Antithrombin
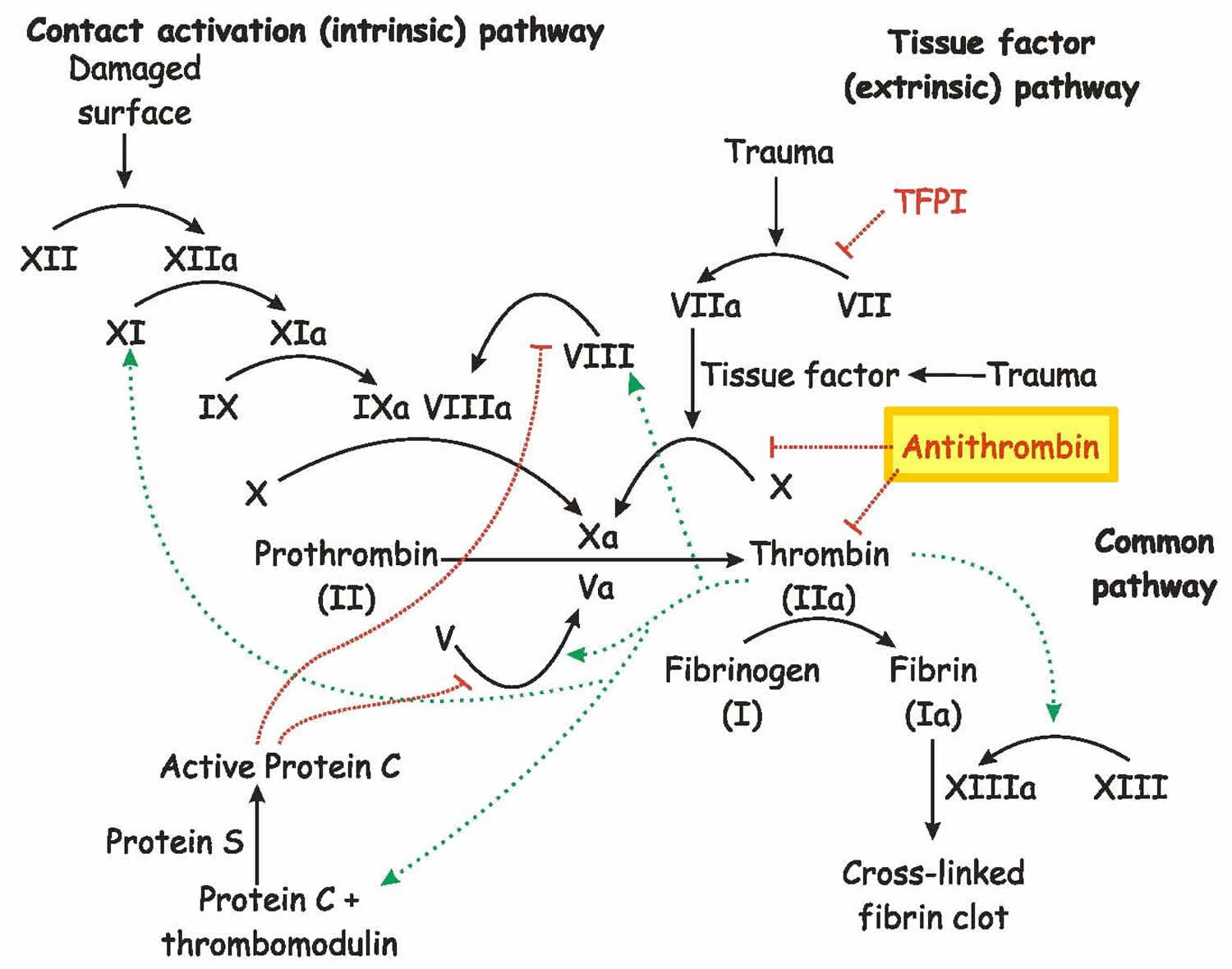
Antithrombin also called antithrombin III, antithrombin 3 or heparin cofactor 1, is a naturally-occurring mild blood thinner protein produced by the liver that helps regulate blood clot formation (coagulation) and antithrombin III is the primary inhibitor of thrombin (factors IIa) including factors IXa, Xa, and XIIa, which is required for the development of blood clots (see Figures 1 and 2) 1, 2, 3, 4, 5, 6, 7, 8, 6, 9, 10, 11, 12. Antithrombin 3 is a protein in the blood, part of a family of serine protease inhibitors known as serpins, that limits the blood’s ability to clot (coagulation) and blocks abnormal blood clots from forming 13, 14, 15. Antithrombin 3 binds to heparin on endothelial cells and forms a complex with thrombin (thrombin-antithrombin complex) thus inhibiting coagulation (see Figure 1). Antithrombin 3 helps the body keep a healthy balance between bleeding and clotting. Normally, when a blood vessel is injured, the body initiates a complex process called hemostasis to form a blood clot and prevent further blood loss. Part of this complex process involves the activation of several proteins called coagulation factors in a series of steps referred to as the coagulation cascade. Antithrombin helps to regulate this process by inhibiting the action of several activated coagulation factors, including thrombin, which is required for the development of blood clots and two clotting factors Xa and IXa, and XIa, that are required for the generation of thrombin, to slow down the process and prevent excessive or inappropriate clotting (thrombosis) (Figure 1).
If antithrombin levels are too high, a person could, theoretically, have a bleeding tendency. However, elevated levels of antithrombin do not appear to cause bleeding or have any clinical significance.
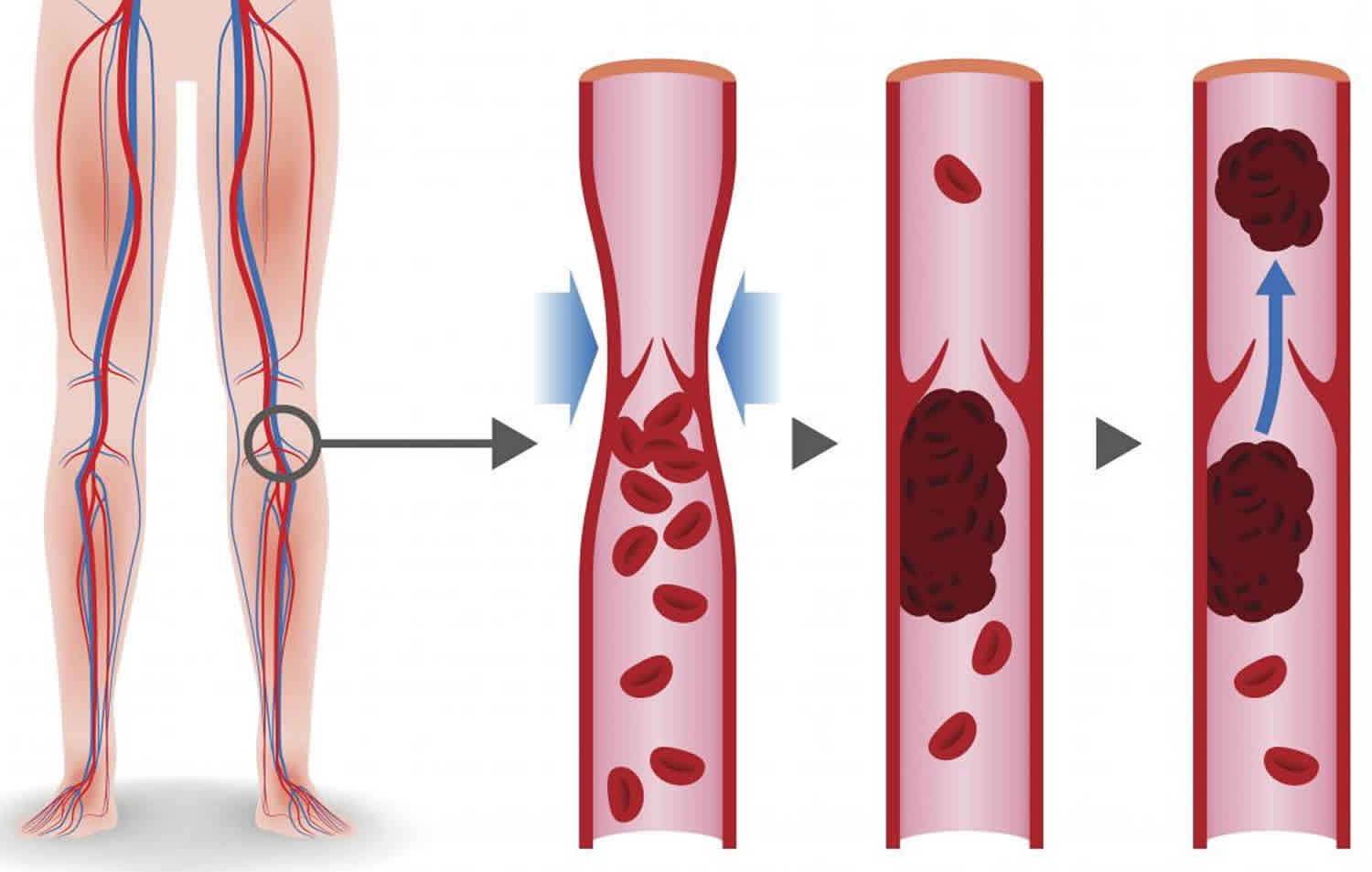
Antithrombin is synthesized in the liver but is not vitamin K-dependent. People with an excessive clotting disorder due to an inherited or acquired antithrombin deficiency are at increased risk of developing blood clots, especially in deep veins such as in the legs known as deep venous thrombosis or DVT. Inherited deficiencies are rare, affecting about 1 in 5,000 people. For people who inherit one defective gene and one normal gene (heterozygous), episodes of inappropriate blood clot formation typically start at about 20 to 30 years of age. Very rarely, a person may inherit two defective antithrombin genes, resulting in severe clotting problems soon after birth.
Acquired antithrombin deficiencies may occur at any age. They are associated with a variety of conditions that cause decreased production, excessive consumption, or loss of antithrombin. These conditions include liver disease, extensive thrombosis, disseminated intravascular coagulation (DIC), blood loss, cancer, and nephrotic syndrome – a form of kidney disease.
There are two types of antithrombin deficiency. With antithrombin deficiency type 1, antithrombin functions normally, but the quantity is insufficient. With antithrombin deficiency type 2, there is a sufficient quantity of antithrombin produced, but it is dysfunctional. These types can be differentiated and assessed by testing:
- Antithrombin activity, which evaluates the function of antithrombin
- Antithrombin antigen, which measures the quantity of antithrombin present
Close consultation with a hematologist is necessary; consultation with a geneticist should be considered as needed. The support of a laboratory equipped to assay antithrombin activity is necessary for patients receiving antithrombin replacement therapy.
In patients with known inherited antithrombin deficiency, management of an acute thrombotic event can be challenging, as these patients may exhibit a variable response to even large doses of heparin. When a therapeutic response to intravenous heparin is not achievable, additional support with an antithrombin concentrate may be necessary 16 Currently, however, direct thrombin inhibitors (eg, argatroban, dabigatran) are recommended. These agents do not require antithrombin for their actions. Anticoagulation can be achieved more easily and without the use of exogenous blood products.
For planned major surgery, correction of antithrombin levels using antithrombin concentrate products is recommended in patients with known antithrombin deficiency. In acute severe trauma, some studies also suggest a beneficial effect with antithrombin replacement.
In contrast to antithrombin concentrates, fresh frozen plasma (FFP) does not have a sufficient concentration of antithrombin to provide adequate replacement in patients who are significantly deficient, so fresh frozen plasma (FFP) should not be used if alternatives are available. The goal of correction should be antithrombin activity of 80% or greater to achieve physiologic function.
Figure 1. Blood clotting pathway
Figure 2. Antithrombin sites of action
Antithrombin 3 deficiency
Antithrombin deficiency is a blood disorder characterized by the tendency to form clots in the veins (thrombosis). There are 2 major causes of antithrombin 3 deficiency: (a) an inherited antithrombin 3 deficiency due to a genetic abnormality (mutation) called hereditary antithrombin deficiency, and (b) an acquired antithrombin 3 deficiency due to some other disease.
In people with inherited antithrombin 3 deficiency or hereditary antithrombin deficiency, there is a reduced amount of antithrombin in the blood due to a genetic abnormality. Antithrombin deficiency may also be acquired or acquired antithrombin 3 deficiency; in such cases, the disorder may be reversible with resoluton/improvement in the disease process responsible for the deficiency.
Acquired antithrombin 3 deficiency
Acquired antithrombin 3 deficiency can occur as a consequence of reduced synthesis of antithrombin (liver damage, acute liver failure, cirrhosis, malnutrition) or increased loss of antithrombin (nephrotic syndrome, enteropathy) or increase in antithrombin consumption through consumption coagulopathies, including disseminated intravascular coagulation syndrome (DIC) due to a severe infection of the blood stream, sepsis, burn, trauma, microangiopathies with thrombosis, malignancies, hematologic transfusion reactions and cardiopulmonary bypass surgery 17, 18.
Levels of antithrombin less than 50 to 60% in sepsis generally have worsening prognostic outcomes, and levels less than 20% correlate with fatal outcomes 18. The loss of antithrombin in sepsis is in part due to the increase in plasma turnover, and the downregulation of antithrombin production 18. The degree of antithrombin deficiency also correlates with the severity of the illness.
Acquired Antithrombin Deficiency
Acquired antithrombin deficiency is not uncommon. Low levels of antithrombin can be found in patients with the conditions listed below. Typically, acquired antithrombin deficiency does not lead to an increased risk of blood clots. This is because in these conditions clotting factors other than antithrombin are frequently also lowered.
Causes of acquired antithrombin deficiency
- Liver failure (such as liver cirrhosis)
- Nephrotic syndrome (a kidney disorder)
- Widespread (metastatic) tumors
- Acute blood clots
- Heparin therapy
- DIC (disseminated intravascular coagulation) – a generalized clotting and bleeding disorder that is often associated with infection in the blood stream (sepsis)
- Severe trauma
- Severe burns
- chemotherapy with Asparaginase
Inherited antithrombin 3 deficiency
Inherited antithrombin 3 deficiency or hereditary antithrombin deficiency is genetic disorder that causes the blood to clot more than normal. Hereditary antithrombin deficiency or Inherited antithrombin 3 deficiency is caused by changes (mutations) in the SERPINC1 gene and many different mutations in this gene are responsible for individual cases of antithrombin deficiency 19. The prevalence inherited antithrombin 3 deficiency (hereditary antithrombin deficiency) is estimated to occur in about 1 in 2,000 to 5,000 individuals 20, 15. Of people who have experienced an abnormal blood clot such as venous thrombosis, about 1 in 20 to 200 have hereditary antithrombin deficiency 18. Therefore, there may be between 60,000 and 600,000 people with hereditary antithrombin 3 deficiency in the U.S.
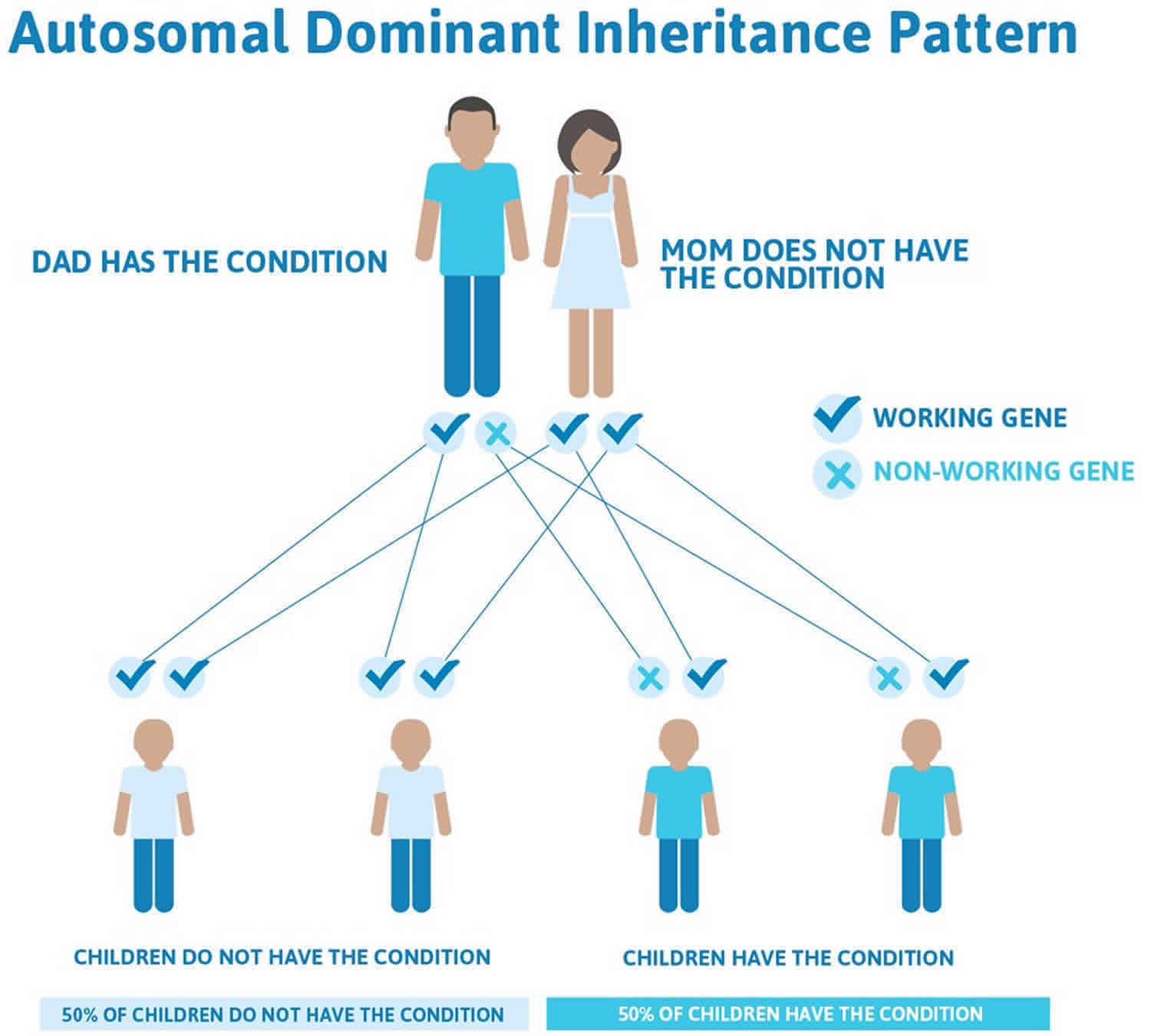
Hereditary antithrombin deficiency is inherited as an autosomal dominant condition. Dominant genetic disorders occur when only a single copy of an altered gene is necessary for the appearance of the disease. Heterozygote is the term used to describe such a person. However, not every person who has the altered gene will develop a blood clot. Geneticists call this variable clinical penetrance. Thus, antithrombin deficiency is an autosomal dominant disorder with variable clinical penetrance. The altered gene can be inherited from either parent, or very rarely can be the result of a new mutation in the affected individual. The risk of passing the altered gene from affected parent to offspring is 50% for each pregnancy. The risk is the same for males and females.
Hereditary antithrombin deficiency is categorize into Type 1 or Type 2. Type 1 Antithrombin Deficiency results in a complete deficiency of antithrombin gene products if in a homozygous state (having inherited two altered genes, one from each of the parents). Homozygous babies with antithrombin deficiency seldom survive though there are rare cases with so-called type IIB mutations. A heterozygous genotype (having inherited only one of the altered gene) results in approximately 50% of functional antithrombin activity. Type 2 Antithrombin Deficiency characteristically demonstrate the production of altered antithrombin protein, which results in the loss of its function 18. There is a decrease in overall antithrombin activity, but the reduction of antithrombin antigen is less likely. The location in which the protein undergoes alteration can affect the reactive site, heparin-binding domain, or both. The lack of antithrombin activity or production most commonly presents as a deep vein thrombosis. However, there is an increased risk of recurring unprovoked thrombosis in unusual sites such as the cerebral or mesenteric veins 18.
People with antithrombin 3 deficiency (hereditary antithrombin deficiency) are at higher than average risk for developing abnormal blood clots, particularly a type of clot that occurs in the deep veins of the legs. This type of clot is called a deep vein thrombosis (DVT). Affected individuals also have an increased risk of developing a pulmonary embolism (PE), which is a clot that travels through the bloodstream and lodges in the lungs. In hereditary antithrombin deficiency, abnormal blood clots usually form only in veins, although they may rarely occur in arteries.
About half of people with antithrombin 3 deficiency (hereditary antithrombin deficiency) will develop at least one abnormal blood clot during their lifetime and carries the highest risk for thrombotic events among the inherited thrombophilias. These clots usually develop after adolescence, peaking around ages 15 to 40. People with hereditary antithrombin deficiency are also likely to have family members who have had a blood clotting problem.
Other factors can increase the risk of abnormal blood clots in people with hereditary antithrombin deficiency. These factors include increasing age, surgery, or immobility. The combination of hereditary antithrombin deficiency and other inherited disorders of blood clotting can also influence risk. Women with hereditary antithrombin deficiency are at increased risk of developing an abnormal blood clot during pregnancy or soon after delivery. They also may have an increased risk for pregnancy loss (miscarriage) or stillbirth.
Hereditary antithrombin deficiency is inherited in a dominant pattern, i.e. there is a 50 % chance that a child will have the disorder if one of the parents has it (see Figure 3 below). Men and women are equally affected. It is independent of blood types. If a person has inherited one defective (mutated) antithrombin gene, he/she is heterozygous. If an individual has inherited 2 defective (mutated) genes, he/she is homozygous. Homozygous individuals rarely survive and the fetus usually dies before birth; a miscarriage results.
Initial treatment for thrombosis in these patients is heparin, and maintenance treatment is generally ongoing with an oral anticoagulant 7. For asymptomatic incidences, primary prophylaxis is currently not recommended due to the increased risk of fatal hemorrhage on long-term anticoagulation versus the lesser risk of fatal venous thromboembolism 18
Type 1 Antithrombin Deficiency
If an individual does not produce enough antithrombin, antigen and activity levels are both low. This is called type 1 deficiency, or a quantitative deficiency. It is either due to an inherited gene defect, or due to an acquired problem, where less antithrombin is made in the liver (such as in liver cirrhosis) or antithrombin is lost in the urine (as may happen in certain kidney diseases). The acquired form of antithrombin deficiency is more prevalent than the congenital form of the disorder.
Type 1 antithrombin deficiency is the most common subtype and is thought to occur in about one in every 3,000 to 5,000 people in the United States and is not limited to any particular ethnic group. It is estimated that approximately 1 percent of people who have venous thrombosis and embolism have congenital antithrombin deficiency.
Type 2 Antithrombin Deficiency
Type IIb deficiency may be the least severe of all antithrombin deficiencies. Some people produce normal amounts of the antithrombin protein, yet the protein has an abnormal structure and, therefore, does not work right. This is called type 2 deficiency. It is due to an inherited defect (mutation). In this type of deficiency, the antigen level is normal, but the activity level is low. A normal antithrombin antigen level, therefore, never fully rules out an antithrombin deficiency. Thus, to fully rule out antithrombin deficiency, one always needs to obtain an antithrombin activity level. The antithrombin activity level is the best initial test to obtain if one suspects that a patient may have antithrombin deficiency. The type 2 defects are further subclassified into type IIa, IIb, and IIc defects, based on the location of the defect in the antithrombin molecule. The conventional wisdom is that it does not matter for clinical management purposes what type of deficiency a person with antithrombin deficiency has. However, there is a subtype of type II deficiency, called type IIb, which is the most common type of antithrombin deficiency. It is caused by an abnormality in the heparin-binding region of the antithrombin molecule. Interestingly, it is associated with only a very low risk for blood clots. Most clinicians do not order specialized testing to clarify exactly what type of antithrombin deficiency a patient has, but it may be important. An individual who has been found to have antithrombin deficiency, but has never had a clot, may want to know whether he/she has type IIb deficiency, or one of the other types. If he/she has type IIb, he/she would have to worry less about the future risk for blood clots.
Antithrombin 3 deficiency causes
Acquired antithrombin 3 deficiency can occur as a consequence of reduced synthesis caused by liver damage or increased loss due to nephrotic syndrome, enteropathy, disseminated intravascular coagulation (DIC) due to a severe infection of the blood stream, sepsis, burn, trauma, microangiopathy, and cardiopulmonary bypass surgery 17. Qualitative defects of antithrombin 3 deficiency (type 2 deficiency) describe mutations which either affect the heparin-binding site, the reactive site or result in pleiotropic effects. Homozygous antithrombin 3 deficiency is incompatible with life unless affecting the heparin-binding site 21. Usually these patients present with venous thrombosis and less likely with arterial thrombosis.
Inherited antithrombin 3 deficiency or hereditary antithrombin deficiency is caused by mutations in the SERPINC1 gene. This gene provides instructions for producing a protein called antithrombin. This protein is found in the bloodstream and is important for controlling blood clotting. Antithrombin blocks the activity of proteins that promote blood clotting, especially a protein called thrombin.
Most of the mutations that cause hereditary antithrombin deficiency change single protein building blocks (amino acids) in antithrombin, which disrupts its ability to control blood clotting. Individuals with this condition do not have enough functional antithrombin to inactivate clotting proteins, which results in the increased risk of developing abnormal blood clots.
Hereditary antithrombin deficiency inheritance pattern
Hereditary antithrombin deficiency is typically inherited in an autosomal dominant pattern, which means one altered copy of the SERPINC1 gene in each cell is sufficient to cause the disorder. Inheriting two altered copies of this gene in each cell is usually incompatible with life; however, a few severely affected individuals have been reported with mutations in both copies of the SERPINC1 gene in each cell.
In cases where the autosomal dominant condition does run in the family, the chance for an affected person to have a child with the same condition is 50% regardless of whether it is a boy or a girl. These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
- When one parent has the abnormal gene, they will pass on either their normal gene or their abnormal gene to their child. Each of their children therefore has a 50% (1 in 2) chance of inheriting the changed gene and being affected by the condition.
- There is also a 50% (1 in 2) chance that a child will inherit the normal copy of the gene. If this happens the child will not be affected by the disorder and cannot pass it on to any of his or her children.
Figure 3. Hereditary antithrombin deficiency autosomal dominant inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Antithrombin 3 deficiency symptoms
People with antithrombin deficiency are at risk of developing a blood clot (thrombus) within a vein (thrombosis). The first episode of thrombosis typically occurs before the age of 40 years. A thrombus is a clump of blood cells (i.e., platelets, clotting factors, fibrin, etc.) that may become attached (adhere) to the interior wall of a blood vessel, usually a deep vein in the leg. This may be brought on by surgery, pregnancy, childbirth, trauma, or use of oral contraceptives. About 40 percent of people with antithrombin deficiency develop a thrombus that pulls away from the wall of a vein in the legs or pelvis (deep vein thrombosis or DVT) and travels through the blood stream to the lungs (pulmonary embolism or PE). Pulmonary emboli are dangerous and hence DVT (deep vein thrombosis) and pulmonary embolism must be treated quickly. Thrombi also occur the superficial veins in the legs (superficial thrombophlebitis). Thrombi may also occur in the veins in the abdomen (mesenteric, portal, hepatic or splenic veins) or around the brain (sinus veins). Clots in the arteries of the heart may lead to heart attack (myocardial infarction) and clots in the arteries of the brain to stroke. However, arterial clots are rare in antithrombin deficiency.
People will usually have symptoms of a blood clot. The most common symptoms of a DVT (deep vein thrombosis) in the arms or legs usually cause swelling, warmth, redness, and pain of the affected arms or legs. When a blood clot breaks off from where it formed and travels to another part of the body, it is called a thromboembolism. Symptoms depend on where the blood clot travels to. A common place is the lung (pulmonary embolism), where the clot can cause a cough, shortness of breath, pain while taking deep breaths (so-called pleuritic chest pain), chest pain, patients can become dizzy, pass out (syncope), or go into shock and in more severe cases death. Blood clots that travel to the brain can cause a stroke.
If a person with an antithrombin deficiency also has other coagulation risks, such as a protein C or S deficiency, a factor V leiden mutation, or oral contraceptive use, then the person may be at a significantly elevated risk of developing a blood clot.
There are several reports in the medical literature of newborn children with antithrombin deficiency who develop blood clots. This occurs rarely however, and may be due to the protective effect of higher levels of a secondary plasma inhibitor of thrombin called alpha-2 macroglobulin.
Antithrombin deficiency can increase the risk of recurrent miscarriage.
People with antithrombin deficiency may need preventive anticoagulation therapy prior to medical or surgical procedures.
Antithrombin concentrates and recombinant antithrombin are now available as a medication to temporarily correct acute or chronic antithrombin deficiencies.
Antithrombin deficiency prevention
Individuals with antithrombin deficiency who experience an unprovoked acute thromboembolic event are candidates for lifelong administration of an oral anticoagulant 22. Discontinuation of oral anticoagulants should be undertaken with great caution and only for essential procedures because of the risk of recurrent thromboembolic events 22. Replacement with antithrombin concentrate may be needed during such times 22.
Patients with known antithrombin deficiency may be considered candidates for antithrombotic prophylaxis during high-risk situations such as surgery and pregnancy.
Nishimura and Takagi 23 reported five patients with antithrombin deficiency who underwent cardiovascular surgery and were administered antithrombin concentrate without postoperative complications such as hemorrhage or thrombosis. The authors concluded that in patients with antithrombin deficiency undergoing cardiac surgery, it is important to perform antithrombin replacement to achieve preoperative antithrombin activity ≥120% and postoperative antithrombin activity ≥80%, while the activated clotting time is maintained at >400 seconds during cardiopulmonary bypass 23.
In a study of 21 women with inherited antithrombin deficiency who received recombinant human antithrombin (rhantithrombin) therapy up to 24 hours before scheduled induction or cesarean delivery, or at the onset of labor, there were no reported cases of venous thromboembolism (VTE) within 7 days of dosing. However, two venous thromboembolism events (one deep vein thrombosis and one pulmonary embolism) occurred 11 and 14 days after discontinuation of rhantithrombin, in patients managed with prophylactic doses of heparin or low-molecular-weight heparin following delivery 24.
Rivaroxaban, a direct oral factor Xa inhibitor, has been shown to be non-inferior to existing treatments (such as warfarin or low-molecular-weight heparin) for preventing the recurrence of symptomatic DVT and pulmonary embolism (PE). Successful perioperative use of rivaroxaban for prevention of thromboembolism in patients with antithrombin deficiency has been reported 25, 26.
Antithrombin 3 deficiency diagnosis
A physical exam may show:
- A swollen leg or arm
- Decreased breath sounds in the lungs
- A rapid heart rate
Your health care provider can also order a blood test to check if you have a low level of antithrombin 3. The best test to determine whether a patient has antithrombin deficiency is a blood test called “antithrombin activity” or “functional antithrombin”. Any physician’s office can order the antithrombin tests and many laboratories can perform them. Two different antithrombin tests can be done, (a) an antithrombin antigen level and (b) an antithrombin activity level (also called “functional test”). The antithrombin antigen test determines how much of the protein is present in the blood. The antithrombin activity test determines whether the antithrombin that is present actually works. There are 2 main types of antithrombin deficiency (antithrombin deficiency), depending on which of these two tests results is low. They are referred to as “type 1” and “type 2” deficiency.
Antithrombin test
Antithrombin testing to evaluate the activity (function) and the amount (quantity) of antithrombin and helps identify antithrombin deficiency. Activity and antigen levels are typically expressed in “percent.” Normal ranges differ from lab to lab, but typically are approximately 80% – 120 %. Healthy newborns have only half the antithrombin levels of adults, but gradually reach the adult levels by 6 months of age. This is important to keep in mind when interpreting the tests of newborn children. Being on birth control pills, hormone replacement therapy, or being pregnant does not change antithrombin test results significantly and results are, thus, reliable. However, being on warfarin (coumadin®, Jantoven®) can increase antithrombin levels; therefore, a normal level while a person is on warfarin does not absolutely rule out the presence of antithrombin deficiency. Once a patient is off warfarin the antithrombin activity test should be repeated. There are many different mutations in the antithrombin gene that can lead to inherited antithrombin deficiency. Genetic testing is, therefore, not possible in routine clinical practice. It is reserved for research studies. Patients with inherited antithrombin deficiency typically have levels in the 40% – 60 % range. It is not well established whether the degree of antithrombin decrease correlates with the risk of thrombosis, i.e. whether patients with lower values have a higher risk for thrombosis.
The antithrombin activity test is performed first, to evaluate whether the total amount of functional antithrombin is normal. If the antithrombin activity is low, then the antithrombin antigen test is performed to determine the quantity of antithrombin present. These two tests can be used to differentiate between type 1 and type 2 antithrombin deficiencies. If a deficiency is detected, both antithrombin tests are typically repeated at a later date to confirm test findings.
Antithrombin testing may sometimes be used to evaluate people who are not responding as expected to heparin. Heparin is an anticoagulant drug that is given to people who have a blood clot or are at an increased risk of forming inappropriate blood clots. The effects of heparin are mediated by antithrombin. Heparin can greatly increase antithrombin activity, thus inhibiting formation of blood clots, but those who are antithrombin-deficient are resistant to heparin treatment.
Decreased antithrombin activity and decreased quantities of antithrombin antigen suggest a type 1 antithrombin deficiency. In this case, the activity is decreased because there is less antithrombin available to participate in clotting regulation.
Reduced antithrombin activity and normal levels of antithrombin antigen suggest a type 2 antithrombin deficiency. This means that there is sufficient antithrombin protein, but it is not functioning as it should. In either case, a deficiency increases the affected person’s risk of developing an inappropriate blood clot.
If the antithrombin activity is normal, then the antithrombin antigen test is usually not performed. In this case, the antithrombin is functioning adequately and the recurrent thrombotic episodes being investigated are likely due to a cause other than an antithrombin deficiency.
Temporarily or chronically decreased antithrombin levels may be seen with conditions that affect its consumption or production, such as:
- DIC (disseminated intravascular coagulation), an acute or chronic condition characterized by the consumption of clotting factors; an affected person may bleed and/or clot.
- DVT (deep vein thrombosis – a blood clot usually in a deep leg vein)
- Liver disease
- Nephrotic syndrome
- Protein-losing condition
- Pulmonary embolism
- Heparin therapy (temporary lower antithrombin level)
- Infants during the first few days of life (about 50% of healthy adult level)
- Estrogen therapy
Increased levels of antithrombin are not usually considered a problem.
Antithrombin 3 deficiency treatment
If you have an acquired antithrombin 3 deficiency that is related to an underlying condition, such as liver disease or nephrotic syndrome, then treating the condition may ease or eliminate the antithrombin 3 deficiency. If needed, your healthcare provider may treat you with antithrombin (as a medication) to temporarily correct the deficiency.
A blood clot is treated with blood-thinning medicines (also called anticoagulants). How long you need to take these drugs depends on how serious the blood clot was and other factors. Many physicians will recommend that an individual with true antithrombin deficiency who has had a blood clot should be on indefinite warfarin (Coumadin®, Jantoven®) therapy. If a person has antithrombin deficiency but has never had a blood clot, it is difficult to decide whether to start long-term warfarin therapy or not. In this case, other factors need to be considered: does the person have additional risk factors for blood clots, such as obesity, smoking, a sedentary lifestyle, presence of an additional clotting disorder or a family history of blood clots. Also, the degree of antithrombin deficiency should be factored in, as should the type of deficiency. Clearly, an individual decision needs to be made whether the person should be on long-term blood thinners or not. Discuss this with your doctor.
Due to a lack of clinical studies, hematologists differ in their opinions regarding the treatment of antithrombin deficiency. Often, intravenous antithrombin concentrates are prescribed when surgery or infant delivery is close at hand. Antithrombin concentrates are also used to prevent venous clots when blood thinners (such as heparin) are not advisable because they may lead to an increased risk of bleeding. This is especially true for neuro-surgery and in severe trauma or at the time of delivery. It is not well established which individuals with antithrombin deficiency need to receive concentrates and which ones not. No guidelines exist as to which patients with antithrombin deficiency should receive antithrombin concentrate. Typically, treatment is given only (a) at times of increased risk for clotting, or (b) when the blood thinner heparin cannot be safely given because it would lead to an increased risk for bleeding. These situations are major surgery, major trauma, and delivery. Two different concentrates of antithrombin concentrate are available in the US. Antithrombin concentrate (Thrombate) is a highly purified and viral-safe product prepared from pooled normal human plasma. Its half-life in the circulation is approximately 2.8 to 4.8 days. A recombinant human antithrombin (Atryn), produced from the milk of transgenic goats, is also available. This product is only approved for use in high-risk situations (eg, surgery, childbirth) in patients with antithrombin deficiency.
For people with very low antithrombin levels, heparin may not work well if administered alone. This is called heparin resistance. In order for heparin to work properly an adequate amount of antithrombin must be present in the blood. If heparin treatment is ineffective, then antithrombin concentrate may be prescribed.
Women with antithrombin deficiency are at particularly high risk for developing clots during pregnancy or after delivery. Reports of the incidence of blot clots during pregnancy in women with antithrombin deficiency range from 3% to 50%. Many recommend the use of subcutaneous low molecular weight heparin injections during pregnancy for women with antithrombin deficiency.
Pregnant women with antithrombin deficiency are at slightly increased risk of losing the fetus without treatment. Pregnancy loss is likely due to blood clots forming in the placenta and cutting off the blood supply and oxygen to the fetus.
As noted above, patients with antithrombin deficiency who undergo surgery are at increased risk of a thromboembolic event unless appropriate preventive measures are taken. The duration of treatment with blood thinners or antithrombin concentrate depends on the type of surgery. In some cases, treatment will last only a few days while in other instances treatment may last for several weeks.
A family in which one or more members have antithrombin deficiency should consult with a hematologist and genetic counselor, who can help the family understand and cope with the disorder.
Sources of antithrombin for use in therapy
- a) Human plasma-derived antithrombin (Thrombate III®) in the U.S.; by Talecris Biopharmaceuticals; several other products in the rest of the world. This is a highly purified, human, blood-derived antithrombin protein concentrate. It is prepared from the blood of tens of thousands of donors, similar to the preparation of clotting factor VIII for hemophilia patients. The blood of each individual donor is screened for hepatitis, HIV, and other viruses, such as parvovirus B-19. The part of the blood called plasma is then highly purified, resulting in an antithrombin concentrate. Any potentially contaminating viruses are inactivated and killed using one or more different methods, such as heat inactivation or special filtration techniques.
- b) Antithrombin is also being produced by recombinant genetic technology (antithrombinryn®; by Lundbeck Inc.). In this technique, the human antithrombin gene has been inserted into the living cells of goats, so that they produce high concentrations of human antithrombin in their milk. The milk is then purified and the antithrombin concentrated. Goats are bred and kept on a security-protected specialized goat farm and are regularly monitored by veterinarians for their health. Similarly, the antithrombin derived from their milk is tested regularly for its safety. antithrombinryn® was FDA approved in the U.S. in March 2009 and is available for clinical use.
Antithrombin 3 Deficiency in Children
Parents of children with antithrombin deficiency need to be aware of the symptoms of blood clots. Blood clots are uncommon in children with antithrombin deficiency, probably because another naturally occurring blood thinner (α2-macroglobulin) is higher during the first two decades of life, protecting most children from blood clots. However, there have been several reports of clots occurring in newborns with antithrombin deficiency. Discussion between the expectant parents, of whom one parent has antithrombin deficiency, and the hematologist (“blood doctor”) and perinatologist (a physician who deals with complications or high risk before or after birth) should be held prior to delivery. Parents need to be aware of the symptoms of blood clots, should they occur in their baby.
Most newborn infants with antithrombin deficiency do not need preventive treatment with heparin or antithrombin concentrate, but may benefit from particularly careful attention to hydration and their kidney and circulatory function. Most children with antithrombin deficiency do not develop blood clots unless there is an additional triggering event, such as surgery, trauma, a catheter, or severe infection. Children known to have antithrombin deficiency may receive preventive therapy with blood thinners around trigger events.
Children with underlying medical conditions that cause acquired antithrombin deficiency, such as nephrotic syndrome (a kidney disorder), protein losing enteropathy (an intestinal disorder) and L-asparaginase chemotherapy for leukemia, have an increased risk of thrombosis. Although it is not clear how much of the thrombotic risk is actually caused by antithrombin deficiency, children who develop clots with such acquired antithrombin deficiency may benefit from antithrombin concentrate to treat the acute clot, and may benefit from blood thinning therapies (including antithrombin) to prevent further blood clots.
Antithrombin 3 Deficiency and Pregnancy
Women with antithrombin deficiency are at particularly high risk for developing clots during pregnancy and after delivery. The exact risk of developing blood clots during pregnancy is impossible to determine accurately. One study showed that only 3 % of pregnancies will be complicated by a blood clot if no concomitant prophylactic blood thinners are given. However, other studies have shown that blood clots occur in up to 50 % of pregnancies. Treatment with heparin injections underneath the skin (“subcutaneously”) during pregnancy should strongly be considered to prevent blood clots. However, no well designed clinical studies exist that allow strong recommendations as to how exactly to treat pregnant women (dose of heparin; treatment with antithrombin concentrate, etc.). Some physicians recommend antithrombin replacement therapy during delivery, when heparin may be contraindicated, since heparin might lead to an increased risk of bleeding. antithrombin concentrate may also be given for a few days after delivery, together with heparin. Warfarin is not used during pregnancy because it may cause birth defects. However, for 6-12 weeks postpartum, warfarin should be considered, because there is a high risk for blood clots in the post-delivery period. A summary of 45 cases of pregnancy in women with antithrombin deficiency with detailed information concerning prophylactic therapy with heparin and/or antithrombin has recently been published. However, no treatment guidelines can be derived from that publication, since many different regimens were used.
Women with antithrombin deficiency also have an increased risk for pregnancy loss, either early (miscarriage) or late (stillbirth) in the pregnancy. This is probably due to blood clots forming in the placenta, leading to blockage of blood flow and oxygen delivery to the fetus. Approximately 1 of 6 pregnancies in women with antithrombin deficiency (17 %) will end with an early fetal loss, and 1 in 40 pregnancies (2.3 %) will end with a stillbirth if no blood thinners are given. Therapy with heparin with or without antithrombin throughout the pregnancy likely decreases that risk.
Guidelines for the prevention of venous thromboembolism during pregnancy and postpartum
Guidelines for the prevention of venous thromboembolism (VTE) during pregnancy and postpartum have been issued by the following organizations:
- American College of Chest Physicians (ACCP)
- American College of Obstetricians and Gynecologists (ACOG)
- Royal College of Obstetricians and Gynaecologists (RCOG)
American College of Obstetricians and Gynecologists (ACOG) recommends testing for inherited thrombophilia in all pregnant women with a history of thrombosis 27. The lack of a strong and consistent evidence base has lead to differing recommendations for women with antithrombin deficiency. For prevention of first venous thromboembolism (VTE), the recommendations are as follows:
- American College of Chest Physicians (ACCP) – Antepartum clinical surveillance. Continue clinical surveillance postpartum if there is no family history of venous thromboembolism (VTE); if there is a family history of VTE, 6 weeks prophylactic or intermediate dose LMWH or vitamin K antagonists targeted at INR 2.0–3.0 28.
- American College of Obstetricians and Gynecologists (ACOG) – Antepartum prophylaxis with low molecular weight heparin (LMWH) or unfractionated heparin (UFH) followed by 6 weeks postpartum anticoagulation 27.
- Royal College of Obstetricians and Gynaecologists (RCOG) – Consider prophylactic-dose LMWH in the presence of more than one thrombophilic defect or other risk factors 29.
Recommendations for the prevention of recurrent venous thromboembolism (VTE) are as follows:
- American College of Chest Physicians (ACCP) – Antepartum and 6 weeks postpartum prophylactic or intermediate dose low molecular weight heparin (LMWH) 28.
- American College of Obstetricians and Gynecologists (ACOG) – Antepartum and 6 weeks postpartum prophylactic or intermediate dose of LMWH or UFH 27.
- Royal College of Obstetricians and Gynaecologists (RCOG) – Consultation with a hematologist and antepartum anti-factor Xa monitoring and higher dose (50-100%) LMWH followed by postpartum higher dose LMWH for 6 weeks or until long-term anticoagulation is started 29.
Antithrombin 3 Deficiency and Surgery or Trauma
Individuals with antithrombin deficiency need very good DVT prophylaxis with blood thinners at times of surgery or major trauma; treatment with antithrombin concentrate during these times can also be considered. Major surgery and trauma are risk factors for blood clots (deep vein thrombosis or pulmonary embolism) in anybody; but they are an even greater risk for the person with antithrombin deficiency. Extra attention to DVT prophylaxis is therefore indicated, typically with one of the heparin drugs. If trauma or excessive risk for bleeding (for example neurosurgery) does not allow physicians to give the blood thinner, antithrombin concentrate is indicated; also, placement of a removable filter into the inferior vena cava, the big vein of the abdomen, may be considered. Such a filter can capture blood clots that have formed in the leg and are traveling upstream on their way to the lung. They can, thus, prevent life-threatening pulmonary embolism. Antithrombin concentrate may be given for the first few days after surgery. Depending on the type and extent of the surgery, prolonged use of blood thinners for several weeks after surgery may be appropriate.
Antithrombin deficiency prognosis
Patients who are heterozygous for type 1 or 2 antithrombin deficiency develop significant thromboembolic complications, generally involving the deep veins. The lifetime risk of developing venous thromboembolism (venous thrombosis) depends on the subtype of antithrombin deficiency. In patients with type 1 inherited antithrombin deficiency, the risk of thrombosis is estimated to be 1% per year, starting at age 15 years 30. The overall lifetime risk of developing a thrombotic event in patients with type 1 inherited antithrombin deficiency is estimated to range from 50% to 85% 30.
In patients with type 2 antithrombin deficiency, the risk of developing venous thrombosis is higher in those patients who have reactive site defects as compared to heparin-binding site defects. The estimated lifetime risk of thrombosis in type 2 mutations has been reported to range from 6 to 20%, depending on the mutation site 18, 31.
Patients may develop recurrent venous thrombosis at an early age and, if the condition is unrecognized or inadequately treated, they may die from such events. Long-term consequences, such as chronic leg ulcerations, severe venous varicosities, and postphlebitic syndrome, are common from repeated episodes of venous thrombosis, which cause significant morbidity. The prognosis of patients with reductions in AT as part of other systemic disorders depends on the underlying disorder.
The frequency of arterial thrombotic complications is low in patients with antithrombin deficiency 30. However, mutations leading to arterial thromboses have been described 30.
The incidence of pregnancy-related venous thrombosis in women with antithrombin deficiency in early reports may have overestimated due to methodologic limitations such as selection bias in family studies. Subsequent studies have suggested a much lower level with ranges between 0.08-15.8% for risk of initial venous thrombosis during 32. However, a family history of venous thrombosis significantly increases risk during pregnancy. venous thrombosis is the leading cause of direct maternal death and thrombotic complications during embryogenesis can lead to a variety of developmental abnormalities 33.
The risk for venous thrombosis is also increased in women taking oral contraceptives (1.2-4.4%) or hormone replacement therapy (2.5-5.1%) 32.
Nephrotic syndrome has been associated with reductions in antithrombin and an increased incidence of venous thrombosis (renal vein, 60%; venous thrombosis, 40%) with only a 3% incidence of arterial thrombosis 30.
References- Olds RJ, Lane DA, Mille B, Chowdhury V, Thein SL. Antithrombin: the principal inhibitor of thrombin. Semin Thromb Hemost. 1994;20(4):353-72. doi: 10.1055/s-2007-1001927
- Rosenberg JS, McKenna PW, Rosenberg RD. Inhibition of human factor IXa by human antithrombin. J Biol Chem. 1975 Dec 10;250(23):8883-8
- Stead N, Kaplan AP, Rosenberg RD. Inhibition of activated factor XII by antithrombin-heparin cofactor. J Biol Chem. 1976 Nov 10;251(21):6481-8.
- Rao LV, Nordfang O, Hoang AD, Pendurthi UR. Mechanism of antithrombin III inhibition of factor VIIa/tissue factor activity on cell surfaces. Comparison with tissue factor pathway inhibitor/factor Xa-induced inhibition of factor VIIa/tissue factor activity. Blood. 1995 Jan 1;85(1):121-9.
- Allingstrup M, Wetterslev J, Ravn FB, Møller AM, Afshari A. Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2016 Apr;42(4):505-520. doi: 10.1007/s00134-016-4225-7
- Olson ST, Richard B, Izaguirre G, Schedin-Weiss S, Gettins PG. Molecular mechanisms of antithrombin-heparin regulation of blood clotting proteinases. A paradigm for understanding proteinase regulation by serpin family protein proteinase inhibitors. Biochimie. 2010 Nov;92(11):1587-96. doi: 10.1016/j.biochi.2010.05.011
- Hsu E, Moosavi L. Biochemistry, Antithrombin III. [Updated 2022 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545295
- Rezaie AR, Giri H. Anticoagulant and signaling functions of antithrombin. J Thromb Haemost. 2020 Dec;18(12):3142-3153. doi: 10.1111/jth.15052
- Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002 Dec;102(12):4751-804. https://doi.org/10.1021/cr010170+
- Damus PS, Hicks M, Rosenberg RD. Anticoagulant action of heparin. Nature. 1973 Dec 7;246(5432):355-7. doi: 10.1038/246355a0
- Carrell R, Skinner R, Jin L, Abrahams JP. Structural mobility of antithrombin and its modulation by heparin. Thromb Haemost. 1997 Jul;78(1):516-9.
- Wiedermann CJ. Clinical review: molecular mechanisms underlying the role of antithrombin in sepsis. Crit Care. 2006 Feb;10(1):209. doi: 10.1186/cc4822
- Ersdal-Badju E, Lu A, Zuo Y, Picard V, Bock SC. Identification of the antithrombin III heparin binding site. J Biol Chem. 1997 Aug 1;272(31):19393-400. doi: 10.1074/jbc.272.31.19393
- Karlaftis V, Sritharan G, Attard C, Corral J, Monagle P, Ignjatovic V. Beta (β)-antithrombin activity in children and adults: implications for heparin therapy in infants and children. J Thromb Haemost. 2014 Jul;12(7):1141-4. doi: 10.1111/jth.12597
- Perry DJ. Antithrombin and its inherited deficiencies. Blood Rev. 1994 Mar;8(1):37-55. doi: 10.1016/0268-960x(94)90006-x
- Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA, Bussières JS, Côté D, Karski J, Martineau R, Robblee JA, Rodger M, Wells G, Clinch J, Pretorius R; BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008 May 29;358(22):2319-31. doi: 10.1056/NEJMoa0802395 Erratum in: N Engl J Med. 2010 Sep 23;363(13):1290.
- Nakashima MO, Rogers HJ. Hypercoagulable states: an algorithmic approach to laboratory testing and update on monitoring of direct oral anticoagulants. Blood Res. 2014 Jun;49(2):85-94
- Maclean PS, Tait RC. Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis and treatment options. Drugs. 2007;67(10):1429-40. doi: 10.2165/00003495-200767100-00005
- Antithrombin Deficiency. https://rarediseases.org/rare-diseases/antithrombin-deficiency
- Hereditary antithrombin deficiency. https://ghr.nlm.nih.gov/condition/hereditary-antithrombin-deficiency
- Johnson CM, Mureebe L, Silver D. Hypercoagulable states: a review. Vasc Endovascular Surg. 2005 Mar-Apr;39(2):123-33
- Antithrombin Deficiency Treatment & Management. https://emedicine.medscape.com/article/198573-treatment#d7
- Nishimura Y, Takagi Y. Strategy for Cardiovascular Surgery in Patients with Antithrombin III Deficiency. Ann Thorac Cardiovasc Surg. 2018 Aug 20;24(4):187-192. doi: 10.5761/atcs.oa.18-00030
- Paidas MJ, Triche EW, James AH, DeSancho M, Robinson C, Lazarchick J, Ornaghi S, Frieling J. Recombinant Human Antithrombin in Pregnant Patients with Hereditary Antithrombin Deficiency: Integrated Analysis of Clinical Data. Am J Perinatol. 2016 Mar;33(4):343-9. doi: 10.1055/s-0035-1564423
- Kawai H, Matsushita H, Kawada H, Ogawa Y, Ando K. The Successful Prevention of Thromboembolism Using Rivaroxaban in a Patient with Antithrombin Deficiency during the Perioperative Period. Intern Med. 2017 Sep 1;56(17):2339-2342. doi: 10.2169/internalmedicine.8487-16
- Minami K, Kumagai K, Sugai Y, Nakamura K, Naito S, Oshima S. Efficacy of Oral Factor Xa Inhibitor for Venous Thromboembolism in a Patient with Antithrombin Deficiency. Intern Med. 2018 Jul 15;57(14):2025-2028. doi: 10.2169/internalmedicine.0483-17
- American College of Obstetricians and Gynecologists Women’s Health Care Physicians. ACOG Practice Bulletin No. 138: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2013 Sep;122(3):706-17. doi: 10.1097/01.AOG.0000433981.36184.4e
- Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, Kearon C, Schunemann HJ, Crowther M, Pauker SG, Makdissi R, Guyatt GH. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl):e351S-e418S. doi: 10.1378/chest.11-2299
- Reducing the Risk of Thrombosis and Embolism during Pregnancy and the Puerperium (Green-top Guideline No. 37a). https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/reducing-the-risk-of-thrombosis-and-embolism-during-pregnancy-and-the-puerperium-green-top-guideline-no-37a
- Antithrombin Deficiency. https://emedicine.medscape.com/article/198573-overview#a3
- Patnaik MM, Moll S. Inherited antithrombin deficiency: a review. Haemophilia. 2008 Nov;14(6):1229-39. doi: 10.1111/j.1365-2516.2008.01830.x
- Stevens SM, Woller SC, Bauer KA, Kasthuri R, Cushman M, Streiff M, Lim W, Douketis JD. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016 Jan;41(1):154-64. doi: 10.1007/s11239-015-1316-1
- Ormesher L, Simcox L, Tower C, Greer IA. Management of inherited thrombophilia in pregnancy. Womens Health (Lond). 2016 Jul;12(4):433-41. doi: 10.1177/1745505716653702