrier integrity and gut immunity and maintain gut homeostasis 94, through different mechanisms, including the inhibition of pathogen colonization, the induction of antimicrobial peptides production and mucus secretion, the increase of IgA production, the down-regulation of the Th17 and pro-inflammatory cytokines such as IL-17F, IL-23, and the upregulation of Tregs production 95.
Moreover, fermentation will lead to the degradation of complex phytochemical molecules into smaller bioactive polyphenols. Studies have shown that polyphenolic compounds found in fermented products are beneficial in microbiota metabolism and growth 96 and can inhibit the production of inflammatory cytokines and suppress inflammatory responses 97. Furthermore, neutralizing free radicals, regulating antioxidant enzyme activities, reducing oxidative stress, and enhancing immune system activity are other potential mechanisms by which plant-based fermented foods and beverages exert health benefits 98.
Different plants, such as fruits, vegetables, tea, grains, legumes, and starchy roots, are used to produce plant-based fermented foods 97. Due to the high content of phenolic compounds with strong antioxidant activity, fermented blueberries and blackberries may protect against chronic inflammatory disorders by decreasing oxidative stress, modulating inflammatory signaling and responses, and improving immunity. Regarding fermented cabbage products, sauerkraut and kimchi, live lactic acid-producing bacteria are the key player in improving health and preventing chronic diseases through improving a healthy gut microbial balance and modulating inflammatory and immune responses. Fermented soybeans are an excellent source of isoflavones with known anti-inflammatory properties. Furthermore, probiotics found in fermented soy products contribute to the health benefits of these nutritious foods. Overall, growing evidence is strongly supporting the health benefits of fermented plant foods. However, existing evidence has been chiefly generated from in vitro (test tube studies) and animal studies, and there exist rare clinical studies in this field. Therefore, the potential role of fermented plant products in human health remains to be determined by randomized, controlled clinical trials.
What is inflammation?
Inflammation is an evolutionarily conserved process characterized by the activation of immune and non-immune cells that protect the host from bacteria, viruses, toxins and infections by eliminating pathogens and promoting tissue repair and recovery 99. Very generally speaking, is the body’s immune system’s response to an irritant, the irritant might be a germ, but it could also be a foreign object, such as a splinter in your finger 100. Inflammation is a normal part of your body’s defense to injury or infection, and, in this way, it is beneficial. But inflammation is damaging when it occurs in healthy tissues or lasts too long. Known as chronic inflammation, it may persist for months or years 101. Furthermore, inflammations don’t always help the body. In some diseases the immune system fights against the body’s own cells by mistake, causing harmful inflammations. Chronic inflammatory diseases contribute to more than half of deaths worldwide 102.
When an inflammation occurs in your body, many different immune system cells may be involved. They release various substances, known as inflammatory mediators. These include the hormones bradykinin and histamine 100. They cause the small blood vessels in the tissue to become wider (dilate), allowing more blood to reach the injured tissue. For this reason, inflamed areas turn red and feel hot. The increased blood flow also allows more immune system cells to be carried to the injured tissue, where they help with the healing process. What’s more, both of these hormones irritate nerves and cause pain signals to be sent to the brain. This has a protective function: If the inflammation hurts, you tend to protect the affected part of the body.
The inflammatory mediators have yet another function: They make it easier for immune system cells to pass out of the small blood vessels, so that more of them can enter the affected tissue. The immune system cells also cause more fluid to enter the inflamed tissue, which is why it often swells up. The swelling goes down again after a while, when this fluid is transported out of the tissue.
Mucous membranes also release more fluid when they are inflamed. For instance, this happens when you have a stuffy nose and the membranes lining your nose are inflamed. Then the extra fluid can help to quickly flush the viruses out of your body.
Depending on the degree and extent of the inflammatory response, including whether it is systemic or local, metabolic and neuroendocrine changes can occur to conserve metabolic energy and allocate more nutrients to the activated immune system 101. Specific biobehavioral effects of inflammation thus include a constellation of energy-saving behaviors commonly known as “sickness behaviors,” such as sadness, anhedonia, fatigue, reduced libido and food intake, altered sleep and social-behavioral withdrawal, as well as increased blood pressure, insulin resistance and dyslipidemia 103. These behavioral changes can be critical for survival during times of physical injury and microbial threat 104.
Inflammation is associated with diseases such as the following:
- Rheumatoid arthritis (RA), where many joints throughout the body are permanently inflamed
- Systemic lupus erythematosus (SLE): Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that causes inflammation in connective tissues, such as cartilage and the lining of blood vessels, which provide strength and flexibility to structures throughout the body.
- Multiple sclerosis (MS): Multiple sclerosis (MS) is an autoimmune disease that affects the brain and spinal cord (central nervous system) that disrupts the flow of information within the brain, and between the brain and body.
- Psoriasis – a chronic skin disease
- Inflammatory bowel diseases like Crohn’s disease or ulcerative colitis
- Cardiovascular diseases like high blood pressure and heart disease
- Lung diseases like asthma
- Mental illnesses like depression 105
- Metabolic diseases like type 2 diabetes
- Neurodegenerative diseases like Parkinson’s disease
- Some types of cancer, like colon cancer 106
Collectively known as chronic inflammatory diseases, these diseases can last for years or even a lifetime. Their severity and level of activity varies. Together, cardiovascular disease, cancer, and diabetes account for almost 70% of all deaths in the United States; these diseases share inflammation as a common link 32, 107.
A normal inflammatory response is characterized by the temporally restricted upregulation of inflammatory activity that occurs when a threat is present and that resolves once the threat has passed 108. However, the presence of certain social, psychological, environmental and biological factors has been linked to the prevention of resolution of acute inflammation and, in turn, the promotion of a state of low-grade, non-infective (that is, ‘sterile’) systemic chronic inflammation that is characterized by the activation of immune components that are often distinct from those engaged during an acute immune response 103. The clinical consequences of systemic chronic inflammation-driven damage can be severe and include increased risk of the metabolic syndrome, which includes the triad of hypertension, hyperglycemia and dyslipidemia 109; type 2 diabetes 110; nonalcoholic fatty liver disease (NAFLD) 111; hypertension 112; cardiovascular disease such as atherosclerosis 113; chronic kidney disease 114; various types of cancer 106; depression 105; neurodegenerative and autoimmune diseases 115, 116; osteoporosis 117 and sarcopenia 114. Empirical evidence that inflammation plays a role in disease onset or progression is strongest for metabolic syndrome, type 2 diabetes and cardiovascular disease. Indeed, it has long been known that patients with autoimmune diseases such as rheumatoid arthritis that are characterized by systemic inflammation have insulin resistance, dyslipidemia and hypertension, and that they have higher rates of metabolic syndrome, type 2 diabetes and cardiovascular disease (particularly ischemic heart disease and stroke) 101, 118. Moreover, the inflammatory biomarker high-sensitivity C-reactive protein (hs-CRP) is a predictor of cardiovascular events in men and women 119. In a recent meta-analysis of data from more than 160,000 people across 54 long-term prospective studies, higher levels of circulating CRP were associated with a relative increase in risk for both coronary heart disease and cardiovascular disease mortality 120. To assess the inflammatory potential of diets, the dietary inflammatory index (DII) was developed 121. Higher dietary inflammatory index (DII) scores indicate greater inflammatory potential of the diet and have been associated with elevated inflammatory biomarker levels interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and C-reactive protein (CRP) 122.
The most compelling evidence for an association between systemic chronic inflammation and disease risk comes from randomized controlled trials that have tested drugs or biologics that target specific pro-inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α. In a recent meta-analysis of eight randomized controlled trials that included a total of 260 participants, anti-TNF-α inhibitor therapy was found to significantly reduce insulin resistance in patients with rheumatoid arthritis and to improve their insulin sensitivity 123. The risk for developing Alzheimer’s disease was also significantly lower among patients with rheumatoid arthritis treated with the TNF-α inhibitor etanercept 124. In addition, a recent double-blind randomized controlled trial of the IL-1β inhibitor canakinumab that assessed more than 10,000 adults with a history of myocardial infarction and elevated circulating C-reactive protein (CRP) levels showed that patients treated with canakinumab subcutaneously every 3 months had lower rates of nonfatal myocardial infarction, nonfatal stroke and cardiovascular disease death compared with those treated with a placebo, despite having no change in LDL cholesterol (low-density lipoprotein or “bad” cholesterol), which is a risk factor for cardiovascular disease. In this trial, canakinumab-treated patients also exhibited a lower likelihood of unstable angina leading to urgent revascularization 125. Along similar lines, a recent study of more than 160,000 people from North Glasgow found that a combination of the inflammatory markers CRP (>10 mg/L), albumin (>35 mg/L) and neutrophil count predicted all-cause mortality over 8 years, in addition to mortality due to cancer, cardiovascular and cerebrovascular disease 126.
Inflammation may result from many factors, such as:
- Environmental chemicals
- Ozone and cardiovascular disease – Exposure to ozone, even at levels lower than the current U.S. Environmental Protection Agency (EPA) air quality standard, may lead to cardiovascular disease 127.
- Injuries like scrapes, insect stings, or a splinter in your finger
- Pathogens (germs) like bacteria, viruses, or fungi
- Radiation
- Nutrition – Diets high in refined grains, alcohol, and processed foods can alter gut microbiota and lead to intestinal and immune changes.
- Microbiome (microbiome refers to the collective genomes of the micro-organisms in a particular environment) – Studies of various microbiome imbalances and disease states show connections to inflammation.
- Social and cultural changes – Disrupted sleep patterns, psychosocial stress, artificial light, and other factors influence the immune system.
- Developmental origins – Childhood obesity, psychological stress, exposure to microbes in infancy, and prenatal conditions are linked to inflammation.
- Physical activity – When skeletal muscles contract, they release proteins that can reduce inflammation throughout the body. Skeletal muscle is an endocrine organ that produces and releases cytokines and other small proteins, called myokines, into the bloodstream. This occurs particularly during muscle contraction and can have the effect of systemically reducing inflammation 128.
Industrialization is thought to have caused a significant overall decrease in physical activity. One study showed that, worldwide, 31% of individuals are considered physically inactive—defined as not meeting the minimum international recommendations for regular physical activity—with levels of inactivity being higher in high-income countries than in low-to-middle-income countries 129. In the United States, these numbers are even higher, with approximately 50% of American adults being considered physically inactive 130.
Low physical activity, therefore, has been found to be directly related to increased anabolic resistance 131 and levels of C-reactive protein (CRP) and pro-inflammatory cytokine levels in healthy individuals 132, as well as in breast cancer survivors 133 and patients with type 2 diabetes 134. These effects can, in turn, promote several inflammation-related pathophysiologic alterations, including insulin resistance, dyslipidemia, endothelial dysfunction, high blood pressure and loss of muscle mass (sarcopenia) 135, that have been found to increase risk for a variety of conditions, including cardiovascular disease, type 2 diabetes, nonalcoholic fatty liver disease (NAFLD), osteoporosis, various types of cancer, depression, dementia and Alzheimer’s disease, in individuals who are chronically inactive 128.
Consistent with these effects, there is strong evidence for an association between physical inactivity and increased risk for age-related diseases and mortality 102. A recent meta-analysis of studies with cohorts from Europe, the United States and the rest of the world that included 1,683,693 participants found that going from physically inactive to achieving the recommended 150 minutes of moderate-intensity aerobic activity per week was associated with lower risk of cardiovascular disease mortality by 23%, cardiovascular disease incidence by 17%, and type 2 diabetes incidence by 26% during an average follow-up period of 12.8 years 136. Moreover, data from 1.44 million participants across several prospective cohort studies revealed that, as compared to individuals exhibiting high levels of leisure-time physical activity (≥90th percentile), those who were physically inactive (≤10th percentile) had a greater risk (>20%) of developing several cancers, including esophageal adenocarcinoma; liver, lung, kidney, gastric cardia and endometrial cancers; and myeloid leukemia, even after adjusting for multiple major risk factors such as adiposity and smoking status (except for lung cancer) 137. Likewise, a meta-analysis of ten studies and 23,345 older adults (70 to 80 years old) who were followed for 3.9–31 years found that individuals meeting the minimum international physical activity recommendations had a 40% lower risk of Alzheimer’s disease as compared to their physically inactive counterparts 138.
Moreover, physical inactivity can increase individuals’ risk for various non-communicable diseases because it is linked to obesity 135 and, in particular, excessive visceral adipose tissue, which is a significant trigger of inflammation 139. Visceral adipose tissue is an active endocrine, immunological and metabolic organ composed of various cells (including immune cells, such as resident macrophages) that expands mostly through adipocyte hypertrophy, which can lead to areas of hypoxia and even cell death, resulting in activation of hypoxia-inducible factor-1α, increased production of reactive oxygen species, and release of damage-associated molecular patterns (DAMPs) (for example, cell-free DNA). These events can induce the secretion of numerous pro-inflammatory molecules, including adipokines, cytokines (for example, IL-1β, IL-6, TNF-α), and chemokines (especially monocyte chemoattractant protein-1) by adipocytes, endothelial cells and resident adipose tissue immune cells (for example, macrophages) 140. This in turn leads to the infiltration of various immune cells in the visceral adipose tissue, including monocytes, neutrophils, dendritic cells, B cells, T cells and natural killer (NK) lymphocytes, and a reduction in T regulatory cells, thereby amplifying inflammation, which can eventually become prolonged and systemic in some individuals 141.
Furthermore, tumor necrosis factor alpha (TNF-α) and other molecules can cause adipocyte insulin resistance, which increases lipolysis, with the resulting spillover of lipids into other organs, such as the pancreas and liver, where they can contribute to beta-cell dysfunction, hepatic insulin resistance and fatty liver 142. Hence, visceral obesity accelerates aging and increases risk for cardiometabolic, neurodegenerative and autoimmune diseases, as well as several types of cancer 143. These dynamics are known to occur in adults and can promote age-related disease risk, but they first emerge during childhood 144. The childhood obesity epidemic might thus be playing a key role in promoting inflammation and age-related disease risk worldwide 145.
In addition to physical inactivity and diet, the industrial revolution and modern era have ushered in changes in social interactions and sleep quality 146 that can promote systemic chronic inflammation 147 and insulin resistance 148, in turn increasing risk for obesity, type 2 diabetes, cardiovascular disease and all-cause mortality 149. Moreover, psychological stressors that are persistently present in some contemporary work environments, such as those characterized by high job demand and low control, can cause physiologic changes 150 that disrupt the ability for glucocorticoids to effectively down-regulate inflammatory activity due to decreased sensitivity caused by chronic elevation in cortisol, leading in turn to systemic chronic inflammation and poor health 151.
Another core feature of modern society that has occurred very recently in human evolutionary history is increased exposure to artificial light, especially the blue spectrum, at atypical biologic times 152. Exposure to blue light, especially after sundown, increases arousal and alertness at night and thus causes circadian rhythm disruption 153, which in turn promotes inflammation 154, and is a risk for multiple inflammation-related diseases 155. As an example, night-shift work has been found to increase risk for the metabolic syndrome and is suspected of being a causal factor in obesity, type 2 diabetes and cardiovascular disease, as well as in breast, ovarian, prostate, colorectal and pancreatic cancer 152.
Despite evidence linking systemic chronic inflammation with disease risk and mortality 126, there are presently no standard biomarkers for indicating the presence of health-damaging chronic inflammation. Studies have shown that biomarkers of acute inflammation predict morbidity and mortality in both cross-sectional and longitudinal studies and may thus be used to index age-related systemic chronic inflammation 156. This approach has notable limitations, though. For example, early work by Roubenoff and colleagues showed that in monocytes from ambulatory individuals, levels of IL-6 and IL-1Ra (but not IL-1β or TNF-α) increased with age 157. However, no difference in IL-1 and IL-6 expression has been found between young and older individuals when the health status of older individuals is strictly controlled 158.
Shifts in the inflammatory response from short- to long-lived can cause a breakdown of immune tolerance 108 and lead to major alterations in all tissues and organs, as well as normal cellular physiology. Systemic chronic inflammation can also impair normal immune function, leading to increased susceptibility to infections and tumors and a poor response to vaccines 159. Furthermore, systemic chronic inflammation during pregnancy and childhood can have serious developmental consequences that include elevating the risk of non-communicable diseases over the life span 160, 144, 161, 162.
Indeed, chronic inflammatory diseases have been recognized as the most significant cause of death in the world today, with more than 50% of all deaths being attributable to inflammation-related diseases such as ischemic heart disease, stroke, cancer, diabetes mellitus, chronic kidney disease, non-alcoholic fatty liver disease (NAFLD) and autoimmune and neurodegenerative conditions5. Evidence is emerging that the risk of developing chronic inflammation can be traced back to early development, and its effects are now known to persist throughout the life span to affect adulthood health and risk of mortality 163, 160, 162.
High inflammatory foods
Dietary patterns high in refined starches, sugar, and saturated and trans-fatty acids, poor in natural antioxidants and fiber from fruits, vegetables, and whole grains, and poor in omega-3 fatty acids may cause an activation of the innate immune system, most likely by excessive production of proinflammatory cytokines associated with a reduced production of anti-inflammatory cytokines 164. It has been found that a higher intake of red and processed meat and fried food and a lower intake of whole grains was associated with higher levels of inflammatory markers (interleukin 6 [IL-6], tumor necrosis factor alpha [TNF-α], and C-reactive protein [CRP]), as well as accelerated cognitive decline in older ages 165. The typical American diet that has become widely adopted in many countries over the past 40 years is relatively low in fruits, vegetables and other fiber- and prebiotic-rich foods 166 and high in refined grains 167, alcohol 168 and ultra-processed foods 169, particularly those containing emulsifiers 170. These dietary factors can alter the gut microbiota composition and function 171 and are linked to increased intestinal permeability 172 and epigenetic changes in the immune system 173 that ultimately cause low-grade endotoxemia and systemic chronic inflammation 174. The influence of diet on inflammation is not confined to these effects, though. For example, orally absorbed advanced glycation and lipoxidation end-products that are formed during the processing of foods or when foods are cooked at high temperatures and in low-humidity conditions are appetite increasing and are linked to overnutrition and hence obesity and inflammation 175. Furthermore, high-glycemic-load foods, such as isolated sugars and refined grains, which are common ingredients in most ultra-processed foods, can cause increased oxidative stress that activates inflammatory genes 176.
When combined with low physical activity, consuming hyperpalatable processed foods that are high in fat, sugar, salt and flavor additives 177 can cause major changes in cell metabolism and lead to the increased production (and defective disposal) of dysfunctional organelles such as mitochondria, as well as to misplaced, misfolded and oxidized endogenous molecules 178. These altered molecules, which increase with age 114, can be recognized as damage-associated molecular patterns (DAMPs) by innate immune cells, which in turn activate the inflammasome machinery, amplify the inflammatory response 112 and contribute to a biological state that has been called “inflammaging,” defined as the “the long-term result of the chronic physiological stimulation of the innate immune system” that occurs in later life 179. As proposed, inflammaging involves changes in numerous organ systems, such as the brain, gut, liver, kidney, adipose tissue and muscle 114 and it is driven by a variety of molecular-age-related mechanisms that have been called the “Seven Pillars of Aging”—namely, adaptation to stress, epigenetics, inflammation, macromolecular damage, metabolism, proteostasis and stem cells and regeneration 180.
Dietary strategies clearly influence inflammation, as documented through both prospective observational studies as well as randomized controlled feeding trials in which participants agree to eat only the food provided to them 32, 181. Studies have shown how various dietary components can modulate key pathways to inflammation including sympathetic activity, oxidative stress, transcription factor nuclear factor kappa B (NF-κB) activation, and proinflammatory cytokine production 182. Circulating markers of inflammation, such as C-reactive protein (CRP), tumor necrosis factor (TNF)-alpha, and some interleukins (IL-6, IL-18), correlate with propensity to develop coronary heart disease and heart attacks 183, 184, 185. Previous studies have also shown that depression is associated with upregulated inflammatory response, characterized by increased levels of pro-inflammatory cytokines and other acute-phase proteins 186.
Other dietary components that are thought to influence inflammation include trans fatty acids 187 and dietary salt. For example, salt has been shown to skew macrophages toward a pro-inflammatory phenotype characterized by the increased differentiation of naive CD4+ T cells into T helper (TH)-17 cells, which are highly inflammatory, and decreased expression and anti-inflammatory activity of T regulatory cells 188. In addition, high salt intake can cause adverse changes in gut microbiota composition, as exemplified by the reduced Lactobacillus population observed in animals and humans fed high-salt diets 188. This specific population is critical for health as it regulates TH17 cells and enhances the integrity of the intestinal epithelial barrier, thus reducing systemic inflammation 188. Consistent with the expected health-damaging effects of consuming foods that are high in trans fats and salt, a recent cohort study of 44,551 French adults who were followed for a median of 7.1 years found that a 10% increase in the proportion of ultra-processed food consumption was associated with a 14% greater risk of all-cause mortality 189.
Several other nutritional factors can also promote inflammation and potentially contribute to the development of systemic chronic inflammation. These factors include deficiencies in micronutrients, including zinc 190 and magnesium 191, which are caused by eating processed or refined foods that are low in vitamins and minerals, and having suboptimal omega-3 levels 192, which impacts the resolution phase of inflammation. Longchain omega-3 fatty acids especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) modulate the expression of genes involved in metabolism and inflammation 192. More importantly, they are precursors to molecules such as resolvins, maresins and protectins that are involved in the resolution of inflammation 193. The main contributors to the growing worldwide incidence of low omega-3 status are a low intake of fish and high intake of vegetable oils that are high in linoleic acid, which displaces omega-3 fatty acids in cell membrane phospholipids 194. In turn, various randomized controlled trials have shown that omega-3 fatty acid supplementation reduces inflammation 195 and may thus have health-promoting effects 194.
Diets that promote inflammation are 196:
- High in Refined Starches or Refined Carbs,
- High in Sugar,
- High in Saturated and Trans-fats,
- High in Salt (sodium),
- Alcohol,
- Food Additives,
- Low in omega-3 fatty acids,
- Low in natural antioxidants,
- Low in fiber from fruits,
- Low in vegetables, and
- Low in whole grains.
Dietary patterns high in refined starches, sugar, and saturated and trans-fatty acids, poor in natural antioxidants and fiber from fruits, vegetables, and whole grains, and poor in omega-3 fatty acids – aka the Average American Diet – may cause an activation of the innate immune system, most likely by an excessive production of proinflammatory cytokines associated with a reduced production of anti-inflammatory cytokines 197.
An Americanization of food habits has been recognized throughout the world, characterized by a high-energy diet, with increasing consumption of industrially processed foods. These foods usually contain large amounts of salt, simple sugars, saturated and trans fats, which food industries offer in response to consumers’ demands. Consequently, the intake of complex carbohydrates, fibres, fruits and vegetables has decreased. The energy and animal proteins consumed largely exceed World Health Organization recommendations, while, generally, a smaller variety of foods is being consumed.
- Most Americans exceed the recommendations for added sugars, saturated fats, and sodium. Most people say that if there is a healthy choice on a menu they will take it. But observations and research show this is generally not the case. Instead, people tend to make choices based on how food tastes. Typically, the more sugar, salt and fat in the food, the more we will like it.
- About three-fourths of the population has an eating pattern that is low in vegetables, fruits, dairy, and oils.
- More than half of the population is meeting or exceeding total grain and total protein foods recommendations, but, are not meeting the recommendations for the subgroups within each of these food groups.
The typical eating patterns currently consumed by many in the United States do not align with the Healthy Dietary Guidelines. As shown in Figure 1, when compared to the healthy eating pattern:
- About three-fourths of the population has an eating pattern that is low in vegetables, fruits, dairy, and oils.
- Most Americans exceed the recommendations for added sugars, saturated fats, and sodium.
Figure 2. The Average American Diet containing foods that can cause inflammation
In addition, the eating patterns of many are too high in calories. Calorie intake over time, in comparison to calorie needs, is best evaluated by measuring body weight status. The high percentage of the population that is overweight or obese suggests that many in the United States over consume calories. More than two-thirds of all adults and nearly one-third of all children and youth in the United States are either overweight or obese.
The typical American diet is about 50% carbohydrate, 15% protein, and 35% fat. The Standard American Diet or “Western-style” dietary patterns with more red meat or processed meat, sugared drinks, sweets, refined carbohydrates, or potatoes-have been linked to obesity 198, 199, 200, 201. The Average American (Western-style) dietary pattern is also linked to increased risk of heart disease, diabetes, and other chronic conditions.
Meal frequency and snacking have increased over the past 30 years in the U.S. 202 on average, children get 27 percent of their daily calories from snacks, primarily from desserts and sugary drinks, and increasingly from salty snacks and candy.
What Americans Eat: Top 10 sources of calories in the U.S. diet
- Grain-based desserts (cakes, cookies, donuts, pies, crisps, cobblers, and granola bars)
- Yeast breads
- Chicken and chicken-mixed dishes
- Soda, energy drinks, and sports drinks
- Pizza
- Alcoholic beverages
- Pasta and pasta dishes
- Mexican mixed dishes
- Beef and beef-mixed dishes
- Dairy desserts(Source: Report of the 2010 Dietary Guidelines Advisory Committee)
For the 20-30 year old the top sources of “Nutrition” are:
- Regular soft drinks 8.8% of total energy
- Pizza 5.1% of total energy
- Beer 3.9%
- Hamburgers and meat loaf 3.4%
- White bread 3.3%
- Cake, doughnuts and pastries 3.3%
- French fries and fried potatos 3.0%
- Potato chips, corn chips and popcorn 2.7%
- Rice 2.6%
- Cheese and cheese spread 2.5%
Since the above list of diets cause inflammation, therefore the main dietary strategy to reduce inflammation should include adequate omega-3 fatty acids intake, reduction of saturated and trans-fats, and consumption of a diet high in fruits, vegetables, nuts, and whole grains and low in refined grains. The whole diet approach seems particularly promising to reduce the inflammation associated with the metabolic syndrome (e.g. high “bad” triglyceride, low “good” HDL cholesterol, belly fat, high blood pressure, high blood sugar/insulin resistance). The choice of healthy sources of carbohydrate, fat, and protein, associated with regular physical activity and avoidance of smoking, is critical to fighting the war against chronic disease. Western dietary patterns warm up inflammation, while healthy dietary patterns cool it down.
Refined Starches or Refined Carbs Inflammatory Foods
The process of refining a food not only removes the fiber, but it also removes much of the food’s nutritional value, including B-complex vitamins, healthy oils and fat-soluble vitamins. Refined carbohydrates are plant-based foods that have the whole grain extracted during processing, where the fibers, starches, vitamins and minerals have been removed, leaving behind refined carbohydrates. Refined carbohydrates sources include bottled fruit juices, juice concentrates, white bread, pastries, sodas, donuts, cakes, cookies, sweets, chips and other highly processed or refined foods. These items contain easily digested carbohydrates that may contribute to weight gain, interfere with weight loss, and promote diabetes and heart disease 203.
Refined carbohydrates are composed of sugars (such as fructose and glucose) which have simple chemical structures composed of only one sugar (monosaccharides) or two sugars (disaccharides). Simple carbohydrates are easily and quickly utilized for energy by the body because of their simple chemical structure, often leading to a faster rise in blood sugar and insulin secretion from the pancreas – which can have negative health effects.
When it comes to carbohydrates, what’s most important is the type of carbohydrate you choose to eat because some sources are healthier than others. The amount of carbohydrate in the diet – high or low – is less important than the type of carbohydrate in the diet. For example, healthy, whole grains such as whole wheat bread, rye, barley and quinoa are better choices than highly refined white bread or French fries. For example, whole grain whole wheat flour.
Typically, foods with highly processed refined carbohydrates (eg, white bread) are digested at a much faster rate and have higher glycemic index (high GI) values than do more compact granules (low-starch gelatinization) and high amounts of viscose soluble fiber (eg, barley, oats, and rye) foods. These refined carbohydrates are more rapidly attacked by digestive enzymes due to grinding or milling that reduces particle size and removes most of the bran and the germ. Numerous epidemiologic studies have found that higher intake of refined carbohydrates (reflected by increased dietary Glycemic Load) is associated with greater risk of type 2 diabetes and heart disease, whereas higher consumption of whole grains protects against these conditions 204.
Table 5. List of refined carbs for more than 100 common foods with their glycemic index and glycemic load, per serving.
| FOOD | Glycemic index (glucose = 100) | Serving size (grams) | Glycemic load per serving |
| BAKERY PRODUCTS AND BREADS | |||
| Banana cake, made with sugar | 47 | 60 | 14 |
| Banana cake, made without sugar | 55 | 60 | 12 |
| Sponge cake, plain | 46 | 63 | 17 |
| Vanilla cake made from packet mix with vanilla frosting (Betty Crocker) | 42 | 111 | 24 |
| Apple muffin, made with rolled oats and sugar | 44 | 60 | 13 |
| Apple muffin, made with rolled oats and without sugar | 48 | 60 | 9 |
| Waffles, Aunt Jemima® | 76 | 35 | 10 |
| Bagel, white, frozen | 72 | 70 | 25 |
| Baguette, white, plain | 95 | 30 | 14 |
| Coarse barley bread, 80% kernels | 34 | 30 | 7 |
| Hamburger bun | 61 | 30 | 9 |
| Kaiser roll | 73 | 30 | 12 |
| Pumpernickel bread | 56 | 30 | 7 |
| 50% cracked wheat kernel bread | 58 | 30 | 12 |
| White wheat flour bread, average | 75 | 30 | 11 |
| Wonder® bread, average | 73 | 30 | 10 |
| Whole wheat bread, average | 69 | 30 | 9 |
| 100% Whole Grain® bread (Natural Ovens) | 51 | 30 | 7 |
| Pita bread, white | 68 | 30 | 10 |
| Corn tortilla | 52 | 50 | 12 |
| Wheat tortilla | 30 | 50 | 8 |
| BEVERAGES | |||
| Coca Cola® (US formula) | 63 | 250 mL | 16 |
| Fanta®, orange soft drink | 68 | 250 mL | 23 |
| Lucozade®, original (sparkling glucose drink) | 95 | 250 mL | 40 |
| Apple juice, unsweetened | 41 | 250 mL | 12 |
| Cranberry juice cocktail (Ocean Spray®) | 68 | 250 mL | 24 |
| Gatorade, orange flavor (US formula) | 89 | 250 mL | 13 |
| Orange juice, unsweetened, average | 50 | 250 mL | 12 |
| Tomato juice, canned, no sugar added | 38 | 250 mL | 4 |
| BREAKFAST CEREALS AND RELATED PRODUCTS | |||
| All-Bran®, average | 44 | 30 | 9 |
| Coco Pops®, average | 77 | 30 | 20 |
| Cornflakes®, average | 81 | 30 | 20 |
| Cream of Wheat® | 66 | 250 | 17 |
| Cream of Wheat®, Instant | 74 | 250 | 22 |
| Grape-Nuts® | 75 | 30 | 16 |
| Muesli, average | 56 | 30 | 10 |
| Oatmeal, average | 55 | 250 | 13 |
| Instant oatmeal, average | 79 | 250 | 21 |
| Puffed wheat cereal | 80 | 30 | 17 |
| Raisin Bran® | 61 | 30 | 12 |
| Special K® (US formula) | 69 | 30 | 14 |
| GRAINS | |||
| Pearled barley, average | 25 | 150 | 11 |
| Sweet corn on the cob | 48 | 60 | 14 |
| Couscous | 65 | 150 | 9 |
| Quinoa | 53 | 150 | 13 |
| White rice, boiled, type non-specified | 72 | 150 | 29 |
| Quick cooking white basmati | 63 | 150 | 26 |
| Brown rice, steamed | 50 | 150 | 16 |
| Parboiled Converted white rice (Uncle Ben’s®) | 38 | 150 | 14 |
| Whole wheat kernels, average | 45 | 50 | 15 |
| Bulgur, average | 47 | 150 | 12 |
| COOKIES AND CRACKERS | |||
| Graham crackers | 74 | 25 | 13 |
| Vanilla wafers | 77 | 25 | 14 |
| Shortbread | 64 | 25 | 10 |
| Rice cakes, average | 82 | 25 | 17 |
| Rye crisps, average | 64 | 25 | 11 |
| Soda crackers | 74 | 25 | 12 |
| DAIRY PRODUCTS AND ALTERNATIVES | |||
| Ice cream, regular, average | 62 | 50 | 8 |
| Ice cream, premium (Sara Lee®) | 38 | 50 | 3 |
| Milk, full-fat, average | 31 | 250 mL | 4 |
| Milk, skim, average | 31 | 250 mL | 4 |
| Reduced-fat yogurt with fruit, average | 33 | 200 | 11 |
| FRUITS | |||
| Apple, average | 36 | 120 | 5 |
| Banana, raw, average | 48 | 120 | 11 |
| Dates, dried, average | 42 | 60 | 18 |
| Grapefruit | 25 | 120 | 3 |
| Grapes, black | 59 | 120 | 11 |
| Oranges, raw, average | 45 | 120 | 5 |
| Peach, average | 42 | 120 | 5 |
| Peach, canned in light syrup | 52 | 120 | 9 |
| Pear, raw, average | 38 | 120 | 4 |
| Pear, canned in pear juice | 44 | 120 | 5 |
| Prunes, pitted | 29 | 60 | 10 |
| Raisins | 64 | 60 | 28 |
| Watermelon | 72 | 120 | 4 |
| BEANS AND NUTS | |||
| Baked beans | 40 | 150 | 6 |
| Black-eyed peas | 50 | 150 | 15 |
| Black beans | 30 | 150 | 7 |
| Chickpeas | 10 | 150 | 3 |
| Chickpeas, canned in brine | 42 | 150 | 9 |
| Navy beans, average | 39 | 150 | 12 |
| Kidney beans, average | 34 | 150 | 9 |
| Lentils | 28 | 150 | 5 |
| Soy beans, average | 15 | 150 | 1 |
| Cashews, salted | 22 | 50 | 3 |
| Peanuts | 13 | 50 | 1 |
| PASTA and NOODLES | |||
| Fettucini | 32 | 180 | 15 |
| Macaroni, average | 50 | 180 | 24 |
| Macaroni and Cheese (Kraft®) | 64 | 180 | 33 |
| Spaghetti, white, boiled, average | 46 | 180 | 22 |
| Spaghetti, white, boiled 20 min | 58 | 180 | 26 |
| Spaghetti, whole-grain, boiled | 42 | 180 | 17 |
| SNACK FOODS | |||
| Corn chips, plain, salted | 42 | 50 | 11 |
| Fruit Roll-Ups® | 99 | 30 | 24 |
| M & M’s®, peanut | 33 | 30 | 6 |
| Microwave popcorn, plain, average | 65 | 20 | 7 |
| Potato chips, average | 56 | 50 | 12 |
| Pretzels, oven-baked | 83 | 30 | 16 |
| Snickers Bar®, average | 51 | 60 | 18 |
| VEGETABLES | |||
| Green peas | 54 | 80 | 4 |
| Carrots, average | 39 | 80 | 2 |
| Parsnips | 52 | 80 | 4 |
| Baked russet potato | 111 | 150 | 33 |
| Boiled white potato, average | 82 | 150 | 21 |
| Instant mashed potato, average | 87 | 150 | 17 |
| Sweet potato, average | 70 | 150 | 22 |
| Yam, average | 54 | 150 | 20 |
| MISCELLANEOUS | |||
| Hummus (chickpea salad dip) | 6 | 30 | 0 |
| Chicken nuggets, frozen, reheated in microwave oven 5 min | 46 | 100 | 7 |
| Pizza, plain baked dough, served with parmesan cheese and tomato sauce | 80 | 100 | 22 |
| Pizza, Super Supreme (Pizza Hut®) | 36 | 100 | 9 |
| Honey, average | 61 | 25 | 12 |
The complete list of the glycemic index and glycemic load for more than 1,000 foods can be found in the article “International tables of glycemic index and glycemic load values: 2008” by Fiona S. Atkinson, Kaye Foster-Powell, and Jennie C. Brand-Miller in the December 2008 issue of Diabetes Care, Vol. 31, number 12, pages 2281-2283 205.
List of refined carbs
Any foods that have been processed for quick consumption.
- Foods made with refined or “white” flour also contain less fiber and protein than whole-grain products.
- Snacks, such as crisps, sausage rolls, pies and pasties
- Granola bars
- Ice cream
- Donuts
- Cakes
- Twinkies
- Pastries
- Canned fruits with added sugar or syrup
- Sweets
- Fruit drinks
- Colas and carbonated sweetened beverages
- Energy drinks
- Sports drink
- Jams
- Crackers
- Dressings
- Sauces
- Cookies
- Fruit chews
- Pizzas
- Apple pies
- Anything with added sugar.
Sugar by Any Other Name
You don’t always see the word “sugar” on a food label. It sometimes goes by another name, like these:
- White sugar
- Brown sugar
- Raw sugar
- Agave nectar
- Brown rice syrup
- Corn syrup
- Corn syrup solids
- Coconut sugar
- Coconut palm sugar
- High-fructose corn syrup
- Invert sugar
- Dextrose
- Anhydrous dextrose
- Crystal dextrose
- Dextrin
- Evaporated cane juice
- Fructose sweetener
- Liquid fructose
- Glucose
- Lactose
- Honey
- Malt syrup
- Maple syrup
- Molasses
- Pancake syrup
- Sucrose
- Trehalose
- Turbinado sugar
Watch out for items that list any form of sugar in the first few ingredients.
Best anti-inflammatory diet
An example of an anti-inflammatory dietary pattern is the Mediterranean diet which is characterized by high intakes of fruit and vegetables, whole grains, nuts, legumes, fish, monounsaturated fat (olive oil); moderate intakes of dairy products and alcohol; and low intakes of meat and meat products and saturated fat 206. A traditional Mediterranean dietary pattern, which typically has a high ratio of monounsaturated (MUFA) and Omega-3 to Omega-6 polyunsaturated fatty acid (PUFAs) and supplies an abundance of fruits, vegetables, legumes, and grains, has been shown to have anti-inflammatory effects when compared with typical North American and Northern European dietary patterns in most observational and interventional studies 207.
The Mediterranean Diet is characterized by 208:
- An abundance of plant food (fruit, vegetables, breads, cereals, potatoes, beans, nuts, and seeds);
- Minimally processed, seasonally fresh, locally grown foods;
- Desserts comprised typically of fresh fruit daily and occasional sweets containing refined sugars or honey;
- Olive oil (high in polyunsaturated fat) as the principal source of fat;
- Daily dairy products (mainly cheese and yogurt) in low to moderate amounts;
- Fish and poultry in low to moderate amounts;
- Up to four eggs weekly;
- Red meat rarely; and
- Wine in low to moderate amounts with meals.
Adherence to a Mediterranean-type diet has been associated with a multitude of health benefits 209 including lower risk of all-cause mortality 210, cancer 211, type 2 diabetes 212, cardiovascular disease 213 and cognitive decline 214. The Mediterranean dietary pattern is high in anti-inflammatory micronutrients (micronutrients are vitamins and minerals needed by your body in very small amounts) and phytochemicals (chemical compounds produced by plants) such as Omega-3 fatty acids, flavonoids, carotenoids, and vitamins C and E, and higher adherence to the Mediterranean diet has been associated with lower levels of inflammatory markers 215, 216. Therefore, greater adherence to a Mediterranean dietary pattern may elicit health benefits through impacts on systemic inflammation 164. Additionally, Mediterranean-style diets have been demonstrated to elicit cardiovascular benefits including improved endothelial function and reductions in serum cholesterol and triglycerides 217. In a six-month randomized controlled trial, breast cancer survivors who adhered to a Mediterranean type diet had beneficial changes in body composition, cholesterol, and glucose levels compared to controls 218.
Mediterranean Diet
The Mediterranean Diet is a way of eating rather than a formal diet plan. The Mediterranean Diet features foods eaten in more than 20 countries bordering the Mediterranean Sea including Greece, Spain, southern Italy, Portugal, Morocco, Cyprus, Croatia and France and each has their own unique culture and cuisine. In reality there is no “one” Mediterranean Diet 219, which in 2010 was recognized by UNESCO as an intangible cultural heritage of humanity. The “Mediterranean diet” encompasses all of them—it’s not one size fits all 220. Despite regional variations, the traditional Mediterranean diet is characterized by a high intake of vegetables, legumes, fruits and nuts and cereals (which in the past were largely unrefined), a high intake of olive oil, but a low intake of saturated lipids, a moderately high intake of fish (depending on the proximity of the sea), a low-to-moderate intake of dairy products (and then mostly in the form of cheese or yoghurt), a low intake of meat and poultry and a regular, but moderate intake of ethanol, primarily in the form of wine and generally during meals 221.
A Mediterranean-style diet typically includes:
- plenty of fruits, vegetables, bread and other grains, potatoes, beans, nuts and seeds are eaten daily and make up the majority of food consumed;
- olive oil as a primary fat source, may account for up to 40% of daily calories; and
- small portions of cheese or yogurt are usually eaten each day, along with a serving of fish, poultry, or eggs.
Fish and poultry are more common than red meat in the Mediterranean diet. The Mediterranean diet also centers on minimally processed, plant-based foods. Wine may be consumed in low to moderate amounts, usually with meals. Fruit is a common dessert instead of sweets.
Main meals consumed daily should be a combination of three elements: cereals, vegetables and fruits, and a small quantity of legumes, beans or other (though not in every meal). Cereals in the form of bread, pasta, rice, couscous or bulgur (cracked wheat) should be consumed as one–two servings per meal, preferably using whole or partly refined grains. Vegetable consumption should amount to two or more servings per day, in raw form for at least one of the two main meals (lunch and dinner). Fruit should be considered as the primary form of dessert, with one–two servings per meal. Consuming a variety of colors of both vegetables and fruit is strongly recommended to help ensure intake of a broad range of micronutrients and phytochemicals. The less these foods are cooked, the higher the retention of vitamins and the lower use of fuel, thus minimizing environmental impact.
The Mediterranean Diet is characterized by 208:
- An abundance of plant food (fruit, vegetables, breads, cereals, potatoes, beans, nuts, and seeds);
- Minimally processed, seasonally fresh, locally grown foods;
- Desserts comprised typically of fresh fruit daily and occasional sweets containing refined sugars or honey;
- Olive oil (high in polyunsaturated fat) as the principal source of fat;
- Daily dairy products (mainly cheese and yogurt) in low to moderate amounts;
- Fish and poultry in low to moderate amounts;
- Up to four eggs weekly;
- Red meat rarely; and
- Wine in low to moderate amounts with meals.
Here are some things you can do to switch from a traditional Western-style diet to a more Mediterranean way of eating.
- Dip bread in a mix of olive oil and fresh herbs instead of using butter.
- Add avocado slices to your sandwich instead of bacon.
- Have fish for lunch or dinner instead of red meat. Brush it with olive oil, and broil or grill it.
- Sprinkle your salad with seeds or nuts instead of cheese.
- Cook with olive or canola oil instead of butter or oils that are high in saturated fat.
- Choose whole-grain bread, pasta, rice, and flour instead of foods made with white flour.
- Add ground flaxseed to cereal, low-fat yogurt, and soups.
- Cut back on meat in meals. Instead of having pasta with meat sauce, try pasta tossed with olive oil and topped with pine nuts and a sprinkle of Parmesan cheese.
- Dip raw vegetables in a vinaigrette dressing or hummus instead of dips made from mayonnaise or sour cream.
- Have a piece of fruit for dessert instead of a piece of cake.
- Use herbs and spices instead of salt to add flavor to foods.
A Mediterranean-style diet can help you achieve the American Heart Association’s recommendations for a healthy dietary pattern that:
- emphasizes vegetables, fruits, whole grains, beans and legumes;
- includes low-fat or fat-free dairy products, fish, poultry, non-tropical vegetable oils and nuts; and
- limits added sugars, sugary beverages, sodium, highly processed foods, refined carbohydrates, saturated fats, and fatty or processed meats.
This style of eating can play a big role in preventing heart disease and stroke and reducing risk factors such as obesity, diabetes, high cholesterol and high blood pressure. There is some evidence that a Mediterranean diet rich in virgin olive oil may help the body remove excess cholesterol from arteries and keep blood vessels open.
The traditional Mediterranean dietary pattern is of particular interest to healthcare providers and dietary scientists, because of observations from the 1960s that populations in countries of the Mediterranean region, such as Greece and Italy, had lower mortality from cardiovascular disease compared with northern European populations or the US, probably as a result of different eating habits.
However, adherence to the Mediterranean diet dietary pattern has been rapidly decreasing in the region since 2000, particularly in Greece, Portugal and Spain – due to the wide dissemination of the fast-food culture. These observations point to a nutrition transition period that encompasses considerable changes in diet and physical activity patterns, which may be leading to an increase in the incidence of chronic and degenerative diseases in the Mediterranean region.
Traditionally characterized by vegetables, legumes, beans, fruits, nuts, seeds, olives, lots of extra virgin olive oil, high-fiber breads and whole grains and fish, this way of eating not only involves a low consumption of processed food, processed carbohydrates, sweets, chocolate and red meat. The recommended foods are rich with monounsaturated fats, fiber, and omega-3 fatty acids.
The Mediterranean Diet is associated with a lower incidence of mortality from all-causes 222 and is also related to lower incidence of cardiovascular diseases 223, type 2 diabetes 224, certain types of cancer 225, and neurodegenerative diseases 226. The Mediterranean diet is now recognized as one of the most healthy food patterns in the world.
Year after year, the Mediterranean diet comes out on top in the U.S. News and World Report annual ranking of best diets. The Mediterranean diet is also touted as one of the healthiest by many health organizations and dietitians 227.
The atmosphere, the state of being and mindset are equally important: people enjoying long, relaxed meals, the warm climate and a sea breeze full of negative ions. This lifestyle also encourages daily exercise — being active. So try to get at least 2½ hours of moderate aerobic activity a week. It’s fine to do blocks of 10 minutes or more throughout your day and week.
Choose exercises that make your heart beat faster and make you breathe harder. For example, go for a swim or a brisk walk or bike ride. You can also get some aerobic activity in your daily routine. Vacuuming, housework, gardening, and yard work can all be aerobic.
The Mediterranean diet is like other heart-healthy diets in that it recommends eating plenty of fruits, vegetables, and high-fiber grains. But in the Mediterranean diet, an average of 35% to 40% of calories can come from fat. Most other heart-healthy guidelines recommend getting less than 35% of your calories from fat. The fats allowed in the Mediterranean diet are mainly from unsaturated oils such as fish oils, olive oil, and certain nut or seed oils (such as canola, soybean, or flaxseed oil) and from nuts (walnuts, hazelnuts, and almonds). These types of oils may have a protective effect on the heart.
Mediterranean Diet Food List
There’s no one “Mediterranean” diet food list because there are more than 20 countries bordering the Mediterranean Sea. Diets vary between these countries and also between regions within a country. Many differences in culture, ethnic background, religion, economy and agricultural production result in different diets. But the common Mediterranean dietary meal plan (source 228) has these characteristics:
- High consumption of fruits, vegetables, bread and other cereals, potatoes, beans, nuts and seeds
- Olive oil is an important monounsaturated fat source
- Dairy products, fish and poultry are consumed in low to moderate amounts, and little red meat is eaten
- Eggs are consumed zero to four times a week
- Wine is consumed in low to moderate amounts
Mediterranean Diet Meal Plan
- Eating a variety of fruits and vegetables each day, such as grapes, blueberries, tomatoes, broccoli, peppers, figs, olives, spinach, eggplant, beans, lentils, and chickpeas.
- Eating a variety of whole-grain foods each day, such as oats, brown rice, and whole wheat bread, pasta, and couscous.
- Choosing healthy (unsaturated) fats, such as nuts, olive oil, and certain nut or seed oils like canola, soybean, and flaxseed. About 35% to 40% of daily calories can come from fat, mainly from unsaturated fats. More than half the fat calories in a Mediterranean diet come from monounsaturated fats (mainly from olive oil). Monounsaturated fat doesn’t raise blood cholesterol levels the way saturated fat does. (source 228).
- Limiting unhealthy (saturated) fats, such as butter, palm oil, and coconut oil. And limit fats found in animal products, such as meat and dairy products made with whole milk.
- Eating mostly vegetarian meals that include whole grains, beans, lentils, and vegetables.
- Eating fish at least 2 times a week, such as tuna, salmon, mackerel, lake trout, herring, or sardines.
- Eating moderate amounts of low-fat dairy products each day or weekly, such as milk, cheese, or yogurt.
- Eating moderate amounts of poultry and eggs every 2 days or weekly.
- Limiting red meat to only a few times a month in very small amounts. For example, a serving of meat is 3 ounces. This is about the size of a deck of cards.
- Limiting sweets and desserts to only a few times a week. This includes sugar-sweetened drinks like soda.
The positive findings have been plentiful:
- A plant-based Mediterranean diet supplemented with extra virgin olive oil or mixed nuts may counteract age-related cognitive decline in older adults, according to a report published online by JAMA Internal Medicine.
- A 2010 meta-analysis published in the The American Journal of Clinical Nutrition found that the Mediterranean diet conferred a significant benefit with regard to the risk of chronic diseases, such as cardiovascular disease.
- In 2014, two meta-analyses found that adherence to a Mediterranean diet was associated with a decreased risk of type 2 diabetes
- Another 2014 systematic review and meta-analysis found that adherence to the Mediterranean diet was associated with a decreased risk of cancer mortality.
- Due to the emphasis on fish and healthy fats which are needed for prostaglandin formation, the Mediterranean diet is beneficial for decreasing inflammation in the body.
- The biggest impact of Mediterranean is on lowering incidences of diabetes.
- The Mediterranean diet is associated with a reduced risk of cancer incidence.
- Other health benefits of Mediterranean diet are prevents heart disease, lowers the risk of a heart attack, lowers cholesterol, prevents type 2 diabetes and prevents metabolic syndrome.
A Mediterranean-style diet might help prevent:
- Stroke.
- Alzheimer’s disease and other dementia.
- Depression.
- Parkinson’s disease.
Anti-inflammatory diet for inflammatory bowel diseases
In studies involving people with chronic inflammatory bowel diseases (IBD) like Crohn’s Disease and Ulcerative Colitis, the anti-Inflammatory diet (IBD-AID) has been used as an adjunct dietary therapy for the treatment of inflammatory bowel diseases (IBD) 229. The goal of the anti-Inflammatory diet (IBD-AID) is to assist with a decreased frequency and severity of flares, obtain and maintain remission in people with inflammatory bowel diseases (IBD). Dysbiosis, or altered bacterial flora, is one of the theories behind the development of anti-Inflammatory diet (IBD-AID), in that certain carbohydrates in the lumen of the gut provide pathogenic bacteria a substrate on which to proliferate 230, 231.
The anti-Inflammatory diet (IBD-AID) has five basic components:
- The first of which is the modification of certain carbohydrates, (including lactose, and refined or processed complex carbohydrates)
- The second places strong emphasis on the ingestion of pre- and probiotics (e.g.; soluble fiber, leeks, onions, and fermented foods) to help restore the balance of the intestinal flora 232, 233, 234
- The third distinguishes between saturated, trans, mono- and polyunsaturated fats 235, 236,
- The fourth encourages a review of the overall dietary pattern, detection of missing nutrients, and identification of intolerances.
- The last component modifies the textures of the foods (e.g.; blenderized, ground, or cooked) as needed (per patient symptomology) to improve absorption of nutrients and minimize intact fiber.
The phases indicated in Table 4 are examples of the modification of texture complexity, so that dietitian and patient can expand the diet as the patient’s tolerance and absorption improves. Some sensitivities common to many patients (not just those with IBD), are eased through supplementation of digestive enzymes or avoidance. A senior dietitian advised the patient and either family or spouse regarding the details of the diet during regular clinic visits. Patients taking supplements (probiotics, vitamin/minerals, omega-3 fatty acids) were advised to continue or discontinue, depending on the needs of the individual and the dietary intake 237.
The anti-Inflammatory diet (IBD-AID) consists of lean meats, poultry, fish, omega-3, eggs, particular sources of carbohydrate, select fruits and vegetables, nut and legume flours, limited aged cheeses (made with active cultures and enzymes), fresh cultured yogurt, kefir, miso and other cultured products (rich with certain probiotics) and honey. Prebiotics, in the form of soluble fiber (containing beta-glucans and inulin, such as bananas, oats, blended chicory root, and flax meal) are suggested. In addition, the patient is advised to begin at a texture phase of the diet matching with symptomology, starting with phase one if in an active flare. Many patients require foods to be softened and textures mechanically altered by pureeing the foods, and avoiding foods with stems and seeds when starting the diet (see phases 1–3 of Table 4), as intact fiber can be problematic for those with strictures and highly active mucosal inflammation. Some patients will require lifelong avoidance of intact fiber. Food irritants are not limited to intact fiber, but may include certain foods, processing agents and flavorings to which IBD patients may be reactive.
Table 6. The Anti-Inflammatory Diet (IBD-AID) Food Phase chart
| Phase type | Phase I | Phase II | Phase III | Phase IV |
|---|---|---|---|---|
| Soft, well-cooked or cooked then pureed foods, no seeds | Soft Textures: well-cooked or pureed foods, no seeds, choose floppy or tender foods | May still need to avoid stems, choose floppy greens or other greens depending on individual tolerance | If in remission with no strictures | |
| Vegetables | Butternut Squash, Pumpkin, Sweet Potatoes, Onions | Carrots, Zucchini, Eggplant, Peas, Snow peas, Spaghetti squash, Green beans, Yellow beans, Microgreens (2 week old baby greens), Watercress, Arugula, Fresh flat leaf parsley and cilantro, Seaweed, Algae | Butter lettuce, Baby spinach, Peeled cucumber, Olives, Leeks Bok Choy, Bamboo shoots, Collard greens, Beet greens, Sweet peppers, Kale, Fennel bulb | Artichokes, Asparagus, Tomatoes, Lettuce, Brussels sprouts, Beets, Cabbage, Kohlrabi, Rhubarb, Pickles, Spring onions, Water chestnuts, Celery, Celeriac, Cauliflower, Broccoli, Radish, Green pepper, Hot pepper |
| Pureed vegetables: Mushrooms, Phase II vegetables (pureed) | Pureed vegetables: all except cruciferous | Pureed vegetables: all from Phase IV, Kimchi | ||
| Fruits | Banana, Papaya, Avocado, Pawpaw | Watermelon (seedless), Mangoes, Honeydew, Cantaloupe, May need to be cooked: Peaches, Plums, Nectarines, Pears, (Phase III fruits are allowed if pureed and seeds are strained out) | Strawberries, Cranberries, Blueberries, Apricots, Cherries, Coconut, Lemons, Limes, Kiwi, Passion fruit, Blackberries, Raspberries, Pomegranate (May need to strain seeds from berries) | Grapes, Grapefruit, Oranges, Currants, Figs, Dates, Apples (best cooked), Pineapple, Prunes |
| Meats and fish | All fish (no bones), Sardines (small bones ok), Turkey and ground beef, Chicken, Eggs | Scallops | Lean cuts of Beef, Lamb, Duck, Goose | Shrimp, Prawns, Lobster |
| Non dairy unsweetened | Coconut milk, Almond milk, Oat milk, Soy milk | |||
| Dairy, unsweetened | Yogurt, Kefir | Farmers cheese (dry curd cottage cheese), Cheddar cheese | Aged cheeses | |
| Nuts/Oils/Legumes/Fats | Miso (refrigerated), Tofu, Olive oil, Canola oil, Flax oil, Hemp oil, Walnut oil, Coconut oil | Almond flour, Peanut flour, Soy flour, Sesame oil, Grapeseed oil, Walnut oil, Pureed nuts, Safflower oil, Sunflower oil | Whole nuts, Soybeans, Bean flours, Nut butters, Well-cooked lentils (pureed), Bean purees (e.g. hummus) | Whole beans and lentils |
| Grains | Ground flax or Chia Seeds (as tolerated) | Steel cut oats (well-cooked as oatmeal) | Rolled well-cooked oats | |
| Spices | Basil, Sage, Oregano, Salt, Nutmeg, Cumin, Cinnamon, Turmeric, Saffron, Mint, Bay leaves, Tamari (wheat free soy sauce), Fenugreek tea, Fennel tea, Vanilla | Dill, Thyme, Rosemary Tarragon, Cilantro, Basil, Parsley | Mint, Ginger, Garlic (minced), Paprika, Chives, Daikon, Mustard | Wasabi, Tamarind, Horseradish, Fenugreek, Fennel |
| Sweeteners | Stevia, Maple syrup, Honey (local), Unsweetened fruit juice | Lemon and lime juice | ||
| Misc. | Capsule or liquid supplements, Cocoa powder | Baking powder (no cornstarch), Baking soda, Unflavored gelatin | Ghee, Light mayonnaise, Vinegar | Ketchup (sugar free), Hot sauce (sugar free) |
Inflammatory bowel disease dietary guidelines
To help patients navigate their nutritional questions, the International Organization of Inflammatory Bowel Diseases recently reviewed the best current evidence to develop expert recommendations regarding dietary measures that might help to control and prevent relapse of inflammatory bowel disease 238. In particular, the International Organization of Inflammatory Bowel Diseases focused on the dietary components and additives that they felt were the most important to consider because they comprise a large proportion of the diets that inflammatory bowel disease patients may follow. Furthermore, the recent International Organization of Inflammatory Bowel Diseases guidelines are an excellent starting point for discussions between patients and their doctors about whether specific dietary changes might be helpful in reducing symptoms and risk of relapse of inflammatory bowel disease. However, all patients with inflammatory bowel disease (IBD) should work with their doctor or a nutritionist, who will conduct a nutritional assessment to check for malnutrition and provide advice to correct deficiencies if they are present.
The International Organization of Inflammatory Bowel Diseases recommendations were developed with the aim of reducing symptoms and inflammation 238. The ways in which altering the intake of particular foods may trigger or reduce inflammation are quite diverse, and the mechanisms are better understood for certain foods than others. For example, fruits and vegetables are generally higher in fiber, which is fermented by bacterial enzymes within the colon. This fermentation produces short-chain fatty acids (SCFAs) that provide beneficial effects to the cells lining the colon. Patients with active IBD have been observed to have decreased short-chain fatty acids, so increasing the intake of plant-based fiber may work, in part, by boosting the production of short-chain fatty acids. However, it is important to note disease-specific considerations that might be relevant to your particular situation. For example, about one-third of Crohn’s disease patients will develop an area of intestinal narrowing, called a stricture, within the first 10 years of diagnosis. Insoluble fiber can worsen symptoms and, in some cases, lead to intestinal blockage if a stricture is present. So, while increasing consumption of fruits and vegetable is generally beneficial for Crohn’s disease, patients with a stricture should limit their intake of insoluble fiber.
The International Organization of Inflammatory Bowel Diseases guidelines include the following recommendations:
| Food | If you have Crohn’s disease | If you have ulcerative colitis |
| Fruits | increase intake | insufficient evidence |
| Vegetables | increase intake | insufficient evidence |
| Red/processed meat | insufficient evidence | decrease intake |
| Unpasteurized dairy products | best to avoid | best to avoid |
| Dietary fat | decrease intake of saturated fats and avoid trans fats | decrease consumption of myristic acid (palm, coconut, dairy fat), avoid trans fats, and increase intake of omega-3 (from marine fish but not dietary supplements) |
| Food additives | decrease intake of maltodextrin-containing foods | decrease intake of maltodextrin-containing foods |
| Thickeners | decrease intake of carboxymethylcellulose | decrease intake of carboxymethylcellulose |
| Carrageenan (a thickener extracted from seaweed) | decrease intake | decrease intake |
| Titanium dioxide (a food colorant and preservative) | decrease intake | decrease intake |
| Sulfites (flavor enhancer and preservative) | decrease intake | decrease intake |
What are specific diets for inflammatory bowel disease?
A number of specific diets have been explored for inflammatory bowel disease (IBD), including the Mediterranean diet, specific carbohydrate diet, Crohn’s disease exclusion diet, autoimmune protocol diet, and a diet low in fermentable oligo-, di-, monosaccharides, and polyols (FODMAPs). Although the International Organization of Inflammatory Bowel Diseases group initially set out to evaluate some of these diets, they did not find enough high-quality trials that specifically studied them 238. Therefore, they limited their recommendations to individual dietary components. Stronger recommendations may be possible once additional trials of these dietary patterns become available. For the time being, patients are to monitor for correlations of specific foods to their symptoms. In some cases, patients may explore some of these specific diets to see if they help.
Summary
There is scientific evidence that dietary factors may influence both the risk of developing inflammatory bowel disease and intestinal mucosal inflammation. However, there is lack of large prospective controlled trials to provide the dietary recommendations patients’ desire. Taken together, studies of exclusive enteral nutrition, exclusion diets, and semi-vegetarian diets suggest that minimizing exposure of the intestinal lumen to selected food items may prolong the remission state of patients with inflammatory bowel disease 239. Even less evidence exists for the efficacy of the specific carbohydrate diet, FODMAP, or Paleo diet. Furthermore, the practicality of maintaining these interventions over long periods of time is doubtful. Patient-targeted dietary recommendations focus on food restrictions and are highly conflicting. High quality dietary intervention studies are needed to facilitate creation of evidence-based dietary guidelines for patients with inflammatory bowel disease. At a practical level, adherence to defined diets may result in an unnecessary financial burden or reduction in overall caloric intake in patients who are already at risk for protein-calorie malnutrition.
Anti-inflammatory diet for arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by synovial inflammation often followed by cartilage and bone erosion 240. Patients with rheumatoid arthritis often ask their physician for specific dietary advice and many report that different food items improve or worsen the disease symptoms (10–12). Red meat, alcohol, and soft drinks are examples of foods reported to worsen symptoms, whereas fish and berries are reported to improve symptoms 241, 242. There are few studies investigating whole diets, but beneficial effects on disease activity have been noted in intervention studies with a Mediterranean diet 243 as well as with fasting followed by a vegetarian diet 244 and gluten-free vegan diet 245. Research on the effects of food components on inflammation and patient-perceived symptoms includes Omega–3 fatty acids, probiotics, vitamin D, and antioxidants. Omega–3 fatty acids seem to have positive effects on several outcomes such as erythrocyte sedimentation rate (ESR) and tender joint count 8 and a number of trials using probiotics have shown positive effects on disease activity 246. There seems to be potential for vitamin D as well to reduce disease activity, but very few studies have been performed 247. Furthermore, several antioxidants and sources of bioactive compounds with antioxidative effects have been evaluated and some studies have obtained a reduction in symptoms as well as lower disease activity with these foods and supplements 248, 249, 250. Many of these dietary interventions in rheumatoid arthritis had small study populations and suffered from a poor design. Nevertheless, they indicate that several foods and food components have potential to reduce rheumatoid arthritis disease activity by lowering grade of inflammation or alleviating symptoms like joint pain. Still, studies investigating the effects of a comprehensive anti-inflammatory portfolio diet are lacking for rheumatoid arthritis.
The Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA) is a diet combining foods with suggested anti-inflammatory effects and food components with promising effects on rheumatoid arthritis disease activity and symptoms 251. Table 1 describes the foods included in detail. In brief, the main meals in the Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA) diet contained fish (mainly salmon) 3–4 times/week and vegetarian dishes with legumes 1–2 times/week. Potatoes, whole-grain cereals, vegetables, yoghurt for sauces, spices, and other flavorings were also included 251. Snacks were composed of fruits, whereas breakfasts contained low-fat dairy, whole-grain cereals, pomegranate and blueberries, nuts, and juice shots with probiotics 251. The probiotic shot used contained Lactobacillus plantarum 299v and was provided to the participants 5 days/week. For the meals not provided, the participants were instructed to limit their intake of meat to ≤3 times/week, to eat ≥5 portions/day of fruit, berries, and vegetables (including those provided), to use oil or margarine for cooking, and to choose low-fat dairy and whole-grain cereals 251.
The total dietary intake during control periods was intended to correspond nutritionally to the average dietary intake in 45- to 64-year-old men and women in Sweden; 17 energy percent (E%) protein, 34 energy percent total fat, 13 energy percent saturated fatty acids, and 43 energy percent carbohydrates 252. The control diet provided by the study contained meat or chicken and refined grains daily, protein bar or quark for snacks, and breakfasts based on either white bread with a butter-based spread and cheese, or a mix of quark and yoghurt with corn flakes and orange juice. Beyond this, the participants were also instructed to consume meat ≥5 times/week; ≤5 portions/day of fruit, berries, and vegetables; seafood ≤1 time/week; use butter for cooking; choose high-fat dairy; and avoid products with probiotics. Before each diet period, the participants received a binder including weekly menus and recipes, and instructions on dietary intake for the meals not provided. Three weekly menus were repeated throughout the diet periods.
One component of the Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA) portfolio diet that distinguishes it from a Mediterranean diet is the probiotics. Patients with rheumatoid arthritis seem to have a less diverse microbiota composition than healthy individuals 253 and probiotics have the ability to alter the intestinal microbiota and downregulate immune response 254. In clinical studies, several strains of probiotics have been used to reduce symptoms in patients with rheumatoid arthritis 254, 255, 256, 257, with conflicting results. Some studies have obtained reduced disease activity with Lactobacillus casei, alone or together with Lactobacillus acidophilus and Bifidobacterium bifidum 256, 254. However, it is not possible to compare Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA) portfolio diet with this research because the strains used differ.
The beneficial effects of omega–3 fatty acids in rheumatoid arthritis have been studied, and supplementation seems to reduce concentrations of mediators of inflammation 258. A recent systematic review and meta-analysis on effects of omega-3 polyunsaturated fatty acids (PUFAs) concluded significant positive effects on several outcomes in rheumatoid arthritis, although not in disease activity score using 28 joint counts (DAS28) 8. Some individual trials have shown positive effects of omage–3 fatty acids, but these have several limitations 259. As an alternative to omega–3 supplements Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA) used fish intake. The Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA) diet was intended to be viable in daily life, and therefore did not aim to reach the amounts of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) used in supplementation trials. Observational data 260 suggest that consuming fish at least twice per week is associated with lower disease activity score using 28 joint counts (DAS28)-CRP, which is further reduced by every additional serving. Moreover, a previous study from another research group showed significant improvements in disease activity score using 28 joint counts (DAS28)-CRP with a mussel diet (low dose of omega–3 fatty acids), comparable with the change during the intervention period in ADIRA 261. In that study, European League Against Rheumatism (EULAR) response criteria 262 differed significantly between the control and intervention periods. This was not the case in Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA), again perhaps because of the lower disease activity at baseline.
This Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA) trial indicates positive effects of a proposed anti-inflammatory diet on disease activity in patients with rheumatoid arthritis 251. However, additional studies are required to determine if Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA) diet can cause clinically relevant improvements 251.
Table 7. The anti-inflammatory portfolio diet in the randomized crossover ADIRA (Anti-inflammatory Diet in Rheumatoid Arthritis) trial
| Foods | Comments | |
| Breakfast | Low-fat fermented dairy | |
| Granola containing nuts, seeds, and berries | ||
| Fiber-enriched oats | ||
| Low-fat milk | ||
| Walnuts | ||
| Blueberries/pomegranate | ||
| Probiotic shot | Juice | |
| Main meal | Salmon | |
| Salmon + cod | Ready-to-eat meal: patties (mixed with cream and egg) served with shellfish sauce (cream and wine), soybeans, and peas | |
| Beans, chickpeas, and/or lentils | Ready-to-eat meals: stew with potatoes and vegetables; stew with bulgur, coconut milk, and vegetables; or patties with vegetables | |
| Wheat berries | ||
| Bulgur, whole grain | ||
| Potatoes | Cooked or oven-baked | |
| Pasta, whole grain | ||
| Yogurt (10% fat) | ||
| Crème fraiche (13% fat) | ||
| Vegetables (e.g., soy beans, spinach, onion, garlic, pepper) | Large amount | |
| Mango | ||
| Rapeseed oil | ||
| Rice vinegar | ||
| Soy sauce | ||
| Honey | ||
| Spices | ||
| Lime, lemon | ||
| Snack | Bananas/apples/pears | Two daily |
Footnote: The participants were provided with foods corresponding to ∼50% of their daily intake, 5 days/wk. For the remaining intake, they were instructed to consume similar foods to those provided.
[Source 251 ]Anti-inflammatory meal plan
By following the US Department of Health food guide, called ChooseMyPlate 263, you can make healthier food choices. The new US Department of Agriculture’s food guide 2015-2020 264 encourages you to eat more fruits and vegetables, whole grains, lean proteins, and low-fat dairy. Using the guide 264, you can learn what type of food you should eat and how much you should eat. You also learn why and how much you should exercise.
There are 5 major food groups that make up a healthy diet:
- Grains
- Vegetables
- Fruits
- Dairy
- Protein foods
You should eat foods from each group every day. How much food you should eat from each group depends on your age, gender, and how active you are.
ChooseMyPlate 263 makes specific recommendations for each type of food group.
1) GRAINS: MAKE AT LEAST HALF OF YOUR GRAINS WHOLE GRAINS
- Whole grains contain the entire grain. Processed grains have had the bran and germ removed. Be sure to read the ingredient list label and look for whole grains first on the list.
- Foods with whole grains have more fiber and protein than food made with processed grains.
- Examples of whole grains are breads and pastas made with whole-wheat flour, oatmeal, bulgur, faro, and cornmeal.
- Examples of processed grains are white flour, white bread, and white rice.
Most children and adults should eat about 5 to 8 servings of grains a day (also called “ounce equivalents”). Children age 8 and younger need about 3 to 5 servings. At least half those servings should be whole grain. An example of one serving of grains includes:
- 1 slice of bread
- Half of a bagel
- 1 cup (30 grams)of cereal
- 1/2 cup (165 grams) cooked rice
- 5 whole-wheat crackers
- 1/2 cup (75 grams) cooked pasta
Eating whole grains can help improve your health by 265:
- Reducing the risk of many chronic diseases.
- Whole grains can help you lose weight. Portion size is still key. Because whole grains have more fiber and protein, they are more filling than refined grains, so you can eat less to get the same feeling of being full. But if you replace vegetables with starches, you’ll gain weight, even if you eat whole grain.
- Whole grains can help you have regular bowel movements.
Ways to eat more whole grains:
- Eat brown rice instead of white rice.
- Use whole-grain pasta instead of regular pasta.
- Replace part of white flour with wheat flour in recipes.
- Replace white bread with whole-wheat bread.
- Use oatmeal in recipes instead of bread crumbs.
- Snack on air-popped popcorn instead of chips or cookies.
2) VEGETABLES: MAKE HALF OF YOUR PLATE FRUITS AND VEGETABLES
- Vegetables can be raw, fresh, cooked, canned, frozen, dried, or dehydrated.
- Vegetables are organized into 5 subgroups based on their nutrient content. The groups are dark-green vegetables, starchy vegetables, red and orange vegetables, beans and peas, and other vegetables.
- Try to include vegetables from each group, try to make sure you aren’t only picking options from the “starchy” group.
Most children and adults should eat between 2 and 3 cups (200 to 300 grams) of vegetables a day. Children age 8 need about 1 to 1 1/2 cups (100 to 150 grams). Examples of a cup include:
- Large ear of corn
- Three 5-inch (13 centimeters) broccoli spears
- 1 cup (100 grams) cooked vegetables
- 2 cups (250 grams) of raw, leafy greens
- 2 medium carrots
- 1 cup (240 milliliters) 100% vegetable juice (carrot, tomato)
Eating vegetables can help improve your health in the following ways:
- Lowers your risk of heart disease, obesity, and type 2 diabetes
- Helps protect you against some cancers
- Helps lower blood pressure
- Reduces the risk of kidney stones
- Helps reduce bone loss
Ways to eat more vegetables:
- Keep plenty of frozen vegetables handy in your freezer.
- Buy pre-washed salad and pre-chopped veggies to cut down on prep time.
- Add veggies to soups and stews.
- Add vegetables to spaghetti sauces.
- Try veggie stir-fries.
- Eat raw carrots, broccoli, or bell pepper strips dipped in hummus or ranch dressing as a snack.
3) FRUITS: MAKE HALF OF YOUR PLATE FRUITS AND VEGETABLES
- Fruits can be fresh, canned, frozen, or dried.
Most adults need 1 1/2 to 2 cups (200 to 250 grams) of fruit a day. Children age 8 and younger need about 1 to 1 1/2 cups (120 to 200 grams). Examples of a cup include:
- 1 small piece of fruit, such as an apple or pear
- 8 large strawberries
- 1/2 cup (130 grams) dried apricots or other dried fruit
- 1 cup (240 milliliters) 100% fruit juice (orange, apple, grapefruit)
- 1 cup (100 grams) cooked or canned fruit
- 1 cup (250 grams) chopped fruit
Eating fruit can help improve your health, they may help to:
- Lower your risk of heart disease, obesity, and type 2 diabetes
- Protect you against some cancers
- Lower blood pressure
- Reduce the risk of kidney stones
- Reduce bone loss
Ways to eat more fruit:
- Put out a fruit bowl and keep it full of fruit.
- Stock up on dried, frozen, or canned fruit, so you always have it available. Choose fruit that is canned in water or juice instead of syrup.
- Buy pre-cut fruit in packages to cut down on prep time.
- Try meat dishes with fruit, such as pork with apricots, lamb with figs, or chicken with mango.
- Grill peaches, apples, or other firm fruit for a healthy, tasty dessert.
- Try a smoothie made with chopped fruit and plain yogurt for breakfast.
- Use dried fruit to add texture to trail mixes.
4) PROTEIN FOODS: CHOOSE LEAN PROTEINS
Protein foods include meat, poultry, seafood, beans and peas, eggs, processed soy products, nuts and nut butters, and seeds. Beans and peas are also part of the vegetable group.
- Choose meats that are low in saturated fat and cholesterol, such as lean cuts of beef and chicken and turkey without skin.
- Most adults need 5 to 6 1/2 servings of protein a day (also called “ounce equivalents”). Children age 8 and younger need about 2 to 4 servings.
Examples of a serving include:
- 1 oz (28 grams) lean meat; like beef, pork, or lamb
- 1 oz (28 grams) poultry; such as turkey or chicken
- 1 large egg
- 1/4 cup (50 grams) tofu
- 1/2 cup (50 grams) cooked beans or lentils
- 1 tablespoon (15 grams) peanut butter
- 1/3 cup (35 grams) nuts
Eating lean protein can help improve your health:
- Seafood high in omega-3 fats, such as salmon, sardines, or trout, can help prevent heart disease.
- Peanuts and other nuts, such as almonds, walnuts, and pistachios, when eaten as part of a healthy diet, can help lower the risk of heart disease.
- Lean meats and eggs are a good source of iron
Ways to include more lean protein in your diet:
- Choose lean cuts of beef, which include sirloin, tenderloin, round, chuck, and shoulder or arm roasts and steaks.
- Choose lean pork, which include tenderloin, loin, ham, and Canadian bacon.
- Choose lean lamb, which includes tenderloin, chops, and leg.
- Buy skinless chicken or turkey, or take the skin off.
- Grill, roast, poach, or broil meats, poultry, and seafood instead of frying.
- Trim all visible fat and drain off any fat when cooking.
- Substitute peas, beans, or soy in place of meat at least once a week. Try bean chili, pea or bean soup, stir-fried tofu, rice and beans, or veggie burgers.
- Include 8 ounces (225 grams) of cooked seafood a week
5) DAIRY: CHOOSE LOW-FAT OR FAT-FREE DAIRY FOODS
Most children and adults should get about 3 cups (720 milliliters) of dairy a day. Children age 2 to 8 need about 2 to 2 1/2 cups (480 to 600 milliliters). Examples of a cup include:
- 1 cup (240 milliliters) milk
- 1 regular container of yogurt
- 1 1/2 ounces (45 grams) hard cheese (such as cheddar, mozzarella, Swiss, Parmesan)
- 1/3 cup (40 grams) shredded cheese
- 2 cups (450 grams) cottage cheese
- 1 cup (250 grams) pudding made with milk or frozen yogurt
- 1 cup (240 milliliters) calcium-fortified soymilk
Eating dairy food can improve your health:
- Consuming dairy foods is important for improving bone health especially during childhood and adolescence, when bone mass is being built.
- Dairy foods have vital nutrients including calcium, potassium, vitamin D, and protein.
- The intake of dairy products is linked to reduced risk of cardiovascular disease, type 2 diabetes, and lower blood pressure in adults.
- Low-fat or fat-free milk products provide little or no solid fat.
Ways to include low-fat foods from the dairy group in your diet:
- Include milk or calcium-fortified soymilk (soy beverage) as a beverage at meals. Choose fat-free or low-fat milk.
- Add fat-free or low-fat milk instead of water to oatmeal and hot cereals.
- Use fat-free or low-fat milk when making condensed cream soups (such as cream of tomato).
- Top casseroles, soups, stews, or vegetables with shredded reduced-fat or low-fat cheese.
- Use lactose-free or lower lactose products if you have trouble digesting dairy products. Also, you can get more calcium from non-dairy sources such as fortified juices, canned fish, soy foods, and green leafy vegetables.
6) OILS: EAT SMALL AMOUNTS OF HEART-HEALTHY OILS
- Oils are not a food group. However, they provide important nutrients and should be part of a healthy diet.
- Fats such as butter and shortening are solid at room temperature. They contain high levels of saturated fats or trans fats. Eating a lot of these fats can increase your risk of heart disease.
- Oils are liquid at room temperature. They contain monounsaturated and polyunsaturated fats. These types of fats are generally good for your heart.
- Children and adults should get about 5 to 7 teaspoons (25 to 35 milliliters) of oil a day. Children age 8 and younger need about 3 to 4 teaspoons (15 to 20 milliliters) a day.
- Choose oils such as olive, canola, sunflower, safflower, soybean, and corn oils.
- Some foods are also high in healthy oils. They include avocados, some fish, olives, and nuts.
7) WEIGHT MANAGEMENT AND PHYSICAL ACTIVITY
ChooseMyPlate 263 also provides information about how to lose excess weight:
- You can use the online SuperTracker to learn what you currently eat and drink. By writing down what you eat and drink every day, you can see where you can make better choices.
- You can use the Daily Food Plan to learn what to eat and drink. You just enter your height, weight, and age to get a personalized eating plan.
- Use the SuperTracker to track your daily activity and food you eat, plus your weight.
- If you have any specific health concerns, such as heart disease or diabetes, be sure to discuss any dietary changes with your doctor or registered dietitian first.
You also learn how to make better choices, such as:
- Eating the right amount of calories to keep you at a healthy weight
- Not overeating and avoiding big portions
- Eating fewer foods with empty calories. These are foods high in sugar or fat with few vitamins or minerals.
- Eating a balance of healthy foods from all 5 food groups
- Making better choices when eating out at restaurants
- Cooking at home more often, where you can control what goes into the foods you eat
- Exercising 150 minutes a week
- Decreasing your screen time in front of the TV or computer
- Getting tips for increasing your activity level.
- Sears, B., & Saha, A. K. (2021). Dietary Control of Inflammation and Resolution. Frontiers in nutrition, 8, 709435. https://doi.org/10.3389/fnut.2021.709435[↩]
- Most, J., Tosti, V., Redman, L. M., & Fontana, L. (2017). Calorie restriction in humans: An update. Ageing research reviews, 39, 36–45. https://doi.org/10.1016/j.arr.2016.08.005[↩]
- Martin, C. K., Das, S. K., Lindblad, L., Racette, S. B., McCrory, M. A., Weiss, E. P., Delany, J. P., Kraus, W. E., & CALERIE Study Team (2011). Effect of calorie restriction on the free-living physical activity levels of nonobese humans: results of three randomized trials. Journal of applied physiology (Bethesda, Md. : 1985), 110(4), 956–963. https://doi.org/10.1152/japplphysiol.00846.2009[↩]
- Das, S. K., Roberts, S. B., Bhapkar, M. V., Villareal, D. T., Fontana, L., Martin, C. K., Racette, S. B., Fuss, P. J., Kraus, W. E., Wong, W. W., Saltzman, E., Pieper, C. F., Fielding, R. A., Schwartz, A. V., Ravussin, E., Redman, L. M., & CALERIE-2 Study Group (2017). Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: a 2-y randomized controlled trial of calorie restriction in nonobese humans. The American journal of clinical nutrition, 105(4), 913–927. https://doi.org/10.3945/ajcn.116.137232[↩]
- Sears B. Anti-inflammatory Diets. J Am Coll Nutr. 2015;34 Suppl 1:14-21. doi: 10.1080/07315724.2015.1080105[↩]
- Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M, Laiglesia LM, Martínez JA, Moreno-Aliaga MJ. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem. 2015 Jun;71(2):341-9. doi: 10.1007/s13105-015-0395-y[↩]
- Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005 Feb;21(2):131-6. doi: 10.1016/j.nut.2004.03.023[↩]
- Gioxari A, Kaliora AC, Marantidou F, Panagiotakos DP. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition. 2018 Jan;45:114-124.e4. doi: 10.1016/j.nut.2017.06.023[↩][↩][↩]
- Dawczynski C, Dittrich M, Neumann T, Goetze K, Welzel A, Oelzner P, Völker S, Schaible AM, Troisi F, Thomas L, Pace S, Koeberle A, Werz O, Schlattmann P, Lorkowski S, Jahreis G. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin Nutr. 2018 Apr;37(2):494-504. doi: 10.1016/j.clnu.2017.02.021[↩][↩]
- Abdulrazaq M, Innes JK, Calder PC. Effect of ω-3 polyunsaturated fatty acids on arthritic pain: A systematic review. Nutrition. 2017 Jul-Aug;39-40:57-66. doi: 10.1016/j.nut.2016.12.003[↩]
- Matsumoto Y, Sugioka Y, Tada M, Okano T, Mamoto K, Inui K, Habu D, Koike T. Monounsaturated fatty acids might be key factors in the Mediterranean diet that suppress rheumatoid arthritis disease activity: The TOMORROW study. Clin Nutr. 2018 Apr;37(2):675-680. doi: 10.1016/j.clnu.2017.02.011[↩]
- Helli, B, Shahi, MM, Mowla, K, Jalali, MT, Haghighian, HK. A randomized, triple-blind, placebo-controlled clinical trial, evaluating the sesamin supplement effects on proteolytic enzymes, inflammatory markers, and clinical indices in women with rheumatoid arthritis. Phytotherapy Research. 2019; 33: 2421– 2428. https://doi.org/10.1002/ptr.6433[↩]
- Batooei M, Tahamoli-Roudsari A, Basiri Z, Yasrebifar F, Shahdoust M, Eshraghi A, Mehrpooya M, Ataei S. Evaluating the Effect of Oral N-acetylcysteine as an Adjuvant Treatment on Clinical Outcomes of Patients with Rheumatoid Arthritis: A Randomized, Double Blind Clinical Trial. Rev Recent Clin Trials. 2018;13(2):132-138. doi: 10.2174/1574887113666180307151937[↩][↩]
- Zhu F, Du B, Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit Rev Food Sci Nutr. 2018 May 24;58(8):1260-1270. doi: 10.1080/10408398.2016.1251390[↩]
- Javadi F, Ahmadzadeh A, Eghtesadi S, Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A, Jazayeri S. The Effect of Quercetin on Inflammatory Factors and Clinical Symptoms in Women with Rheumatoid Arthritis: A Double-Blind, Randomized Controlled Trial. J Am Coll Nutr. 2017 Jan;36(1):9-15. doi: 10.1080/07315724.2016.1140093[↩][↩]
- Ghavipour M, Sotoudeh G, Tavakoli E, Mowla K, Hasanzadeh J, Mazloom Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur J Clin Nutr. 2017 Jan;71(1):92-96. doi: 10.1038/ejcn.2016.151[↩][↩]
- Lo Gullo A, Mandraffino G, Bagnato G, Aragona CO, Imbalzano E, D’Ascola A, Rotondo F, Cinquegrani A, Mormina E, Saitta C, Versace AG, Sardo MA, Lo Gullo R, Loddo S, Saitta A. Vitamin D Status in Rheumatoid Arthritis: Inflammation, Arterial Stiffness and Circulating Progenitor Cell Number. PLoS One. 2015 Aug 4;10(8):e0134602. doi: 10.1371/journal.pone.0134602[↩]
- Hale LP, Chichlowski M, Trinh CT, Greer PK. Dietary supplementation with fresh pineapple juice decreases inflammation and colonic neoplasia in IL-10-deficient mice with colitis. Inflamm Bowel Dis. 2010 Dec;16(12):2012-21. doi: 10.1002/ibd.21320[↩]
- Pandey S, Cabot PJ, Shaw PN, Hewavitharana AK. Anti-inflammatory and immunomodulatory properties of Carica papaya. J Immunotoxicol. 2016 Jul;13(4):590-602. doi: 10.3109/1547691X.2016.1149528[↩]
- Müller, S., März, R., Schmolz, M., Drewelow, B., Eschmann, K. and Meiser, P. (2013), Placebo-controlled Randomized Clinical Trial on the Immunomodulating Activities of Low- and High-Dose Bromelain after Oral Administration – New Evidence on the Antiinflammatory Mode of Action of Bromelain. Phytother. Res., 27: 199-204. https://doi.org/10.1002/ptr.4678[↩]
- Aryaeian N, Shahram F, Mahmoudi M, Tavakoli H, Yousefi B, Arablou T, Jafari Karegar S. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active Rheumatoid Arthritis. Gene. 2019 May 25;698:179-185. doi: 10.1016/j.gene.2019.01.048[↩][↩]
- Daily JW, Yang M, Park S. Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Med Food. 2016 Aug;19(8):717-29. doi: 10.1089/jmf.2016.3705[↩]
- Chandran, B. and Goel, A. (2012), A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phytother. Res., 26: 1719-1725. https://doi.org/10.1002/ptr.4639[↩]
- Bang JS, Oh DH, Choi HM, Sur BJ, Lim SJ, Kim JY, Yang HI, Yoo MC, Hahm DH, Kim KS. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther. 2009;11(2):R49. doi: 10.1186/ar2662[↩][↩]
- Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M, Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin Nutr. 2015 Dec;34(6):1101-8. doi: 10.1016/j.clnu.2014.12.019[↩]
- Alghadir AH, Gabr SA, Al-Eisa ES. Green tea and exercise interventions as nondrug remedies in geriatric patients with rheumatoid arthritis. J Phys Ther Sci. 2016 Oct;28(10):2820-2829. doi: 10.1589/jpts.28.2820[↩]
- Ohishi T, Goto S, Monira P, Isemura M, Nakamura Y. Anti-inflammatory Action of Green Tea. Antiinflamm Antiallergy Agents Med Chem. 2016;15(2):74-90. doi: 10.2174/1871523015666160915154443[↩]
- Sung S, Kwon D, Um E, Kim B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules. 2019 Apr 22;24(8):1589. doi: 10.3390/molecules24081589[↩]
- Salehi-Abargouei A, Saraf-Bank S, Bellissimo N, Azadbakht L. Effects of non-soy legume consumption on C-reactive protein: a systematic review and meta-analysis. Nutrition. 2015 May;31(5):631-9. doi: 10.1016/j.nut.2014.10.018[↩]
- Helli, B, Shahi, MM, Mowla, K, Jalali, MT, Haghighian, HK. A randomized, triple-blind, placebo-controlled clinical trial, evaluating the sesamin supplement effects on proteolytic enzymes, inflammatory markers, and clinical indices in women with rheumatoid arthritis. Phytotherapy Research. 2019; 33: 2421– 2428. https://doi.org/10.1002/ptr.6433[↩]
- Bustamante MF, Agustín-Perez M, Cedola F, Coras R, Narasimhan R, Golshan S, Guma M. Design of an anti-inflammatory diet (ITIS diet) for patients with rheumatoid arthritis. Contemp Clin Trials Commun. 2020 Jan 21;17:100524. doi: 10.1016/j.conctc.2020.100524[↩]
- The effects of diet on inflammation: emphasis on the metabolic syndrome. Giugliano D, Ceriello A, Esposito K. J Am Coll Cardiol. 2006 Aug 15; 48(4):677-85. https://www.ncbi.nlm.nih.gov/pubmed/16904534/[↩][↩][↩][↩][↩][↩]
- Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Circulation. 2002 Oct 15; 106(16):2067-72. http://circ.ahajournals.org/content/106/16/2067.long[↩]
- Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis. 2008;18:283–90. https://www.ncbi.nlm.nih.gov/pubmed/17449231[↩]
- Priebe M, van Binsbergen J, de Vos R, Vonk RJ. Whole grain foods for the prevention of type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No.: CD006061. DOI: 10.1002/14651858.CD006061.pub2.[↩]
- Pereira MA, O’Reilly E, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med. 2004;164:370–376. https://www.ncbi.nlm.nih.gov/pubmed/14980987[↩]
- Liu S. Whole-grain foods, dietary fiber, and type 2 diabetes: searching for a kernel of truth. The American journal of clinical nutrition. 2003;77:527–529. http://ajcn.nutrition.org/content/77/3/527.long[↩]
- King DE. Dietary fiber, inflammation, and cardiovascular disease. Mol Nutr Food Res. 2005;49:594–600. https://www.ncbi.nlm.nih.gov/pubmed/15884088[↩]
- Ajani UA, Ford ES, Mokdad AH. Dietary fiber and C-reactive protein: findings from national health and nutrition examination survey data. The Journal of nutrition. 2004;134:1181–1185. http://jn.nutrition.org/content/134/5/1181.long[↩]
- King DE, Egan BM, Geesey ME. Relation of dietary fat and fiber to elevation of C-reactive protein. Am J Cardiol. 2003;92:1335–1339. https://www.ncbi.nlm.nih.gov/pubmed/14636916[↩]
- Ma Y, Griffith JA, Chasan-Taber L, et al. Association between dietary fiber and serum C-reactive protein-. The American journal of clinical nutrition. 2006;83(4):760-766. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1456807/[↩]
- King DE, Egan BM, Woolson RF, Mainous AG, 3rd, Al-Solaiman Y, Jesri A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch Intern Med. 2007;167:502–506. https://www.ncbi.nlm.nih.gov/pubmed/17353499[↩]
- Inflammatory disease processes and interactions with nutrition. Br J Nutr. 2009 May;101 Suppl 1:S1-45. doi: 10.1017/S0007114509377867. https://www.ncbi.nlm.nih.gov/pubmed/19586558/[↩][↩]
- Dietary patterns and mortality in Danish men and women: a prospective observational study. British Journal of Nutrition (2001), 85, 219-225. DOI: 10.1079/BJN2000240. https://www.cambridge.org/core/services/aop-cambridge-core/content/view/2C582B42A8FEC3F37A7EEE62875078B1/S0007114501000307a.pdf[↩][↩]
- Diets and cardiovascular disease. An evidence-based assessment. J Am Coll Cardiol, 45 (2005), pp. 1379-1387.[↩]
- The diet-heart hypothesis: a critique. J Am Coll Cardiol, 43 (2004), pp. 731-733.[↩]
- Optimal diets for prevention of coronary heart disease. JAMA, 288 (2002), pp. 2569-2578.[↩]
- Diet, nutrition, and coronary heart disease. P.S. Douglas (Ed.), Cardiovascular Health and Disease in Women (2nd edition.), WB Saunders, Philadelphia, PA (2002), pp. 71-92.[↩]
- Ross R. Atherosclerosis, an inflammatory disease. N Engl J Med 1999;340:115-26. https://www.ncbi.nlm.nih.gov/pubmed/9887164[↩]
- Brown AA, Hu FB. Dietary modulation of endothelial function: implications for cardiovascular disease. Am J Clin Nutr 2001;73:673-86. http://ajcn.nutrition.org/content/73/4/673.full[↩]
- Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Am J Clin Nutr. 2004 Oct; 80(4):1029-35. http://ajcn.nutrition.org/content/80/4/1029.long[↩][↩]
- In humans, serum polyunsaturated fatty acid levels predict the response of proinflammatory cytokines to psychologic stress. Maes M, Christophe A, Bosmans E, Lin A, Neels H. Biol Psychiatry. 2000 May 15; 47(10):910-20. https://www.ncbi.nlm.nih.gov/pubmed/10807964/[↩]
- Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Circulation. 2003 Jul 15; 108(2):155-60. http://circ.ahajournals.org/content/108/2/155.long[↩]
- Depressive symptoms, omega-6:omega-3 fatty acids, and inflammation in older adults. Kiecolt-Glaser JK, Belury MA, Porter K, Beversdorf DQ, Lemeshow S, Glaser R. Psychosom Med. 2007 Apr; 69(3):217-24. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2856352/[↩]
- Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. J Clin Endocrinol Metab. 2006 Feb; 91(2):439-46. https://www.ncbi.nlm.nih.gov/pubmed/16234304/[↩]
- Inflammatory disease processes and interactions with nutrition. Calder PC, Albers R, Antoine JM, Blum S, Bourdet-Sicard R, Ferns GA, Folkerts G, Friedmann PS, Frost GS, Guarner F, Løvik M, Macfarlane S, Meyer PD, M’Rabet L, Serafini M, van Eden W, van Loo J, Vas Dias W, Vidry S, Winklhofer-Roob BM, Zhao J. Br J Nutr. 2009 May; 101 Suppl 1():S1-45. https://www.ncbi.nlm.nih.gov/pubmed/19586558/[↩][↩]
- Effect of dietary antioxidants on postprandial endothelial dysfunction induced by a high-fat meal in healthy subjects. Esposito K, Nappo F, Giugliano F, Giugliano G, Marfella R, Giugliano D. Am J Clin Nutr. 2003 Jan; 77(1):139-43. http://ajcn.nutrition.org/content/77/1/139.long[↩]
- Gunawardena, Dhanushka; Karunaweera, Niloo; Lee, Samiuela; Van Der Kooy, Frank; Harman, David G.; Raju, Ritesh; Bennett, Louise; Gyengesi, Erika; Sucher, Nikolaus J.; Münch, Gerald. Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts – identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015 Mar;6(3):910-9. doi: 10.1039/c4fo00680a. https://www.ncbi.nlm.nih.gov/pubmed/25629927[↩][↩][↩][↩]
- Wszolek ZK, Herkes GK, Lagerlund TD, Kokmen E (1992) Comparison of EEG background frequency analysis, psychologic test scores, short test of mental status, and quantitative SPECT in dementia. J Geriatr Psychiatry Neurol 5:22-30.[↩]
- Anti-inflammatory Diets. Journal of the American College of Nutrition Vol. 34 , Iss. sup1,2015. http://dx.doi.org/10.1080/07315724.2015.1080105[↩]
- Holub BJ. Clinical nutrition: 4. Omega-3 fatty acids in cardiovascular care. Hoffer LJ, Jones PJ, eds. CMAJ: Canadian Medical Association Journal. 2002;166(5):608-615. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC99405/[↩]
- Rodriguez-Leyva D, Bassett CM, McCullough R, Pierce GN. The cardiovascular effects of flaxseed and its omega-3 fatty acid, alpha-linolenic acid. The Canadian Journal of Cardiology. 2010;26(9):489-496. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2989356/[↩]
- Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts – identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015 Mar;6(3):910-9. doi: 10.1039/c4fo00680a. https://www.ncbi.nlm.nih.gov/pubmed/25629927[↩][↩][↩][↩][↩][↩]
- Bennett PN, Brown MJ. New Delhi: Churchill Livingstone; 2005. Clinical pharmacology. [↩]
- Kim OY, Jo SH, Jang Y, Chae JS, Kim JY, Hyun YJ, Lee JH (2009) G allele at RAGE SNP82 is associated with proinflammatory markers in obese subjects. Nutr Res 29:106-113.[↩]
- Finlay WJ, Cunningham O, Lambert MA, Darmanin-Sheehan A, Liu X, Fennell BJ, Mahon CM, Cummins E, Wade JM, O’Sullivan CM, Tan XY, Piche N, Pittman DD, Paulsen J, Tchistiakova L, Kodangattil S, Gill D, Hufton SE (2009) Affinity maturation of a humanized rat antibody for anti-RAGE therapy: comprehensive mutagenesis reveals a high level of mutational plasticity both inside and outside the complementarity-determining regions. J Mol Biol 388:541-558.[↩]
- van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der Poll T (2009) Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 31:280-284.[↩]
- Dorsch W, Schneider E, Bayer T, Breu W, Wagner H (1990) Anti-inflammatory effects of onions: inhibition of chemotaxis of human polymorphonuclear leukocytes by thiosulfinates and cepaenes. Int Arch Allergy Appl Immunol 92:39-42.[↩]
- Wagner H, Dorsch W, Bayer T, Breu W, Willer F (1990) Antiasthmatic effects of onions: inhibition of 5-lipoxygenase and cyclooxygenase in vitro by thiosulfinates and “Cepaenes”. Prostaglandins Leukot Essent Fatty Acids 39:59-62.[↩]
- Baldasseroni S, Mannucci E, Orso F, Di Serio C, Pratesi A, Bartoli N, Marella GA, Colombi C, Foschini A, Valoti P, Mossello E, Fumagalli S, Marchionni N, Tarantini F (2012) Adiponectin in outpatients with coronary artery disease: independent predictors and relationship with heart failure. Nutr Metab Cardiovasc Dis 22:292-299.[↩]
- Cheng WW, Li YS, Gong XW, Zhao LL, Wang JG, Deng P, Jiang Y (2008) [Construction of different mutants of HA-tagged human RAGE gene and their eukaryotic expression]. Nan Fang Yi Ke Da Xue Xue Bao 28:1779-1781.[↩]
- Kwon HK, Hwang JS, So JS, Lee CG, Sahoo A, Ryu JH, Jeon WK, Ko BS, Im CR, Lee SH, Park ZY, Im SH (2010) Cinnamon extract induces tumor cell death through inhibition of NFkappaB and AP1. BMC Cancer 10:392.[↩]
- Boulanger E, Daroux M (2008) Peritoneal aging during PD: implication of RAGE, the receptor for AGEs. Nefrologia 28 Suppl 6:5-10.[↩]
- Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts – identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. http://pubs.rsc.org/en/content/getauthorversionpdf/C4FO00680A[↩]
- Rasic-Milutinovic Z, Perunicic G, Pljesa S, Gluvic Z, Ilic M, Stokic E (2007) Metabolic syndrome in HD patients: association with body composition, nutritional status, inflammation and serum iron. Intern Med 46:945-951.[↩]
- Kano M, Takayanagi T, Harada K, Makino K, Ishikawa F (2005) Antioxidative activity of anthocyanins from purple sweet potato, Ipomoera batatas cultivar Ayamurasaki. Biosci Biotechnol Biochem 69:979-988.[↩]
- Petta S, Camma C, Cabibi D, Di Marco V, Craxi A (2011) Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 34:757-766.[↩]
- Yoo BC, Kim SH, Cairns N, Fountoulakis M, Lubec G (2001) Deranged expression of molecular chaperones in brains of patients with Alzheimer’s disease. Biochem Biophys Res Commun 280:249-258.[↩]
- Zheng W, Xin N, Chi ZH, Zhao BL, Zhang J, Li JY, Wang ZY (2009) Divalent metal transporter 1 is involved in amyloid precursor protein processing and Abeta generation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 23:4207-4217.[↩]
- Liu B, Gao HM, Hong JS (2003) Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect 111:1065-1073.[↩]
- Dominguez RO, Marschoff ER, Guareschi EM, Repetto MG, Famulari AL, Pagano MA, Serra JA (2008) Insulin, glucose and glycated hemoglobin in Alzheimer’s and vascular dementia with and without superimposed Type II diabetes mellitus condition. J Neural Transm 115:77-84.[↩]
- Ujcic-Voortman JK, Baan CA, Verhoeff AP, Krol A, Seidell JC (2011) Ethnic differences in systemic inflammation: an investigation of C-reactive protein levels among Moroccan, Turkish and Dutch groups in the Netherlands. Atherosclerosis 218:511-516. [↩]
- Shahbazi, R., Sharifzad, F., Bagheri, R., Alsadi, N., Yasavoli-Sharahi, H., & Matar, C. (2021). Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients, 13(5), 1516. https://doi.org/10.3390/nu13051516[↩][↩][↩]
- Bull, M. J., & Plummer, N. T. (2014). Part 1: The Human Gut Microbiome in Health and Disease. Integrative medicine (Encinitas, Calif.), 13(6), 17–22. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4566439[↩][↩][↩]
- Neish A. S. (2009). Microbes in gastrointestinal health and disease. Gastroenterology, 136(1), 65–80. https://doi.org/10.1053/j.gastro.2008.10.080[↩]
- Vyas, U., & Ranganathan, N. (2012). Probiotics, prebiotics, and synbiotics: gut and beyond. Gastroenterology research and practice, 2012, 872716. https://doi.org/10.1155/2012/872716[↩]
- Rath, C. M., & Dorrestein, P. C. (2012). The bacterial chemical repertoire mediates metabolic exchange within gut microbiomes. Current opinion in microbiology, 15(2), 147–154. https://doi.org/10.1016/j.mib.2011.12.009[↩]
- Rinninella, E., Raoul, P., Cintoni, M., Franceschi, F., Miggiano, G., Gasbarrini, A., & Mele, M. C. (2019). What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms, 7(1), 14. https://doi.org/10.3390/microorganisms7010014[↩]
- Pandiyan, P., Bhaskaran, N., Zou, M., Schneider, E., Jayaraman, S., & Huehn, J. (2019). Microbiome Dependent Regulation of Tregs and Th17 Cells in Mucosa. Frontiers in immunology, 10, 426. https://doi.org/10.3389/fimmu.2019.00426[↩]
- Shahbazi, R., Yasavoli-Sharahi, H., Alsadi, N., Ismail, N., & Matar, C. (2020). Probiotics in Treatment of Viral Respiratory Infections and Neuroinflammatory Disorders. Molecules (Basel, Switzerland), 25(21), 4891. https://doi.org/10.3390/molecules25214891[↩]
- Bell, V., Ferrão, J., Pimentel, L., Pintado, M., & Fernandes, T. (2018). One Health, Fermented Foods, and Gut Microbiota. Foods (Basel, Switzerland), 7(12), 195. https://doi.org/10.3390/foods7120195[↩][↩]
- Zhao J., Gong L., Wu L., She S., Liao Y., Zheng H., Zhao Z., Liu G., Yan S. Immunomodulatory effects of fermented fig (Ficus carica L.) fruit extracts on cyclophosphamide-treated mice. J. Funct. Foods. 2020;75:104219. doi: 10.1016/j.jff.2020.104219[↩]
- Westfall S., Lomis N., Kahouli I., Dia S.Y., Singh S.P., Prakash S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. CMLS. 2017;74:3769–3787. doi: 10.1007/s00018-017-2550-9[↩]
- Feng Y., Huang Y., Wang Y., Wang P., Song H., Wang F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE. 2019;14:e0218384. doi: 10.1371/journal.pone.0218384[↩]
- Zhang S., Chen D.C. Facing a new challenge: The adverse effects of antibiotics on gut microbiota and host immunity. Chin. Med. J. 2019;132:1135–1138. doi: 10.1097/CM9.0000000000000245[↩]
- Selhub E.M., Logan A.C., Bested A.C. Fermented foods, microbiota, and mental health: Ancient practice meets nutritional psychiatry. J. Physiol. Anthropol. 2014;33:2. doi: 10.1186/1880-6805-33-2[↩]
- Feng Y., Zhang M., Mujumdar A.S., Gao Z. Recent research process of fermented plant extract: A review. Trends Food Sci. Technol. 2017;65:40–48. doi: 10.1016/j.tifs.2017.04.006[↩][↩]
- Kim B., Hong V.M., Yang J., Hyun H., Im J.J., Hwang J., Yoon S., Kim J.E. A Review of Fermented Foods with Beneficial Effects on Brain and Cognitive Function. Prev. Nutr. Food Sci. 2016;21:297–309. doi: 10.3746/pnf.2016.21.4.297[↩]
- Kotas, M. E., & Medzhitov, R. (2015). Homeostasis, inflammation, and disease susceptibility. Cell, 160(5), 816–827. https://doi.org/10.1016/j.cell.2015.02.010[↩]
- InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. What is an inflammation? 2010 Nov 23 [Updated 2018 Feb 22]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279298[↩][↩]
- Straub, R. H., & Schradin, C. (2016). Chronic inflammatory systemic diseases: An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evolution, medicine, and public health, 2016(1), 37–51. https://doi.org/10.1093/emph/eow001[↩][↩][↩]
- Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., Ferrucci, L., Gilroy, D. W., Fasano, A., Miller, G. W., Miller, A. H., Mantovani, A., Weyand, C. M., Barzilai, N., Goronzy, J. J., Rando, T. A., Effros, R. B., Lucia, A., Kleinstreuer, N., & Slavich, G. M. (2019). Chronic inflammation in the etiology of disease across the life span. Nature medicine, 25(12), 1822–1832. https://doi.org/10.1038/s41591-019-0675-0[↩][↩]
- Straub RH. The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat Rev Rheumatol. 2017 Dec;13(12):743-751. doi: 10.1038/nrrheum.2017.172[↩][↩]
- Slavich GM Psychoneuroimmunology of stress and mental health in The Oxford Handbook of Stress and Mental Health (eds Harkness K & Hayden EP) (Oxford University Press, in the press; ).[↩]
- Miller, A. H., & Raison, C. L. (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews. Immunology, 16(1), 22–34. https://doi.org/10.1038/nri.2015.5[↩][↩]
- Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018 May;18(5):309-324. doi: 10.1038/nri.2017.142[↩][↩]
- Inflammation and cancer: how hot is the link ? Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Biochem Pharmacol. 2006 Nov 30; 72(11):1605-21. https://www.ncbi.nlm.nih.gov/pubmed/16889756/[↩]
- Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016 Aug;15(8):551-67. doi: 10.1038/nrd.2016.39[↩][↩]
- Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017 Feb 8;542(7640):177-185. doi: 10.1038/nature21363[↩]
- Jin C, Henao-Mejia J, Flavell RA. Innate immune receptors: key regulators of metabolic disease progression. Cell Metab. 2013 Jun 4;17(6):873-882. doi: 10.1016/j.cmet.2013.05.011[↩]
- Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019 Mar;16(3):145-159. doi: 10.1038/s41575-018-0082-x[↩]
- Furman, D., Chang, J., Lartigue, L., Bolen, C. R., Haddad, F., Gaudilliere, B., Ganio, E. A., Fragiadakis, G. K., Spitzer, M. H., Douchet, I., Daburon, S., Moreau, J. F., Nolan, G. P., Blanco, P., Déchanet-Merville, J., Dekker, C. L., Jojic, V., Kuo, C. J., Davis, M. M., & Faustin, B. (2017). Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nature medicine, 23(2), 174–184. https://doi.org/10.1038/nm.4267[↩][↩]
- Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017 Jun;13(6):368-380. doi: 10.1038/nrneph.2017.51[↩]
- Ferrucci, L., & Fabbri, E. (2018). Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature reviews. Cardiology, 15(9), 505–522. https://doi.org/10.1038/s41569-018-0064-2[↩][↩][↩][↩]
- Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014 Jul;14(7):463-77. doi: 10.1038/nri3705[↩]
- Bennett, J. M., Reeves, G., Billman, G. E., & Sturmberg, J. P. (2018). Inflammation-Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Frontiers in medicine, 5, 316. https://doi.org/10.3389/fmed.2018.00316[↩]
- Straub, R. H., Cutolo, M., & Pacifici, R. (2015). Evolutionary medicine and bone loss in chronic inflammatory diseases–A theory of inflammation-related osteopenia. Seminars in arthritis and rheumatism, 45(2), 220–228. https://doi.org/10.1016/j.semarthrit.2015.04.014[↩]
- Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. 2014 Sep 2;130(10):837-44. doi: 10.1161/CIRCULATIONAHA.114.009990[↩]
- Ridker PM. A Test in Context: High-Sensitivity C-Reactive Protein. J Am Coll Cardiol. 2016 Feb 16;67(6):712-723. doi: 10.1016/j.jacc.2015.11.037[↩]
- Emerging Risk Factors Collaboration, Kaptoge, S., Di Angelantonio, E., Lowe, G., Pepys, M. B., Thompson, S. G., Collins, R., & Danesh, J. (2010). C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet (London, England), 375(9709), 132–140. https://doi.org/10.1016/S0140-6736(09)61717-7[↩]
- Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R., & Hébert, J. R. (2014). Designing and developing a literature-derived, population-based dietary inflammatory index. Public health nutrition, 17(8), 1689–1696. https://doi.org/10.1017/S1368980013002115[↩]
- Hayden, K. M., Beavers, D. P., Steck, S. E., Hebert, J. R., Tabung, F. K., Shivappa, N., Casanova, R., Manson, J. E., Padula, C. B., Salmoirago-Blotcher, E., Snetselaar, L. G., Zaslavsky, O., & Rapp, S. R. (2017). The association between an inflammatory diet and global cognitive function and incident dementia in older women: The Women’s Health Initiative Memory Study. Alzheimer’s & dementia : the journal of the Alzheimer’s Association, 13(11), 1187–1196. https://doi.org/10.1016/j.jalz.2017.04.004[↩]
- Burska, A. N., Sakthiswary, R., & Sattar, N. (2015). Effects of Tumour Necrosis Factor Antagonists on Insulin Sensitivity/Resistance in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. PloS one, 10(6), e0128889. https://doi.org/10.1371/journal.pone.0128889[↩]
- Chou, R. C., Kane, M., Ghimire, S., Gautam, S., & Gui, J. (2016). Treatment for Rheumatoid Arthritis and Risk of Alzheimer’s Disease: A Nested Case-Control Analysis. CNS drugs, 30(11), 1111–1120. https://doi.org/10.1007/s40263-016-0374-z[↩]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017 Sep 21;377(12):1119-1131. doi: 10.1056/NEJMoa1707914[↩]
- Proctor, M. J., McMillan, D. C., Horgan, P. G., Fletcher, C. D., Talwar, D., & Morrison, D. S. (2015). Systemic inflammation predicts all-cause mortality: a glasgow inflammation outcome study. PloS one, 10(3), e0116206. https://doi.org/10.1371/journal.pone.0116206[↩][↩]
- Day, D. B., Xiang, J., Mo, J., Li, F., Chung, M., Gong, J., Weschler, C. J., Ohman-Strickland, P. A., Sundell, J., Weng, W., Zhang, Y., & Zhang, J. J. (2017). Association of Ozone Exposure With Cardiorespiratory Pathophysiologic Mechanisms in Healthy Adults. JAMA internal medicine, 177(9), 1344–1353. https://doi.org/10.1001/jamainternmed.2017.2842[↩]
- Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, Izquierdo M, Ruilope LM, Lucia A. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018 Dec;15(12):731-743. doi: 10.1038/s41569-018-0065-1[↩][↩]
- Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U; Lancet Physical Activity Series Working Group. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012 Jul 21;380(9838):247-57. doi: 10.1016/S0140-6736(12)60646-1[↩]
- Katzmarzyk PT, Lee IM, Martin CK, Blair SN. Epidemiology of Physical Activity and Exercise Training in the United States. Prog Cardiovasc Dis. 2017 Jun-Jul;60(1):3-10. doi: 10.1016/j.pcad.2017.01.004[↩]
- Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, Atherton PJ, Phillips SM. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. 2013 Jun;98(6):2604-12. doi: 10.1210/jc.2013-1502[↩]
- Fedewa MV, Hathaway ED, Ward-Ritacco CL. Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br J Sports Med. 2017 Apr;51(8):670-676. doi: 10.1136/bjsports-2016-095999. Epub 2016 Jul 21[↩]
- Meneses-Echávez JF, Correa-Bautista JE, González-Jiménez E, Schmidt Río-Valle J, Elkins MR, Lobelo F, Ramírez-Vélez R. The Effect of Exercise Training on Mediators of Inflammation in Breast Cancer Survivors: A Systematic Review with Meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016 Jul;25(7):1009-17. doi: 10.1158/1055-9965.EPI-15-1061[↩]
- Hayashino Y, Jackson JL, Hirata T, Fukumori N, Nakamura F, Fukuhara S, Tsujii S, Ishii H. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Metabolism. 2014 Mar;63(3):431-40. doi: 10.1016/j.metabol.2013.08.018[↩]
- Booth, F. W., Roberts, C. K., & Laye, M. J. (2012). Lack of exercise is a major cause of chronic diseases. Comprehensive Physiology, 2(2), 1143–1211. https://doi.org/10.1002/cphy.c110025[↩][↩]
- Wahid, A., Manek, N., Nichols, M., Kelly, P., Foster, C., Webster, P., Kaur, A., Friedemann Smith, C., Wilkins, E., Rayner, M., Roberts, N., & Scarborough, P. (2016). Quantifying the Association Between Physical Activity and Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. Journal of the American Heart Association, 5(9), e002495. https://doi.org/10.1161/JAHA.115.002495[↩]
- Moore, S. C., Lee, I. M., Weiderpass, E., Campbell, P. T., Sampson, J. N., Kitahara, C. M., Keadle, S. K., Arem, H., Berrington de Gonzalez, A., Hartge, P., Adami, H. O., Blair, C. K., Borch, K. B., Boyd, E., Check, D. P., Fournier, A., Freedman, N. D., Gunter, M., Johannson, M., Khaw, K. T., … Patel, A. V. (2016). Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA internal medicine, 176(6), 816–825. https://doi.org/10.1001/jamainternmed.2016.1548[↩]
- Santos-Lozano A, Pareja-Galeano H, Sanchis-Gomar F, Quindós-Rubial M, Fiuza-Luces C, Cristi-Montero C, Emanuele E, Garatachea N, Lucia A. Physical Activity and Alzheimer Disease: A Protective Association. Mayo Clin Proc. 2016 Aug;91(8):999-1020. doi: 10.1016/j.mayocp.2016.04.024[↩]
- Pérez, L. M., Pareja-Galeano, H., Sanchis-Gomar, F., Emanuele, E., Lucia, A., & Gálvez, B. G. (2016). ‘Adipaging’: ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. The Journal of physiology, 594(12), 3187–3207. https://doi.org/10.1113/JP271691[↩]
- Frasca, D., Blomberg, B. B., & Paganelli, R. (2017). Aging, Obesity, and Inflammatory Age-Related Diseases. Frontiers in immunology, 8, 1745. https://doi.org/10.3389/fimmu.2017.01745[↩]
- Grant, R. W., & Dixit, V. D. (2015). Adipose tissue as an immunological organ. Obesity (Silver Spring, Md.), 23(3), 512–518. https://doi.org/10.1002/oby.21003[↩]
- Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013 Jan;93(1):359-404. doi: 10.1152/physrev.00033.2011[↩]
- Himbert, C., Delphan, M., Scherer, D., Bowers, L. W., Hursting, S., & Ulrich, C. M. (2017). Signals from the Adipose Microenvironment and the Obesity-Cancer Link-A Systematic Review. Cancer prevention research (Philadelphia, Pa.), 10(9), 494–506. https://doi.org/10.1158/1940-6207.CAPR-16-0322[↩]
- Singer, K., & Lumeng, C. N. (2017). The initiation of metabolic inflammation in childhood obesity. The Journal of clinical investigation, 127(1), 65–73. https://doi.org/10.1172/JCI88882[↩][↩]
- NCD Risk Factor Collaboration (NCD-RisC) (2017). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet (London, England), 390(10113), 2627–2642. https://doi.org/10.1016/S0140-6736(17)32129-3[↩]
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018 Oct;14(10):576-590. doi: 10.1038/s41574-018-0059-4[↩]
- Tobaldini E, Fiorelli EM, Solbiati M, Costantino G, Nobili L, Montano N. Short sleep duration and cardiometabolic risk: from pathophysiology to clinical evidence. Nat Rev Cardiol. 2019 Apr;16(4):213-224. doi: 10.1038/s41569-018-0109-6[↩]
- Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018 Jul;84:56-66. doi: 10.1016/j.metabol.2018.02.010[↩]
- Kivimäki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018 Apr;15(4):215-229. doi: 10.1038/nrcardio.2017.189[↩]
- Chandola, T., Brunner, E., & Marmot, M. (2006). Chronic stress at work and the metabolic syndrome: prospective study. BMJ (Clinical research ed.), 332(7540), 521–525. https://doi.org/10.1136/bmj.38693.435301.80[↩]
- Cohen, S., Janicki-Deverts, D., Doyle, W. J., Miller, G. E., Frank, E., Rabin, B. S., & Turner, R. B. (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences of the United States of America, 109(16), 5995–5999. https://doi.org/10.1073/pnas.1118355109[↩]
- Lunn, R. M., Blask, D. E., Coogan, A. N., Figueiro, M. G., Gorman, M. R., Hall, J. E., Hansen, J., Nelson, R. J., Panda, S., Smolensky, M. H., Stevens, R. G., Turek, F. W., Vermeulen, R., Carreón, T., Caruso, C. C., Lawson, C. C., Thayer, K. A., Twery, M. J., Ewens, A. D., Garner, S. C., … Boyd, W. A. (2017). Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. The Science of the total environment, 607-608, 1073–1084. https://doi.org/10.1016/j.scitotenv.2017.07.056[↩][↩]
- Hatori, M., Gronfier, C., Van Gelder, R. N., Bernstein, P. S., Carreras, J., Panda, S., Marks, F., Sliney, D., Hunt, C. E., Hirota, T., Furukawa, T., & Tsubota, K. (2017). Global rise of potential health hazards caused by blue light-induced circadian disruption in modern aging societies. NPJ aging and mechanisms of disease, 3, 9. https://doi.org/10.1038/s41514-017-0010-2[↩]
- Leproult, R., Holmbäck, U., & Van Cauter, E. (2014). Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes, 63(6), 1860–1869. https://doi.org/10.2337/db13-1546[↩]
- Touitou Y, Reinberg A, Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017 Mar 15;173:94-106. doi: 10.1016/j.lfs.2017.02.008[↩]
- Arai, Y., Martin-Ruiz, C. M., Takayama, M., Abe, Y., Takebayashi, T., Koyasu, S., Suematsu, M., Hirose, N., & von Zglinicki, T. (2015). Inflammation, But Not Telomere Length, Predicts Successful Ageing at Extreme Old Age: A Longitudinal Study of Semi-supercentenarians. EBioMedicine, 2(10), 1549–1558. https://doi.org/10.1016/j.ebiom.2015.07.029[↩]
- Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998 Jan;53(1):M20-6. doi: 10.1093/gerona/53a.1.m20[↩]
- Ahluwalia N, Mastro AM, Ball R, Miles MP, Rajendra R, Handte G. Cytokine production by stimulated mononuclear cells did not change with aging in apparently healthy, well-nourished women. Mech Ageing Dev. 2001 Sep;122(12):1269-79. doi: 10.1016/s0047-6374(01)00266-4[↩]
- Verschoor, C. P., Lelic, A., Parsons, R., Evelegh, C., Bramson, J. L., Johnstone, J., Loeb, M. B., & Bowdish, D. (2017). Serum C-Reactive Protein and Congestive Heart Failure as Significant Predictors of Herpes Zoster Vaccine Response in Elderly Nursing Home Residents. The Journal of infectious diseases, 216(2), 191–197. https://doi.org/10.1093/infdis/jix257[↩]
- Fleming, T. P., Watkins, A. J., Velazquez, M. A., Mathers, J. C., Prentice, A. M., Stephenson, J., Barker, M., Saffery, R., Yajnik, C. S., Eckert, J. J., Hanson, M. A., Forrester, T., Gluckman, P. D., & Godfrey, K. M. (2018). Origins of lifetime health around the time of conception: causes and consequences. Lancet (London, England), 391(10132), 1842–1852. https://doi.org/10.1016/S0140-6736(18)30312-X[↩][↩]
- Olvera Alvarez, H. A., Kubzansky, L. D., Campen, M. J., & Slavich, G. M. (2018). Early life stress, air pollution, inflammation, and disease: An integrative review and immunologic model of social-environmental adversity and lifespan health. Neuroscience and biobehavioral reviews, 92, 226–242. https://doi.org/10.1016/j.neubiorev.2018.06.002[↩]
- Renz H, Holt PG, Inouye M, Logan AC, Prescott SL, Sly PD. An exposome perspective: Early-life events and immune development in a changing world. J Allergy Clin Immunol. 2017 Jul;140(1):24-40. doi: 10.1016/j.jaci.2017.05.015[↩][↩]
- Miller, G. E., Chen, E., & Parker, K. J. (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological bulletin, 137(6), 959–997. https://doi.org/10.1037/a0024768[↩]
- Casas, R., Sacanella, E., & Estruch, R. (2014). The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocrine, metabolic & immune disorders drug targets, 14(4), 245–254. https://doi.org/10.2174/1871530314666140922153350[↩][↩]
- Chmurzynska, A., Muzsik, A., Krzyżanowska-Jankowska, P., Walkowiak, J., & Bajerska, J. (2019). The Effect of Habitual Fat Intake, IL6 Polymorphism, and Different Diet Strategies on Inflammation in Postmenopausal Women with Central Obesity. Nutrients, 11(7), 1557. https://doi.org/10.3390/nu11071557[↩]
- Carrera-Bastos P, Fontes-Villalba M, O’Keefe JH, Lindeberg S & Cordain L The western diet and lifestyle and diseases of civilization. Res. Rep. Clin. Cardiol 2, 15–35 (2011).[↩]
- Bentley J U.S. trends in food availability and a dietary assessment of loss-adjusted food availability, 1970–2014. EIB-166, U.S. Department of Agriculture, Economic Research Service (2017).[↩]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001-2002 to 2012-2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017 Sep 1;74(9):911-923. doi: 10.1001/jamapsychiatry.2017.2161[↩]
- Martínez Steele E, Baraldi LG, Louzada ML, Moubarac JC, Mozaffarian D, Monteiro CA. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. 2016 Mar 9;6(3):e009892. doi: 10.1136/bmjopen-2015-009892[↩]
- Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017 Aug;66(8):1414-1427. doi: 10.1136/gutjnl-2016-313099[↩]
- Sonnenburg ED, Sonnenburg JL. The ancestral and industrialized gut microbiota and implications for human health. Nat Rev Microbiol. 2019 Jun;17(6):383-390. doi: 10.1038/s41579-019-0191-8[↩]
- Bishehsari, F., Magno, E., Swanson, G., Desai, V., Voigt, R. M., Forsyth, C. B., & Keshavarzian, A. (2017). Alcohol and Gut-Derived Inflammation. Alcohol research : current reviews, 38(2), 163–171. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513683[↩]
- Richards, J. L., Yap, Y. A., McLeod, K. H., Mackay, C. R., & Mariño, E. (2016). Dietary metabolites and the gut microbiota: an alternative approach to control inflammatory and autoimmune diseases. Clinical & translational immunology, 5(5), e82. https://doi.org/10.1038/cti.2016.29[↩]
- Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev. 2015 Jun;14(6):479-89. doi: 10.1016/j.autrev.2015.01.009[↩]
- Vlassara, H., & Striker, G. E. (2011). AGE restriction in diabetes mellitus: a paradigm shift. Nature reviews. Endocrinology, 7(9), 526–539. https://doi.org/10.1038/nrendo.2011.74[↩]
- Dickinson S, Hancock DP, Petocz P, Ceriello A, Brand-Miller J. High-glycemic index carbohydrate increases nuclear factor-kappaB activation in mononuclear cells of young, lean healthy subjects. Am J Clin Nutr. 2008 May;87(5):1188-93. doi: 10.1093/ajcn/87.5.1188[↩]
- Hall K. D. (2018). Did the Food Environment Cause the Obesity Epidemic?. Obesity (Silver Spring, Md.), 26(1), 11–13. https://doi.org/10.1002/oby.22073[↩]
- van Niekerk G, du Toit A, Loos B, Engelbrecht AM. Nutrient excess and autophagic deficiency: explaining metabolic diseases in obesity. Metabolism. 2018 May;82:14-21. doi: 10.1016/j.metabol.2017.12.007[↩]
- Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and ‘Garb-aging’. Trends Endocrinol Metab. 2017 Mar;28(3):199-212. doi: 10.1016/j.tem.2016.09.005[↩]
- Kennedy, B. K., Berger, S. L., Brunet, A., Campisi, J., Cuervo, A. M., Epel, E. S., Franceschi, C., Lithgow, G. J., Morimoto, R. I., Pessin, J. E., Rando, T. A., Richardson, A., Schadt, E. E., Wyss-Coray, T., & Sierra, F. (2014). Geroscience: linking aging to chronic disease. Cell, 159(4), 709–713. https://doi.org/10.1016/j.cell.2014.10.039[↩]
- Health effects of trans-fatty acids: experimental and observational evidence. Mozaffarian D, Aro A, Willett WC. Eur J Clin Nutr. 2009 May; 63 Suppl 2():S5-21. https://www.ncbi.nlm.nih.gov/pubmed/19424218/[↩]
- Kiecolt-Glaser JK. Stress, Food, and Inflammation: Psychoneuroimmunology and Nutrition at the Cutting Edge. Psychosomatic medicine. 2010;72(4):365-369. doi:10.1097/PSY.0b013e3181dbf489. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2868080/[↩]
- Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation, 101 (2000), pp. 1767-1772.[↩]
- Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation, 101 (2000), pp. 2149-2153.[↩]
- Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation, 106 (2002), pp. 24-30.[↩]
- Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Howren MB, Lamkin DM, Suls J. Psychosom Med. 2009 Feb; 71(2):171-86. https://www.ncbi.nlm.nih.gov/pubmed/19188531/[↩]
- Mozaffarian D, Aro A, Willett WC. Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr. 2009 May;63 Suppl 2:S5-21. doi: 10.1038/sj.ejcn.1602973[↩]
- Müller DN, Wilck N, Haase S, Kleinewietfeld M, Linker RA. Sodium in the microenvironment regulates immune responses and tissue homeostasis. Nat Rev Immunol. 2019 Apr;19(4):243-254. doi: 10.1038/s41577-018-0113-4[↩][↩][↩]
- Schnabel, L., Kesse-Guyot, E., Allès, B., Touvier, M., Srour, B., Hercberg, S., Buscail, C., & Julia, C. (2019). Association Between Ultraprocessed Food Consumption and Risk of Mortality Among Middle-aged Adults in France. JAMA internal medicine, 179(4), 490–498. https://doi.org/10.1001/jamainternmed.2018.7289[↩]
- Bonaventura P, Benedetti G, Albarède F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015 Apr;14(4):277-85. doi: 10.1016/j.autrev.2014.11.008[↩]
- Nielsen FH. Effects of magnesium depletion on inflammation in chronic disease. Curr Opin Clin Nutr Metab Care. 2014 Nov;17(6):525-30. doi: 10.1097/MCO.0000000000000093[↩]
- Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017 Oct 15;45(5):1105-1115. doi: 10.1042/BST20160474[↩][↩]
- Serhan, C. N., & Levy, B. D. (2018). Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. The Journal of clinical investigation, 128(7), 2657–2669. https://doi.org/10.1172/JCI97943[↩]
- Calder PC. Very long-chain n-3 fatty acids and human health: fact, fiction and the future. Proc Nutr Soc. 2018 Feb;77(1):52-72. doi: 10.1017/S0029665117003950[↩][↩]
- AbuMweis S, Jew S, Tayyem R, Agraib L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: a meta-analysis of randomised placebo-control human clinical trials. J Hum Nutr Diet. 2018 Feb;31(1):67-84. doi: 10.1111/jhn.12493[↩]
- The effects of diet on inflammation: emphasis on the metabolic syndrome. Giugliano D, Ceriello A, Esposito K. J Am Coll Cardiol. 2006 Aug 15; 48(4):677-85. https://www.ncbi.nlm.nih.gov/pubmed/16904534[↩]
- The Effects of Diet on Inflammation: Emphasis on the Metabolic Syndrome. Journal of the American College of Cardiology Volume 48, Issue 4, 15 August 2006, Pages 677-685. http://www.sciencedirect.com/science/article/pii/S0735109706013350[↩]
- Schulze MB, Fung TT, Manson JE, Willett WC, Hu FB. Dietary patterns and changes in body weight in women. Obesity (Silver Spring). 2006;14:1444-53. https://www.ncbi.nlm.nih.gov/pubmed/16988088[↩]
- Newby PK, Muller D, Hallfrisch J, Andres R, Tucker KL. Food patterns measured by factor analysis and anthropometric changes in adults. Am J Clin Nutr. 2004;80:504-13. https://www.ncbi.nlm.nih.gov/pubmed/15277177[↩]
- Schulz M, Nothlings U, Hoffmann K, Bergmann MM, Boeing H. Identification of a food pattern characterized by high-fiber and low-fat food choices associated with low prospective weight change in the EPIC-Potsdam cohort. J Nutr. 2005;135:1183-9. https://www.ncbi.nlm.nih.gov/pubmed/15867301[↩]
- Newby PK, Muller D, Hallfrisch J, Qiao N, Andres R, Tucker KL. Dietary patterns and changes in body mass index and waist circumference in adults. Am J Clin Nutr. 2003;77:1417-25. https://www.ncbi.nlm.nih.gov/pubmed/12791618[↩]
- Popkin BM, Duffey KJ. Does hunger and satiety drive eating anymore? Increasing eating occasions and decreasing time between eating occasions in the United States. Am J Clin Nutr. 2010;91:1342-7. https://www.ncbi.nlm.nih.gov/pubmed/20237134[↩]
- Harvard University, Harvard School of Public Health. Carbohydrates. https://www.hsph.harvard.edu/nutritionsource/carbohydrates/[↩]
- Optimal diets for prevention of coronary heart disease. Hu FB, Willett WC. JAMA. 2002 Nov 27; 288(20):2569-78. https://www.ncbi.nlm.nih.gov/pubmed/12444864/[↩]
- Diabetes Care 2008 Dec; 31(12): 2281-2283. https://doi.org/10.2337/dc08-1239. International Tables of Glycemic Index and Glycemic Load Values: 2008. http://care.diabetesjournals.org/content/31/12/2281.full[↩]
- Martínez-González MA, Fernández-Jarne E, Serrano-Martínez M, Wright M, Gomez-Gracia E. Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. Eur J Clin Nutr. 2004 Nov;58(11):1550-2. doi: 10.1038/sj.ejcn.1602004[↩]
- Diet and Inflammation. Nutrition in Clinical Practice Vol 25, Issue 6, pp. 634 – 640. First published date: December-07-2010. 10.1177/0884533610385703[↩]
- F.B. Hu. The Mediterranean Diet and mortality—olive oil and beyond. N Engl J Med, 348 (2003), pp. 2595-2596[↩][↩]
- Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018 Jan;72(1):30-43. doi: 10.1038/ejcn.2017.58[↩]
- Sofi F, Macchi C, Abbate R, et al. (2014) Mediterranean diet and health status: an updated metaanalysis and a proposal for a literature-based adherence score. Public Health Nutr 17:2769–2782. doi: 10.1017/S1368980013003169[↩]
- Schwingshackl, L., Schwedhelm, C., Galbete, C., & Hoffmann, G. (2017). Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients, 9(10), 1063. https://doi.org/10.3390/nu9101063[↩]
- Jannasch F, Kröger J, Schulze MB (2017) Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr 147:1174–1182. doi: 10.3945/jn.116.242552[↩]
- Shen J, Wilmot KA, Ghasemzadeh N, et al. (2015) Mediterranean dietary patterns and cardiovascular health. Annu Rev Nutr 35:425–449. doi: 10.1146/annurev-nutr-011215-025104[↩]
- Lourida I, Soni M, Thompson-Coon J, et al. (2013) Mediterranean diet, cognitive function, and dementia. Epidemiology 24:479–489. doi: 10.1097/EDE.0b013e3182944410[↩]
- Sureda, A., Bibiloni, M., Julibert, A., Bouzas, C., Argelich, E., Llompart, I., Pons, A., & Tur, J. A. (2018). Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients, 10(1), 62. https://doi.org/10.3390/nu10010062[↩]
- Lahoz, C., Castillo, E., Mostaza, J. M., de Dios, O., Salinero-Fort, M. A., González-Alegre, T., García-Iglesias, F., Estirado, E., Laguna, F., Sanchez, V., Sabín, C., López, S., Cornejo, V., de Burgos, C., Garcés, C., & Investigators of the SPREDIA-2 Group (2018). Relationship of the Adherence to a Mediterranean Diet and Its Main Components with CRP Levels in the Spanish Population. Nutrients, 10(3), 379. https://doi.org/10.3390/nu10030379[↩]
- Vincent-Baudry S, Defoort C, Gerber M, Bernard MC, Verger P, Helal O, Portugal H, Planells R, Grolier P, Amiot-Carlin MJ, Vague P, Lairon D. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr. 2005 Nov;82(5):964-71. doi: 10.1093/ajcn/82.5.964[↩]
- Skouroliakou M, Grosomanidis D, Massara P, Kostara C, Papandreou P, Ntountaniotis D, Xepapadakis G. Serum antioxidant capacity, biochemical profile and body composition of breast cancer survivors in a randomized Mediterranean dietary intervention study. Eur J Nutr. 2018 Sep;57(6):2133-2145. doi: 10.1007/s00394-017-1489-9[↩]
- Altomare, R., Cacciabaudo, F., Damiano, G., Palumbo, V. D., Gioviale, M. C., Bellavia, M., Tomasello, G., & Lo Monte, A. I. (2013). The mediterranean diet: a history of health. Iranian journal of public health, 42(5), 449–457. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3684452[↩]
- Castro-Quezada, I., Román-Viñas, B., & Serra-Majem, L. (2014). The Mediterranean diet and nutritional adequacy: a review. Nutrients, 6(1), 231–248. https://doi.org/10.3390/nu6010231[↩]
- Serra-Majem L., Bach A., Roman B. Recognition of the Mediterranean diet: Going step further. Public Health Nutr. 2006;9:101–102. doi: 10.1079/PHN2005929[↩]
- Sofi F., Macchi C., Abbate R., Gensini G.F., Casini A. Mediterranean diet and health. Biofactors. 2013;39:335–342. doi: 10.1002/biof.1096[↩]
- Estruch R., Ros E., Salas-Salvadó J., Covas M.I., Corella D., Arós F., Gómez-Gracia E., Ruiz-Gutiérrez V., Fiol M., Lapetra J., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303[↩]
- Mitrou P.N., Kipnis V., Thiébaut A.C., Reedy J., Subar A.F., Wirfält E., Flood A., Mouw T., Hollenbeck A.R., Leitzmann M.F., et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: Results from the NIH-AARP Diet and Health Study. Arch. Intern. Med. 2007;167:2461–2468. doi: 10.1001/archinte.167.22.2461[↩]
- Couto E., Boffetta P., Lagiou P., Ferrari P., Buckland G., Overvad K., Dahm C.C., Tjønneland A., Olsen A., Clavel-Chapelon F., et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br. J. Cancer. 2011;104:1493–1499. doi: 10.1038/bjc.2011.106[↩]
- Sofi F., Abbate R., Gensini G.F., Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010;92:1189–1196. doi: 10.3945/ajcn.2010.29673[↩]
- Widmer, R. J., Flammer, A. J., Lerman, L. O., & Lerman, A. (2015). The Mediterranean diet, its components, and cardiovascular disease. The American journal of medicine, 128(3), 229–238. https://doi.org/10.1016/j.amjmed.2014.10.014[↩]
- American Heart Association – Mediterranean Diet – http://www.heart.org/HEARTORG/HealthyLiving/HealthyEating/Nutrition/Mediterranean-Diet_UCM_306004_Article.jsp[↩][↩]
- Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report . Nutrition Journal. 2014;13:5. doi:10.1186/1475-2891-13-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3896778/[↩][↩]
- Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8:357–363. doi: 10.1016/j.cgh.2010.01.001. https://www.ncbi.nlm.nih.gov/pubmed/20096379[↩]
- Bosscher D, Breynaert A, Pieters L, Hermans N. Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects. J Physiol Pharmacol. 2009;60(Suppl 6):5–11. https://www.ncbi.nlm.nih.gov/pubmed/20224145[↩]
- Jia W, Whitehead RN, Griffiths L, et al. Is the abundance of Faecalibacterium prausnitzii relevant to Crohn’s disease? Ross F, ed. Fems Microbiology Letters. 2010;310(2):138-144. doi:10.1111/j.1574-6968.2010.02057.x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2962807/[↩]
- McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World Journal of Gastroenterology : WJG. 2010;16(18):2202-2222. doi:10.3748/wjg.v16.i18.2202. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2868213/[↩]
- Lorea Baroja M, Kirjavainen PV, Hekmat S, Reid G. Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clinical and Experimental Immunology. 2007;149(3):470-479. doi:10.1111/j.1365-2249.2007.03434.x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2219330/[↩]
- Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. https://www.ncbi.nlm.nih.gov/pubmed/21468064[↩]
- Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutrition reviews. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. https://www.ncbi.nlm.nih.gov/pubmed/20500789[↩]
- Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutrition Journal. 2014;13:5. doi:10.1186/1475-2891-13-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3896778/[↩]
- Levine A, Rhodes JM, Lindsay JO, Abreu MT, Kamm MA, Gibson PR, Gasche C, Silverberg MS, Mahadevan U, Boneh RS, Wine E, Damas OM, Syme G, Trakman GL, Yao CK, Stockhamer S, Hammami MB, Garces LC, Rogler G, Koutroubakis IE, Ananthakrishnan AN, McKeever L, Lewis JD. Dietary Guidance From the International Organization for the Study of Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020 May;18(6):1381-1392. doi: 10.1016/j.cgh.2020.01.046[↩][↩][↩][↩]
- Borrelli O, Cordischi L, Cirulli M, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol. 2006 Jun;4(6):744–53. https://www.ncbi.nlm.nih.gov/pubmed/16682258[↩]
- Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford). 2012 Jul;51 Suppl 5:v3-11. doi: 10.1093/rheumatology/kes113[↩]
- Berenbaum, F., Chauvin, P., Hudry, C., Mathoret-Philibert, F., Poussiere, M., De Chalus, T., Dreuillet, C., Russo-Marie, F., Joubert, J. M., & Saraux, A. (2014). Fears and beliefs in rheumatoid arthritis and spondyloarthritis: a qualitative study. PloS one, 9(12), e114350. https://doi.org/10.1371/journal.pone.0114350[↩]
- Tedeschi, S. K., Frits, M., Cui, J., Zhang, Z. Z., Mahmoud, T., Iannaccone, C., Lin, T. C., Yoshida, K., Weinblatt, M. E., Shadick, N. A., & Solomon, D. H. (2017). Diet and Rheumatoid Arthritis Symptoms: Survey Results From a Rheumatoid Arthritis Registry. Arthritis care & research, 69(12), 1920–1925. https://doi.org/10.1002/acr.23225[↩]
- Forsyth C, Kouvari M, D’Cunha NM, Georgousopoulou EN, Panagiotakos DB, Mellor DD, Kellett J, Naumovski N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: a systematic review of human prospective studies. Rheumatol Int. 2018 May;38(5):737-747. doi: 10.1007/s00296-017-3912-1[↩]
- Kjeldsen-Kragh J, Haugen M, Borchgrevink CF, Laerum E, Eek M, Mowinkel P, Hovi K, Førre O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet. 1991 Oct 12;338(8772):899-902. doi: 10.1016/0140-6736(91)91770-u[↩]
- Hafström I, Ringertz B, Spångberg A, von Zweigbergk L, Brannemark S, Nylander I, Rönnelid J, Laasonen L, Klareskog L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: the effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology (Oxford). 2001 Oct;40(10):1175-9. doi: 10.1093/rheumatology/40.10.1175[↩]
- Aqaeinezhad Rudbane SM, Rahmdel S, Abdollahzadeh SM, Zare M, Bazrafshan A, Mazloomi SM. The efficacy of probiotic supplementation in rheumatoid arthritis: a meta-analysis of randomized, controlled trials. Inflammopharmacology. 2018 Feb;26(1):67-76. doi: 10.1007/s10787-017-0436-y[↩]
- Franco, A. S., Freitas, T. Q., Bernardo, W. M., & Pereira, R. (2017). Vitamin D supplementation and disease activity in patients with immune-mediated rheumatic diseases: A systematic review and meta-analysis. Medicine, 96(23), e7024. https://doi.org/10.1097/MD.0000000000007024[↩]
- Khojah HM, Ahmed S, Abdel-Rahman MS, Elhakeim EH. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: a clinical study. Clin Rheumatol. 2018 Aug;37(8):2035-2042. doi: 10.1007/s10067-018-4080-8[↩]
- Thimóteo NSB, Iryioda TMV, Alfieri DF, Rego BEF, Scavuzzi BM, Fatel E, Lozovoy MAB, Simão ANC, Dichi I. Cranberry juice decreases disease activity in women with rheumatoid arthritis. Nutrition. 2019 Apr;60:112-117. doi: 10.1016/j.nut.2018.10.010[↩]
- Shishehbor F, Rezaeyan Safar M, Rajaei E, Haghighizadeh MH. Cinnamon Consumption Improves Clinical Symptoms and Inflammatory Markers in Women With Rheumatoid Arthritis. J Am Coll Nutr. 2018 May 3:1-6. doi: 10.1080/07315724.2018.1460733[↩]
- Vadell, A., Bärebring, L., Hulander, E., Gjertsson, I., Lindqvist, H. M., & Winkvist, A. (2020). Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)-a randomized, controlled crossover trial indicating effects on disease activity. The American journal of clinical nutrition, 111(6), 1203–1213. https://doi.org/10.1093/ajcn/nqaa019[↩][↩][↩][↩][↩][↩][↩]
- Amcoff E, Edberg A, Barbieri HE, Lindroos AK, Nälsén C, Pearson M, Lemming EW. Riksmaten – vuxna 2010–11. Livsmedels- och näringsintag bland vuxna i Sverige (Riksmaten – adults 2010–11. Food- and nutrient intake in the Swedish adult population). Available from: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2011/riksmaten_2010_20111.pdf (in Swedish).[↩]
- Guerreiro, C. S., Calado, Â., Sousa, J., & Fonseca, J. E. (2018). Diet, Microbiota, and Gut Permeability-The Unknown Triad in Rheumatoid Arthritis. Frontiers in medicine, 5, 349. https://doi.org/10.3389/fmed.2018.00349[↩]
- Alipour B, Homayouni-Rad A, Vaghef-Mehrabany E, Sharif SK, Vaghef-Mehrabany L, Asghari-Jafarabadi M, Nakhjavani MR, Mohtadi-Nia J. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. Int J Rheum Dis. 2014 Jun;17(5):519-27. doi: 10.1111/1756-185X.12333[↩][↩][↩]
- Mandel, D. R., Eichas, K., & Holmes, J. (2010). Bacillus coagulans: a viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC complementary and alternative medicine, 10, 1. https://doi.org/10.1186/1472-6882-10-1[↩]
- Zamani B, Golkar HR, Farshbaf S, Emadi-Baygi M, Tajabadi-Ebrahimi M, Jafari P, Akhavan R, Taghizadeh M, Memarzadeh MR, Asemi Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. Int J Rheum Dis. 2016 Sep;19(9):869-79. doi: 10.1111/1756-185X.12888[↩][↩]
- Pineda, M., Thompson, S. F., Summers, K., de Leon, F., Pope, J., & Reid, G. (2011). A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Medical science monitor : international medical journal of experimental and clinical research, 17(6), CR347–CR354. https://doi.org/10.12659/msm.881808[↩]
- Jiang, J., Li, K., Wang, F., Yang, B., Fu, Y., Zheng, J., & Li, D. (2016). Effect of Marine-Derived n-3 Polyunsaturated Fatty Acids on Major Eicosanoids: A Systematic Review and Meta-Analysis from 18 Randomized Controlled Trials. PloS one, 11(1), e0147351. https://doi.org/10.1371/journal.pone.0147351[↩]
- Veselinovic, M., Vasiljevic, D., Vucic, V., Arsic, A., Petrovic, S., Tomic-Lucic, A., Savic, M., Zivanovic, S., Stojic, V., & Jakovljevic, V. (2017). Clinical Benefits of n-3 PUFA and ɤ-Linolenic Acid in Patients with Rheumatoid Arthritis. Nutrients, 9(4), 325. https://doi.org/10.3390/nu9040325[↩]
- Tedeschi, S. K., Bathon, J. M., Giles, J. T., Lin, T. C., Yoshida, K., & Solomon, D. H. (2018). Relationship Between Fish Consumption and Disease Activity in Rheumatoid Arthritis. Arthritis care & research, 70(3), 327–332. https://doi.org/10.1002/acr.23295[↩]
- Lindqvist, H. M., Gjertsson, I., Eneljung, T., & Winkvist, A. (2018). Influence of Blue Mussel (Mytilus edulis) Intake on Disease Activity in Female Patients with Rheumatoid Arthritis: The MIRA Randomized Cross-Over Dietary Intervention. Nutrients, 10(4), 481. https://doi.org/10.3390/nu10040481[↩]
- van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998 Oct;41(10):1845-50. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K[↩]
- https://www.choosemyplate.gov/[↩][↩][↩]
- USDA Dietary Guidelines for Americans 2015-2020. 8th Edition. https://health.gov/dietaryguidelines/2015/[↩][↩]
- U.S. National Library of Medicine, MedlinePlus. Food guide plate. https://medlineplus.gov/ency/article/002093.htm[↩]