Duplex kidney
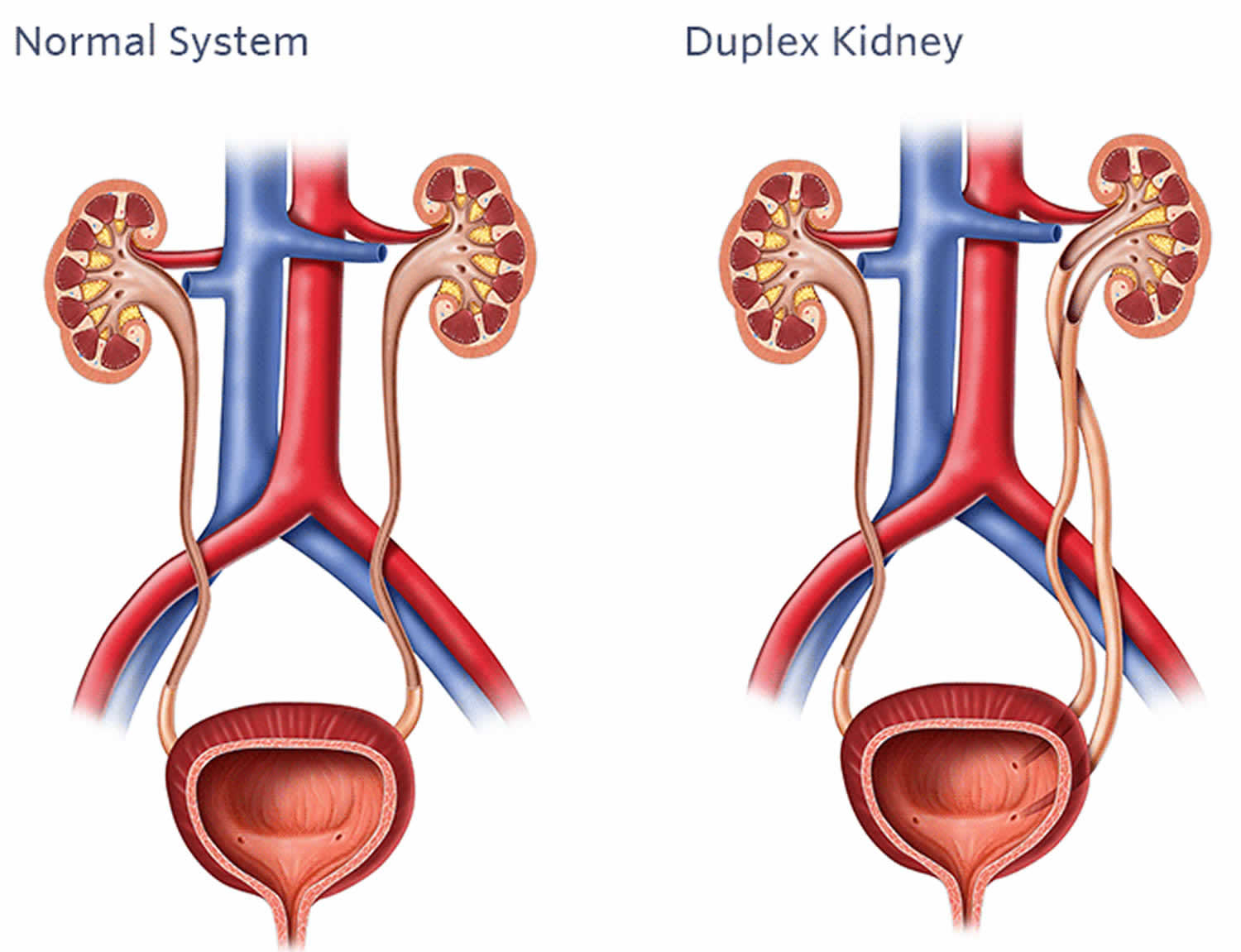
Duplex kidney also called duplicated renal collecting system, is a developmental condition in which one or both kidneys have two ureter tubes to drain urine into the bladder, rather than a single tube. Duplex kidneys can occur in one or both kidneys. Duplex kidneys are a normal variant, meaning that they occur commonly enough in healthy children to be considered normal and most cause no medical problems and will require no treatment. However, about 40% of patients with duplex kidneys have been reported to be associated with conditions that require treatment by a urologist, including flow of urine back into the kidney instead of to the bladder and obstruction of urine flow through the ureter tube 1. Furthermore, because duplex kidneys are frequently asymptomatic and therefore predominantly detected in patients who seek medical assistance, the actual percentage of patients with symptoms is likely to be lower. Symptoms associated with duplex kidneys can include pain, hematuria, dysuria (painful urination) and difficulty or abnormal frequency of micturition 1. Specific manifestation of the pathology depends on the anatomy of each duplication event 2. Furthermore, duplex kidneys are linked to a number of renal disorders, including pelvi-calyceal dilatation, cortical scarring, vesicoureteral reflux (VUR), hydronephrosis, ureterocoeles on the non-duplex side, caliculi or yo-yo reflux (in the incomplete duplication cases) 1.
Other duplex kidneys can be associated with the following:
- Vesicoureteral reflux (VUR): Vesicoureteral reflux (VUR) occurs when urine flows back to the kidneys from the bladder, rather than from the bladder and out of the body through the ureter tubes. The valve that normally prevents urine reflux is called the vesicoureteral valve. The danger for children with VUR is that a resulting urinary tract infection (UTI) weakens the urinary tract’s ability to prevent bacteria from entering the kidney. In turn, this can result in kidney damage and infection. Serious conditions can even result in death due to acute infection and scarring of the kidney (reflux nephropathy).
- Ectopic insertion of the ureter: An ectopic ureter is when the ureter does not attach correctly to the bladder and urine empties outside of the bladder rather than inside it. In cases of duplex kidney, one ureter drains properly into the bladder while the duplicate ureter does not. The duplicate ureter is the ectopic ureter. In boys, the ectopic ureter drains into the urethra near the prostate; in girls it drains into the urethra or reproductive organs.
- Ureterocele: A ureterocele occurs when the ureter balloons just inside where it connects to the bladder. A ureterocele may also extend outside the bladder neck and the urethra. The ballooning of the ureter causes an obstruction of urine flow due to narrowing. Ureteroceles can vary in size from very small to almost filling up the bladder.
Duplex kidney occurs in about 1 percent of children and females are affected twice as frequently as males 1. The reasons for this sex bias are unknown 3.
Duplex kidney classification
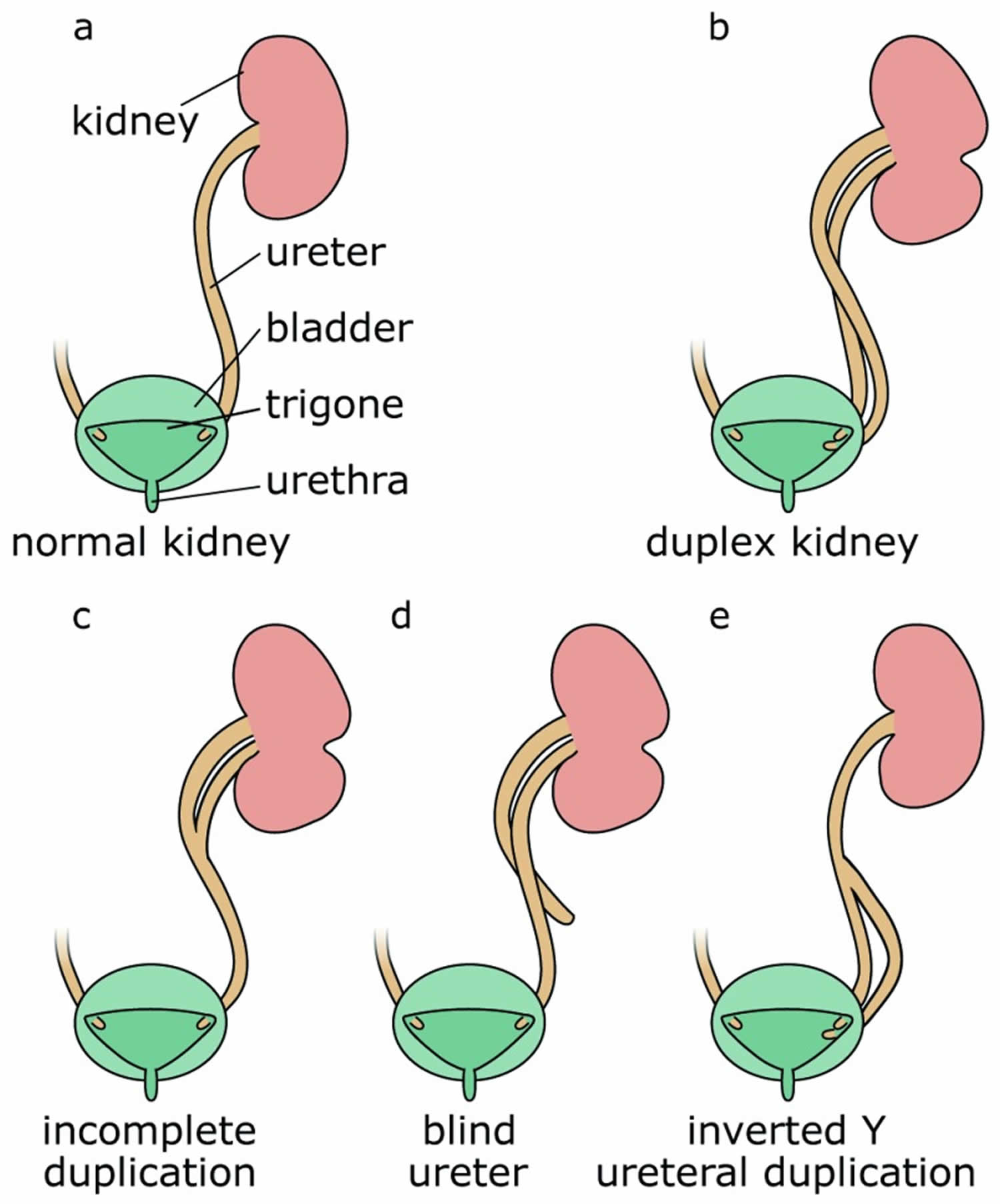
Duplex systems can have a variety of phenotypes, and multiple classification systems have been proposed to categorize Duplex kidney (Figure 1) 4. In incomplete duplication, the two poles of a duplex kidney share the same ureteral orifice of the bladder. Such duplex kidneys with a bifid pelvis or ureter arise when an initially single ureteric bud bifurcates before it reaches the ampulla. This is likely caused by a premature first branching event that occurred before the ureter has reached the metanephric mesenchyme. Much more frequent are complete duplications, which occur when two ureteric buds emerge from the nephric duct. In most cases, the lower pole of the kidney is normal and the upper pole is abnormal 5, an observation explained by the fact that the ectopic ureteric bud frequently emerges anteriorly to the position of the normal ureteric bud and drives the formation of the upper pole of a duplex kidney. Inverted Y-ureteral duplication is a rare condition in which two ureteral orifices drain from a single normal kidney. Inverted Y-ureteral duplication is believed to be caused by the merging of two independent ureteric buds just before or as they reach the kidney anlagen 6. A very rare H-shaped ureter has also been reported 7. Although the vast majority of cases involve a simple duplication, multiplex ureters with up to six independent buds have also been described 8. In some cases, the additional ureter or ureters are ectopic and fail to connect to the bladder or the kidney (blind ending ureter) 8.
Figure 1. Duplex kidney classification
Footnote: Compared with a normal kidney (a), complete duplication produces a duplex kidney with two poles that drain into two ureters (b). Incomplete duplication leads to a Y-shaped ureter (c). Blind ureters do not drain into the bladder (d). In the rare case of inverted Y-ureteral duplication, two ureters fuse before entering the kidney (e).
[Source 3 ]Duplex kidney causes
The cause of most duplex kidneys can be traced back to the very first induction steps of the ureter. In the majority of cases, an additional ureteric bud emerges in a rostral (anterior) position to the normal outgrowth. By contrast, in adults, the upper (abnormal) kidney pole drains into the bladder at a site distal to the orifice of the lower kidney pole 9. This paradoxical phenomenon, known as the Weigert–Meyer rule 10, can be explained by the significant amount of remodelling occurring at the future ureter–bladder junction during development. As apoptosis eliminates the common nephric duct, the ureter inserts into the developing bladder and moves upwards (Figure 2) 11. An initially anteriorly positioned ureter thus ends up with a more distal insertion site in the bladder, a model that has been proposed by Mackie and Stephens 12. Correct positioning of the ureter into the bladder is important to allow formation of a normal trigone (the triangle formed by the two ureter orifices and the urethra) and prevent ureter reflux caused by a malfunctioning valve or a too-short ureter tunnel. Because the vast majority of duplex kidneys arise from an ectopic bud in a rostral position, it is usually the upper pole of the kidney that is affected by vesicoureteral reflux (VUR) and hydronephrosis.
To understand the cause of duplex kidneys, it is important to consider how the urinary system forms. From a developmental point of view, the urogenital tract derives from two independent germ layers with kidneys and ureters arising from the intermediate mesoderm and the bladder and urethra developing from cloacal endoderm 11. Accordingly, malformations of the urinary system can be further classified into congenital abnormalities of the upper and lower urinary tract (sometimes abbreviated as CALUT). Despite this developmental distinction, it should be noted that some authors group malformations of the ureter as part of congenital abnormalities of the lower urinary tract.
Kidney development in mammals commences with the formation of the nephric duct at the anterior (rostral) pole of the intermediate mesoderm 3. As development proceeds, epithelial cells of the nephric duct proliferate and actively migrate towards the caudal end of the nephrogenic cord 13. Eventually, the nephric duct fuses with the cloaca, a process that involves dedicated apoptosis and requires GATA3 and LHX1 as well as retinoic acid and RET and FGF signalling 14.
As the nephric duct elongates caudally, a series of tubules forms within the nephrogenic cord. The most anteriorly positioned pronephric tubules are considered an evolutionary remnant and are non-functional in mammals. Subsequently, a wave of mesonephric tubules develop that fall into two groups. While rostrally positioned tubules are connected to the nephric duct and serve as an embryonic kidney, more caudally located tubules do not drain into the nephric duct and are non-functional 15. Both pronephros and mesonephros are transitory structures in the mammalian embryo and disappear (pronephros) or are remodelled (mesonephros) at later stages of development.
The metanephros represents the permanent kidney in mammals and develops at the most caudal position of the intermediate mesoderm. Metanephros development is first detectable as a population of slightly condensed mesenchymal cells within the nephrogenic cord which express a set of molecular markers (HOX11, SIX2, GDNF, EYA1) 16. In normal development, signalling from the metanephric mesenchyme induces the formation and outgrowth of a single ureteric bud from the nephric duct, which will invade the metanephric mesenchyme and undergo a first stereotypic dichotomous branching event (T-shaped ureter). The collecting duct system (ureteric tree) forms through further rounds of branching that often include tri-tips, which, however, eventually resolve into ureter bifurcations 17. In return, signals released from the ureter will induce the metanephric mesenchyme to differentiate into nephrons, the functional units of the kidney 18.
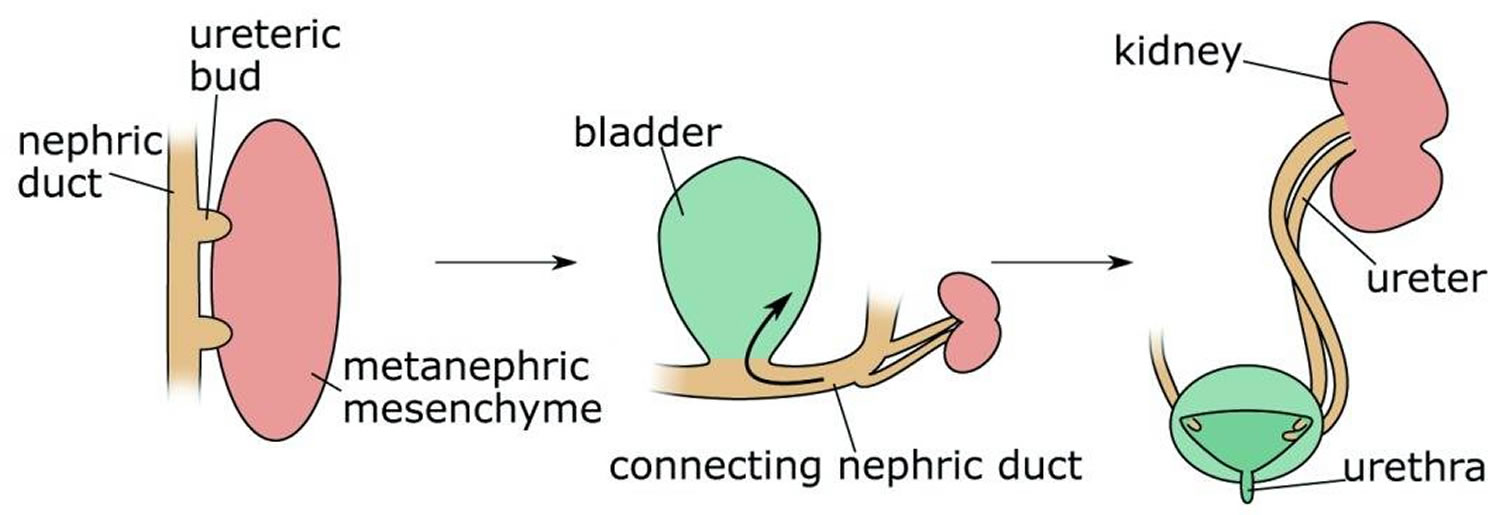
Development of the urinary system is not restricted to kidney formation but also involves extensive developmental remodeling of the lower tract. An excellent and detailed description of this complex process can be found in 11. In brief, the emerging ureteric bud is initially connected to the cloaca via the distal part of the nephric duct, also termed the common nephric duct. Downgrowth of the urorectal septum leads to a separation of the cloaca into a ventrally located urogenital sinus and a dorsally positioned anorectal sinus 11. The cranial urogenital sinus will further elongate to develop into the bladder, whereas its posterior portion will form the urethra. As development proceeds, apoptosis eliminates the common nephric duct, leading to the fusion of the ureter with the future bladder, thus creating the ureterovesical junction 19.
Figure 2. Duplex kidney formation
Footnote: Duplex kidneys form through the induction of two ureteric buds from the nephric duct that will invade the metanephric mesenchyme. Subsequently, apoptosis of the common nephric duct (CND) leads to the insertion of both ureters into the developing bladder with the orifice of the initially posteriorly positioned ureteric bud ending up in a superior position.
[Source 3 ]Duplex kidney symptoms
Most duplex kidneys are asymptomatic and diagnosed incidentally during imaging studies. However, where symptoms do occur (infection, reflux or obstruction), the patient is likely to have completely duplicated ureters. Occasionally, hydronephrosis can be severe enough to result in flank discomfort or even a palpable mass.
Duplex kidney symptoms may include:
- Urinary tract infections (UTIs).
- An obstruction of the urinary tract resulting in poor urine flow.
- Urinary incontinence is marked by frequent leaking of urine.
- In girls, one symptom is tissue protruding from the urethra opening in the vagina.
Duplex kidney complications
Duplex kidney may result in urine flowing back into the kidney rather into the bladder and also may cause obstruction of urine.
Obstruction of urine
Two conditions related to duplex kidney that can result in obstruction of urine flow are ureterocele and an ectopic ureter.
Duplex kidney diagnosis
Ultrasound images before birth often detect duplex kidney. Aside from the ultrasound detection (using an x-ray-like machine that emits high-frequency sound waves), the symptoms above are caused by the following complications associated with duplex kidney.
Duplex kidney treatment
Since most duplex kidneys are a normal finding, no treatment is necessary. If a duplex renal collecting system is associated with vesicoureteral reflux (VUR), an ectopic ureter or a ureterocele, then they are treated according to the underlying condition.
Treatment for vesicoureteral reflux
Vesicoureteral reflux (VUR) may go away with no treatment in some children, which is more likely in younger children and in less severe cases. Antibiotics are generally the first treatment to stop the urinary tract infection (UTI).
Surgery can reconstruct the area where the ureter connects to the bladder. This lengthens the ureter tunnel and allows it to act as a valve that closes as the bladder fills, preventing urine reflux.
Treatment for ureteroceles
Ureteroceles may require simple management of symptoms or surgery, depending on the size of the ballooning, the functioning of the kidney and bladder, and the degree of urine obstruction.
- Endoscopic surgery involves a cytoscope, a lighted tube with a camera that generates images a urologist can view. The cytoscope is put into the urethral opening and if a ureterocele is found, it can be punctured with a small incision during the procedure.
- Ureteral reimplantation is a procedure in which a doctor connects the ectopic ureter correctly to the bladder.
- Partial nephrectomy is the removal of the upper portion of the duplex kidney that has the ectopic ureter.
Treatment for ectopic ureter
Surgical treatments include:
- Ureteral reimplantation is a procedure in which a doctor connects the ectopic ureter correctly to the bladder.
- Cutaneous distal ureterostomy involves bringing the ectopic ureter of a newborn to the skin’s surface so it drains externally (into the diaper). After about 18 months, the ureter is correctly implanted into the bladder.
- Partial nephrectomy is the removal of the upper portion of the duplex kidney that has the ectopic ureter.
- Ureteropyelostomy is an option when the ectopic ureter in the upper section of a duplex kidney can be connected to the lower section of the duplex kidney that is functioning normally.
- Doery AJ, Ang E, Ditchfield MR: Duplex kidney: not just a drooping lily. J Med Imaging Radiat Oncol. 2015;59(2):149–53. 10.1111/1754-9485.12285
- Didier RA, Chow JS, Kwatra NS, et al. : The duplicated collecting system of the urinary tract: embryology, imaging appearances and clinical considerations. Pediatr Radiol. 2017;47(11):1526–38. 10.1007/s00247-017-3904-z
- Kozlov VM, Schedl A. Duplex kidney formation: developmental mechanisms and genetic predisposition. F1000Res. 2020;9:F1000 Faculty Rev-2. Published 2020 Jan 6. doi:10.12688/f1000research.19826.1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6945105
- Glassberg KI, Braren V, Duckett JW, et al. : Suggested Terminology for Duplex Systems, Ectopic Ureters and Ureteroceles. J Urol. 1984;132(6):1153–4. 10.1016/s0022-5347(17)50072-5
- Zissin R, Apter S, Yaffe D, et al. : Renal duplication with associated complications in adults: CT findings in 26 cases. Clin Radiol. 2001;56(1):58–63. 10.1053/crad.2000.0639
- Britt DB, Borden TA, Woodhead DM: Inverted Y Ureteral Duplication with a Blind-Ending Branch. J Urol. 1972;108(3):387–8. 10.1016/s0022-5347(17)60749-3
- Akbulut F, Savun M, Ucpinar B, et al. : Duplicated Renal System with H Shaped Ureter: An Extraordinary Anomaly. Case Rep Urol. 2016;2016:4062515. 10.1155/2016/4062515
- Senel U, Tanriverdi HI, Ozmen Z, et al. : Ectopic Ureter Accompanied by Duplicated Ureter: Three Cases. J Clin Diagn Res. 2015;9(9):PD10–2. 10.7860/JCDR/2015/13933.6446
- Privett JTJ, Jeans WD, Roylance J: The incidence and importance of renal duplication. Clin Radiol. 1976;27(4):521–30. 10.1016/s0009-9260(76)80121-3
- Weigert C: Ueber einige Bildungsfehler der Ureteren. Virchows Arch. 1877;70(4):490–501. 10.1007/BF01935232
- Georgas KM, Armstrong J, Keast JR, et al. : An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development. 2015;142(10):1893–908. 10.1242/dev.117903
- Mackie GG, Stephens FD: Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. J Urol. 1975;114(2):274–80. 10.1016/s0022-5347(17)67007-1
- Attia L, Schneider J, Yelin R, et al. : Collective cell migration of the nephric duct requires FGF signaling. Dev Dyn. 2015;244(2):157–67. 10.1002/dvdy.24241
- Hoshi M, Reginensi A, Joens MS, et al. : Reciprocal Spatiotemporally Controlled Apoptosis Regulates Wolffian Duct Cloaca Fusion. J Am Soc Nephrol. 2018;29(3):775–783. 10.1681/ASN.2017040380
- Mugford JW, Sipilä P, Kobayashi A, et al. : Hoxd11 specifies a program of metanephric kidney development within the intermediate mesoderm of the mouse embryo. Dev Biol. 2008;319(2):396–405. 10.1016/j.ydbio.2008.03.044
- Taguchi A, Kaku Y, Ohmori T, et al. : Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14(1):53–67. 10.1016/j.stem.2013.11.010
- Lefevre JG, Short KM, Lamberton TO, et al. : Branching morphogenesis in the developing kidney is governed by rules that pattern the ureteric tree. Development. 2017;144(23):4377–85. 10.1242/dev.153874
- O’Brien LL: Nephron progenitor cell commitment: Striking the right balance. Semin Cell Dev Biol. 2019;91:94–103. 10.1016/j.semcdb.2018.07.017
- Liaw A, Cunha GR, Shen J, et al. : Development of the human bladder and ureterovesical junction. Differentiation. 2018;103:66–73. 10.1016/j.diff.2018.08.004