Camptocormia
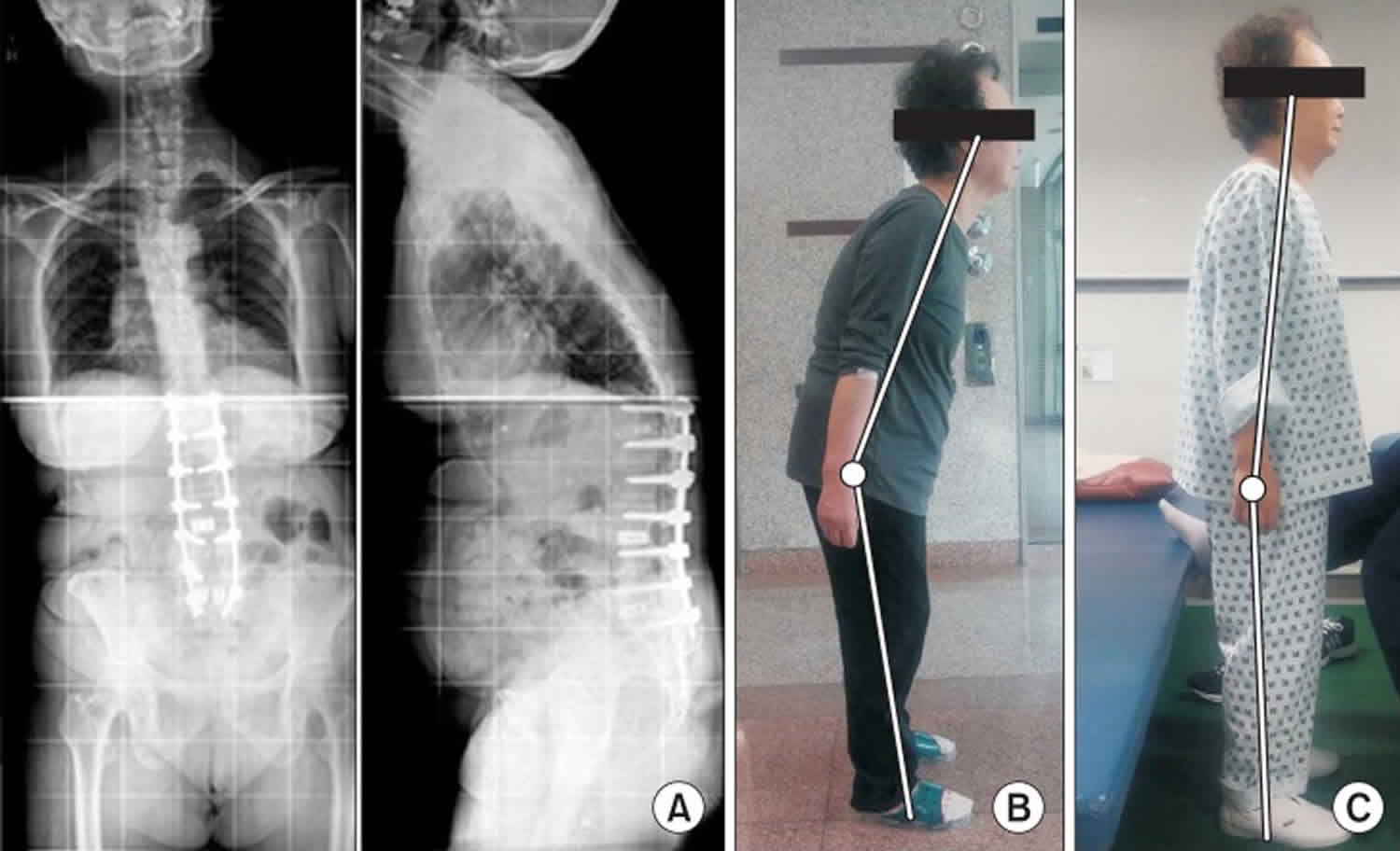
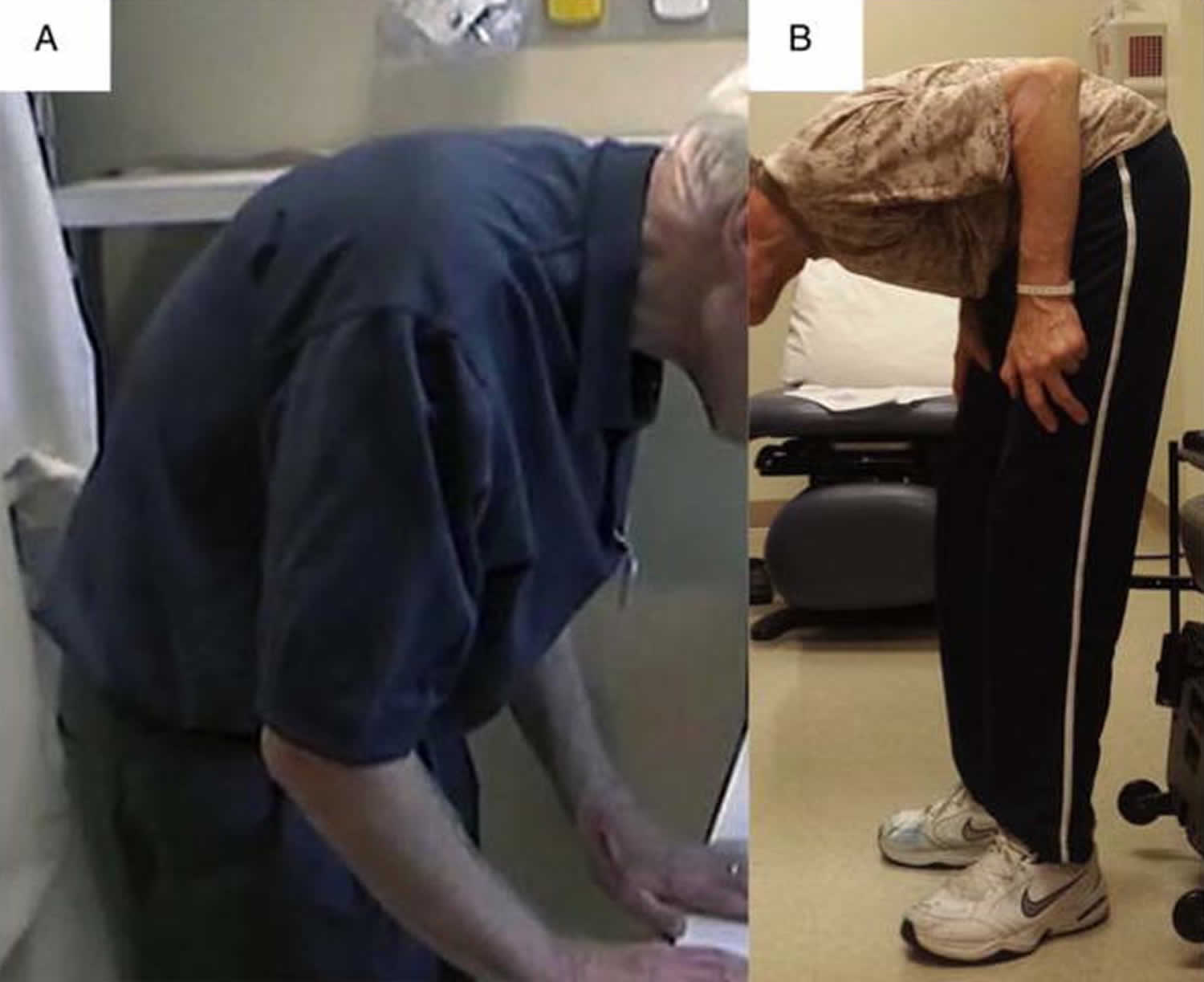
Camptocormia also known as “bent spine syndrome” or “cyphose hystérique”, is a disabling pathological, involuntary, non-fixed forward bending of the thoracolumbar spine with weight-bearing such as during standing, walking or sitting, which completely disappears when laying down, and is due to isolated atrophy of the paraspinal muscles 1. The term camptocormia is derived from the Greek word “kamptein” = to bend and “kormos” = trunk 2. There is no consensus on the degree of thoracolumbar flexion to define camptocormia 3. Most studies use a forward bending angle of between 15° to 45° when the individual is standing or walking to define camptocormia 4. Some authors subclassified camptocormia into upper and lower camptocormia with upper camptocormia defined as abnormal truncal flexion at a point between the lower thoracic and upper lumbar spine (Figure 1A) and lower camptocormia defined as flexion at the hip joint (figure 1B) 5. A large number of studies use only a descriptive term without a bending angle, indicating the difficulties in defining camptocormia 6. Based on a control group of patients with Parkinson’s disease who disclaimed suffering from camptocormia, a recent study demonstrated that the stooped posture of advanced Parkinson’s disease does not exceed a forward bending angle of 25°. Oeda et al. 7 found a similar forward bending angle distribution in Parkinson’s disease patients without camptocormia. Furthermore, analysis of the group of photo-documented Parkinson’s disease camptocormia patients (n = 145) showed that the bending angle as the sole criterion is insufficient to define camptocormia because a third of the patients who suffered subjectively from camptocormia had an angle of less than 30° 4. Others have defined camptocormia by a score of ≥2 of item 28 (posture) of the Unified Parkinson Disease Rating Scale part III (UPDRS III) 8. This definition does not differentiate between the stooped posture of advanced Parkinson’s disease and camptocormia. Even the revised MDS-UPDRS item “posture” cannot differentiate between stooped posture and camptocormia. The ability of a patient to straighten up temporarily does not rule out camptocormia and forward bending by orthopedic diseases of the spine must be excluded.
Camptocormia is a very disabling syndrome that frequently causes impairment in activities of daily living as well as back pain and social isolation of patients 9.
Characteristic complaints are the inability to drive a car because of the inability to turn the body backwards, inability to look people in the eyes, inability to carry something in front of the body, inability to pick up things that lay higher than the table or the shoulder position and problems in eating and swallowing or dyspnoea due to body position 10. Camptocormia syndrome was first described by Henry Earle in 1815 11 and reported by James Parkinson in some of his cases in 1817 12. The term was coined by the French neurologist A. Souques in 1915 to describe an “incurvation du tronc” in soldiers of World War 1 indicating a “cyphose hystérique” 13. Until the 1980 s, camptocormia was considered to be a psychiatric condition. Kiuru & Iivanainen 14 and Laroche et al. 15 were the first to describe camptocormia in association with organic diseases.
Camptocormia can be due to central nervous system diseases, such as Parkinson’s disease, dystonia, multisystem atrophy or Alzheimer’s disease, due to peripheral nervous system diseases, such as primary myopathy, secondary myopathy, motor neuron disease, myasthenia, or chronic inflammatory demyelinating polyneuropathy, due to side effects of drug treatment, due to disc herniation, arthritis or spinal trauma, or due to paraneoplasia 16. Camptocormia is most frequently encountered in movement disorders (Parkinson’s disease and dystonia) and muscles diseases (myositis and myopathy, mainly facio-scapulo-humeral muscular dystrophy) 4. Only rarely may camptocormia be attributable to psychiatric disease.
Camptocormia diagnosis is based on clinical findings, imaging of the cerebrum or spine, needle electromyography (EMG) or muscle biopsy 16.
Currently, there is no gold standard treatment available, treatment options are limited and frequently futile and rely on conservative measures, such as psychotherapy, physiotherapy, use of orthoses (such as plaster corset, low-slung backpack, high-frame walker with forearm support, thoraco-pelvic anterior distraction orthosis or taping), drugs, injection of botulinum toxin, withdrawal of causative drugs, electroconvulsive therapy, or invasive measures, such as surgical correction or deep brain stimulation. The outcome is generally fair. Some patients profit from therapy whereas others do not respond to treatment and become progressively immobile 16.
In summary, camptocormia is an involuntary pathological flexion of the thoracolumbar spine that is passively reversible (recumbent position) and that causes relevant impairment in daily life as well as back pain in most cases. The definition and the clinical diagnostic criteria should focus on the following aspects: Involuntary flexion of the thoracolumbar spine, reversibility in recumbent position, forward bending angle, individual complaints, back pain, hardening of the paraspinal muscle and the course during the day. Camptocormia in the vast majority of cases is an organic disorder, either a manifestation of central nervous system disorders or due to affection of the peripheral nerves or the skeletal muscle. Only rarely is camptocormia caused by a psychiatric disorder. Drugs, trauma, or orthopedic problems may have a contributing effect. Since camptocormia is due to a number of various different disorders, the initial step in the management of camptocormia is detection of the underlying cause. Treatment should generally be directed towards the underlying etiology and pathomechanism. General measures, such as physiotherapy, orthoses, or botulinum toxin may be helpful in single cases. Only if the underlying cause is effectively treated can a sub-stantial therapeutic effect be expected. If treatment of camptocormia is ineffective, patients sooner or later re-quire walking devices and lastly a wheelchair. Since the ability to characterize the pathophysiology of camptocormia in Parkinson’s disease with the available technologies is limited, an animal model of abnormal posturing is required to fully understand the postural phenomena and to develop effective treatment.
Figure 1. Camptocormia Parkinson’s disease
Footnote: Patients with Parkinson’s disease presenting with truncal flexion greater than 45° while standing. (A) Upper camptocormia defined as abnormal truncal flexion at a point between the lower thoracic and upper lumbar spine. (B) Lower camptocormia defined as flexion at the hip joint.
[Source 3 ]Camptocormia causes
Possible causes of camptocormia 3:
- Neurodegenerative diseases
- Dystonias
- Amyotrophic lateral sclerosis 23
- Inherited myopathies
- Acquired myopathies
- Myasthenia gravis 33
- Chronic inflammatory demyelinating polyradiculoneuropathy 34
- Medication-induced
- Lumbar disc herniation 40
- Lentricular lesion due to stroke 41
- Esophageal hiatal hernia 42
- Radiotherapy-induced 43
- Paraneoplastic process 44
- Familial cerebellar hypoplasia 45
Since the cause of camptocormia is multifactorial, its prevalence in general has not been studied. However, there was a study conducted by Laroche and Cintas 46 to evaluate the causes of camptocormia in 63 cases. The results showed that 40 of 63 cases were diagnosed as delayed-onset isolated paraspinal myopathy, including 4 cases concomitant with the diagnosis of Parkinson’s disease. Twenty-three of 63 cases were diagnosed with various aetiologies including camptocormia due to Parkinson’s disease (4 cases without evidence of paraspinal myopathy), combination of paraspinal myopathy and bilateral glutaeus medius myopathy (2 cases), limb girdle muscular dystrophy of unknown cause (8 cases), myotonic dystrophy (3 cases), facioscapulomuscular dystrophy (2 cases), inclusion body myositis (2 cases), polymyositis (1 case) and adult-onset progeria (1 case). Therefore, according to this study 46, only 8 of 63 cases (12.7%) were diagnosed as Parkinson’s disease, of which 4 cases showed paraspinal myopathy. Azher and Jankovic 47 investigated the aetiology of 16 patients with camptocormia and found that 11 cases (68.8%) were compatible with a diagnosis of Parkinson’s disease. The prevalence of camptocormia in Parkinson’s disease has also been studied. The largest study involving 1453 patients with Parkinson’s disease was conducted by Yoritaka et al 48 and reported a 9.5% rate of camptocormia. However, the prevalence of camptocormia in idiopathic Parkinson’s disease from all studies ranged from 3.0% to 17.7% 48. Most of these studies defined camptocormia using a minimum of 45° of thoracolumbar flexion; however, several studies did not mention the degree of spinal flexion 49.
Parkinson’s disease camptocormia
The pathogenesis of camptocormia in Parkinson’s disease is not clearly understood, and central and peripheral mechanisms have both been proposed. Most explanations are derived from observations in patients with Parkinson’s disease who responded to some type of treatment. However, the possible pathogenesis of camptocormia in Parkinson’s disease can be subdivided into four groups 3:
- Part of the disease progression seen in Parkinson’s disease;
- A form of dystonia occurring with Parkinson’s disease;
- A consequence of paraspinal myopathy due to the pathophysiology of Parkinson’s disease or concomitantly occurring with Parkinson’s disease;
- Caused by medications that were used in patients with Parkinson’s disease.
Camptocormia is a part of the disease progression seen in Parkinson’s disease
Camptocormia associated with Parkinson’s disease usually emerges as the disease progresses 50. There are several lines of evidence supporting this contention. First, camptocormia is likely to develop in Parkinson’s disease with a long duration of disease, high score on the Unified Parkinson’s disease Rating Scale (UPDRS) part III, and advanced Hoehn and Yahr stage. Second, camptocormia was reported in patients with Parkinson’s disease who had no evidence of paraspinal myopathy proven by electromyography or histopathology 46. Third, there have been a few cases showing that camptocormic symptoms could be reversed following levodopa 51. Fourth, camptocormic symptoms were also improved by high-frequency subthalamic nucleus 52 or globus pallidus interna deep brain stimulation 53 together with improved motor symptoms in patients with Parkinson’s disease. The argument against this idea is that most cases of camptocormia in Parkinson’s disease did not respond to either levodopa 47 or deep brain stimulation (DBS) 17. However, as is the nature of axial motor symptoms of Parkinson’s disease, such as freezing of gait, some cases respond to either dopaminergic medications or deep brain stimulation but some cases do not respond to any of these treatments. Furthermore, the roles of non-dopaminergic systems in Parkinson’s disease with camptocormia are also unclear. Evidence has shown that anticholinergics, benzodiazepines and baclofen, failed to provide benefit for most patients with camptocormia 22. Such mixed evidence about neurotransmitter systems makes it difficult to draw any conclusions, but does suggest that there is not a simple relationship. Investigations of which structures in the central nervous system are involved in the pathogenesis of camptocormia in Parkinson’s disease, such as measuring brain metabolism or functional connectivity, are limited because the camptocormic symptoms disappear while lying in the scanner. However, in a structural brain MRI study of camptocormia in patients with Parkinson’s disease, the severity of the camptocormia was negatively correlated with the normalised sagittal surface of the pons and whole brain volume 54. There was no difference in [123I] β-CIT SPECT in Parkinson’s disease with and without camptocormia 55. Therefore, scientists cannot specify which parts of the brain play a role in the pathogenesis of camptocormia in Parkinson’s disease.
Camptocormia is a form of dystonia occurring with Parkinson’s disease
Dystonia can occur in any part of the body in patients with Parkinson’s disease. Many features of camptocormia are compatible with the definition of dystonia. Camptocormia presents and usually worsens while walking or exercising. This is compatible with action-induced dystonia. Dystonia can cause an abnormal posture in any body part, which is similar to camptocormia in terms of abnormal spinal flexion that might be the effect of strong abdominal muscle contraction 56. There are several manoeuvres to alleviate camptocormic symptoms that might be similar to ‘sensory tricks’ that present in dystonia such as standing against a wall or wearing a low-slung backpack. In axial dystonia such as cervical dystonia, previous studies have shown that patients with cervical dystonia have an internal postural perceptive distortion. When asking patients to direct their heads forward, patients with cervical dystonia had a greater deviation of their head position compared with normal patients 57. Similarly, patients with early camptocormia might have a distorted concept of what an erect spine is. Finally, in isolated primary dystonic camptocormia, treating with GPi-DBS might show clinical improvement as rated by the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS), ranging from 33% to 100% after surgery 58. There were case reports of dopa-responsive dystonia presenting with camptocormia in which the symptom totally disappeared after a low dose of levodopa; no neurological disorders appeared during follow-up many years later 21. However, this hypothesis still lacks supportive physiological studies. Cortical inhibition such as short-intracortical inhibition, longintracortical inhibition and cortical silent period (investigated by transcranial magnetic stimulation), and blink reflex recovery cycle, that show abnormalities in dystonia 59, have not been explored in camptocormia.
Camptocormia is a consequence of paraspinal myopathy due to the pathophysiology of Parkinson’s disease or concomitantly occurs with Parkinson’s disease
The possibility of a myopathy causing camptocormia has been controversial. In this regard, camptocormia might be considered analogous to dropped head syndrome, also thought sometimes due to a myopathy. There are two hypotheses to explain the thoracolumbar paraspinal myopathy in camptocormia caused by Parkinson’s disease itself 60. First, camptocormia might be a consequence of overusing paraspinal muscles due to rigidity in patients with Parkinson’s disease. However, the results of muscle biopsies in camptocormia due to overuse myopathy were different compared to camptocormia in patients with Parkinson’s disease. Muscle biopsy of the former condition revealed marked fibre necrosis, inflammation and macrophage reaction compared to the latter, which lacked an inflammatory process. The second hypothesis is proprioceptive dysregulation. Patients with Parkinson’s disease have a poor ability to estimate the amplitude of joint motion in terms of accuracy as a result of abnormal proprioception compared to normal controls; the abnormal proprioception can also occur in axial musculature. According to the proprioceptive dysregulation hypothesis, inappropriate proprioceptive information will be sent back to supraspinal areas; at that point, supraspinal control provides inappropriate feed forward information to spinal interneuron circuits for adjusting the tone of axial muscles resulting in inappropriate muscle loading that might cause rigidity and myopathy, and, eventually, camptocormia. In addition, impaired proprioception of the axial musculature in Parkinson’s disease correlated with the severity of the Unified Parkinson’s disease Rating Scale (UPDRS) part III 60. Additional evidence that might support the role of proprioceptive dysregulation causing paraspinal myopathy, which would be the proximate cause of camptocormia, comes from experimental Achilles tenotomy in rats 61. Tenotomy can alter the proprioceptive function of the muscle that attaches to the tendon. Histopathological findings of soleus muscle in the rat, following tenotomy, showed core-like lesions in centre and periphery of type 1 fibres with reducing activity of oxidative enzymes including succinate dehydrogenase and ATPase while increasing activity of acid phosphatase. These histopathological findings were similar to the typical biopsy of paraspinal muscles of Parkinson’s disease with camptocormia 60. In this regard, camptocormia might develop due to secondary paraspinal myopathy that is influenced by proprioceptive dysregulation. Another possible pathophysiology of camptocormia in Parkinson’s disease is that there is a concomitant myopathy not necessarily directly related to Parkinson’s disease. There are many reports that both inherited and acquired myopathies could both be an aetiologies of camptocormia and these myopathies could occur as a concomitant condition in patients with Parkinson’s disease. In this circumstance, some scientists consider that myopathy is a primary aetiology of camptocormia without any correlation to the pathophysiology of Parkinson’s disease. However, there are strong arguments against camptocormia being due to paraspinal myopathy. First, there is no good evidence of truncal weakness in patients with camptocormia that should be present with myopathy. Second, the oedema of paraspinal muscles seen with muscle MRI is not specific and cannot confirm a myopathy 50. According to the cited evidence, the main pathogenesis of camptocormia in patients with Parkinson’s disease does not appear to be solely explained by myopathy.
Camptocormia is caused by medications that are used in Parkinson’s disease
To date, there is only one study suggesting that camptocormia in Parkinson’s disease is the result of administering a dopaminergic agent 39. Galati et al 39 reported a patient who was initially well controlled with 4 mg daily of ropinirole extended release tablets. However, she slowly developed a combination of camptocormia and Pisa syndrome. Levodopa was added and her motor symptoms improved; however, her abnormal posture worsened. The authors decided to withdraw ropinirole without modifying the levodopa dose. After withdrawing ropinirole, her posture returned to nearly normal within 3 months. Other cases of medication-induced camptocormia were reported in a patient with vascular parkinsonism who received pramipexole 38 and severe anxiety depression in a patient who received multiple antipsychotic medications including olanzapine and clozapine 36. In the former case, the patient’s camptocormia improved within a month after discontinuation of pramipexole. In the latter case, after her depression was controlled by multiple sessions of electroconvulsive therapy and antipsychotic medications were stopped, the patient’s posture was totally upright and she did not show any abnormal posture within the 6-month follow-up period. The possible explanation for antipsychotics-induced camptocormia might be related to their extrapyramidal side effects that might cause truncal flexion. However, the reason dopaminergic agents would induce camptocormia is unknown.
Camptocormia symptoms
Camptocormia is clinically characterized by an excessive involuntary trunk flexion due to progressive weakness of the extensor vertebral muscles 62. In more severe cases, the patient might present with an anthropoid posture (severe flexion is defined as having the head and trunk parallel with the ground with arms swinging normally) 63. Camptocormia is enhanced during standing and walking 64 and relieved in recumbent or supine position 65. In quite a number of cases, camptocormia is associated with lower back pain 66) but in others it is painless 67. Alleviating manoeuvres are sitting, lying in a recumbent position, standing against a wall or using walking support. Camptocormia may be associated with concomitant weakness of the gluteus maximus and hip and genuflexion 68. Camptocormia is associated with dropped head syndrome only in single cases 69, such as in patients with myotonic dystrophy type 1 62, multiple system atrophy, or postencephalitic Parkinson syndrome 70. Nearly all patients with camptocormia have spondylarthrosis, rendering it a risk factor for developing camptocormia 71. In a study of 16 patients, mean age at onset of camptocormia was 65 years and mean age at onset of neurological abnormalities was 52 years 72. Almost 69% of the patients had Parkinson’s disease, 25% had dystonia, and one Gilles de la Tourette syndrome 72. The family history may be positive for muscle disease in up to 50% of the cases 73.
Previous studies showed that camptocormia usually presented following a diagnosis of Parkinson’s disease with the disease duration ranging from 6 to 8 years 74. In addition, some patients with Parkinson’s disease reported that their symptoms were aggravated by stress, fatigue and strenuous exercise 75.
Camptocormia diagnosis
Since the cause of camptocormia is quite heterogeneous, investigations in different directions have to be carried out at the beginning of the diagnostic work-up. Generally, the diagnosis may be established upon clinical findings, blood chemical investigations, imaging of the cerebrum, EMG, or muscle biopsy. There are a number of blood chemical parameters which are useful in the diagnostic work-up of camptocormia. Among these are the blood sedimentation rate (ESR), C-reactive protein (CRP), electrolytes, such as calcium and phosphorus, creatine kinase, aldolase, or vitamin D 16. For the diagnosis of metabolic myopathies, determination of lactate and pyruvate during standardized exercise can be of additional help.
Imaging studies
Cerebral CT scans may show atrophy, basal ganglia calcification, basal ganglia lacunas, lenticular lesions, or reduced volume of the midbrain or pons 76. Cranial MRI may show signal abnormalities of the basal ganglia in a small number of patients with camptocormia and Parkinson’s disease 77. MRI of the vertebral muscles may show features of a circumscribed myopathy, such as variable degrees of atrophy and fatty replacement of the thoracolumbar paraspinal muscles 78. These alterations are similar to those seen in muscular dystrophy 79. Localized changes from edema with contrast enhancement are considered to be an early sign, whereas atrophy or fatty degeneration are considered as late changes 80. Some authors interpret these changes rather as secondary than the cause of camptocormia 81. CT scans of the spinal muscles may show atrophy and hypodensity of the muscles being interpreted as fatty involution 82.
Needle electromyography
Depending on the underlying cause, EMG may be normal, neurogenic or myogenic. A myogenic pattern may be recorded even in patients with Parkinson’s disease 83. Needle EMG of the paravertebral muscles may also reveal abundant fibrillations, positive sharp waves, orbizarre high-frequency discharges 84.
Muscle biopsy
Muscle biopsy may be normal or may show mild myopathic features, inflammatory features suggesting focal inflammatory myopathy (focal myositis), or dystrophic features 79. There may also be extensive diffuse or lobulated fibrosis as the only variant finding in camptocormia patients as compared to controls 80. Muscle biopsy in Parkinson’s disease patients with camptocormia may be divided into three groups, i.e. necrotizing myopathy, inflammatory myopathy, or mitochondrial myopathy 85.
Myopathic changes in patients with Parkinson’s disease include abnormal fiber size variation, increase of internal nuclei, increase of connective tissue, or myofiber disarray, or fatty degeneration 83. In patients with advanced Parkinson’s disease and camptocormia muscle biopsy may show end-stage myopathy with autophagic vacuoles, chronic inf lammatory myopathy, non-specific myopathic changes, or mitochondrial myopathy 84. Single cases may also show amyloid deposition and ragged red fibers.
Gait analysis
Kinematic, kinetic and biomechanical analysis may reveal exaggerated anterior pelvic tilt during terminal stance when walking in an upright posture. In a forward-bent posture, however, the anterior pelvic tilt may be significantly less 86. Some authors assume that the extreme forward-bent posture is a compensatory mechanism to reduce the excessive pelvic tilt 86.
Camptocormia treatment
Since there are a variety of causes for camptocormia, treatment involves treating the underlying causes. Such treatment might be divided into three categories; conservative non-pharmacological, pharmacological and surgical approaches including deep brain stimulation. Conservative measures include psychotherapy, physiotherapy, application of drugs, injection of botulinum toxin, withdrawal of causative drugs, or electroconvulsive therapy. Invasive therapeutic measures include surgical methods or deep brain stimulation. Treatment of choice is the therapy of the underlying disorder and in case no disease-modifying agents are available orthoses, physiotherapy, and eventually analgesics are the only choice 87.
Patients with drug-induced camptocormia usually respond to withdrawal of antipsychotics or reduction of the daily doses. Single cases with inflammatory myopathy of the paraspinal muscles may profit from administration of steroids 71. Steroids for camptocormia in Parkinson’s disease, on the contrary, failed to show a beneficial effect 64. Application of anticholinergics, amantadine, dopamine agonists, muscle relaxants or tetrabenazine is usually ineffective 81.
Psychotherapy
There are a number of psychotherapeutic techniques which can be applied to patients with camptocormia with a psychogenic origin. These include psychoeducation regarding secondary gain, suggestions to improve posture, positive reinforcement, or behavioral therapy 88. Persuasive re-education was particularly applied in WW1 cases but this psychological therapy was rather additive than persuasive 89.
Physiotherapy and orthoses
Classical orthoses and physiotherapy often provide little correction, are often poorly tolerated 87 and are quickly abandoned 90. Application of a thoracopelvic anterior distraction orthosis, however, results in a quality of life increase by 90% 87. In single cases, physiotherapy and orthoses may relieve lower back pain 91.
Levodopa
In the majority of cases with advanced Parkinson’s disease, camptocormia is levodopa (L-DOPA)-resistant 92. However, in single cases, camptocormia associated with Parkinson’s disease, dystonia, or multiple system atrophy, administration of L-DOPA has been shown to be beneficial 93. Depending on the investigated cohort, up to 20% of the Parkinson patients with camptocormia profit from L-DOPA therapy 68. In a patient with multiple system atrophy with predominant parkinsonism, camptocormia and Parkinson’s disease markedly improved under L-DOPA 92. In a patient with Parkinson’s disease, adjustment of dopaminergic therapy by carbidopa-levodopa and entacarpone resulted not only in improvement of Parkinson’s disease but also of camptocormia 94.
Immunoglobulins
Little data have been published demonstrating a beneficial effect of immunoglobulins (IVIG) in camptocormia 69. An 81-year-old male with confirmed inflammatory myopathy of the paraspinal muscles experienced dramatic improvement to treatment with IVIG 69. IVIG seem to be effective only in cases with inflammatory myopathy.
Botulinum toxin
Injection of botulinum toxin into the rectus abdominis muscles has been shown to be beneficial in single cases in which camptocormia was due to focal dystonia 95. Injections of botulinum toxin into the iliopsoas muscles may also relieve camptocormia 96. In other patients with Parkinson-associated camptocormia, however, injection of botulinum toxin into the iliopsoas muscle was ineffective 97. Botulinum toxin may not only be effective in patients with focal dystonia but also in patients with Parkinson’s disease 98.
Electroconvulsive therapy
In a single patient with camptocormia induced by olanzapine, discontinuation of the drug and application of L-DOPA was hardly effective, but electroconvulsive therapy was tried with success 99.
Deep brain stimulation
In single cases in which camptocormia is associated with Parkinson’s disease or segmental dystonia, bilateral pallidal high-frequency deep brain stimulation 100 or bilateral subthalamic nucleus stimulation 101 may have a beneficial effect. The therapeutic effect of deep brain stimulation suggests that, at least in single cases, camptocormia is indeed a CNS disease due to affection of the striatum and its reticulospinal and thalamic projections 71. In a patient with longstanding crippling Parkinson’s disease, camptocormia improved dramatically after bilateral subthalamic deep brain stimulation 102. For pallidal stimulation deep brain stimulation electrodes are stereotactically implanted to target the internal globus pallidus 103. Long-term pallidal stimulation results in significant functional improvement in the ab-sence of any treatment-related adverse effects 103.
Surgery
In cases where conservative measures are unsuccessful, patients may profit from posterior thoracolumbar fixation, which may need to be augmented with anterior interbody fusion 104.
Camptocormia Parkinson’s treatment
Potential treatment modalities for treating camptocormia in Parkinson’s disease.
- Non-pharmacological approaches
- Plaster corset
- Low-slung backpack with weight
- High-frame walker with forearm support
- Thoraco-pelvic anterior distraction orthosis
- Physiotherapies
- Proprioceptive and tactile stimulation
- Stretching
- Postural re-education
- Kinesiotaping on thoracolumbar paraspinal muscle
- Pharmacological approaches
- Levodopa
- Botulinum neurotoxin injection
- Lidocaine injection
- Surgical approaches
- Orthopaedic spinal surgical correction
- Unilateral pallidotomy
- Bilateral high-frequency deep brain stimulation
- Subthalamic nucleus
- Globus pallidus interna
- Repetitive trans-spinal magnetic stimulation (immediate and short-lasting effect)
Non-pharmacological approaches
Historically, during World War 1, Rosanoff-Saloff and Souques described a French soldier who was diagnosed with painful camptocormia with almost a 90° thoracolumbar flexion due to conversion disorder; the symptoms were improved by applying a plaster corset 105. Subsequently, there was a case report of a patient with Parkinson’s disease who developed camptocormia resistant to dopaminergic treatment. His camptocormia totally disappeared while the patient wore a 6 kg low-slung backpack and returned after the backpack was removed 106. Another manoeuvre that was reported to alleviate camptocormia was using a high-frame walker with forearm support 107. Three patients with idiopathic Parkinson’s disease, using the high-frame walker, improved their walking distances; using the high-frame walker also reduced the degree of camptocormia and lessened these patients’ back pain. The evidence of wearing a corset, carrying a weighted backpack and using high-frame walker with forearm support has only been reported in single cases or small case series. However, de Sèze et al 108 conducted a prospective study with 15 camptocormic patients using a thoracopelvic anterior distraction orthosis and a physiotherapy programme; 2 of 15 were diagnosed with Parkinson’s disease. The authors showed that an orthosis improved pain and quality of life (QoL), as assessed with a visual analogue scale. Average pain scores were reduced by 69% and 70% on days 30 and 90, respectively, when compared to day 0. The average improvement of QoL was 87% and 92% on days 30 and 90, respectively, when compared with day 0. Current evidence from a meta-analysis showed that physiotherapy could improve motor symptoms, especially gait and balance, in patients with Parkinson’s disease 109. However, no strong evidence is available concerning the efficacy of physiotherapy for postural abnormalities in patients with Parkinson’s disease. However, there has been a recent single-blind, randomised controlled trial that compared efficacy between postural rehabilitation (n=7), postural rehabilitation plus using kinesiotaping (KT) on thoracolumbar paraspinal muscles (n=6) and no intervention (n=7) involving 20 patients with Parkinson’s disease with anterior and/or lateral trunk bending 110. Postural rehabilitation was targeted on proprioceptive and tactile stimulation, stretching and postural re-education through active movement execution. At the end of the first month, the physiotherapy groups, either postural rehabilitation or postural rehabilitation plus using kinesiotaping, showed significant improvement in anterior trunk bending, gait and balance compared with pretreatment, and also showed significant improvement in anterior and lateral trunk bending, gait and balance compared with the no intervention group 110. Therefore, physiotherapy might be an option for improving the postural abnormality in patients with Parkinson’s disease. Recently, there has been a randomised, single-blind, crossover, placebo-controlled study of 37 patients using repetitive trans-spinal magnetic stimulation (rTSMS). Eight 1 s trains of 5 Hz stimulation were given with intertrain interval of 10 s. The stimulation was delivered with a circular coil over the area of maximal thoracolumbar flexion. The primary outcome showed that rTSMS produced immediate relief of camptocormia in term of reduction of the degree of thoracolumbar flexion compared with sham stimulation (mean of 10.9° vs −0.1°, respectively). However, the authors measured the outcome only immediately after completing stimulation and did not investigate a longer lasting effect. Therefore, the value of rTSMS for treating camptocormia is not clear 111.
Pharmacological approaches
The efficacy of oral levodopa for alleviating camptocormic symptoms is uncertain. Reports have indicated that oral levodopa could attenuate camptocormic symptoms in some cases of dopa-responsive dystonia 21, Parkinson’s disease 51 and multiple system atrophy 112. However, for Parkinson’s disease, the effect of levodopa for reducing camptocormic symptoms was completely unpredictable. Bloch et al 113 reported that approximately 20% of patients with Parkinson’s disease with camptocormia received some benefit from oral levodopa. There was no report of other dopaminergic medications that improved camptocormic symptoms in patients with Parkinson’s disease. Other oral antidystonic and antispasmodic medications, including trihexiphenidyl, baclofen, amantadine, biperiden, tetrabenazine, clonazepam and bromazepam were also disappointing 22.
Botulinum neurotoxin (BoNT) injection and lidocaine have been used to treat camptocormia. In summary of this literature, botulinum neurotoxin serotype A, including abobotulinumtoxin A 114, onabotulinumtoxin A58 115 and incobotulinumtoxin A 116, was studied in patients with Parkinson’s disease with camptocormia. Two studies used ultrasound-guided BoNT injection, one study used CT-guided BoNT injection and one study used a blind injection technique. Rectus abdominis and iliopsoas muscles were the main muscles injected. Several outcome measurements including objective 115 and subjective 116 outcome measurements were used to evaluate the efficacy of BoNT injection. Overall, the efficacy of BoNT injection is controversial. It is premature to draw the conclusion that Botulinum neurotoxin (BoNT) injection is ineffective. There are many reasons, including small sample sizes, not injecting BoNT into other muscles (eg, abdominal external and internal oblique muscles) that might contribute to camptocormia, insufficient data for appropriate doses and types of BoNT, and lack of a standard clinical outcome measurement.
Another injection agent investigated was lidocaine (with rehabilitation). Furusawa et al 117 conducted a study using 50 mg of 1% lidocaine, which was injected bilaterally into the abdominal external oblique muscles under ultrasound guidance to treat 12 Parkinson’s disease with upper camptocormia followed by rehabilitation, which emphasised truncal extension. Initially, a single injection was used and then repeated once daily for 4–5 days in all patients. The result showed that eight patients showed significant improvement in posture after a single injection together with rehabilitation. However, the effect diminished in several days. Repeated intervention produced long-term improvement in nine patients while eight of these patients revealed a lasting effect during the 90-day follow-up period. However, the data need to be reproduced in a randomised study involving a large population.
Surgical approaches
Surgical approaches to treat camptocormic symptoms in Parkinson’s disease are orthopaedic surgical correction, pallidotomy, and deep brain stimulation (DBS) targeting the subthalamic nucleus and globus pallidus interna. For orthopaedic surgical correction, all reports showed some benefit from surgery in terms of pain reduction and postural correction compared to the preoperative stage 118. Only one report showed excellent results and the benefits were maintained at least 29 months after surgery 119. However, the surgical procedure was complicated in all patients. There was a case report of a patient with Parkinson’s disease with 60° of camptocormia who received a unilateral right-sided pallidotomy 2 years after the onset of camptocormia that significantly improved her posture and gait 120. The patient reported that the benefit of the pallidotomy immediately occurred after surgery and lasted at least 6 months. At present, DBS is an option to treat symptoms of various types of dystonia and Parkinson’s disease. However, there is no solid evidence that DBS is an appropriate treatment for camptocormia. The evidence for DBS to treat camptocormia either as an outcome of various types of dystonia or associated with Parkinson’s disease came from case reports or a series of small cases. Recently, there was a case series of 16 patients (3 cases from the authors and 13 cases from the literature) who presented with camptocormia due to generalised dystonia, segmental dystonia or isolated camptocormia, and who received bilateral globus pallidus interna-DBS 58. The results showed that globus pallidus interna-DBS improved clinical outcome in terms of improved total and trunk subscores for the BFMDRS. These improved scores ranged from 33% to 100% for the total score and 50%–100% for the trunk subscore. The time of last follow-up ranged from 6 to 60 months after surgery. In Parkinson’s disease with camptocormia, subthalamic nucleus-DBS 121 and globus pallidus interna-DBS 53 have both been used. In summary, 56 patients with Parkinson’s disease with camptocormia who received DBS to treat their camptocormic symptoms have been reported in the literature. Fifty-one patients received subthalamic nucleus-DBS whereas five patients received globus pallidus interna-DBS. Thirty-four of 56 patients (61%) noted that their posture had improved following DBS. However, the efficacy of subthalamic nucleus-DBS or globus pallidus interna-DBS to treat camptocormia in patients with Parkinson’s disease should be measured cautiously because there are a variety of outcome measurement tools in every study. Therefore, the exact efficacy of subthalamic nucleus-DBS and globus pallidus interna-DBS on camptocormia in patients with Parkinson’s disease is still inconclusive but it might be considered an option to treat levodopa non responsive camptocormia in patients with Parkinson’s disease.
Camptocormia physical therapy
In this study 122, therapeutic exercise such as low-slung backpack-wearing, core muscle strengthening exercise, back extensor muscles strengthening exercise and pelvic tilting exercise and anterior spinal hyperflexion brace have been tested and shown to improve activities of daily living and motor symptoms present in Parkinson’s disease with camptocormia. In the future, a conservative treatment study with a larger group of patients is needed.
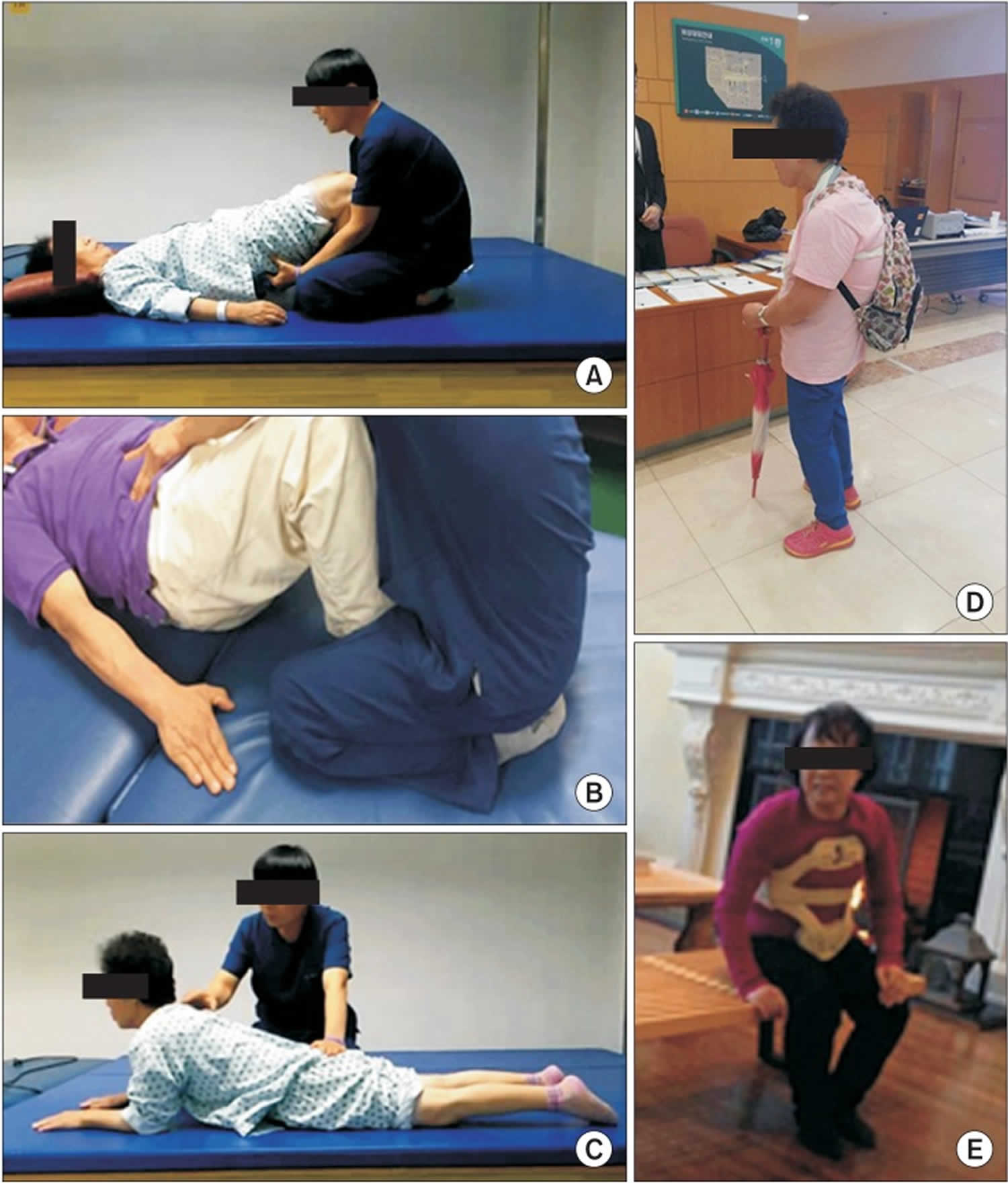
Six inpatients received a 30-minute treatment twice a day for 5 weeks, for an average of 34 days (range, 23–44 days) 122. Treatment sessions were composed of 20 minutes of physiotherapy-core muscle strengthening exercise, pelvic tilting exercise, back extensor strengthening exercise and bending side muscle stretching exercise, more focused on strengthening 122. Then 10 minutes of treatment comprised ambulation while wearing a low-slung backpack 122. The weight of the backpack started at 1.5 kg. Depending on tolerability, we increased the weight of the backpack until camptocormia was corrected in a standing position 122. Patients were educated to wear the low-slung backpack at least 2 hours daily while ambulating. Anterior spinal hyperflexion brace was applied to 1 patient who was not able to tolerate the low-slung backpack due to its weight 122.
Figure 2. Camptocormia physical therapy
Footnote: Various modalities of conservative management for camptocormia. (A) Core muscle strengthening exercise. (B) Pelvic tilting exercise. (C) Back extensor strengthening exercise. (D) Low-slung backpack. (E) Anterior spinal hyperflexion brace.
[Source 122 ] References- Schulz-Schaeffer WJ, Margraf NG, Munser S, Wrede A, Buhmann C, Deuschl G, & Oehlwein C (2015) Effect of neurostimulation on camptocormia in Parkinson’s disease depends on symptom duration. Mov Disord, 30, 368–372.
- Pérez-Sales P. Camptocormia. Br J Psychiatry. 1990;157:765–7.
- Srivanitchapoom P, Hallett M. Camptocormia in Parkinson’s disease: definition, epidemiology, pathogenesis and treatment modalities. J Neurol Neurosurg Psychiatry. 2016;87(1):75-85. doi:10.1136/jnnp-2014-310049 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5582594
- Margraf NG, Wrede A, Deuschl G, Schulz-Schaeffer WJ. Pathophysiological Concepts and Treatment of Camptocormia. J Parkinsons Dis. 2016;6(3):485-501. doi:10.3233/JPD-160836 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5008234
- Furusawa Y, Mukai Y, Kobayashi Y, et al. Role of the external oblique muscle in upper camptocormia for patients with Parkinson’s disease. Mov Disord. 2012;27:802–3.
- Asahi T, Taguchi Y, Hayashi N, Hamada H, Dougu N, Takashima S, Tanaka K, & Endo S (2011) Bilateral subthalamic deep brain stimulation for camptocormia associated with Parkinson’s disease. Stereotact Funct Neurosurg, 89, 173–177.
- Oeda T, Umemura A, Tomita S, Hayashi R, Kohsaka M, & Sawada H (2013) Clinical factors associated with abnormal postures in Parkinson’s disease. PLoS One, 8, e73547
- Umemura A, Oka Y, Ohkita K, Yamawaki T, & Yamada K (2010) Effect of subthalamic deep brain stimulation on postural abnormality in Parkinson disease. J Neurosurg, 112, 1283–1288.
- Abe K, Uchida Y, & Notani M (2010) Camptocormia in Parkinson’s disease. Parkinsons Dis, pii: 267640
- Margraf NG, Granert O, Hampel J, Wrede A, Schulz-Schaeffer WJ, Deuschl G. Clinical Definition of Camptocormia in Parkinson’s Disease. Mov Disord Clin Pract. 2016;4(3):349-357. Published 2016 Oct 11. doi:10.1002/mdc3.12437 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6174367
- Earle H (1815) Reply to the review of Mr. Bayrton’s essay on the cure of crooked spine. Edinburgh Med Surg J, 11, 35–51.
- Parkinson J (2002) An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci, 14, 223–236.
- Souques A (1916) Reformes, incapacites, gratifications dans la camptocormie. Rev Neurol, 23, 757–758.
- Kiuru S, & Iivanainen M (1987) Camptocormia, a new side effect of sodium valproate. Epilepsy Res, 1, 254–257.
- Laroche M, Rousseau H, Mazieres B, Bonafe A, Joffre F, & Arlet J (1989) [Value of x-ray computed tomography in muscular pathology. Personal cases and review of the literature]. Rev Rhum Mal Osteoartic, 56, 433–439.
- Finsterer, Josef & Strobl, Walter. (2010). Presentation, Etiology, Diagnosis, and Management of Camptocormia. European neurology. 64. 1-8. 10.1159/000314897
- Schäbitz WR, Glatz K, Schuhan C, et al. Severe forward flexion of the trunk in Parkinson’s disease: focal myopathy of the paraspinal muscles mimickingcamptocormia. Mov Disord. 2003;18:408–14.
- Diederich NJ, Goebel HH, Dooms G, et al. Camptocormia associated with focal myositis in multiple-system atrophy. Mov Disord. 2006;21:390–4.
- Gavrylova N, Limousin N, Belin J, et al. Camptocormia as presenting sign in dementia with Lewy bodies. Clin Neurol Neurosurg. 2013;115:2397–8.
- Brucki S, Nitrini R. Camptocormia in Alzheimer’s disease: an association? Mov Disord. 2008;23:156–7.
- Micheli F, Pardal MM. Dopa-responsive dystonic camptocormia. Neurology. 2007;68:1543.
- Capelle HH, Schrader C, Blahak C, et al. Deep brain stimulation for camptocormia in dystonia and Parkinson’s disease. J Neurol. 2011;258:96–103.
- Castrillo Sanz A, Rodríguez Vico J, Hernández Barral M, et al. Early appearance of camptocormia in motor neuron disease: an association? J Clin Neuromuscul Dis. 2013;15:43–4.
- Papadopoulos C, Papadimas GK, Spengos K, et al. Bent spine syndrome in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2011;43:615.
- Dupeyron A, Stober N, Gelis A, et al. Painful camptocormia: the relevance of shaking your patient’s hand. Eur Spine J. 2010;19(Suppl 2):S87–90.
- Findlay AR, Lewis S, Sahenk Z, et al. Camptocormia as a late presentation in a manifesting carrier of duchenne muscular dystrophy. Muscle Nerve. 2013;47:124–7.
- Kemta Lekpa F, Chevalier X, Dubourg O, et al. Isolated camptocormia revealing sporadic late onset nemaline myopathy. Presse Med. 2013;42:1142–4.
- Renard D, Castelnovo G, Fernandez C, et al. Camptocormia as presenting sign in myofibrillar myopathy. Neuromuscul Disord. 2012;22:987–9.
- Sakiyama Y, Okamoto Y, Higuchi I, et al. A new phenotype of mitochondrial disease characterized by familial late-onset predominant axial myopathy and encephalopathy. Acta Neuropathol. 2011;121:775–83.
- Zenone T, Streichenberger N, Puget M. Camptocormia as a clinical manifestation of polymyositis/systemic sclerosis overlap myositis associated with anti-Ku. Rheumatol Int. 2013;33:2411–15.
- Kim JM, Song EJ, Seo JS, et al. Polymyositis-like syndrome caused by hypothyroidism, presenting as camptocormia. Rheumatol Int. 2009;29:339–42.
- Ma H, McEvoy KM, Milone M. Sporadic inclusion body myositis presenting with severe camptocormia. J Clin Neurosci. 2013;20:1628–9.
- Devic P, Choumert A, Vukusic S, et al. Myopathic camptocormia associated with myasthenia gravis. Clin Neurol Neurosurg. 2013;115:1488–9.
- Terashima M, Kataoka H, Sugie K, et al. Coexistence of chronic inflammatory demyelinating polyneuropathy and camptocormia. J Neurol Neurosurg Psychiatry. 2009;80:1296–7.
- Kiuru S, Iivanainen M. Camptocormia, a new side effect of sodium valproate. Epilepsy Res. 1987;1:254–7.
- Vela L, Jiménez Morón D, Sánchez C, et al. Camptocormia induced by atypical antipsychotics and resolved by electroconvulsive therapy. Mov Disord. 2006;21:1977–80.
- Robert F, Koenig M, Robert A, et al. Acute camptocormia induced by olanzapine: a case report. J Med Case Rep. 2010;4:192.
- Nakayama Y, Miwa H. Drug-induced camptocormia: a lesson regarding vascular Parkinsonism. Intern Med. 2012;51:2843–4.
- Galati S, Möller JC, Städler C. Ropinirole-induced pisa syndrome in Parkinson disease. Clin Neuropharmacol. 2014;37:58–9.
- Duman I, Baklaci K, Tan AK, et al. Unusual case of camptocormia triggered by lumbar-disc herniation. Clin Rheumatol. 2008;27:525–7.
- Nieves AV, Miyasaki JM, Lang AE. Acute onset dystonic camptocormia caused by lentricular lesions. Mov Disord. 2001;16:177–80.
- Jang W, Kwon HS, Kim JS, et al. A Parkinson’s disease patient with camptocormia caused by an esophageal hiatal hernia. Mov Disord. 2012;27:922–3.
- Kelly L, Perju-Dumbrava LD, Thyagarajan D, et al. Delayed postirradiation camptocormia. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-200083. pii: bcr2013200083
- Zwecker M, Iancu I, Zeilig G, et al. Camptocormia: a case of possible paraneoplastic aetiology. Clin Rehabil. 1998;12:157–60.
- Turkmen S, Hoffmann K, Demirhan O, et al. Cerebellar hypoplasia, with quadrupedal locomotion, caused by mutations in the very low-density lipoprotein receptor gene. Eur J Hum Genet. 2008;16:1070–4.
- Laroche M, Cintas P. Bent spine syndrome (camptocormia): a retrospective study of 63 patients. Joint Bone Spine. 2010;77:593–6.
- Azher SN, Jankovic J. Camptocormia: pathogenesis, classification, and response to therapy. Neurology. 2005;65:355–9.
- Yoritaka A, Shimo Y, Takanashi M, et al. Motor and non-motor symptoms of 1453 patients with Parkinson’s disease: prevalence and risks. Parkinsonism Relat Disord. 2013;19:725–31.
- Lepoutre AC, Devos D, Blanchard-Dauphin A, et al. A specific clinical pattern of camptocormia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:1229–34.
- Jankovic J. Camptocormia, head drop and other bent spine syndromes: heterogeneous etiology and pathogenesis of Parkinsonian deformities. Mov Disord. 2010;25:527–8.
- Ho B, Prakash R, Morgan JC, et al. A case of levodopa-responsive camptocormia associated with advanced Parkinson’s disease. Nat Clin Pract Neurol. 2007;3:526–30.
- Lyons M, Boucher O, Patel N, et al. Long-term benefit of bilateral subthalamic deep brain stimulation on camptocormia in Parkinson’s disease. Turk Neurosurg. 2012;22:489–92.
- Thani NB, Bala A, Kimber TE, et al. High-frequency pallidal stimulation for camptocormia in Parkinson disease: case report. Neurosurgery. 2011;68:E1501–5.
- Bonneville F, Bloch F, Kurys E, et al. Camptocormia and Parkinson’s disease: MR imaging. Eur Radiol. 2008;18:1710–19.
- Holler I, Dirnberger G, Pirker W, et al. Camptocormia in idiopathic Parkinson’s disease: [(123)I]beta-CIT SPECT and clinical characteristics. Eur Neurol. 2003;50:118–20.
- Kataoka H, Tonomura Y, Eura N, et al. Painful abdominal contractions in patients with Parkinson disease. J Clin Neurosci. 2012;19:624–7.
- Müller SV, Gläser P, Tröger M. Disturbed ergocentric space representation in cervical dystonia. Mov Disord. 2005;20:58–63.
- Reese R, Knudsen K, Falk D, et al. Motor outcome of dystonic camptocormia treated with pallidal neurostimulation. Parkinsonism Relat Disord. 2014;20:176–9.
- Edwards MJ, Talelli P, Rothwell JC. Clinical applications of transcranial magnetic stimulation in patients with movement disorders. Lancet Neurol. 2008;7:827–40.
- Wrede A, Margraf NG, Goebel HH, et al. Myofibrillar disorganization characterizes myopathy of camptocormia in Parkinson’s disease. Acta Neuropathol. 2012;123:419–32.
- Karpati G, Carpenter S, Eisen AA. Experimental core-like lesions and nemaline rods: a correlative morphological and physiological study. Arch Neurol. 1972;27:237–51.
- Kocaaga Z, Bal S, Turan Y, Gurgan A, Esme-li F: camptocormia and dropped head syn-drome as a clinic picture of myotonic myop-athy. Joint Bone Spine 2008; 75: 730–733.
- Sandler SA. Camptocormia, or the functional bent back. Psychosom Med. 1947;9:197–204.
- Diaz-Guzman J, Nunez-Enamorado N, Ruiz-Jimenez J, Garcia E, Diez-Torres I, Ricoy-Campo JR: Parkinsonism and camp-tocormia with focal spinal myopathy: case report and responsiveness to treatment. Rev Neurol 2006; 43: 466–469.
- Kuo SH, Vullaganti M, Jimenez-Shahed J, Kwan JY: Camptocormia as a presentation of generalized inf lammatory myopathy. Mus-cle Nerve 2009; 40: 1059–1063.
- Dupeyron A, Stober N, Gelis A, Castelnovo G, Labauge P, Pélissier J: Painful camptocor-mia: the relevance of shaking your patient’s hand. Eur Spine J 2009 (in press
- Melamed E, Djaldetti R: Camptocormia in Parkinson’s disease. J Neurol 2006; 253(suppl 7):VII14–VII16.
- Bloch F, Houeto JL, Tezenas du Montcel S, Bonnev i l le F, Etchepa re F, Welter ML , Rivaud-Pechoux S, Hahn-Barma V, Mai-sonobe T, Behar C, Lazennec JY, Kurys E, Arnulf I, Bonnet AM, Agid Y: Parkinson’s disease with camptocormia. J Neurol Neuro-surg Psychiatry 2006; 77: 1223–1228.
- Dominick J, Sheean G, Schleimer J, Wixom C: Response of the dropped head/bent spine syndrome to treatment with intravenous im-munoglobulin. Muscle Nerve 2006; 33: 824–826.
- Umapathi T, Chaudhry V, Cornblath D, Drachman D, Griffin J, Kuncl R: Head drop and camptocormia. J Neurol Neurosurg Psy-chiatry 2002; 73: 1–7.
- Djaldetti R, Melamed E: Camptocormia in Parkinson’s disease: new insights. J Neurol Neurosurg Psychiatry 2006; 77: 1205.
- Azher SN, Jankovic J: camptocormia: patho-genesis, classification, and response to ther-apy. Neurology 2005; 65: 355–359.
- Ricq G, Laroche M: Acquired lumbar kyphosis caused in adults by primary paraspinal myopathy. Epidemiology, computed tomog-raphy findings, and outcomes in a cohort of 23 patients. Joint Bone Spine 2000; 67: 528–532.
- Spuler S, Krug H, Klein C, et al. Myopathy causing camptocormia in idiopathic Parkinson’s disease: a multidisciplinary approach. Mov Disord. 2010;25:552–9.
- Margraf NG, Wrede A, Rohr A, et al. Camptocormia in idiopathic Parkinson’s disease: a focal myopathy of the paravertebral muscles. Mov Disord. 2010;25:542–51.
- Lavault S, Bloch F, Houeto JL, Konofal E, Welter ML, Agid Y, Arnulf I: Periodic leg movements and REM sleep without atonia in Parkinson’s disease with camptocormia. Mov Disord 2009; 24: 2419–2423.
- Lepoutre AC, Devos D, Blanchard-Dauphin A, Pardessus V, Maurage CA, Ferriby D, Hurtevent JF, Cotten A, Destée A, Defebvre L: A specific clinical pattern of camptocor-mia in Parkinson’s disease. J Neurol Neuro-surg Psychiatry 2006; 77: 1229–1234.
- Haig AJ, Tong HC, Kendall R: The bent spine syndrome: myopathy + biomechanics = symptoms. Spine J 2006; 6: 190–194.
- Laroche M, Delisle MB, Aziza R, Lagarrigue J, Mazieres B: Is camptocormia a primary muscular disease? Spine (Phila Pa 1976) 1995; 20: 1011–1016.
- Delisle MB, Laroche M, Dupont H, Rochaix P, Rumeau JL: Morphological analyses of paraspinal muscles: comparison of progres-sive lumbar kyphosis (camptocormia) and narrowing of lumbar canal by disc protru-sions. Neuromuscul Disord 1993; 3: 579–582.
- Duman I, Baklaci K, Tan AK, Kalyon TA: Unusual case of camptocormia triggered by lumbar-disc herniation. Clin Rheumatol 2008; 27: 525–527.
- Hilliquin P, Menkès CJ, Laoussadi S, Job-D e s l a nd re C , S er r at r ic e G : C a mpto c or m i a i n the elderly. A new entity by paravertebral muscle involvement? Rev Rhum Mal Osteo-artic 1992; 59: 169–175.
- Margraf NG, Wrede A, Rohr A, Schulz-Schaeffer WJ, Raethjen J, Eymess A, Volk-mann J, Mehdorn MH, Jansen O, Deuschl G: Camptocormia in idiopathic Parkinson’s disease: a focal myopathy of the paraverte-bral muscles. Mov Disord 2010; 25: 542–551.
- Schäbitz WR, Glatz K, Schuhan C, Sommer C, Berger C, Schwaninger M, Hartmann M, Hilmar Goebel H, Meinck HM: Severe for-ward f lexion of the trunk in Parkinson’s dis-ease: focal myopathy of the paraspinal mus-cles mimicking camptocormia. Mov Disord 2003; 18: 408–414.
- Gdynia HJ, Sperfeld AD, Unrath A, Ludolph AC, Sabolek M, Storch A, Kassubek J: Histo-pathological analysis of skeletal muscle in patients with Parkinson’s disease and ‘dropped head’/’bent spine’ syndrome. Par-kinsonism Relat Disord 2009; 15: 633–639.
- Abdulhadi HM, Kerrigan DC: Camptocor-mia: a biomechanical analysis. A case report. Am J Phys Med Rehabil 1996; 75: 310 –313.
- De Sèze MP, Creuzé A, de Sèze M, Mazaux JM: An orthosis and physiotherapy pro-gramme for camptocormia: a prospective case study. J Rehabil Med 2008; 40: 761–765.
- Micheli F, Pardal MM: DOPA-responsive dystonic camptocormia. Neurology 2007; 68: 1543.
- Macleod AD: Head drop and camptocormia. J Neurol Neurosurg Psychiatry 2003; 74: 692.
- Pardessus V, Compere S, Tiffreau V, Blanchard A, Thevenon A: Leather corset for the treatment of camptocormia: 31 cases. Ann Readapt Med Phys 2005; 48: 603–609.
- Shinjo SK, Torres SC, Radu AS: Camptocor-mia: a rare axial myopathy disease. Clinics (São Paulo) 2008; 63: 416–417.
- Song IU, Kim JS, Lee KS: DOPA-responsive camptocormia in a patient with multiple sys-tem atrophy. Parkinsonism Relat Disord 2008; 14: 161–163.
- Van Gerpen JA: DOPA-responsive dystonic camptocormia. Neurology 2006; 66: 1779.
- Ho B, Prakash R, Morgan JC, Sethi KD: A case of levodopa-responsive camptocormia associated with advanced Parkinson’s dis-ease. Nat Clin Pract Neurol 2007; 3: 526–530.
- Mahjneh I, Edström B, Sandström G: Bent spine straightens up – a case of camptocor-mia. Duodecim 2009; 125: 1889–1893.
- Colosimo C, Salvatori FM: Injection of the iliopsoas muscle with botulinum toxin in camptocormia. Mov Disord 2009; 24: 316 –317.
- Von Coelln R, Raible A, Gasser T, Asmus F: Ultrasound-guided injection of the iliopsoas muscle with botulinum toxin in camptocor-mia. Mov Disord 2008; 23: 889–892.
- Fietzek UM, Schroeteler FE, Ceballos-Bau-mann AO: Goal attainment after treatment of parkinsonian camptocormia with botu-linum toxin. Mov Disord 2009; 24: 2027–2028.
- Zwecker M, Iancu I , Zeilig G, Ohry A: Camptocormia: a case of possible paraneoplastic aetiology. Clin Rehabil 1998; 12: 157–160.
- Fukaya C, Otaka T, Obuchi T, Kano T, Na-gaoka T, Kobayashi K, Oshima H, Yamamo-to T, Katayama Y: Pallidal high-frequency deep brain stimulation for camptocormia: an experience of three cases. Acta Neurochir Suppl 2006; 99: 25–28.
- Umemura A, Oka Y, Ohkita K, Yamawaki T, Yamada K: Effect of subthalamic deep brain stimulation on postural abnormality in Par-kinson disease. J Neurosurg 2009 (in press).
- Hellmann MA, Djaldetti R, Israel Z, Melamed E: Effect of deep brain subthalam-ic stimulation on camptocormia and postur-al abnormalities in idiopathic Parkinson’s disease. Mov Disord 2006; 21: 2008–2010.
- Nandi D, Parkin S, Scott R, Winter JL, Joint C, Gregory R, Stein J, Aziz TZ: Camptocor-mia treated with bilateral pallidal stimula-tion: case report. Neurosurg Focus 2002; 12:ECP2.
- Peek AC, Quinn N, Casey AT, Etherington G: Thoracolumbar spinal fixation for camp-tocormia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2009; 80: 1275–1278.
- Finsterer J, Strobl W. Presentation, etiology, diagnosis, and management of camptocormia. Eur Neurol. 2010;64:1–8.
- Gerton BK, Theeler B, Samii A. Backpack treatment for camptocormia. Mov Disord. 2010;25:247–8.
- Schroeteler FE, Fietzek UM, Ziegler K, et al. Upright posture in parkinsonian camptocormia using a high-frame walker with forearm support. Mov Disord. 2011;26:1560–1.
- de Sèze MP, Creuzé A, de Sèze M, et al. An orthosis and physiotherapy programme for camptocormia: a prospective case study. J Rehabil Med. 2008;40:761–5.
- Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev. 2013;9:CD002817
- Capecci M, Serpicelli C, Fiorentini L, et al. Postural rehabilitation and Kinesio taping for axial postural disorders in Parkinson’s disease. Arch Phys Med Rehabil. 2014;95:1067–75.
- Arii Y, Sawada Y, Kawamura K, et al. Immediate effect of spinal magnetic stimulation on camptocormia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1221–6.
- Sławek J, Derejko M, Lass P, et al. Camptocormia or Pisa syndrome in multiple system atrophy. Clin Neurol Neurosurg. 2006;108:699–704.
- Bloch F, Houeto JL, Tezenas du Montcel S, et al. Parkinson’s disease with camptocormia. J Neurol Neurosurg Psychiatry. 2006;77:1223–8.
- von Coelln R, Raible A, Gasser T, et al. Ultrasound-guided injection of the iliopsoas muscle with botulinum toxin in camptocormia. Mov Disord. 2008;23:889–92.
- Colosimo C, Salvatori FM. Injection of the iliopsoas muscle with botulinum toxin in camptocormia. Mov Disord. 2009;24:316–17.
- Fietzek UM, Schroeteler FE, Ceballos-Baumann AO. Goal attainment after treatment of parkinsonian camptocormia with botulinum toxin. Mov Disord. 2009;24:2027–8.
- Furusawa Y, Mukai Y, Kawazoe T, et al. Long-term effect of repeated lidocaine injections into the external oblique for upper camptocormia in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:350–4.
- Wadia PM, Tan G, Munhoz RP, et al. Surgical correction of kyphosis in patients with camptocormia due to Parkinson’s disease: a retrospective evaluation. J Neurol Neurosurg Psychiatry. 2011;82:364–8.
- Peek AC, Quinn N, Casey AT, et al. Thoracolumbar spinal fixation for camptocormia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2009;80:1275–8.
- Sławek J, Derejko M, Lass P. Camptocormia as a form of dystonia in Parkinson’s disease. Eur J Neurol. 2003;10:107–8.
- Schulz-Schaeffer WJ, Margraf NG, Munser S, et al. Effect of neurostimulation on camptocormia in Parkinson’s disease depends on symptom duration. Mov Disord. 2015;30:368–72.
- Lee KH, Kim JM, Kim HS. Back Extensor Strengthening Exercise and Backpack Wearing Treatment for Camptocormia in Parkinson’s Disease: A Retrospective Pilot Study. Ann Rehabil Med. 2017;41(4):677-685. doi:10.5535/arm.2017.41.4.677 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5608676