What is epispadias
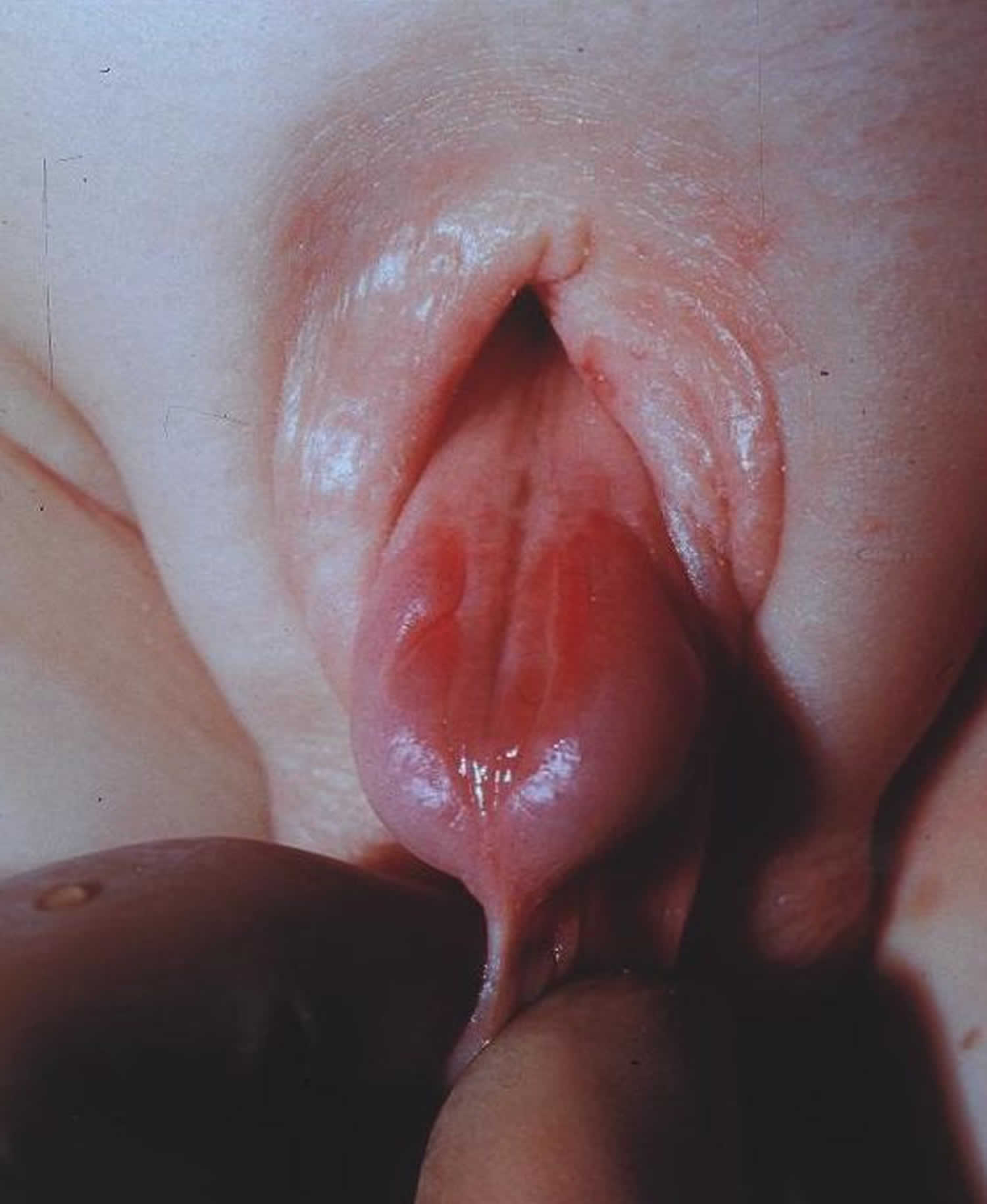
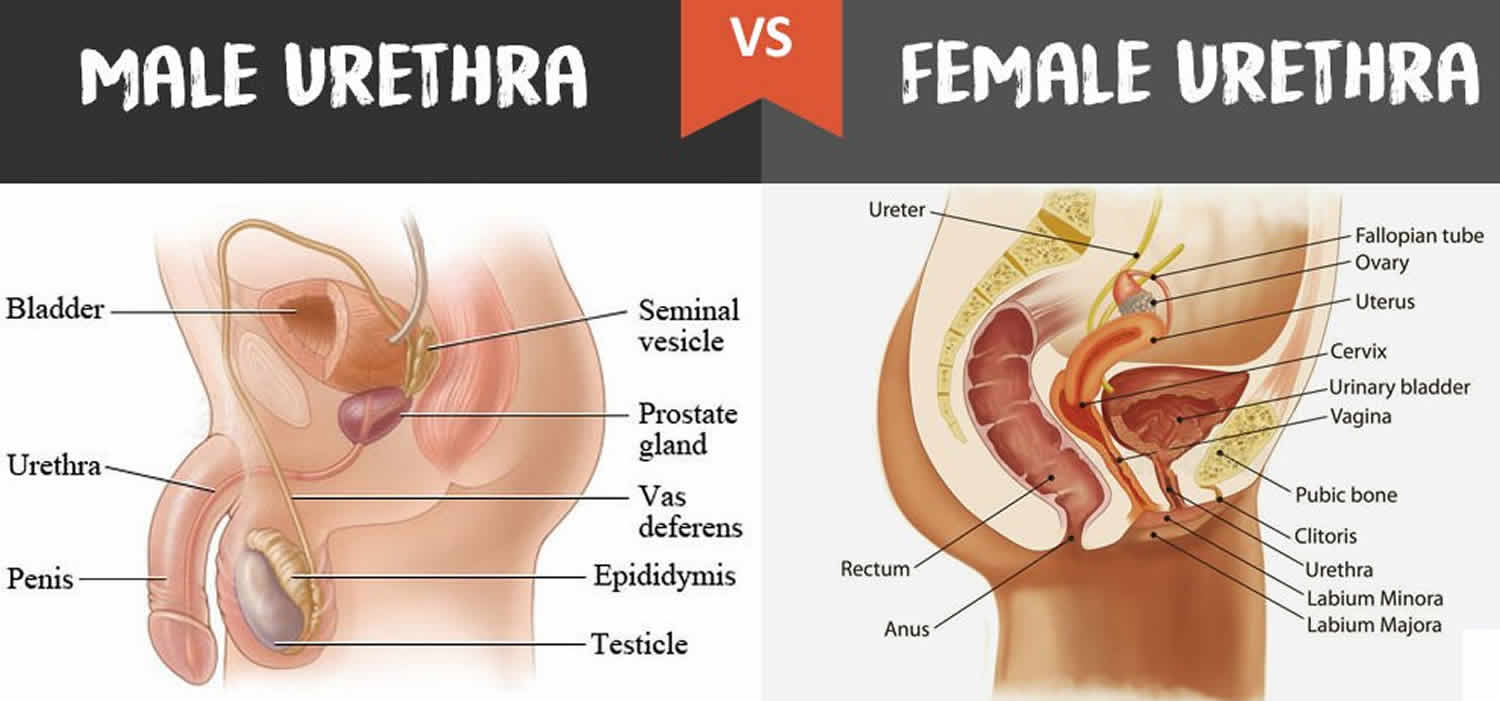
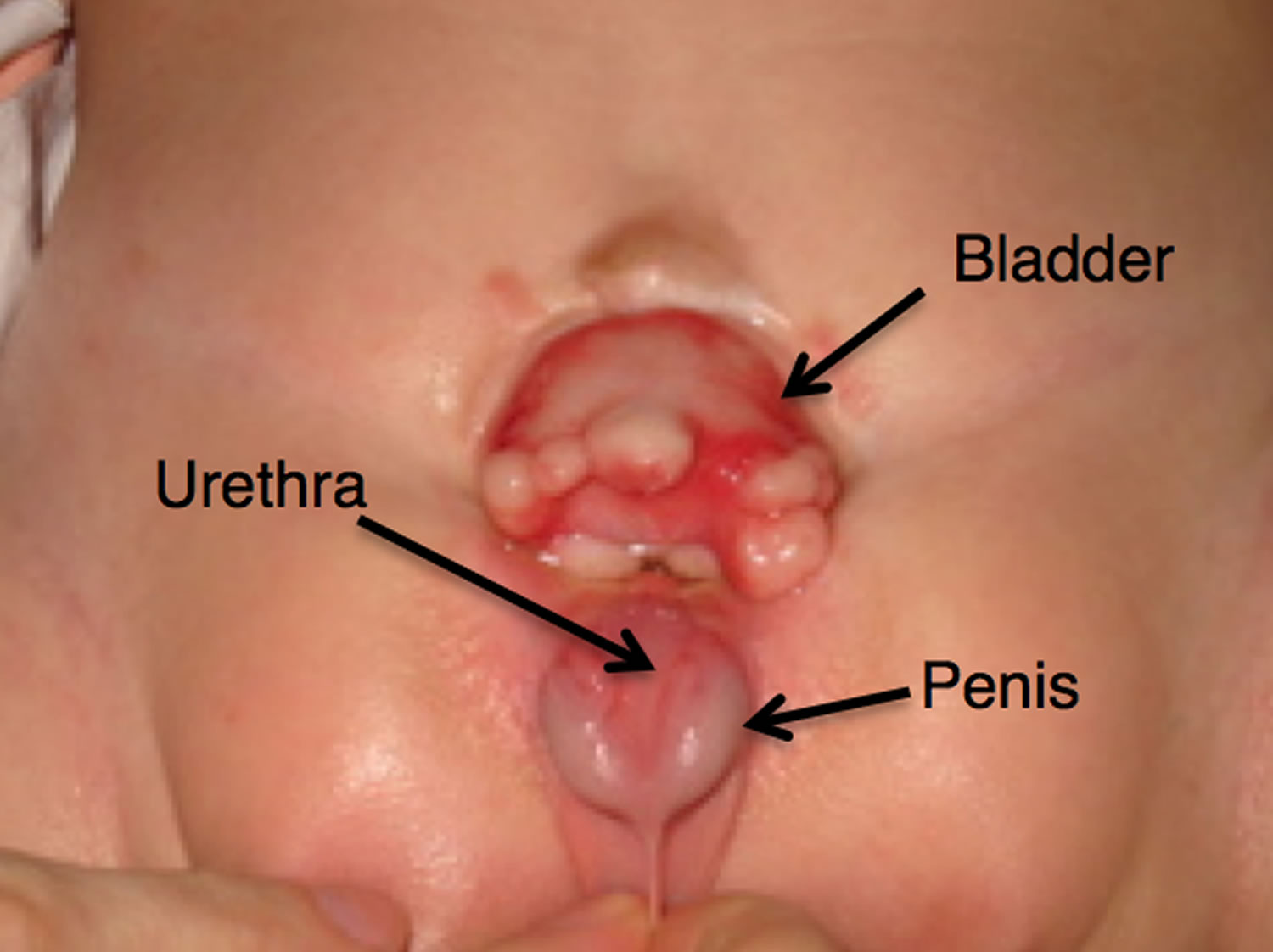
Epispadias is a rare congenital (present at birth) anomaly involving the development of the urethra (the tube that carries the urine from the bladder to an external opening) (Figure 1). Normal urethra in boys, that tube goes to the tip of the penis and also carries semen. Epispadias is a problem both boys and girls can have. The urethra does not develop into a full tube and the urine exits the body from an abnormal location. In girls with epispadias, the urethral opening is in the belly area instead of between the clitoris and labia. In boys with epispadias, the urethra generally opens on the top or side of the penis rather than the tip. However, it is possible for the urethra to be open the entire length of the penis. From the meatal opening to the tip of the penis, the penis is split and is opened, forming a gutter. The penis also usually has some degree of curvature or chordee.
The causes of epispadias are unknown at this time. It may be related to improper development of the pubic bone. Epispadias is associated with bladder exstrophy, an uncommon birth defect in which the bladder is inside out, and sticks through the abdominal wall. Nearly all boys with bladder exstrophy will also have epispadias. Most girls with exstrophy also have epispadias. Epispadias can occur in both boys and girls who are otherwise healthy with no other abnormalities.
Epispadias occurs in 1 in 117,000 newborn boys and 1 in 484,000 newborn girls. The condition is usually diagnosed at birth or shortly thereafter, based on a physical examination. Very mild cases may be missed at birth and not be diagnosed until later, if the child (usually female) leaks urine after toilet training.
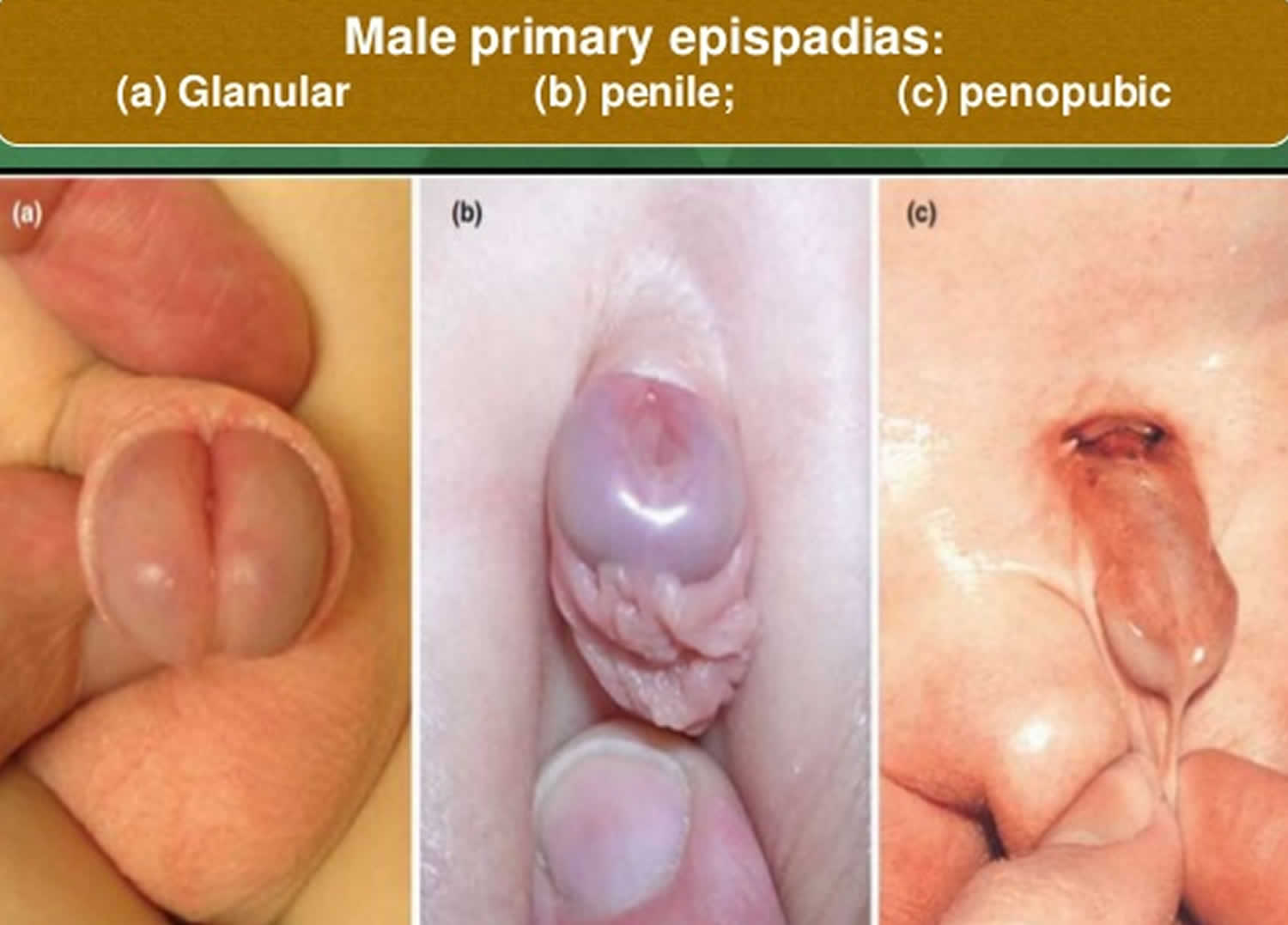
Classification of epispadias is based on the location of the meatus (opening where the urine comes out) on the penis. It can be positioned on the glans (glanular), along the shaft of the penis (penile) or near the pubic bone (penopubic) (Figure 2). The position of the meatus is important because it predicts the degree to which the bladder can store urine (continence). The closer the meatus is to the base of the penis, the more likely the bladder will not hold urine.
Surgery can help the person control the flow of urine. It will also fix the appearance of the genitals. Leakage of urine (incontinence) can often be repaired at the same time. However, a second surgery may be needed.
Some people with epispadias may continue to have urinary incontinence, even after surgery. Ureter and kidney damage and infertility may occur.
Figure 1. Urethra – male and female
Figure 2. Epispadias male
Footnote: Boys with epispadias. Distal epispadias is seen in (A), while the more typical severe type is shown on the right (C), called peno-pubic epispadias. In patients with peno-pubic epispadias, the urinary opening is at the base of the penis and the partial foreskin on the underside is being used to pull the penis down towards the feet, since the penis is often curved and tethered upwards towards the belly. Reconstruction involves moving the urinary opening to the tip of the penis, straightening the penis, and performing either a circumcision or foreskin reconstruction.
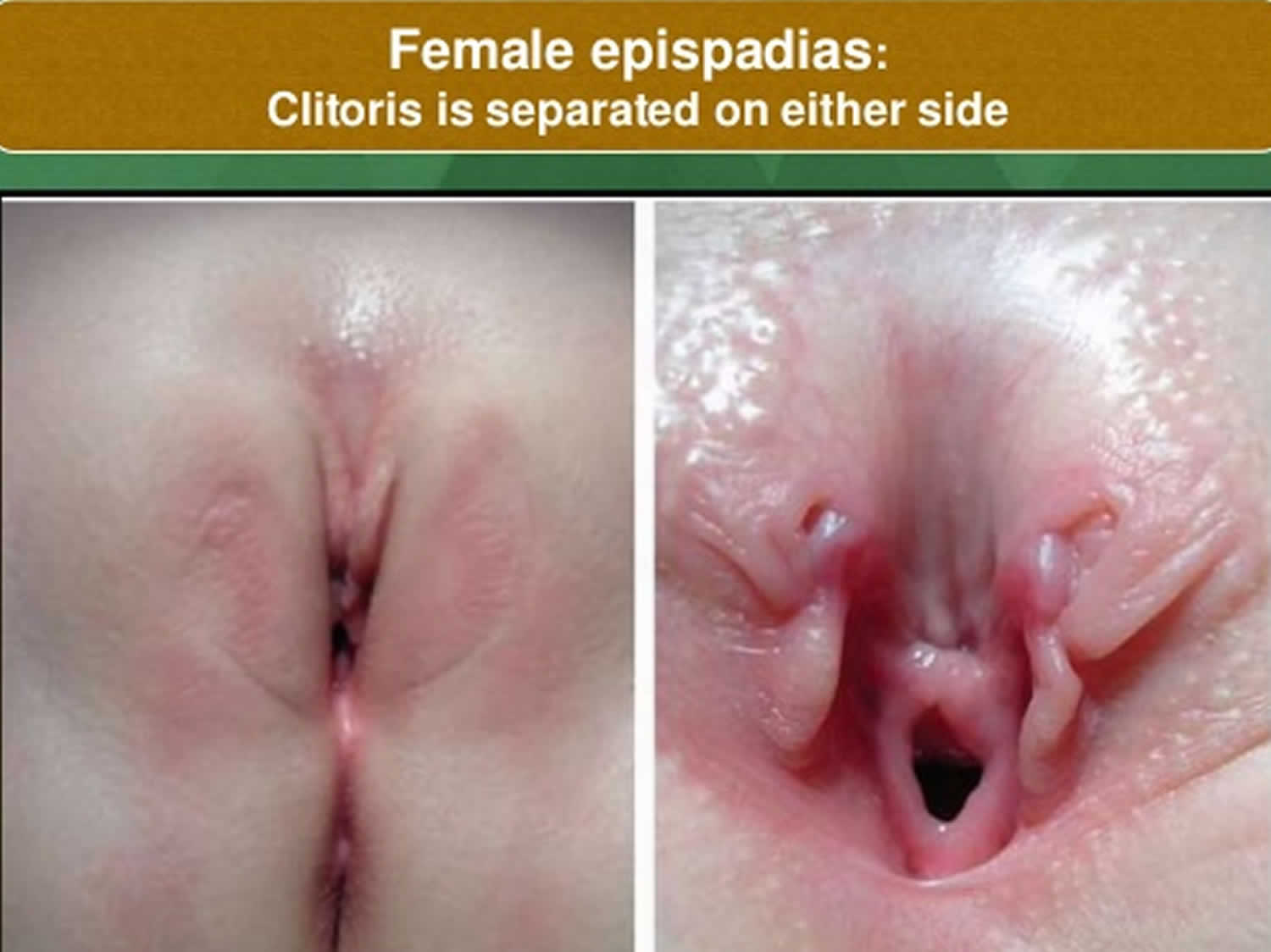
Figure 3. Epispadias female
Exstrophy epispadias complex
Exstrophy-epispadias complex refers to a spectrum of birth defects that includes epispadias, classical bladder exstrophy, and exstrophy of the cloaca and several variants 1. Exstrophy-epispadias complex is characterized by a visible defect of the lower abdominal wall and other problems. In normal development, the cloacal membrane temporarily separates the cloaca (final part of the intestine) into urogenital and anal regions, and it ruptures after fusing with a structure known as the urogenital septum, which is made up of the tissue that will form the abdominal muscles (mesoderm). If the cloacal membrane does not fuse correctly with the urogenital septum, it does not form the mesoderm and, as a result, the abdominal muscles do not form. The exact timing of the rupture determines whether the child is born with isolated epispadias, classic bladder exstrophy, or cloacal exstrophy. Depending on severity, exstrophy-epispadias complex may involve the urinary system, musculoskeletal system, pelvis, pelvic floor, abdominal wall, genitalia, and sometimes the spine and anus. There is no known cause for exstrophy-epispadias complex 2. Treatment may involve several surgeries to repair the abdominal wall and any associated malformations 3.
Bladder exstrophy is a rare condition in which the bladder does not form properly and is flattened and exposed on the abdominal wall. The condition is more common in males (3:1 male to female ratio), and is associated with epispadias, a condition in which the urethra is opened dorsally as a plate.
In males, the penis is short and broad with upward curvature (dorsal chordee). In females, the clitoris is separated into 2 segments (bifid) associated with divergent labia and an anteriorly displaced vagina.
Bladder exstrophy is a condition that requires a staged reconstructive approach by an experienced surgeon in order to maximize the child’s chances of achieving urinary continence and the ability to urinate spontaneously. The initial stage of reconstruction is performed in the newborn period and includes closure of the bladder plate and reapproximation of the pubic bones. The epispadias repair is usually performed at 6 to 9 months of age, although it is sometimes performed in conjunction with the bladder closure at birth, and the continence procedure (bladder neck repair) is performed at approximately 5 years of age if the bladder is big enough and the child is willing to participate in a voiding program.
Exstrophy patients require lifelong follow-up due to the complexity of their surgical reconstruction and the medical issues they are prone to develop throughout life.
Figure 4. Epispadias with bladder exstrophy
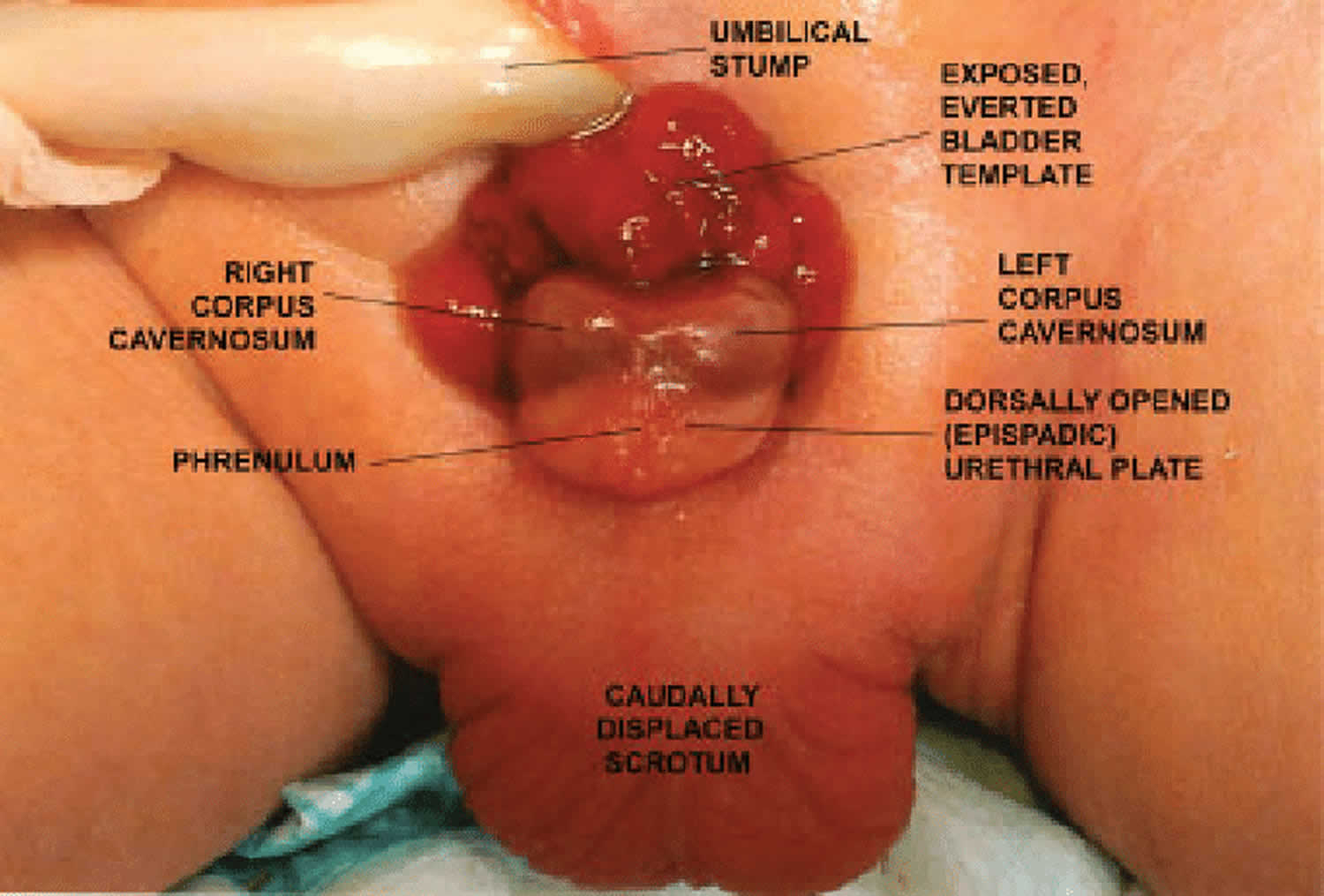
Figure 5. Bladder exstrophy (classic bladder exstrophy)
Footnote: Male newborn with classic bladder exstrophy. Exposed, everted bladder template is clearly visible immediately below umbilical stump; a completely dorsally opened (epispadic) urethral plate runs from bladder neck down to the open glans; left and right corpora cavernosa are visible beneath and alongside urethral plate; the scrotum is caudally displaced.
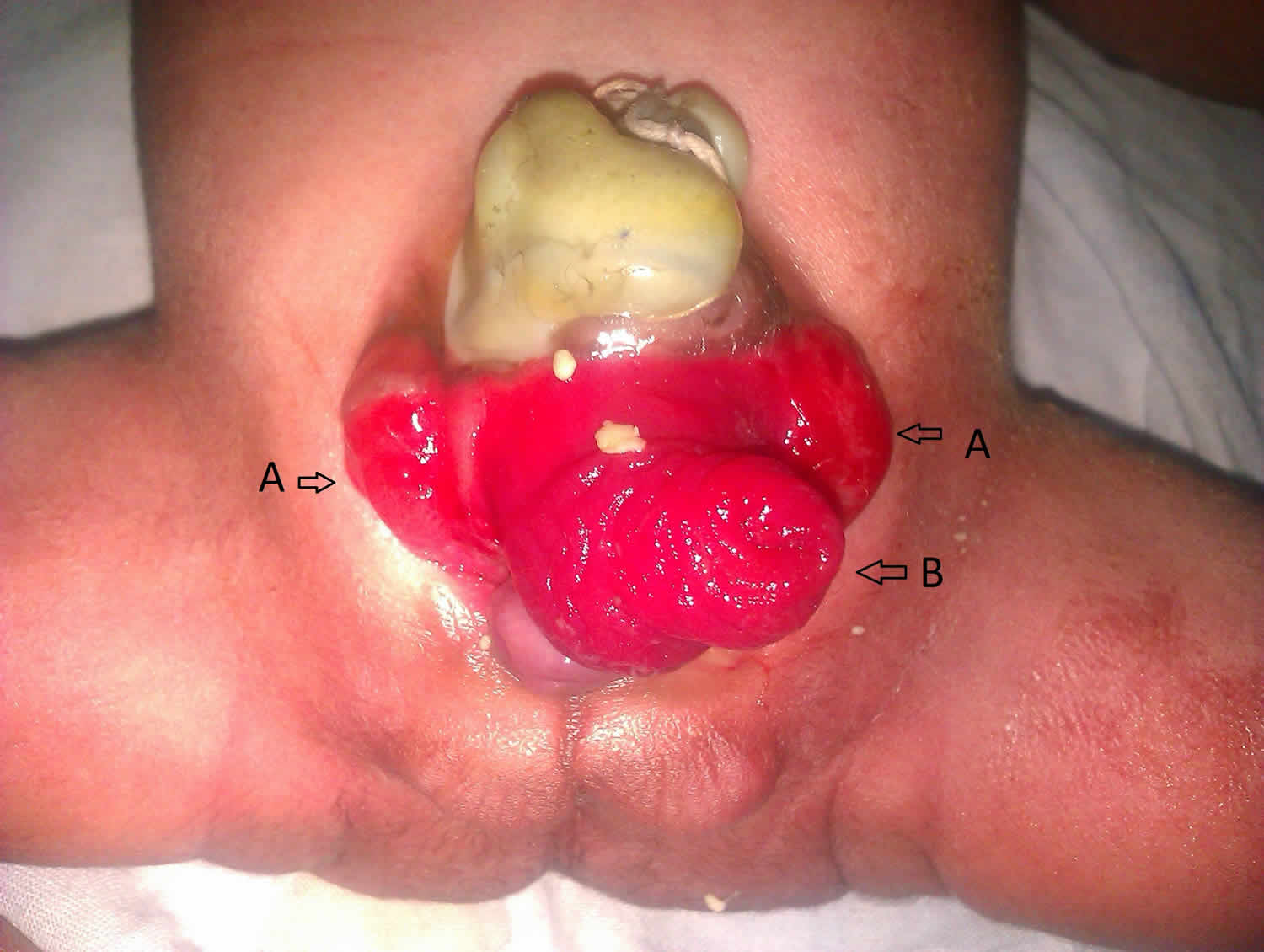
[Source 4 ]Figure 6. Cloacal exstrophy
Footnote: A newborn baby of 35 completed weeks of gestation, of undetermined sex and birth weight of 1700 grams was brought to us on day 1 of life. The newborn had an abdominal wall defect just below the umbilicus with a red mass protruding out of it which looked like an elephant head with a central tubular trunk like structure {prolapsed ileum – B} and two lateral ear like structures {exstrophised bladder – A}. There was imperforate anus, a swelling over the left sacral area probably a meningocele with an underlying defect of the left sacral bone. The child was moving both the lower limbs but had equinovarus defect in the left foot. It is a rare anomaly with no sex predilection. The defect occurs as an abnormal large cloacal membrane gives way before the urorectal septum has partitioned the cloacal pouch, thus, the cloaca itself exstrophies, resulting in two half of the exstrophised bladder separated by exstrophised ileocecal bowel area. On the rostral side, the ileum prolapses appearing as a long proboscis of small bowel mucosa. Inferiorly an orifice leads to a blind ending and short colonic segment. Cloacal exstrophy. may be associated with multiple anomalies. Management is primarily surgical.
[Source 5 ]Exstrophy-epispadias complex causes
In normal development, the cloacal membrane temporarily separates the urogenital and anal structures and them breaks when tissue that will form abdominal muscles begins to grow in its place. The bladder exstrophy-epispadias-cloacal exstrophy complex is caused by a developmental abnormality that occurs 4-5 weeks after conception, during the development of the embryo (the name given to the baby during this stage), in which the cloacal membrane is not replaced by tissue that will form the abdominal muscles. The cause and nature of the faulty development is not exactly certain. It is 4 to 5 weeks after conception that the various organs and different types of muscles and tissues of the body begin to form from layers of cells that separate, divide and fold. One theory suggests that something goes wrong during this early folding and separation, causing the cloacal membrane to fail to close, leaving the bladder outside of the abdominal wall. A second theory proposes that the layer of skin which forms over the bladder at this stage is thin and unable to hold in the bladder. It pulls apart, again leaving the bladder inside out.
The birth prevalence of classic bladder exstrophy has been estimated to be between 1 in 10,000 and 1 in 50,000 livebirths 6. Males are affected 2-3 times more often than females. Isolated epispadias occurs in approximately 1 in 112,000 live male births and 1 in 400,000 live female births. Cloacal exstrophy occurs in approximately 1 in 400,000 live births 6.
Exstrophy-epispadias complex signs and symptoms
The bladder-exstrophy-epispadias-cloacal exstrophy complex can take many forms depending on the extent of the developmental abnormality that causes it. The mildest form is when there is an opening in the urethra (epispadias). The most severe form is when there is an opening in the urethra, bladder and bowel (cloacal exstrophy).
The most common form is classic bladder exstrophy in which the bladder and related structures are turned inside out through an opening in the abdominal wall. Classic bladder exstrophy is intermediate in severity and the bladder is open from the top of the bladder through the urethra and to the tip of the penis.
Boys with epispadias have a urethra that is extremely short and split and the opening is on the upper surface of the penis. Girls with epispadias have a urethral opening located between a split clitoris and labia minor.
Cloacal exstrophy is a severe birth defect in which there is usually a membrane-covered area on the abdominal wall that contains the abdominal contents (omphalocele). The bladder is divided in two halves and males have a penis split in two halves. Females have a clitoris divided in two halves and may have two vaginal openings. The opening of the rectum to the outside of the body is usually missing or abnormally small.
Other abnormalities are sometimes associated with the complex. These include a separation of the pubic bones, absence of the lower portion of the bladder causing lack of bladder control (incontinence) and abnormal position of the tubes that carry urine from the kidneys to the bladder (ureters) causing back up of urine in the kidneys (reflux),
Exstrophy-epispadias complex diagnosis
Prenatal ultrasound examination of a fetus with the bladder-exstrophy-epispadias-cloacal exstrophy complex may reveal absence of bladder filling, low-set umbilical cord, separation of pubic bones, small genitals and an abdominal mass that increases in size as the pregnancy progresses. But unborn babies pee often, making the bladder hard to see, and it is easy to miss seeing bladder-exstrophy. That is why many babies are diagnosed after they are born. Because the bladder and other structures are exposed on the outer surface of the body, bladder exstrophy is seen right after birth. Sometimes, the diagnosis of exstrophy is not made right away, because it is a rare defect that most healthcare providers have not seen before. Sometimes, it will take a specialist to confirm the diagnosis and to tell if the baby is a boy or a girl.
Exstrophy-epispadias complex treatment
The treatment of bladder exstrophy consists of a series of corrective surgeries performed over several years. The first surgery is closure of the bladder to allow it to hold urine, placement of the bladder inside the pelvis and closure of the abdominal wall. In some cases, children with bladder exstrophy may also require a series of surgical procedures to reconstruct the external genitalia. These surgeries are usually performed before the age of 2 years. Bladder neck reconstruction is performed at approximately 5 years of age to allow control of urine and ureters are repositioned to prevent urine from backing up into the kidneys.
The outlook for maintaining normal kidney function after surgical correction and reconstruction is good. However, some individuals with this disorder may experience long-term urinary problems such as kidney stones, kidney infections, and varying degrees of urinary incontinence. Other treatment is symptomatic and supportive.
Exstrophy-epispadias complex prognosis
Continence results and long-term complications after functional reconstruction
Though countless publications on exstrophy-epispadias complex exist, surgical outcome data have mostly been ascertained retrospectively, as single-center or single-surgeon experiences. Definitions of successful outcome, observation periods and end-points, as well as evaluation of complications and, in particular, terminology focusing on the terms “continence” or “social continence” diverge immensely. Woodhouse was the first who revealed that bladder function in exstrophy-epispadias complex is not stable over time, and late failure with muscular atony may occur 7. Nowadays, it is reasonable to expect continence rates of about 80% in childhood 8. Within this concept, however, though most exstrophic bladders can be preserved, spontaneous voiding is not guaranteed and, especially after childhood, an increasing number of patients need bladder augmentation or self catheterization either via the urethra or via a catherizable stoma. In our first 100 one-stage functional reconstructed exstrophy-epispadias complex patients, 47 underwent a primary and 53 a redo reconstruction with a mean observation period of 11.1 years 9. Complete continence after primary reconstruction with spontaneous voiding was possible in 72.3% of the patients; whereas reliable continence dropped after redo bladder neck plasty to only 41.5% 9. These outcome data are comparable to other high-volume exstrophy-epispadias complex centers 10. If primary closure fails, only 60% obtain adequate capacity for a planned bladder neck reconstruction in a staged concept. If the second closure fails, only 40% will have adequate capacity for a bladder neck reconstruction and only 20% will become dry 11. Numerous possible complications (such as recurrent urinary tract infections, recurrent epididymitis, residual urine and therefore urinary calculi formation, etc.) may complicate the course of the disease and require meticulous long-term care.
Reconstruction failure after functional reconstruction
Reconstruction failure is usually assessed clinically, by endoscopy and with urodynamics. Identifying the medical problem, with simultaneous consideration of the individual and family history, should lead to further therapeutic recommendations. If bladder storage is impaired, the bladder can be augmented with bowel, preferentially with ileum or sigma. After augmentation, sufficient bladder emptying must be provided either through catheterization per urethram or through a catheterizable channel according to the Mitrofanoff principle. If the bladder neck resistance is low, injectable materials like dextranomer/hyaluronic acid can enforce urethral resistance 12. This minimally invasive approach allows quite reasonable success in order to improve continence, but success will be only durable after at least 3 injections 12. A definitive solution is bladder neck closure with creation of a catheterizable channel, but reliable compliance of patients and parents are of fundamental importance for success. In cases with bad bladder development, upper tract deterioration and continence is not achievable over a reasonable period and a well-balanced benefit-effort-analysis urinary diversion should be performed. Patient age, social background and life style should be taken into consideration to decide whether a catheterizable pouch or a sigma-rectum-pouch is chosen for urinary diversion.
Continence results and long-term complications after urinary diversion
In the literature, urinary diversion provides very high primary continence rates. Thirty eight children with a mean age of 5 years were reported to be completely continent during the day and only 8.6% used pads during the night 13. However, gaining anal continence in childhood after urinary diversion is an individual process until the child is about 5-7 years old. Another advantage of this method is the fact that the upper urinary tract is protected due to the modified low-pressure reservoirs. Using the new antirefluxive ureteral implantation techniques: 15.8% had episodes of pyelonephritis, and 14.5% needed ureteral reimplantation (due to stenosis in 10.1% and reflux in 4.4%) 14. Sixty nine percent of patients need alkalizing drugs to prevent hyperchloremic acidosis, and therefore potentially impaired bone mineralization and growth deficiency. On the other hand, severe long-term complications must be considered like the development of adenocarcinoma at the ureterointestinal anastomosis after 15-25 years. The incidence of these mostly adenocarcinomas has been estimated to be 3.5 up to 19% 15, and is 8-550 times more frequent in patients with ureterosigmoidostomy compared with the incidence of colorectal cancer in age-matched controls. Recent data showed that colonic adenomas can be securely managed with local excision and during the observation period no recurrence occurred as yet. Therefore, annual rectoscopy is highly recommended after the 10th postoperative year 15.
Male exstrophy-epispadias complex patients: fertility and genital outcome
Nowadays, modern reconstruction techniques enable acceptable functionality and cosmetics in the exstrophy-epispadias complex. Current and future efforts reflect that congenital genitourinary anomalies have tremendous impact on adult life 16. A fulfilled sexual life, being married and having offspring represent main indicators for a successful genital rehabilitation 17. Naturally, interest in sexual activity is normal. Most striking for the male exstrophy-epispadias complex patients are penile size and deviation, as well as anxiety about and avoidance of sexual interaction. Despite these severe restrictions, about 50% of male exstrophy-epispadias complex patients practice sexual intercourse. A positive attitude towards micropenis and the male gender role can be achieved by patients and parents, but mental success mainly depends on parental enthusiasm, openness, and sufficient knowledge about the anomaly. Due to these restrictions, close and long lasting relationships were the consequence 17. In his literature review, Woodhouse 18 found at least some kind of ejaculation in 75% of the exstrophy-epispadias complex patients, regardless of the reconstruction method, and concluded that about 50% of the male exstrophy-epispadias complex patients were able to father children. Recent long-term results regarding fertility in the exstrophy-epispadias complex do sparsely exist 19. There is no consent as to whether primary diversion or functional reconstruction will allow better semen transport or fertility 17. Complications of reconstructive surgery and postinfectious effects, however, seem to be disastrous to fertility in male exstrophy-epispadias complex patients. Recently, incidence of primary spermatogenesis failure, especially in the azoospermia group, was reported to be about 20% 17. Therefore, pathogenesis of the impaired fertility in exstrophy-epispadias complex is probably multifactorial 17. Long-term data suggest that functional bladder neck reconstruction with a consequent anatomical placement of the colliculus seminalis in the posterior urethra, however, allows antegrade ejaculations in 94.1% of the patients 17. So not only for continence, but also for ejaculation and fertility, the primary successful and anatomically correct approach to the bladder neck seems to be the key point.
Female exstrophy-epispadias complex patients: fertility and genital outcome
In general, female exstrophy-epispadias complex patients require comparatively little surgery with mostly acceptable cosmetic outcome. Due to normal internal genitalia, mainly not affected by the reconstructive bladder surgery, fertility should usually be normal. Furthermore, as a consequence of a low cervical insertion an even a higher chance for pregnancy is assumed. Additionally, Woodhouse stated that, compared to males, female exstrophy patients have fewer problems with sexuality and sexual intercourse 20. Thirty four of his 42 female patients were able to participate in sexual intercourse; 12 of them did not even require vaginoplasty; and 32 were married or maintained a steady partnership. In this group, 22 pregnancies resulted in 19 healthy babies; only three pregnancies were terminated for therapeutic reasons not related to exstrophy-epispadias complex 20. Stein reported 14 adult female patients older than 18 years after urinary diversion: 93% were married; only 3 reported unpleasant sexual activity 21. However, Matthews reported a series of 83 female exstrophy-epispadias complex patients, who had a late onset of sexual activity with a mean age of 20.2 years despite appropriate sexual desire 22. Six patients complained about dyspareunia; five refused sexual intercourse because of unsatisfying cosmetics; and only 12 experienced orgasms 22. Due to the less complex female reconstruction, comparatively little attention is drawn to the outcome and so, unsatisfactory reconstructed genitalia often impair female self-esteem 23. Thus, gender-related outcome seems to be of fundamental impact and warrants physicians’ empathy and commitment 24. However, in adulthood, vaginal or uterine prolapse is the most striking problem. Still, there is a paucity of knowledge about pelvic floor anatomy after reconstruction and sparse reports have failed to determine risk factors for this major complication. Inadequate pelvic ring adaptation and therefore pelvic floor adaptation in combination with removal of the bladder template may be risk factors for uterine prolapse 25. More recently, there is some evidence that restoration of the pelvic floor and therefore pelvic adaptation or osteotomy might prohibit uterine prolapse 25. Established treatment strategies of uterine prolapse include sacrofixation, uteri- or hysterectomy. Only sparse long-term data exist, but benefit in our experience is only for short periods. Complications like vault prolapse occur, and this might be a result of the finally unclear pathophysiology.
Most available data about psychosocial and psychosexual development in exstrophy-epispadias complex refer to well-adjusted adults who have already passed through puberty and adolescence. Standard questionnaires provide evidence for a normal quality of life, a usually high social adaptation level with good school performance and education standards. In adulthood, many exstrophy-epispadias complex patients have a so-called ordinary life including marriage, sexual relationships, family relations, children of their own, and professional success. Some of our own adult patients, however, express their wish to erase the memory of those challenging times and complain about loneliness in certain periods of life (e.g., puberty). Health status was usually derived from continence status. Impairment of daily life and self-esteem is common in 25% of cases, contacts with peers were present, but a lot effort was put into hiding the anomaly in daily life 23. Exstrophy-epispadias complex patients themselves stated that openness about the exstrophy-epispadias complex, regular upbringing, sufficient information, and a supportive parental attitude regarding self-esteem and autonomy as the best strategies for successful coping. Predictive factors for mental health were parental warmth, urinary continence and genital appearance 26. Hence, some reports state a certain prevalence of psychiatric diagnoses consisting mainly of internalized conflicts and emotional problems such as marked anxiousness, sadness, depression, low self-esteem, poor body concept, isolation and withdrawal, others deny the evidence of psychopathology in relation to exstrophy-epispadias complex 26. Attainment of continence at a later age consequently leads to more externalized struggles with low adaptive behavior scores. Due to their specific developmental implication, genitourinary malformations may create vulnerabilities to psychosexual dysfunction due to prolonged incontinence, residual genital defects and postsurgical genital appearance 26. Continence is often achieved by several operations, and is not a result of learning and developing processes 26. Parental overprotection and physical handicaps like incontinence – sometimes present until late school age – may hold back children at school or during social activities with peers.
For the parents, the first year of life of the exstrophy-epispadias complex child is a major challenge, sometimes with definite impairment of the child-parent relationship and severe problems with parental coping strategies. Thus, parents should be offered psychological support as soon as possible. As a consequence, support from a multidisciplinary team, helping these affected individuals and parents through the whole of childhood and adolescence, is mandatory. A prospective analysis of clinical predictive factors in gender-related long-term outcome is needed to provide an individualized flexible treatment strategy with predictable success and quality of life. Besides the pediatric urologist, this must also comprise the pediatric orthopedic surgeon, the pediatrician, the pediatric psychologist experienced in urology, experienced pediatric nurses and urotherapists.
Risk of malignancy in the exstrophic bladder
At birth, hamartomatous polyps are visible on the exstrophic bladder surface in about 50% of the cases 27. These polyps have been interpreted as reactive, potential pre-malignant environmental changes. Therefore, closure of the bladder template within the first few hours of life is widely recommended. However, no direct proof was made that bladder cancer is definitely developing from a polyp or a coexistent glandular metaplasia 27. After several operative attempts to the bladder, epithelial damage in terms of glandular cystitis or intestinal metaplasia was more commonly found within the exstrophy-epispadias complex 27. Until now, natural history of this intestinal metaplasia is still unclear and cannot be ruled out as a strong risk factor for adenocarcinoma or other urothelial malignancy in long-term follow-up 27. There are some reports about adenocarcinomas and squamous cell carcinomas occurring in unreconstructed, environment-exposed exstrophic bladders 28. Astonishingly, neoplasia was found in the exstrophic bladder remnant, even when early cystectomy had been performed 28. So, the estimated risk for bladder carcinoma in the exstrophy-epispadias complex population was 700 times higher than the age-matched general population 28.
Unresolved questions
Taking all treatment perspectives together, the most serious problem is the lack of any histological or clinical data allowing a reliable prognosis of future bladder growth and long-term storage and voiding function after birth 29. Therefore, the outcome and outcome-related prognostic factors are still unclear 29. Prospective outcome analysis is mandatory to further improve treatment strategies. In addition, current long-term outcome analysis now allows judgments to be made about treatment strategies implemented 20-30 years ago. A standardized follow-up program as a result of long-term outcome studies will definitely help to improve the final results and therefore lifelong outcome success.
Epispadias vs Hypospadias
Hypospadias is a birth defect (congenital condition) in which a baby boy’s urethra is located on the under side of his penis rather than at the tip 30. There are different degrees of hypospadias, depending on how far away the end of the urethra is from the tip of the penis. Hypospadias may cause a curvature of the penis, called chordee. Some cases of hypospadias result in a man who will be unable to urinate while standing up, perform sexual intercourse, and/or procreate. Children with hypospadias should not be circumcised because the foreskin, which is removed during circumcision, is a source of tissue that surgeons use to rebuild the missing part of the urethra.
In boys with hypospadias, the urethra forms abnormally during weeks 8–14 of pregnancy. The abnormal opening can form anywhere from just below the end of the penis to the scrotum.
Hypospadias is common and doesn’t cause difficulty in caring for your infant. The urethra is the tube through which urine drains from your bladder and exits your body.
Hypospadias is fairly common birth defect affecting about 1 in 200 to 1 in 300 male newborns 31. Hypospadias is often readily corrected through outpatient surgery. Hypospadias also occurs in girls, but it’s extremely rare (affecting an estimated one in 500,000 babies) and a vastly different condition. If your daughter is born with hypospadias, your child’s specialist will be your best source of information and support.
In most cases, the exact cause of hypospadias is unknown. Sometimes, hypospadias is genetic, but environment also may play a role. Hypospadias is slightly more common in boys whose father or brother also had the condition.
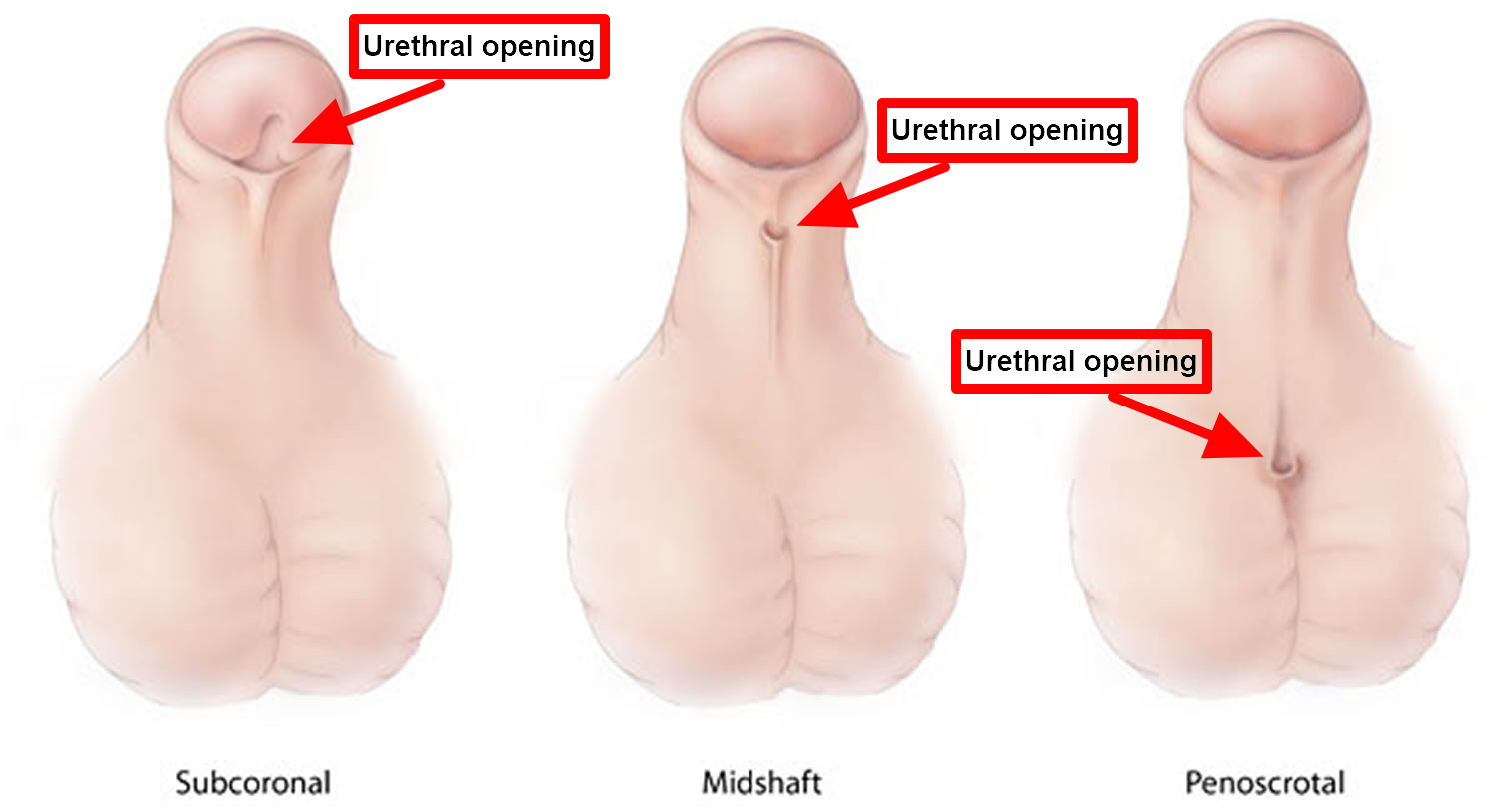
In hypospadias, the urethral opening can be located at any point along the underside of the penis (also called the “ventral aspect”) (see Figure 1). Where the opening falls will determine how severe the condition is, and how your child’s medical team will approach repairing it.
There are different degrees of hypospadias; some can be minor and some more severe.
- Subcoronal hypospadias – anterior or distal (near the tip of the penis): The opening of the urethra is located somewhere near the head of the penis. This is the mildest form of hypospadias, occurring in about 50 percent of cases.
- Midshaft hypospadias – middle (midway up the penis): The opening of the urethra is located along the shaft of the penis. Considered moderate hypospadias, this accounts for about 30 percent of cases.
- Penoscrotal hypospadias – posterior or proximal (at the scrotum or perineum): The opening of the urethra is located where the penis and scrotum meet. This is the most severe kind of hypospadias, and occurs in 20 percent of cases.
Some parents may confuse hypospadias with epispadias, in which the urethra opens along the top of the penis, but these are two separate and distinct conditions with very different treatments.
Boys with hypospadias can sometimes have a curved penis. They could have problems with abnormal spraying of urine and might have to sit to urinate. In some boys with hypospadias, the testicle has not fully descended into the scrotum (cryptorchidism).
While some children with very mild forms of this condition may not require surgery, if your son has hypospadias you should seek an evaluation from a pediatric urologic surgeon.
Surgery usually restores the normal appearance of your child’s penis. The outlook for infants who undergo this operation is extremely good: In most instances, they make a full recovery and have a normal-looking, fully functional penis within about six months. With successful treatment of hypospadias, most males can have normal urination and reproduction.
If left untreated, more severe forms of hypospadias can interfere with sexual intercourse when your child is an adult.
Figure 7. Hypospadias types
Risk factors for hypospadias
Although the cause of hypospadias is usually unknown, these factors may be associated with the condition:
- Family history. This condition is more common in infants with a family history of hypospadias.
- Genetics. Certain gene variations may play a role in disruption of the hormones that stimulate formation of the male genitals.
- Maternal age over 35 and weight. Some research suggests that there may be an increased risk of hypospadias in infant males born to women older than 35 years and who were considered obese had a higher risk of having a baby with hypospadias 32.
- Fertility treatments: Women who used assisted reproductive technology to help with pregnancy had a higher risk of having a baby with hypospadias 33.
- Exposure to certain substances during pregnancy. There is some speculation about an association between hypospadias and a mother’s exposure to certain hormones 34 or certain compounds such as pesticides or industrial chemicals, but further studies are needed to confirm this.
Hypospadias symptoms
In hypospadias, the opening of the urethra is located on the underside of the penis instead of at the tip. In most cases, the opening of the urethra is within the head of the penis. Less often, the opening is at the middle or the base of the penis. Rarely, the opening is in or beneath the scrotum.
Signs and symptoms of hypospadias may include:
- Opening of the urethra at a location other than the tip of the penis
- Downward curve of the penis (chordee)
- Hooded appearance of the penis because only the top half of the penis is covered by foreskin
- Abnormal spraying during urination or a downward urinary spray (in older children with more severe hypospadias, this may mean he has to sit down to urinate)
- An abnormal appearance of the tip of the penis (the glans)
- In some cases, boys born with hypospadias may also have undescended testicles and/or inguinal hernias (that is, hernias of the groin).
Hypospadias won’t cause your son physical pain or block his urination (though if it goes untreated it can make it difficult for him to direct his urine spray).
Hypospadias complications
If hypospadias is not treated, it can result in:
- Abnormal appearance of the penis
- Problems learning to use a toilet
- Abnormal curvature of the penis with erection
- Problems with impaired ejaculation
Hypospadias diagnosis
Your child’s pediatrician can diagnose hypospadias based on a physical exam. He or she will likely refer you to a surgeon who specializes in genital and urinary conditions (pediatric urologist) for further evaluation. Medical centers with specialty teams can help you evaluate options and can provide expert treatment.
When the opening of the urethra is abnormal and the testicles cannot be felt on exam, the genitals may be difficult to identify as clearly male or female (ambiguous genitalia). In this case, further evaluation with a multidisciplinary team is recommended.
Hypospadias treatment
If your son has a very mild case, he may not require surgery because his condition will not have a large impact on his life. However, sometimes parents of boys born with minor abnormalities still opt for surgery for cosmetic reasons, like straightening the penis and removing excess foreskin. However, treatment usually involves surgery to reposition the urethral opening and, if necessary, straighten the shaft of the penis. Surgery is usually done between the ages of 6 and 12 months.
If the penis looks abnormal, circumcision should not be done. If hypospadias is found during circumcision, the procedure should be completed. In either case, referral to a pediatric urologist is recommended.
Epispadias causes
No one knows for certain what causes hypospadias or epispadias but several possibilities have been explored. Researchers have found some evidence of genetic causes, such as a larger incidence of the condition in twins and within families. Babies with mothers who were exposed to increased levels of progesterone, a hormone commonly used during in vitro fertilization, have higher rates of hypospadias. Also, exposure to estrogen during pregnancy (exposure can happen when a pregnant mom eats fruits and vegetables with pesticides on them or drinks milk from pregnant cows) may be a risk factor. Hypospadias is more common in babies of Jewish and Italian descent.
Epispadias symptoms
Males will have a short, wide penis with an abnormal curve. The urethra most often opens on the top or side of the penis instead of the tip. However, the urethra may be open along the whole length of the penis.
Females have an abnormal clitoris and labia. The urethral opening is often between the clitoris and the labia, but it may be in the belly area. They may have trouble controlling urination (urinary incontinence).
Signs include:
- Abnormal opening from the bladder neck to the area above the normal urethra opening
- Backward flow of urine into the kidney (reflux nephropathy, hydronephrosis)
- Urinary incontinence
- Urinary tract infections
- Widened pubic bone
Epispadias diagnosis
Epispadius diagnosis is usually made clinically by inspection after birth.
Epispadias tests may include:
- Blood test
- Intravenous pyelogram (IVP), a special x-ray of the kidneys, bladder, and ureters
- MRI and CT scans, depending on the condition
- Pelvic x-ray
- Ultrasound of the urinary system and genitals
After birth, ultrasound baseline examination of the kidneys is mandatory for all epispadias newborn infants. Later on, irrespective of the method of reconstruction, kidney ultrasound is a perfect screening method for distinguishing any upper urinary tract changes during follow-up.
Epispadias treatment
Surgical repair of epispadias is recommended in patients where the epispadias is more than mild, which usually is performed at 6-12 months of age. Your surgical team includes urologists and an orthopaedic surgeon who work together to do this repair. In severe cases, the best option may be for your healthcare team to perform the surgery while the child is still in utero. Research has shown that having an orthopaedist do osteotomies during the first surgery makes a difference in long-term continence, especially for children with epispadias.
Leakage of urine (incontinence) is common in children with epispadias. Even in distal penile shaft epispadias with only a mild genital defect, urinary incontinence occurs in up to 75% of cases 29. A second operation or operations may be necessary to correct incontinence. During cystoscopy, a defect of the external sphincter can be identified as a longitudinal attenuated tissue strip from the bladder neck through to the urethral sphincter. This urethral tissue must be surgically removed. The urethra must be retubularized to an adequate size and the external sphincter and the pelvic floor musculature must completely be readapted. In epispadias with relevant urinary incontinence and a wide sphincter defect, a complete bladder neck procedure is needed; in mild defects, approximation of the pelvic floor during penile procedure might be sufficient. Very often the bladder wall is thin in epispadias, so potential muscular support for the bladder neck is only minor and therefore operative outcome restricted. Osteotomy, however, is hardly recommended in epispadias.
Long-term care
As your child nears adulthood, it is especially important that the care they receive remains effective and streamlined.
References- Exstrophy and Epispadias. https://emedicine.medscape.com/article/1014971-overview
- What is Cloacal Exstrophy? http://www.urologyhealth.org/urologic-conditions/cloacal-exstrophy
- Bladder Exstrophy-Epispadias-Cloacal Exstrophy Complex. https://rarediseases.org/rare-diseases/bladder-exstrophy-epispadias-cloacal-exstrophy-complex/
- Valerio, Enrico & Vanzo, Valentina & Zaramella, Patrizia & Salvadori, Sabrina & Castagnetti, Marco & Baraldi, Eugenio. (2015). Exstrophy–Epispadias Complex in a Newborn: Case Report and Review of the Literature. American Journal of Perinatology Reports. 5. 10.1055/s-0035-1556759.
- https://doi.org/10.7199/ped.oncall.2012.21
- Bladder Exstrophy-Epispadias-Cloacal Exstrophy Complex https://rarediseases.org/rare-diseases/bladder-exstrophy-epispadias-cloacal-exstrophy-complex/
- Standing the test of time: long-term outcome of reconstruction of the exstrophy bladder. Woodhouse CR, North AC, Gearhart JP. World J Urol. 2006 Aug; 24(3):244-9.
- Long-term results of bladder neck reconstruction for incontinence in children with classical bladder exstrophy or incontinent epispadias. Mouriquand PD, Bubanj T, Feyaerts A, Jandric M, Timsit M, Mollard P, Mure PY, Basset T. BJU Int. 2003 Dec; 92(9):997-1001; discussion 1002.
- Rösch WH, Schott G, Scheuering S, Schrott KM. Long-term outcome analysis of the first 100 complete reparis of bladder exstrophy in Erlanger-technique. BJU. 2001;87:24.
- Contemporary outcomes in bladder exstrophy. Gargollo PC, Borer JG. Curr Opin Urol. 2007 Jul; 17(4):272-80.
- The multiple reoperative bladder exstrophy closure: what affects the potential of the bladder? Gearhart JP, Ben-Chaim J, Sciortino C, Sponseller PD, Jeffs RD. Urology. 1996 Feb; 47(2):240-3.
- Injectable polydimethylsiloxane for treating incontinence in children with the exstrophy-epispadias complex: long-term results. Burki T, Hamid R, Ransley PG, Mushtaq I, Duffy PG. BJU Int. 2006 Oct; 98(4):849-53.
- Urinary diversion in bladder exstrophy and incontinent epispadias: 25 years of experience. Stein R, Fisch M, Stöckle M, Hohenfellner R. J Urol. 1995 Sep; 154(3):1177-81.
- Rectosigmoid pouch (Mainz Pouch II) in children. Pahernik S, Beetz R, Schede J, Stein R, Thüroff JW. J Urol. 2006 Jan; 175(1):284-7.
- Schröder A, Stein R, Thüroff JW. Bladder exstrophy and ureterosigmoidostomy – High risk for late complications and secondary malignancies. J Urol. 2006;175:154. doi: 10.1016/S0022-5347(06)00055-3
- Somatic function, mental health and psychosocial functioning in 22 adolescents with bladder exstrophy and epispadias. Diseth TH, Bjordal R, Schultz A, Stange M, Emblem R. J Urol. 1998 May; 159(5):1684-9; discussion 1689-90.
- Genital and reproductive function in males after functional reconstruction of the exstrophy-epispadias complex–long-term results. Ebert AK, Bals-Pratsch M, Seifert B, Reutter H, Rösch WH. Urology. 2008 Sep; 72(3):566-9; discussion 569-70.
- Prospects for fertility in patients born with genitourinary anomalies. Woodhouse CR. J Urol. 2001 Jun; 165(6 Pt 2):2354-60.
- The inheritance of the exstrophy-epispadias complex. Shapiro E, Lepor H, Jeffs RD. J Urol. 1984 Aug; 132(2):308-10.
- The anatomy and reconstruction of the adult female genitalia in classical exstrophy. Woodhouse CR, Hinsch R. Br J Urol. 1997 Apr; 79(4):618-22.
- The fate of the adult exstrophy patient. Stein R, Stöckle M, Fisch M, Nakai H, Müller SC, Hohenfellner R. J Urol. 1994 Nov; 152(5 Pt 1):1413-6.
- Urogynaecological and obstetric issues in women with the exstrophy-epispadias complex. Mathews RI, Gan M, Gearhart JP. BJU Int. 2003 Jun; 91(9):845-9.
- Psychosocial and psychosexual development in childhood and adolescence within the exstrophy-epispadias complex. Ebert A, Scheuering S, Schott G, Roesch WH. J Urol. 2005 Sep; 174(3):1094-8.
- Gender-associated differences in the psychosocial and developmental outcome in patients affected with the bladder exstrophy-epispadias complex. Lee C, Reutter HM, Grässer MF, Fisch M, Noeker M. BJU Int. 2006 Feb; 97(2):349-53.
- Pelvic-floor imaging using three-dimensional ultrasonography and magnetic resonance imaging in the long term follow-up of the bladder-exstrophy-epispadias complex. Ebert AK, Falkert A, Brandl R, Hirschfelder H, Koller M, Rösch WH. BJU Int. 2010 Jan; 105(2):248-53.
- Gearhart JP. The bladder exstrophy-epispadias-cloacal exstrophy complex. In: Gearhart JP, Rink RC, Mouriquand PDE, editor. Pediatric Urology. Chapter 32. Philadelphia: W. B. Saunders Co; 2001. pp. 511–546.
- Polyps in the exstrophic bladder. A cause for concern? Novak TE, Lakshmanan Y, Frimberger D, Epstein JI, Gearhart JP. J Urol. 2005 Oct; 174(4 Pt 2):1522-6; discussion 1526.
- Neoplasia in adult exstrophy patients. Smeulders N, Woodhouse CR. BJU Int. 2001 May; 87(7):623-8.
- Ebert AK, Reutter H, Ludwig M, Rösch WH. The exstrophy-epispadias complex. Orphanet J Rare Dis. 2009;4:23. Published 2009 Oct 30. doi:10.1186/1750-1172-4-23 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2777855/
- Hypospadias: anatomy, etiology, and technique. Baskin LS, Ebbers MB. J Pediatr Surg. 2006 Mar; 41(3):463-72. https://www.ncbi.nlm.nih.gov/pubmed/16516617/
- Perovic S, editor. Atlas of Congenital Anomalies of the External Genitalia. Refot-Arka: Belgrad, Yugoslavia; 1999
- Carmichael SL, Shaw GM, Laurent C, Olney RS, Lammer EJ, and the National Birth Defects Prevention Study. Maternal reproductive and demographic characteristics as risk factors for hypospadias. Paediatr Perinat Epidemiol. 2007; 21: 210–218.
- Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA, and the National Birth Defects Prevention Study. Assisted reproductive technology and major structural birth defects in the United States. Human Rep. 2009; 24:360–366
- Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med. 2005;159: 957–962