Eosinophilic fasciitis
Eosinophilic fasciitis also known as Shulman syndrome, is a very rare autoimmune rheumatic disorder in which the tough band of fibrous tissue, called the fascia, underneath the skin becomes inflamed and swollen and gradually harden in the arms and legs 1. Approximately 300 cases have been reported in the medical literature 2. Eosinophilic fasciitis occurs mostly in middle-aged men but can occur in women and children 3. Eosinophilic fasciitis is more common in people ages 30 to 60. Rapid swelling can occur in the hands, arms, legs, and feet 4. People with eosinophilic fasciitis have a buildup of eosinophils, a type of white blood cell, in the affected fascia and muscles. Eosinophilic fasciitis eventually causes the skin to swell and slowly thicken and harden (induration). Eosinophilic fasciitis is similar in appearance to scleroderma but is not related 1. Corticosteroids and other immune-suppressing medications are used to relieve the symptoms.
The onset of eosinophilic fasciitis is characterized by acute or subacute development of pitting edema and erythema (see Figure 2). As the disease progresses, edema is gradually replaced by a ‘peau d’orange’ aspect as deep sclerosis starts to develop (see Figure 2). Most commonly eosinophilic fasciitis involves the extremities, both arms and legs, and the fingers, feet and face are spared. Sparing of these sites serves to distinguish eosinophilic fasciitis from scleroderma where finger involvement is often one of the first signs. Neck and truncal involvement is reported in widespread disease. Cutaneous involvement may be accompanied by general symptoms such as weight loss, myalgia and asthenia 5. Patients with long-standing eosinophilic fasciitis may develop joint contractures (loss of joint motion due to the hardening of the skin in areas adjacent to the joint) resulting in impaired mobility.
The exact cause of eosinophilic fasciitis is unknown. Eosinophilic fasciitis is considered to be on a continuum with morphea (localized scleroderma) 6. Studies show localized morphea can develop in 28–65% of eosinophilic fasciitis patients 7, 8, 6. Coexisting eosinophilic fasciitis and morphea lesions both respond to treatment for eosinophilic fasciitis. However, the presence of morphea correlates with higher risk of resistant disease 7, 9. Furthermore, patients with morphea can have increased morbidity due to increased risk of residual disease damage 6.
Multiple triggers of eosinophilic fasciitis have been have been proposed in case reports. Up to 28% of eosinophilic fasciitis patients have a history of extreme physical activity or trauma days to weeks prior to presentation 10, 7. Two case series, consisting of 52 5 and 63 patients 11, reported exercise to be suggestive for disease induction in 46 and 28% of the patients, respectively. However, the mechanisms leading to clinical phenotype remain unknown.
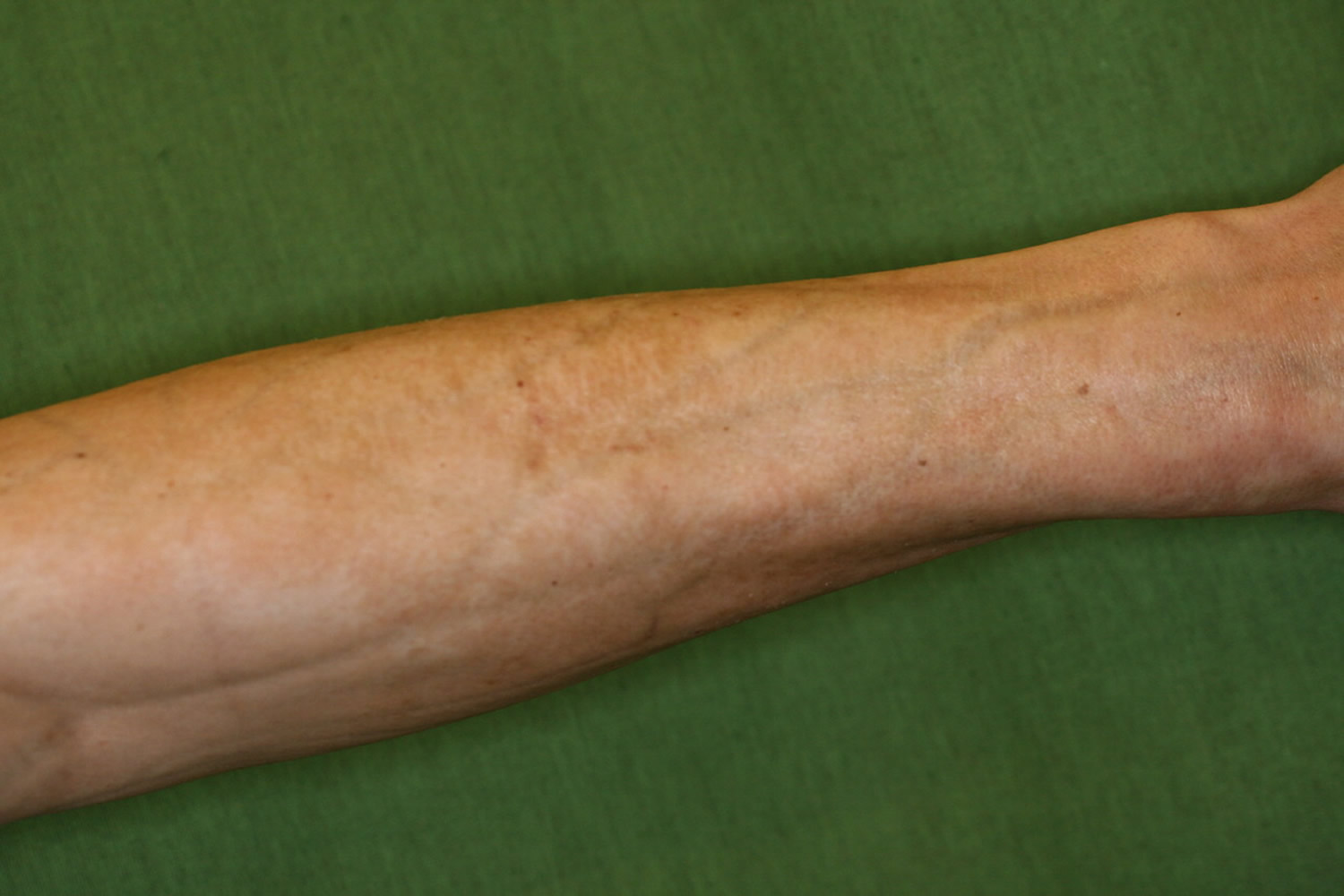
A diagnosis of eosinophilic fasciitis is suspected based upon a detailed patient history, a thorough clinical evaluation, and various laboratory studies. Characteristic skin grooves called venous grooving (groove sign) are typically present (see Figure 1 and 3). The prayer sign (when someone tries to join hands as in prayer but, cannot press the hands flat so they remain curved with just the fingers touching) is indicative of problems with joint mobility and is associated with eosinophilic fasciitis.
About 10-20% of people with eosinophilic fasciitis recover spontaneously without treatment. For those who do not, glucocorticoids (0.5–1 mg/kg/day), such as prednisone, are the mainstay therapy. Even with treatment, improvement in symptoms can take weeks or months. Glucocorticoids are successful in treating eosionophilic fasciitis in over 70% of cases 12. If glucocorticoids are unsuccessful, methotrexate at low doses (15–25 mg once weekly) is probably the most favored second-line treatment, especially in people with reddish to purpleish (morphea-like) skin lesions 13. Other treatment options include nonsteroidal anti-inflammatory drugs (NSAIDs), D-penicillamine, chloroquine, cimetidine, azathioprine, cyclosporin A, infliximab, UVA-1, and bath PUVA 12. Physical therapy may help improve joint mobility and decrease contractures. Surgical release has been used in some severe cases to manage significant joint contractures 14.
Figure 1. Eosinophilic fasciitis
Footnote: Classical symptoms of eosinophilic fasciitis with venous furrowing on the arms of the patient.
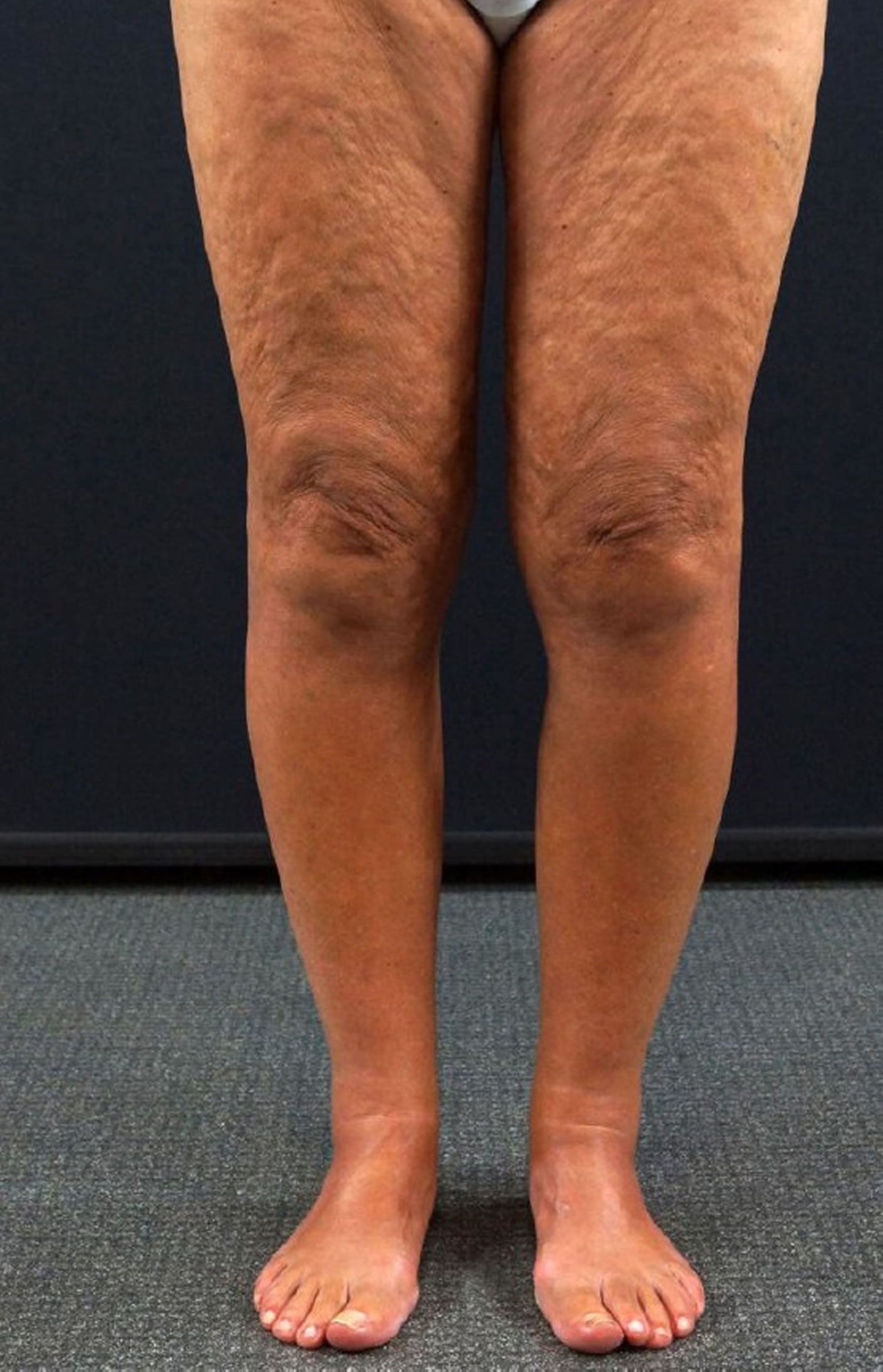
[Source 15 ]Figure 2. Eosinophilic fasciitis with Peau d’orange sign (“orange skin”, a phenomenon in which hair follicles become buried in edema, giving the skin an orange peel appearance)
Footnote: Eosinophilic fasciitis in a 64-year-old woman. Peau d’orange sign
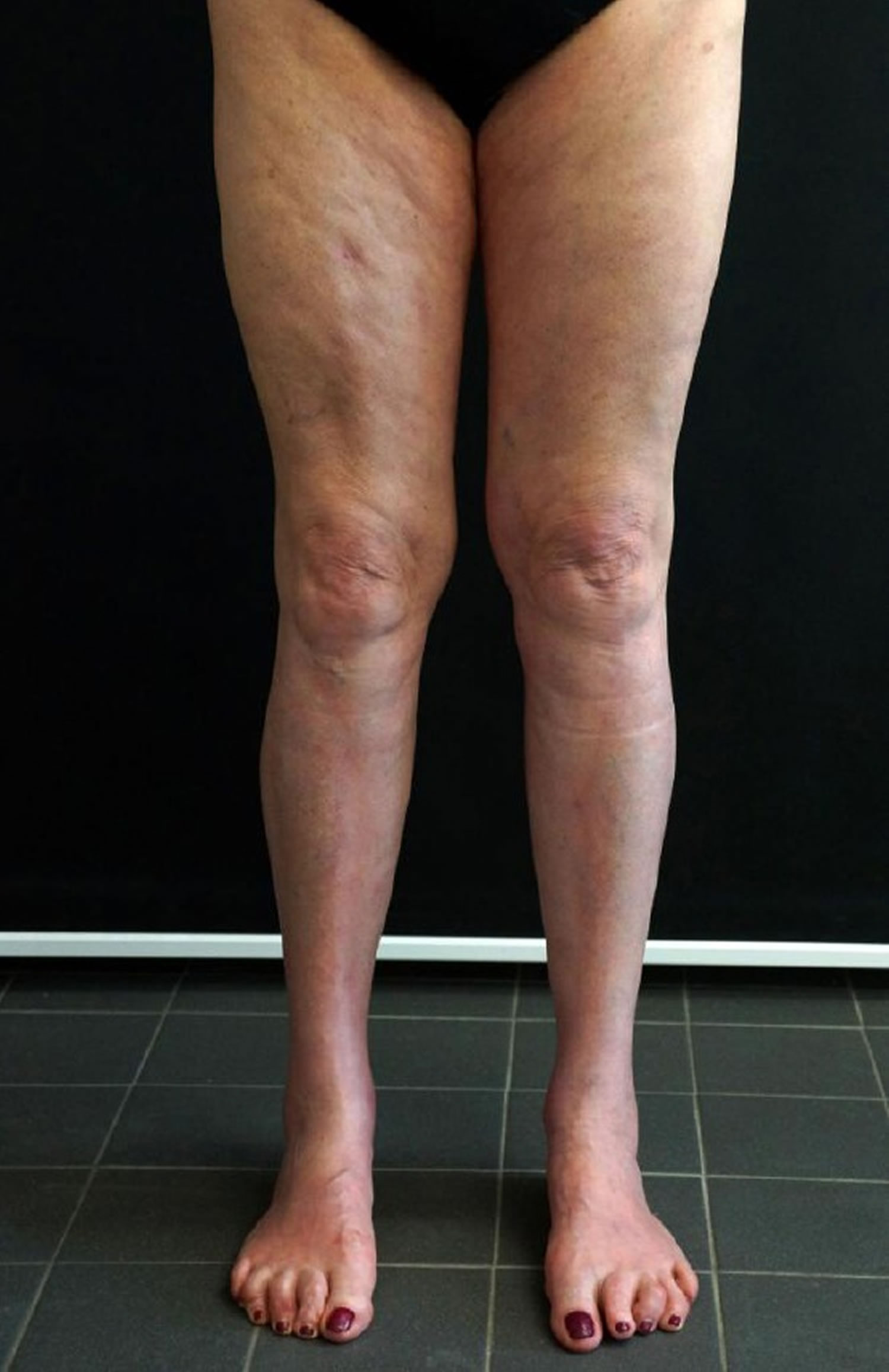
[Source 16 ]Figure 3. Eosinophilic fasciitis groove sign (linear depression along the veins)
Footnote: Eosinophilic fasciitis in a 65-year-old woman. Positive groove sign on her leg
[Source 16 ]Eosinophilic fasciitis causes
The cause of eosinophilic fasciitis is unknown. In people with eosinophilic fasciitis, white blood cells, called eosinophils, build up in the muscles and tissues. Eosinophils are linked to allergic reactions. Classified as an autoimmune disorder, eosinophilic fasciitis was first described in 1974 by Shulman 17 and is also known to be complicated by other autoimmune diseases, such as scleroderma, polymyositis, hypereosinophilic syndrome, and Churg-Strauss vasculitis 18.
Some scientists believe that eosinophilic fasciitis may be a variant of scleroderma or morphea (localized scleroderma). These rare disorders are characterized by the hardening and thickening of skin and surrounding tissue, often due to the malfunction of the immune system.
Researchers believe that eosinophilic fasciitis results due to a nonspecific triggering event that causes an abnormal immune system response, specifically an abnormal allergic or inflammatory reaction. This abnormal response causes the overproduction and accumulation of eosinophils and other white blood cells in certain tissues of the body. The exact reason for this overproduction and accumulation is not fully understood.
Several possible triggers have been reported with some consistency. A preceding history of vigorous exercise (exercise-induced eosinophilic fasciitis) or trauma has been reported in 30%-50% of patients 19. Multiple drugs have also been implicated, including simvastatin, atorvastatin, and phenytoin 20.
Historically, eosinophilic fasciitis was reported to develop after consumption of dietary supplement contaminated L-tryptophan (eosinophilia–myalgia syndrome), or other chemicals 21. Tryptophan is an essential amino acid found in numerous foods including poultry and was used as a sleep aid or to treat depression. In 1989 L-tryptophan ingestion was linked to an epidemic of a disorder known as eosinophilia-myalgia syndrome. Despite this, there is no consistent association between L-tryptophan or other dietary exposure and eosinophilic fasciitis. As evidence, L-tryptophan use was significantly associated with dyspnea, an uncommon finding in eosinophilic fasciitis cases. In another instance, a patient with eosinophilic fasciitis had used L-tryptophan for several years but had started a formal exercise program 2 weeks prior to disease onset.
A similar syndrome occurs in European patients exposed to Borrelia infection 22, 23, 24. Most of these factors have also been reported in association with morphea [i.e., Borrelia burgdorferi infection, radiation therapy 25 and insect bites 26].
Some researchers believe eosinophilic fasciitis is one of several disorders that should be grouped under the designation fasciitis-panniculitis syndrome (FPS). Disorders under this designation are characterized by hardening and thickening of the skin due to inflammation and fibrosis.
Eosinophilic fasciitis has been associated with several diseases 27. Hematologic diseases have been consistently reported and are supported by large case series and case reports 28. The spectrum of associated hematologic disease is broad and includes aplastic anemia and hemolytic anemia, thrombocytopenia, myeloproliferative disorders, myelodysplastic disorders, lymphoma, leukemia, monoclonal gammopathy of undetermined significance (MGUS), and multiple myeloma 29.
An association with thyroid disease has been reported in several cases 30. Eosinophilic fasciitis has rarely been linked to solid-organ tumors and primary biliary cirrhosis, in addition to several other diseases. These disease associations may suggest a shared pathophysiology of cellular dysregulation and/or autoimmunity.
Eosinophilic fasciitis pathology
Although the cause of eosinophilic fasciitis is unknown, studies have shed light on some of the mechanisms involved in its pathogenesis. In general, the pathophysiology underlying eosinophilic fasciitis is postulated to involve an inflammatory response resulting in an activated inflammatory cell infiltrate of affected tissues and subsequent dysregulation of extracellular matrix production by lesional fibroblasts 31. Dermal fibroblasts are hyperactive overexpressing type 1 collagen and fibronectin 32.
Viallard et al 33 demonstrated that, when stimulated, peripheral blood mononuclear cells of eosinophilic fasciitis patients produce significantly higher amounts of five cytokines, including interleukin (IL)–5 and interferon (IFN)–gamma. IL-5 is known to activate mature eosinophils and to stimulate eosinophil chemotaxis, growth, and differentiation. IFN-gamma activates tissue macrophages and T cells. The findings of Dziadzio et al 34 support increased levels of IL-5 in eosinophilic fasciitis, in addition to increased levels of transforming growth factor (TGF)–beta, another fibrogenic cytokine.
Toquet et al 35 investigated the phenotype of the lesional inflammatory cell infiltrate in patients with eosinophilic fasciitis and demonstrated a predominance of macrophages, CD8+ lymphocytes, and few eosinophils. Pathologic specimens from patients with eosinophilic fasciitis demonstrate increased numbers of eosinophils, especially early in the disease course.
Taken together, the findings of these studies suggest a mechanistic framework marked by a proinflammatory and fibrogenic cytokine response with resultant tissue inflammatory cell infiltration.
In the tissues, the end effector cell of fibrosis is the fibroblast. Fibroblasts from lesional tissue of patients with eosinophilic fasciitis produce excess collagen in vitro and display elevated TGF-beta and type 1 collagen mRNA levels when examined via in situ hybridization with specific cDNA 36, 37. Therefore, the pathogenesis appears to involve the concomitant increase in the expression of genes for TGF-beta and extracellular matrix proteins in fibroblasts in the affected tissues.
Mori et al 38 suggested that an autocrine stimulatory loop involving major basic protein, a product of eosinophil degranulation, IL-6, which enhances collagen production and is induced my major basic protein, and TGF-beta could account for the progressive fibrosis seen in several eosinophil prominent disorders.
Other studies showed elevated levels of serum manganese superoxide dismutase and tissue metalloproteinase 1 (TIMP-1) in eosinophilic fasciitis, suggesting a role in pathogenesis and providing a possible marker of disease activity 39, 40.
Fasciitis may be a common manifestation of various pathophysiologic processes associated with eosinophilia. The existence of primary and secondary forms of fasciitis has recently been suggested.
Understanding the mechanisms involved in the development of fascial inflammation and fibrosis in these conditions may yield insights into the pathogenesis of other fibrotic skin diseases.
Eosinophilic fasciitis symptoms
Although researchers have been able to establish a clear syndrome with characteristic or “core” symptoms, much about eosinophilic fasciitis is not fully understood. Several factors including the small number of identified cases, the lack of large clinical studies, and the possibility of other unknown influencing factors prevent physicians from developing an accurate picture of associated symptoms and prognosis. Therefore, it is important to note that affected individuals may not have all of the symptoms discussed below.
Eosinophilic fasciitis symptoms may include:
- Tenderness and swelling of the skin on the arms, legs, or sometimes the joints (most often on both sides of the body)
- Arthritis
- Carpal tunnel syndrome (a condition caused by compression of median nerveaffecting one or both hands. It is characterized by a sensation of numbness, tingling, burning and/or pain in the hand and wrist. Symptoms are slowly progressive and may eventually make it difficult to form a fist or grasp small objects).
- Muscle pain
- Thickened skin that looks puckered
Classically, patients with eosinophilic fasciitis present with symmetric swelling of the skin associated with an aching of the affected extremities, which may develop acutely over a period of days to weeks. Eosinophilic fasciitis may also manifest subacutely. In addition, if patients present later in their disease course, they are more likely to have symptoms of induration or fibrosis of the affected areas.
The onset of illness is not accompanied by fever or other systemic symptoms. In up to half of all patients, disease onset follows an episode of strenuous physical exercise or activity 41.
Neither Raynaud phenomenon nor symptoms of respiratory, gastrointestinal, or cardiac involvement are typically present.
Inflammatory arthritis has been reported and manifests as joint pain, swelling, and morning stiffness 19.
With progressive fibrosis, patients may experience limited range of motion due to joint contractures and paresthesias in a distribution pattern consistent with carpal tunnel syndrome.
Cutaneous manifestations include the following 19:
- The cutaneous manifestations of eosinophilic fasciitis evolve as the disease progresses. In the acute inflammatory stage, cutaneous changes include erythematous swelling and nonpitting edema. These findings are later replaced by skin induration, and, eventually, fibrosis predominates. The affected skin is taut and firmly adherent to underlying tissues. Dimpling, peau d’orange, and venous furrowing, or the “groove sign,” can be seen.
- Other cutaneous changes reported include urticaria, bullae, alopecia, lichen sclerosus et atrophicus, vitiligo, and hyperpigmentation.
- Cutaneous manifestations are generally bilateral and symmetric. The upper extremity, proximal and distal to the elbow, and the lower extremity, proximal and distal to the knee, are most commonly involved. The trunk and neck can also be involved. Face and hand involvement are rare.
- A concurrent localized lesion of morphea may be seen in 25% of patients.
Extracutaneous manifestations include the following:
- Joint contractures represent the most common extracutaneous manifestation of eosinophilic fasciitis, occurring in 50%-75% of patients, and can affect elbows, wrists, ankles, knees, and shoulders 41. Extensive truncal fibrosis may limit chest expansion. A clawlike deformity of the hand has been described.
- Inflammatory arthritis was reported in roughly 40% of patients in two series 19. The knees, wrists, hands, and feet appear to be most commonly involved.
- Carpal tunnel syndrome is seen in 16%-23% of patients 19.
- Clinically significant visceral involvement is rare, limited to case reports. If present, significant visceral involvement should prompt investigation of an alternative diagnosis. When pursued, specific testing with pulmonary function testing, esophagogastroduodenoscopy and electromyelography (EMG) may demonstrate subtle or nonspecific abnormalities 42.
Some affected individuals may develop nonspecific symptoms including fatigue, weight loss, fever, and a general feeling of ill health (malaise) or a general lack of strength (asthenia). Although muscle strength is usually unaffected, muscle pain (myalgia) and inflammation of the joints (arthritis) often occurs. Bone pain has also been reported.
Eosinophilic fasciitis possible complications
Musculoskeletal complications (arthralgia, contractures, and arthritis) are reported in up to 40% of morphea and eosinophilic fasciitis patients 43, 44. Eosinophilic fasciitis manifests secondary to malignancy, such as myeloproliferative disorders, myelodysplastic disorders, lymphoma, leukemia, monoclonal gammopathy of undetermined significance (MGUS), and multiple myeloma, in a minority of patients (5–10%). The outlook is much worse if blood diseases occur.
Eosinophilic fasciitis diagnosis
Eosinophilic fasciitis is diagnosed using clinical appearance, characteristic histology, and other laboratory findings.
Tests that may be done include:
- Complete blood count (CBC) with differential
- Gamma globulins (a type of immune system protein)
- Erythrocyte sedimentation rate (ESR)
- MRI
- Muscle biopsy
- Skin biopsy (the biopsy needs to include the deep tissue of the fascia)
Diagnostic criteria for eosinophilic fasciitis have been proposed by different groups of investigators 45:
- Major criteria
- (a) symmetric or asymmetric diffuse or localised swelling, induration and thickening of the skin and subcutaneous tissue
- (b) Histology showing fascial thickening with an accumulation of lymphocytes and macrophages with or without eosinophils
- Minor criteria
- (a) Peripheral eosinophilia (> 0.5 x 109/L)
- (b) Hypergammaglobulinemia (> 1.5 g/L)
- (c) Muscular weakness and/or elevated serum aldolase
- (d) Groove sign and/or peau d’orange appearance of skin
- (e) T2-weighted MRI showing hyperintense fascia
- Exclusion criterion
- Diagnosis of systemic scleroderma
Japanese diagnostic criteria of eosinophilic fasciitis 46:
- Major criterion:
- Symmetrical plate-like sclerotic lesions are present on the four limbs.
- However, this condition lacks Raynaud’s phenomenon, and systemic sclerosis can be excluded.
- Minor criteria 1:
- The histology of a skin biopsy that incorporates the fascia shows fibrosis of the subcutaneous connective tissue, with thickening of the fascia and cellular infiltration of eosinophils and monocytes.
- Minor criteria 2:
- Thickening of the fascia is seen using imaging tests such as magnetic resonance imaging (MRI).
- A definitive diagnosis is made when a patient has the major criterion and one of the minor criteria, or the major criterion and two of the minor criteria.
A full-thickness biopsy, containing fascia and muscle, is the golden standard for the diagnosis of eosinophilic fasciitis 6. The specimen should include the skin, fat, fascia, and superficial muscle in continuity. Biopsy is especially important in an atypical presentation 47.
Histology typically displays a thickened fascia infiltrated by lymphocytes accompanied by eosinophils, plasma cells, and macrophages 48. The presence of eosinophils is transient and may be absent if patients have prolonged disease or receive systemic corticosteroids or immunosuppressive drugs 6. Adjacent myositis is frequently observed 49. The presence of thickened dermal collagen fibers may reflect the presence of concomitant superficial morphea 48. More recently, magnetic resonance imaging (MRI) 50, ultrasound 51, and positron emission tomography (PET) 52 have been reported to be helpful in establishing the diagnosis by decreasing the likelihood of sampling errors for deep biopsies or by visualizing the fasciitis.
Characteristic laboratory findings of eosinophilic fasciitis include the following:
- Peripheral blood eosinophilia is present in 61%-83% of patients. The degree of eosinophilia is variable over time, even in the absence of specific therapy 53.
- Hypergammaglobulinemia is characteristic, although this finding varies widely by case series, occurring in 18%-67% of patients. It is most often due to a polyclonal increase in immunoglobulin G 54.
- An increase in the erythrocyte sedimentation rate (ESR) is found in 29%-70% of cases 54.
Additional laboratory findings of eosinophilic fasciitis include the following 19:
- Serum creatine kinase and aldolase levels are generally normal.
- Rheumatoid factor and antinuclear antibodies are occasionally positive.
- Hematologic abnormalities and disease are associated with eosinophilic fasciitis. Aplastic anemia, although rare, is the most frequent common associated hematological complication, but cases have been described with thrombocytopenia, hemolytic anemia, pernicious anemia, lymphoma, and leukemia 55
- Borrelia serology or polymerase chain reaction (PCR) findings are occasionally positive and may suggest a treatable etiology. However, as discussed above, the exact correlation between eosinophilic fasciitis and Borrelia remains unclear 56
- Metalloproteinase 1 (TIMP-1) may be a new serological marker of disease activity 57
Imaging studies
Magnetic resonance imaging (MRI) is the imaging modality of choice. MRI of the involved areas shows characteristic findings of fascial thickening, abnormal signal intensity, and contrast enhancement. Additionally, MRI aids in making the diagnosis, locating the biopsy site, and monitoring the response to treatment 58.
Although it has not been used frequently or studied extensively in eosinophilic fasciitis, one case report has shown that ultrasonography can aid in early diagnosis 59. According to a study by Kissin et al 60 that included 12 patients with eosinophilic fasciitis, a 12-MHz, B-mode ultrasound may be used to measure subcutaneous compressibility and thereby serve as an adjunctive tool to distinguish eosinophilic fasciitis from diffuse systemic sclerosis, especially when tissue sampling is less feasible or when the result of tissue sampling is equivocal.
Eosinophilic fasciitis differential diagnosis
Symptoms of the following disorders can be similar to those of eosinophilic fasciitis. Comparisons may be useful for a differential diagnosis.
Eosinophilic fasciitis differential diagnosis include the following:
- The localized forms of scleroderma, morphea, and linear forms of scleroderma
- Limited and diffuse cutaneous systemic sclerosis
- Other localized cutaneous fibrosing disorders, eg, nephrogenic systemic fibrosis, scleromyxedema, and scleredema
Scleroderma is a rare connective tissue disorder characterized by abnormally increased production and accumulation of collagen, the body’s major structural protein, in skin and other organs of the body. There are systemic and localized forms of scleroderma. Systemic scleroderma is characterized by hardening (induration) and thickening of the skin and abnormal degenerative changes and formation of fibrous tissue (fibrosis) in certain organs of the body including the lungs, heart, kidneys, and gastrointestinal tract. Associated symptoms, which may vary widely from case to case, may include abnormal discoloration of and pain affecting the fingers and toes upon exposure to cold temperatures (Raynaud’s phenomenon); abnormal tightness, thickening, “waxiness,” and loss of elasticity of the skin; shortness of breath; difficulty swallowing; muscle weakness; joint pain; heart abnormalities including irregular heartbeats (palpitations); kidney (renal) abnormalities; and/or other symptoms and findings. In individuals with localized scleroderma, involvement is restricted to the skin, tissue under the skin (subcutaneous tissue), and, in some cases, underlying muscle and bone. Linear scleroderma is a localized form of scleroderma that may involve only certain areas of the body, such as an arm, a leg, or a portion of the face. It is characterized by multiple lesions of the skin, abnormally increased or decreased skin pigmentation (hyper- or hypopigmentation), and associated atrophy of the skin, subcutaneous tissue, muscle, and bone. Although the exact cause of scleroderma is unknown, researchers suggest that the disorder represents an abnormal autoimmune response.
Morphea also known as localized scleroderma, is a rare skin disorder that encompasses a group of idiopathic sclerotic skin diseases characterized by the accumulation of collagen in the skin and subcutaneous tissues 6. Affected tissue may thicken and harden in response to inflammation caused by collagen accumulation. Individuals with morphea may not have any apparent symptoms (asymptomatic). Skin discoloration and joint pain (arthralgia) may occur in some cases. The exact cause of morphea is unknown, although it is believed to be an autoimmune disorder.
Eosinophilia-myalgia syndrome occurred as an epidemic in 1989 associated with the ingestion of contaminated L-tryptophan, a dietary supplement widely sold at that time. The contaminant remains unknown. This syndrome abruptly caused severe, disabling, chronic muscle pain (myalgia); skin symptoms such as inflammation of the tough band of fibrous tissue beneath the skin (fascia); and other neurotoxic reactions. Affected individuals had elevated levels of certain white blood cells (eosinophils) in various tissues of the body (eosinophilia). Since the epidemic, cases of eosinophilia-myalgia syndrome have occurred rarely. Diagnosis is not easy and depends on finding unusually high levels of eosinophils (circulating white blood cells) associated with severe myalgia (and the findings noted above).
The Spanish toxic oil syndrome due to ingestion of contaminated rapeseed oil occurred as an epidemic in 1981 and caused skin involvement similar to eosinophilic fasciitis. The epidemic abruptly ceased once the contaminated rapeseed oil was no longed consumed.
Eosinophilic disorder is a general term for any disorder characterized by infiltration of the skin and tissue by a certain type of white blood cell called eosinophils, including disease resulting from arthropod bites, infections, and drug reactions. Churg-Strauss syndrome, hypereosinophilic syndrome and eosinophilic cellulitis are examples of disorders characterized by elevated levels of eosinophils.
Eosinophilic fasciitis treatment
First-line therapy for eosinophilic fasciitis is with systemic corticosteroids and the drug prednisone is often prescribed 61. Prednisone therapy may be required for two months or longer. In many cases, high doses of corticosteroids are used at first and slowly tapered off over a period of months to years 4. However, there is higher risk of eosinophilic fasciitis reoccurrence once prednisone is discontinued 7. Methotrexate can be used concomitantly with prednisone or as second-line monotherapy 10, 62, 63. Additionally, some suggest dual treatment with methotrexate and prednisone should be considered mainstay therapy for patients with morphea-like lesions, as these patients often have decreased response to systemic corticosteroid monotherapy 9.
Although patients may require prolonged therapy, it should be noted that up to one third of eosinophilic fasciitis cases may spontaneously resolve 64. It is known that concurrent morphea is associated with a 1.4 to 3 times higher risk of resistance to systemic corticosteroid therapy 65.
Case reports describe the use of a number of agents for second-line therapy. No consensus exists on which agent is best for that purpose.
Other drugs that have been occasionally used are mycophenolate mofetil, cyclosporin A, cyclophosphamide, infliximab, dapsone, azathioprine, sulfasalazine, tumour necrosis factor-inhibitors, sirolimus, immunoglobulins, and D-penicillamine. Photo(chemo)therapy with either UVA1, PUVA (psoralen ultraviolet A) or extracorporeal photochemotherapy has also been reported 66. There are some case reports about the successful but off-label use of interleukin-6 antagonist tocilizumab 67, anti-CD-antibody rituximab 68, and Janus kinase inhibitor tofacitnib 69. Recently, two drugs, mepolizumab and reslizumab, were approved for the treatment of eosinophil associated asthma. These drugs abolish eosinophils from the blood by neutralizing interleukin-5. Another agent, benralizumab, directly kills eosinophils and is under trial for treatment of eosinophilic asthma. All of these agents appear promising as candidates for treatment of eosinophilic fasciitis. In some of the cases, these new drugs have been used in combination with either methotrexate or prednisolone.
Lastly, the following alternative treatments have been reported in case reports and series: intravenous immune globulin (IVIG) 70 and bone marrow transplantation 71.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and H2 blockers also have been used to treat eosinophilic fasciitis.
Physical therapy should be initiated to improve joint mobility and to decrease contractures 72. Surgical release has been used in some cases to manage significant joint contractures 73.
Dermatologists, rheumatologists, and surgeons (for the skin-muscle biopsy) are consulted most often for management of these cases.
Eosinophilic fasciitis prognosis
In most cases, eosinophilic fasciitis goes away within 1 to 3 years. However, symptoms may last longer or come back. A retrospective review found that clinical factors associated with persistent fibrosis included presence of morphealike skin lesions, younger age at onset, truncal involvement, and presence of dermal fibrosclerosis on histopathologic specimen 74.
Loss of edema is usually the first clinical sign of improvement and can occur within 4 weeks of commencing treatment. Concurrently, the skin becomes softer, but 3-6 months may elapse before maximal reduction in induration and contractures is achieved 19.
While total resolution of the clinical signs can occur, some degree of induration remaining even after many months of corticosteroid therapy is not unusual.
A direct correlation does not always exist between clinical disease activity and laboratory findings. The eosinophilia and ESR usually return to reference ranges within 6-8 weeks, although the ESR and hypergammaglobulinemia may remain abnormal for up to 12 months 19.
Eventually, corticosteroid therapy can be withdrawn in many of the patients, without relapse occurring.
The development of aplastic anemia is a rare but grave complication 55. One study reported on 4 patients with eosinophilic fasciitis and severe aplastic anemia. In 3 cases, the aplastic anemia was refractory to conventional immunosuppressive therapy with antithymocyte globulin and cyclosporine. However, in 1 patient, rituximab displayed significant efficacy for both the skin and hematologic symptoms. In an additional 19 cases of eosinophilic fasciitis and aplastic anemia, corticosteroid regimens improved skin symptoms in 5 of 12 cases but were ineffective in the treatment of aplastic anemia in all but 1 case. Aplastic anemia was profound in 13 cases and was the cause of death in 8 cases. Only 5 patients achieved long-term remission 75.
References- Eosinophilic fasciitis. https://medlineplus.gov/ency/article/000447.htm
- Eosinophilic Fasciitis. https://rarediseases.org/rare-diseases/eosinophilic-fasciitis/
- Eosinophilic Fasciitis. https://www.msdmanuals.com/professional/musculoskeletal-and-connective-tissue-disorders/autoimmune-rheumatic-disorders/eosinophilic-fasciitis
- Mazori DR, Femia AN, Vleugels RA. Eosinophilic fasciitis: an updated review on diagnosis and treatment. Curr Rheumatol Rep. 2017;19:74. doi: 10.1007/s11926-017-0700-6
- Lakhanpal S, Ginsburg WW, Michet CJ, Doyle JA, Moore SB. Eosinophilic fasciitis: clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum. 1988 May;17(4):221-31. doi: 10.1016/0049-0172(88)90008-x
- Mertens JS, Seyger MMB, Thurlings RM, Radstake TRDJ, de Jong EMGJ. Morphea and Eosinophilic Fasciitis: An Update. Am J Clin Dermatol. 2017 Aug;18(4):491-512. doi: 10.1007/s40257-017-0269-x
- Berianu F, Cohen MD, Abril A, Ginsburg WW. Eosinophilic fasciitis: clinical characteristics and response to methotrexate. Int J Rheum Dis. 2015;18:91–98. doi: 10.1111/1756-185X.12499
- Lebeaux D, Sène D. Eosinophilic fasciitis (Shulman disease). Best Pract Res Clin Rheumatol. 2012 Aug;26(4):449-58. doi: 10.1016/j.berh.2012.08.001
- Lebeaux D, Francès C, Barete S, et al. Eosinophilic fasciitis (Shulman disease): new insights into the therapeutic management from a series of 34 patients. Rheumatology (Oxford) 2012;51:557–561. doi: 10.1093/rheumatology/ker366
- Wright NA, Mazori DR, Patel M, Merola JF, Femia AN, Vleugels RA. Epidemiology and treatment of eosinophilic fasciitis: an analysis of 63 Patients from 3 tertiary care centers. JAMA Dermatol. 2016;152:97–99. doi: 10.1001/jamadermatol.2015.3648
- Wright NA, Mazori DR, Patel M, Merola JF, Femia AN, Vleugels RA. Epidemiology and Treatment of Eosinophilic Fasciitis: An Analysis of 63 Patients From 3 Tertiary Care Centers. JAMA Dermatol. 2016 Jan;152(1):97-9. doi: 10.1001/jamadermatol.2015.3648
- Danis R, Akbulut S, Altintas A, Ozmen S, Ozmen CA. Unusual presentation of eosinophilic fasciitis: two case reports and a review of the literature. J Med Case Rep. 2010;4:46. Published 2010 Feb 8. doi:10.1186/1752-1947-4-46 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2830980
- Pinal-Fernandez I, Selva-O’ Callaghan A, Grau JM. Diagnosis and classification of eosinophilic fasciitis. Autoimmun Rev. 2014 Apr-May; 13(4-5):379-82.
- Eosinophilic Fasciitis. https://emedicine.medscape.com/article/329515-overview
- Eosinophilic Fasciitis Associated with Mycoplasma arginini Infection. Pálma Silló, Dóra Pintér, Eszter Ostorházi, Mercedes Mazán, Norbert Wikonkál, Katinka Pónyai, Dmitriy V. Volokhov, Vladimir E. Chizhikov, Susan Szathmary, Laszlo Stipkovits, Sarolta Kárpáti. Journal of Clinical Microbiology Feb 2012, 50 (3) 1113-1117; DOI: 10.1128/JCM.05568-11
- Wollina U, Hansel G, Schönlebe J, Heinig B, Temelkova I, Tchernev G, Vojvocic A, Lotti T. Eosinophilic Fasciitis – Report of Three Cases and Review of the Literature. Open Access Maced J Med Sci. 2019 May 15;7(18):2964-2968. doi: 10.3889/oamjms.2019.296
- Shulman LE. Diffuse fasciitis with hypergammaglobulinemia and eosinophilia: a new syndrome? J Rheumatol I: 46, 1974.
- Bischoff L, Derk C. Eosinophilic fasciitis: demographics, disease pattern and response to treatment: report of 12 cases and review of the literature. Int J Dermatol 47: 29-35, 2008.
- Bischoff L, Derk CT. Eosinophilic fasciitis: demographics, disease pattern and response to treatment: report of 12 cases and review of the literature. Int J Dermatol. 2008 Jan. 47(1):29-35.
- DeGiovanni C, Chard M, Woollons A. Eosinophilic fasciitis secondary to treatment with atorvastatin. Clin Exp Dermatol. 2006 Jan. 31(1):131-2.
- Blauvelt A, Falanga V. Idiopathic and L-tryptophan-associated eosinophilic fasciitis before and after L-tryptophan contamination. Arch Dermatol. 1991 Aug. 127(8):1159-66.
- Bischoff L, Derk CT. Eosinophilic fasciitis: demographics, disease pattern and response to treatment: report of 12 cases and review of the literature. Int J Dermatol. 2008;47:29–35. doi: 10.1111/j.1365-4632.2007.03544.x
- Antic M, Lautenschlager S, Itin PH. Eosinophilic fasciitis 30 years after- what do we really know?: report of 11 patients and review of the literature. Dermatology. 2006;213:93–101. doi: 10.1159/000093847
- Granter SR, Barnhill RL, Duray PH. Borrelial fasciitis: diffuse fasciitis and peripheral eosinophilia associated with Borrelia infection. Am J Dermatopathol. 1996 Oct;18(5):465-73. doi: 10.1097/00000372-199610000-00004
- Sherber NS, Wigley FM, Paget SA. Diffuse fasciitis with eosinophilia developing after local irradiation for breast cancer. Clin Rheumatol. 2009 Jun;28(6):729-32. doi: 10.1007/s10067-009-1122-2
- Mallepalli JR, Quinet RJ, Sus R. Eosinophilic fasciitis induced by fire ant bites. Ochsner J. 2008 Fall;8(3):114-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3096327
- Lee P. Eosinophilic fasciitis: new associations and current perspectives [editorial]. J Rheumatol. 1981 Jan-Feb. 8(1):6-8.
- Doyle JA, Connolly SM, Hoagland HC. Hematologic disease in scleroderma syndromes. Acta Derm Venereol. 1985. 65(6):521-5.
- Masuoka H, Kikuchi K, Takahashi S, Kakinuma T, Hayashi N, Furue M. Eosinophilic fasciitis associated with low-grade T-cell lymphoma. Br J Dermatol. 1998 Nov. 139(5):928-30.
- Hur JW, Lee HS, Uhm WS, et al. Eosinophilic fasciitis associated with autoimmune thyroiditis. Korean J Intern Med. 2005 Jun. 20(2):180-2.
- Eosinophilic Fasciitis. https://emedicine.medscape.com/article/329515-overview#a5
- Jinnin M, Ihn H, Yamane K, Asano Y, Yazawa N, Tamaki K. Serum levels of tissue inhibitor of metalloproteinase-1 and 2 in patients with eosinophilic fasciitis. Br J Dermatol. 2004;151(2):407–12. https://doi.org/10.1111/j.1365-2133.2004.06062.x
- Viallard JF, Taupin JL, Ranchin V, Leng B, Pellegrin JL, Moreau JF. Analysis of leukemia inhibitory factor, type 1 and type 2 cytokine production in patients with eosinophilic fasciitis. J Rheumatol. 2001 Jan;28(1):75-80.
- Dziadzio L, Kelly EA, Panzer SE, Jarjour N, Huttenlocher A. Cytokine abnormalities in a patient with eosinophilic fasciitis. Ann Allergy Asthma Immunol. 2003 Apr;90(4):452-5. doi: 10.1016/S1081-1206(10)61832-7
- Toquet C, Hamidou MA, Renaudin K, Jarry A, Foulc P, Barbarot S, Laboisse C, Mussini JM. In situ immunophenotype of the inflammatory infiltrate in eosinophilic fasciitis. J Rheumatol. 2003 Aug;30(8):1811-5.
- Kähäri VM, Heino J, Niskanen L, Fräki J, Uitto J. Eosinophilic fasciitis. Increased collagen production and type I procollagen messenger RNA levels in fibroblasts cultured from involved skin. Arch Dermatol. 1990 May;126(5):613-7.
- Peltonen J, Kähäri L, Jaakkola S, Kähäri VM, Varga J, Uitto J, Jimenez SA. Evaluation of transforming growth factor beta and type I procollagen gene expression in fibrotic skin diseases by in situ hybridization. J Invest Dermatol. 1990 Mar;94(3):365-71. doi: 10.1111/1523-1747.ep12874491
- Mori Y, Kahari VM, Varga J. Scleroderma-like cutaneous syndromes. Curr Rheumatol Rep. 2002 Apr;4(2):113-22. doi: 10.1007/s11926-002-0006-0
- Jinnin M, Ihn H, Yamane K, Asano Y, Yazawa N, Tamaki K. Serum levels of tissue inhibitor of metalloproteinase-1 and 2 in patients with eosinophilic fasciitis. Br J Dermatol. 2004 Aug;151(2):407-12. doi: 10.1111/j.1365-2133.2004.06062.x
- Lebeaux D, Francès C, Barete S, Wechsler B, Dubourg O, Renoux J, Maisonobe T, Benveniste O, Gatfossé M, Bourgeois P, Amoura Z, Cacoub P, Piette JC, Sène D. Eosinophilic fasciitis (Shulman disease):new insights into the therapeutic management from a series of 34 patients. Rheumatology (Oxford) 2012;51(3):557–61. https://doi.org/10.1093/rheumatology/ker366
- Lakhanpal S, Ginsburg WW, Michet CJ, et al. Eosinophilic fasciitis: clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum. 1988 May. 17(4):221-31.
- Caspi D, Fishel R, Varon M, et al. Multisystem presentation of eosinophilic fasciitis. Rheumatol Rehabil. 1982 Nov. 21(4):218-21.
- Christen-Zaech S, Hakim MD, Afsar FS, Paller AS. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008 Sep;59(3):385-96. doi: 10.1016/j.jaad.2008.05.005
- Zulian F, Vallongo C, Woo P, Russo R, Ruperto N, Harper J, Espada G, Corona F, Mukamel M, Vesely R, Musiej-Nowakowska E, Chaitow J, Ros J, Apaz MT, Gerloni V, Mazur-Zielinska H, Nielsen S, Ullman S, Horneff G, Wouters C, Martini G, Cimaz R, Laxer R, Athreya BH; Juvenile Scleroderma Working Group of the Pediatric Rheumatology European Society (PRES). Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005 Sep;52(9):2873-81. doi: 10.1002/art.21264
- Pinal-Fernandez I, Selva-O’Callaghan A, Grau JM. Diagnosis and classification of eosinophilic fasciitis. Autoimmun Rev. 2014;13(4-5):379–82. https://doi.org/10.1016/j.autrev.2014.01.019
- Jinnin M, Yamamoto T, Asano Y, Ishikawa O, Sato S, Takehara K, Hasegawa M, Fujimoto M, Ihn H. Diagnostic criteria, severity classification and guidelines of eosinophilic fasciitis. J Dermatol. 2018;45(8):881–90. https://doi.org/10.1111/1346-8138.14160
- Daniel RS, Brown AN. Case report of unilateral eosinophilic fasciitis in a Vietnamese woman. Am J Med Sci. 2009 Feb. 337(2):153-4.
- Lebeaux D, Frances C, Barete S, Wechsler B, Dubourg O, Renoux J, et al. Eosinophilic fasciitis (Shulman disease): new insights into the therapeutic management from a series of 34 patients. Rheumatology (Oxford, England). 2012;51(3):557–61. doi:10.1093/rheumatology/ker366
- Huang KW, Chen XH. Pathology of eosinophilic fasciitis and its relation to polymyositis. Can J Neurol Sci. 1987 Nov;14(4):632-7.
- Kirchgesner T, Dallaudière B, Omoumi P, Malghem J, Vande Berg B, Lecouvet F, Houssiau F, Galant C, Larbi A. Eosinophilic fasciitis: typical abnormalities, variants and differential diagnosis of fasciae abnormalities using MR imaging. Diagn Interv Imaging. 2015 Apr;96(4):341-8. doi: 10.1016/j.diii.2014.06.018
- Mondal S, Goswami RP, Sinha D, Ghosh A. Ultrasound is a useful adjunct in diagnosis of eosinophilic fasciitis. Rheumatology (Oxford, England) 2015;54(11):2041. doi:10.1093/rheumatology/kev290
- Marie I, Sauvetre G. Fluorodeoxyglucose positron emission tomography in eosinophilic fasciitis. Joint Bone Spine. 2014 Dec;81(6):541. doi: 10.1016/j.jbspin.2014.07.001
- Falanga V, Medsger TA Jr. Frequency, levels, and significance of blood eosinophilia in systemic sclerosis, localized scleroderma, and eosinophilic fasciitis. J Am Acad Dermatol. 1987 Oct. 17(4):648-56.
- Antic M, Lautenschlager S, Itin PH. Eosinophilic fasciitis 30 years after – what do we really know? Report of 11 patients and review of the literature. Dermatology. 2006. 213(2):93-101.
- Kim SW, Rice L, Champlin R, Udden MM. Aplastic anemia in eosinophilic fasciitis: responses to immunosuppression and marrow transplantation. Haematologia (Budap). 1997. 28(3):131-7.
- Antón E. Failure to demonstrate Borrelia burgdorferi-specific DNA in lesions of eosinophilic fasciitis. Histopathology. 2006 Jul. 49(1):88-90.
- Jinnin M, Ihn H, Yamane K, Asano Y, Yazawa N, Tamaki K. Serum levels of tissue inhibitor of metalloproteinase-1 and 2 in patients with eosinophilic fasciitis. Br J Dermatol. 2004 Aug. 151(2):407-12.
- Sugimoto T, Nitta N, Kashiwagi A. Usefulness of magnetic resonance imaging in eosinophilic fasciitis. Rheumatol Int. 2007 Jun. 27(8):791-2.
- Dybowski F, Neuen-Jacob E, Braun J. Eosinophilic fasciitis and myositis: use of imaging modalities for diagnosis and monitoring. Ann Rheum Dis. 2008 Apr. 67(4):572-4.
- Kissin EY, Garg A, Grayson PC, Dubreuil M, Vradii D, York M, et al. Ultrasound assessment of subcutaneous compressibility: a potential adjunctive diagnostic tool in eosinophilic fasciitis. J Clin Rheumatol. 2013 Oct. 19(7):382-5.
- Wright NA, Mazori DR, Patel M, Merola JF, Femia AN, Vleugels RA. Epidemiology and Treatment of Eosinophilic Fasciitis: An Analysis of 63 Patients From 3 Tertiary Care Centers. JAMA Dermatol. 2016 Jan 1. 152 (1):97-9.
- Lakhanpal S, Ginsburg WW, Michet CJ, Doyle JA, Moore SB. Eosinophilic fasciitis: clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum. 1988;17:221–231. doi: 10.1016/0049-0172(88)90008-X
- Mertens JS, Thurlings RM, Kievit W, Seyger MMB, Radstake TRD, de Jong EMGJ. Long-term outcome of eosinophilic fasciitis: a cross-sectional evaluation of 35 patients. J Am Acad Dermatol. 2017;77:512–517. doi: 10.1016/j.jaad.2017.05.018
- Manzini C, Sebastiani M, Giuggioli D, Manfredi A, Colaci M, Cesinaro A, et al. D-penicillamine in the treatment of eosinophilic fasciitis: case reports and review of the literature. Clin Rheumatol. 2011 Oct 12.
- Endo Y, Tamura A, Matsushima Y, Iwasaki T, Hasegawa M, Nagai Y, Ishikawa O. Eosinophilic fasciitis:report of two cases and a systematic review of the literature dealing with clinical variables that predict the outcome. Clin Rheumatol. 2007;26(9):1445–51. https://doi.org/10.1007/s10067-006-0525-6
- Tull R, Hoover WD 3rd, De Luca JF, Huang WW, Jorizzo JL. Eosinophilic fasciitis: a case series with an emphasis on therapy and induction of remission. Drugs Context. 2018 Oct 2;7:212529. doi: 10.7573/dic.212529
- Espinoza F, Jorgensen C, Pers YM. Efficacy of Tocilizumab in the treatment of Eosinophilic fasciitis:Report of one case. Joint Bone Spine. 2015;82(6):460–1. https://doi.org/10.1016/j.jbspin.2015.02.008
- Nahhas AF, Alam M, Lim HW. Rituximab as a therapeutic consideration for refractory eosinophilic fasciitis. Int J Dermatol. 2018;57(5):614–615. https://doi.org/10.1111/ijd.13940
- Kim SR, Charos A, Damsky W, Heald P, Girardi M, King BA. Treatment of generalized deep morphea and eosinophilic fasciitis with the Janus kinase inhibitor tofacitinib. JAAD Case Rep. 2018 Apr 30;4(5):443-445. doi: 10.1016/j.jdcr.2017.12.003
- Pimenta S, Bernardes M, Bernardo A, Brito I, Castro L, Simões-Ventura F. Intravenous immune globulins to treat eosinophilic fasciitis: a case report. Joint Bone Spine. 2009 Oct;76(5):572-4. doi: 10.1016/j.jbspin.2009.06.001
- Cetkovský P, Koza V, Cetkovská P, Svojgrová M. Successful treatment of severe Shulman’s syndrome by allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998 Mar;21(6):637-9. doi: 10.1038/sj.bmt.1701137
- Mazori DR, Femia AN, Vleugels RA. Eosinophilic Fasciitis:an Updated Review on Diagnosis and Treatment. Curr Rheumatol Rep. 2017;19(12):74. https://doi.org/10.1007/s11926-017-0700-6
- Suzuki G, Itoh Y, Horiuchi Y. Surgical management of eosinophilic fasciitis of the upper extremity. J Hand Surg Br. 1997 Jun. 22(3):405-7.
- Endo Y, Tamura A, Matsushima Y, Iwasaki T, Hasegawa M, Nagai Y. Eosinophilic fasciitis: report of two cases and a systematic review of the literature dealing with clinical variables that predict outcome. Clin Rheumatol. 2007 Sep. 26(9):1445-51.
- de Masson A, Bouaziz JD, Peffault de Latour R, Benhamou Y, Moluçon-Chabrot C, Bay JO, et al. Severe aplastic anemia associated with eosinophilic fasciitis: report of 4 cases and review of the literature. Medicine (Baltimore). 2013 Mar. 92(2):69-81.