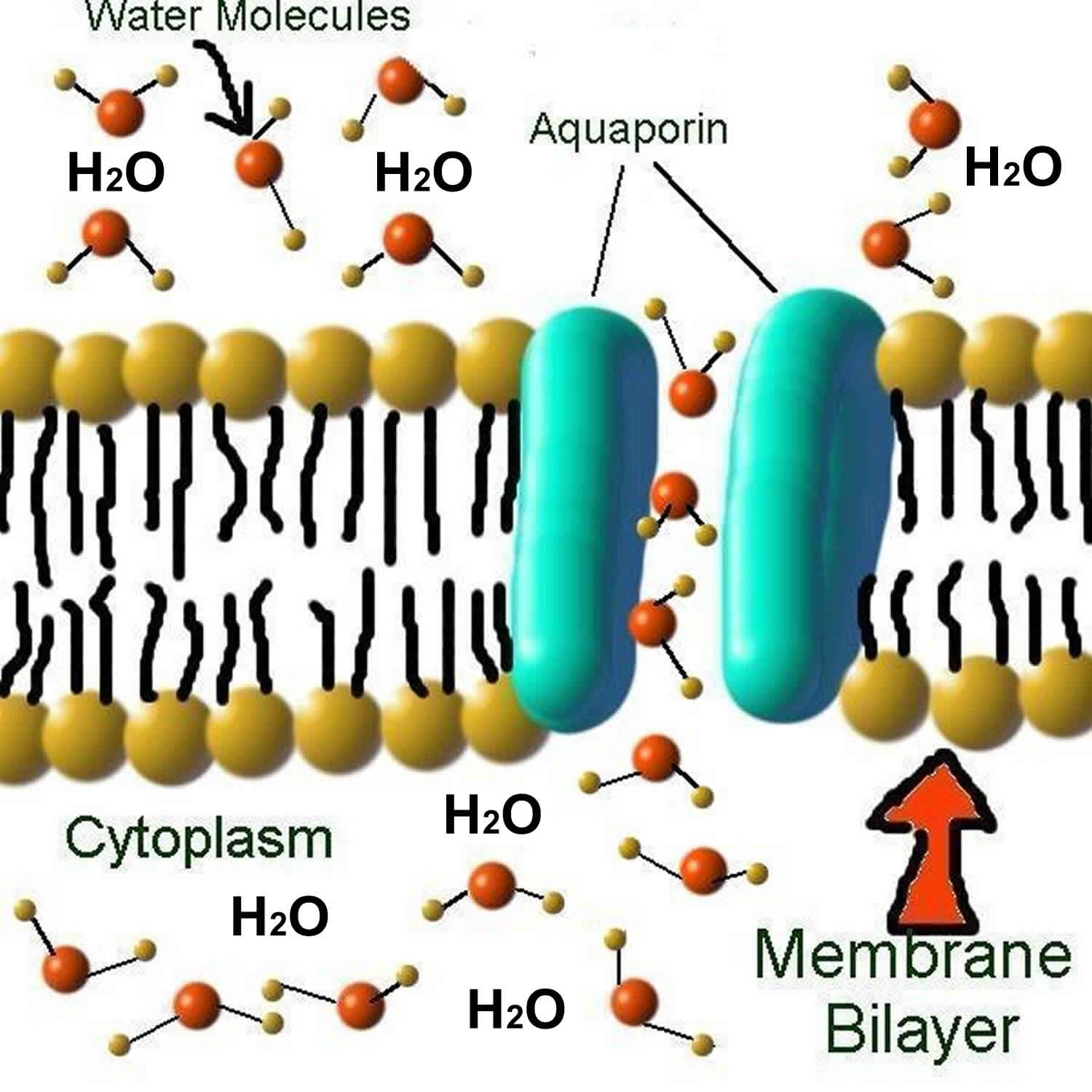
What are aquaporins
Aquaporins are small (~30 kDa monomers) integral membrane proteins that serve as channels in the transfer of water and in some cases, small solutes across cell membranes in reaction to osmotic gradients in cells 1. Water movement across cell membranes is driven by osmotic and hydrostatic forces, but the speed of this process can be influenced by the presence of specific aquaporin channels. With aquaporins present, water can “gush” through the membrane at the extraordinary rate of three billion water molecules per second per aquaporin channel 2. In their absence, water only trickles across the hydrophobic lipid bilayers of cell membranes. Aquaporins are conserved in bacteria, plants, and animals. Aquaporins are specifically active in endothelial and epithelial tissues. Structural analyses of aquaporins molecules have revealed the presence of a pore in the center of each aquaporin molecule. Aquaporins are hydrophobic integral trans-membrane channel proteins implicated in cellular proliferation and water transport in various cancers. In mammalian cells, more than 13 isoforms (AQP0-AQP12) have been identified so far 1. According to their primary structure, aquaporins have been classified into three subfamilies. Water-selective aquaporins include aquaporin-0 (AQP0), aquaporin-1 (AQP1), aquaporin-2 (AQP2), aquaporin-4 (AQP4), aquaporin-5 (AQP5), aquaporin-6 (AQP6), and aquaporin-8 (AQP8), which are also known as orthodox aquaporins. Aquaglyceroporins, including aquaporin-3 (AQP3), aquaporin-7 (AQP7), aquaporin-9 (AQP9), and aquaporin-10 (AQP10), are permeable to water and some small uncharged solutes, such as glycerol and urea 3. Aquaporin-3 (AQP3) aquaporin-8 (AQP8), and aquaporin-9 (AQP9) have been demonstrated to transport hydrogen peroxide (H2O2) in mammalian cells 4. The third subfamily named superaquaporins, including aquaporin-11 (AQP11) and aquaporin-12 (AQP12) 5, has low homology at its amino acid level with other classical aquaporins. Due to a wide spectrum of pathophysiological function in balancing water homeostasis, modulating intracellular signaling, and regulating cell proliferation and oxidative stress response, aquaporins have been proven to participate in renal diseases, dermatosis, bowel disease, and cancer 6.
Aquaporins are differentially expressed in many types of cells and tissues in the body. Aquaporin-0 (AQP0) is abundant in the lens. Aquaporin-1 (AQP1) is found in the blood vessels, kidney proximal tubules, eye, and ear. Aquaporin-2 (AQP2) is expressed in the kidney collecting ducts, where it shuttles between the intracellular storage sites and the plasma membrane under the control of antidiuretic hormone (ADH). Mutations of AQP2 result in diabetes insipidus. Aquaporin-3 (AQP3) is present in the kidney collecting ducts, epidermis, urinary, respiratory, and digestive tracts. Aquaporin-3 (AQP3) in organs other than the kidney may be involved in the supply of water to them. Aquaporin-4 (AQP4) is present in the brain astrocytes, eye, ear, skeletal muscle, stomach parietal cells, and kidney collecting ducts. Aquaporin-4 (AQP4) water channels play a central role in brain water regulation in neurologic disorders. Aquaporin-5 (AQP5) is in the secretory cells such as salivary, lacrimal, and sweat glands. AQP5 is also expressed in the ear and eye. Aquaporin-6 (AQP6) is localized intracellular vesicles in the kidney collecting duct cells. Aquaporin-7 (AQP7) is expressed in the adipocytes, testis, and kidney. Aquaporin-8 (AQP8) is expressed in the kidney, testis, and liver. Aquaporin-9 (AQP9) is present in the liver and leukocytes. Aquaporin-10 (AQP10) is expressed in the intestine. The diverse and characteristic distribution of aquaporins in the body suggests their important and specific roles in each organ.
Virtually all mammalian cells incorporate aquaporins into their cell membranes, and many cells produce multiple aquaporins, each with a specific function. It is therefore not surprising that malfunctions have important clinical conditions. Aquaporinopathies include nephrogenic diabetes insipidus (which is caused by a loss-of-function mutation in aquaporin-2 [AQP2]) and neuromyelitis optica (which is caused by the development of aquaporin-4 [AQP4]-targeted autoantibodies).
High-resolution X-ray crystal structures have been determined for several mammalian aquaporins. Each ~30-kDa aquaporin monomer is made up of six membrane-spanning helical domains (H1–H6) and two short helical segments (HB and HE) that surround cytoplasmic and extracellular vestibules, respectively 7. These vestibules are connected by a narrow aqueous pore of ~25 Å (1 Å=0.1 nm) in length 8. Four aquaporin monomers assemble to form tetramers in the plasma membrane. Tetramers of one of the aquaporins, aquaporin-4 [AQP4], further assemble into supramolecular square arrays – called orthogonal arrays of particles – which are maintained by inter-tetrameric N-terminal interactions involving specific residues 9.
Structural data on aquaporins, together with mutagenesis and molecular dynamics simulations, have indicated that single-file transport occurs through a narrow pore in each monomer, where water selectivity is conferred by electrostatic and steric factors 10. Glycerol-transporting aquaporins, called aquaglyceroporins, have a less-constricted pore compared with that of water-selective aquaporins (diameter of 3.4 Å compared with 2.8 Å, respectively), with relatively more hydrophobic residues lining the pore.
Many mammalian aquaporins, including aquaporin-1, aquaporin-2, aquaporin-4, aquaporin-5 and aquaporin-8, function primarily as bidirectional water-selective transporters. Cells expressing aquaporins on their plasma membrane have an ~5- to 50-fold higher osmotic water permeability than membranes that do not 11. Water transport through single-file pores poses a biophysical limitation on the efficiency with which aquaporins can transport water, so that aquaporins must be present in the membrane at a high density to increase membrane water permeability substantially 12. Aquaporin-expressing cells generally contain several thousands, or more, aquaporins per μm² of membrane, as compared with ten or fewer ion channels per μm² of membrane.
A subset of the aquaporins, the aquaglyceroporins aquaporin-3, aquaporin-7 and aquaporin-9, transport both water and glycerol, and studies have suggested that aquaporin-9 can also transport other small polar solutes, including amino acids, sugars and even arsenite 13. Aquaporin-6 has been proposed to function as an intracellular chloride channel 14. The functions and cellular localization of aquaporins 10–13 are not clear 15. In addition to their well-established water- and glycerol-transporting functions, aquaporins have also been proposed to transport other small molecules and gases, including carbon dioxide, ammonia, nitric oxide and hydrogen peroxide 16. Although some gases are theoretically small enough to pass through the aqueous pore of the aquaporin, compelling evidence is lacking for physiologically relevant gas transport, partly because the intrinsic lipid-mediated membrane permeability to most gases is high 17. Current evidence suggests that most, if not all, significant biological functions of the mammalian aquaporins, can be attributed to aquaporin-facilitated water and/or glycerol transport.
Because most aquaporins are constitutively expressed at the cell plasma membrane, regulation of their function occurs mainly at the level of transcription. There are numerous descriptive studies of up- and down-regulation by various aquaporins in response to various stresses, such as aquaporin-4 upregulation in brain trauma and inflammation 18. With the exception of aquaporin-2, the biological significance of transcriptional regulation of aquaporin expression is unclear. Transcription and post-translational regulation of aquaporin2 expression are particularly important for the mechanism of urine concentration 19. Similar to the process of GLUT4 translocation to the membrane in response to insulin, targeting of aquaporin2 to the apical plasma membrane in the collecting duct of the kidney is controlled by the antidiuretic hormone vasopressin through a mechanism involving regulated vesicular trafficking of aquaporin-2-containing endosomes.
Aquaporins function
Aquaporins primarily function as water-conducting channels and are considered to be “the plumbing system for cells”, although some are also permeable to small solutes. Aquaporins are usually specific for water permeability and exclude the passage of other solutes. A type of aquaporin known as aquaglyceroporins can also conduct some very small uncharged solutes such as glycerol, CO2, ammonia, and urea across the membrane. However, all aquaporins are impermeable to charged solutes. Water molecules traverse the aquaporin channel in single file.
Aquaporins are involved in many physiological functions, including renal water balance, epithelial fluid secretion, cell migration, brain oedema and metabolism in adipocytes. Modulators of aquaporin function are predicted to have broad clinical indications in edema, cancer, obesity, brain injury, glaucoma, epilepsy and inflammation.
Table 1. Aquaporins primarily function
| Major Sites of Expression | Comments | |
|---|---|---|
| Aquaporin-0 | Eye: lens fiber cells | Fluid balance within the lens |
| Aquaporin-1 | Red blood cells | Osmotic protection |
| Kidney: proximal tubule | Concentration of urine | |
| Eye: ciliary epithelium | Production of aqueous humor | |
| Brain: choriod plexus | Production of cerebrospinal fluid | |
| Lung: alveolar epithelial cells | Alveolar hydration state | |
| Aquaporin-2 | Kidney: collecting ducts | Mediates antidiuretic hormone activity |
| Aquaporin-3 * | Kidney: collecting ducts | Reabsorbtion of water into blood |
| Trachea: epithelial cells | Secretion of water into trachea | |
| Aquaporin-4 | Kidney: collecting ducts | Reabsorbtion of water |
| Brain: ependymal cells | CSF fluid balance | |
| Brain: hypothalamus | Osmosensing function? | |
| Lung: bronchial epithelium | Bronchial fluid secretion | |
| Aquaporin-5 | Salivary glands | Production of saliva |
| Lacrimal glands | Production of tears | |
| Aquaporin-6 | Kidney | Very low water permeability; function? |
| Aquaporin-7 * | Fat cells | Transports glycerol out of adipocytes |
| Testis and sperm | ||
| Aquaporin-8 | Testis, pancreas, liver, others | |
| Aquaporin-9 * | Leukocytes |
The identification of useful small-molecule aquaporin inhibitors has been slow, in part because of technical challenges in assaying the water-transporting function of aquaporins and challenges associated with targeting the compact, pore-containing aquaporin molecule.
Mice lacking aquaporin-5, which is expressed in salivary and airway submucosal glands, show defective secretion of saliva 20 and airway mucus 21. Aquaporin-facilitated fluid secretion has also been found in the ocular ciliary epithelium 22, which produces aqueous fluid, and in the brain choroid plexus 23, which produces cerebrospinal fluid (CSF). There is a caveat, however: aquaporins appear to be required for active transepithelial fluid transport only when fluid transport rate, normalized to epithelial surface area, is very high, as it is in the proximal tubule and salivary gland. By contrast, in the lung alveolus, deletion of aquaporin-5 in mice does not impair active fluid absorption, although it is expressed in alveolar epithelial cells and is responsible for ~90% of osmotically driven water transport 24. This is because the area-normalized rate of fluid absorption by the alveolus is more than 100-fold lower than that in the proximal tubule. The basal (aquaporin independent) water permeability of the alveolar epithelium is sufficient to support relatively slow fluid absorption.
Aquaporins in cell migration
The involvement of aquaporins in cell migration was discovered following the observation of impaired tumor angiogenesis in aquaporin-1-null mice and the characterization of endothelial cell cultures derived from wild-type and aquaporin-1-null mice 25. Motivated by the high expression of aquaporin-1 in tumor microvessels 26, aquaporin-1 deletion in mice reduces the growth and vascularity of implanted tumors 25. Cultured aortic endothelial cells from aquaporin-1-null mice migrated slower than cells from wild-type mice in response to a chemotactic stimulus, and cell migration was increased when various cells not normally expressing aquaporins were transfected with aquaporins. Further evidence, including aquaporin polarization to the leading edge of migrating cells, increased lamellipodial dynamics in aquaporin-expressing cells and aquaporin-dependent cell migration in different cells types and with different aquaporins, suggested a mechanism for aquaporin-facilitated migration 27.
On the basis of these results, it’s been proposed that actin depolymerization and ion influx increase cytoplasmic osmolality at the leading edge of a migrating cell, driving water influx through the plasma membrane 25. This idea of water flow into and out of migrating cells is supported by data showing that migration can be modulated by changes in extracellular osmolality and transcellular osmotic gradients 28. A model postulates that water influx causes expansion of the adjacent plasma membrane by increased hydrostatic pressure, which is followed by actin repolymerization to stabilize the cell membrane protrusion. In support of this idea is the observation that regional hydrostatic pressure changes within cells do not equilibrate throughout the cytoplasm, on a distance scale of 10 μm and a time scale of 10 seconds, and could thus contribute to the formation of localized cell membrane protrusions 29. However, this mechanism remains unproven and other mechanisms, such as aquaporin-dependent changes in cell volume during migration and interaction of aquaporins with other proteins, are potential alternatives.
Regardless of the exact biophysical mechanism, aquaporin-facilitated cell migration appears to be a general phenomenon relevant not only in angiogenesis but also in tumor spread, wound healing and immune cell chemotaxis. aquaporin expression in tumor cells increases their ability to extravasate across blood vessels and to invade locally 30, which might account for the high level of aquaporin expression in many tumor types and the correlation between aquaporin expression and tumor grade in some tumors, such as glioblastomas 31. Expression of aquaporin4 in brain astrocytes increases their migration towards a chemotaxic stimulus and increases glial scarring 32, and expression of aquaporin-3 in skin and cornea facilitates wound healing 33.
Although the evidence remains preliminary, aquaporins might also be involved in intracellular vesicular transport processes, such as pancreatic granule exocytosis and astrocyte cytokine secretion 34, perhaps by a mechanism involving rapid aquaporin-dependent changes in cell volume, which might accompany vesicle fusion.
Aquaporin-4 in brain swelling
Water movement across barriers also occurs in the brain, but it is the astrocytes rather than epithelial cells that are involved. The aquaporin-4 water channel is expressed in astrocytes throughout the central nervous system, particularly at brain–fluid interfaces at the blood–brain and ependymal–CSF barriers. In cytotoxic (cellular) brain edema, water moves into the brain through an intact blood–brain barrier in response to osmotic driving forces. For example, the acute serum hyponatremia that occurs during water intoxication causes brain swelling by a simple osmotic mechanism. Mice lacking aquaporin4 show improved outcome and reduced brain water accumulation compared with that in wild-type mice in models of cytotoxic brain edema, such as water intoxication, ischemic stroke and bacterial meningitis 35.
In contrast to cytotoxic edema, vasogenic (leaky-vessel) brain edema involves water movement into the brain by a bulk-fluid flow mechanism, through a leaky blood–brain barrier, and exit from the brain through the aquaporin4-rich glia limitans, which lines the ventricles and the surface of the brain. When these water exit routes are blocked, such as in obstructive hydrocephalus, water also moves out of the brain through microvessels at the blood–brain barrier. Mice lacking aquaporin-4 have a worse clinical outcome and greater brain water accumulation in models of vasogenic brain edema, including intra-parenchymal fluid infusion, cortical-freeze injury, brain tumor and brain abscess 36, and in a model of obstructive hydrocephalus 37. Therefore, as a bidirectional water channel, aquaporin-4 facilitates brain water accumulation in cytotoxic edema and the clearance of excess brain water in vasogenic edema. aquaporin-4 appears to have a similar role in the spinal cord, reducing cytotoxic swelling and improving the clinical outcome following spinal cord compression injury 38, whereas increasing vasogenic swelling following spinal cord contusion injury 39.
Aquaporin-4 in neural signaling
Aquaporins are expressed in electrically excitable tissues in supportive cells adjacent to excitable cells (e.g. in astrocytes but not neurons in the brain, in Müller cells but not bipolar cells in the retina, in supportive cells but not hair cells in the inner ear, and in support cells but not olfactory receptor neurons in the olfactory epithelium). Electrophysiological measurements show impaired vision 40, hearing 41 and olfaction 42 in aquaporin-4-null mice. In the brain, the seizure threshold is reduced and seizure duration prolonged by an aquaporin-4 deficiency 43. Possible mechanisms for altered neuroexcitation in aquaporin-4 deficiency include slower K+ re-uptake into astrocytes following neuroexcitation 43 and mild expansion of the extracellular space surrounding these cells 44.
How aquaporin-4 alters K+ re-uptake following neuroexcitation is the subject of ongoing investigation. It had been postulated that the interaction between aquaporin4 and the inwardly rectifying K+ channel Kir4.1 was responsible. However, patch-clamp analysis has indicated that aquaporin-4 deficiency does not affect Kir4.1 K+ channel function in brain astrocytes or retinal Müller cells 45. It was proposed that aquaporin-4-dependent water permeability enhances K+ transport by a ‘pseudo-solvent drag’ mechanism. Neuroexcitation involves ion buffering in the extracellular space, which is the small aqueous volume surrounding cells (~20% of the volume in brain). Excess K+ released by neurons during excitation is taken up and ‘siphoned’ largely by astrocytes. It was postulated that aquaporin-4-facilitated water transport in astrocytes in the brain is an important determinant of both water and ion movement between cells and the extracellular space during neuroexcitation. Re-uptake of K+ following neuroexcitation results in osmotic water influx into aquaporin-4-expressing astrocytes and consequent extracellular space shrinkage, which maintains the electrochemical driving force for K+ re-uptake (by maintaining elevated K+ concentrations in the extracellular space). The diminished astrocyte water permeability that occurs in aquaporin4 deficiency would reduce extracellular space contraction and hence slow K+ re-uptake. This hypothesis, although unproven as yet, is attractive because it relates neuroexcitation phenotypes to the water-transporting role of aquaporin-4.
Aquaglyceroporin aquaporin-3 in skin hydration
The functional significance of glycerol transport by the aquaglyceroporins, such as aquaporin-3 in skin and aquaporin-7 in adipocytes was, for many years, unclear. However, more recent studies have indicated roles for these channels in skin hydration and fat metabolism. aquaporin3-facilitated glycerol transport in skin is an important determinant of epidermal and stratum corneum hydration 46. Mice lacking aquaporin-3, which is normally expressed in the basal layer of proliferating keratinocytes in the epidermis, manifest reduced stratum corneum hydration and skin elasticity. This is caused by reduced epidermal cell glycerol permeability, resulting in reduced glycerol content in the stratum corneum and epidermis, where it acts as a ‘humectant’, or water-retaining osmolyte 47. Topical or systemic administration of glycerol normalized stratum corneum glycerol content and corrected the skin-hydration defect in aquaporin-3 deficiency 48. These findings provided a rationale for the common use of glycerol in cosmetics and various skin medical formulations and generated interest in the involvement of aquaporin-3 in skin diseases 49.
Aquaporin-3 in cell proliferation
An unanticipated role of aquaporin-3 in cell proliferation was observed in several aquaporin-3-expressing tissues, including skin, colon and cornea. Aquaporin-3-deficient mice manifest impaired cutaneous and corneal wound healing 33 and colonic epithelial cell regeneration 50. In addition, a remarkable tumor phenotype was found in aquaporin-3-null mice, which showed complete resistance to the formation of skin tumors in response to a tumor initiator-promoter protocol that produces multiple tumors in wild-type mice 51. Biochemical studies in epidermal cells of these aquaporin-3-deficient mice showed impaired cellular glycerol metabolism and biosynthesis, with reduced ATP content and impaired MAPK signaling. Thus, it was proposed that aquaporin-3-facilitated glycerol transport is a key determinant of cell proliferation through a mechanism involving reduced epidermal glycerol concentration in aquaporin3 deficiency, impaired lipid biosynthesis, reduced glycerol metabolism and ATP, impaired MAPK signaling (particularly p38 kinase) and, finally, reduced cell proliferation. Further investigation of glycerol metabolism in aquaporin-3-expressing cells is needed to verify this proposed mechanism. In any case, the possibility of aquaporin3 inhibition being used to prevent or treat certain tumors is intriguing because aquaporin-3 inhibition is predicted to reduce both migration and proliferation in tumor cells.
Aquaglyceroporin aquaporin-7 in fat metabolism
A quite unexpected role of an aquaporin in obesity has been discovered. Aquaporin-7 is expressed in the plasma membrane of adipocytes. Aquaporin-7-null mice manifest a remarkable progressive increase in fat mass and adipocyte hypertrophy, accumulating glycerol and triglycerides in their adipocytes, as they age 52. Biochemical studies suggested that this adipocyte hypertrophy is the consequence of reduced plasma membrane glycerol permeability, resulting in cellular glycerol and triacylglycerol accumulation, and glycerol kinase upregulation. These results focus attention on adipocyte glycerol permeability as a novel regulator of adipocyte size and whole-body fat mass, suggesting that the modulation of adipocyte aquaporin-7 expression and/or function can alter fat mass. aquaporin9 has been suggested as an important route for hepatic glycerol uptake 53, and aquaporin-7 and aquaporin9 as key metabolic regulators in diabetes and obesity 54. However, additional work is needed to validate these ideas.
Aquaporins in kidney
Eight aquaporins, including aquaporin-1, aquaporin-2, aquaporin-3, aquaporin-4, aquaporin-5, aquaporin-6, aquaporin-7, and aquaporin-11, are expressed in different segments and various cells in the kidney to maintain normal urine concentration function 55. Aquaporin-2 is critical in regulating urine concentrating ability. The expression and function of aquaporin-2 are regulated by a series of transcriptional factors and post-transcriptional phosphorylation, ubiquitination, and glycosylation. Mutation or functional deficiency of aquaporin-2 leads to severe nephrogenic diabetes insipidus. Nephrogenic diabetes insipidus, the inability to produce concentrated urine, can result from several different malfunctions in the aquaporin-2 (AQP2) system controlled by anti-diuretic hormone (ADH).
In the kidney, eight aquaporins, including aquaporin-1, aquaporin-2, aquaporin-3, aquaporin-4, aquaporin-5, aquaporin-6, aquaporin-7, and aquaporin-11, are expressed in different segments and various cells to maintain normal urine concentration function, tissue development and substance metabolism 56 (Figure 1).
Figure 1. Aquaporins in kidney
Footnote: Expression localization of aquaporins in kidney. aquaporin-1 is located in proximal tubule, descending thin limbs of Henle, and vasa recta; aquaporin-2, aquaporin-3, aquaporin-4, aquaporin-5 and aquaporin-6 are in the collecting duct; aquaporin-7 and aquaporin-11 are expressed in proximal tubule.
[Source 55 ]Aquaporin-1 is the first discovered water channel, and is located in the apical and basolateral plasma membrane of the proximal tubule, descending thin limbs of Henle, and descending vasa recta to mediate water reabsorption 57. Aquaporin-1 is a highly selective water-permeable channel. Mice defective with aquaporin-1 exhibit polyuria, indicating its key role in the formation of hypertonicity 58.
Aquaporin-2 is one of the most important channel proteins involved in regulating urine concentration, and is located at the apical membrane of principal cells in the collecting duct 59. The water reabsorption function of aquaporin-2 is mainly regulated by arginine vasopressin (ADH) via increasing intracellular production of cyclic adenosine monophosphatase (cAMP) and further phosphorylation of aquaporin-2 at Ser256 and Ser269 to stimulate the intracellular trafficking of aquaporin-2 to the plasma membrane 60. Besides arginine vasopressin (ADH), researchers have demonstrated that activation of bile acid receptor TGR5 and hydrogen sulfide (H2S) stimulates the expression of aquaporin-2 via cAMP-protein kinase A (PKA) signaling pathway and attenuates the defection of urinary concentration in mice 61. In fact, aquaporin-2 expression, phosphorylation, and trafficking are not only activated by canonical arginine vasopressin (ADH)/cAMP/PKA signaling but also other signaling. Erlotinib, an epidermal growth factor receptor (EGFR) inhibitor, has been reported to enhance aquaporin-2 accumulation in plasma membrane and water reabsorption through increasing phosphorylation of aquaporin-2 at Ser-256 and Ser-269 and reducing endocytosis of aquaporin-2 without affecting classic PKA signaling 62. Administration of Wnt5a alleviated decreased urine osmolality and upregulation of aquaporin-2 via activation of calcium/calmodulin/calcineurin signaling 63. Additionally, the expression of aquaporin-2 can be regulated by several transcription factors, such as AP-1, NF-κB, and NFAT 64. cAMP-responsive element binding protein (CREB) has been recognized as a major regulator of aquaporin-2 expression. However, recent evidence identified that C/EBPβ is pertinent to transcriptional regulation of aquaporin-2 and the relationship between cAMP-responsive element binding protein (CREB) and aquaporin-2 is indirect 65. Notably, normal expression of aquaporin-2 in apical plasma membrane plays a critically determinant role in renal urine concentration and body water balance. Deletion or mutation of the aquaporin-2 gene causes severe water disorders and triggers the initiation of nephrogenic diabetes insipidus (nephrogenic diabetes insipidus). Urinary excretion of aquaporin-2 has been recognized as a useful marker for diagnosis of renal diseases 65.
Aquaporin-3 is constitutively located in the basolateral membrane of principle cells in the cortex and outer medullar collecting duct and is regulated by thirst, ADH (antidiuretic hormone), and aldosterone. Aquaporin-4 is mostly distributed in the basolateral membrane of principle cells of the medullary segment of the collecting duct. Protein kinase C and dopamine rather than ADH (antidiuretic hormone) affect phosphorylation of aquaporin-4 to regulate water permeability. aquaporin-3 and aquaporin-4 could export water entering cytoplasm via aquaporin-2. Of note, aquaporin-3 also facilitates glycerol and hydrogen peroxide transport through the cell membrane, which regulates a series of intracellular signaling and affects cellular functions, such as cell proliferation, apoptosis and migration 66. Aquaporin-3-null mice showed nephrogenic diabetes insipidus-like phenotype, while the absence of aquaporin-4 only presented mild urinary concentration defect. However, the double knockout mice of aquaporin-3/aquaporin-4 have a greater impairment of urinary function than aquaporin-3-null mice 67, which may be due to their similar localization and water permeability in the urinary tract.
A few years ago, scientists firstly reported that aquaporin-5 is located in type B intercalated cells of collecting ducts 68. Aquaporin-6 is localized in the intracellular vesicles of intercalated cells and colocalized with H+-ATPase 69. Aquaporin-6 hardly transports water from the membrane unless at a low pH value. The function of aquaporin-5 and aquaporin-6 in the kidney is still not clear.
Aquaporin-7 is expressed in the brush border of the S3 segment of the proximal tubule, and shows great effect on metabolism by regulating the transportation of glycerol. Defective aquaporin-7 expression has little effect on water permeability of proximal tubules, but is associated with significant metabolism disorders, like obesity and insulin resistance 70.
Aquaporin-11 is uniquely localized in the membrane of endoplasmic reticulum (ER) of proximal tubular cells. The transport function of aquaporin-11 is controversial regarding whether it transports water and glycerol or only glycerol 71. Aquaporin-11 knockout mice develop uremia due to the renal cysts derived from the proximal tubule.
Nowadays, the pathophysiologic functions of aquaporin-s in renal-specific cell types and liquid homeostasis have been deeply studied to provide the therapeutic targets. The data showed that aquaporin-s might be an ideal biomarker for renal diseases 72.
Human aquaporin diseases
In humans, loss-of-function mutations in aquaporins exist but are rare. As mentioned above, mutations in aquaporin2 produce non-X-linked nephrogenic diabetes insipidus by a recessive mechanism involving defective aquaporin2 protein folding and retention in the endoplasmic reticulum (ER), as well as by a dominant mechanism, which results from interactions between wild-type and mutant aquaporin-2 in the endoplasmic reticulum and/or Golgi that prevent plasma membrane targeting of wild-type aquaporin2 73. The incidence of nephrogenic diabetes insipidus caused by aquaporin-2 mutations is fewer than one in 20 million births.
For other aquaporins, only a handful of subjects have been identified with loss-of-function mutations. A few subjects that lack functional aquaporin1, which were identified by blood-group screening, are phenotypically normal but manifest defective urinary concentrating function when deprived of water 74. Because of the rarity of aquaporin1-deficient individuals, as well as a few subjects that apparently lack functional aquaporin-3 or aquaporin-7 75, and because of wide phenotype variations in humans, little useful information is available about the roles of these aquaporins in humans.
Mutations in aquaporin-0 in lens fiber cause congenital cataracts by a mechanism that is speculated to involve defective cell–cell adhesion rather than impaired water transport 76. Neither disease-causing mutations of other aquaporins in humans nor strong associations between aquaporin polymorphisms and disease have so far been described.
The neuroinflammatory demyelinating disease neuromyelitis optica is an interesting aquaporinopathy that has recently received considerable attention. It is a relatively rare variant of multiple sclerosis that primarily affects the optic nerve and the spinal cord, causing blindness, paralysis and death, and it has a poor prognosis, even with aggressive immunosuppressive therapy. A defining feature of neuromyelitis optica is the presence of serum antibodies directed against extracellular epitopes on aquaporin-4 (autoantibodies) 77. Neuromyelitis optica autoantibodies (NMO-IgG) are thought to mediate their pathogenic effect by binding to aquaporin-4 on astrocytes, which activates complement- and cell-mediated cytotoxicity, elaborates inflammatory mediators and stimulates leukocyte infiltration 78. It is not known what triggers the production of neuromyelitis optica autoantibodies (NMO-IgG), nor how they enter the CNS or why lesions are largely absent in brain and in peripheral aquaporin4-expressing tissues. Active research is being carried out to establish useful animal models of neuromyelitis optica 79 and to develop new therapeutic approaches, such as small-molecule inhibitors or monoclonal antibodies (‘aquaporumabs’) that block the binding of neuromyelitis optica-IgG to aquaporin-4.
References- Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem. 2004;39(1):1-83. https://www.ncbi.nlm.nih.gov/pubmed/15242101
- A brief survey of aquaporins and their implications for renal physiology. Clin Lab Sci. 2006 Spring;19(2):70-9. https://www.ncbi.nlm.nih.gov/pubmed/16749243
- Soveral, G.; Casini, A. Aquaporin modulators: A patent review (2010–2015). Expert Opin. Ther. Pat. 2017, 27, 49–62.
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016, 471, 191–197
- Ishibashi, K.; Hara, S.; Kondo, S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 2009, 13, 107–117
- Chen, Y.; Jin, S.; Teng, X.; Hu, Z.; Zhang, Z.; Qiu, X.; Tian, D.; Wu, Y. Hydrogen Sulfide Attenuates LPS-Induced Acute Kidney Injury by Inhibiting Inflammation and Oxidative Stress. Oxid. Med. Cell. Longev. 2018
- Aquaporins at a glance. Alan S. Verkman. J Cell Sci 2011 124: 2107-2112; doi: 10.1242/jcs.079467
- Walz, T., Fujiyoshi, Y. and Engel, A. (2009). The AQP structure and functional implications. Handb. Exp. Pharmacol. 190, 31-56.
- Crane, J. M. and Verkman, A. S. (2009). Determinants of aquaporin-4 assembly in orthogonal arrays revealed by live-cell single-molecule fluorescence imaging. J. Cell Sci. 122, 813-821.
- Hub, J. S., Grubmuller, H. and de Groot, B. L. (2009). Dynamics and energetics of permeation through aquaporins. What do we learn from molecular dynamics simulations? Handb. Exp. Pharmacol. 190, 57-76
- Verkman, A. S. and Mitra, A. K. (2000). Structure and function of aquaporin water channels. Am. J. Physiol. 278, F13-F28
- Yang, B. and Verkman, A. S. (1997). Water and glycerol permeabilities of aquaporins 1-5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J. Biol. Chem. 272, 16140-16146
- Carbrey, J. M., Song, L., Zhou, Y., Yoshinaga, M., Rojek, A., Wang, Y., Liu, Y., Lujan, H. L., DiCarlo, S. E., Nielsen, S., et al. (2009). Reduced arsenic clearance and increased toxicity in aquaglyceroporin-9-null mice. Proc. Natl. Acad. Sci. USA 106, 15956-15960.
- Yasui, M., Hazama, A., Kwon, T. H., Nielsen, S., Guggino, W. B. and Agre, P. (1999). Rapid gating and anion permeability of an intracellular aquaporin. Nature 402, 184-187.
- Ishibashi, K. (2009). New members of mammalian aquaporins: AQP10-AQP12. Handb. Exp. Pharmacol. 190, 251-262.
- Miller, E. W., Dickinson, B. C. and Chang, C. J. (2010). Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 107, 15681-15686
- Tornroth-Horsefield, S., Hedfalk, K., Fischer, G., Lindkvist-Petersson, K. and Neutze, R. (2010). Structural insights into eukaryotic aquaporin regulation. FEBS Lett. 584, 2580-2588.
- Tomura, S., Nawashiro, H., Otani, N., Uozumi, Y., Toyooka, T., Ohsumi, A. and Shima, K. (2011). Effect of decompressive craniectomy on aquaporin-4 expression after lateral fluid percussion injury in rats. J. Neurotrauma 28, 237-243
- Noda, Y., Sohara, E., Ohta, E. and Sasaki, S. (2010). Aquaporins in kidney pathophysiology. Nat. Rev. Nephrol. 6, 168-178.
- Ma, T., Song, Y., Gillespie, A., Carlson, E. J., Epstein, C. J. and Verkman, A. S. (1999). Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J. Biol. Chem. 274, 20071-20074
- Song, Y. and Verkman, A. S. (2001). Aquaporin-5 dependent fluid secretion in airway submucosal glands. J. Biol. Chem. 276, 41288-41292
- Verkman, A. S., Ruiz-Ederra, J. and Levin, M. (2008b). Functions of aquaporins in the eye. Prog. Retin. Eye Res. 27, 420-433
- Oshio, K., Watanabe, H., Song, Y., Verkman, A. S. and Manley, G. T. (2005). Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel aquaporin-1. FASEB J. 19, 76-78
- Ma, T., Fukuda, N., Song, Y., Matthay, M. A. and Verkman, A. S. (2000a). Lung fluid transport in aquaporin-5 knockout mice. J. Clin. Invest. 105, 93-100
- Saadoun, S., Papadopoulos, M. C., Hara-Chikuma, M. and Verkman, A. S. (2005a). Impairment of angiogenesis and cell migration by targeted of aquaporin-1 gene disruption. Nature 434, 786-792.
- Endo, M., Jain, R. K., Witwer, B. and Brown, D. (1999). Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc. Res. 58, 89-98
- Loitto, V. M., Karlsson, T. and Magnusson, K. E. (2009). Water flux in cell motility: expanding the mechanisms of membrane protrusion. Cell Motil. Cytoskeleton 66, 237-247.
- Saadoun, S., Papadopoulos, M. C., Watanabe, H., Yan, D., Manley, G. T. and Verkman, A. S. (2005b). Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J. Cell. Sci. 118, 5691-5698
- Charras, G. T., Yarrow, J. C., Horton, M. A., Mahadevan, L. and Mitchison, T. J. (2005). Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435, 365-369
- Hu, J. and Verkman, A. S. (2006). Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 20, 1892-1894
- Verkman, A. S., Hara-Chikuma, M. and Papadopoulos, M. C. (2008a). Aquaporins – new players in cancer biology. J. Mol. Med. 86, 523-529
- Auguste, K. I., Jin, S., Uchida, K., Yan, D., Manley, G. T., Papadopoulos, M. C. and Verkman, A. S. (2007). Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 21, 108-116
- Hara-Chikuma, M. and Verkman, A. S. (2008b). Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J. Mol. Med. 96, 523-529
- Li, L., Zhang, H., Varrin-Doyer, M., Zamvil, S. S. and Verkman, A. S. (2011). Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB J. 25, 1556-1566
- Papadopoulos, M. C. and Verkman, A. S. (2005). Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J. Biol. Chem. 280, 13906-13912
- Bloch, O., Papadopoulos, M. C., Manley, G. T. and Verkman, A. S. (2005). Aquaporin-4 gene deletion in mice increases focal edema associated with brain abscess. J. Neurochem. 95, 254-262.
- Bloch, O., Manley, G. T. and Verkman, A. S. (2006). Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4 deficient mice. J. Cereb. Blood Flow. Metab. 26, 1527-1537
- Saadoun, S., Bell, B. A., Verkman, A. S. and Papadopoulos, M. C. (2008). Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain 131, 1087-1098
- Kimura, A., Hsu, M., Seldin, M., Verkman, A. S., Scharfman, H. E. and Binder, D. K. (2010). Protective role of aquaporin-4 water channels after contusion spinal cord injury. Ann. Neurol. 67, 794-801
- Li, J., Patil, R. V. and Verkman, A. S. (2002). Mildly abnormal retinal function in transgenic mice without Muller cell aquaporin-4 water channels. Invest. Ophthalmol. Vis. Sci. 43, 573-579
- Li, J. and Verkman, A. S. (2001). Impaired hearing in mice lacking aquaporin-4 water channels. J. Biol. Chem. 276, 31233-31237
- Lu, D., Zhang, H., Zador, Z. and Verkman, A. S. (2008). Impaired olfaction in mice lacking aquaporin-4 water channels. FASEB J. 22, 3216-3223
- Binder, D. K., Yao, X., Sick, T. J., Verkman, A. S. and Manley, G. T. (2006). Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia 53, 631-636
- Zhang, H. and Verkman, A. S. (2010b). Microfiberoptic measurement of extracellular space volume in brain and tumor slices based on fluorescent dye partitioning. Biophys. J. 99, 1284-1291
- Ruiz-Ederra, J., Zhang, H. and Verkman, A. S. (2007). Evidence against functional interaction between aquaporin-4 water channels and Kir4.1 K+ channels in retinal Müller cells. J. Biol. Chem. 282, 21866-21872
- Ma, T., Hara, M., Sougrat, R., Verbavatz, J. M. and Verkman, A. S. (2002). Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J. Biol. Chem. 277, 17147-17153
- Hara, M., Ma, T. and Verkman, A. S. (2002). Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J. Biol. Chem. 277, 46616-46621
- Hara, M. and Verkman, A. S. (2003). Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc. Natl. Acad. Sci. USA 100, 7360-7365
- Hara-Chikuma, M. and Verkman, A. S. (2008c). Roles of aquaporin-3 in epidermis. J. Invest. Dermatol. 128, 2145-2151
- Thiagarajah, J. R., Zhao, D. and Verkman, A. S. (2007). Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. Gut 56, 1529-1535
- Hara-Chikuma, M. and Verkman, A. S. (2008a). Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol. Cell. Biol. 28, 326-332
- Hara-Chikuma, M., Sohara, E., Rai, T., Ikawa, M., Okabe, M., Sasaki, S., Uchida, S. and Verkman, A. S. (2005). Progressive adipocyte hypertrophy in aquaporin-7 deficient mice: Adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 280, 15493-15496
- Carbrey, J. M., Gorelick-Feldman, D. A., Kozono, D., Praetorius, J., Nielsen, S. and Agre, P. (2003). Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc. Natl. Acad. Sci. USA 100, 2945-2950.
- Maeda, N., Hibuse, T. and Funahashi, T. (2009). Role of aquaporin-7 and aquaporin-9 in glycerol metabolism; involvement in obesity. Handb. Exp. Pharmacol. 190, 233-249
- Aquaporins in Renal Diseases. Int. J. Mol. Sci. 2019, 20(2), 366; https://doi.org/10.3390/ijms20020366
- Li, Y.; Wang, W.; Jiang, T.; Yang, B. Aquaporins in Urinary System. Adv. Exp. Med. Biol. 2017, 969, 131–148.
- Chou, C.L.; Knepper, M.A.; Hoek, A.N.; Brown, D.; Yang, B.; Ma, T.; Verkman, A.S. Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1 null mice. J. Clin. Investig. 1999, 103, 491–496
- Verkman, A.S.; Yang, B. Aquaporin gene delivery to kidney. Kidney Int. 2002, 61, S120–S124
- Ren, H.; Yang, B.; Molina, P.A.; Sands, J.M.; Klein, J.D. NSAIDs Alter Phosphorylated Forms of AQP2 in the Inner Medullary Tip. PLoS ONE 2015, 10, e141714.
- Kavanagh, C.; Uy, N.S. Nephrogenic Diabetes Insipidus. Pediatr. Clin. N. Am. 2019, 66, 227–234.
- Li, S.; Qiu, M.; Kong, Y.; Zhao, X.; Choi, H.J.; Reich, M.; Bunkelman, B.H.; Liu, Q.; Hu, S.; Han, M.; et al. Bile Acid G Protein-Coupled Membrane Receptor TGR5 Modulates Aquaporin 2-Mediated Water Homeostasis. J. Am. Soc. Nephrol. 2018, 29, 2658–2670
- Ando, F.; Sohara, E.; Morimoto, T.; Yui, N.; Nomura, N.; Kikuchi, E.; Takahashi, D.; Mori, T.; Vandewalle, A.; Rai, T.; et al. Wnt5a induces renal AQP2 expression by activating calcineurin signalling pathway. Nat. Commun. 2016, 7, 13636
- Kortenoeven, M.L.; Trimpert, C.; van den Brand, M.; Li, Y.; Wetzels, J.F.; Deen, P.M. In mpkCCD cells, long-term regulation of aquaporin-2 by vasopressin occurs independent of protein kinase A and CREB but may involve EPAC. Am. J. Physiol. Ren. Physiol. 2012, 302, F1395–F1401
- Jung, H.J.; Raghuram, V.; Lee, J.W.; Knepper, M.A. Genome-Wide Mapping of DNA Accessibility and Binding Sites for CREB and C/EBPbeta in Vasopressin-Sensitive Collecting Duct Cells. J. Am. Soc. Nephrol. 2018, 29, 1490–1500
- Krais, A.M.; Andersen, C.; Eriksson, A.C.; Johnsson, E.; Nielsen, J.; Pagels, J.; Gudmundsson, A.; Lindh, C.H.; Wierzbicka, A. Excretion of Urinary Metabolites of the Phthalate Esters DEP and DEHP in 16 Volunteers after Inhalation and Dermal Exposure. Int. J. Environ. Res. Public Health 2018, 15
- Hara-Chikuma, M.; Satooka, H.; Watanabe, S.; Honda, T.; Miyachi, Y.; Watanabe, T.; Verkman, A.S. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-kappaB signalling in keratinocytes and development of psoriasis. Nat. Commun. 2015, 6, 7454
- Kortenoeven, M.L.; Fenton, R.A. Renal aquaporins and water balance disorders. Biochim. Biophys. Acta 2014, 1840, 1533–1549.
- Procino, G.; Mastrofrancesco, L.; Sallustio, F.; Costantino, V.; Barbieri, C.; Pisani, F.; Schena, F.P.; Svelto, M.; Valenti, G. AQP5 is expressed in type-B intercalated cells in the collecting duct system of the rat, mouse and human kidney. Cell. Physiol. Biochem. 2011, 28, 683–692.
- Promeneur, D.; Kwon, T.H.; Yasui, M.; Kim, G.H.; Frokiaer, J.; Knepper, M.A.; Agre, P.; Nielsen, S. Regulation of AQP6 mRNA and protein expression in rats in response to altered acid-base or water balance. Am. J. Physiol. Ren. Physiol. 2000, 279, F1014–F1026
- Rodriguez, A.; Catalan, V.; Gomez-Ambrosi, J.; Fruhbeck, G. Aquaglyceroporins serve as metabolic gateways in adiposity and insulin resistance control. Cell Cycle 2011, 10, 1548–1556
- Tanaka, Y.; Watari, M.; Saito, T.; Morishita, Y.; Ishibashi, K. Enhanced Autophagy in Polycystic Kidneys of AQP11 Null Mice. Int. J. Mol. Sci. 2016, 17, 1993
- Rossi, L.; Nicoletti, M.C.; Carmosino, M.; Mastrofrancesco, L.; Di Franco, A.; Indrio, F.; Lella, R.; Laviola, L.; Giorgino, F.; Svelto, M.; et al. Urinary Excretion of Kidney Aquaporins as Possible Diagnostic Biomarker of Diabetic Nephropathy. J. Diabetes Res. 2017
- Bichet, D. G. (2006). Hereditary polyuric disorders: new concepts and differential diagnosis. Semin. Nephrol. 26, 224-233
- King, L. S., Choi, M., Fernandez, P. C., Cartron, J. P. and Agre, P. (2001). Defective urinary-concentrating ability due to a complete deficiency of aquaporin-1. N. Engl. J. Med. 345, 175-179
- Roudier, N., Ripoche, P., Gane, P., Le Pennec, P. Y., Daniels, G., Cartron, J. P. and Bailly, P. (2002). AQP3 deficiency in humans and the molecular basis of a novel blood group system, GIL. J. Biol. Chem. 277, 45854-45859
- Chepelinsky, A. B. (2009). Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts. Handb. Exp. Pharmacol. 190, 265-297
- Lennon, V. A., Kryzer, T. J., Pittock, S. J., Verkman, A. S. and Hinson, S. R. (2005). IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473-477
- Wingerchuk, D. M., Lennon, V. A., Lucchinetti, C. F., Pittock, S. J. and Weinshenker, B. G. (2007). The spectrum of neuromyelitis optica. Lancet Neurol. 6, 805-815.
- Saadoun, S., Waters, P., Bell, B. A., Vincent, A., Verkman, A. S. and Papadopoulos, M. C. (2010). Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133, 349-361