Cholestatic pruritus
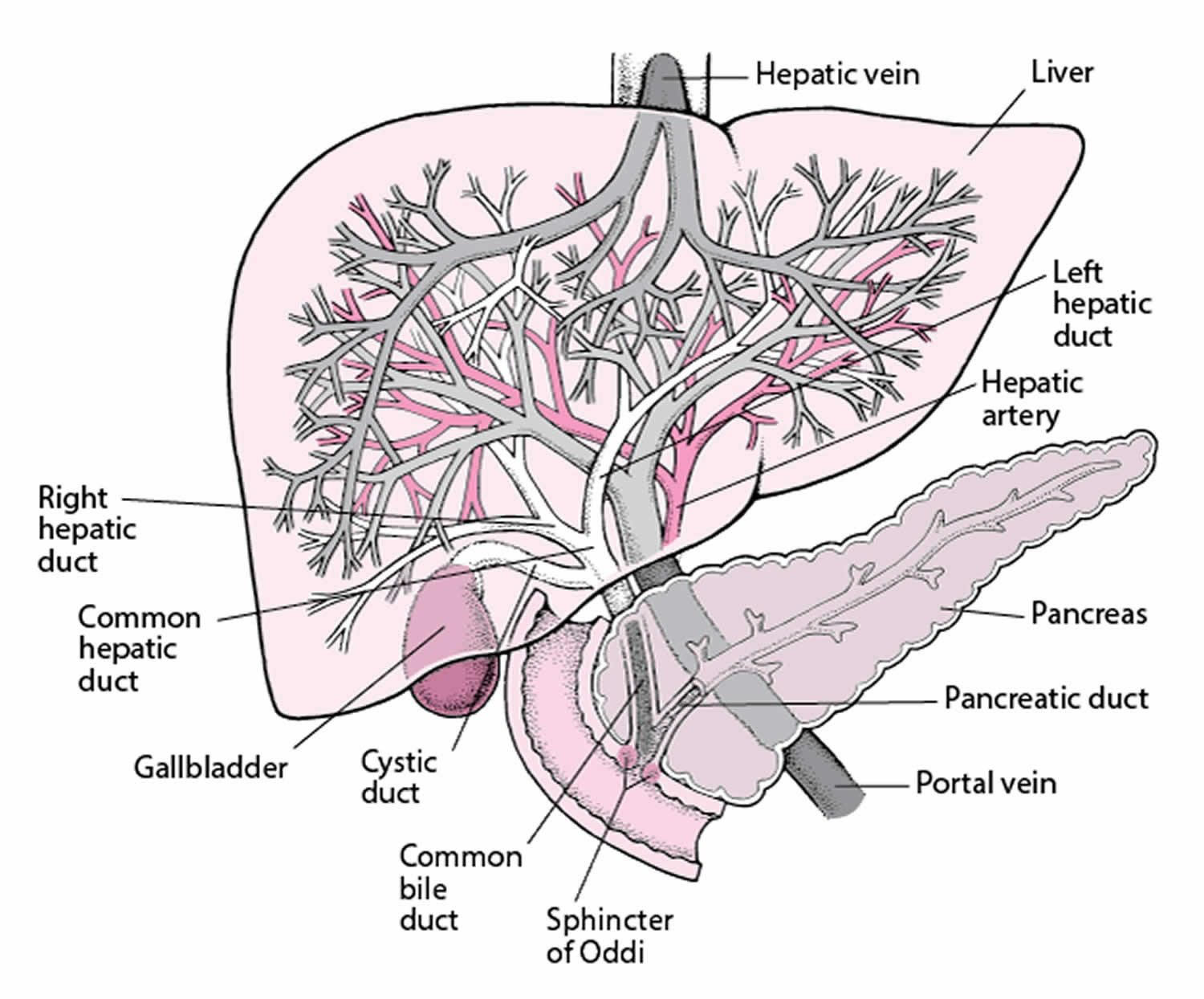
Cholestatic pruritus is the sensation of itch (pruritus) due to nearly any liver disease. Cholestasis is the reduction or stoppage of bile flow. Bile is the digestive fluid produced by the liver. Disorders of the liver, bile duct, or pancreas can cause cholestasis, but the most commonly associated entities are primary biliary cirrhosis, primary sclerosing cholangitis, obstructive choledocholithiasis, carcinoma of the bile duct, drug-induced and chronic hepatitis C viral infection and other forms of viral hepatitis 1.
With cholestasis, the flow of bile is impaired at some point between the liver cells (which produce bile) and the duodenum (the first segment of the small intestine). When bile flow is stopped, the pigment bilirubin (a waste product formed when old or damaged red blood cells are broken down) escapes into the bloodstream and accumulates. Normally, bilirubin joins with bile in the liver, moves through the bile ducts into the digestive tract, and is eliminated from the body. Most bilirubin is eliminated in stool, but a small amount is eliminated in urine.
The causes of cholestasis are divided into two groups: those originating within the liver (intrahepatic cholestasis) and those originating outside the liver (extrahepatic cholestasis). Cholestatic pruritus is more common in intrahepatic cholestasis than extrahepatic cholestasis.
- Within the liver (intrahepatic cholestasis): Causes of intrahepatic cholestasis include acute hepatitis, alcoholic liver disease, primary biliary cholangitis with inflammation and scarring of the bile ducts, cirrhosis due to viral hepatitis B or C (also with inflammation and scarring of the bile ducts), certain drugs (for example, amoxicillin/clavulanate, chlorpromazine, azathioprine, and oral contraceptives), hormonal effects on bile flow during pregnancy (a condition called cholestasis of pregnancy), and cancer that has spread to the liver.
- Outside the liver (extrahepatic cholestasis): Causes extra-hepatic cholestasis include a stone in a bile duct, narrowing (stricture) of a bile duct, cancer of a bile duct, cancer of the pancreas, and inflammation of the pancreas (pancreatitis).
Cholestasis is thought to release toxic substances from the liver, which stimulates neural itch fibers in the skin. Characteristically, cholestatic pruritus is most severe at night; it tends to affect the hands, feet and areas where clothes are rubbing on the skin.
Cholestatic pruritus may be generalized or localized at the limbs (mostly at the palms of the hands and soles of the feet) 2. Cholestatic pruritus can occur at any stage of the disease and may lessen with the development of end-stage liver disease 3. Fluctuations are characteristic for cholestatic pruritus over time during the course of the disease and in one day, it shows a typical circadian rhythm with a peak in the evening and early night 4. Once pruritus occurs, the severity can diminish over time 5. The intensity of pruritus may also be exacerbated by psychologic stress, heat and contact with wool 6. Cool temperatures often lead to improvement. Furthermore, pruritus is more common in women than in men. Female cholestatic patients may also experience worsening of pruritus in the progesterone phase of the menstrual cycle, in the late pregnancy or during hormone replacement therapy, suggesting a role for female sex hormones 4.
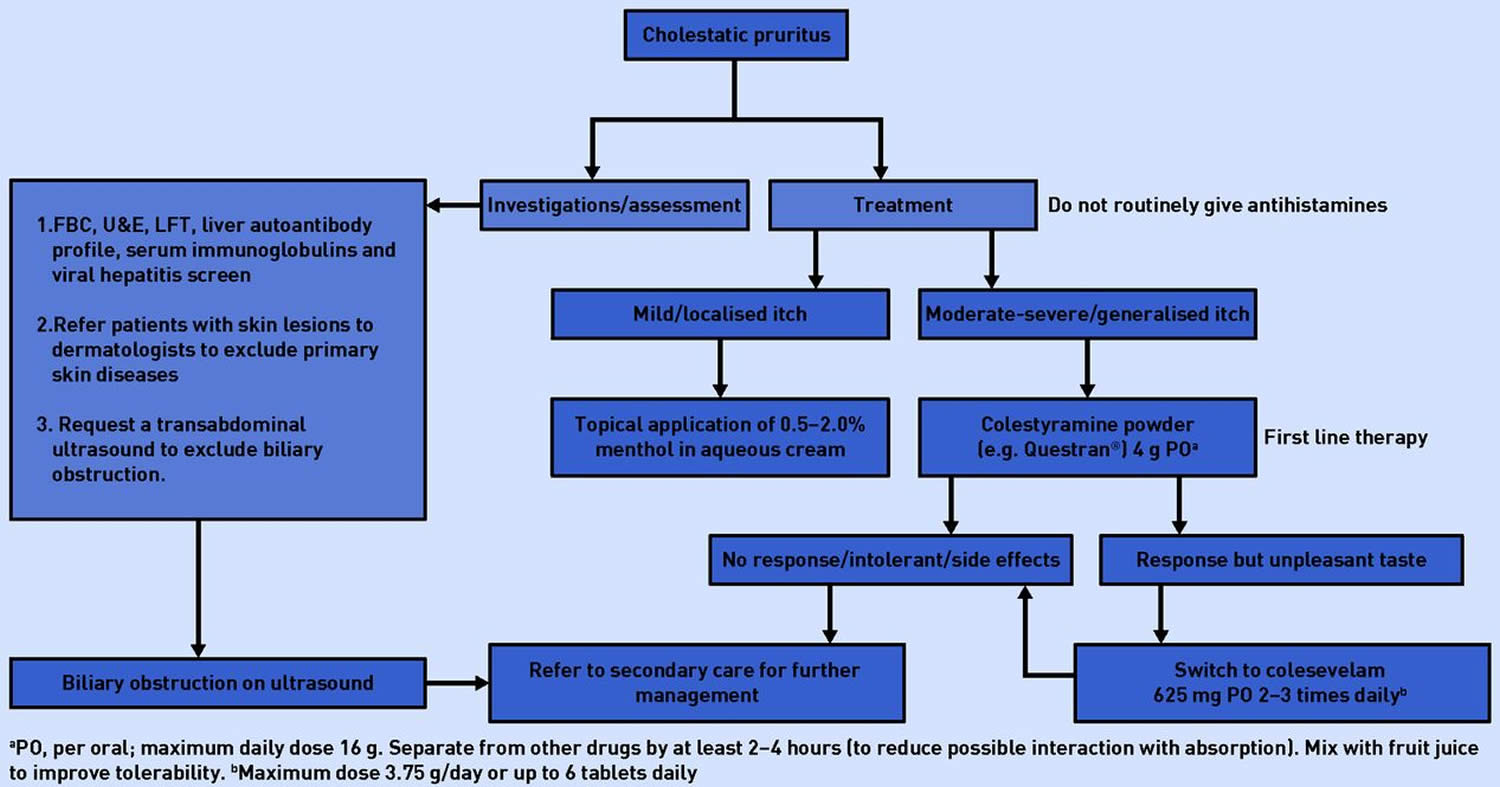
Clinical examination of a patient with cholestatic pruritus may be completely normal. Jaundice is absent in the majority of patients and its presence usually suggests advanced stage of underlying liver disease or severe biliary obstruction 7. Figure 1 shows a suggested approach to the assessment and management of cholestatic pruritus.
- A blockage of the bile ducts can usually be treated with surgery or endoscopy (using a flexible viewing tube with surgical instruments attached).
- A blockage within the liver may be treated in various ways depending on the cause. If a drug is the suspected cause, the doctor stops its use. If acute hepatitis is the cause, cholestasis and jaundice usually disappear when hepatitis has run its course. People with cholestasis are advised to avoid or stop using any substance that is toxic to the liver, such as alcohol and certain drugs.
- Cholestyramine, taken by mouth, can be used to treat itchiness. This drug binds with certain bile products in the intestine, so they cannot be reabsorbed to irritate the skin.
- Unless the liver is severely damaged, taking vitamin K can improve blood clotting.
- Supplements of calcium and vitamin D are often taken if the cholestasis persists, but they are not very effective in preventing loss of bone tissue.
Figure 1. Cholestatic pruritus diagnostic and management algorithm
[Source 7 ]Cholestatic pruritus causes
The precise pathogenesis of pruritus in cholestasis still remains unsolved 8. There is still no substance that is considered as causative pruritogen in cholestasis 6. The perception of itch depends on a complex interplay of pruritogens, receptors, neuronal and cerebral pathways 9. Itch and pain are highly associated perceptions and involve the activity of the same receptor, transient receptor potential cation channel subfamily V member 1 (TRPV1); however itch is transmitted by itch-specific unmyelinated C-fibers, transmitting signals from the skin to the spinal cord, to the thalamus, activating the cortex, leading to scratch 10. The possible ligands and receptors for the itch sensation remain unclear and the question remains whether sensory neurons exist exclusively mediating itch sensation 10. Probably the itch-causing molecules in cholestasis are (biotrans) formed in liver and/or gut, are secreted into bile, accumulate in the systemic circulation and do centrally also affect the endogenous opioid and serotoninergic system 11. Several hypotheses as underlying mechanism have been proposed, including peripheral mechanism such as bile acid accumulation, progesterone metabolites and central mechanism such as increased endogenous opioids and elevations in lysophosphatidic acid levels 12. The current understanding of the potential pruritogens is mirrored by the success of the different therapeutic approaches 13. But not all patients with cholestasis report pruritus, so there may also be a subject-dependent factor, such as a genetic factor, like a polymorphism for multidrug resistance associated protein 2 (MRP2) 14.
It has been inferred that the pruritogen(s) that mediates cholestatic pruritus is made in the liver and excreted in bile and that, as a result of cholestasis, accumulates in body tissues and by some mechanism triggers the sensation of itch 15. The following observations support a liver origin of the pruritogen(s):
- In patients with cholestasis, the cessation of itch portends liver failure 16
- Patients with pruritus report the disappearance of their symptom after liver transplantation 17 and
- Relief of cholestasis from mechanical obstruction (e.g., common hepatic duct stones) is associated with decrease or disappearance of pruritus 18. The nature of the pruritogen, however, is unknown.
Bile acids accumulate in the tissues of patients with cholestasis 19. Under experimental conditions, bile acids were reported to trigger local “itch” when injected intracutaneously in normal volunteers 20; this, however, is not a model of the cholestatic itch. The oral administration of cholylsarcosine, a synthetic bile acid, to four patients with primary biliary cirrhosis was reported to be associated with pruritus in one patient and worsening pruritus in another 21. As pruritus is intermittent in cholestasis, this observation cannot be interpreted as evidence in support of a role of bile acids in the pruritus of cholestasis because pruritus tends to be intermittent; in addition, the intake of certain foodstuffs, usually patient specific, is sometimes reported to increase or to worsen the pruritus.
Three observations do not support a role of bile acids in the mediation of the pruritus of cholestasis:
- In liver failure, when bile acids are maximally elevated, pruritus tends to disappear 16
- Not all patients with cholestasis report pruritus, in spite of marked elevations of serum bile acids, and
- Pruritus can fluctuate independently from the serum concentration of bile acids.
It is possible, however, that a certain profile of serum bile acids is necessary for these substances to mediate the pruritus. A recent clinical study published in abstract form reported that the administration of obeticholic acid, in contrast to the placebo drug, to patients with primary biliary cirrhosis was associated with pruritus, was severe in some patients, for which the drug had to be stopped, and in others sufficiently inconvenient to require dose reduction. Obeticholic acid is a synthetic derivative of chenodeoxycholic acid that is an agonist at the farsenoid nuclear receptor and that has choleretic properties 22. Farsenoid nuclear receptor is a bile acid sensor associated with a decrease in bile acid production 23. The relevance of this observation in the pathogenesis of the pruritus of cholestasis is unknown.
Histamine was reported to be increased in patients with liver disease and pruritus 24; 16% of patients with primary biliary cirrhosis who participated in the internet survey reported that hydroxyzine, an antihistamine, was frequently prescribed to treat their pruritus, and provided some relief in some patients 25. The skin of patients with cholestasis and pruritus is devoid of the classic histamine mediated reaction consisting of erythema and edema, which does not support a major role of this pruritogen in the mediation of this type of pruritus; however, the sedative effect of antihistamines, and not an antipruritic effect, may be responsible for some of the relief in association with this type of drugs.
Substance P is an excitatory neurotransmitter that acts through the NK-1 receptor synthesized by primary afferent nociceptors and released into the spinal cord after noxious stimuli 26. The central administration of substance P is associated with scratching behavior 27. In addition, in animal studies, increased opioidergic tone secondary to the administration of morphine activates mechanisms that promote pain (i.e., nociception), instead of analgesia, mediated, in part, by the NK-1 receptor 28; furthermore, the expression of substance P in the dorsal root ganglia, which are involved in the transmission of nociceptive stimuli 26, is increased in association with prolonged administration of opiates 26. Analogous to the activation of mechanisms that promote pain mediated by substance P associated with chronic opiate administration, the increased opioidergic tone, which is associated with cholestasis 29, may also contribute to a state of enhanced nociception that may be perceived as pruritus mediated, in part, by substance P. In this context, the mean serum concentration of substance P was 12-fold higher in patients with liver disease and pruritus than in patients with liver disease without pruritus, and that of a control group of subjects 30. These data suggest that substance P may mediate some of the manifestations of liver disease, including pruritus; however, in primates, which seem to respond to the central administration of pruritogens similarly to human beings, the administration of substance P was not associated with scratching 31.
In animal studies, it has been reported that lipophosphatidic acid (LPA) induces nociception through substance P release from peripheral nerve endings 32 and it has been implicated as a mediator in animal and in in vitro neural models that explore signals associated with pain 33. A role of lipophosphatidic acid and the enzyme that generates its production, autotaxin, has been recently proposed in the pruritus of cholestasis 34. The concentration of several lipophosphatidic acid (LPA) species was reported to be significantly higher in the sera from patients with cholestasis of pregnancy than in the sera from pregnant women matched for gestation term 35. In C57BL/6J female mice, LPA injections were reported to cause scratching behavior, which was interpreted as evidence for a pruritogenic role of lipophosphatidic acid (LPA) in cholestasis 35; however, this finding is not relevant in the study of the pruritus of cholestasis as scratching behavior in association with the intradermal injection, as given in this study, of substances, including LPA, is not a model of scratching in cholestasis. The activity of autotaxin (ATX) was reported to be higher in the serum from women with cholestasis of pregnancy, and that of patients with cholestasis and pruritus from liver diseases and not in the serum of patients with pruritus from uremia, Hodgkin’s “disease,” and atopic dermatitis 34. The serum activity of autotaxin (ATX) was also reported to have decreased in the serum of patients with liver disease and pruritus who had responded to the antibiotic rifampicin 34. Autotaxin activity was not decreased in the serum from patients with cholestasis and pruritus who had participated in a controlled trial of colesevalan, a nonabsorbable resin that binds bile acids in the gut, versus placebo, in which the resin was reported not to relieve pruritus. The activity of autotaxin was also reported to correlate with a decrease in the severe pruritus of patients who had undergone dialysis with Molecular Adsorbent Recirculating System (MARSTM), or nasobiliary drainage 34. Nasobiliary drainage facilitates bile flow, and the MARSTM procedure is associated with removal of vasoactive substances and with important changes in blood flow, which may also increase bile flow 36; the effects of these two interventions thus, may decrease the degree of cholestasis and hence the pruritus associated with it. Autotaxin was not found in the bile of patients treated with nasobiliary drainage and who had responded to the treatment with a decrease in pruritus, although it was reported to have returned to pretreatment serum levels when the pruritus recurred 34. The changes in the activity of autotaxin in relation with improvement or worsening of the pruritus may reflect changes in the degree of cholestasis and do not necessarily imply a pruritogenic effect of this enzymatic pathway. The antibiotic rifampicin induces drug-metabolizing enzymes and transporters, through activation of the pregnane X receptor (PXR) 37. The idea that the reported antipruritic effect of this drug results from either the enhanced metabolism of the “pruritogens” or its transport has been suggested 38. The inhibition of the expression of autotaxin by rifampicin in vitro, in HepG2 hepatoma cells, and in hepatoma cells overexpressing pregnane X receptor (PXR), and not on cells in which PXR had been knocked out, was interpreted to suggest that a decrease in autotaxin by rifampicin may be the mechanism by which this antibiotic decreases pruritus in patients with cholestasis 39; however, the lack of effect of rifampicin in some patients was not explained in this context 40. The effect of rifampicin in vitro as measured in this study 34 cannot be interpreted in the context of the pruritus of cholestasis as the model does not reflect the cholestatic milieu in human beings. It was concluded from these studies that autotaxin may be a therapeutic target for the treatment of the pruritus of liver disease 34. Whether autotaxin or LPA mediate the pruritus of cholestasis and the potential neurophysiologic and/or neuropathophysiologic mechanisms by which these substance may mediate pruritus are unknown.
Another hypothesis in the pathogenesis of cholestatic pruritus is an elevated level of endogenous opioids, such as Met-enkephalins as a central mechanism of pruritus 9. In patients with chronic liver diseases endogenous opioid levels are elevated (via an uncertain mechanism) and a series of reports have shown a beneficial effect of opioid antagonists in cholestatic pruritus, although there is no strict correlation between itch perception and serum opioid levels 41. It is also described that intrathecal administrations of morphine lead to pruritus 14. A possible explanation for the working mechanism is the intimate interaction between itch and pain; pain has a strong negative modulating effect on itch (hence the scratch), and the transmission of both involve the transient receptor potential cation channel subfamily V member 1 (TRPV1) 11.
Not all patients with cholestasis report pruritus 25; this observation may suggest a genetic predisposition to experience this sensation in the context of cholestasis. There are maybe also genetic, environmental and dietary factors in the pathogenesis of cholestatic itch. The interindividual differences in susceptibility for cholestatic pruritus and the genetic background for intrahepatic cholestasis of pregnancy advocate genetic factors that contribute to itching 12. Consumption of specific food leads to aggravation of itch in some patients, suggesting influence of environmental and dietary factors in pathogenesis 12.
Cholestatic pruritus symptoms
Jaundice, dark urine, light-colored stools, and generalized itchiness are characteristic symptoms of cholestasis. Jaundice is a yellow color of the skin and eyes that results from excess bilirubin deposited in the skin, and dark urine results from excess bilirubin excreted by the kidneys. The skin itches, possibly because bile products accumulate in the skin. Scratching can damage the skin. Stools may become light-colored because the passage of bilirubin into the intestine is blocked, preventing it from being eliminated from the body in stool. Stools may contain too much fat (a condition called steatorrhea) because bile cannot enter the intestine to help digest fat in foods. Fatty stools may be foul-smelling.
Cholestatic pruritus may be generalized or localized at the limbs (mostly at the palms of the hands and soles of the feet) 2. Cholestatic pruritus can occur at any stage of the disease and may lessen with the development of end-stage liver disease 3. Fluctuations are characteristic for cholestatic pruritus over time during the course of the disease and in one day, it shows a typical circadian rhythm with a peak in the evening and early night 4. Once pruritus occurs, the severity can diminish over time 5. The intensity of pruritus may also be exacerbated by psychologic stress, heat and contact with wool 6. Cool temperatures often lead to improvement. Furthermore, pruritus is more common in women than in men. Female cholestatic patients may also experience worsening of pruritus in the progesterone phase of the menstrual cycle, in the late pregnancy or during hormone replacement therapy, suggesting a role for female sex hormones 4.
The lack of bile in the intestine also means that calcium and vitamin D are poorly absorbed. If cholestasis persists, a deficiency of these nutrients can cause loss of bone tissue. Vitamin K, which is needed for blood clotting, is also poorly absorbed from the intestine, causing a tendency to bleed easily.
Prolonged jaundice due to cholestasis produces a muddy skin color and fatty yellow deposits in the skin. Whether people have other symptoms, such as abdominal pain, loss of appetite, vomiting, or fever, depends on the cause of cholestasis.
Cholestatic pruritus diagnosis
A doctor suspects cholestasis in people who have jaundice and tries to determine whether the cause is within or outside the liver on the basis of symptoms and the results of a physical examination.
Recent use of drugs that can cause cholestasis suggests a cause within the liver. Small spiderlike blood vessels visible in the skin (called spider angiomas), an enlarged spleen, and accumulation of fluid within the abdomen (ascites)—which are signs of chronic liver disease—also suggest a cause within the liver.
Findings that suggest a cause outside the liver include certain kinds of abdominal pain (such as intermittent pain in the upper right side of the abdomen and sometimes also in the right shoulder) and an enlarged gallbladder (felt during the physical examination or detected by imaging studies).
Some findings do not indicate whether the cause is within or outside the liver. They include consumption of large amounts of alcohol, loss of appetite, nausea, and vomiting.
Typically, blood tests are done to measure levels of two enzymes (alkaline phosphatase and gamma-glutamyl transpeptidase) that are very high in people with cholestasis. A blood test that measures the level of bilirubin indicates the severity of the cholestasis but not its cause.
An imaging study, usually ultrasonography, is almost always done if blood test results are abnormal. Computed tomography (CT) or sometimes magnetic resonance imaging (MRI) may be done in addition to or instead of ultrasonography. If the cause appears to be within the liver, a liver biopsy may be done and usually establishes the diagnosis.
If the cause appears to be blockage of the bile ducts, more precise images of these ducts are usually needed. Typically, one of the following is done:
- Endoscopic retrograde cholangiopancreatography (ERCP): A flexible viewing tube (endoscope) is inserted through the mouth and into the small intestine, and a radiopaque contrast agent (which can be seen on x-rays) is injected through the tube into the bile and pancreatic ducts. Then, x-rays are taken.
- Magnetic resonance cholangiopancreatography (MRCP): Magnetic resonance cholangiopancreatography is MRI of the bile and pancreatic ducts, with specialized techniques that are used to make the fluid in the ducts appear bright and the surrounding tissues appear dark.
- Endoscopic ultrasonography: Images are obtained via an ultrasound probe inserted with a flexible viewing tube (endoscope) through the mouth and into the small intestine.
Cholestatic pruritus treatment
The unknown pathogenesis of cholestatic pruritus also precludes the development of effective therapy 41. The treatment options are limited and do not yet provide relief for all patients 6. A step by step recommendation is based on the European Association for the Study of the Liver (EASL) guidelines 42.
The first step in treating patients with cholestatic pruritus is to treat the underlying hepatobiliarydiseaseand to exclude bile duct obstruction 42. In biliary obstruction endoscopic, radiological or surgical correction must be obtained, such as endoscopic treatment of dominant strictures in PSC or bile duct stenting in malignant extrahepatic biliary obstruction 9. In drug-induced cholestasis discontinuation of offending medication is the treatment of choice 14.
In intrahepatic cholestasis of pregnancy and in primary biliary cholangitis (PBC) ursodeoxycholic acid (UDCA), a naturally occurring dihydroxy bile acid is the first treatment option, in a dose of 10-15 mg/kg/daily 5. Ursodeoxycholic acid (UDCA) is a disease-modifying therapy, but there is no evidence that ursodeoxycholic acid (UDCA) has any effect on pruritus, except in intrahepatic cholestasis of pregnancy 43. In primary biliary cholangitis it improves biochemical parameters, delays progression to liver cirrhosis and enhances survival 9. Also in intrahepatic cholestasis of pregnancy UCDA is associated with decreased levels of endogenous bile acids, normalization of alanine aminotransferase levels, and improved fetal outcomes 9. The working mechanism of ursodeoxycholic acid (UDCA) may be explained by competition for intestinal absorption, by increasing hepatic clearance of endogenous bile acids or due to bile detoxification or an anti-apoptotic effect 9. Still about 40% of the primary biliary cholangitis patients don’t respond to UDCA 44. New pharmacological approaches for non-responders are reported, such as obeticholic acid (OCA) and fibrates, targeting nuclear receptors which regulate the intracellular concentration of biliary constituents. Obeticholic acid (OCA) is a semi-synthetic hydrophilic bile acid analogue that is selective for Farnesoid X receptor (FXR). Farnesoid X receptor (FXR) represses bile acid uptake (by inhibiting sodium taurocholate co-transporting polypeptide NTCP), represses bile acid synthesis (by inhibiting cholesterol-7alpha-hydroxylase CYP7A1), stimulates biliary secretion (via induction of canalicular transporters), leads to detoxification and has antifibrotic effects 45. Obeticholic acid (OCA) leads to significant improvement in serum alkaline phosphatase in primary biliary cholangitis 3, but it can exacerbate pruritus in a dose dependent relationship by an unknown mechanism 46. Another option is adding fibrates to ursodeoxycholic acid (UDCA), which has an anti-cholestatic, anti-inflammatory, anti-fibrotic and anti-pruritic effect, through the activation of peroxisome proliferator-activated receptors (PPAR) by stimulating FXR, promoting bile acid secretion (by multidrug resistance protein 3 (MDR3) expression), downregulation of bile acid synthesis and regulating detoxification 3. Particularly bezafibrate 400mg/day, a PPAR agonist, leads to biochemical improvement, improvement of the noninvasive measures of liver fibrosis and alleviation of pruritus and fatigue 47. In the placebo-controlled trial, recently published in the New England 47, one third of the patients in the bezafibrate group compared with no one in the placebo group reached normal levels of the main biochemical markers of the disease at 24 months. Increase in serum creatinine and myalgia are described as adverse events 47. Longer and larger trials will be required to assess the effect of bezafibrate on hard outcomes such as liver transplantation and death and to know the side effects on the long term 47.
General measures (e.g., cold water, emollients with menthol or aqueous cream) may be helpful for pruritus, though studies evaluating their efficacy are lacking 48. In cholestasis-associated pruritus antihistamines are mostly ineffective and should not be prescribed, however antihistamines are frequently used because of sedative effects in patients with nocturnal pruritus 6. If the underlying hepatobiliary disease can’t be corrected, starting systematic treatment focusing entirely on pruritus itself in a stepwise approach should be considered, as suggested in the recommendations of 2009 EASL guidelines for the management of pruritus in cholestatic patients 42. There are four goals in the therapy for cholestatic pruritus. The first goal is removing pruritogens from the enterohepatic cycle by bile acid sequestrants (cholestyramine). Managing the metabolism of pruritogens by the Pregnane X-receptor (PXR) agonist, rifampicin, an enzyme-inducer is the second goal. The third and fourth goal is influencing the itch perception with μ-opioid antagonist or selective serotonin reuptake inhibitors (SSRI) 42. In treatment-refractory pruritus elimination of possible pruritogens from the systemic circulation with MARS or plasmapheresis can be attempted 49.
The widely used first-line treatment are non-absorbable anion exchange resins cholestyramine, a ratio 4 gram up to four times daily, based on their bile salt binding properties, preventing the reuptake in the terminal ileum 42. It is the first-line treatment despite its limited evidence. It was already widespread before the era of evidence based medicine. Its beneficial effect is reported in small uncontrolled case series 49. Resins should be spaced away at least 4 hours from other drugs to prevent binding and drug interactions and morning dose is preferred 5. Poor tolerance is often an issue due to bad taste and complains as constipation and bloating 9. Colesevelam (Cholestagel) is a novel and often better tolerated bile sequestrant, with higher affinity for bile salts. However, a placebo-controlled trial revealed 50% reduction in bile salts concentration without improvement of pruritus 9.
If resins are ineffective, the second-line treatment is rifampicin, a pregnane X-receptor (PXR) agonist and potent inducer of key enzymes in the hepatic and intestinal detoxification machinery (such as CYP3A4, CYP2D, UGT1A1, SULT2A1) and export pump MRP2 9. It stimulates excretion of pruritogens, detoxification and alters the intestinal metabolism by an antimicrobial effect 9. In vitro studies also found that rifampicin reduce autotaxin (ATX) expression at the transcriptional level in human hepatoma cells by a PXR dependent mechanism 50. Rifampicin is the only proven drug in randomized placebo-controlled trials and in two meta-analyses 51. In the meta-analysis of Tandon et al 51, four clinical trials with variable quality with a total of 57 patients were included. The other meta-analysis included a total of 61 participants from 3 double-blind randomized prospective studies and 2 randomized controlled cross-over trails 52. Both meta-analyses concluded that rifampicin was safe and effective, leading to a relief of pruritus in up to 77% of the patients as compared with placebo or alternatives 52, 52. Low dose initiation (150 mg) with serial monitoring of serum liver tests and blood count before dose escalation (up to maximum of 600 mg daily) is recommended 42, because of drug-induced hepatitis and significant liver dysfunction is reported in up to 12% of patients with cholestasis 53. In a recent retrospective review with 105 patients under rifampicin, only 5% developed hepatitis, recovering after drug cessation, so it can be concluded that in 95% rifampicin is safe and drug cessation in rifampicin-induced hepatitis is effective 54. Even in patients with jaundice and advanced liver disease rifampicin was safe 54. Rifampicin can also affect vitamin K metabolism, increasing the INR in icteric patients 3. Other side effects as hemolytic anemia, thrombocytopenia, renal impairment and many drug interactions are reported 5. Patients should be informed about the occurrence of discoloration of urine, tears and other body secretions when initiating this therapy 42.
If rifampicin is ineffective or intolerable within 2 weeks, oral opioid antagonist, such as naltrexone, is proposed as next agent 42, reducing itch at a dose of 50 mg daily 51. Naltrexone should be started at low dose of 12.5 mg increasing by a quarter every 3-7 days or this oral therapy should be preceded by Naloxone intravenous for 3 days because of the self-limited withdrawal-like syndrome in the first days of treatment 5. Since naloxone ejects the opioids from their receptors in the brain, causing a withdrawal-like syndrome. To prevent a break-through phenomenon during long term therapy, interrupting the treatment for 2 days a week has been suggested 55. Following the liver biochemistry on naloxone therapy is also recommended because naltrexone hepatotoxicity is uncommon but has been reported 5.
The selective serotonin reuptake inhibitor (SSRI), Sertraline, may be considered as fourth-line treatment for patients resistant to above mentioned treatments, starting at 25 mg daily, increasing gradually to 75-100 mg daily 56. SSRIs probably act by altering neurotransmitter-concentrations within the central nervous system 57. The uncommon adverse events are dry mouth, nausea, dizziness, diarrhea, visual hallucinations or fatigue 3.
Other therapies have also been tried. The use of Ondansetron, Phenobarbital and Propofol (in hypnotic dose 15 mg intravenously) are not recommended because of lack of efficacy and side-effect profile 5.
In therapy-refractory pruritus, ultraviolet B (UVB) photo-therapy can be considered, presumably working by influencing the itch-specific nerve endings or by chemical modification of pruritogens in the skin 58. It is a well-tolerated and promising therapy in hepatogenic pruritus, but further studies are needed 58. Also invasive physical approaches can be considered, for example alleviating pruritus by transient therapeutic interruption of the enterohepatic circulation, such as MARS (molecular adsorbents recirculating system), an extracorporeal albumin dialysis capable of removing albumin-bound molecules 59. Furthermore, plasma-pheresis 60 and bile duct drainage 61 have been reported to remove pruritogens accumulating in the plasma or bile 42. The temporary success of these therapies is their main limitation, but supports that the putative pruritogens in cholestasis accumulates in the blood and undergo an enterohepatic circulation 9. Intractable pruritus may become an indication for liver transplantation when all the previous therapeutic efforts have failed 14, even in the absence of liver failure 9. Transplantation is highly effective to control cholestatic itch with rapid reduction (frequently within 24 hours) 62.There is a lot of active research in cholestatic pruritus, with several experimental agents and approaches being developed 3. Rifampicin, a PXR agonist, remains the most evidence-based treatment for cholestatic pruritus 44. Drugs targeting nuclear receptors involved in regulation of bile formation, bile acid homeostasis and defense pathways may lead to new treatment options, such as FXR ligands (Obeticholic acid), PPAR ligands (Fibrates), GR/PXR ligands (budesonide) 63. Fibrates, especially targeting PPAR-alpha, are an attractive treatment option for primary biliary cholangitis because of the antipruritic and anticholestatic effects, but long-term placebo controlled studies are still needed 64. A study with the Seladelpar, a selective PPAR-delta agonist, improves alkaline phosphatase, but increases aminotransferases, leading to early discontinuation of the study 65. Novel therapies including bile acid reuptake inhibitors and drugs targeting the autotaxin/lypophosphatidic acid pathway, are in development 6. In a cross-over fase 2a study bile acid transporter inhibitor GSK2330672 in primary biliary cholangitis patients showed good results on pruritus and is well tolerated (most common adverse event is diarrhea) 66. A protein variant of Fibroblast growth factor 19 (FGF19) from the ileum, NGM282, suppressor of bile acid synthesis is also under study 64.
References- Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. ISBN 978-1-4160-2999-1
- KREMER AE, WENNIGER LM, OUDE ELFERINK RP, BEUERS UH. Stand van zaken: Pruritus bij leverziekten: pathogenenese en behandeling. Ned Tijdschr Geneeskd, 2011, 155 : A4045
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol., 2017, 67 :145-172.
- KREMER AE, OUDE ELFERINCK RP, BEUERS U. Pathophysiology and current management of pruritus in liver disease. Clinics and research in hepatology and gastroenterology, 2011,35 : 89-97.
- AASLD practice guidelines. LINDOR KD, GERSKIN ME, POUPON R, KAPLAN M, BERGASA NV, HEATHCOTE EJ. Primary Biliairy Cirrhosis. Hepatology, 2009, 50 : 291-308.
- HEGADE VS, BOLIER R, OUDE ELFERICK RP, BEUERS U, KENDRICK S, JONES DE. A systematic approach to the managment of cholestatic pruritus in primary biliary cirrhosis. Frontline Gastroenterol., 2016, 7(3) : 158-166.
- Itch and liver: management in primary care. Vinod S Hegade, Stuart FW Kendrick, Jahangir Rehman, David EJ Jones. British Journal of General Practice 2015; 65 (635): e418-e420. DOI: 10.3399/bjgp15X685477
- De Vloo C, Nevens F. Cholestatic pruritus : an update. Acta Gastroenterol Belg. 2019 Jan-Mar;82(1):75-82. https://www.ageb.be/Articles/Volume%2082%20(2019)/Fasc1/12-De_Vloo.pdf
- KREMER AE, BEUERS U, OUDE-ELFERINK RP, PUSL T. Pathogenesis and treatment of pruritus in cholestasis. Drugs, 2008, 68 : 2163-2182.
- KREMER AE, BOLIER R, VAN DIJCK R, OUDE ELFERINK RP, BEUERS U. Advances in pathogenesis and management of pruritus in cholestasis. Dig Dis., 2014, 32 : 637-645.
- BEUERS U, KREMER AE, BOLIER R, OUDE ELFERINK. Pruritus in cholestasis: facts and fiction: Hepatology, 2014, 60 : 399-407.
- BOLIER R, OUDE ELFERINK RP, BEUERS U. Advances in pathogenesis and treatment of pruritus. Clin Liver Dis., 2013, 17 : 319-329.
- KREMER AE, FERAMISCO J, REEH PW, BEUERS U, OUDE ELFERINKRP. Receptors, cells and circuits involved in pruritus of systemic disorders. Biochmica et biophysica acta, 2014, 869-892.
- BERGASA NV. The pruritus of cholestasis. J Hepatol., 2005, 43 :1078-1088.
- Bergasa NV. Pruritus of Cholestasis. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 6. Available from: https://www.ncbi.nlm.nih.gov/books/NBK200923
- Lloyd-Thomas H. G, Sherlock S. Testosterone therapy for the pruritus of obstructive jaundice. Br. Med. J. 1952;2 (4797):1289–1291.
- Mezey E, Burns C, Burdick J. F, Braine H. G. A case of severe benign intrahepatic cholestasis treated with liver transplantation. Am. J. Gastroenterol. 2002;97 (2):475–477
- Jarnagin W. R, Burke E, Powers C, Fong Y, Blumgart L. H. Intrahepatic biliary enteric bypass provides effective palliation in selected patients with malignant obstruction at the hepatic duct confluence. Am. J. Surg. 1998;175 (6):453–460.
- Stiehl A. Bile acids and bile acid sulfates in the skin of patients with cholestasis and pruritus. Z. Gastroenterol. 1974;12 (2):121–124.
- Varadi D. P. Pruritus induced by crude bile and purified bile acids. Experimental production of pruritus in human skin. Arch. Dermatol. 1974;109 (5):678–681.
- Ricci P, Hofmann A. F, Hagey L. R, Jorgensen R. A, Dickson E. R, Lindort K. D. Adjuvant cholylsarcosine during ursodeoxycholic acid treatment of primary biliary cirrhosis. Dig. Dis. Sci. 1998;43 (6):1292–1295.
- Fiorucci S, Clerici C, Antonelli E, Orlandi S, Goodwin B, Sadeghpour B. M, Sabatino G. et al. Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis. J. Pharmacol. Exp. Ther. 2005;313 (2):604–612.
- Mencarelli A, Fiorucci S. FXR an emerging therapeutic target for the treatment of atherosclerosis. J. Cell Mol. Med. 2010;14 (1–2):79–92.
- Gittlen S. D, Schulman E. S, Maddrey W. C. Raised histamine concentrations in chronic cholestatic liver disease. Gut. 1990;31 (1):96–99.
- Rishe E, Azarm A, Bergasa N. V. Itch in primary biliary cirrhosis: A patients’ perspective. Acta Derm. Venereol. 2008;88 (1):34–37.
- Ossipov M. H, Lai J, King T, Vanderah T. W, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers. 2005;80:319–324.
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur. J. Pharmacol. 1995;275:229–233.
- King T, Gardell L, Wang R, Vardanyan A, Ossipov M. H, Malan T. P. J, Vanderah T. W. et al. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005;116:276–288.
- Jones E. A, Bergasa N. V. The pruritus of cholestasis: From bile acids to opiate agonists. Hepatology. 1990;11 (5):884–887.
- Trivedi M, Bergasa N. V. Serum concentrations of substance P in cholestasis. Ann. Hepatol. 2010;9 (2):177–180.
- Ko M. C, Naughton N. N. Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. J. Pain. 2009;10 (5):509–516.
- Renback K, Inoue M, Ueda H. Lysophosphatidic acid-induced, pertussis toxin-sensitive nociception through a substance P release from peripheral nerve endings in mice. Neurosci. Lett. 1999;270 (1):59–61.
- Ahn D. K, Lee S. Y, Han S. R, Ju J. S, Yang G. Y, Lee M. K, Youn D. H, Bae Y. C. Intratrigeminal ganglionic injection of LPA causes neuropathic pain-like behavior and demyelination in rats. Pain. 2009;146 (1–2):114–120.
- Kremer A. E, van Dijk R, Leckie P, Schaap F. G, Kuiper E. M, Mettang T, Reiners K. S. et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56 (4):1391–1400.
- Kremer A. E, Martens J. J, Kulik W, Rueff F, Kuiper E. M, van Buuren H. R, van Erpecum K. J. et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2012;139 (3):1008–1018.
- Laleman W, Wilmer A, Evenepoel P, Elst I. V, Zeegers M, Zaman Z, Verslype C, Fevery J, Nevens F. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit. Care. 2006;10 (4):R108.
- Timsit Y. E, Negishi M. CAR and PXR: The xenobiotic-sensing receptors. Steroids. 2007;72 (3):231–246.
- Bergasa N. V. The pruritus of cholestasis. J. Hepatol. 2005;43 (6):1078–1088.
- Bachs L, Parés A, Elena M, Piera C, Rodés J. Comparison of rifampicin with phenobarbitone for treatment of pruritus in biliary cirrhosis. Lancet. 1989;1 (8638):574–576.
- Woolf G. M, Reynold T. B. Failure of rifampicin to relieve pruritus in chronic liver disease. J. Clin. Gastroenterol. 1990;12:174–177.
- OUDE ELFERINK RP, KREMER AE, BEUERS UH. Mediators of pruritus during cholestasis. Curr Opin Gastroenterol., 2011, 27 : 289-293.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol., 2009, 51 :237-67.
- CARBONE M, MELLS GF, PELLS G, DAWWAS MF, NEWTON JL, HENEGHAN MA, et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic Acid. Gastroenterology, 2013, 144 : 560-569.
- BOLIER R, DE VRIES ES, PARES A, HELDER J, KEMPER EM, ZWINDERMAN K. et al.Fibratess for the treatment of cholestatic itch (FITCH): study protocol for a randomized controlled trial. Trail, 2017 : 18 :230.
- ZOLLNER G, TRAUNER M. Nuclear receptors as therapeutic targets in cholestatic liver diseases. British Journal of Pharmacology, 2009, 156 : 7-27.
- NEVENS F, ANDREONE P, MAZZELLA G, STRASSER SI, BOWLUS C, INVERNIZZI P, et al.A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Eng J Med., 2016, 375 : 631-643.
- CORPECHOT C, CHAZOUILLERES O, ROUSSEAU A, LE GRUYER A, HABERSETZER F, MATHURIN P.et al.A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med., 2018, 378 : 2171-2181.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol., 2017, 67 :145-172.
- KREMER AE, BOLIER R, VAN DIJCK R, OUDE ELFERINK RP, BEUERS U. Advances in pathogenesis and management of pruritus in cholestasis. Dig Dis., 2014, 32 : 637-645.
- KREMER AE, VAN DIJK R, LECKIE P, SCHAAP R, KUIPER EM, METTANT T. et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology,2012, 56 : 1391-1400.
- TANDON P, ROWE BH, VANDERMEER B, BAIN VG. The efficacy and safety of bile Acid binding agents, opioid antagonists, or rifampin in the treatment of cholestasis-associated pruritus. Am J Gastroenterol., 2007, 102 :1528-1536.
- KHURANA S, SINGH P. Rifampin is safe for treatment of pruritus due to chronic cholestasis : a meta-analysis of prospective randomized-controlled trials. Liver Int., 2006, 26 : 943-948.
- PRINCE MI, BURT AD, JONES DEJ. Hepatitis and liver dysfunction with rifampicin therapy for pruritus in primary biliary cirrhosis. Gut, 2002, 50 :436-439.
- WEBB GJ, RAHMAN SR, LEVY C, HIRSCHFIELD GM. Low risk of hepatotoxicity from rifampicin when used for cholestatic pruritus: a cross- disease cohort study. Aliment Pharmacol Ther., 2018, 47 : 1213-1219.
- CARSON KL, TRAN TT, COTTON P. et al. Pilot study of the use of naltrexone to treat the severe pruritus of cholestatic liver disease. Am J Gastroenterol., 1996, 91(5) : 1022-1023.
- MAYO MJ, HANDEM I, SALDANA S, JACOBE H, GETACHEW Y, RUSH AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology, 2007, 45 : 666-674.
- BROWNING J, COMBES B, MAYO MJ. Long-term efficacy of sertraline as a treatment for cholestatic pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol., 2003, 98 : 2736-2741.
- DECOCK S, ROELANDTS R, STEENBERGEN WV, LALEMAN W, CASSIMAN D, VERSLYPE C. et al. Cholestasis-induced pruritus treated with ultraviolet B phototherapy: an observational case series study. J Hepatol., 2012, 57 : 637-641.
- PARES A, HERRERA M, AVILES J, SANZ M, MAS A. Treatment of resistant pruritus from cholestatis with albumin dialysis: combined analysis of patients from three centers. Journal of hepatology, 2010, 57 : 307-312.
- ALALLAM A, BARTH D, HEATHCOTE EJ. Role of plasmapheresis in the treatment of severe pruritus in pregnant patients with primary biliary cirrhosis: case reports. Can J Gastroenterol., 2008, 22 : 505-507.
- BEUERS U, GERKEN G, PUSL T. Biliary drainage transiently relieves intractable pruritus in primary biliary cirrhosis. Hepatology, 2006, 44 : 280-281.
- GROSS CR, MALINCHOC M, KIM WR, EVANS RW, WIESNER RH, PETZ JL, et al. Quality of life before and after liver transplantation for cholestatic liver disease. Hepatology, 1999, 29 : 356-364.
- CHAZOUILLERES O. Novel aspects in the management of cholestatic liver diseases. Dig Dis., 2016, 345 : 340-346.
- FLOREANI A, SUN Y, ZOU ZS, LI B, CAZZAGON N, BOWLUS C. et al.Propesed therapies in priamy biliary cholangitis. Expert Rev Gastroenterol Hepatol., 2016, 10(3) :371-382.
- JONES D, BOUDES PF, SWAIN MG, BOWLUS CL, GALAMBOS MR, BACON BR, et al. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol., 2017, 2(10) :716-726.
- HEGADE VS, KENDRICK SF, DOBBINS RL, MILLER SR, THOMPSON D, RICHARDS D, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis : a double-blind, randomised, placebocontrolled, crossover, phase 2a study. Lancet, 2017, 389 :1114-1123.