Funisitis
Funisitis is an inflammatory process involving the umbilical cord (umbilical vein, umbilical artery, and the Wharton’s jelly) 1. Wharton’s jelly is the gelatinous extracellular matrix contained within the umbilical cord that serves to protect the umbilical vessels 2. It is accepted that funisitis is a hallmark of fetal inflammatory response syndrome (FIRS), a condition characterized by an elevation in fetal plasma concentrations of interleukin-6, associated with the impending onset of preterm labor, a higher rate of neonatal morbidity (after adjustment for gestational age), and multi-organ fetal involvement 3. The fetal inflammatory response syndrome (FIRS) is the counterpart of the systemic inflammatory response syndrome (SIRS) in adults; however, in fetuses, it is a risk factor for short- and long-term complications (i.e. neonatal sepsis, bronchopulmonary dysplasia, periventricular leukomalacia, and cerebral palsy) 4.
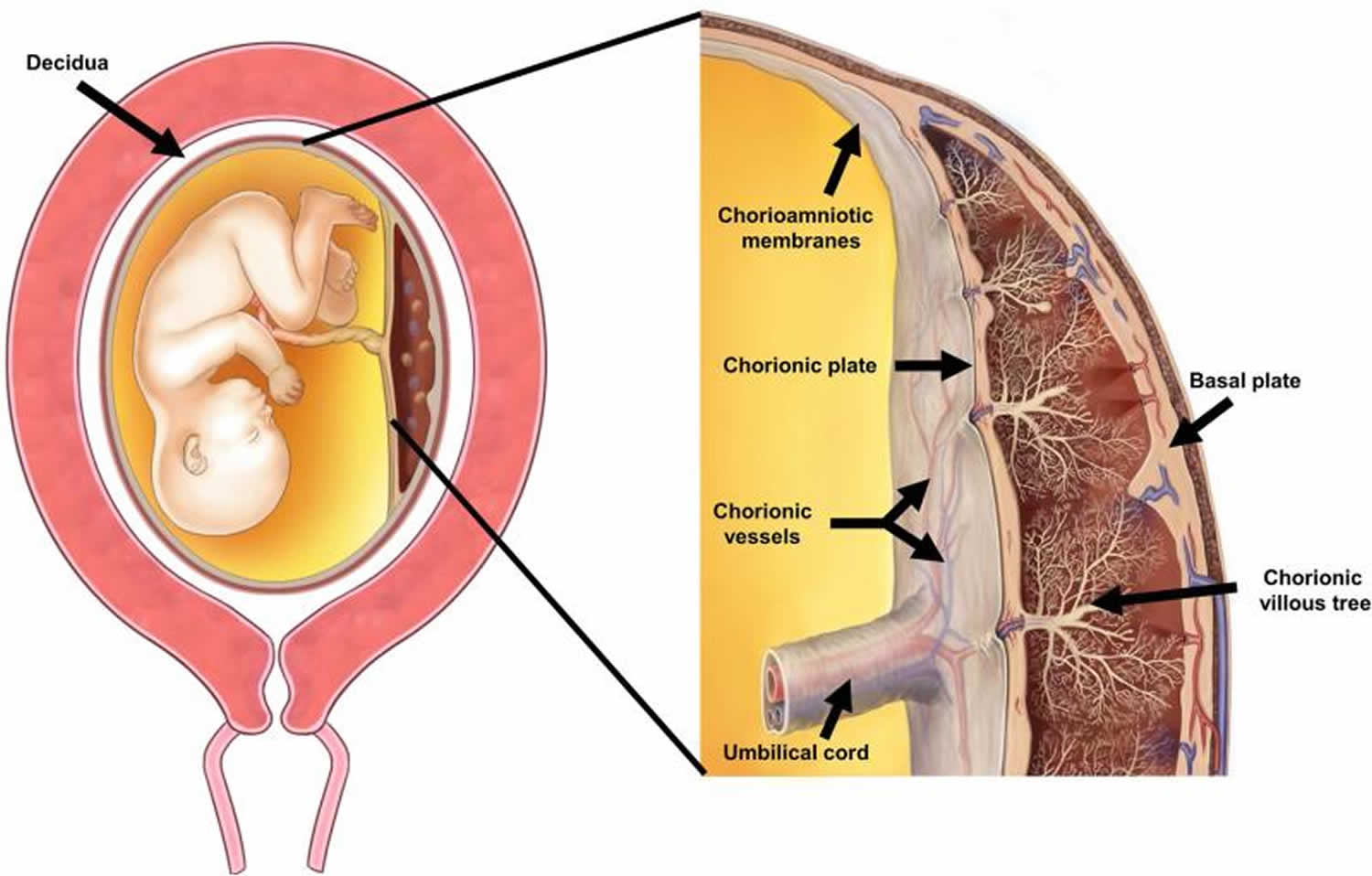
The placenta is composed of three major structures: the placental disc, the chorioamniotic membranes, and the umbilical cord (Figure 1) 4. Acute inflammatory lesions of the placenta are characterized by the infiltration of neutrophils in each of these structures 5. Specifically, when the inflammatory process affects the chorion and amnion, this is termed acute chorioamnionitis 5; if it affects the villous tree, this represents acute villitis 5. If the inflammatory process involves the umbilical cord (umbilical vein, umbilical artery, and the Wharton’s jelly), this is referred to as funisitis, the histological counterpart of the fetal inflammatory response syndrome 6.
Funisitis is diagnosed by the marked presence of neutrophils in the wall of umbilical vessels with or without Wharton jelly infiltration 7. Inflammation in the umbilical vein (phlebitis) can be seen separately from the umbilical arteries (arteritis). Established or long standing infections can cause tissue necrosis and accumulation of cellular debris is called necrotizing funisitis. Inflammation located at the periphery or surface of the umbilical cord may be the primary pattern seen is called peripheral funisitis. Necrotizing funisitis is a very severe form of funisitis that occurs when the funisitis has been present for a long time. Debris from inflammatory cells accumulate and the umbilical cord becomes calcified. Treatment with IV antibiotics is necessary for necrotizing funisitis, with a minimum of 7 days. This can occur in healthy born infants; the infection can also occurs in the days and weeks following birth. With IV antibiotic treatment and early management, outcomes are good 8.
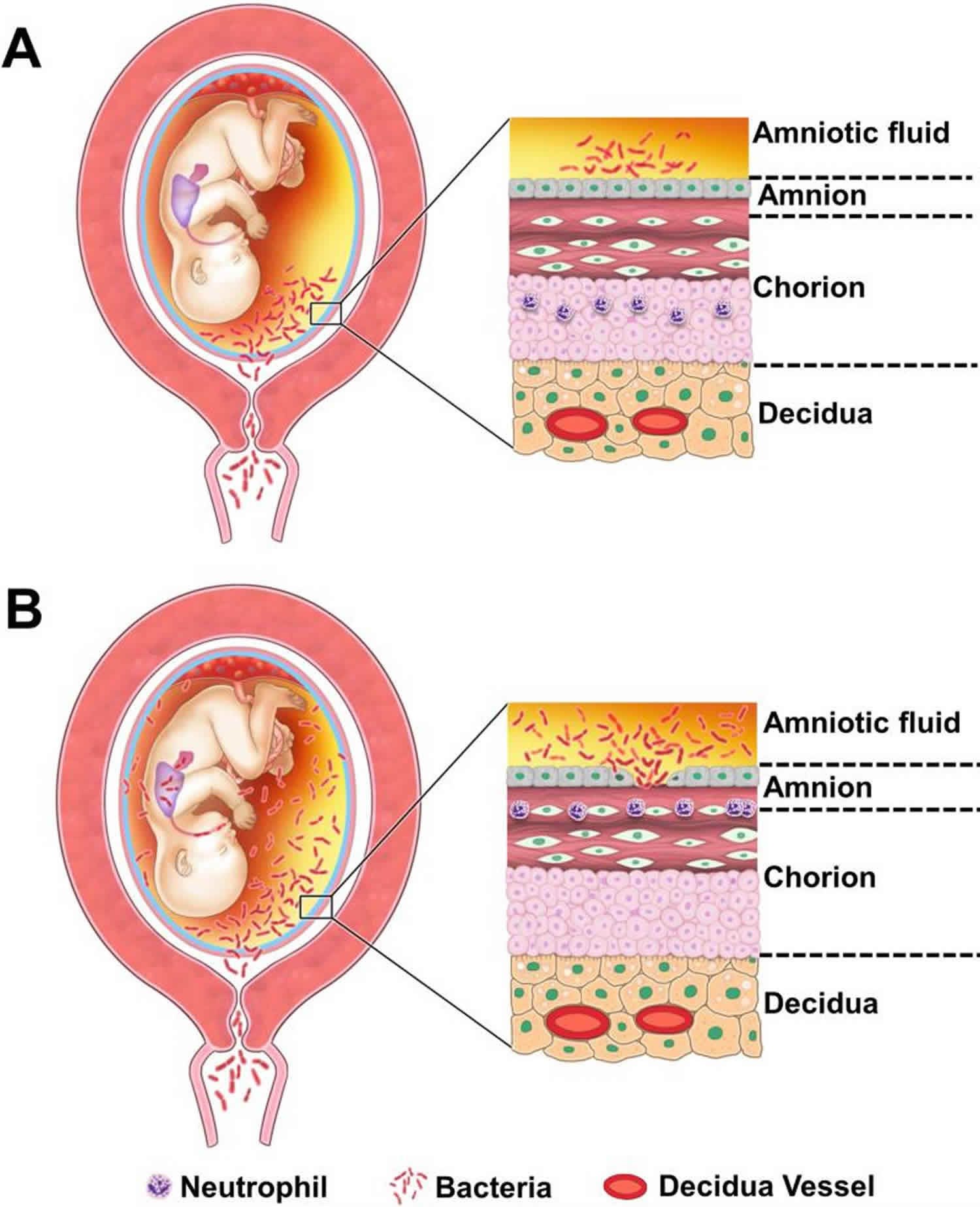
Systematic studies of the umbilical cord suggest that acute funisitis begins as multiple, discrete foci, along the umbilical cord, which then merge with the progression of the inflammatory process 9. Figure 2 illustrates the topography of the inflammatory process in several umbilical cords serially sectioned at 1 mm intervals. The chemotactic gradient attracting neutrophils from the lumen of the umbilical vessels into the Wharton’s jelly is thought to be dependent on elevated concentrations of chemokines in the amniotic fluid. The severity of funisitis correlates with fetal plasma IL-6 concentrations (an indicator of the severity of the systemic fetal inflammatory response) and amniotic fluid IL-6 – the latter reflects the intensity of the intra-amniotic inflammatory response 10.
Typically, acute funisitis begins as inflammation of the umbilical vein (umbilical phlebitis), followed by umbilical arteritis involving theumbilical arteries (Figure 2). Progression of inflammation along the length of the umbilical cord. The initial phase is multifocal, as demonstrated by the yellow/orange rings in the second umbilical cord from left to right (Figure 2). Subsequently,the areas of inflammation coalesce, and funisitis affects the entire umbilical cord.
In an attempt to define the stages of the spectrum of umbilical cord infection, some experts have separated the infection into distinct categories: category 1, funisitis and umbilical discharge (shaggy unhealthy umbilical stump, malodorous or purulent discharge); category 2, omphalitis with abdominal wall cellulitis (periumbilical erythema and superficial tenderness in addition to findings in category 1); category 3, omphalitis with systemic sepsis; and category 4, omphalitis with fasciitis (umbilical necrosis with extensive local disease, periumbilical ecchymosis, crepitus bullae, and evidence of involvement of superficial and deep fascia)
The presence of funisitis is significantly associated with interventional delivery and other adverse outcomes including an Apgar score <7 at 1 min, clinical evidence of sepsis and admission to the neonatal intensive care unit 11.
Figure 1. Anatomy of the pregnant uterus with an emphasis on the placenta
Footnote: The upper part of the figure illustrates the fetus, umbilical cord and placenta. The chorioamniotic membranes include the amnion and chorion. Decidua is of maternal origin (secretory endometrium) and is adjacent to the myometrium. The lower part of the figure represents a cross-section of the human placenta, including the chorionic plate, chorioamniotic membranes, umbilical cord, and the intervillous space. The basal plate of the placenta is formed of decidua, and is traversed by the spiral arteries, which bring maternal blood into the intervillous space. The villous circulation (fetal) is illustrated in a cross-section of the stem villi. The fetal vessels on the surface of the chorionic plate include arteries and veins, which coalesce to form the umbilical vein and umbilical arteries.
[Source 4 ]Figure 2. Inflammatory process in the umbilical cord
Footnote: Topography of the inflammatory process in the umbilical cord. (A) Typically, acute funisitis begins as inflammation of the umbilical vein (umbilical phlebitis; the blue vessel represents the umbilical vein), followed by umbilical arteritis involving the umbilical arteries (red). (B) Progression of inflammation along the length of the umbilical cord. The initial phase is multi-focal, as demonstrated by the yellow/orange rings in the second umbilical cord from left to right in figure 3B. Subsequently, the areas of inflammation coalesce, and funisitis affects the entire umbilical cord.
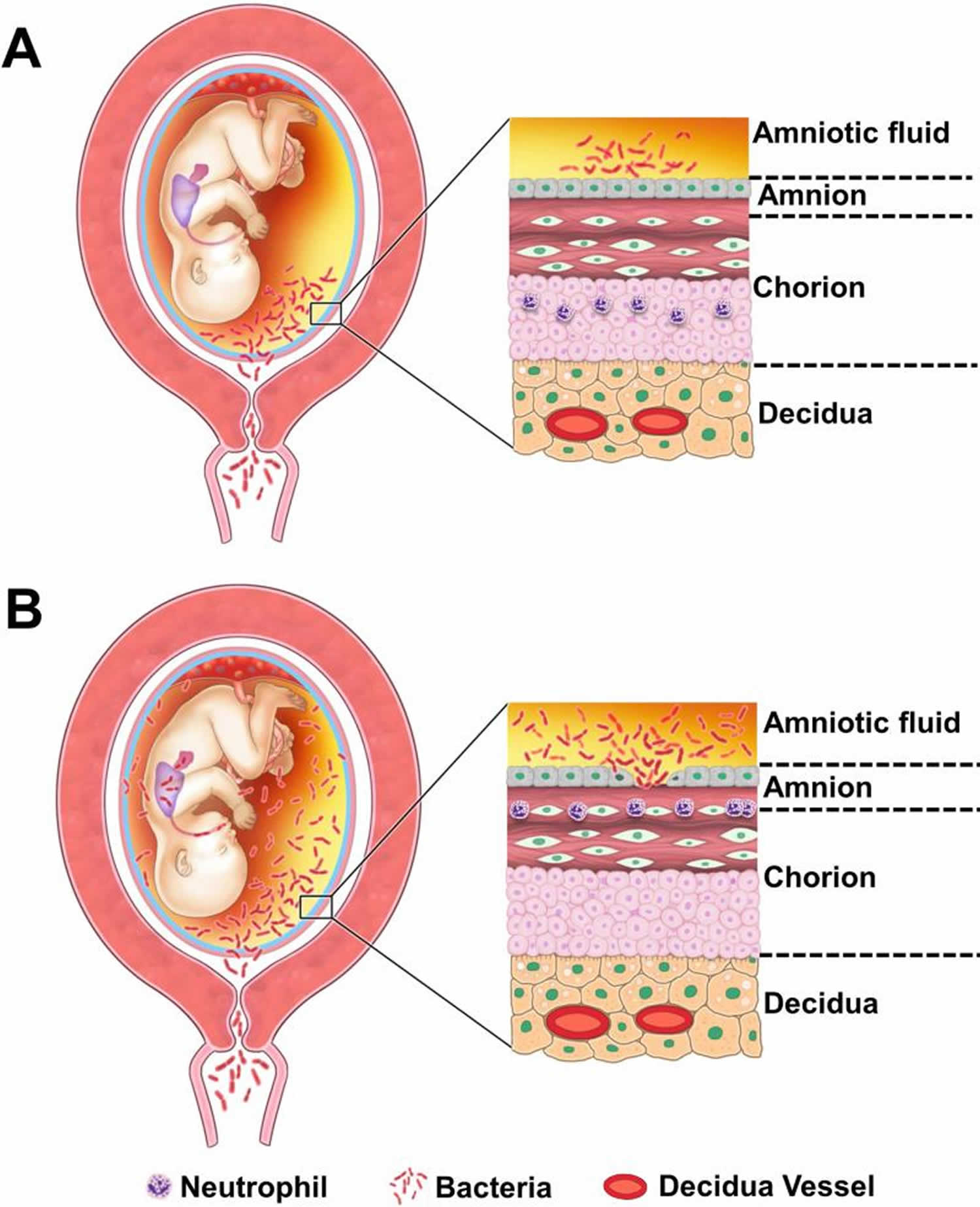
[Source 4 ]Figure 3. Pathways of intra-amniotic infection
Footnote: (A) Most cases of microbial invasion of the amniotic cavity are the result of ascending infection from the vagina and cervix. (B) Extensive microbial invasion of the amniotic cavity can result in fetal infection (bacteria are located in the fetal lung) and damaged chorioamniotic membranes (i.e. necrotizing chorioamnionitis). The destruction of the amnion epithelium is a cardinal feature of necrotizing chorioamnionitis.
[Source 4 ]Funisitis causes
Funisitis is the migration of fetal neutrophils out of the bloodstream and into the umbilical cord. This process of migration initiates with the release of neutrophil chemokines, such as interleukin-8 and granulocyte chemotactic protein. Funisitis is most commonly present in the setting of intraamniotic infection, specifically chorioamnionitis, and is part of the fetal inflammatory response syndrome (FIRS), which indicates a high risk of preterm labor and increased neonatal morbidity. This process is identified microscopically after delivery, but due to the need for mature neutrophils within fetal blood, it is not typically present before 20 weeks of gestation 12.
Under normal conditions, the amniotic cavity is sterile for microorganisms using cultivation 13 and molecular microbiologic techniques, based on the detection of the 16S rRNA gene (present in all bacteria, but not in mammalian cells). Four pathways have been proposed whereby microorganisms reach the amniotic cavity 14:
- Ascending from the lower genital tract 15;
- Hematogenous 16;
- Accidental introduction at the time of amniocentesis, percutaneous umbilical cord blood sampling, fetoscopy, or another invasive procedure 17;
- Retrograde seeding from the peritoneal cavity from the fallopian tubes 18. However, there is limited evidence in support of the latter pathway.
Ascending microbial invasion from the lower genital tract appears to be the most frequent pathway for intra-amniotic infection 19. While all pregnant women have microorganisms in the lower genital tract, most do not have intra-amniotic infection. The mucus plug represents an anatomical and functional barrier to ascending infection during pregnancy 20. In the non-pregnant state, the endometrial cavity is not sterile 21, but the decidua is thought to be sterile during pregnancy.
A hematogenous pathway can operate during the course of blood-born maternal infections 16. Microorganisms such as Listeria monocytogenes 22, Treponema pallidum, Yersinia pestis, Cytomegalovirus, Plasmodium species, and others can gain access through the maternal circulation to the intervillous space, from where they invade the villi and the fetal circulation 19. Bacteria involved in periodontal disease may use this pathway to reach the amniotic cavity 23.
Intra-amniotic infection has been documented in patients with preterm labor with intact membranes 24, prelabor rupture of membranes 25, cervical insufficiency 26, an asymptomatic short cervix 27, idiopathic vaginal bleeding 28, placenta previa 29 and clinical chorioamnionitis at term 30. Rupture of membranes is not necessary for bacteria to reach the amniotic cavity – indeed, there is experimental evidence that bacteria can cross intact membranes 31. Most of these infections are subclinical in nature, and therefore, they occur in the absence of clinical chorioamnionitis 32. Hence, most of these infections are undetected unless the amniotic fluid is analyzed. The most frequent microorganisms found in the amniotic cavity are genital mycoplasmas 33 and in particular, Ureaplasma species 34, Gardnerella vaginalis 35, Fusobacteria species, etc. 36. Fungi can also be found – women who became pregnant with intrauterine contraceptive devices are at high risk for intra-amniotic infection with Candida albicans 37. Polymicrobial invasion of the amniotic cavity is present in approximately 30% of cases 38. Table 1 describes the frequency of microbial invasion of the amniotic cavity in different obstetrical syndromes. Table 2 demonstrates the microorganisms detected in amniotic cavity in patients with preterm labor with intact membranes 39 and clinical chorioamnionitis at term 30.
The frequency of microbial invasion of the amniotic cavity is similar in patients with spontaneous labor at term and those with preterm labor and intact membranes who subsequently deliver a preterm neonate (17% vs. 22%, respectively) 40. Yet, preterm neonates born to mothers with microbial invasion of the amniotic cavity have a higher frequency of neonatal sepsis, a systemic inflammatory response (defined as an elevated umbilical cord IL-6 concentration), and funisitis than those born to mothers at term with microbial invasion of the amniotic cavity. Why? Microbial invasion of the amniotic cavity in women in spontaneous labor at term is of shorter duration and can occur after the initiation of parturition 41. For example, bacteria can be introduced when the chorioamniotic membranes are exposed to the vaginal microbiota during the course of digital examinations performed during labor to determine cervical dilatation and effacement. Such microbial invasion typically has a low inoculum size which elicits a mild intra-amniotic inflammatory response and rarely leads to fetal microbial invasion (hence, the low frequency of funisitis and neonatal sepsis).
On the other hand, in preterm labor with intact membranes or preterm PROM, microbial invasion is established before the initiation of preterm labor. Such infections have a higher microbial burden than those observed in most women in spontaneous labor at term, have probably lasted longer, and therefore, result in a more intensive intra-amniotic inflammatory response 41. Given the longer duration of infection, the likelihood of a fetal attack is higher, and thus, not surprisingly, the rate of congenital neonatal sepsis is greater in preterm than in term neonates (2.27-5.14/1000 in preterm neonates versus 0.04-0.89/1000 term neonates) 42.
Recently, scientists reported a new type of intra-amniotic inflammation termed “sterile inflammation”, which is more frequent than intra-amniotic infection (microbial-associated intra-amniotic inflammation) in patients with preterm labor with intact membranes 43, preterm PROM 44 and an asymptomatic short cervix 45. Interestingly, sterile intra-amniotic inflammation is associated with acute histologic chorioamnionitis (40-60% of cases) 30. Moreover, acute inflammatory lesions of the placenta are present in a small subset of patients without intraamniotic inflammation in the context of preterm labor 46, preterm PROM 44, short cervix 47 and clinical chorioamnionitis 30. Potential explanations are: 1) inflammation of chorioamniotic membranes is a non-specific mechanism of host defense against “danger signals” of non-microbial origin; 2) extra-amniotic infection, which is probably rare; 3) non-viable microorganisms which may release chemotactic factors leading to inflammation 15. These organisms may have invaded the amniotic cavity and been cleared by the immune system.
The observation that acute histologic chorioamnionitis is present without demonstrable intra-amniotic infection is now well-established 30. Roberts et al 48 reported, using both cultivation and molecular microbiologic techniques, that only 4% of patients with acute histologic chorioamnionitis at term have microorganisms in the placenta. Therefore, acute histologic chorioamnionitis should not be considered synonymous with amniotic fluid infection. The characterization of any biological fluid as “sterile” is dependent on the sensitivity of the assays used to detect microorganisms. Cultivation can be very sensitive, and even one microorganism can grow into a colony under optimal conditions; however, such conditions are rarely provided in clinical laboratories. Molecular microbiologic techniques are considered more sensitive; yet, sufficient microbial DNA must be present for this methodology to provide a positive result. PCR assays with specific primers for a microorganism are considered superior to broad range PCR assays based on conserved regions of the bacterial genome (e.g. 16S gene). The use of deep sequencing can change what is known about the microbiologic landscape of biological fluids. Extreme caution must be used when interpreting the results of sequencing studies, as contamination during metagenomics can occur.
Table 1. Frequency of chorioamnionitis according to gestational age at delivery
| Weeks of gestation | Chorioamnionitis (n) | Total number of patients | Percent (%) |
|---|---|---|---|
| 21–24 | 17 | 18 | 94.4 |
| 25–28 | 19 | 48 | 39.6 |
| 29–32 | 34 | 96 | 35.4 |
| 33–36 | 53 | 497 | 10.7 |
| 37–40 | 233 | 6139 | 3.8 |
| 41–44 | 36 | 707 | 5.1 |
| Total | 392 | 7505 | 5.2 |
Table 2. Microorganisms detected in the amniotic fluid of patients with spontaneous preterm labor with intact membranes and patients with clinical chorioamnionitis at term using cultivation and molecular microbiologic technique
| Patients with spontaneous preterm labor with intact membranes 39 | Patients with clinical chorioamnionitis at term 30 |
|---|---|
| Fusobacterium nucleatum | Ureaplasma species |
| Sneathia sanguinegens | Gardnerella vaginalis |
| Ureaplasma species | Mycoplasma hominis |
| Streptococcus mitis | Streptococcus agalactiae |
| Gardnerella vaginalis | Lactobacillus species |
| Peptostreptococcus species | Bacteroides species |
| Leptotrichia amnionii | Acinetobacter species |
| Mycoplasma hominis | Sneathia |
| Streptococcus agalactiae | Streptococcus viridans |
| Lactobacillus species | Porphyromonas species |
| Bacillus species | Veillonella species |
| Coagulase-negative Staphylococcus species | Peptostreptococcus species |
| Prevotella species | Escherichia coli |
| Others: Uncultivated Bacteroidetes, Delftia acidovorans, Neisseria cinerea | Pseudomonas aeruginosa |
| Staphylococcus aureus | |
| Eubacterium species | |
| Gram (–) bacilli | |
| Enterococcus species | |
| Others: Fusobacterium species, Candida species, Abiotrophia defective, Micrococcus luteus, Staphylococcus epidermidis, Firmicute, Propionibacterium acnes | |
Funisitis diagnosis
Presence of any neutrophilic infiltrate involving the umbilical vein, umbilical arteries, cord substance (Wharton jelly) or peripheral umbilical cord. Qualifying the location and degree of inflammation is encouraged over the generic use of the term “funisitis,” historically used to inadequately describe the presence of any inflammation in any location.
Funisitis treatment
The primary management of funisitis is antibiotic therapy, particularly if accompanying symptoms of choriamnionitis are present. The most common antibiotics used are ampicillin and gentamicin. Alternative antibiotics include clindamycin, cefazolin, and vancomycin in women allergic to penicillin. After delivery, the current recommendation is to administer one additional dose with a cesarean section but no additional antibiotics for vaginal deliveries. Additional broad-spectrum antibiotics may be required, depending on the clinical status 50.
Funisitis prognosis
Severe inflammation has been linked to periventricular leukomalacia 51. More severe inflammatory response seen when funisitis is present in preterm infants, possibly accounting for some differences in gestational age related morbidity 52
References- Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11(1):18–25.
- Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21(1):50-60.
- Jessop FA, Lees CC, Pathak S, Hook CE, Sebire NJ. Funisitis is associated with adverse neonatal outcome in low-risk unselected deliveries at or near term. Virchows Arch. (2016) 468:503–7. doi: 10.1007/s00428-015-1899-0
- Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29-S52. doi:10.1016/j.ajog.2015.08.040 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4774647
- Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6(5):435–48.
- Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11(1):18–25
- Naeye RL. Effects of maternal cigarette smoking on the fetus and placenta. Br J ObstetGynaecol. (1978) 85:732–7. 10.1111/j.1471-0528.1978.tb15593.x
- Care of the umbilicus and management of umbilical disorders. https://www.uptodate.com/contents/care-of-the-umbilicus-and-management-of-umbilical-disorders?search=care-of-the-umbilicus-and-management-of-umbilical-disorders1~47%26usage_type%3Ddefault%26display_rank%3D1&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Kim CJ, Yoon BH, Kim M, Park JO, Cho SY, Chi JG. Histo-topographic distribution of acute inflammation of the human umbilical cord. Pathol Int. 2001;51(11):861–5.
- Kim CJ, Yoon BH, Romero R, Moon JB, Kim M, Park SS, et al. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. Am J Obstet Gynecol. 2001;185(2):496–500.
- Jessop FA, Lees CC, Pathak S, Hook CE, Sebire NJ. Funisitis is associated with adverse neonatal outcome in low-risk unselected deliveries at or near term. Virchows Arch. 2016;468(4):503-507. doi:10.1007/s00428-015-1899-0 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4830890
- Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015 Oct;213(4 Suppl):S29-52.
- Harris JW, Brown H. Bacterial content of the uterus at cesarean section. Am J Obstet Gynecol. 1927;(13):133.
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–5.
- Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166(5):1382–8.
- Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect Immun. 1999;67(11):5958–66.
- Hamoda H, Chamberlain PF. Clostridium welchii infection following amniocentesis: a case report and review of the literature. Prenat Diagn. 2002;22(9):783–5.
- Benirschke K. Routes and types of infection in the fetus and the newborn. AMA J Dis Child. 1960;99:714–21.
- Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31(3):553–84.
- Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol. 2001;185(3):586–92.
- Mitchell CM, Haick A, Nkwopara E, Garcia R, Rendi M, Agnew K, et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol. 2015;212(5):611, e1–9
- Mazor M, Froimovich M, Lazer S, Maymon E, Glezerman M. Listeria monocytogenes. The role of transabdominal amniocentesis in febrile patients with preterm labor. Arch Gynecol Obstet. 1992;252(2):109–12.
- Leon R, Silva N, Ovalle A, Chaparro A, Ahumada A, Gajardo M, et al. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2007;78(7):1249–55.
- Combs CA, Garite TJ, Lapidus JA, Lapointe JP, Gravett M, Rael J, et al. Detection of microbial invasion of the amniotic cavity by analysis of cervicovaginal proteins in women with preterm labor and intact membranes. Am J Obstet Gynecol. 2015;212(4):482, e1–e12.
- Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2015:1–8.
- Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38(3):261–8.
- Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, et al. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202(5):433, e1–8.
- Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18(1):31–7.
- Madan I, Romero R, Kusanovic JP, Mittal P, Chaiworapongsa T, Dong Z, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010;38(3):275–9.
- Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43(1):19–36.
- Galask RP, Varner MW, Petzold CR, Wilbur SL. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol. 1984;148(7):915–28.
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39.
- Allen-Daniels MJ, Serrano MG, Pflugner LP, Fettweis JM, Prestosa MA, Koparde VN, et al. Identification of a gene in Mycoplasma hominis associated with preterm birth and microbial burden in intraamniotic infection. Am J Obstet Gynecol. 2015
- Jacobsson B, Aaltonen R, Rantakokko-Jalava K, Morken NH, Alanen A. Quantification of Ureaplasma urealyticum DNA in the amniotic fluid from patients in PTL and pPROM and its relation to inflammatory cytokine levels. Acta Obstet Gynecol Scand. 2009;88(1):63–70.
- Hillier SL, Krohn MA, Cassen E, Easterling TR, Rabe LK, Eschenbach DA. The role of bacterial vaginosis and vaginal bacteria in amniotic fluid infection in women in preterm labor with intact fetal membranes. Clin Infect Dis. 1995;20(Suppl 2):S276–8.
- Digiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64(1):38–57.
- Kim SK, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Mazaki-Tovi S, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD). J Perinat Med. 2010;38(1):45–53.
- Jalava J, Mantymaa ML, Ekblad U, Toivanen P, Skurnik M, Lassila O, et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol. 1996;103(7):664–9.
- Digiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056
- Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38(7):543–8.
- Yoon BH, Romero R, Moon J, Chaiworapongsa T, Espinoza J, Kim YM, et al. Differences in the fetal interleukin-6 response to microbial invasion of the amniotic cavity between term and preterm gestation. J Matern Fetal Neonatal Med. 2003;13(1):32–8.
- Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1):21–47.
- Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72(5):458–74.
- Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2014:1–16.
- Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014:1–17.
- Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71(4):330–58.
- Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014:1–17.
- Roberts DJ, Celi AC, Riley LE, Onderdonk AB, Boyd TK, Johnson LC, et al. Acute histologic chorioamnionitis at term: nearly always noninfectious. PLoS One. 2012;7(3):e31819
- Russell P, Inflammatory lesions of the human placenta I, The American journal of diagnostic gynecology and obstetrics 1979; 1: 127-137
- Committee on Obstetric Practice. Committee Opinion No. 712: Intrapartum Management of Intraamniotic Infection. Obstet Gynecol. 2017 Aug;130(2):e95-e101.
- Wharton KN, Pinar H, Stonestreet BS, et al. Severe umbilical cord inflammation-a predictor of periventricular leukomalacia in very low birth weight infants. Early Hum Dev. 2004;77(1-2):77-87. doi:10.1016/j.earlhumdev.2004.02.001
- Kim CJ, Yoon BH, Park SS, Kim MH, Chi JG. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol. 2001;32(6):623-629. doi:10.1053/hupa.2001.24992